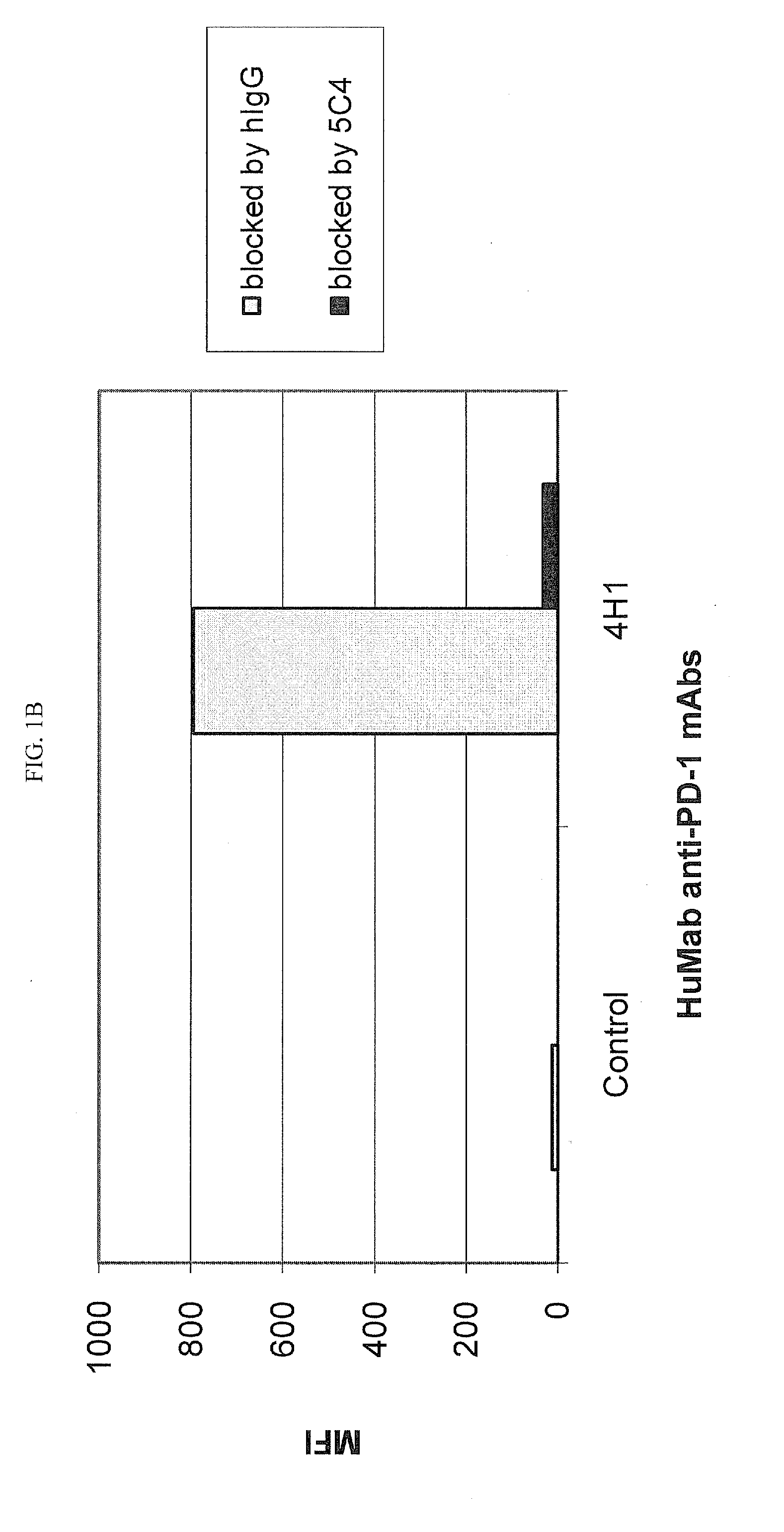
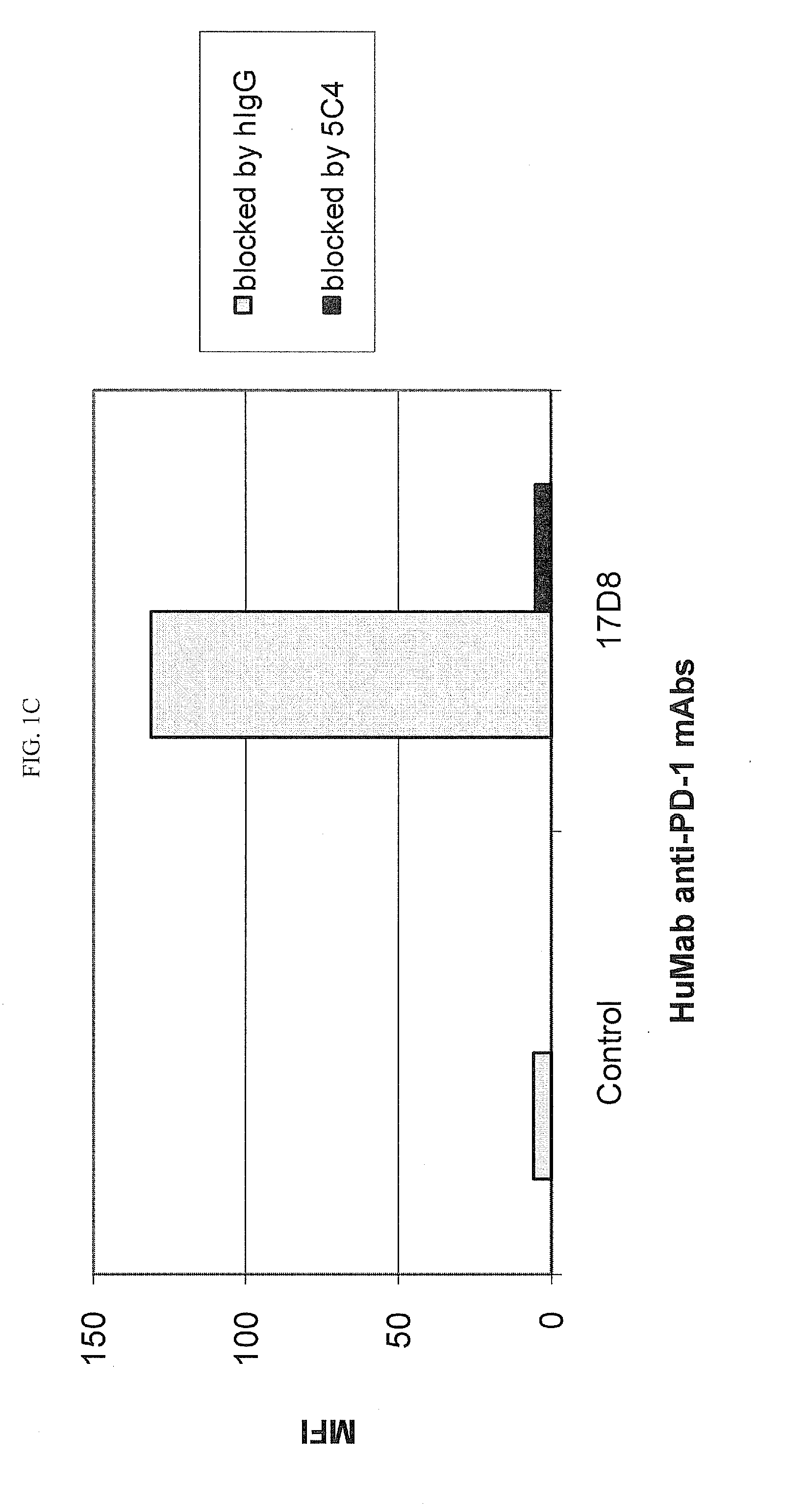
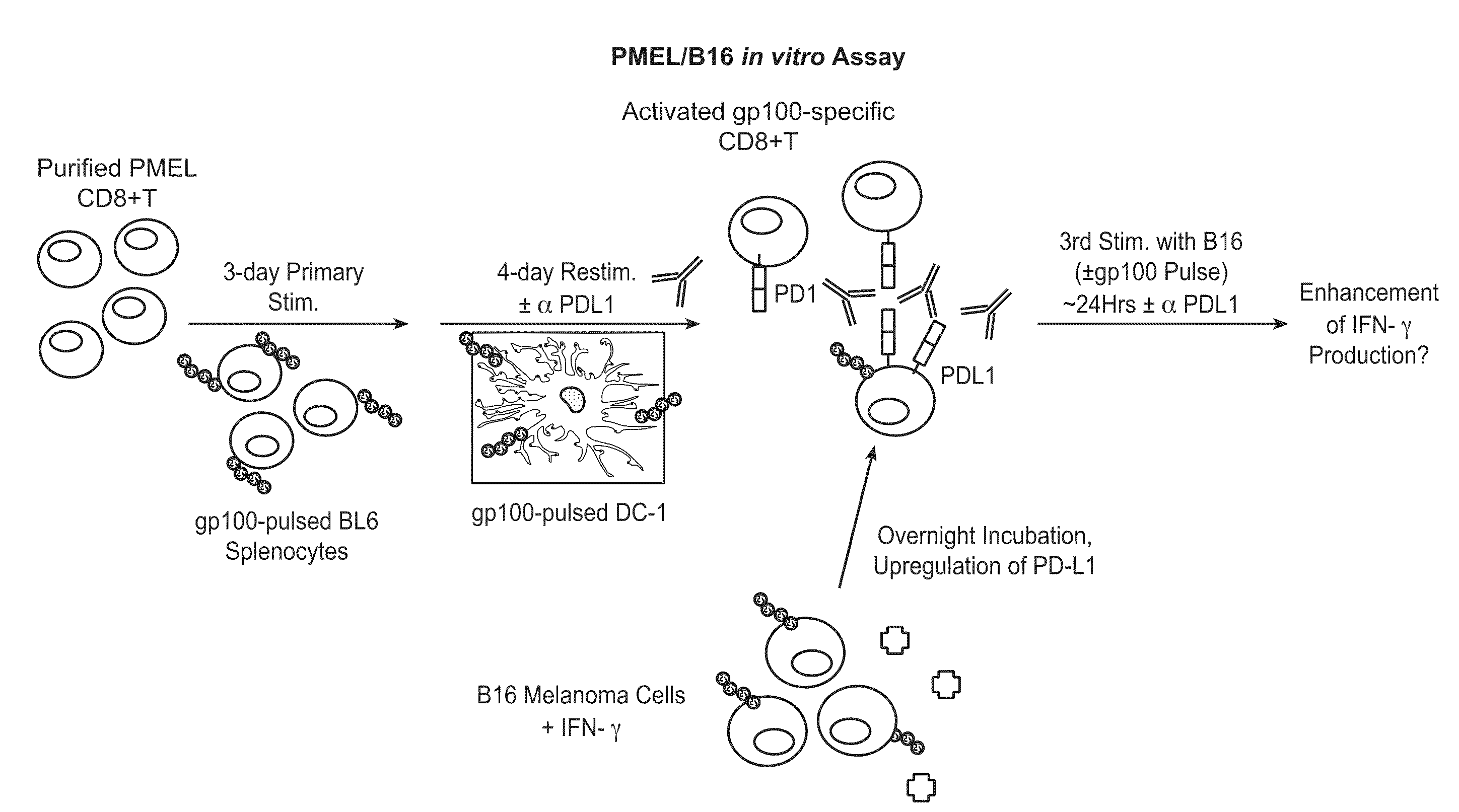
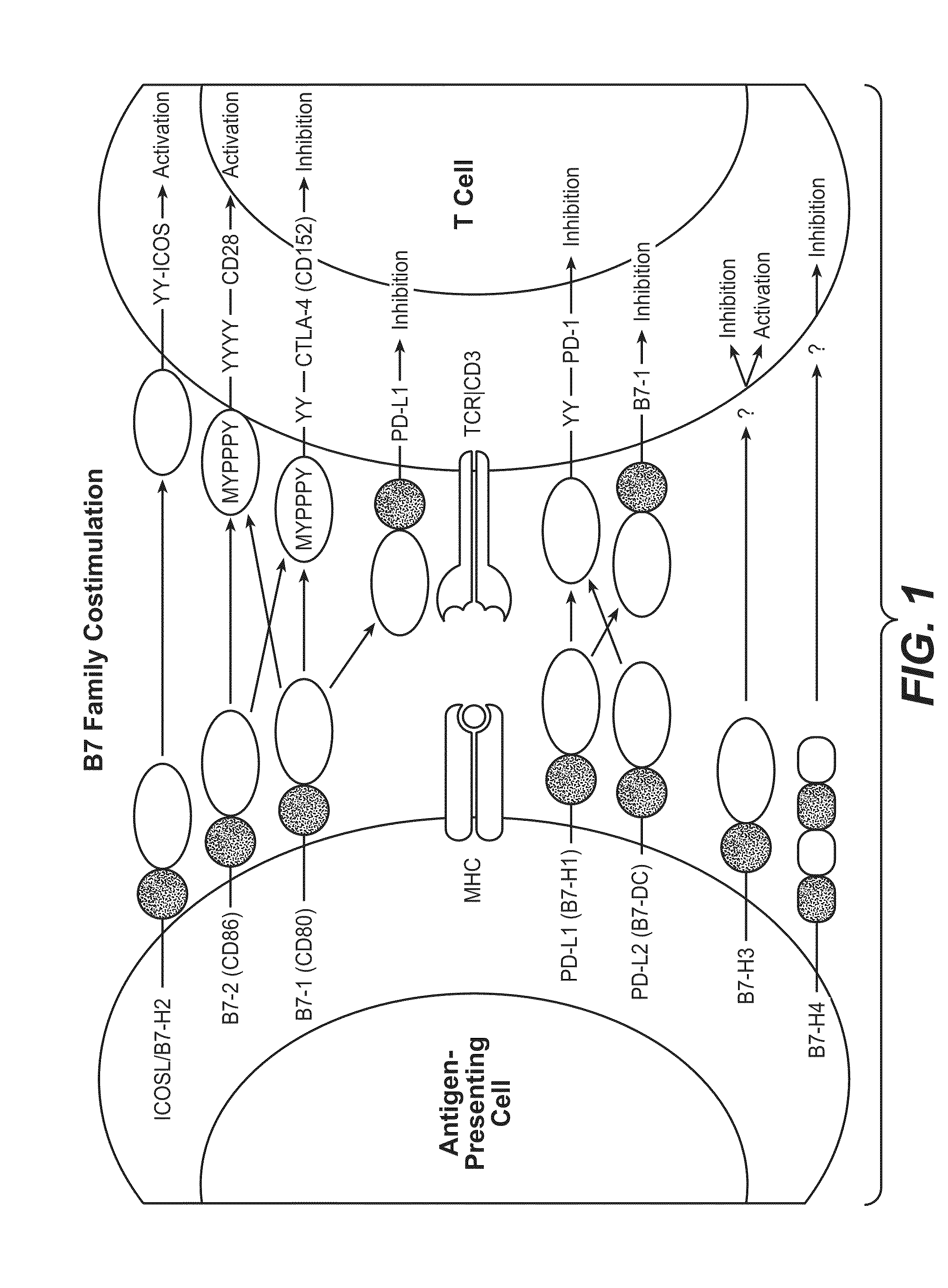
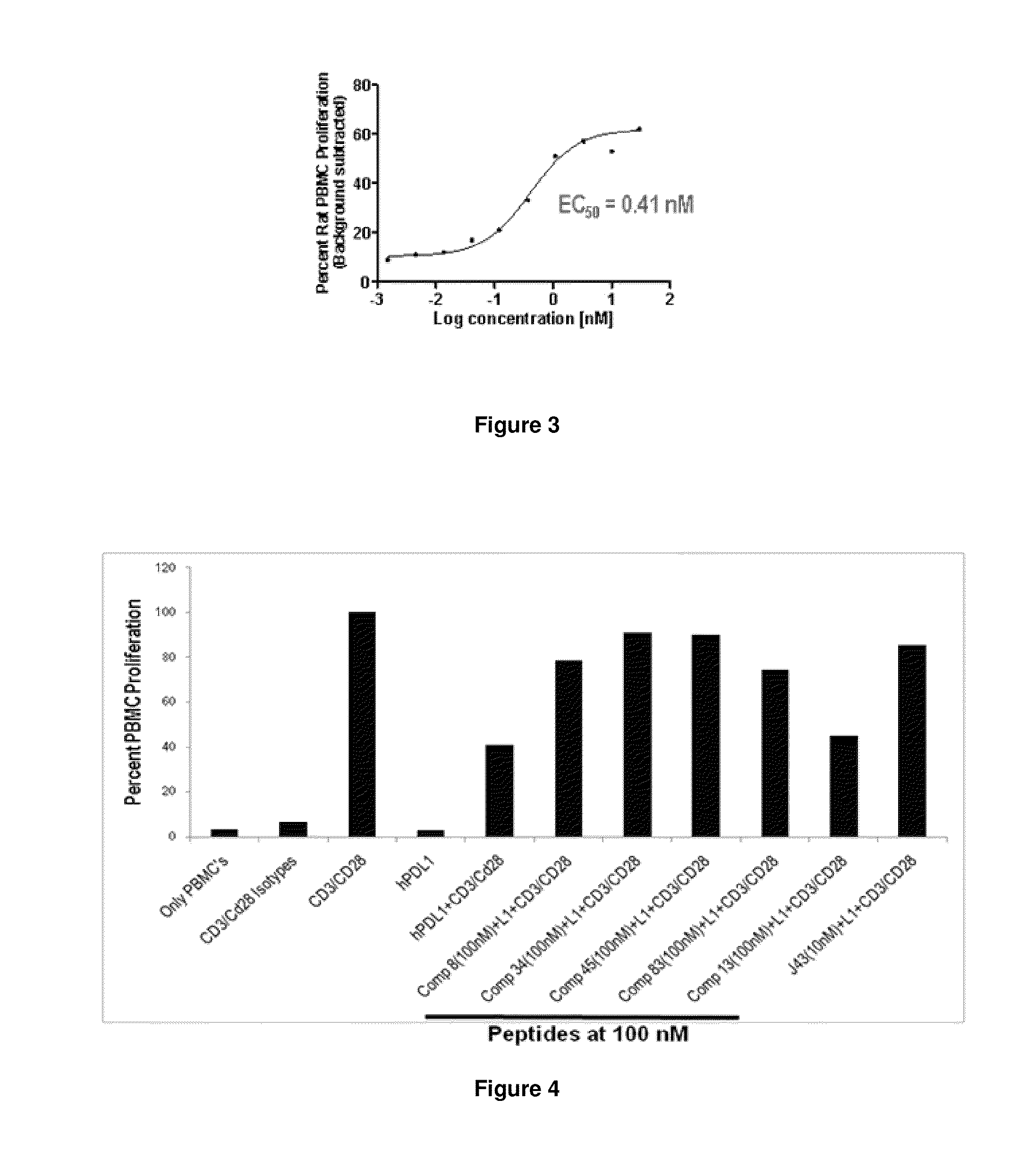
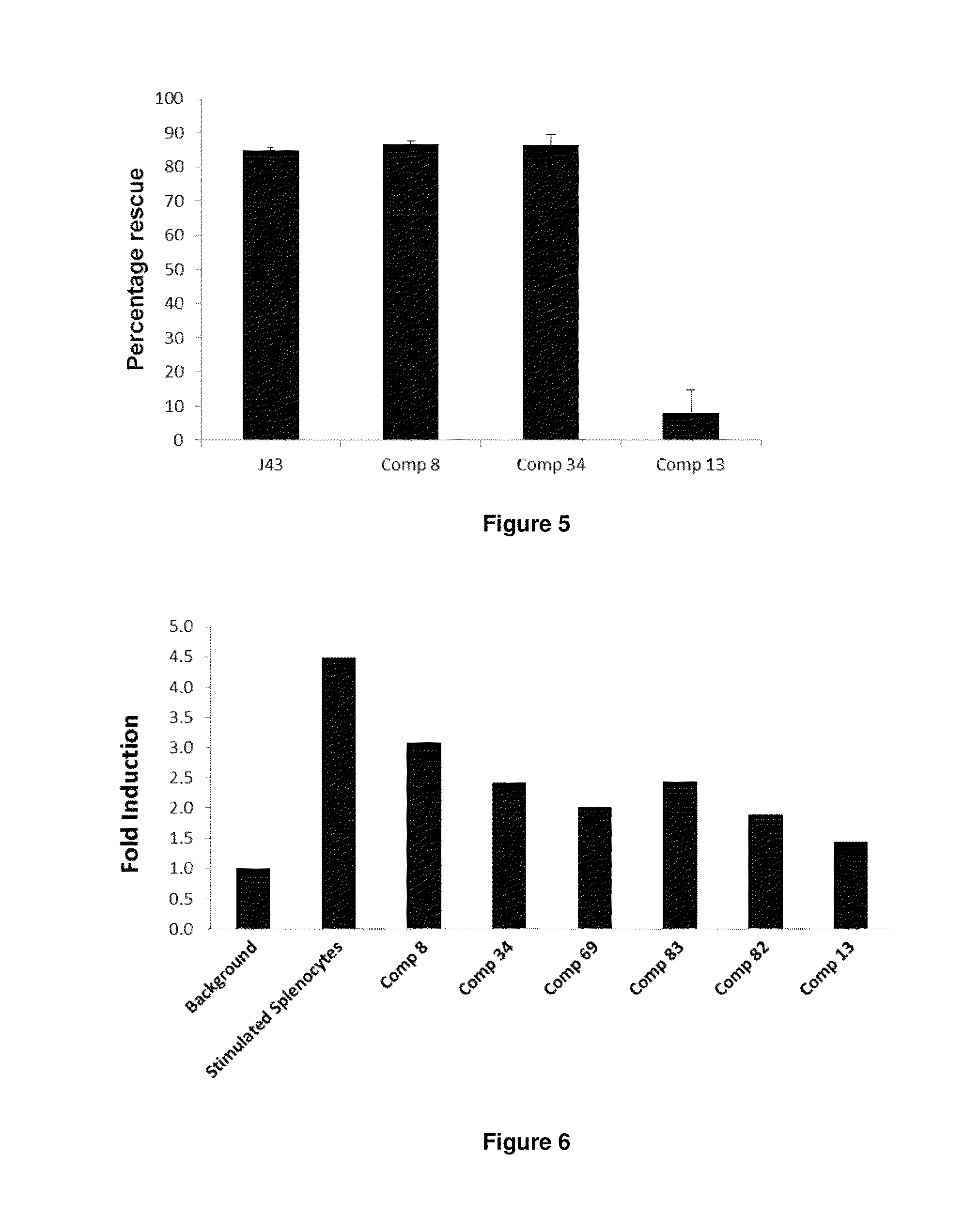
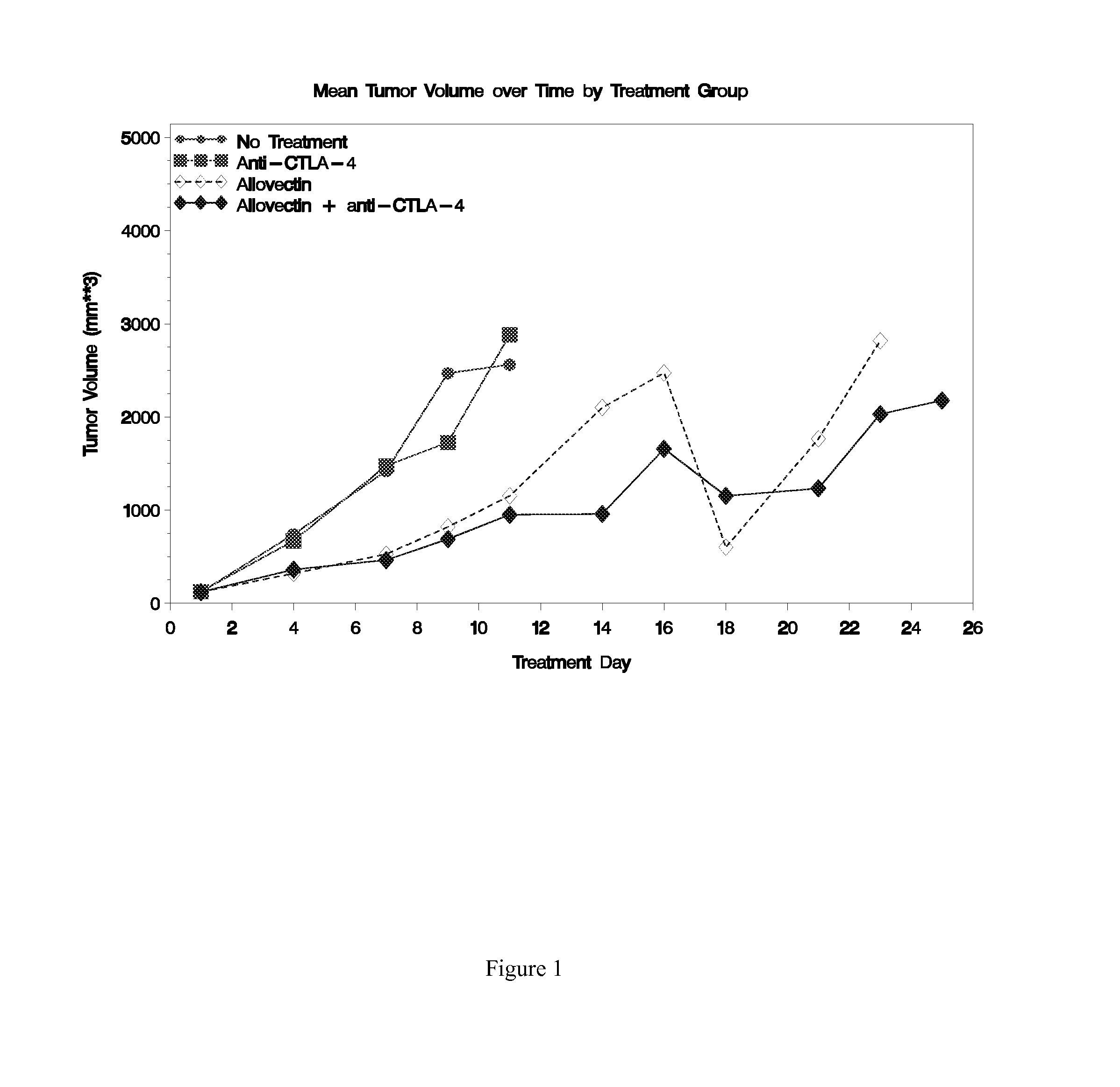
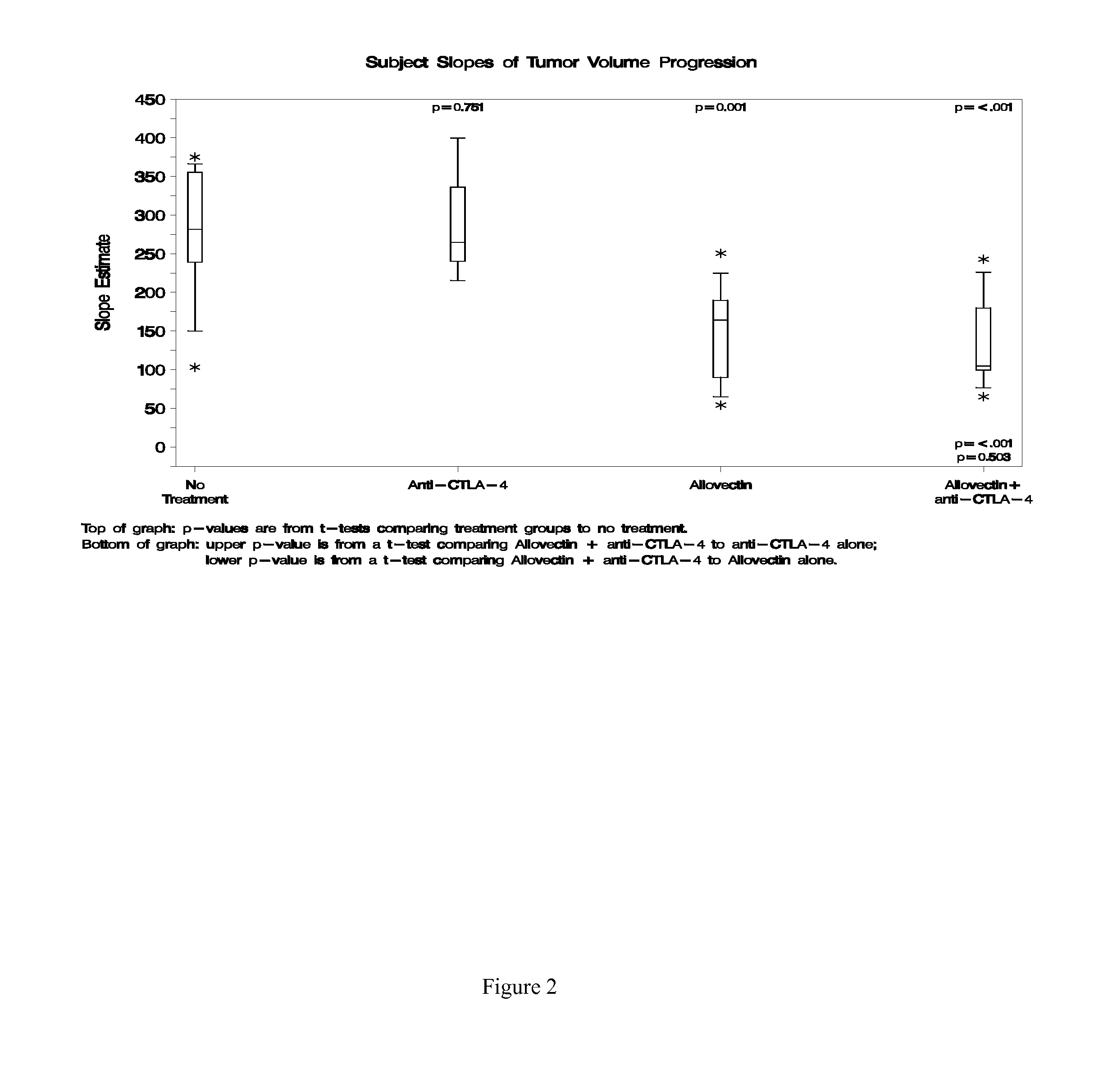
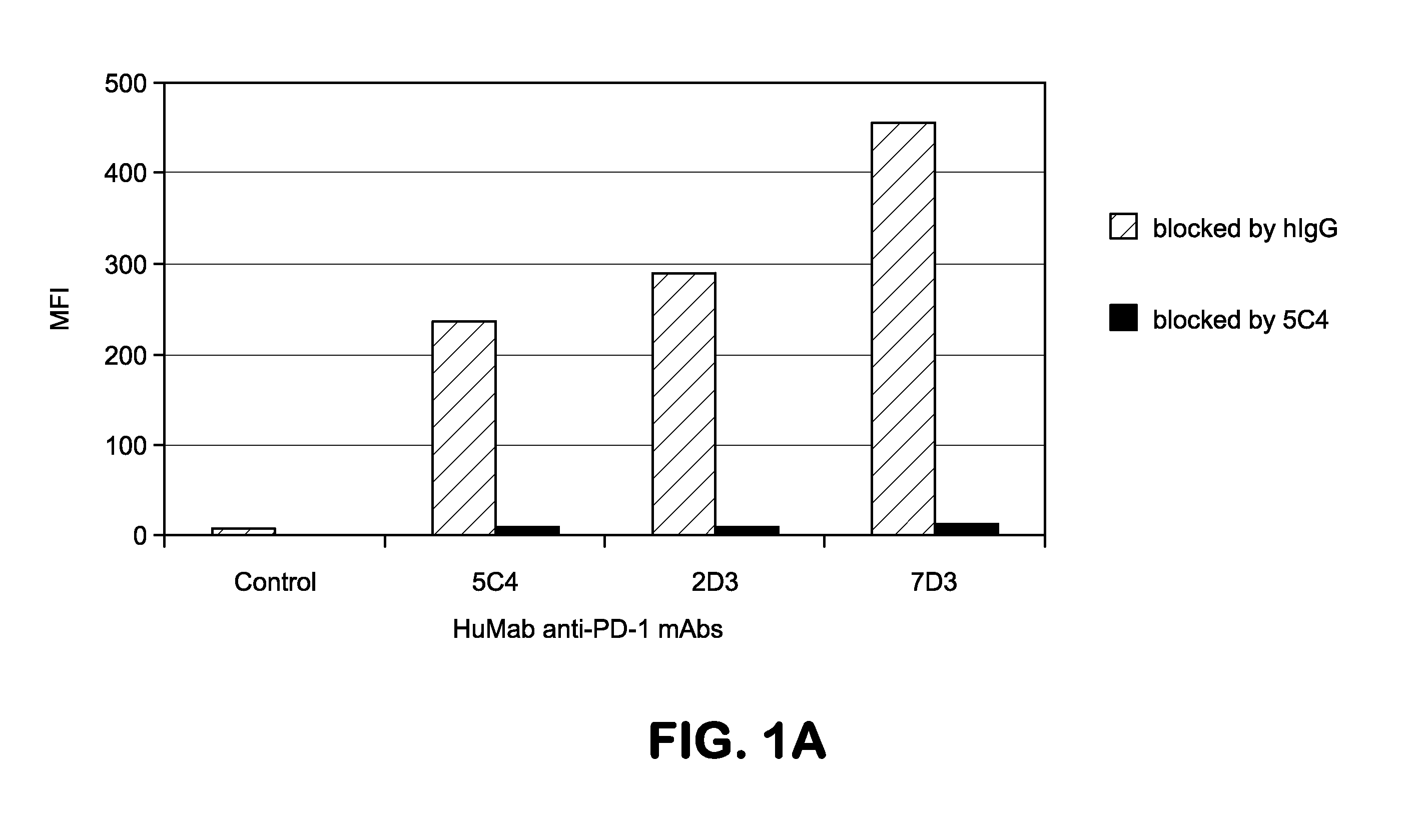
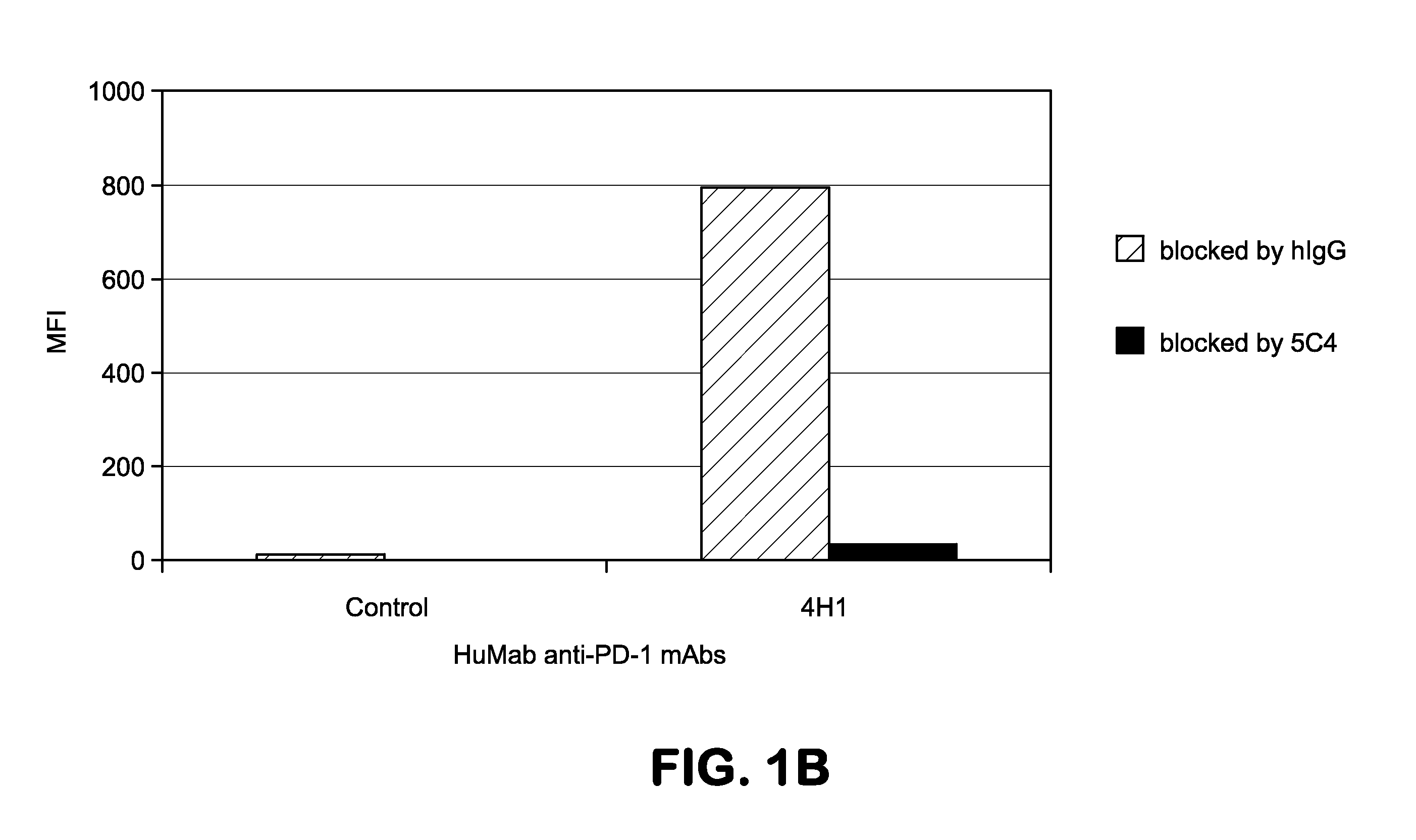
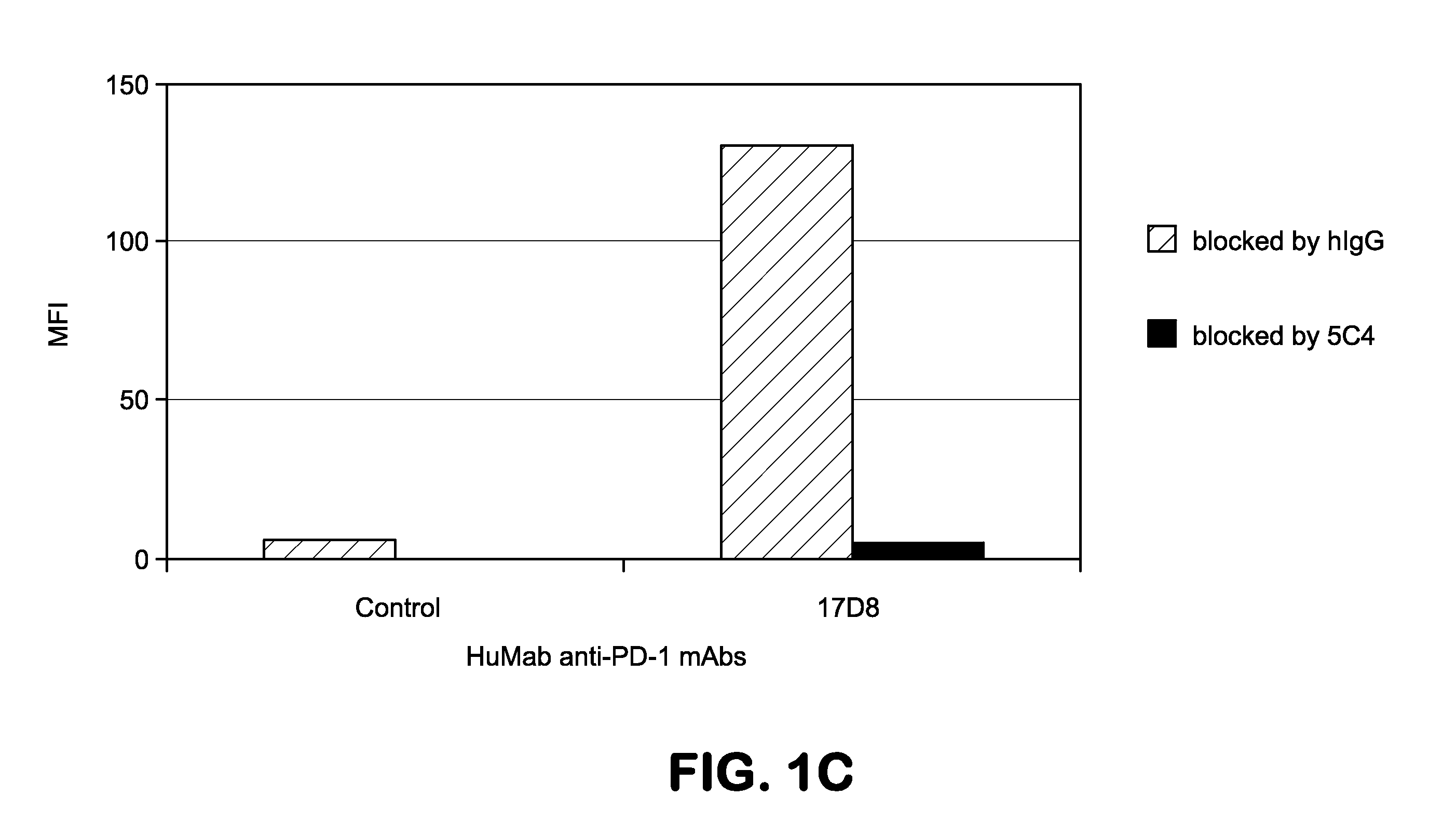
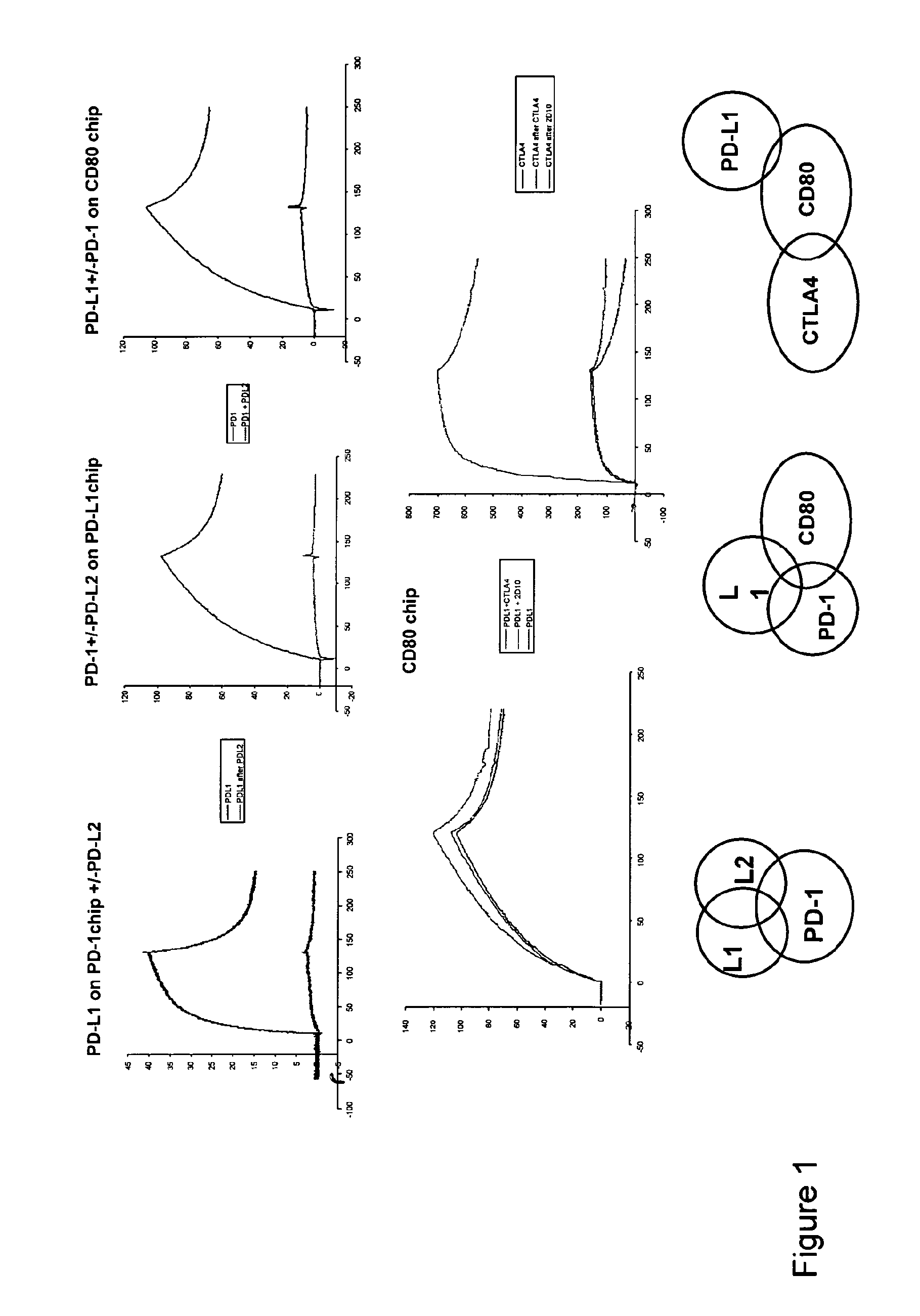
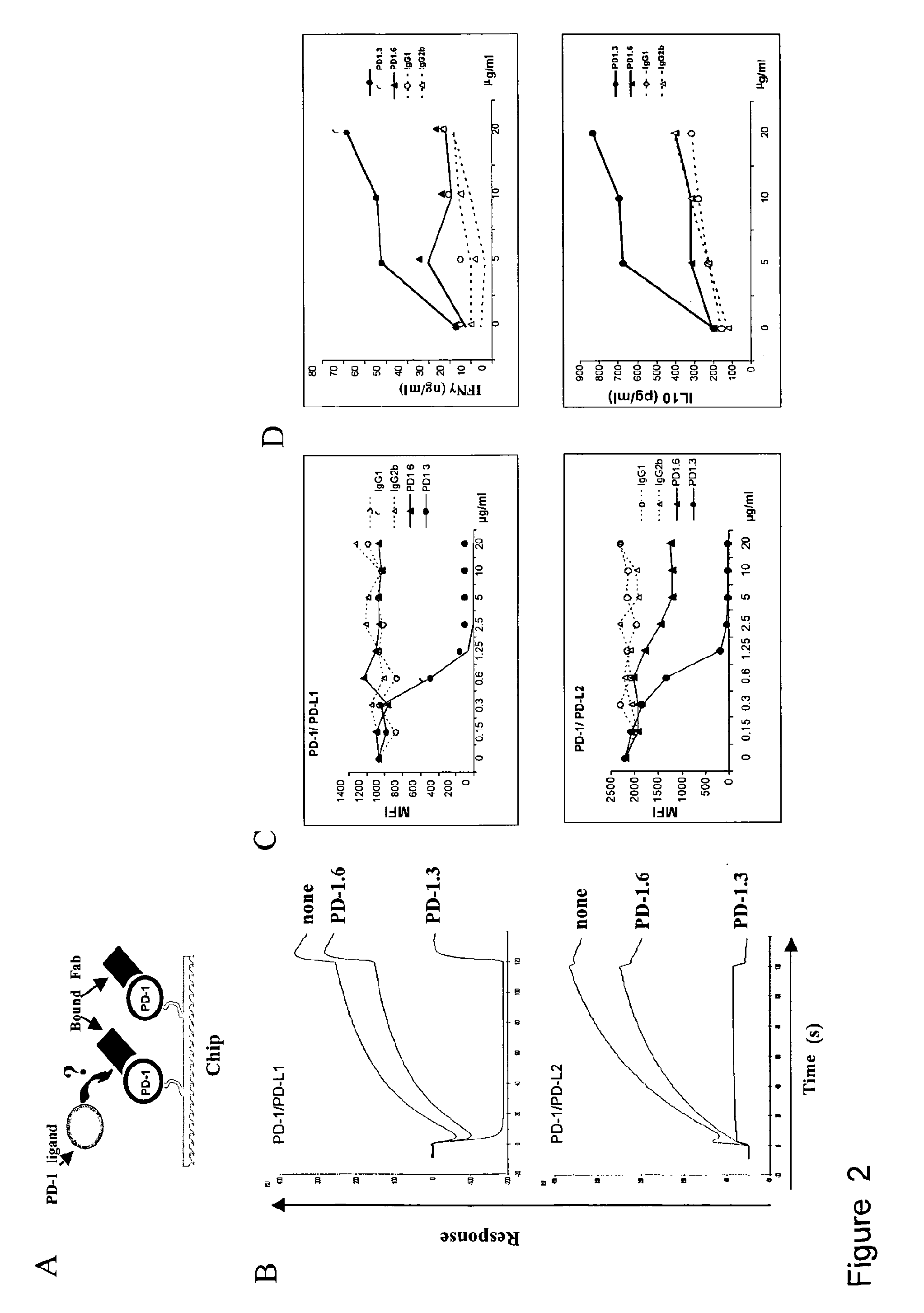
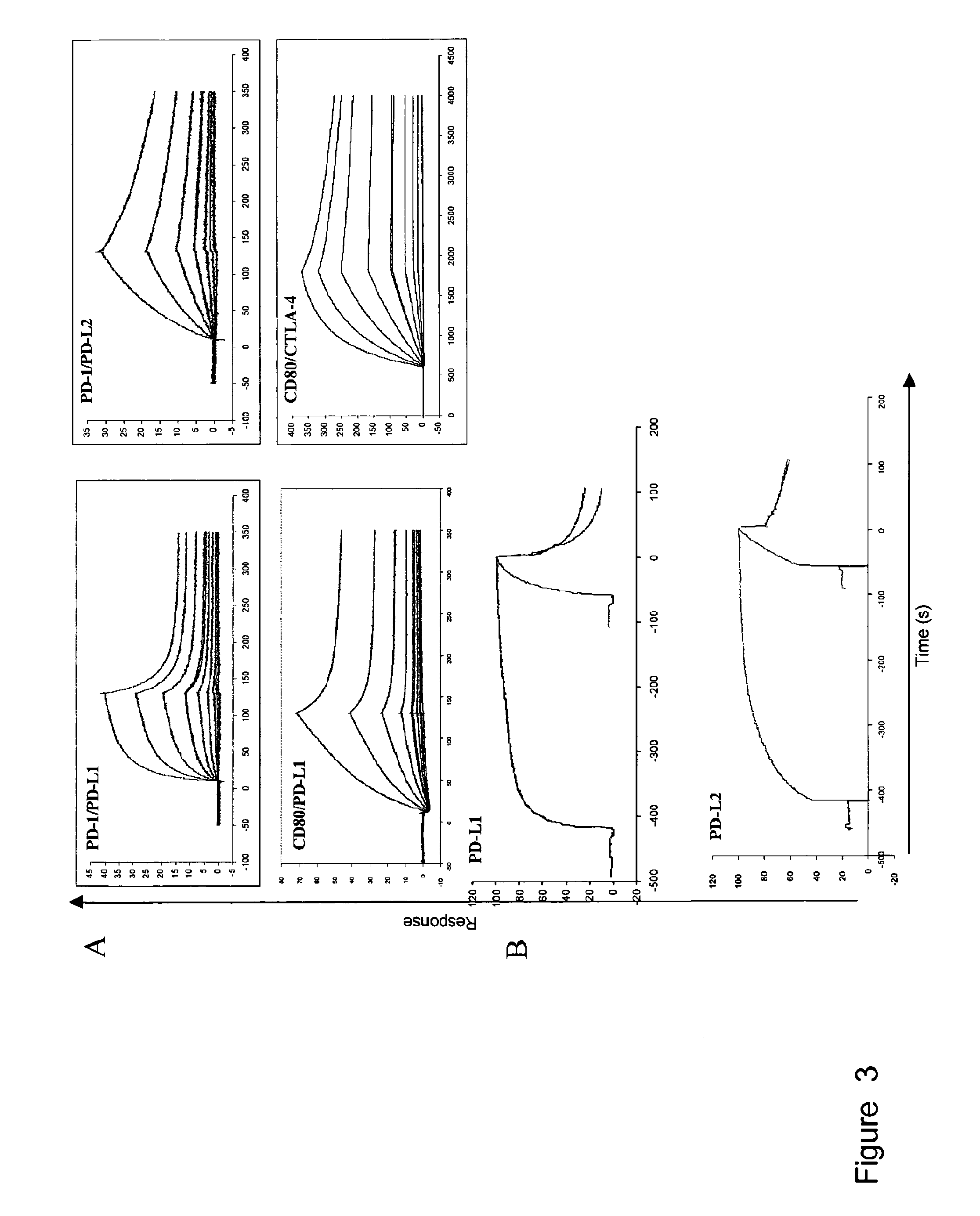
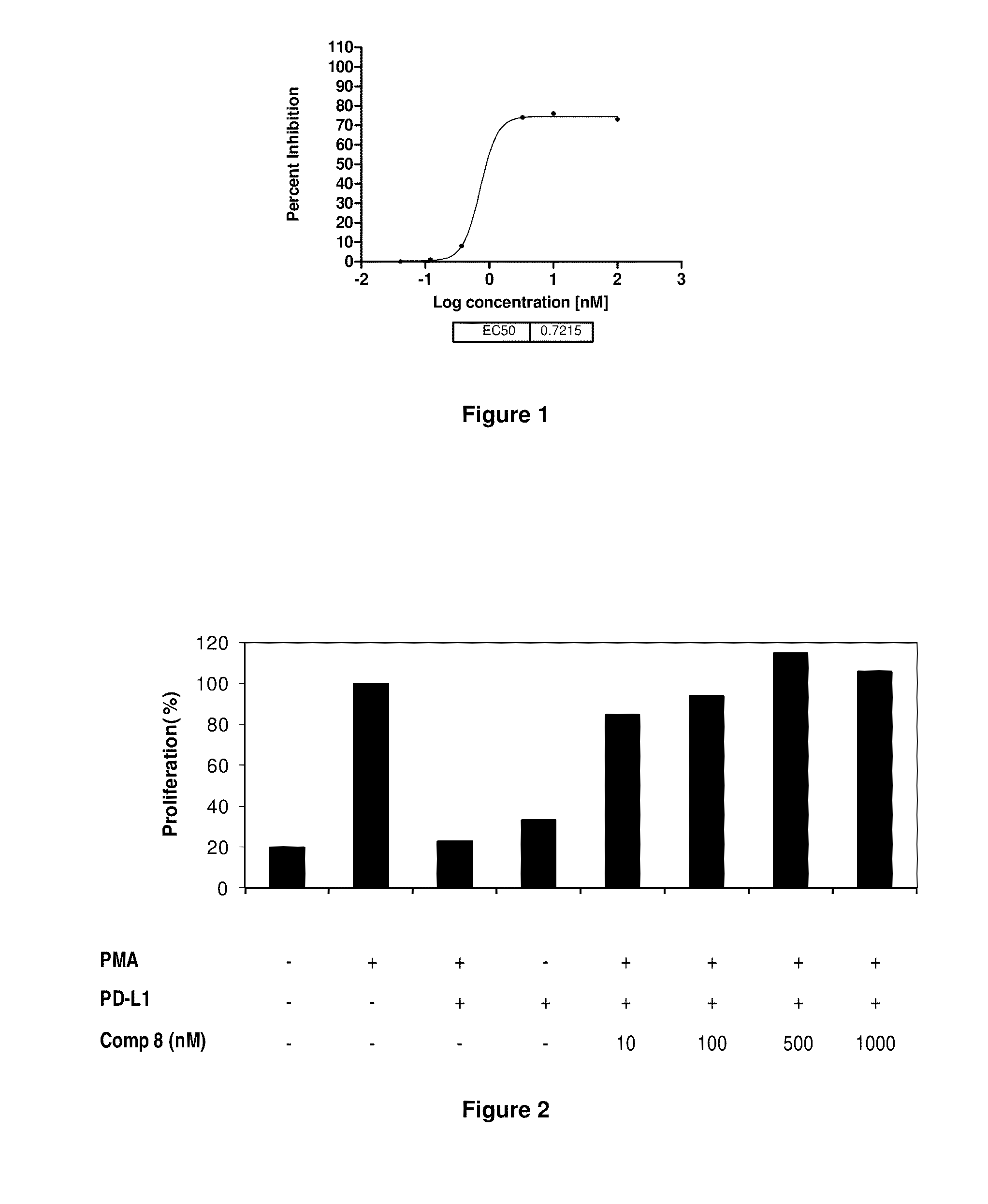
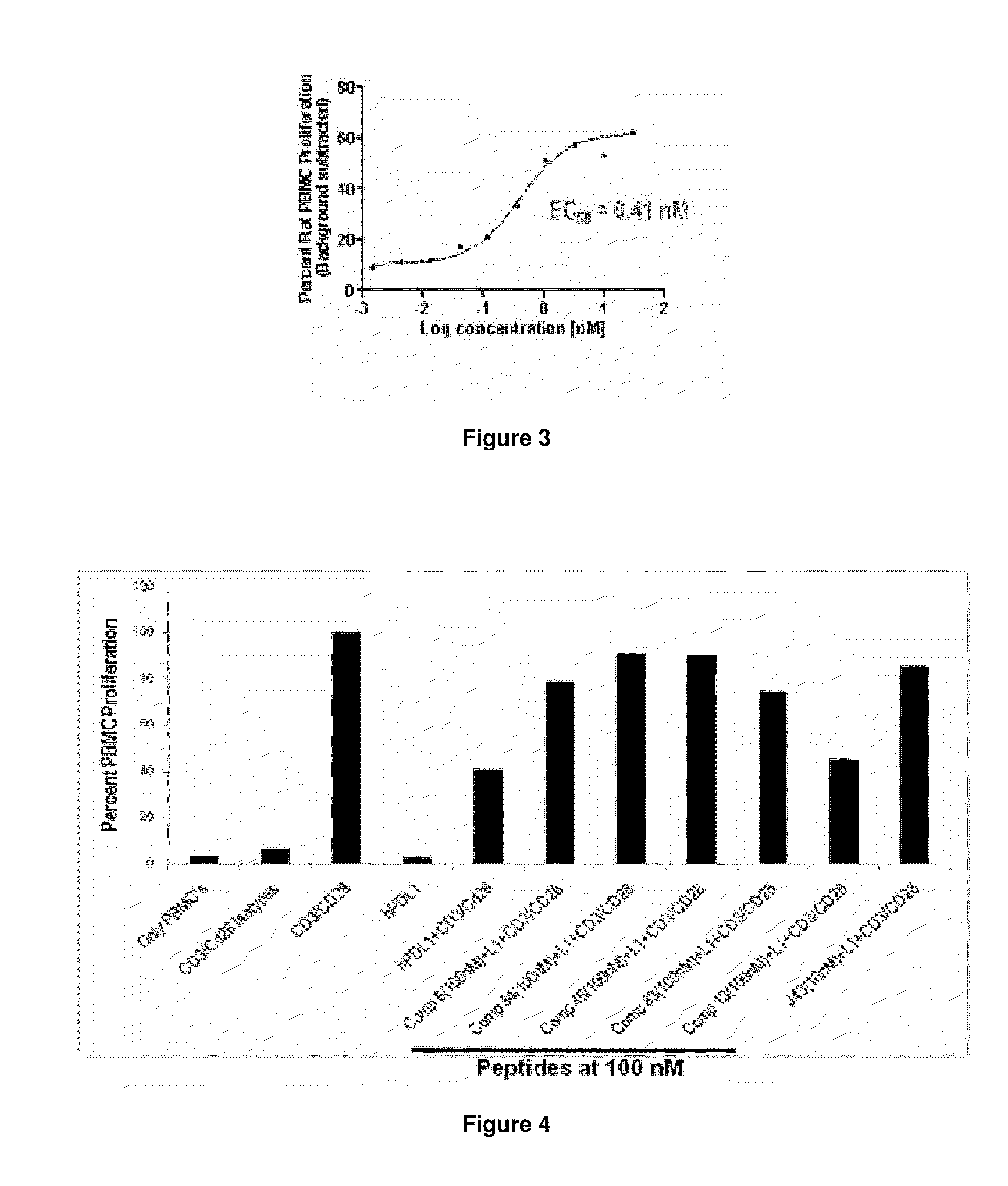
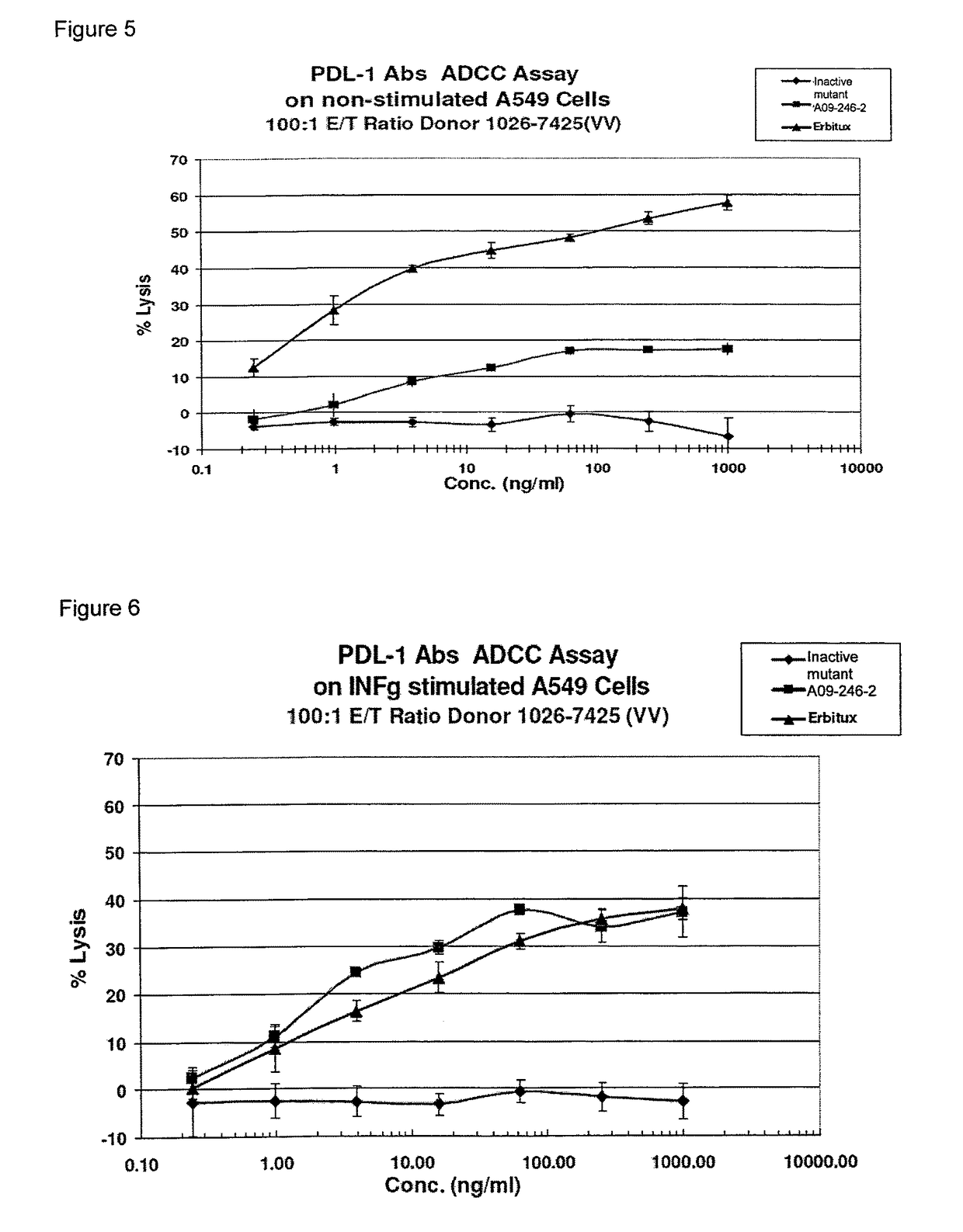
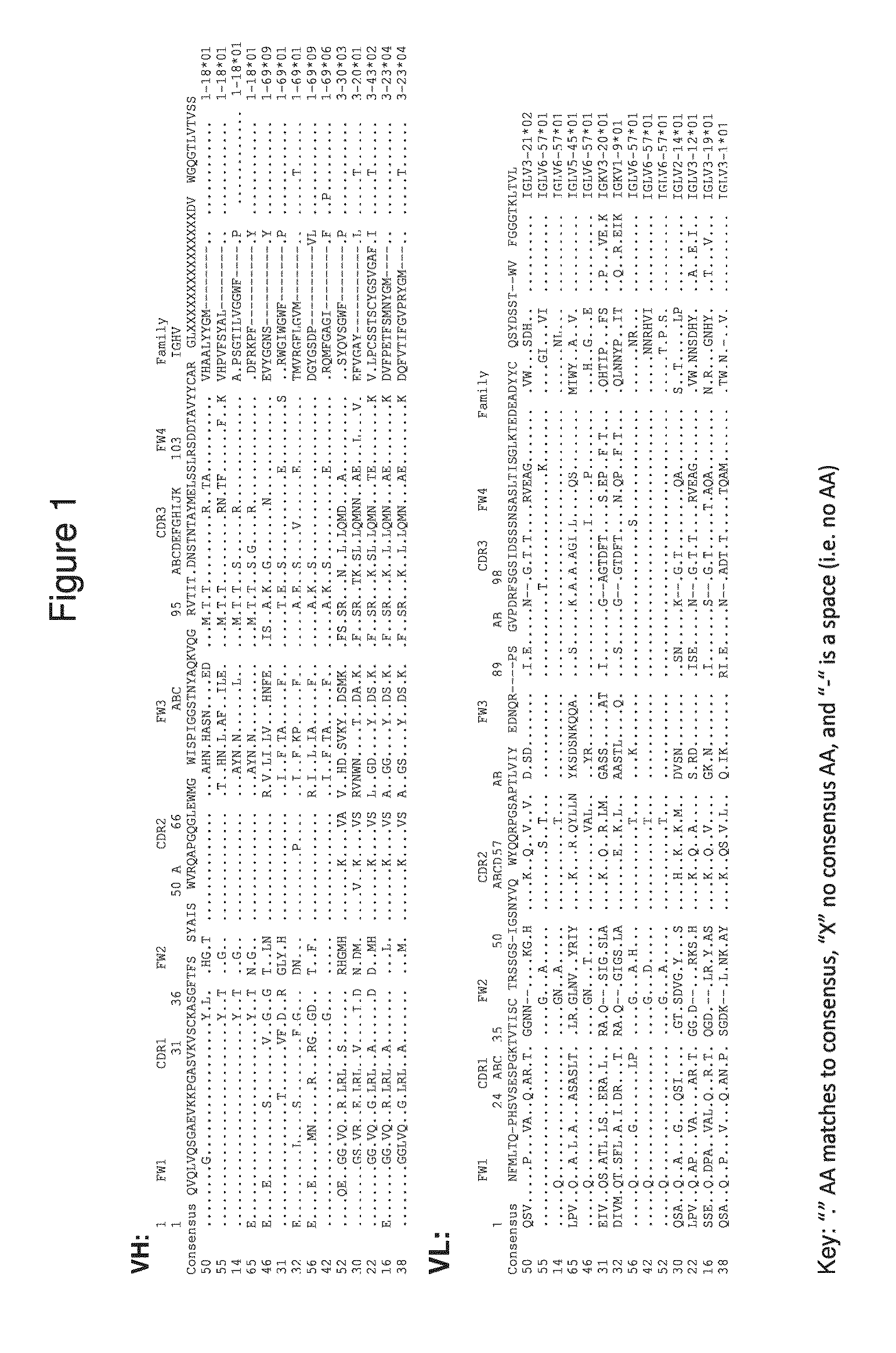
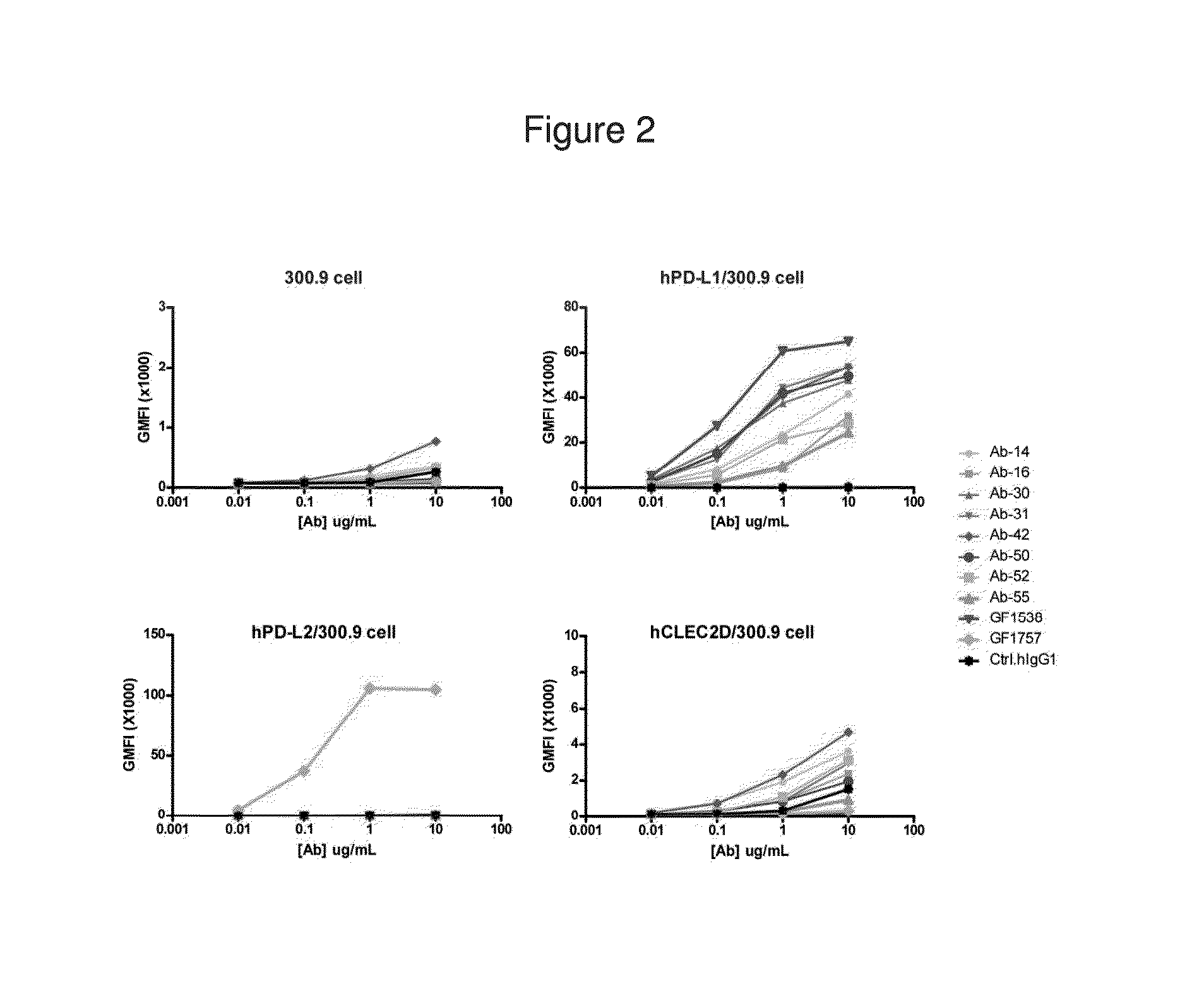
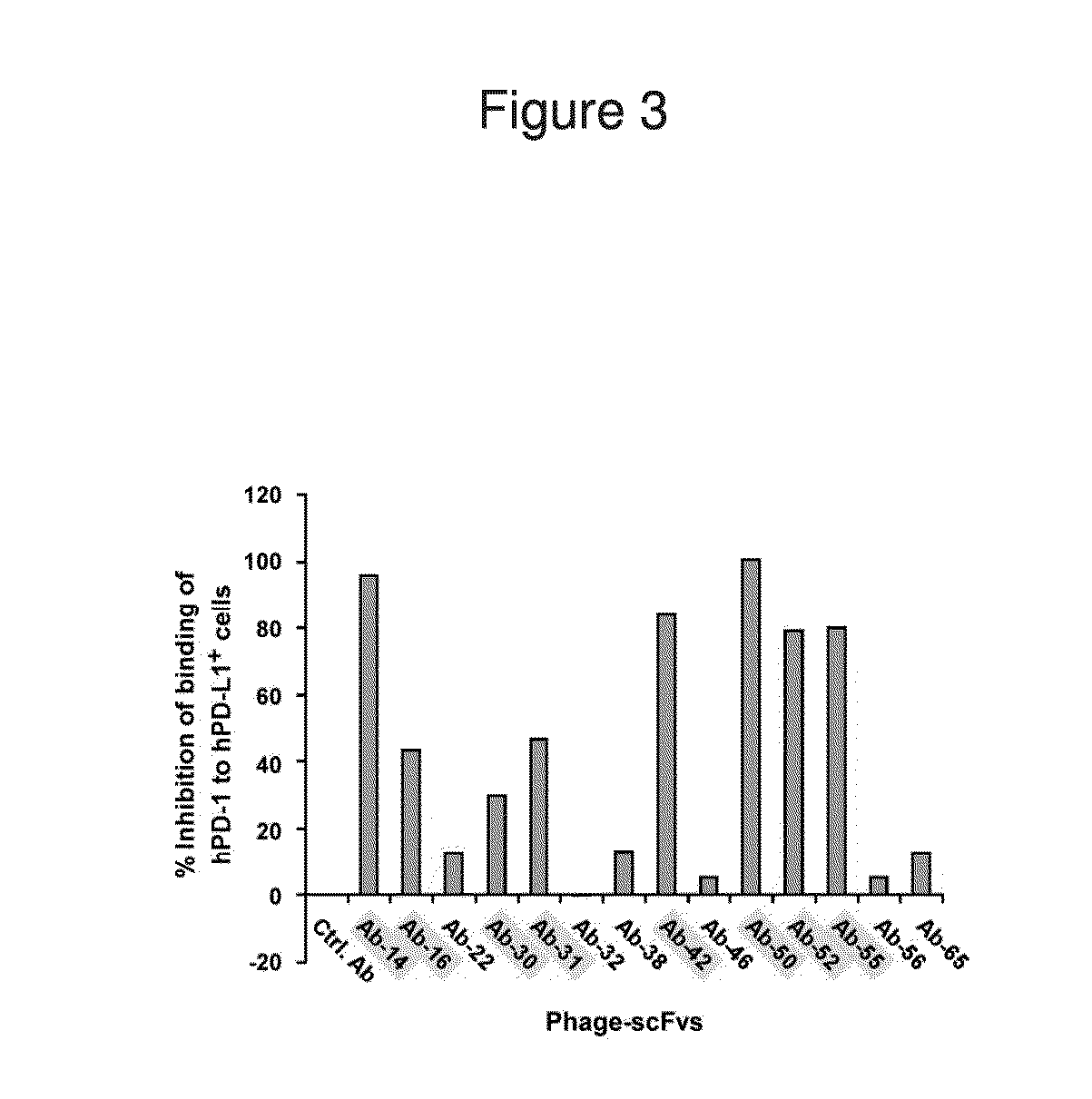
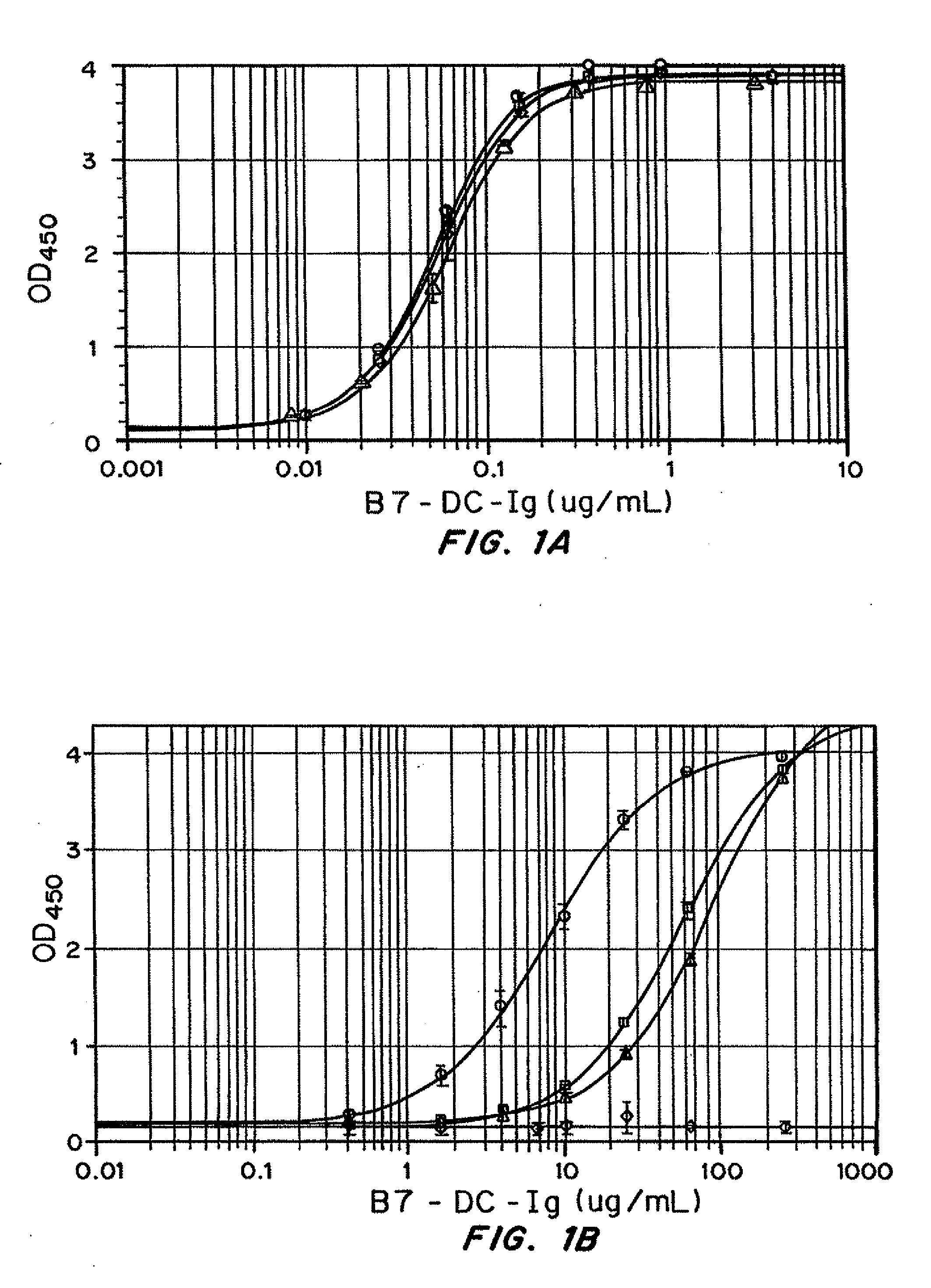
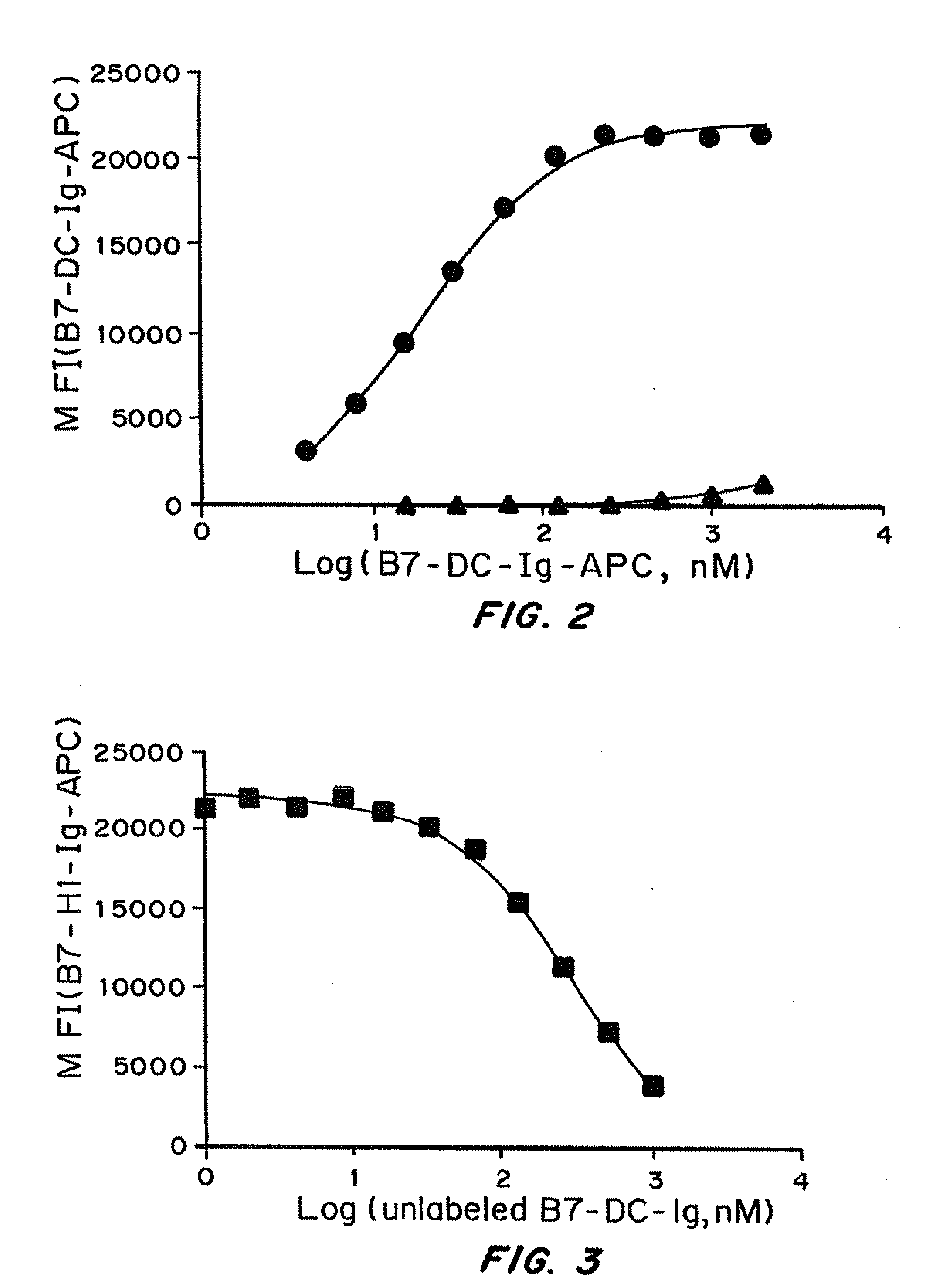
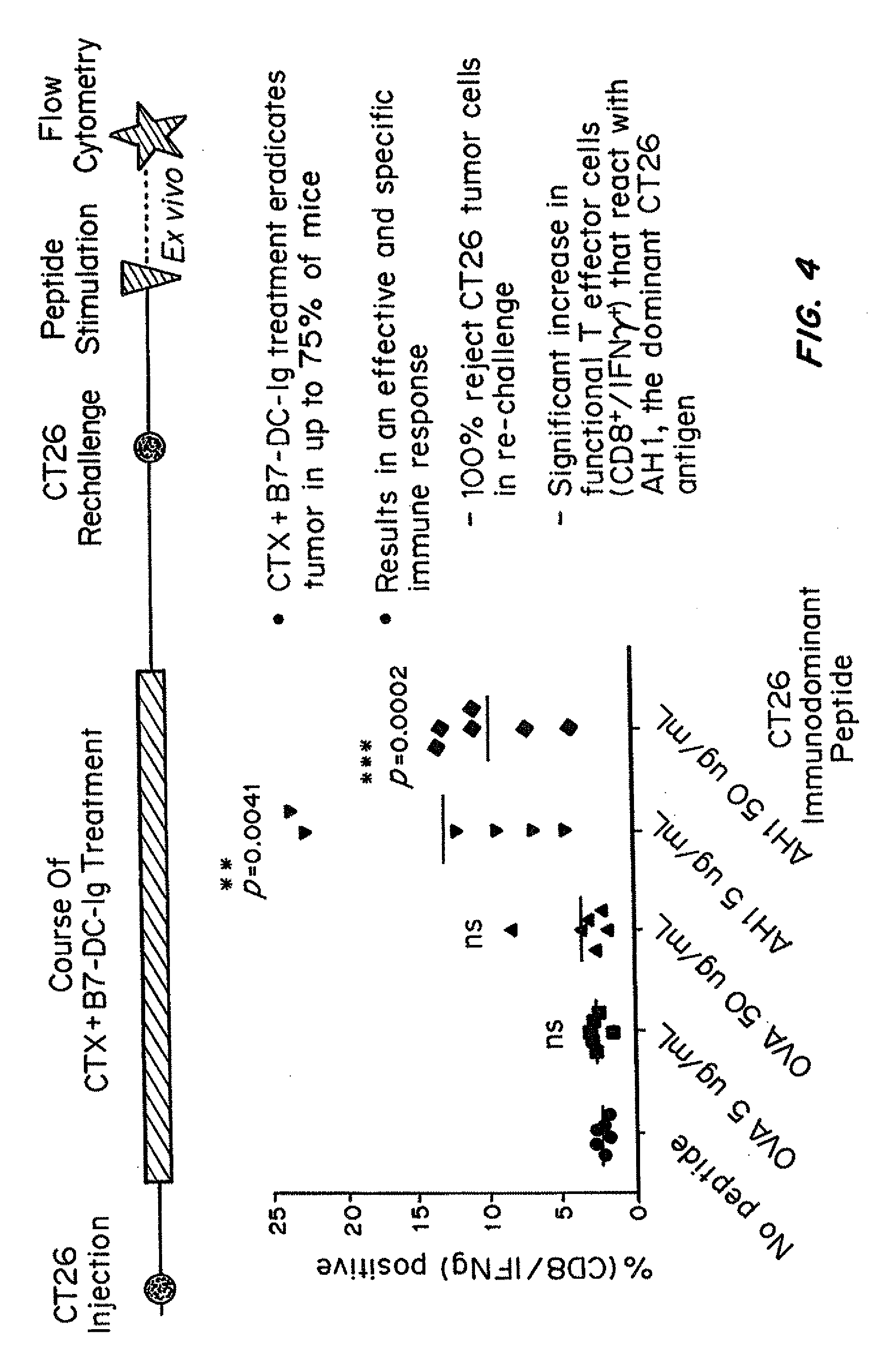
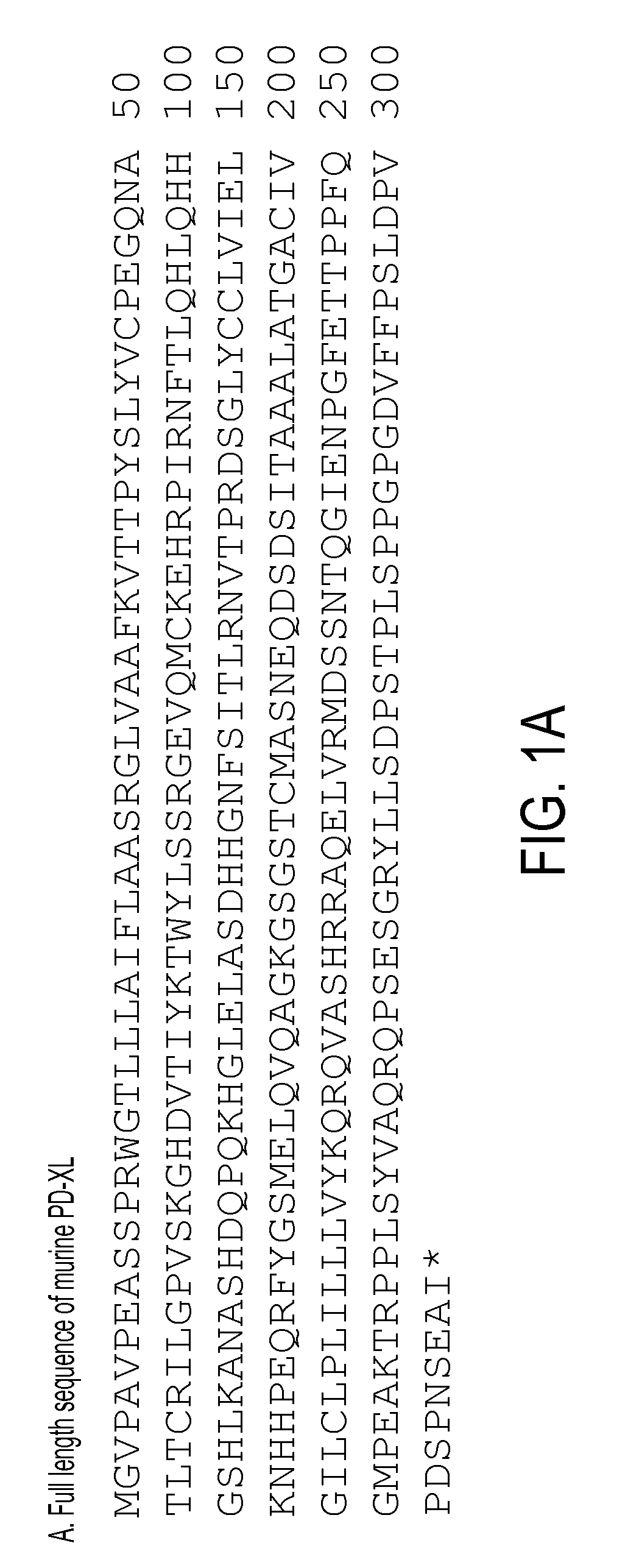
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
394 results about "PD-L1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
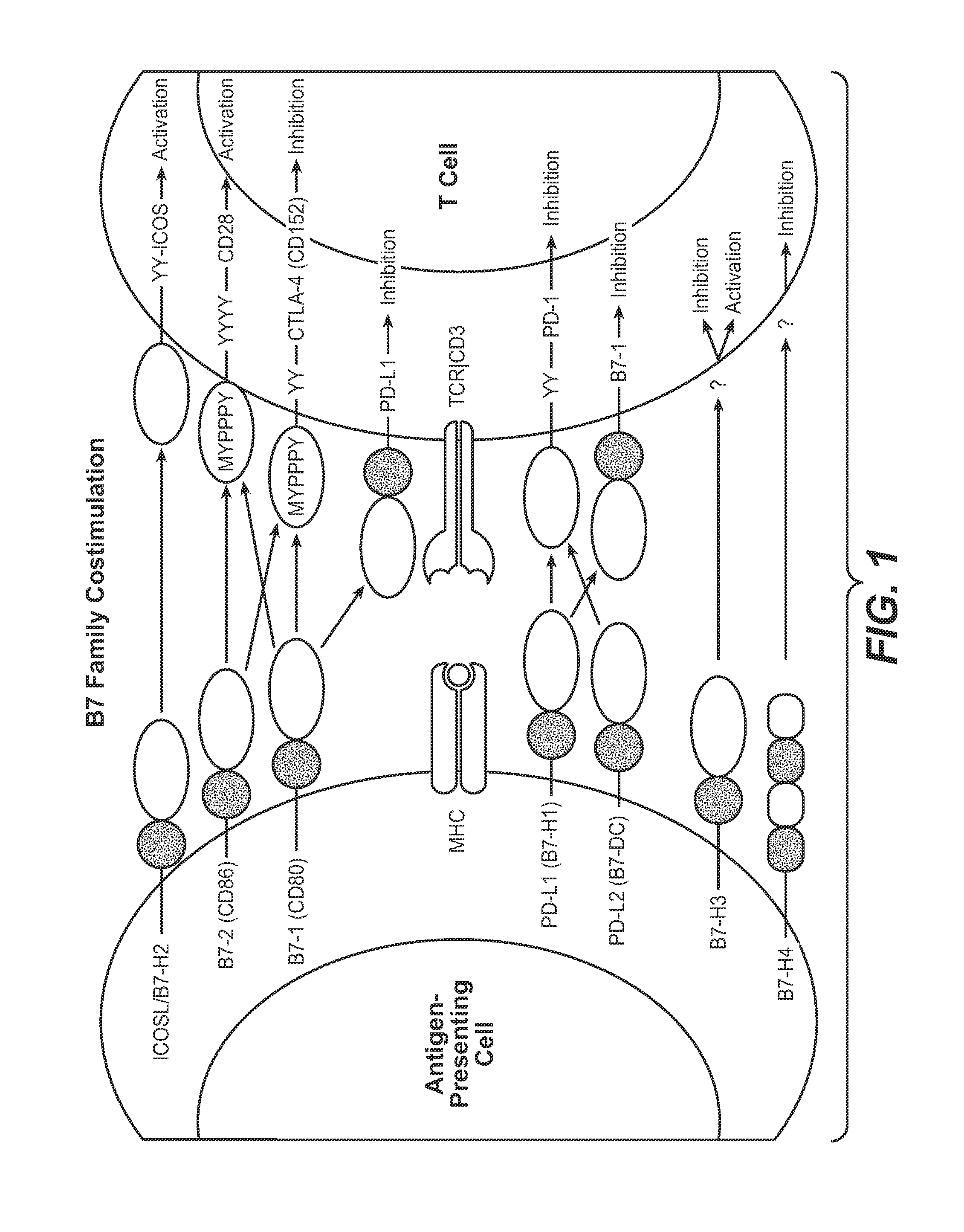
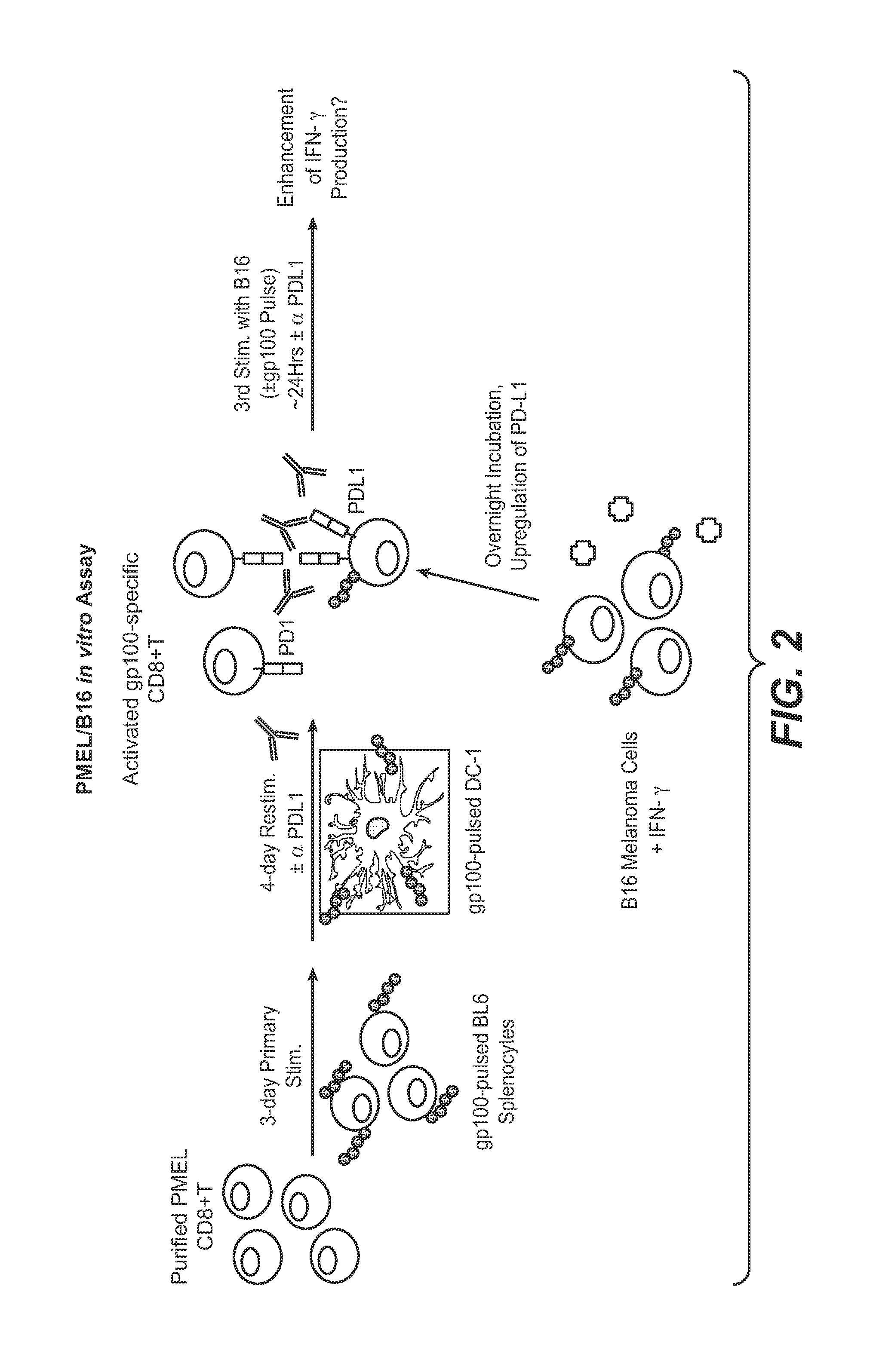
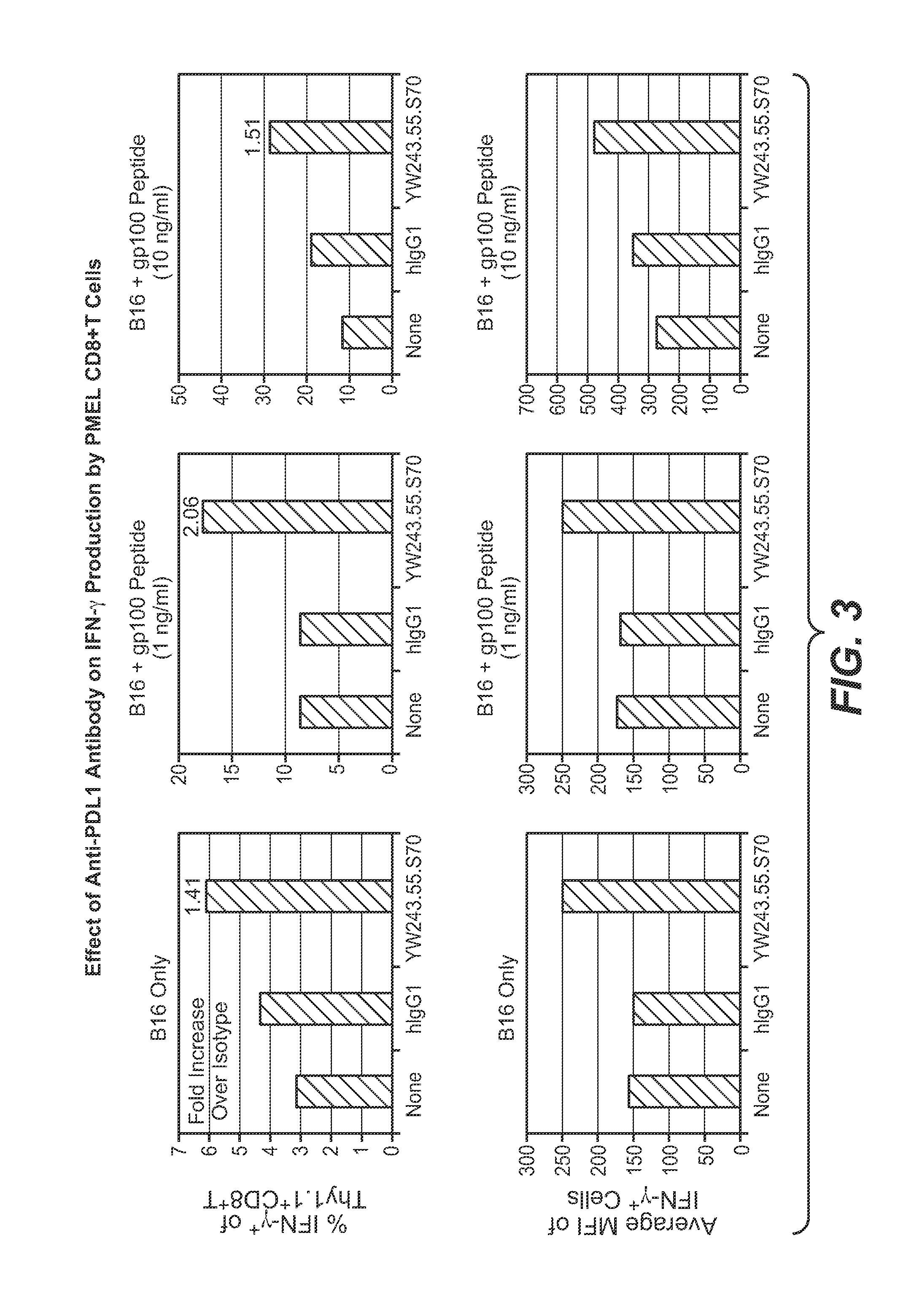
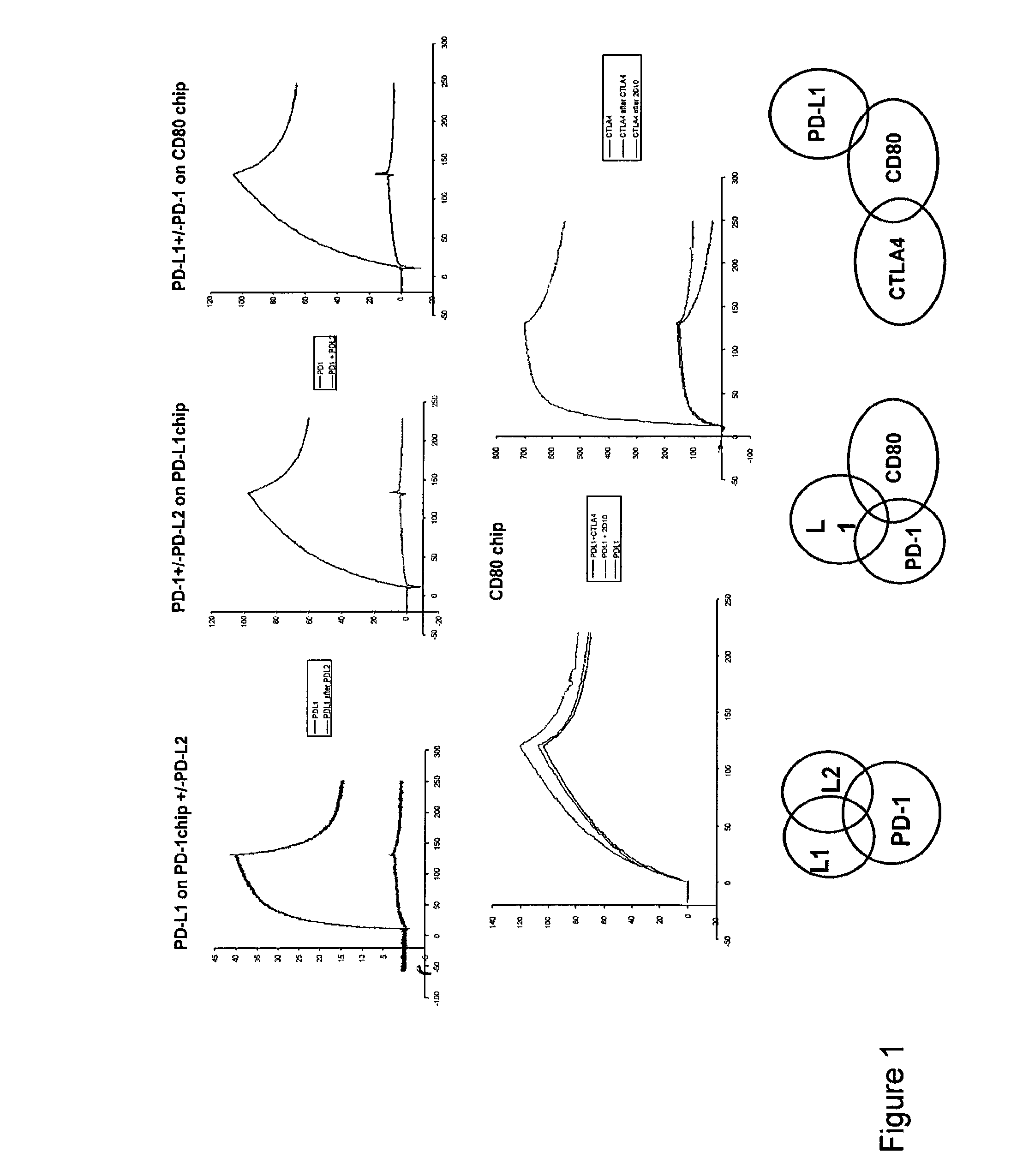
Programmed death-ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a protein that in humans is encoded by the CD274 gene. Programmed death-ligand 1 (PD-L1) is a 40kDa type 1 transmembrane protein that has been speculated to play a major role in suppressing the adaptive arm of immune system during particular events such as pregnancy, tissue allografts, autoimmune disease and other disease states such as hepatitis. Normally the adaptive immune system reacts to antigens that are associated with immune system activation by exogenous or endogenous danger signals. In turn, clonal expansion of antigen-specific CD8+ T cells and/or CD4+ helper cells is propagated. The binding of PD-L1 to the inhibitory checkpoint molecule PD-1 transmits an inhibitory signal based on interaction with phosphatases (SHP-1 or SHP-2) via Immunoreceptor Tyrosine-Based Switch Motif (ITSM) motif . This reduces the proliferation of antigen-specific T-cells in lymph nodes, while simultaneously reducing apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells) - further mediated by a lower regulation of the gene Bcl-2.
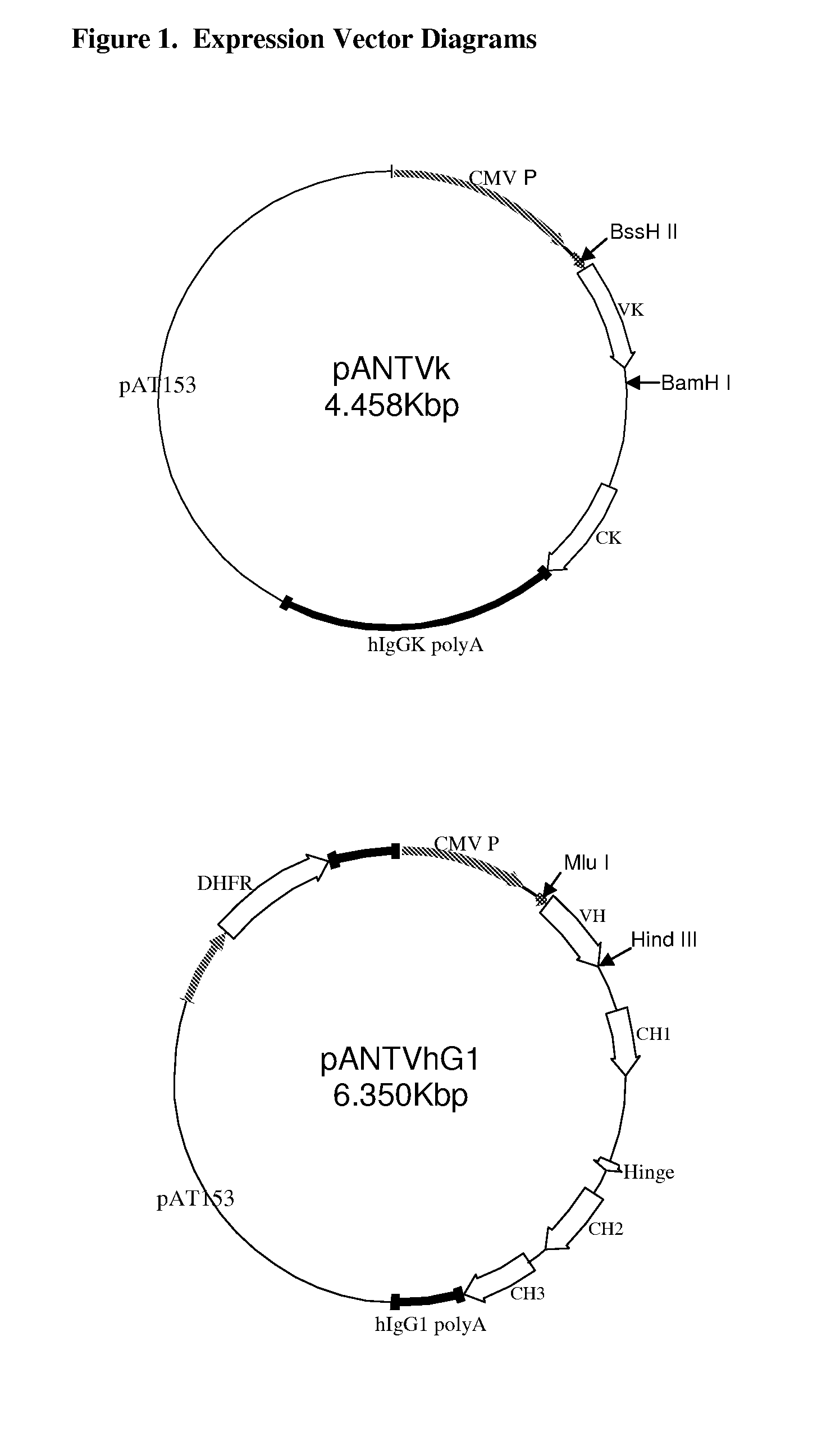
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
PD-1 binding proteins
ActiveUS8168757B2Regulating T cell responsesImprove immunityAntibody mimetics/scaffoldsAntibody ingredientsHost immunitySignalling pathways
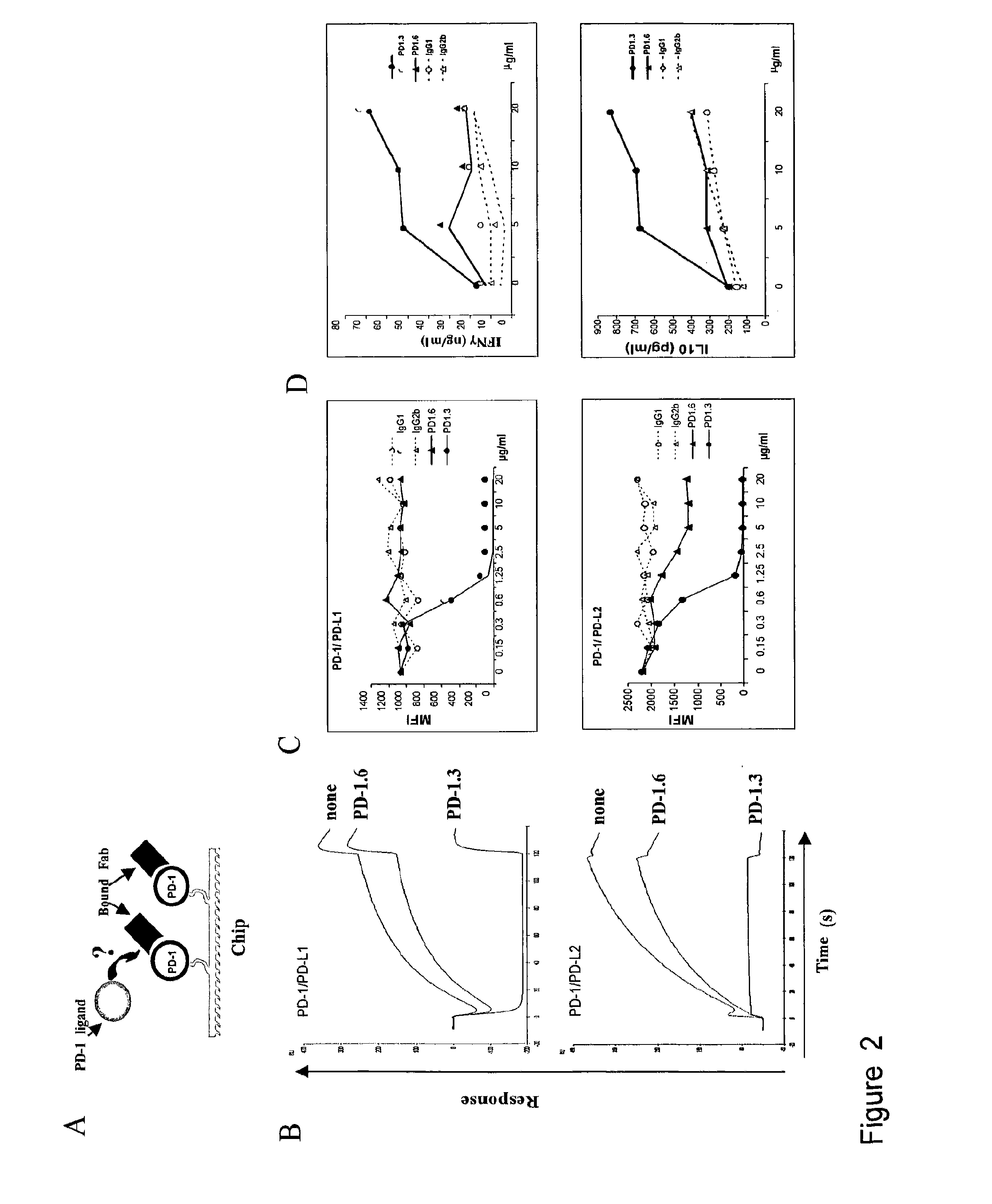
The present invention features PD-1 binding proteins, a subset of which inhibits binding of PD-L1 to the PD-1 receptor. These binding proteins can be employed to modulate the immune system through the manipulation of the PD-1 signaling pathway, enhancing host immunity to treat infections and cancer.
Owner:MERCK SHARP & DOHME LLC
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
PD-1 Antibodies and PD-L1 Antibodies and Uses Thereof
ActiveUS20120039906A1Reduced activityStrong cytotoxicityAntibacterial agentsAnimal cellsPD-L1Antibody
Owner:INST JEAN PAOLI & IRENE CALMETTES +2
Human Anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor
ActiveUS20110271358A1Reduced antigen binding affinityLess immunogenicAntibacterial agentsAntipyreticTransplant rejectionAutoimmune disease
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Cancer immunotherapy by disrupting pd-1/pd-l1 signaling
ActiveUS20130309250A1Reduces and suppresses signalingReliable responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
The disclosure provides a method for immunotherapy of a subject afflicted with cancer, comprises administering to the subject a composition comprising a therapeutically effective amount of an antibody that inhibits signaling from the PD-1 / PD-L1 signaling pathway. This disclosure also provides a method for immunotherapy of a subject afflicted with cancer comprising selecting a subject that is a suitable candidate for immunotherapy based on an assessment that the proportion of cells in a test tissue sample from the subject that express PD-L1 on the cell surface exceeds a predetermined threshold level, and administering a therapeutically effective amount of an anti-PD-1 antibody to the selected subject. The invention additionally provides rabbit mAbs that bind specifically to a cell surface-expressed PD-L1 antigen in a FFPE tissue sample, and an automated IHC method for assessing cell surface expression in FFPE tissues using the provided anti-PD-L1 Abs.
Owner:BRISTOL MYERS SQUIBB CO
Anti-pd-l1 antibodies and their use to enhance t-cell function
ActiveUS20100203056A1Reduce level of pathogenChronic infectionAntibacterial agentsOrganic active ingredientsT-cell dysfunctionPD-L1
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
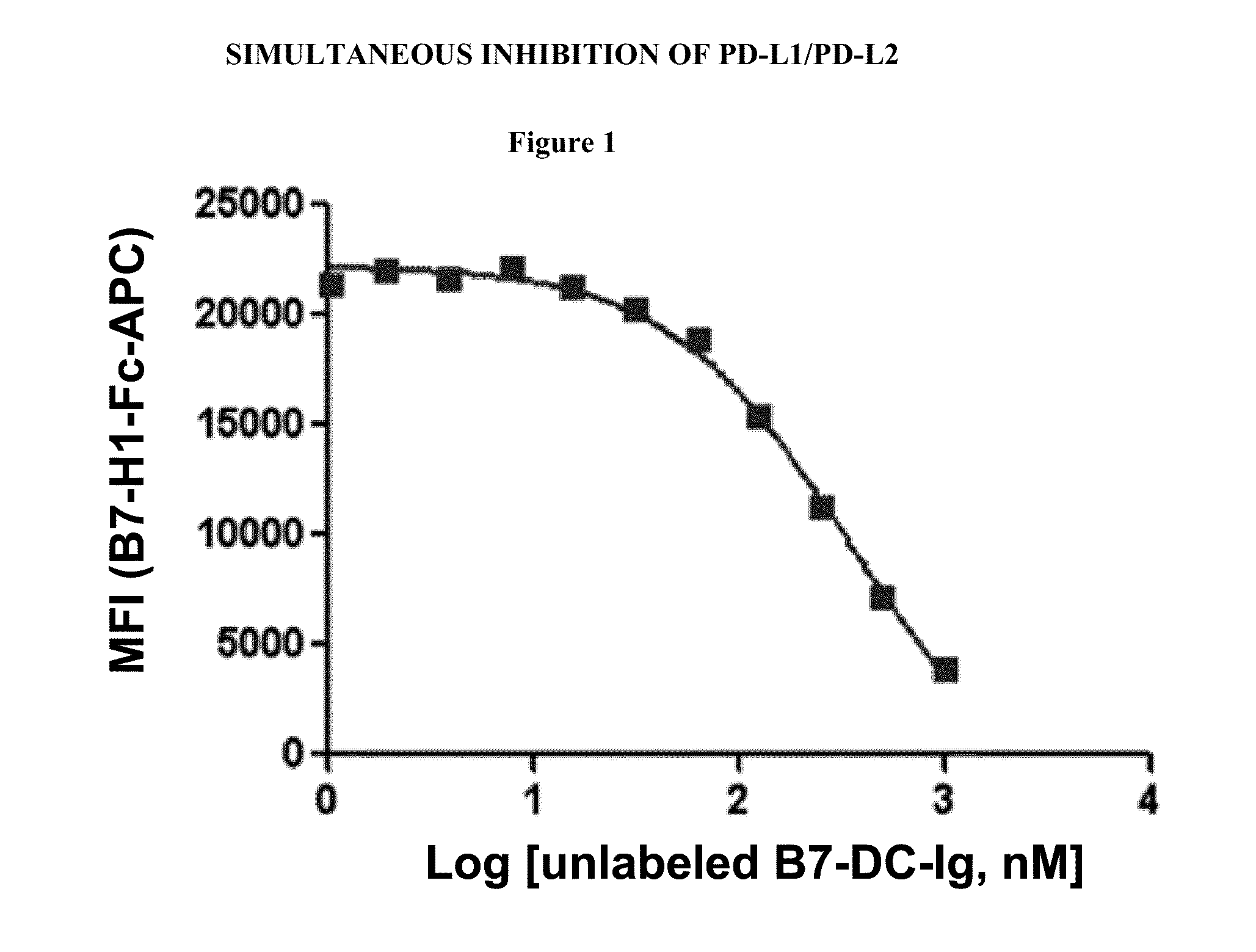
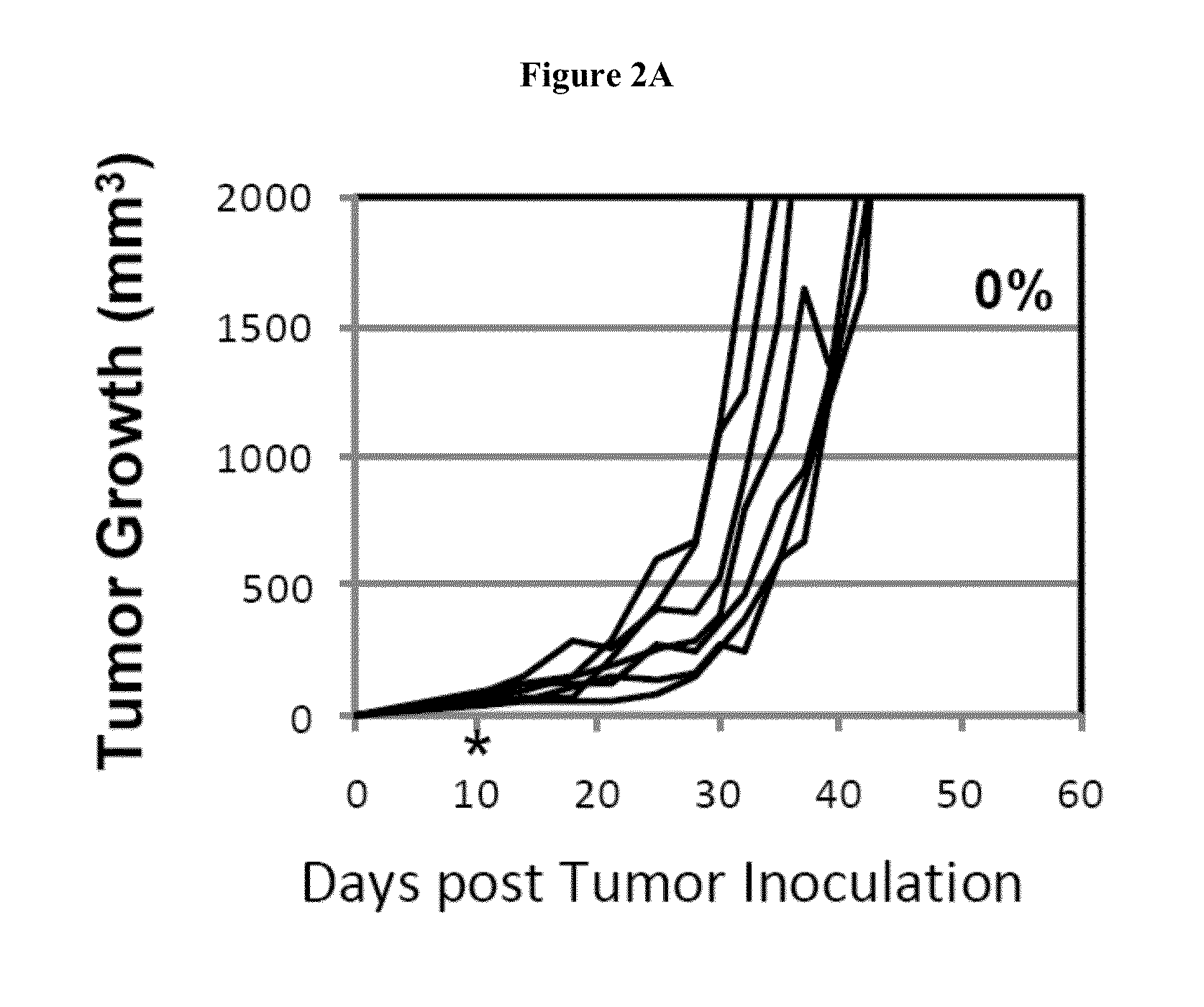
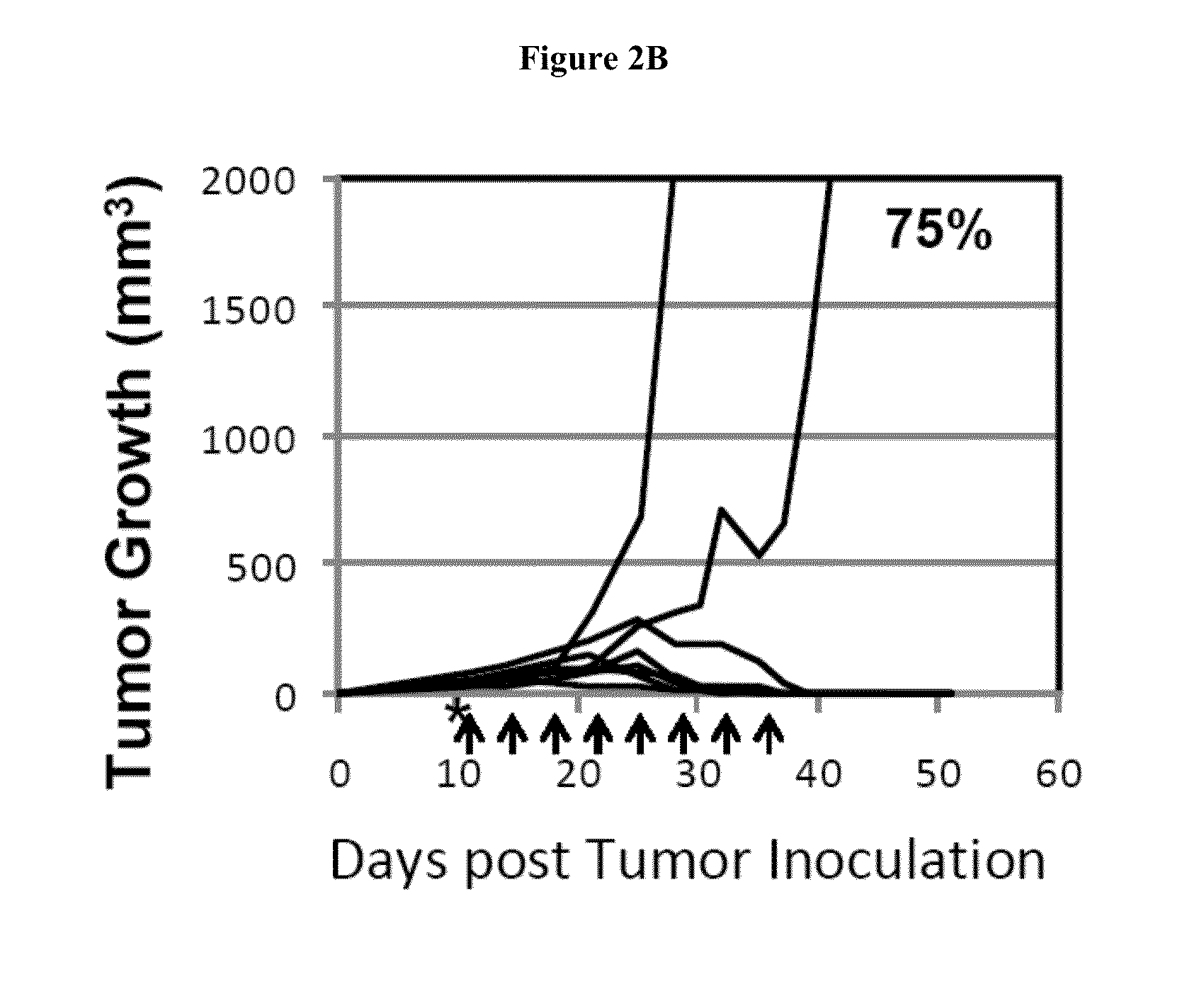
Simultaneous inhibition of pd-l1/pd-l2
InactiveUS20130017199A1Increase frequencyIncrease percentageAntibacterial agentsAntimycoticsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by simultaneously inhibiting the PD-1 ligands, PD-L1 and PD-L2. The immune response can be modulated by providing antagonists which bind with different affinity, by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again. In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
Immunosuppression modulating compounds
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
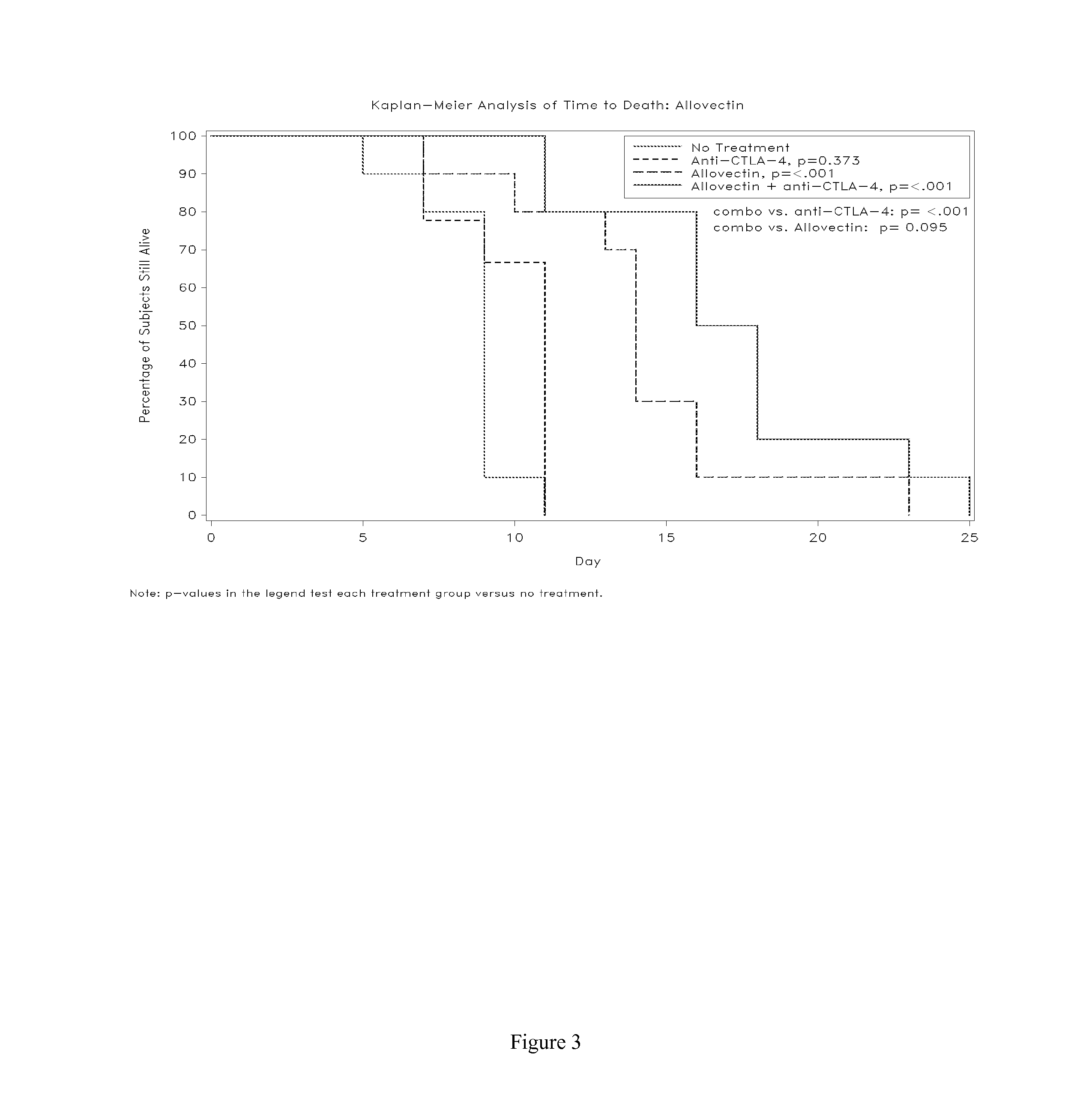
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130071403A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentIrritation
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Antibodies that bind PD-L1 and uses thereof
ActiveUS9212224B2Reduces and suppresses signalingReliable responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
Owner:BRISTOL MYERS SQUIBB CO
Cancer immunotherapy by disrupting pd-1/pd-l1 signaling
ActiveUS20150125463A1Reliable responseReduces and suppresses signalingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
The disclosure provides a method for immunotherapy of a cancer patient, comprises administering to the patient an Ab that inhibits signaling from the PD-1 / PD-L1 signaling pathway, or a combination of such Ab and an anti-CTLA-4 Ab. This disclosure also provides a method for immunotherapy of a cancer patient comprising selecting a patient who is a suitable candidate for immunotherapy based on an assessment that the proportion of cells in a test tissue sample from the patient that express PD-L1 on the cell surface exceeds a predetermined threshold level, and administering an anti-PD-1 Ab to the selected subject. The disclosure additionally provides rabbit mAbs that bind specifically to a cell surface-expressed PD-L1 antigen in a FFPE tissue sample, and an automated IHC method for assessing cell surface expression in FFPE tissues using the provided anti-PD-L1 Abs.
Owner:BRISTOL MYERS SQUIBB CO
PD-1 antibodies and PD-L1 antibodies and uses thereof
ActiveUS8741295B2Improve binding affinity and other biological propertyReduced activityAntibacterial agentsAntimycoticsPD-L1Antibody
Owner:INST JEAN PAOLI & IRENE CALMETTES +2
Immunosuppression modulating compounds
InactiveUS20110318373A1Reduce the binding forceAntibacterial agentsBiocideDiseaseSignalling pathways
The present invention provides immunosuppression compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signaling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides an application of the compositions containing the peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of peptide moieties as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
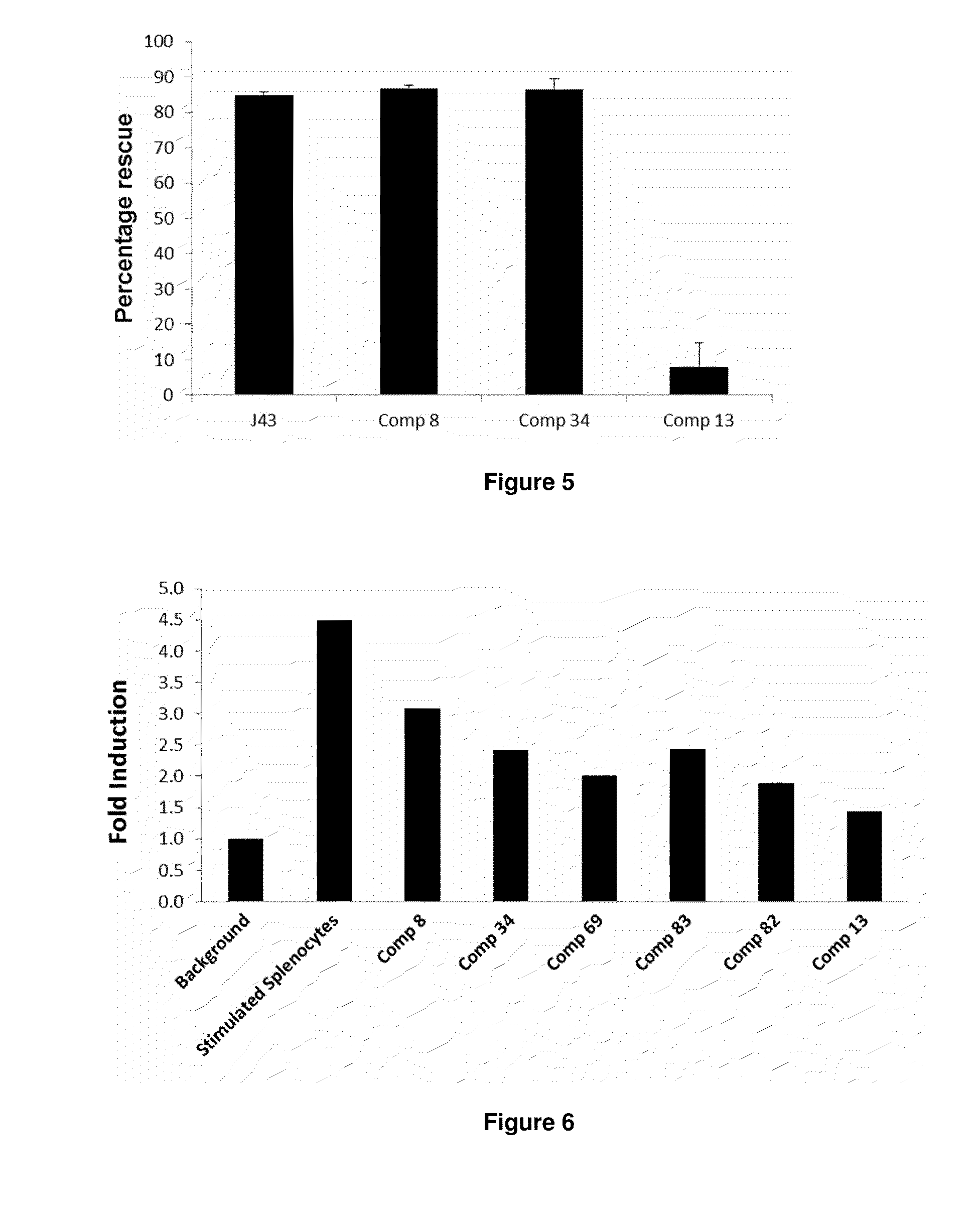
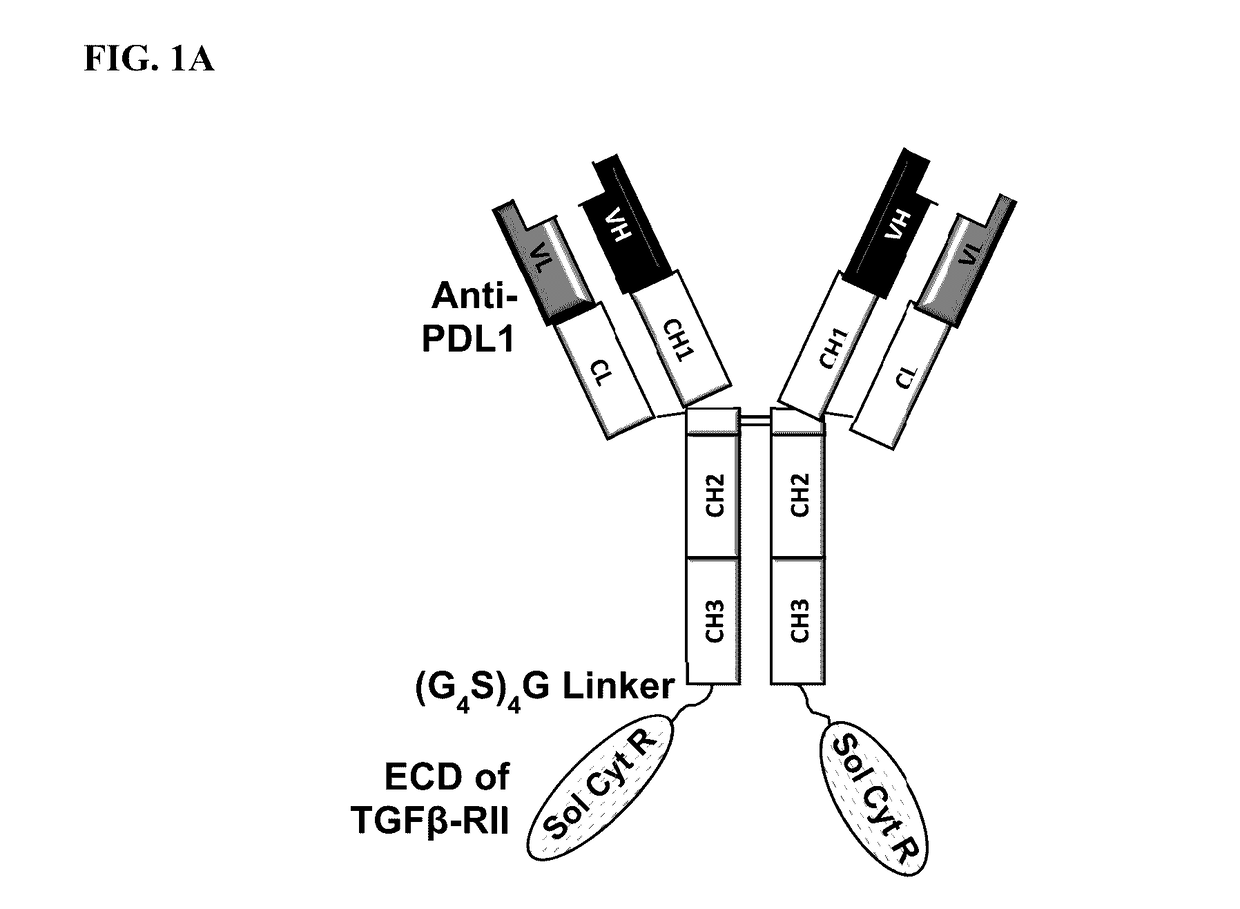
Targeted tgfß inhibition
ActiveUS20150225483A1Effective therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAntigen Binding Fragment
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
Targeted TGFβ inhibitors
ActiveUS9676863B2Effective therapyNervous disorderPeptide/protein ingredientsAntigenAntigen Binding Fragment
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
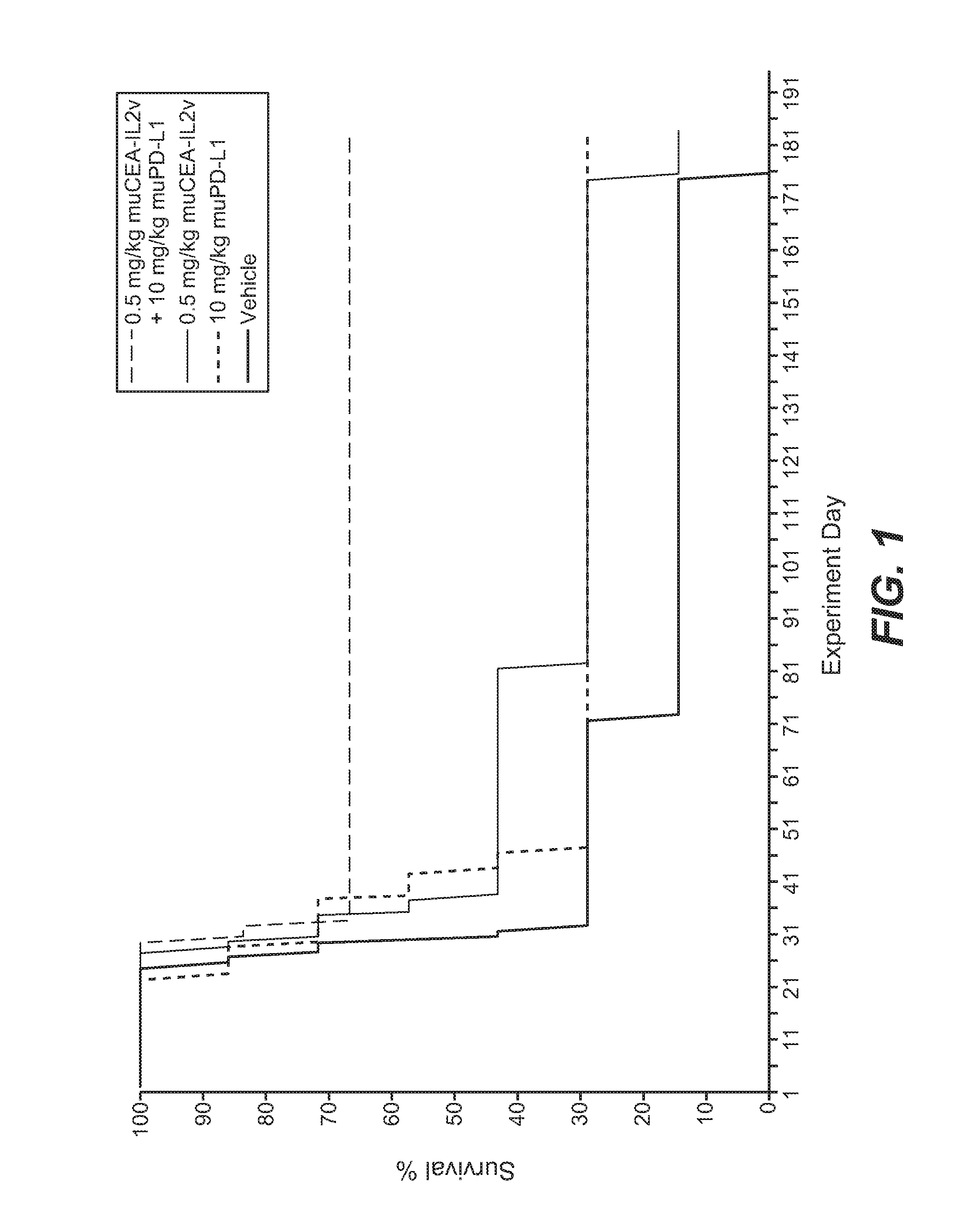
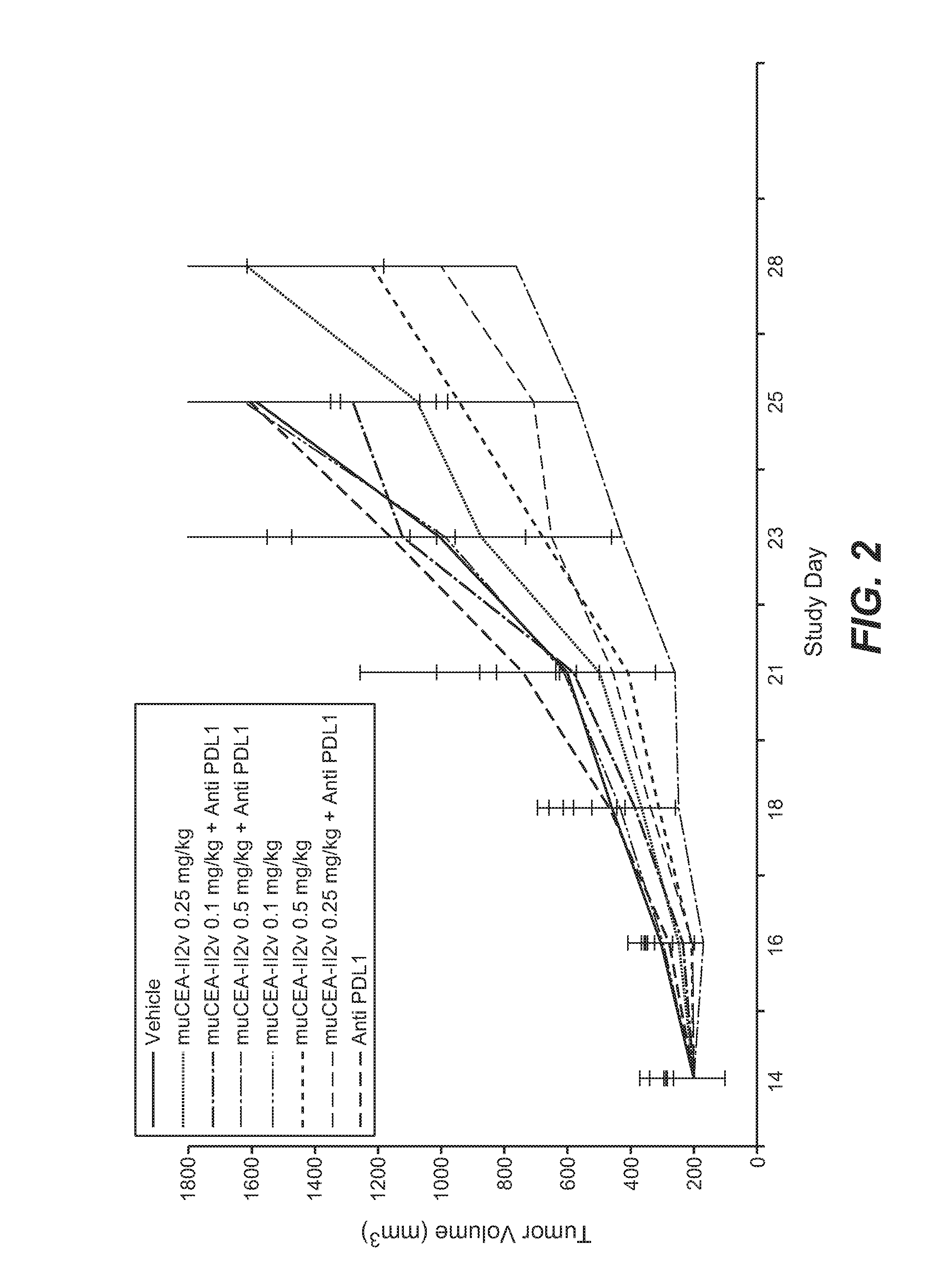
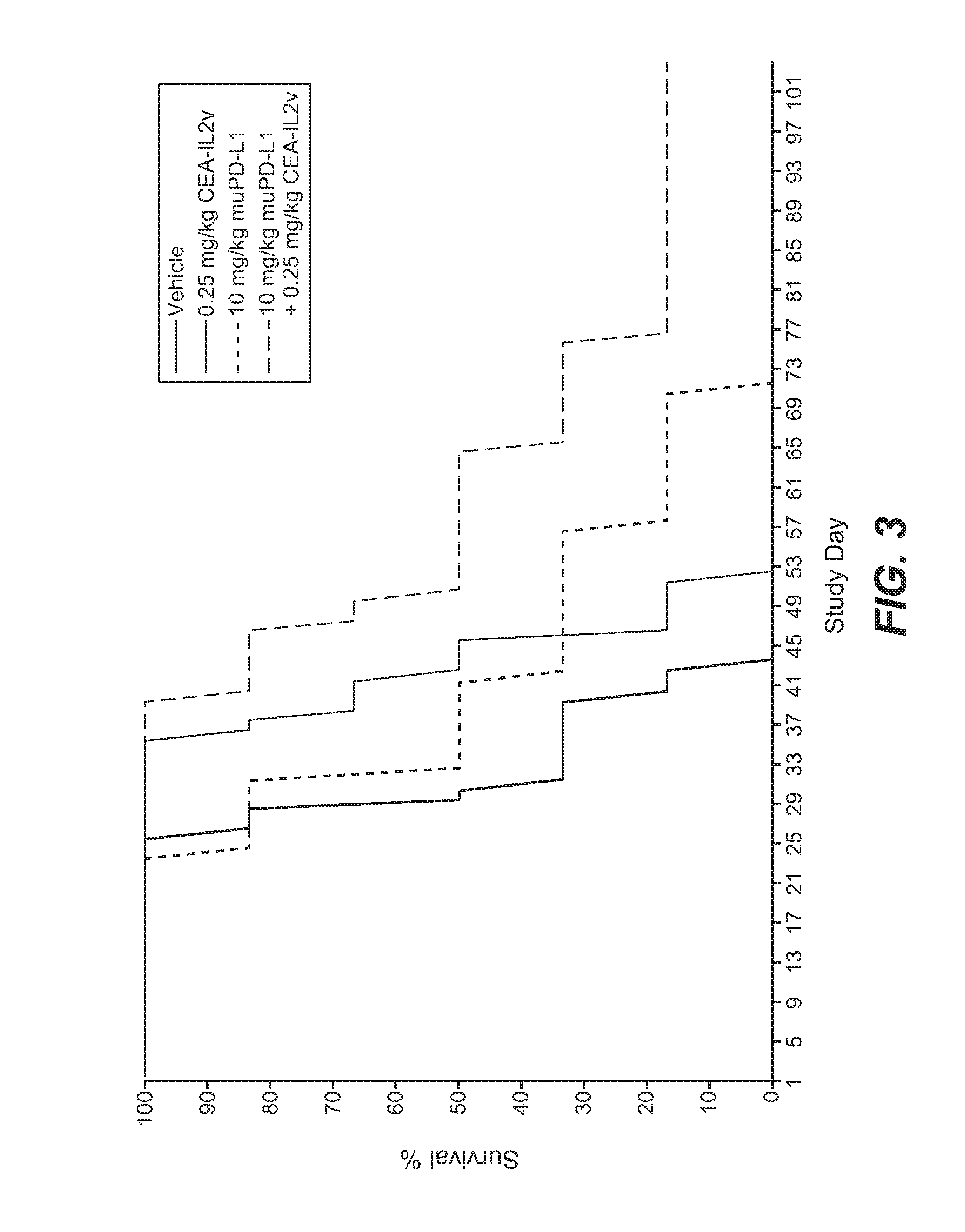
Combination therapy of tumor-targeted il-2 variant immunocytokines and antibodies against human pd-l1
InactiveUS20160175397A1Enhancing median and overall survivalImprove survivalNervous disorderPeptide/protein ingredientsTumor targetPD-L1
The present invention relates to the combination therapy of specific tumor-targeted IL-2 variant immunocytokines with specific antibodies which bind human PD-L1.
Owner:F HOFFMANN LA ROCHE INC
Anti-PD-L1 antibodies and uses thereof
ActiveUS9624298B2Function increaseUpregulate cell-mediated immune responsesNervous disorderPeptide/protein ingredientsAntigen Binding FragmentPD-L1
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Use of PD-L3 proteins and PD-L3 specific antibodies or antibody fragments to regulate CD4+ and CD8+ T cell immunity
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNF. Proteins, antibodies, activated T cells and methods for using the same are disclosed.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
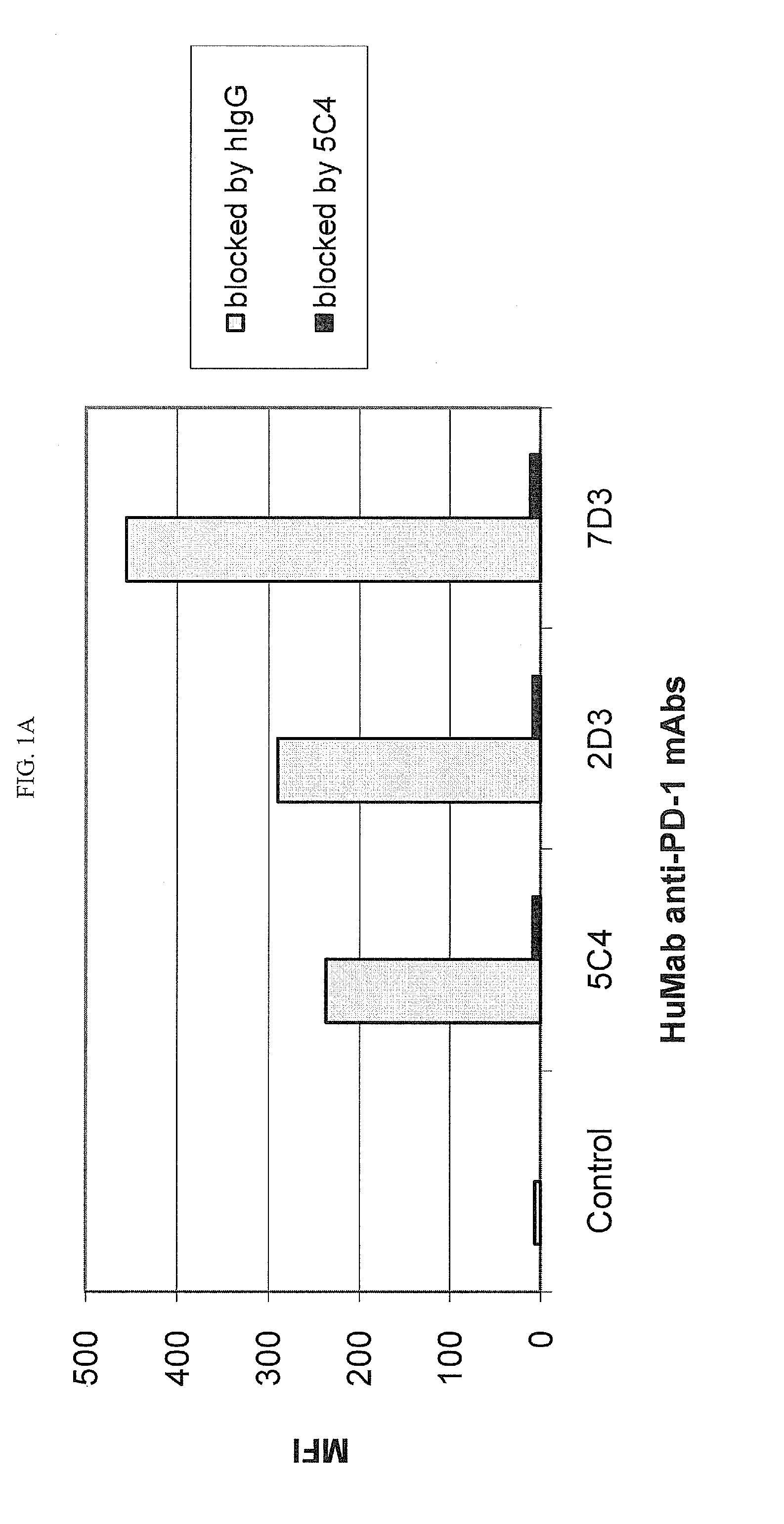
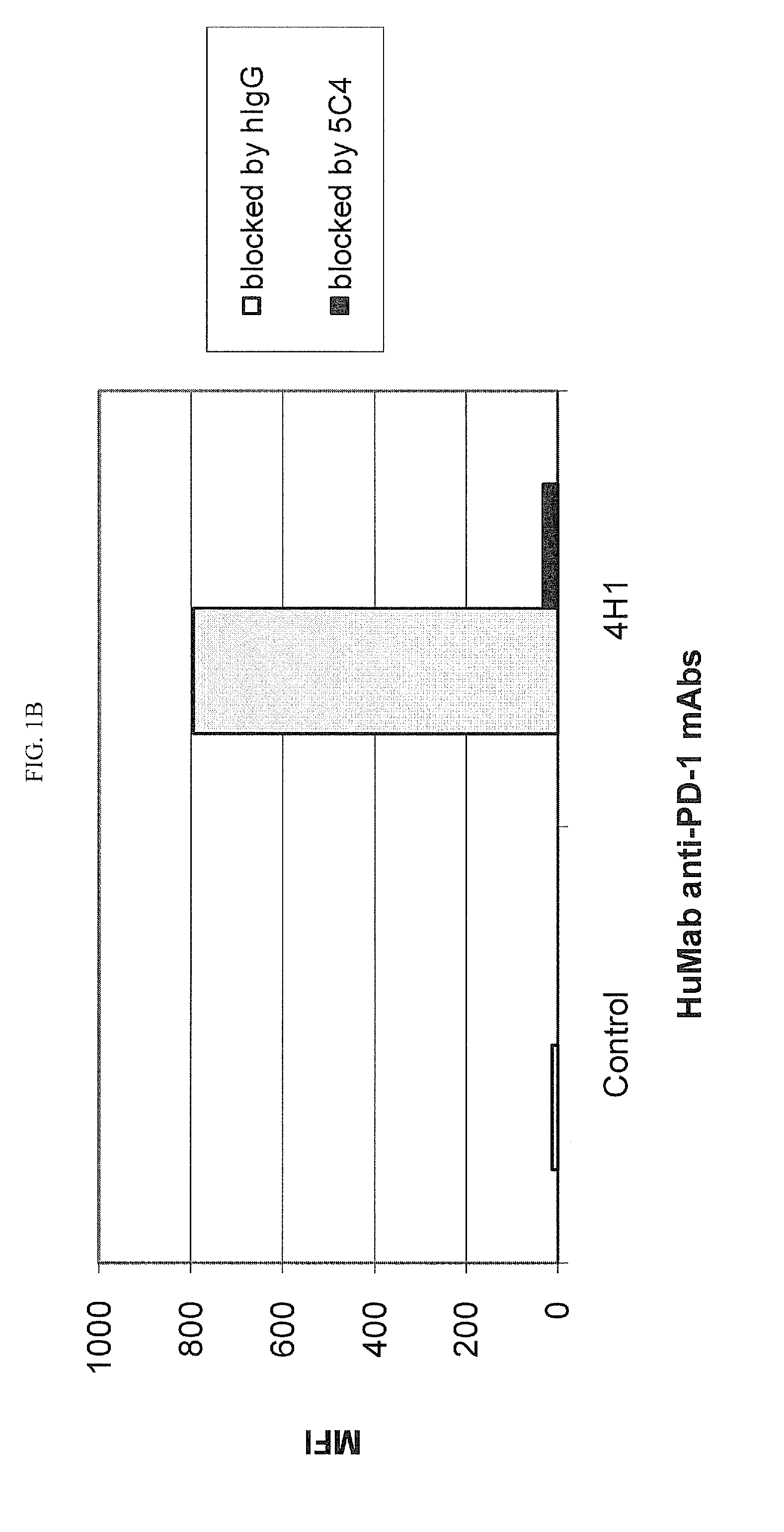
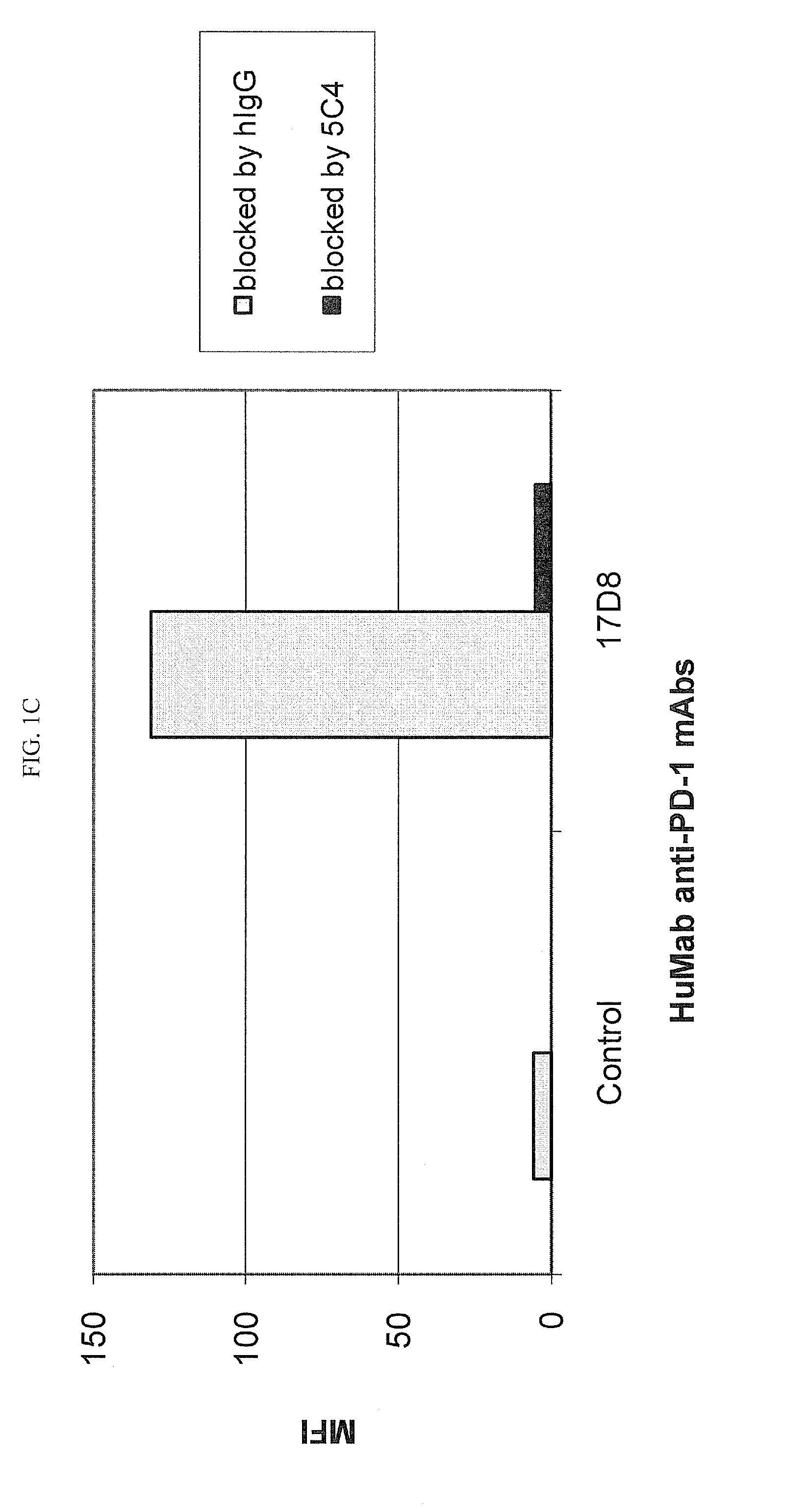
Human monoclonal Anti-pd-l1 antibodies and methods of use
ActiveUS20150274835A1Enhance immune responseIncrease in antigen specific T cell activityImmunoglobulins against cytokines/lymphokines/interferonsAntiviralsPD-L1Programmed death
The present invention comprises human monoclonal antibodies that bind to PD-L1 (also known as programmed death ligand 1 or B7H1). Binding of the invented antibody to PD-L1 inhibits binding to its receptor, PD1 (programmed death 1), and ligand-mediated activities and can be used to treat cancer and chronic viral infections.
Owner:DANA FARBER CANCER INST INC
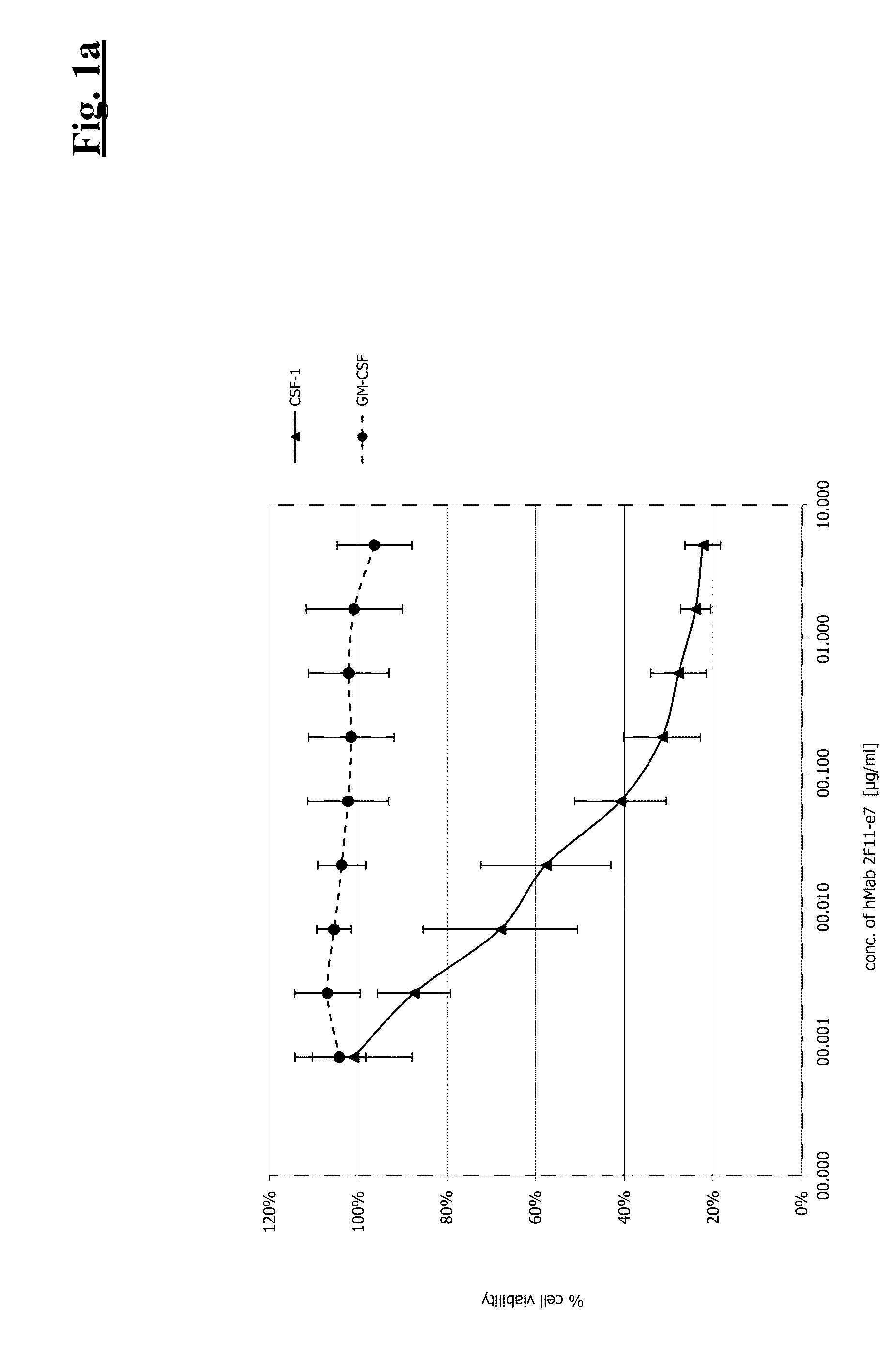
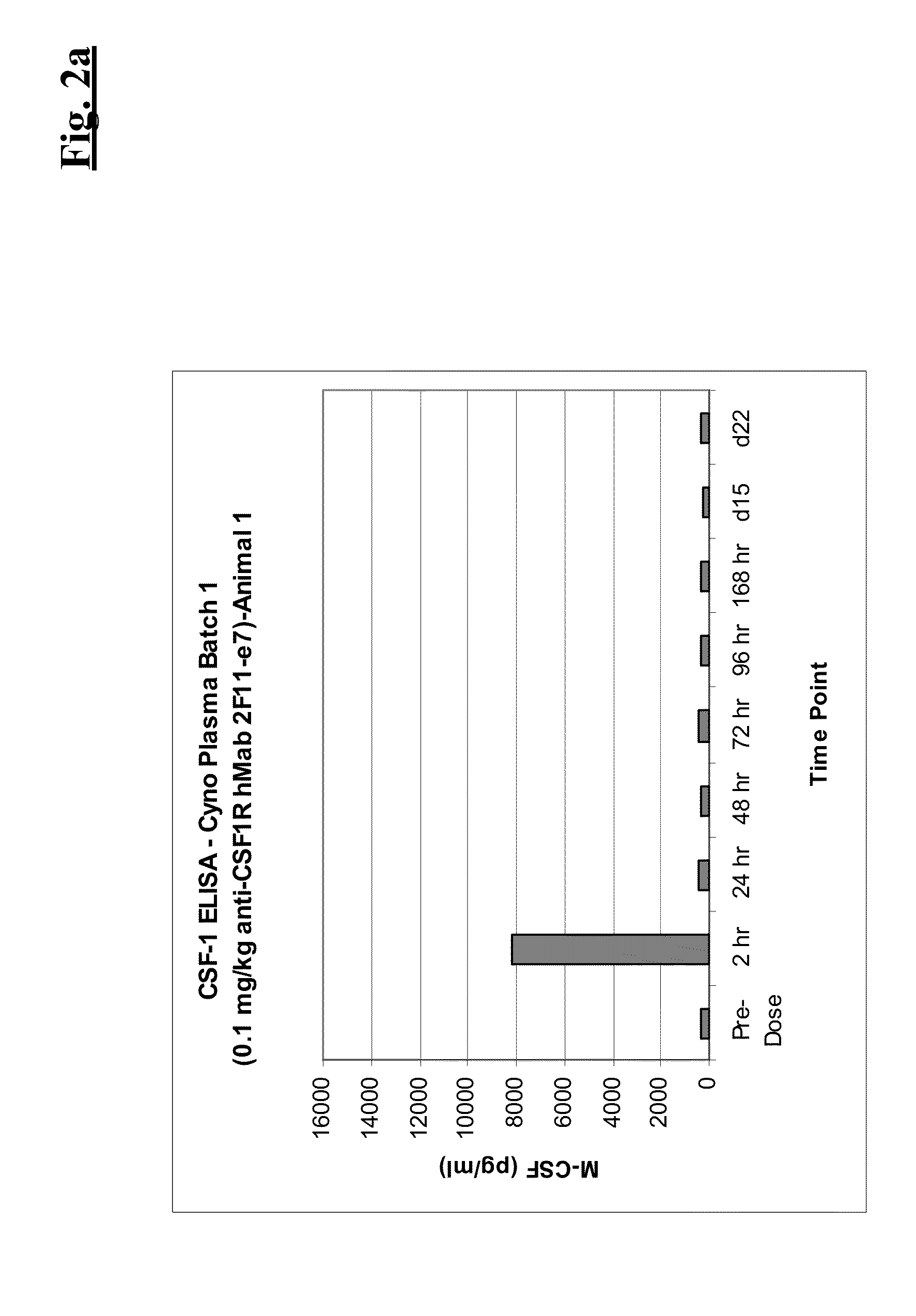
Combination therapy of antibodies against human CSF-1R and antibodies agains human PD-L1
InactiveUS20150073129A1Efficient antiproliferative activityUseful in treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPD-L1Combination therapy
The present invention relates to the combination therapy of specific antibodies which bind human CSF-1R with specific antibodies which bind human PD-L1.
Owner:F HOFFMANN LA ROCHE INC
PD-L1 Antibodies and Uses Thereof
ActiveUS20150346208A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentPD-L1
Provided herein are novel PD-L1 antibodies and methods for using the same for diagnosing a medical condition associated with elevated PD-L1 levels (e.g., cancer) in subjects in need thereof and antigen binding fragments thereof. The PD-L1 antibodies and antigen binding fragments are also useful in evaluating the efficacy of a particular therapeutic regime in a subject diagnosed as having a PD-L1-related medical condition.
Owner:VENTANA MEDICAL SYST INC
Method for Treating and Diagnosing Hematologic Malignancies
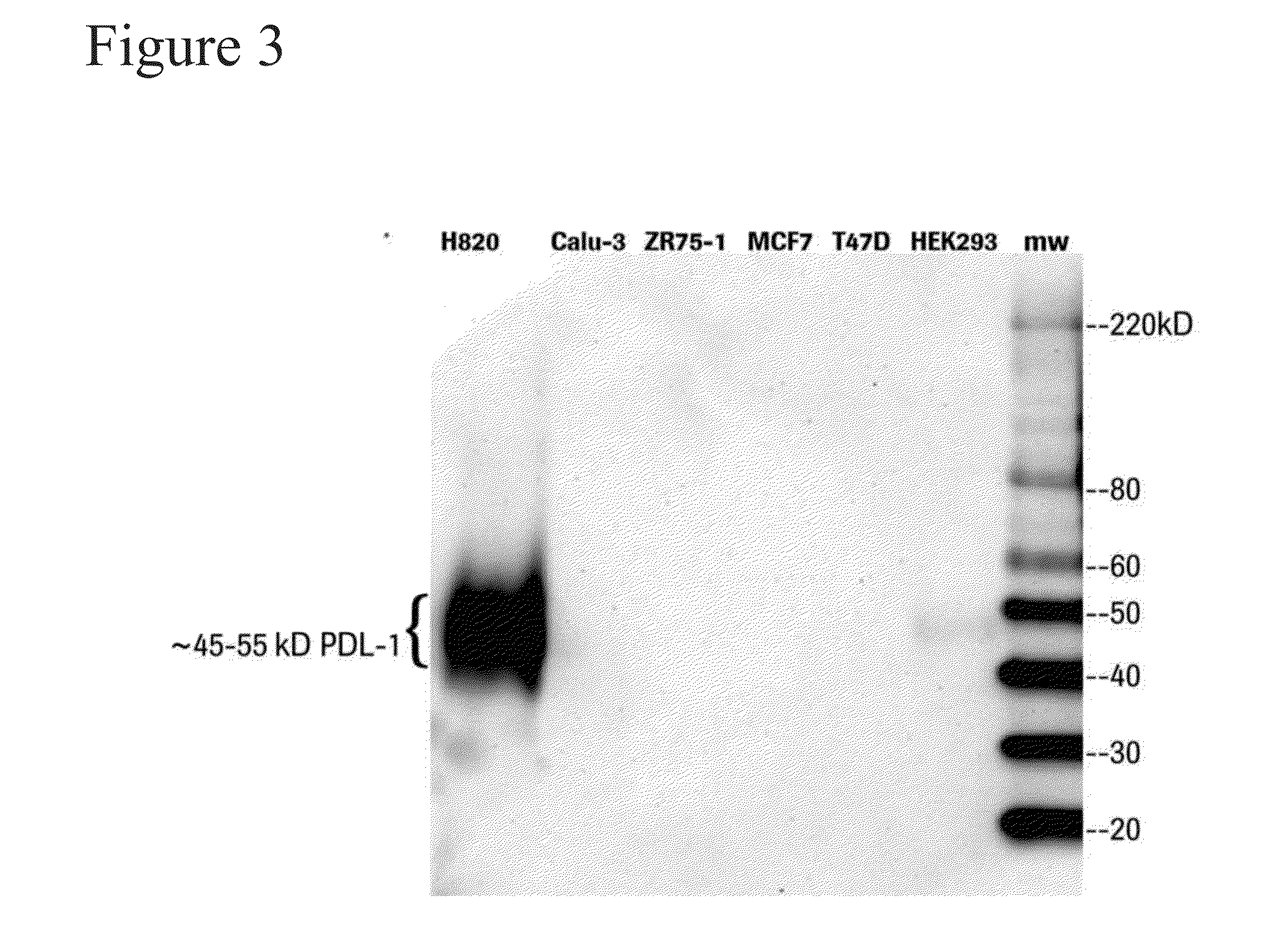
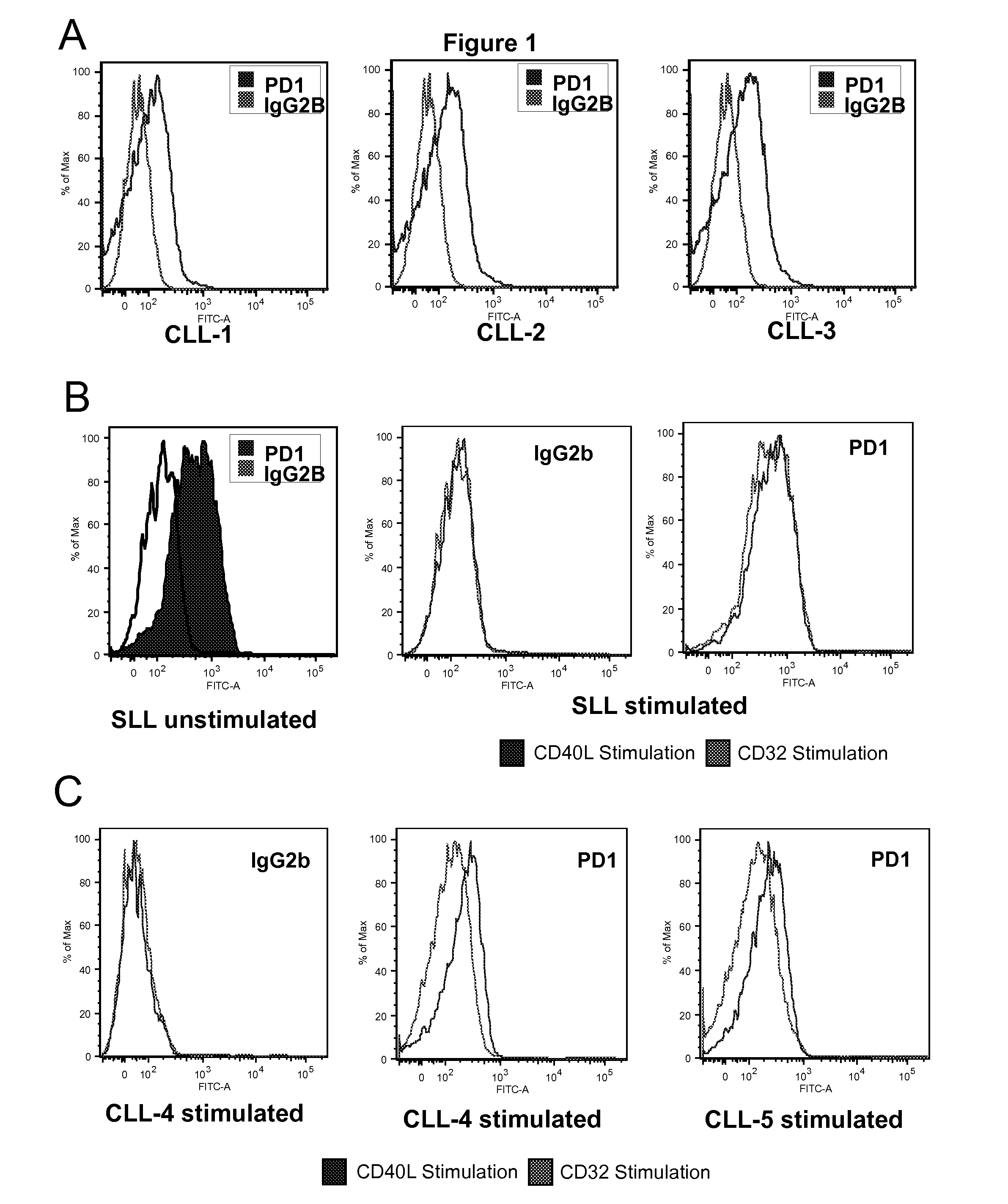
ActiveUS20110177088A1Avoid overactivationStrong cytotoxicityAnimal cellsPeptide/protein ingredientsPD-L1Oncology
The invention relates to methods for treating and diagnosing hematologic malignancies, Chronic lymphocytic leukemia and Small Lymphocytic Lymphoma in particular, using PD-1 ligands (PD-L1, PD-L2 or anti-PD-1 antibodies).
Owner:OLIVE DANIEL +2
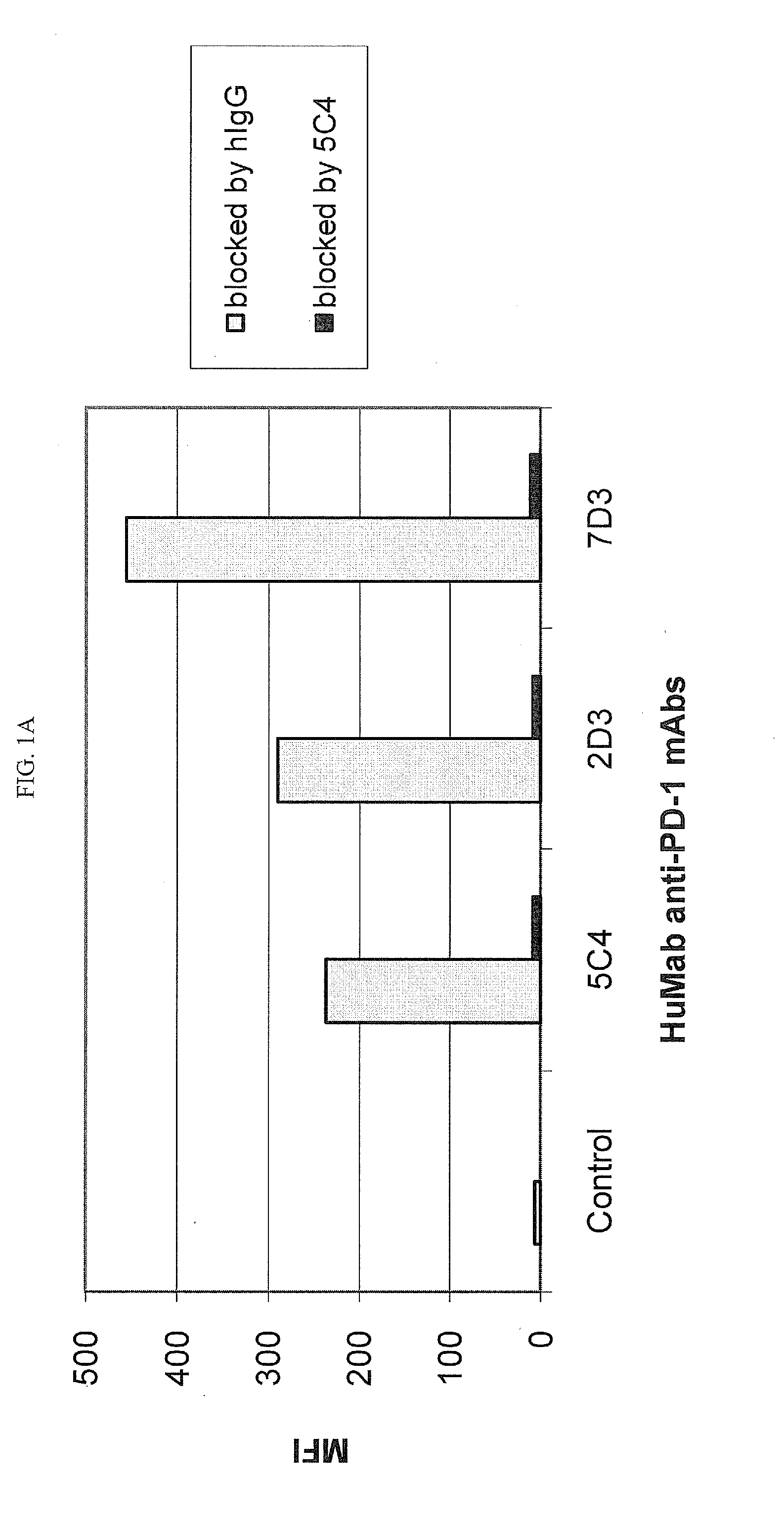
Anti-PD-1 humanized monoclonal antibody and application thereof
ActiveCN105175544AHigh affinityPlay effectivelyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyNucleotide
The invention discloses an anti-PD-1 humanized monoclonal antibody and application thereof, belonging to the technical field of molecular immunology. The anti-PD-1 humanized monoclonal antibody provided by the invention contains a light chain and a heavy chain, wherein the amino acid sequence of the light chain is as shown in SEQ ID NO.2, and the amino acid sequence of the heavy chain is as shown in SEQ ID NO.4 or SEQ ID NO.6. Meanwhile, the invention also provides a nucleotide sequence coding the light chain and heavy chain. The anti-PD-1 humanized monoclonal antibody provided by the invention has very high affinity with human PD-1 protein, and can interdict PD-1 / PD-L1 combination so as to promote T cell proliferation and IFN-gamma secretion.
Owner:ANHUI RUBIOX VISION BIOTECH
Pd-1 antagonists and methods for treating infectious disease
InactiveUS20110159023A1Rapid induction of protectionRobust effector responseAntibacterial agentsOrganic active ingredientsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by binding to and blocking PD-1 to prevent or reduce inhibitory signal transduction, or by binding to ligands of PD-1 such as PD-L1, thereby preventing (in whole or in part) the ligand from binding to PD-1 to deliver an inhibitory signal. The immune response can be modulated by providing antagonists which bind with different affinity (i.e., more or less as required), by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again (similar to what occurs with antigen elicitation using priming and boosting). In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
Regulatory t cell mediator proteins and uses thereof
ActiveUS20110027278A1Eliminate the effects ofPeptide/protein ingredientsAntibody mimetics/scaffoldsRegulatory T cellAllergy
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNF. Proteins, antibodies, activated T cells and methods for using the same are disclosed.In particular methods of using these proteins and compounds, preferably antibodies, which bind or modulate (agonize or antagonize) the activity of these proteins, as immune modulators and for the treatment of cancer, autoimmune disease, allergy, infection and inflammatory conditions, e.g. multiple sclerosis is disclosed
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Therapeutic compounds for immunomodulation
The present invention provides Immunosuppressive compounds capable of inhibiting the programmed cell death 1 (PD1) signalling pathway. The present invention further provides peptide based compositions for treatment of cancer or treatment of infections via immunopotentiation caused by inhibition of immunosuppressive signalling induced by PD-1, PD-L1, or PD-L2 and therapies using them, immunopotentiative substrates included as the active ingredient. Further, the invention provides pharmaceutical compositions comprising the Immunosuppressive peptide compounds or modified peptide moieties for preventive and / or therapeutic agents for cancer, cancer metastasis, immunodeficiency, an infectious disease or the like and an application of PD-1 or PD-L1 as a testing or diagnostic agent or a research agent for such a disease.
Owner:AURIGENE DISCOVERY TECH
Regulatory T Cell Mediator Proteins and Uses Thereof
The present invention relates to novel regulatory T cell proteins. One protein, designated PD-L3, resembles members of the PD-L1 family, and co-stimulates αCD3 proliferation of T cells in vitro. A second, TNF-like, protein has also been identified as being upregulated upon αCD3 / αGITR stimulation. This protein has been designated Treg-sTNE Proteins, antibodies, activated T cells and methods for using the same are disclosed.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
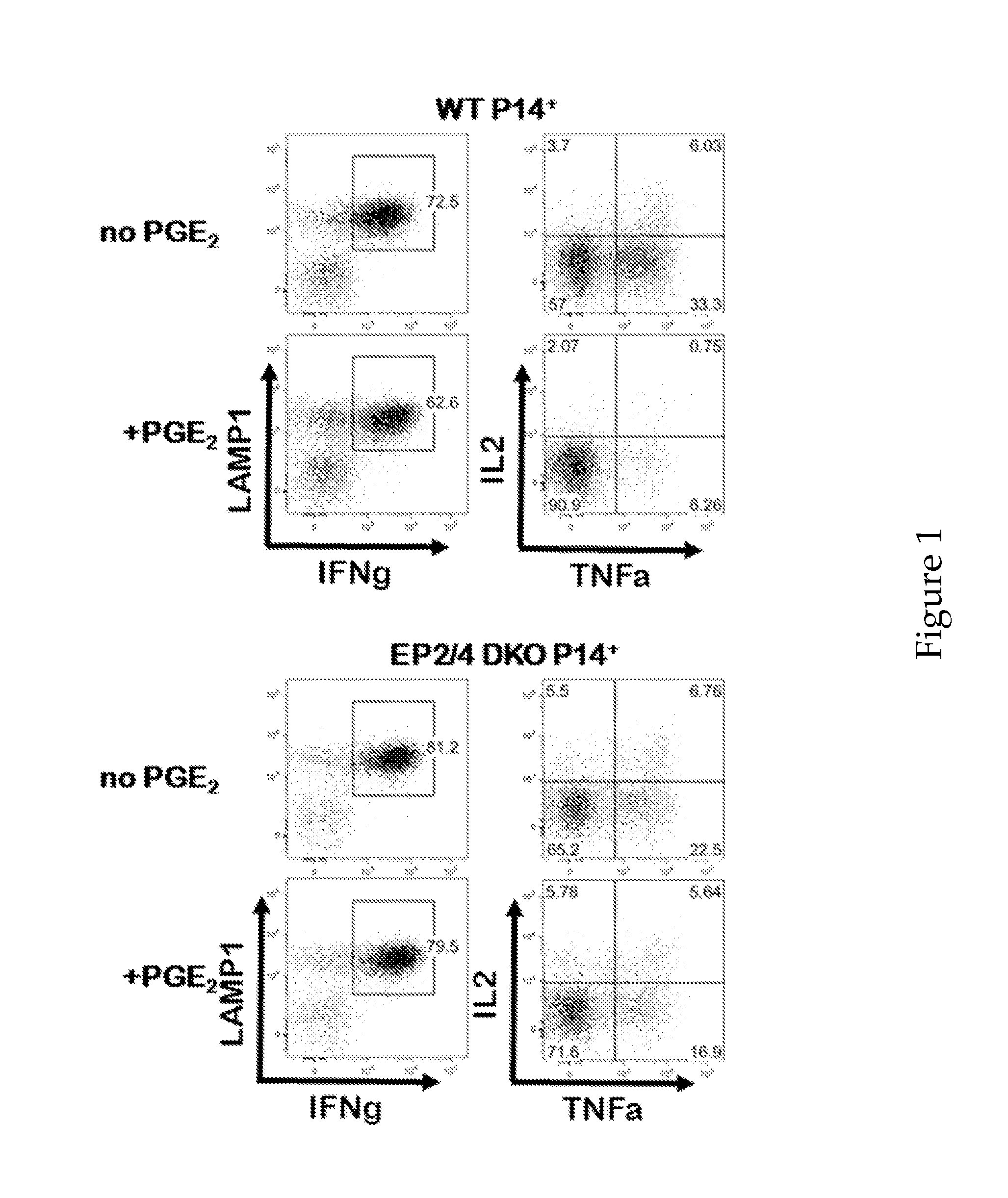
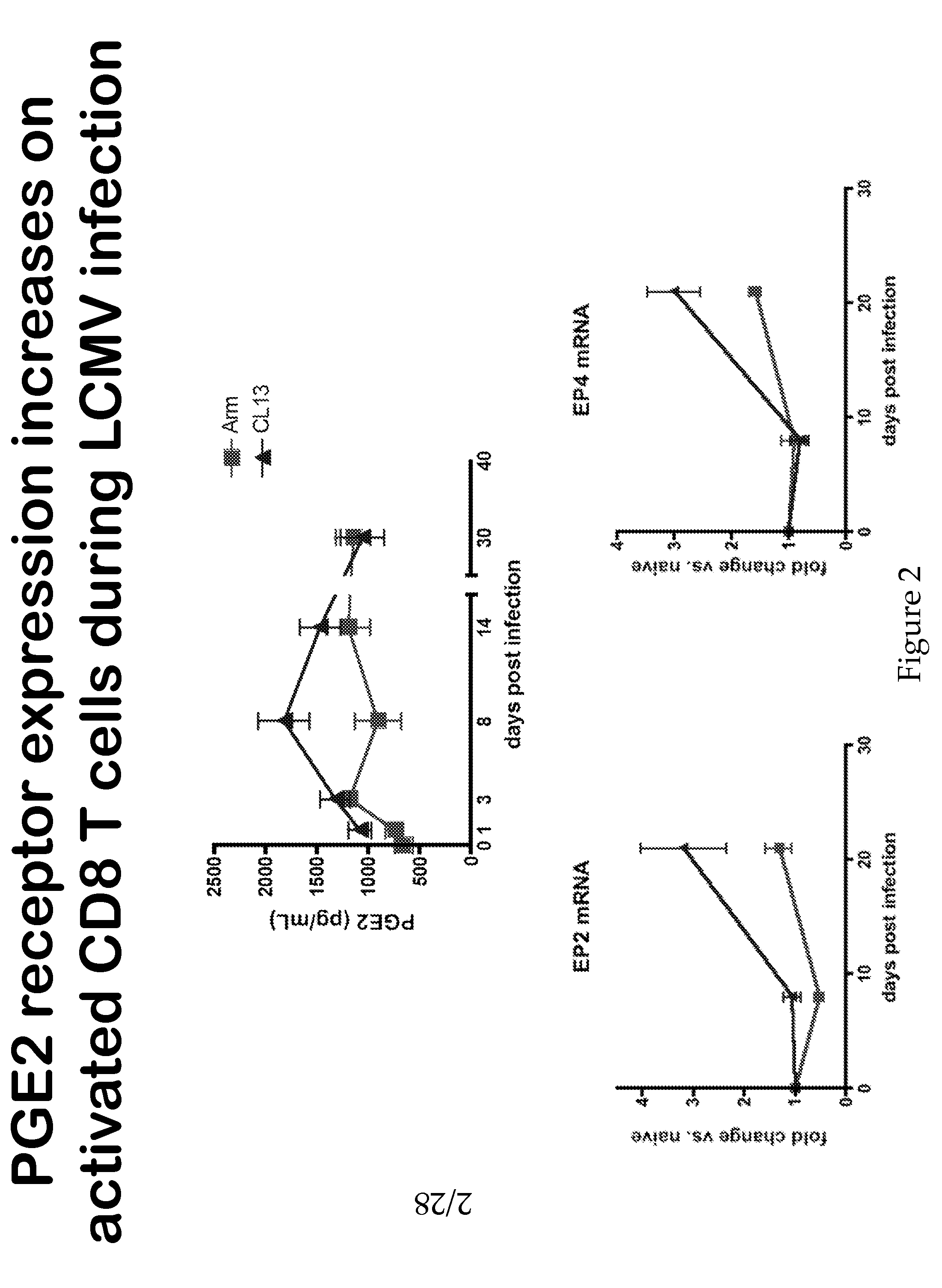
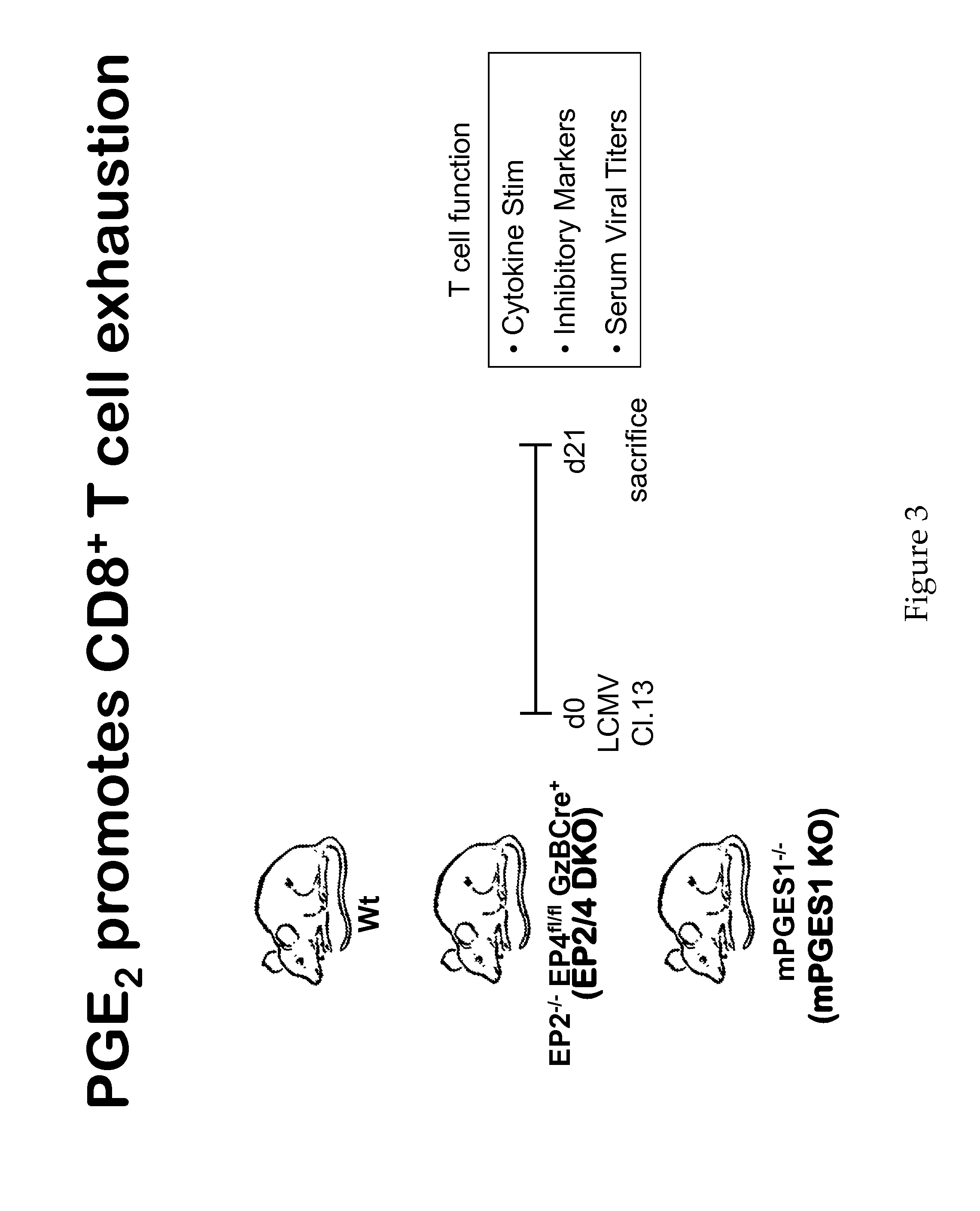
Compositions and Methods for Reducing CTL Exhaustion
InactiveUS20150004175A1Preventing CTL exhaustionReduce exhaustBiocideOrganic active ingredientsPD-L1Viral infection
The compositions and methods described herein are useful for diminishing CTL exhaustion in a subject in need thereof, during an immune response to a viral infection or during an immune response to cancer, thereby leading to a greater CTL response against the viral infection or cancer. The invention relates to compositions and methods for the therapeutic intervention of signaling through EP2 and EP4, by inhibiting at least one of EP2, EP4, PGE2, or combinations thereof. The invention also relates to compositions and methods for the therapeutic intervention of signaling through EP2 and EP4, in combination with the therapeutic intervention of signaling through PD-1, by inhibiting at least one of EP2, EP4, PGE2, or combinations thereof, in combination with inhibiting at least one of PD-1, PD-L1, PD-L2, and combinations thereof.
Owner:YALE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com