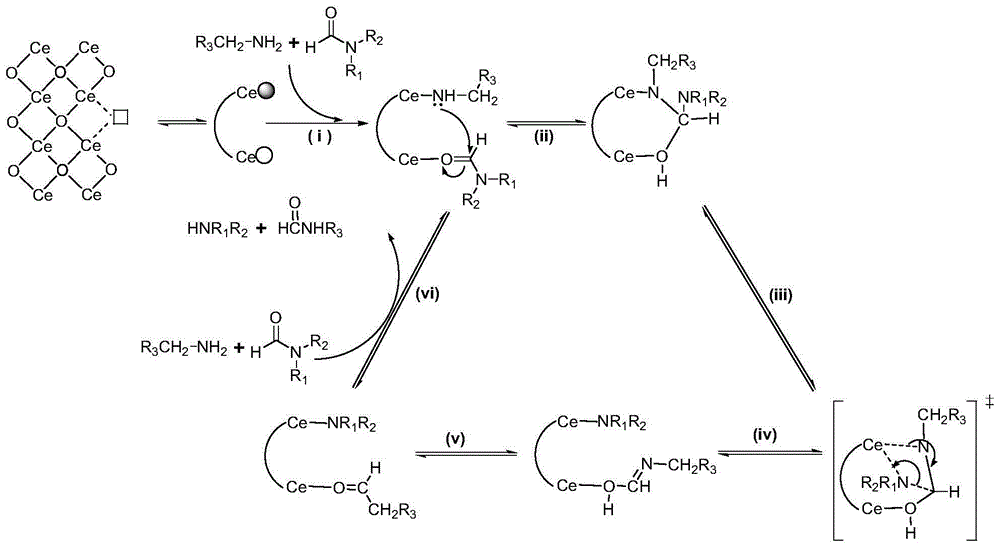
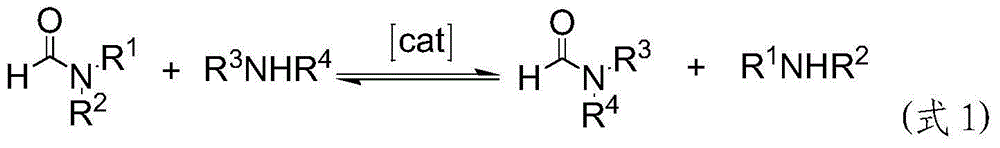Preparation method of methanamide
A formamide and secondary amine technology, applied in the field of formamide preparation, can solve the problems of environmental pollution, complex catalyst preparation process, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
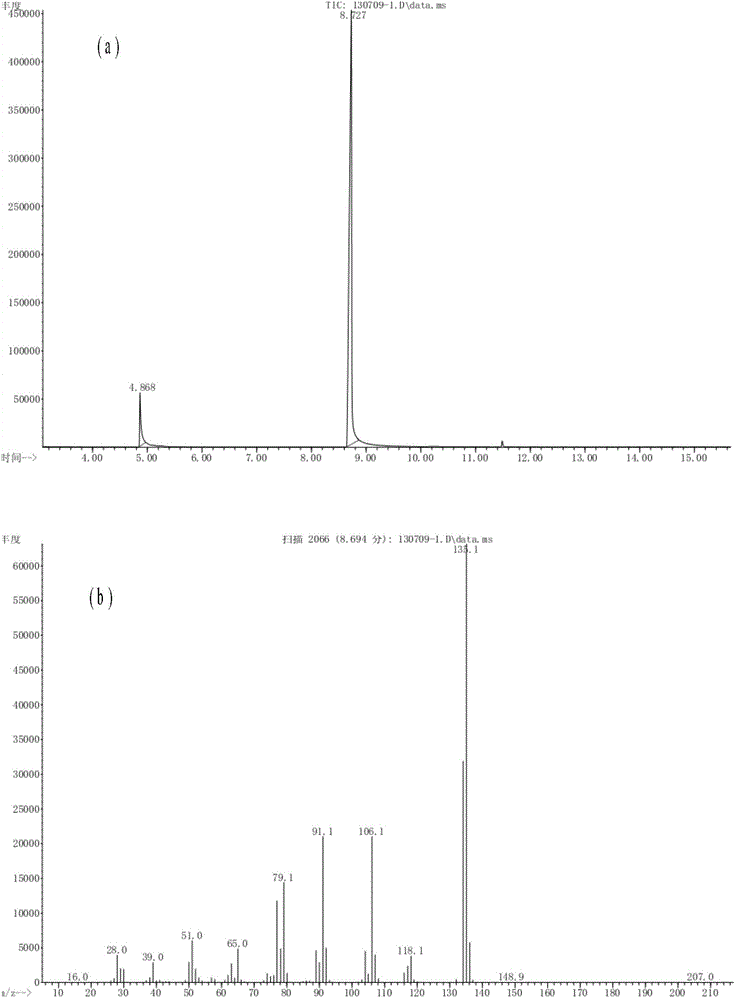
[0028] CeO obtained by roasting method 2 The process is as follows: directly roast cerium ammonium nitrate at 650°C for 2 hours, and the obtained CeO 2 , denoted as CEO-1, applied to the reaction of benzylamine with N,N-dimethylformamide. In a 150ml Teflon-lined reactor, add 15mmol benzylamine and 20ml N,N-dimethylformamide respectively, weigh 1g CEO-1 to catalyze the reaction, stir the reaction at 100°C for 12h, after the reaction , the chromatographic detection product, its conversion rate and selectivity are shown in Table 1.
Embodiment 2
[0030] CeO obtained by co-precipitation method 2 The process is as follows: dissolve cerium nitrate in water, adjust the pH to 11 with ammonia water, filter and separate, dry the filter cake at 100°C overnight, and roast at 500°C for 4 hours to obtain CeO 2 Denoted as CEO-2, it is used in the hydrolysis reaction of benzylamine and N,N-dimethylformamide. In a 150ml Teflon-lined reactor, add 15mmol benzylamine and 20ml N,N-dimethylformamide respectively, weigh 1g CEO-2 to catalyze the reaction, stir the reaction at 100°C for 12h, after the reaction , the chromatographic detection product, its conversion rate and selectivity are shown in Table 1.
Embodiment 3
[0032] Dissolve cerium ammonium nitrate and polyvinylpyrrolidone in ethylene glycol, reflux at 190°C for 24 hours, filter and separate, vacuum-dry the filter cake at 80°C, and roast at 600°C to obtain CeO 2 Denoted as CEO-3, it is used in the hydrolysis reaction of benzylamine and N,N-dimethylformamide. In a 150ml Teflon-lined reactor, add 15mmol benzylamine and 20ml N,N-dimethylformamide respectively, weigh 1g CEO-3 to catalyze the reaction, stir the reaction at 100°C for 12h, after the reaction , the chromatographic detection product, its conversion rate and selectivity are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com