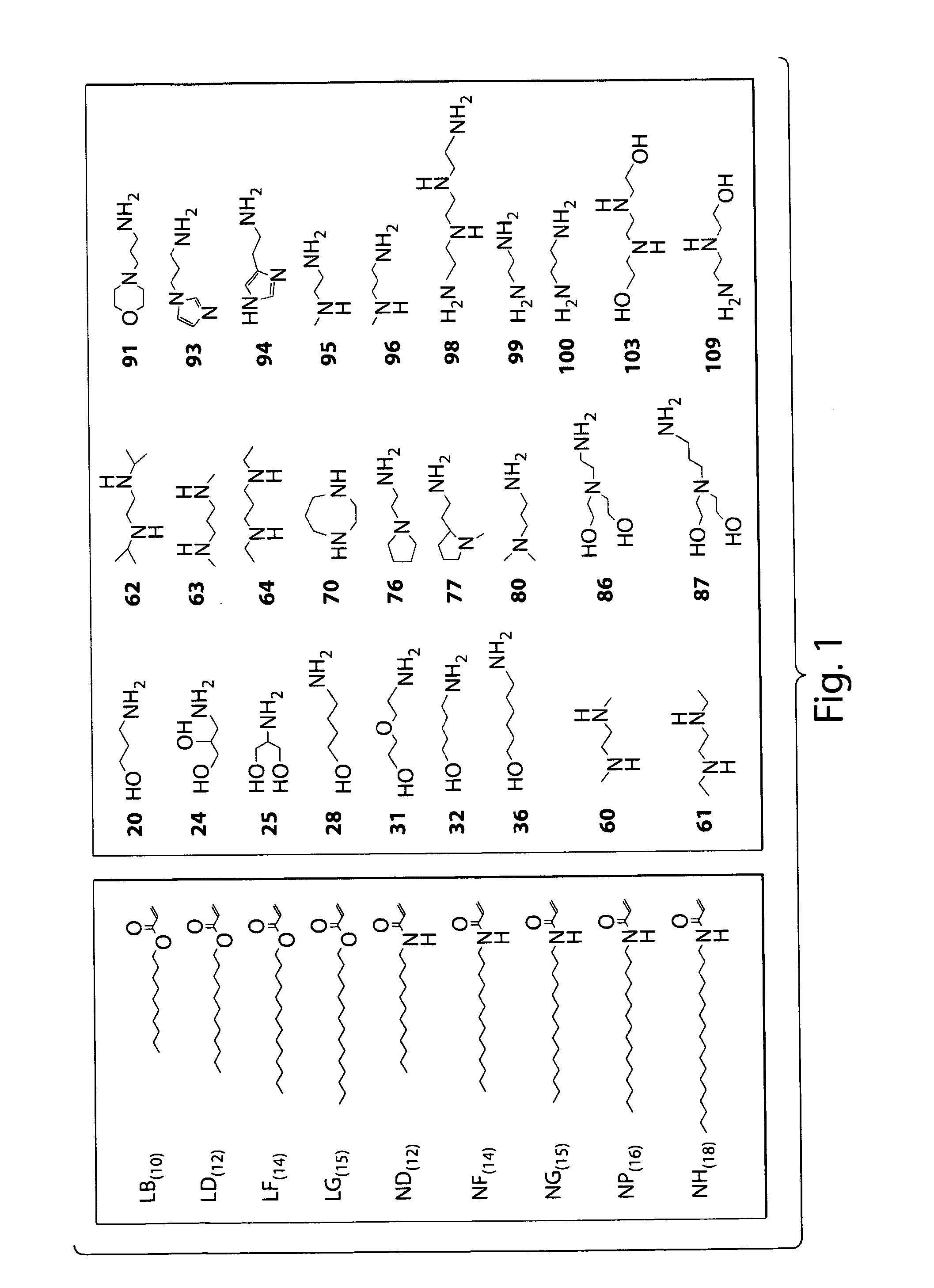
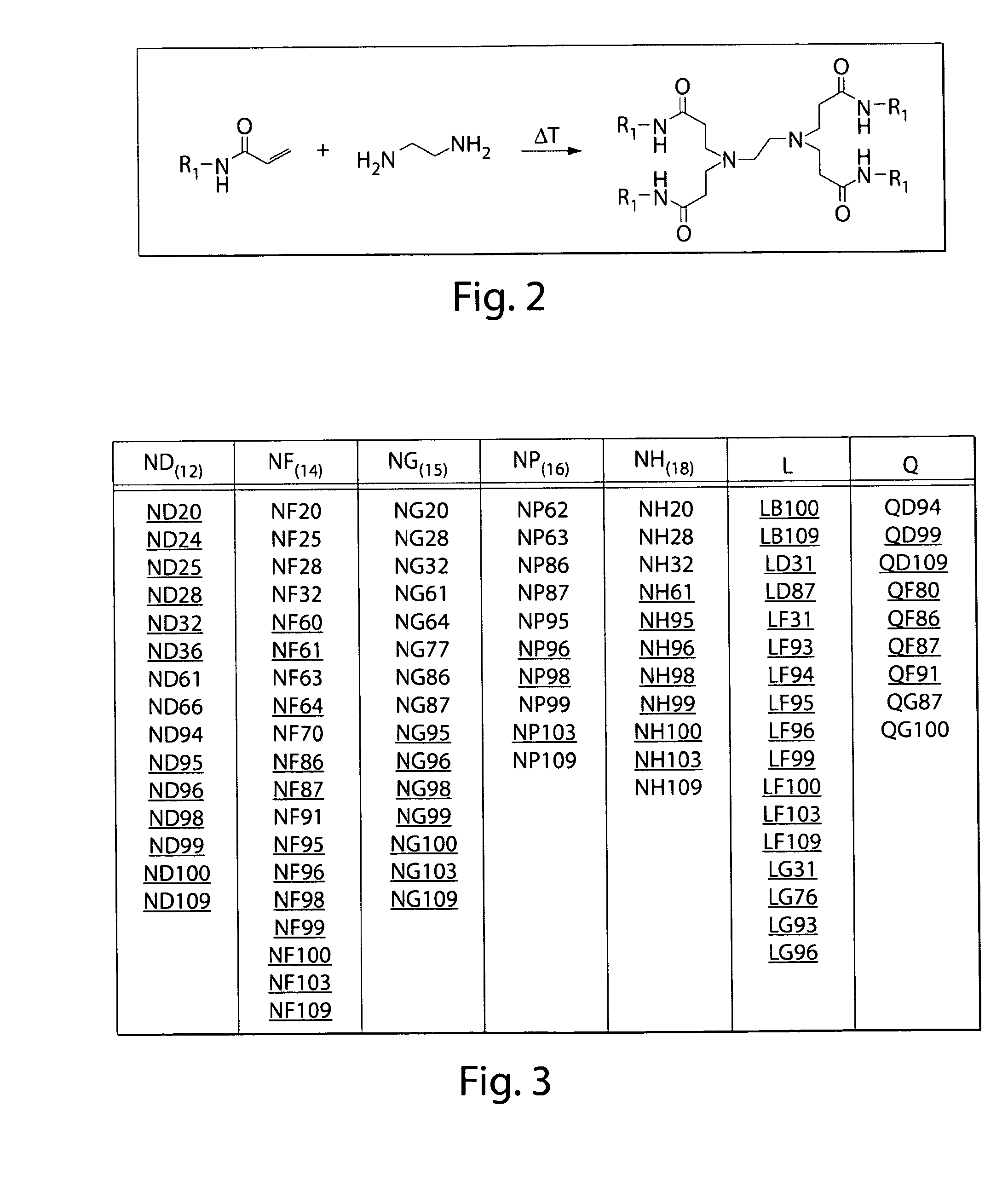
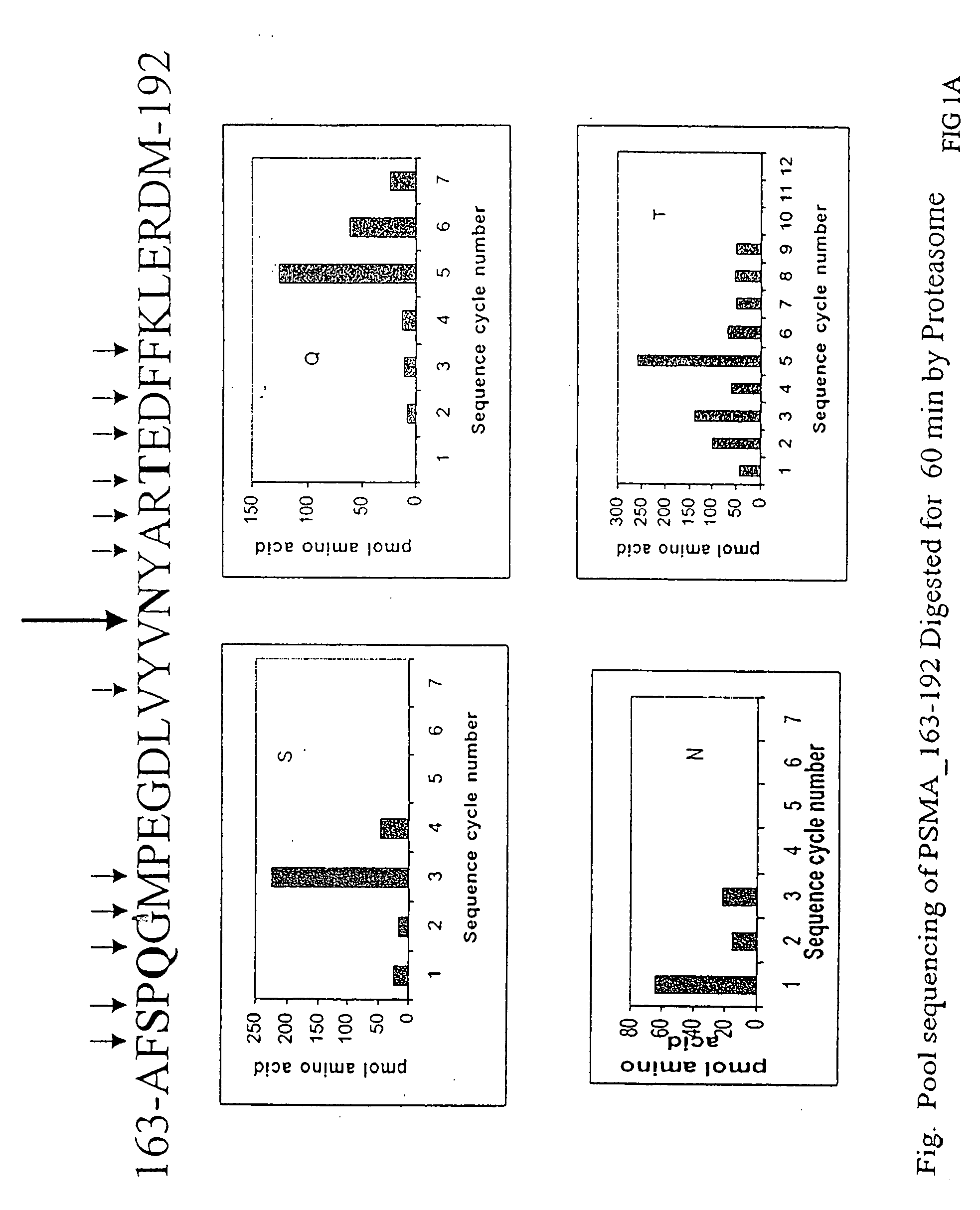
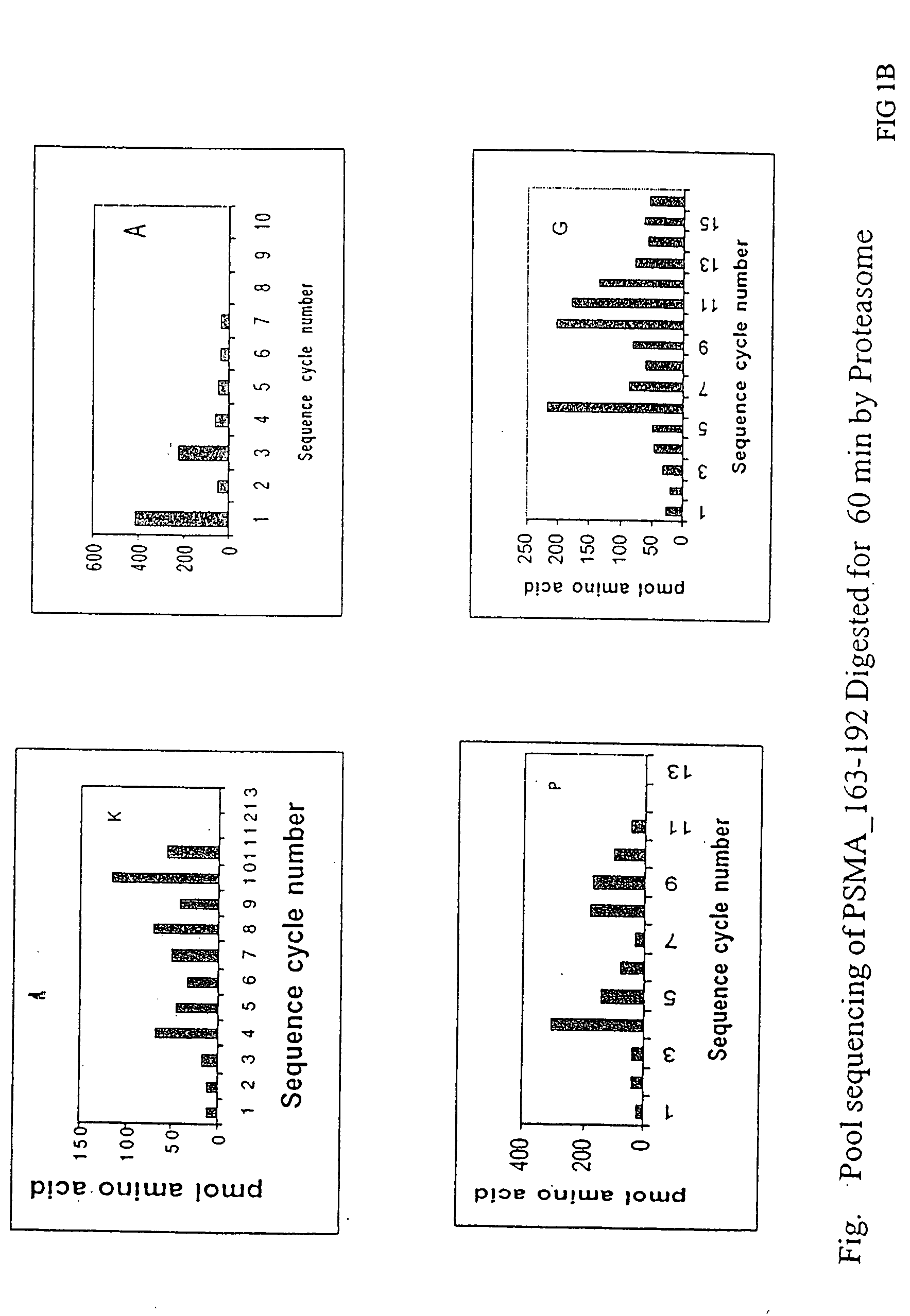
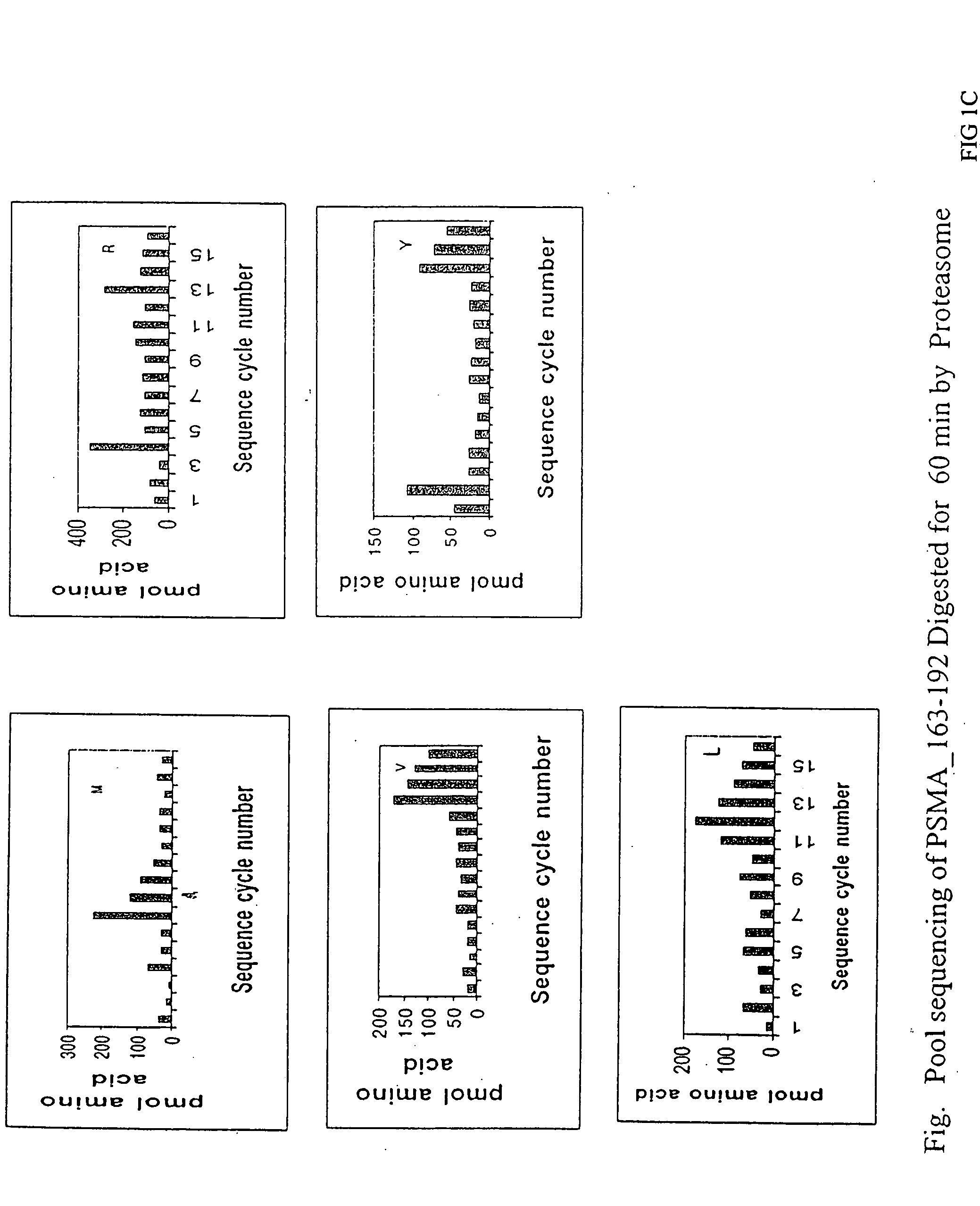
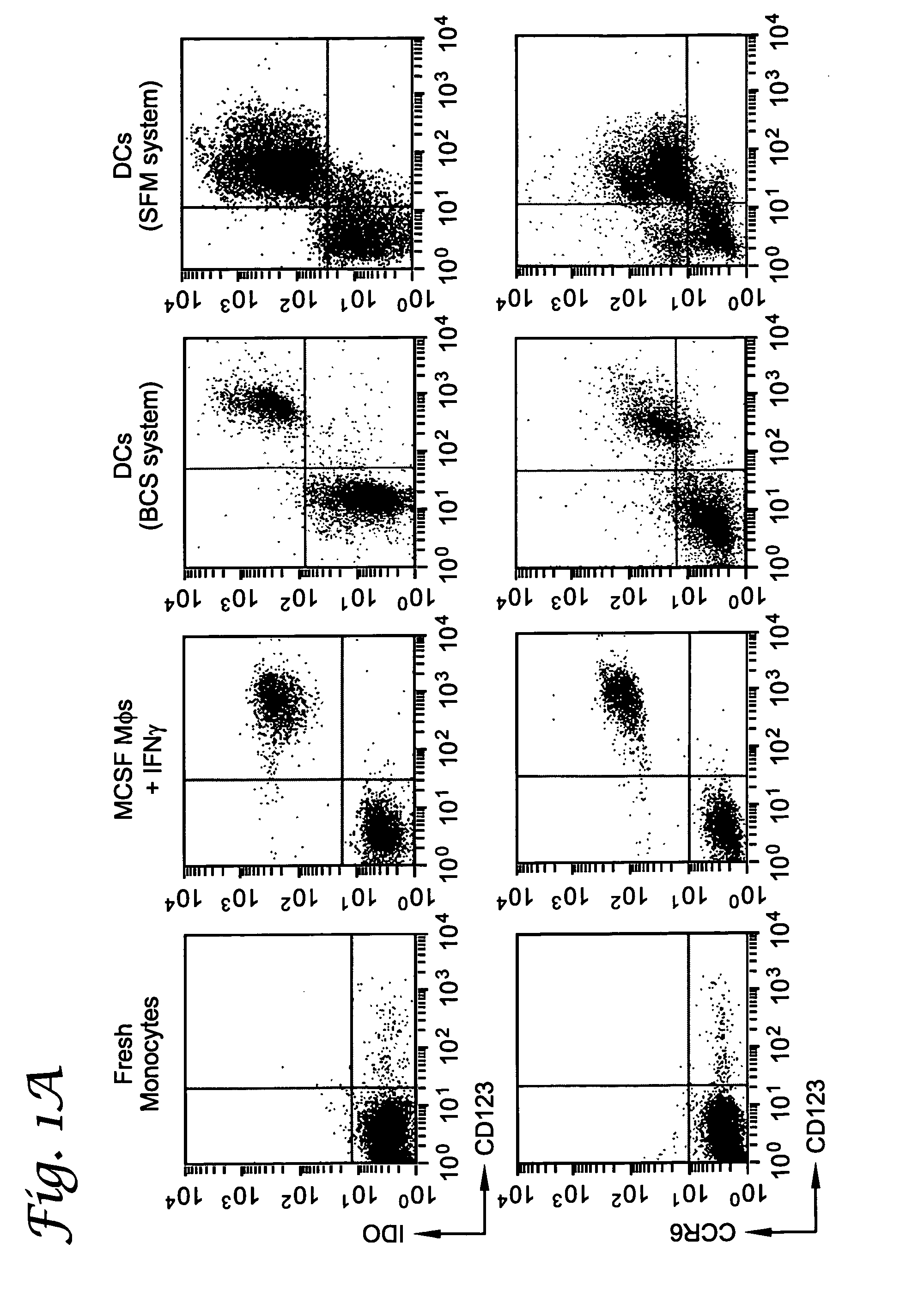
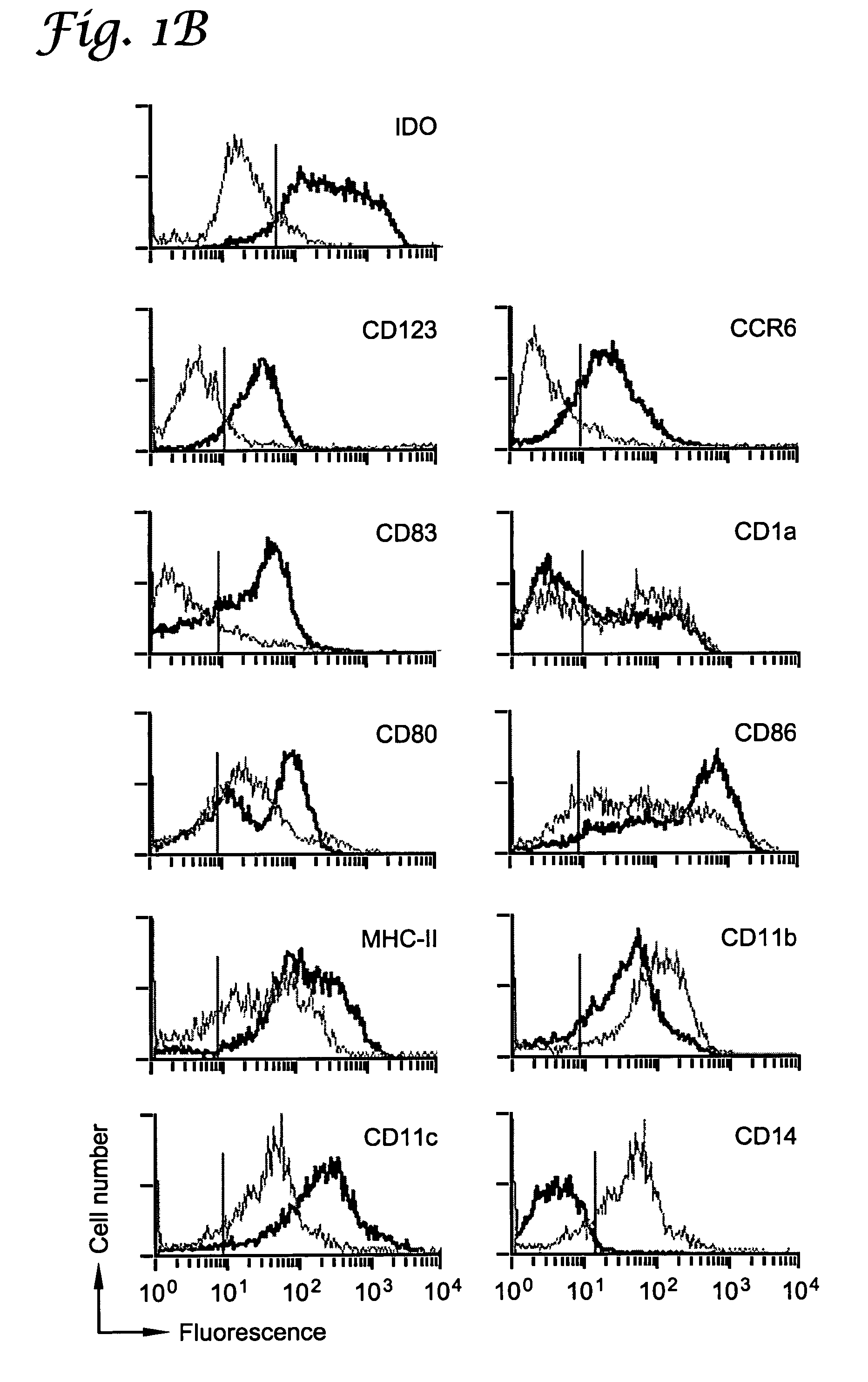
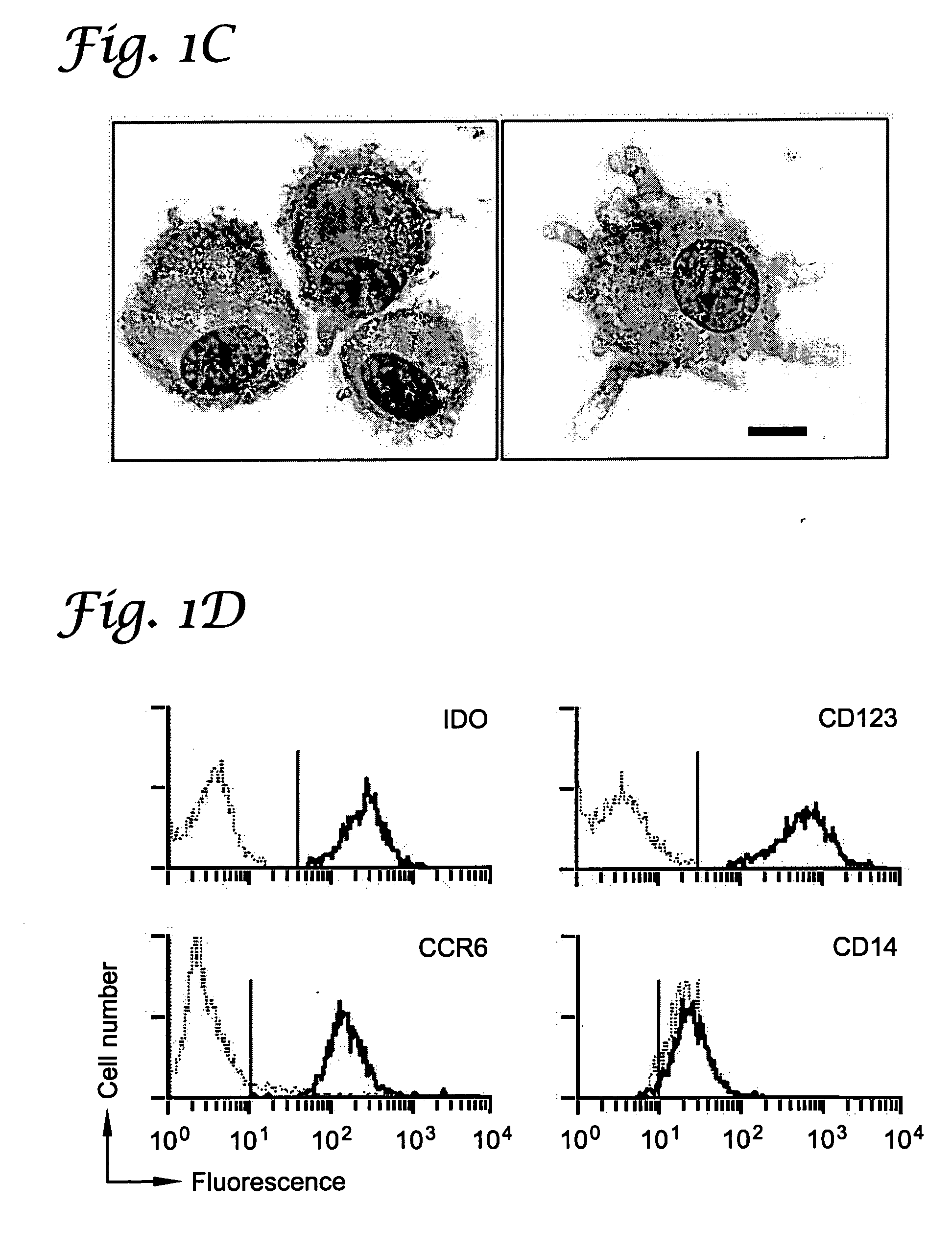
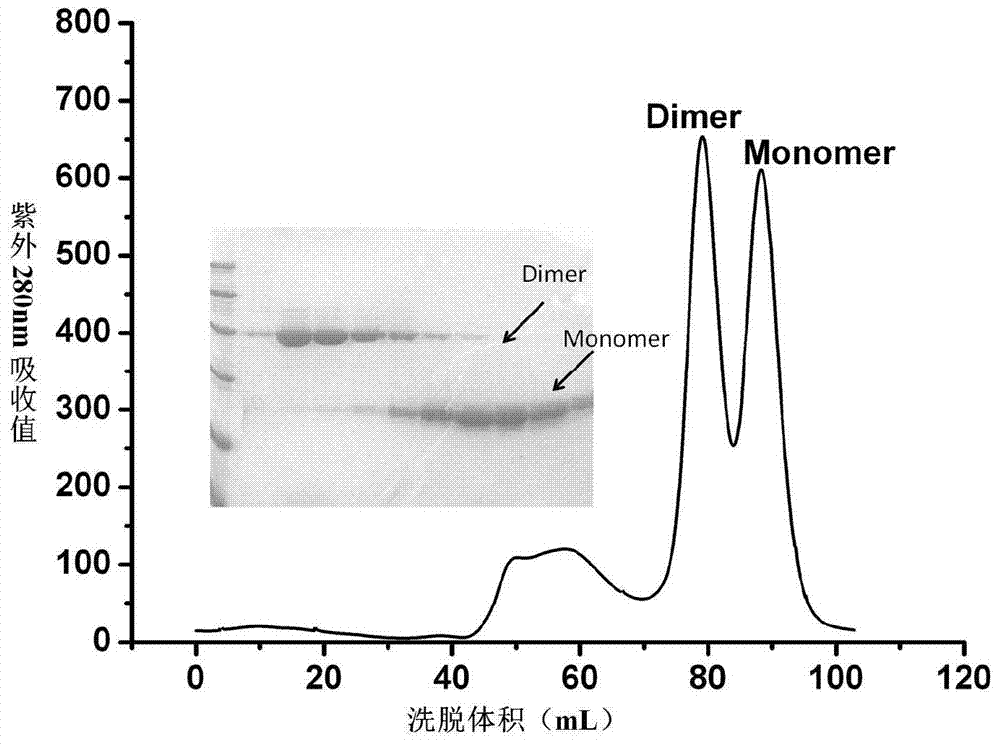
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
214 results about "Cell mediated immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cell-Mediated Immunity. Meaning. The humoral immunity is associated with the B-lymphocytes and is responsible for destroying the pathogens by producing antibodies against it. The cell-mediated immunity is associated with the T-lymphocytes and is responsible for destroying the pathogens or microorganism which have invaded the cells.
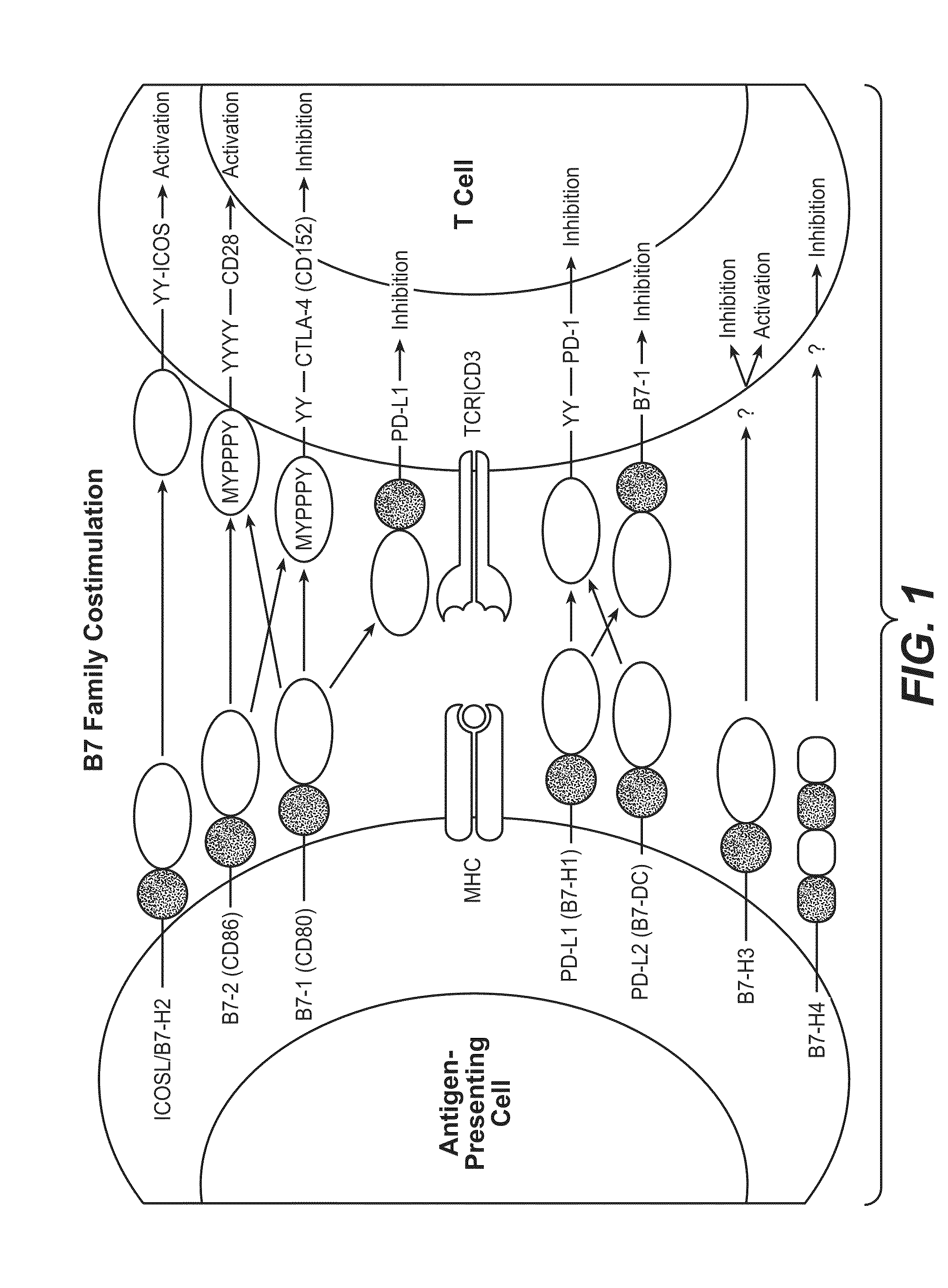
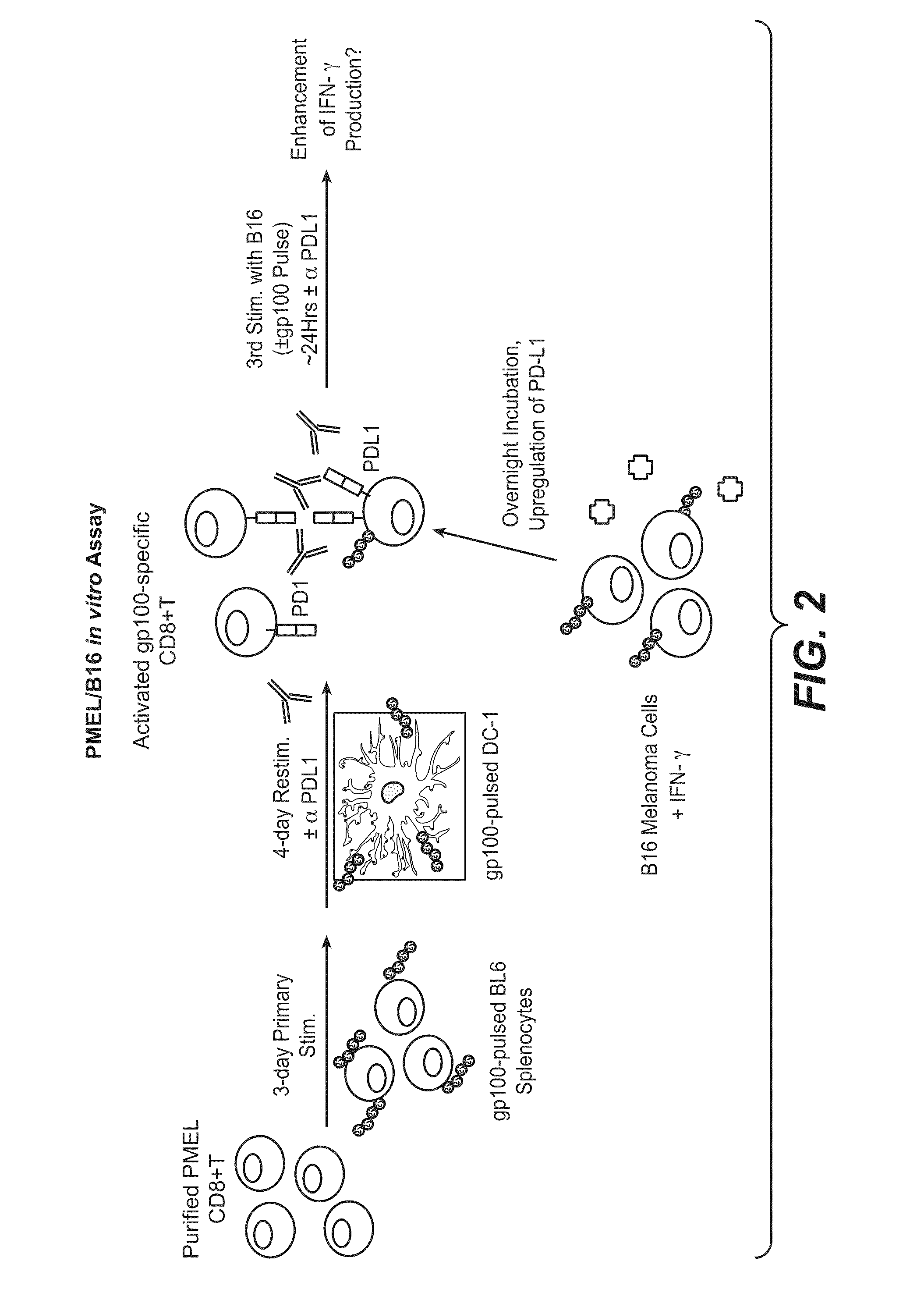
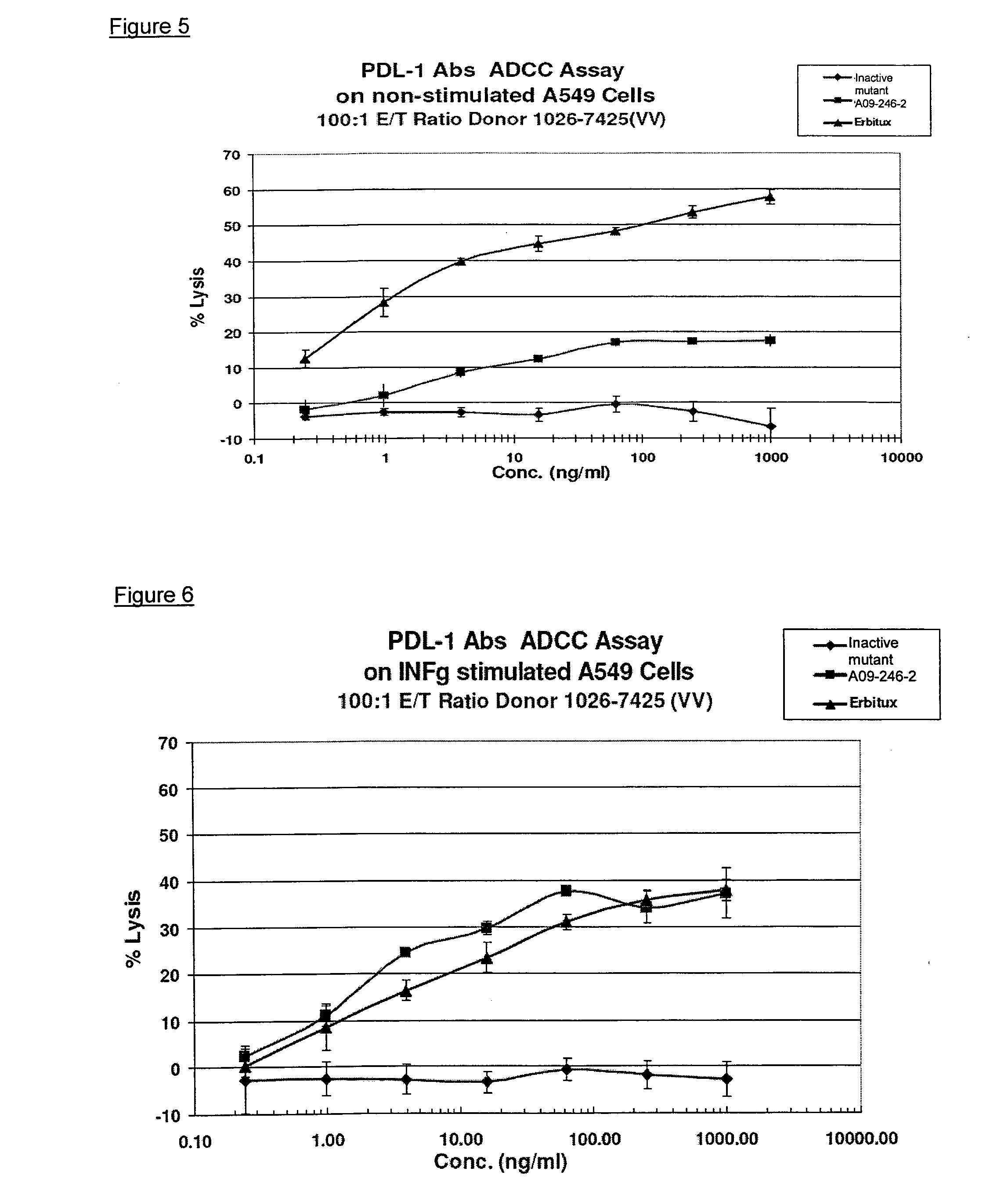
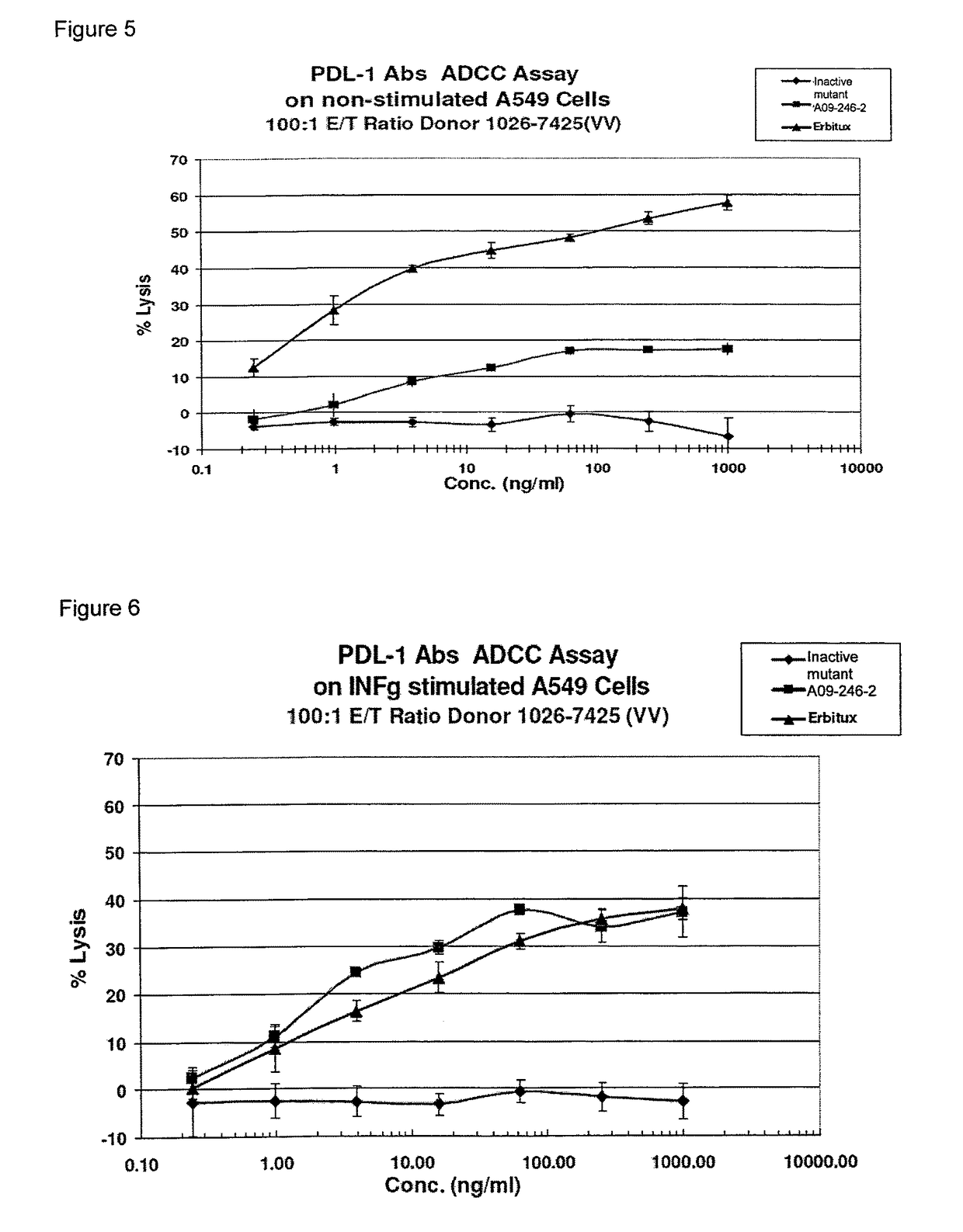
Anti-PD-L1 antibodies, compositions and articles of manufacture
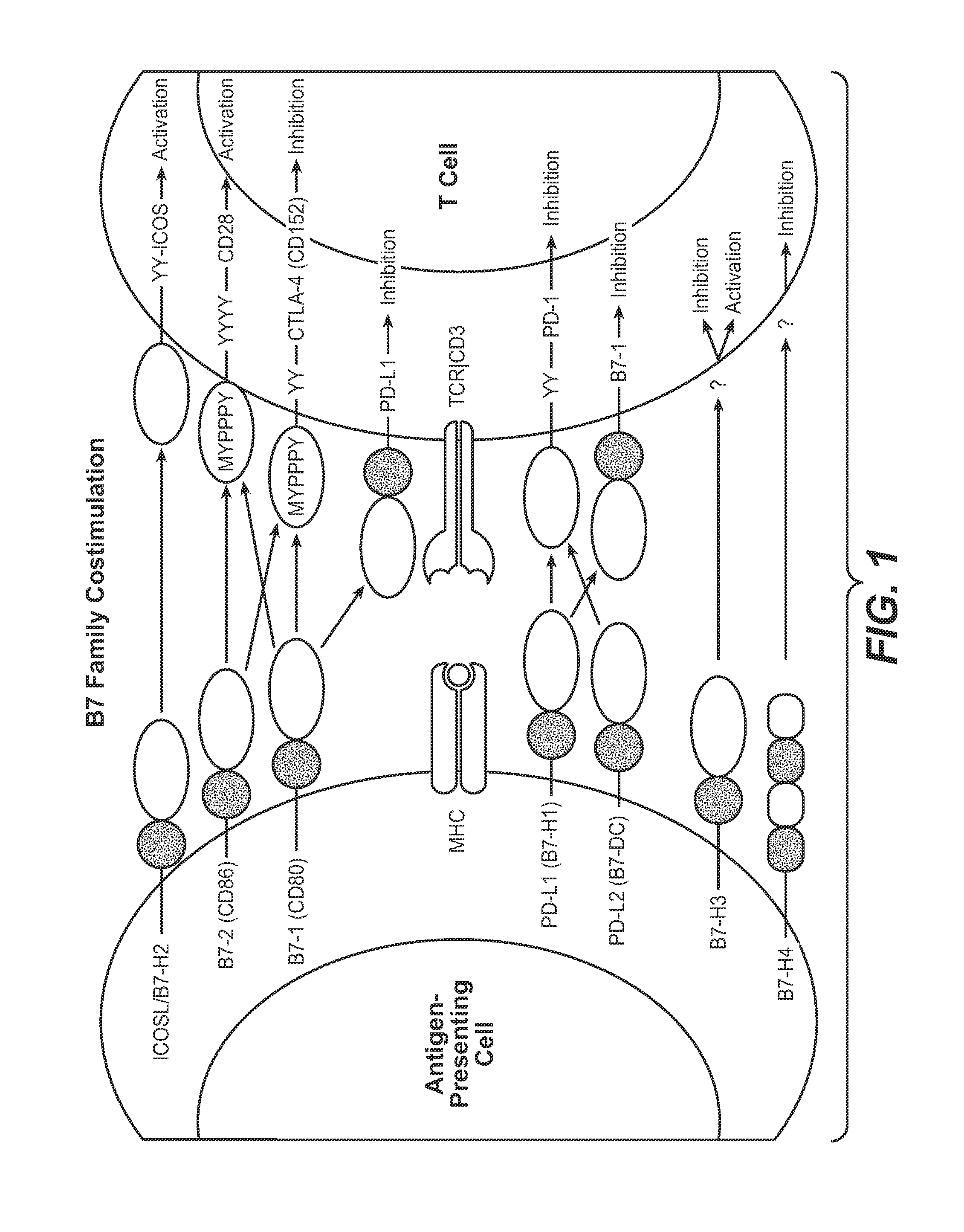
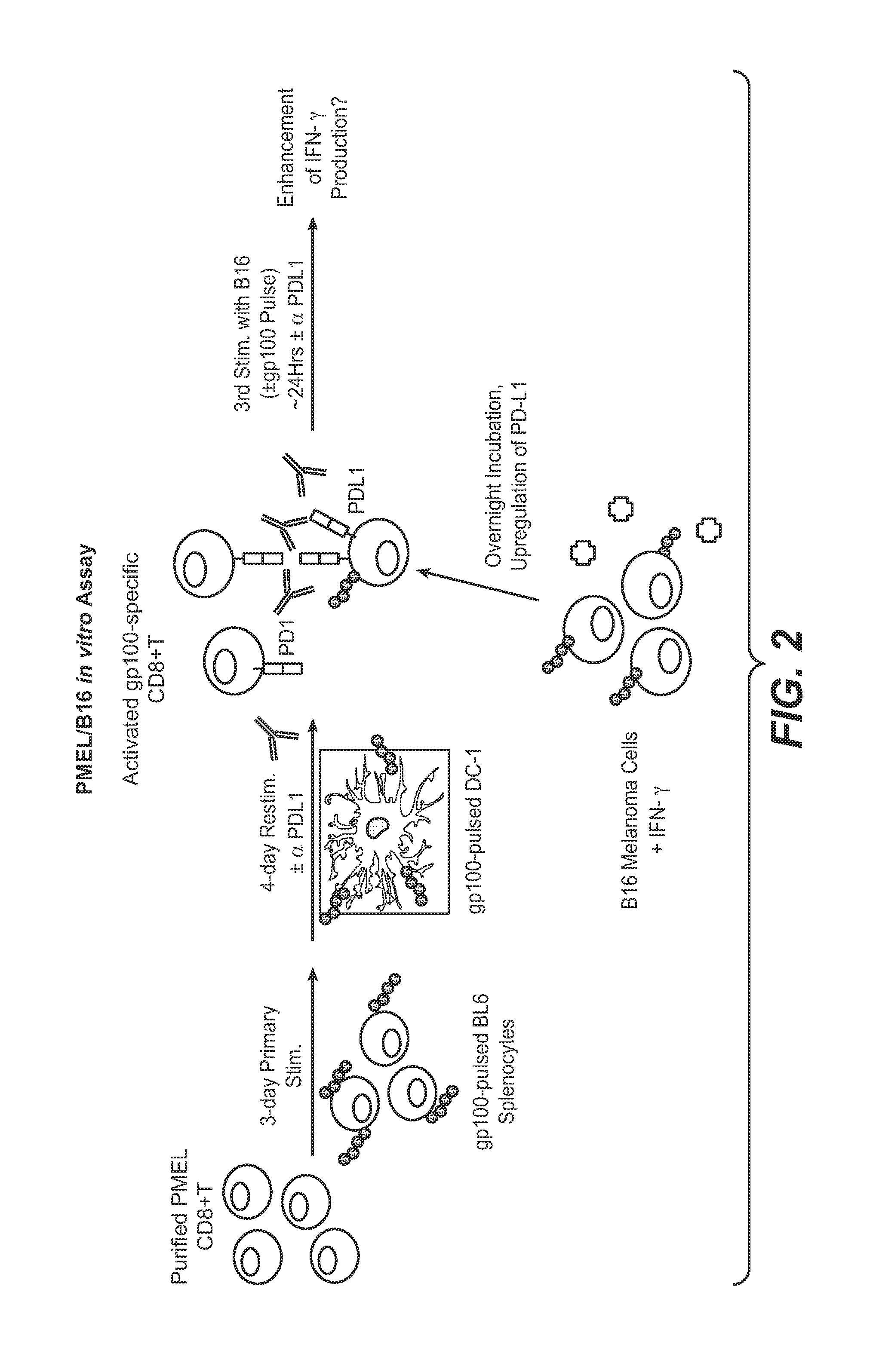
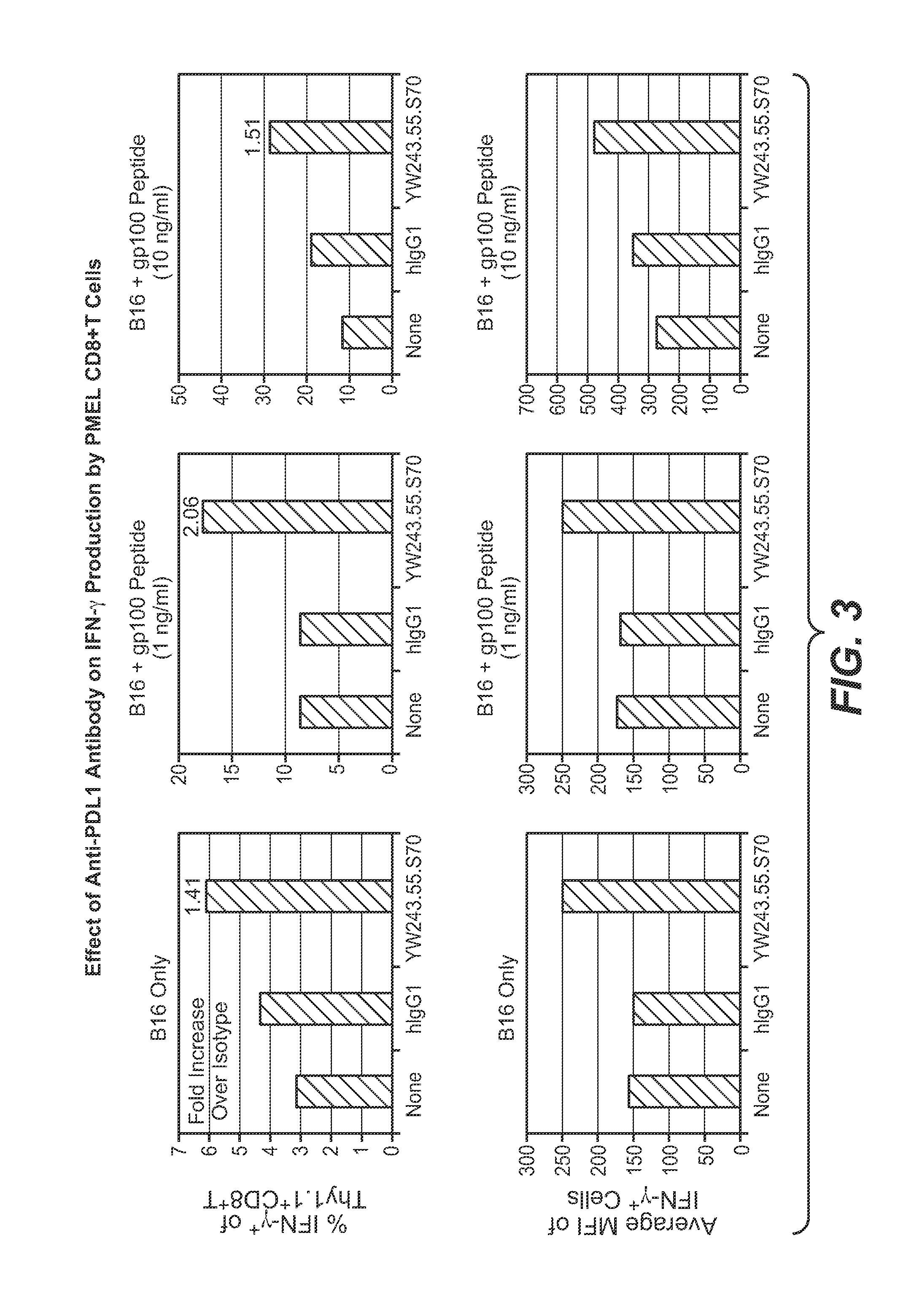
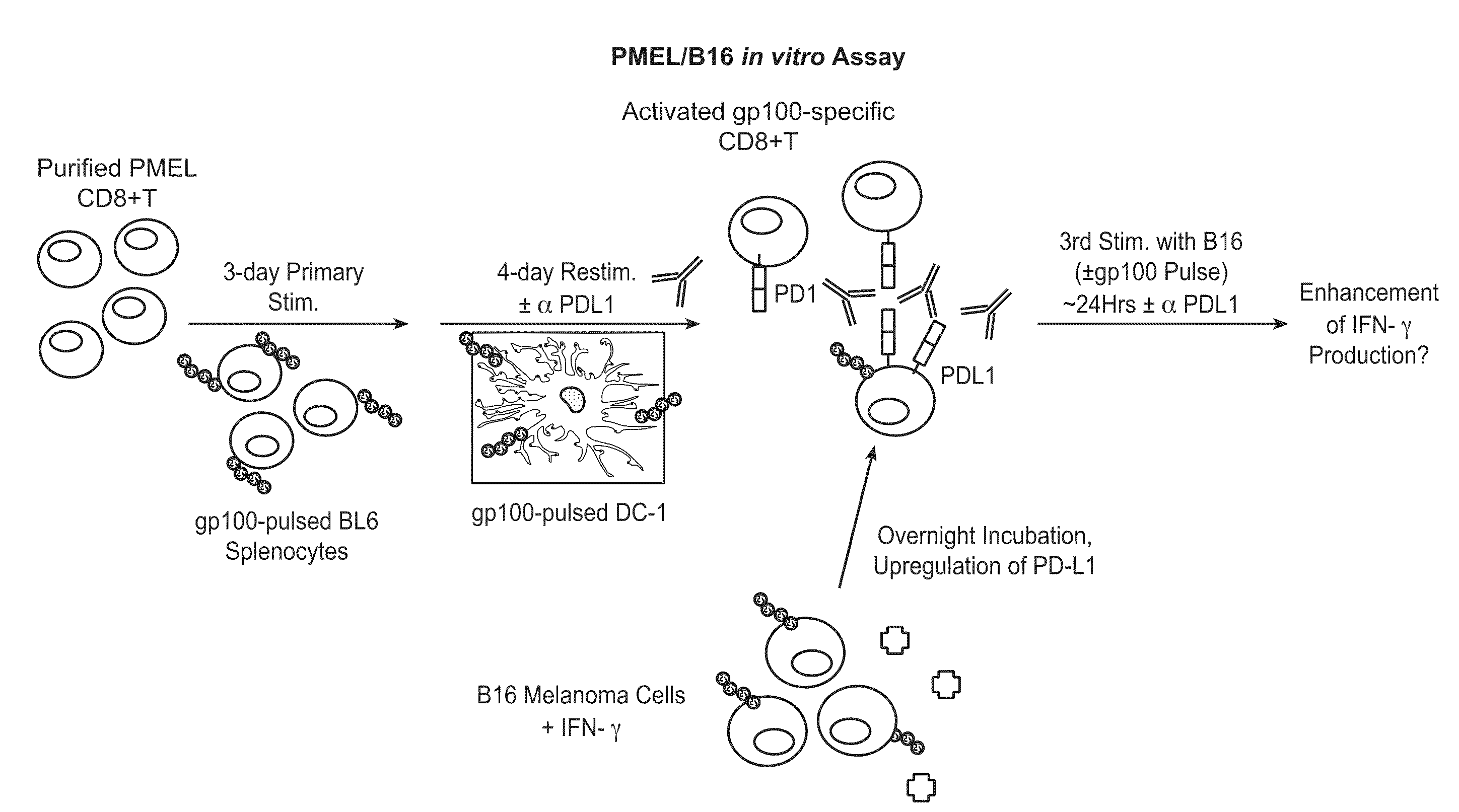
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-pd-l1 antibodies and their use to enhance t-cell function
ActiveUS20100203056A1Reduce level of pathogenChronic infectionAntibacterial agentsOrganic active ingredientsT-cell dysfunctionPD-L1
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
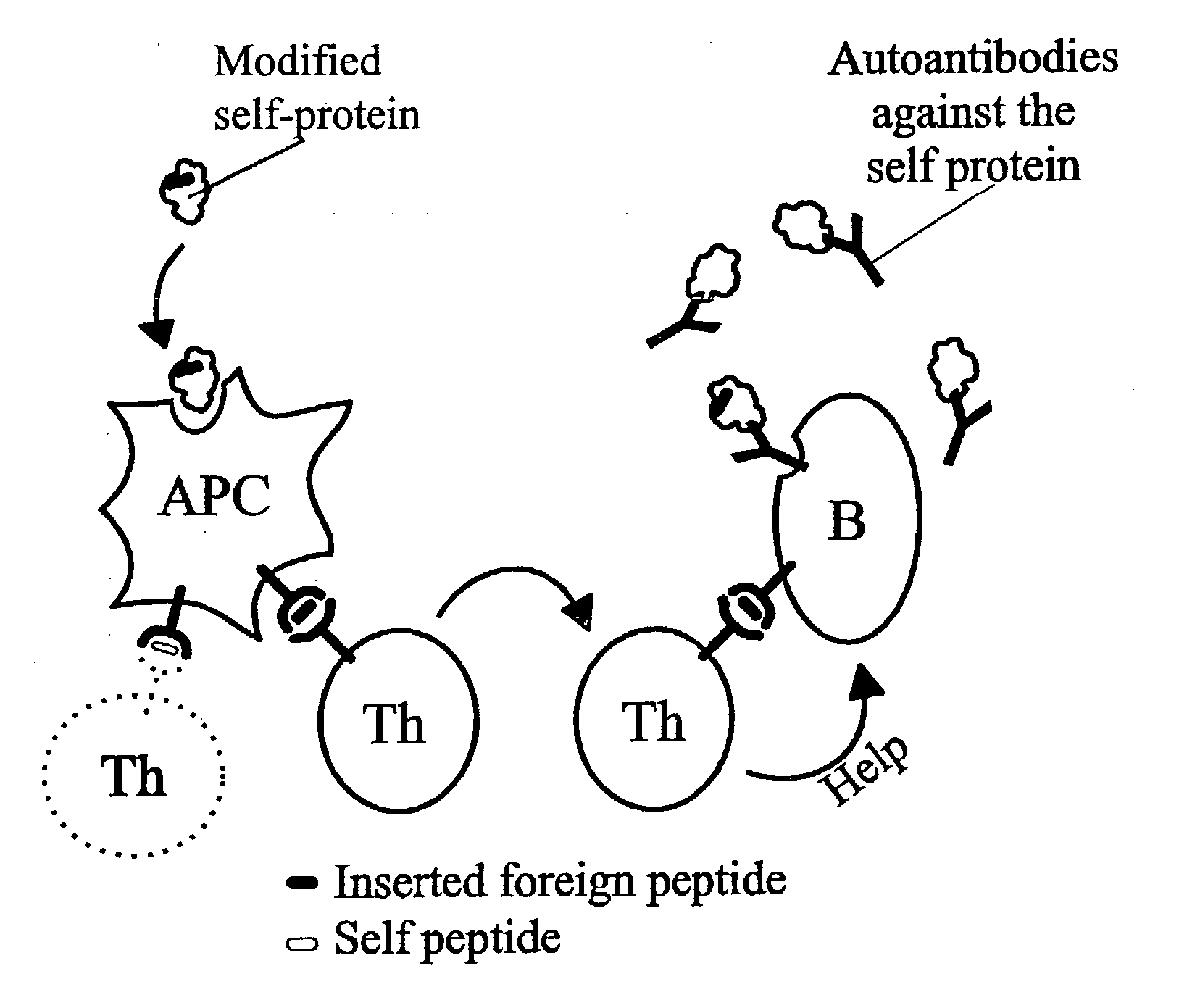
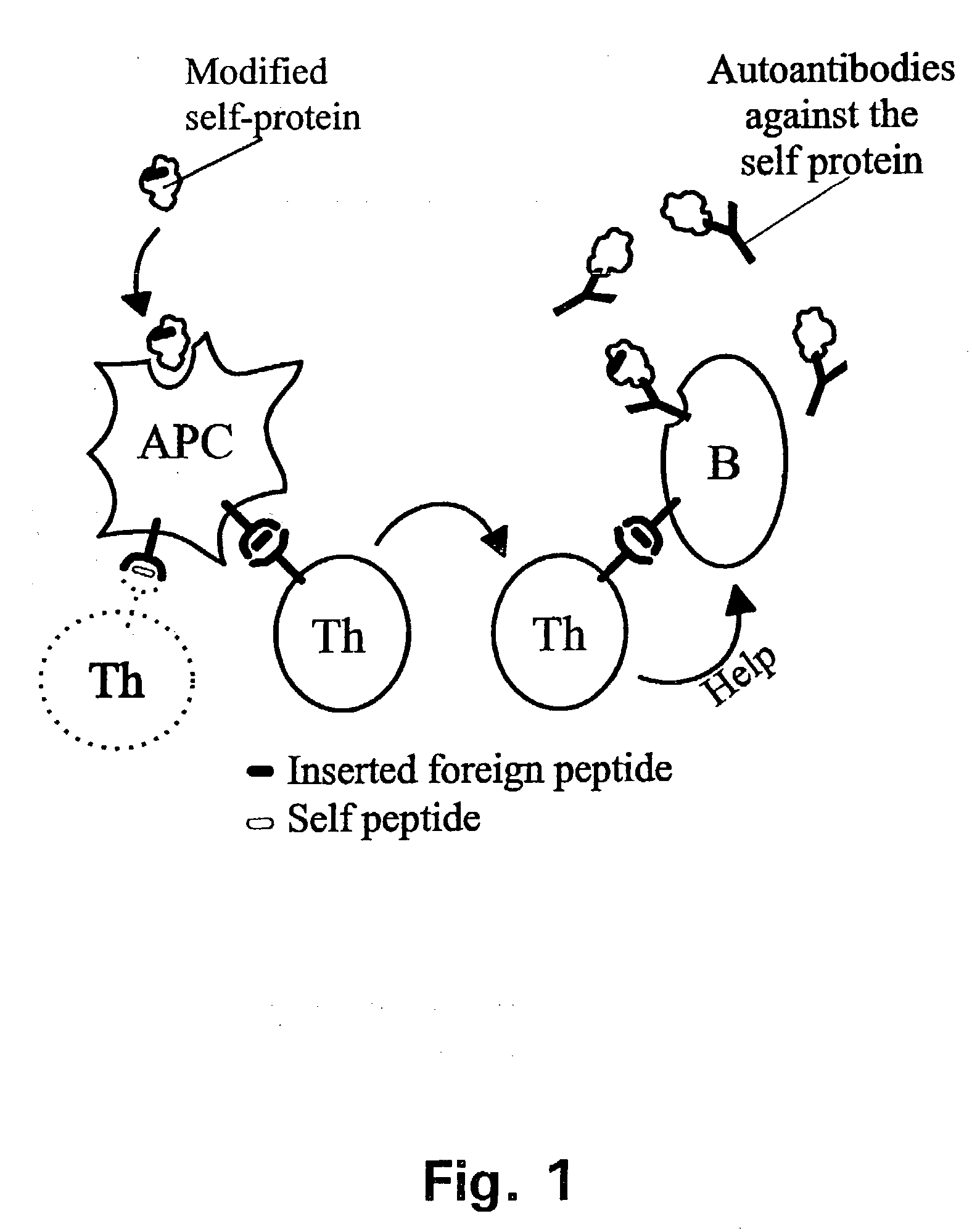
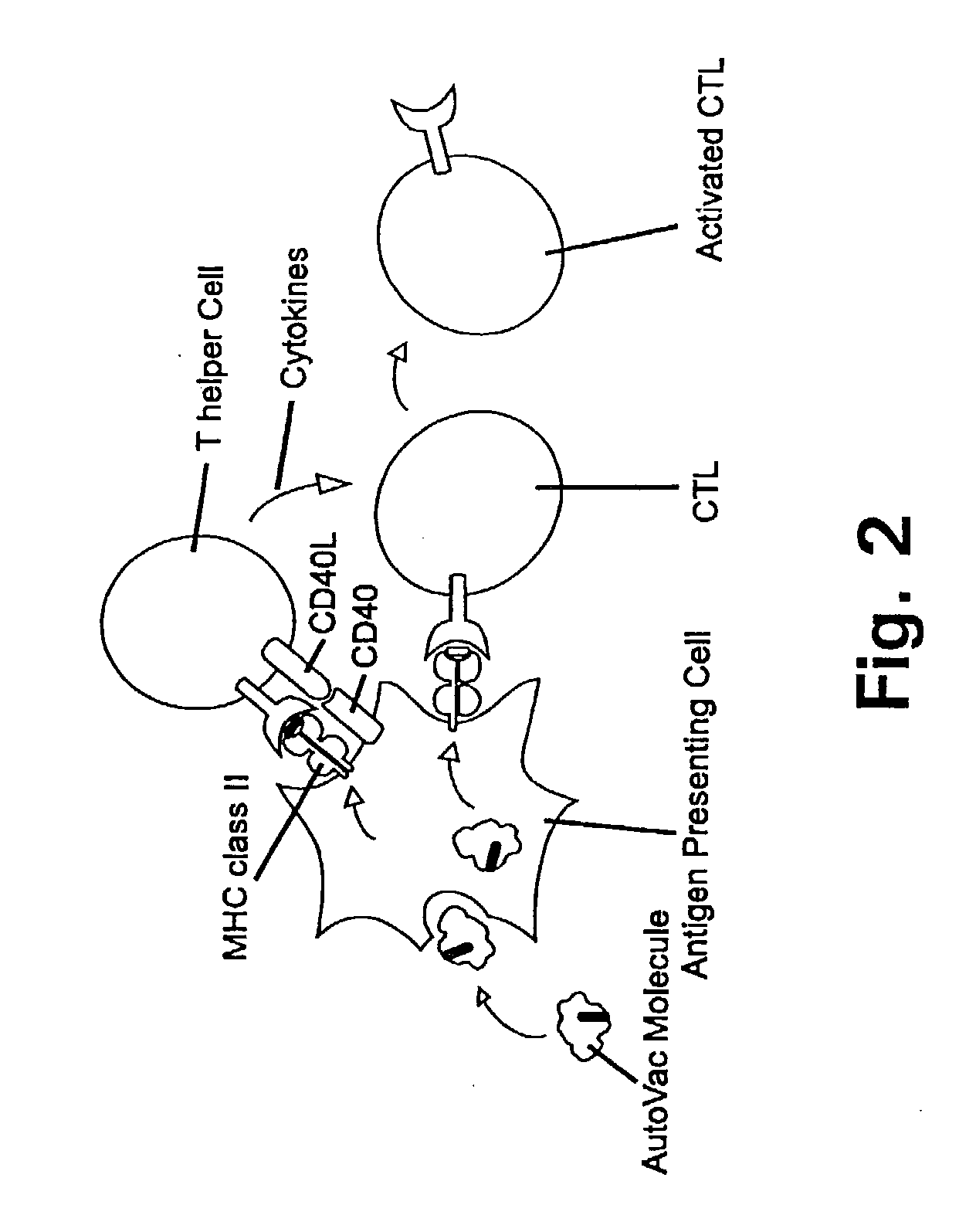
Novel methods for therapeutic vaccination
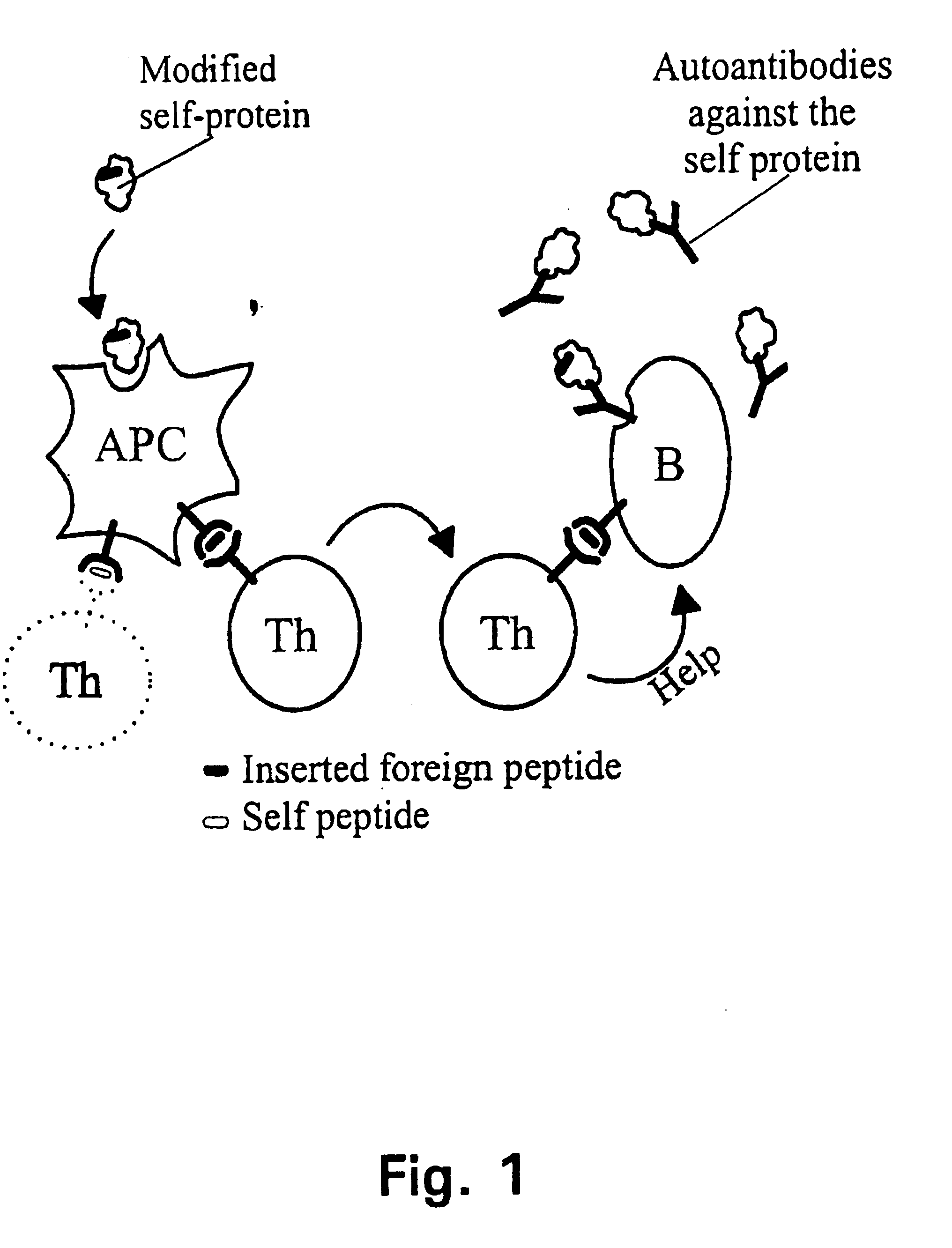
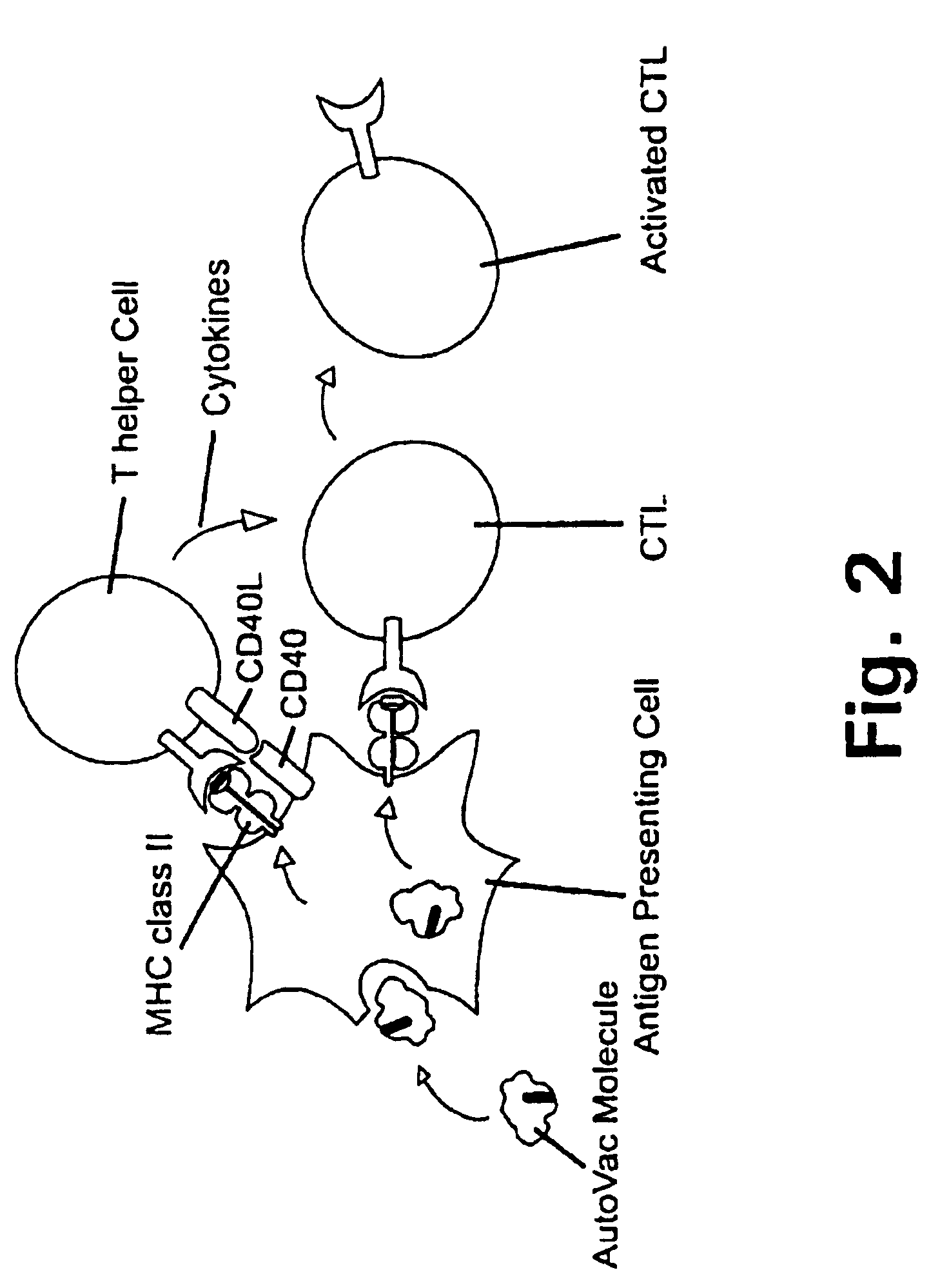
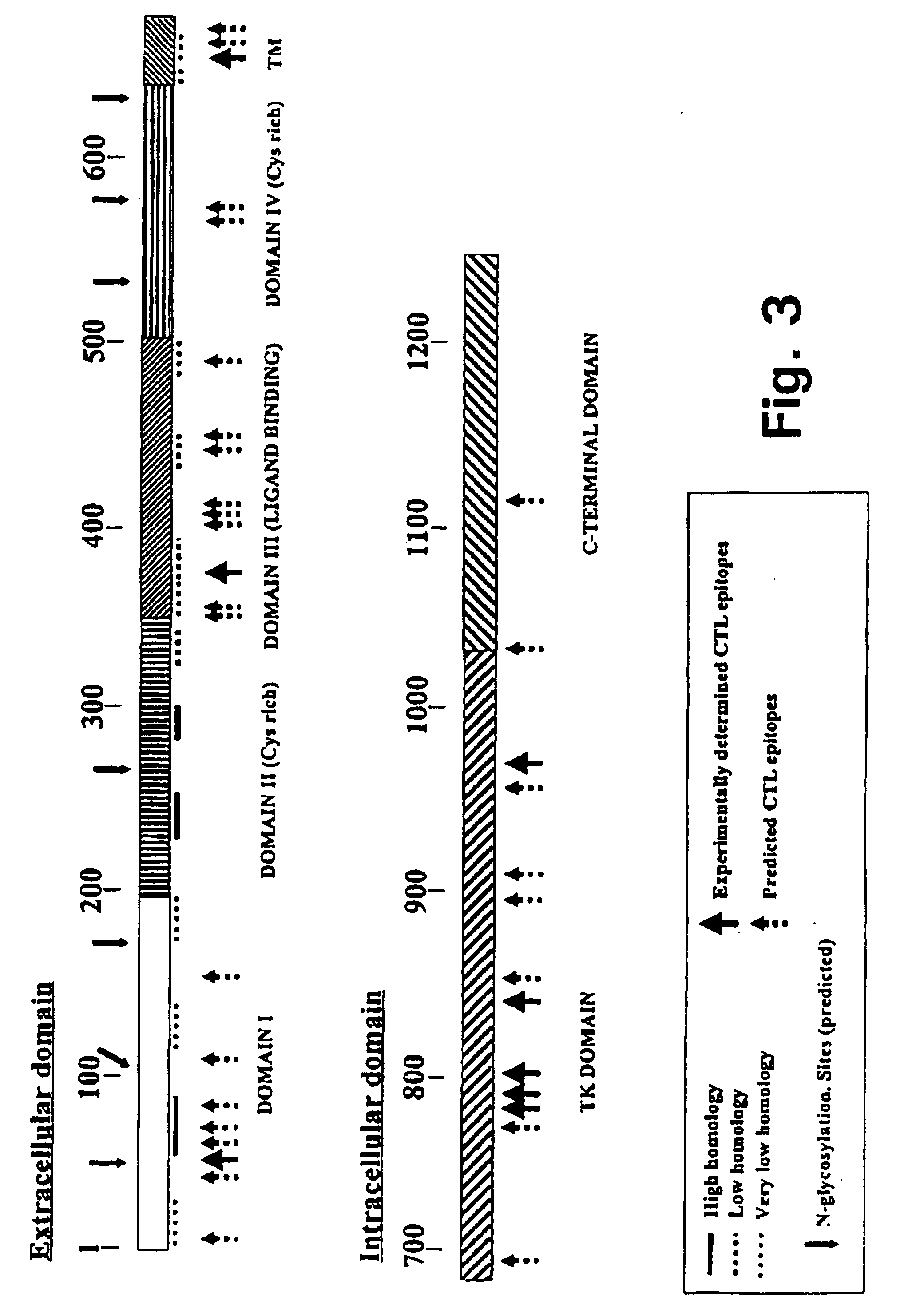
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Modulation of the immune response
InactiveUS20120009222A1SsRNA viruses negative-senseOrganic active ingredientsCell mediated immunityImmune Stimulation
The present invention provides lipidoids that can be used to modulate the immune response in a subject. Lipidoids are prepared by the conjugate addition of an amine to an acrylate to acrylamide. The lipidoids form complexes or particles with an immunostimulatory polynucleotide, which are then administerd to a subject. Such compositions have been found to stimulate the production of cytokines and increase both humoral and cell-mediate immune response. The invention also provides pharmaceuti-cal compositions thereof and methods for using the same.
Owner:MASSACHUSETTS INST OF TECH
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Anti-PD-L1 antibodies and uses thereof
ActiveUS9624298B2Function increaseUpregulate cell-mediated immune responsesNervous disorderPeptide/protein ingredientsAntigen Binding FragmentPD-L1
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Methods for the Treatment of Autoimmune Disorders Using Immunosuppressive Monoclonal Antibodies with Reduced Toxicity
ActiveUS20080095766A1Reduce the possibilityIncreasing concentration of antibodySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
Anti-neovasculature preparations for cancer
InactiveUS20050260234A1RapidityPeptide/protein ingredientsMicrobiological testing/measurementResearch modelCell mediated immunity
Disclosed herein are immunogenic compositions, methods of designing immunogenic compositions, methods of treatment using immunogenic compositions, methods of evaluating cell-mediated immunity resulting from immunogenic compositions, research models, and methods of making research models, all of which relate to targeting tumor vasculature.
Owner:MANNKIND CORP
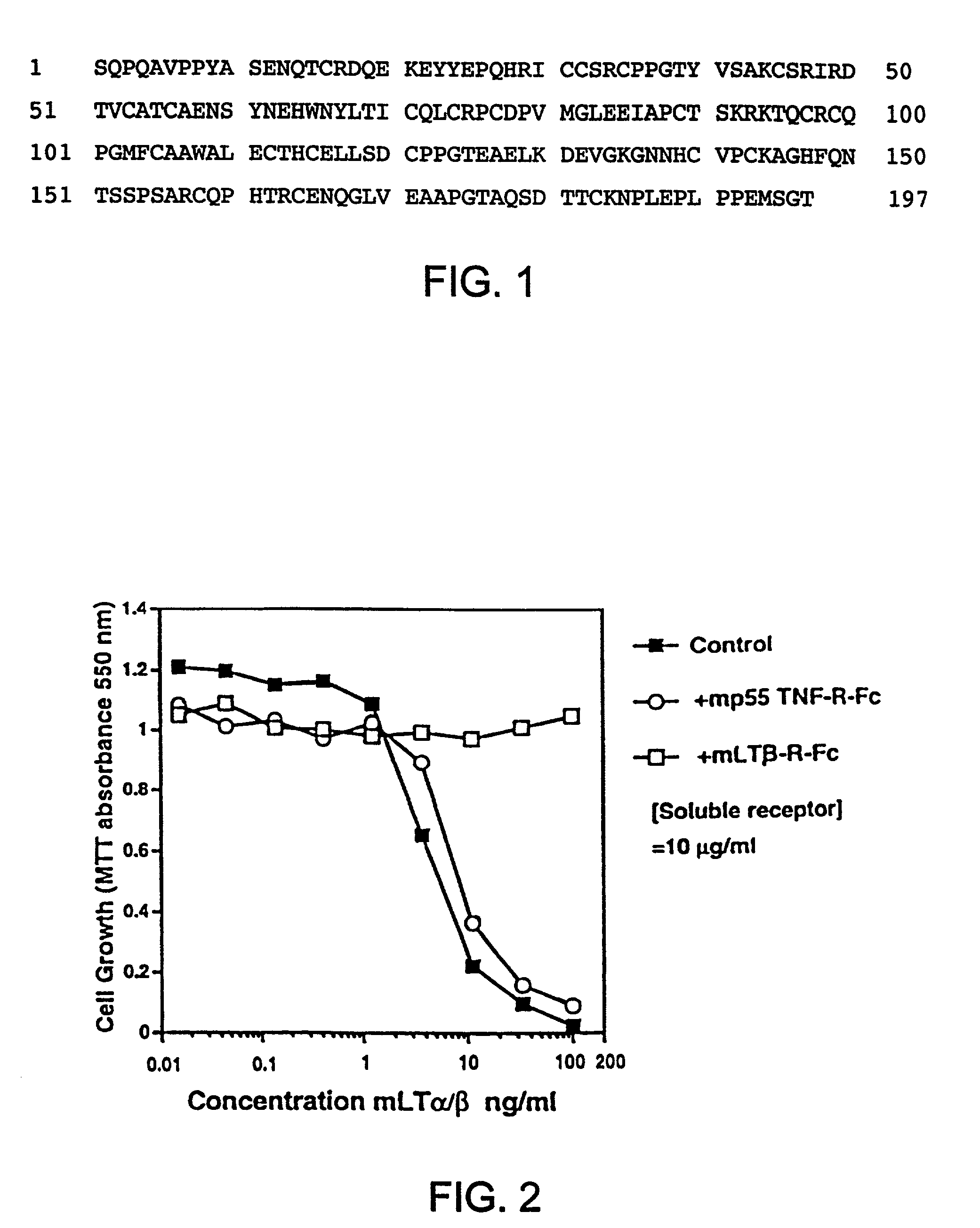
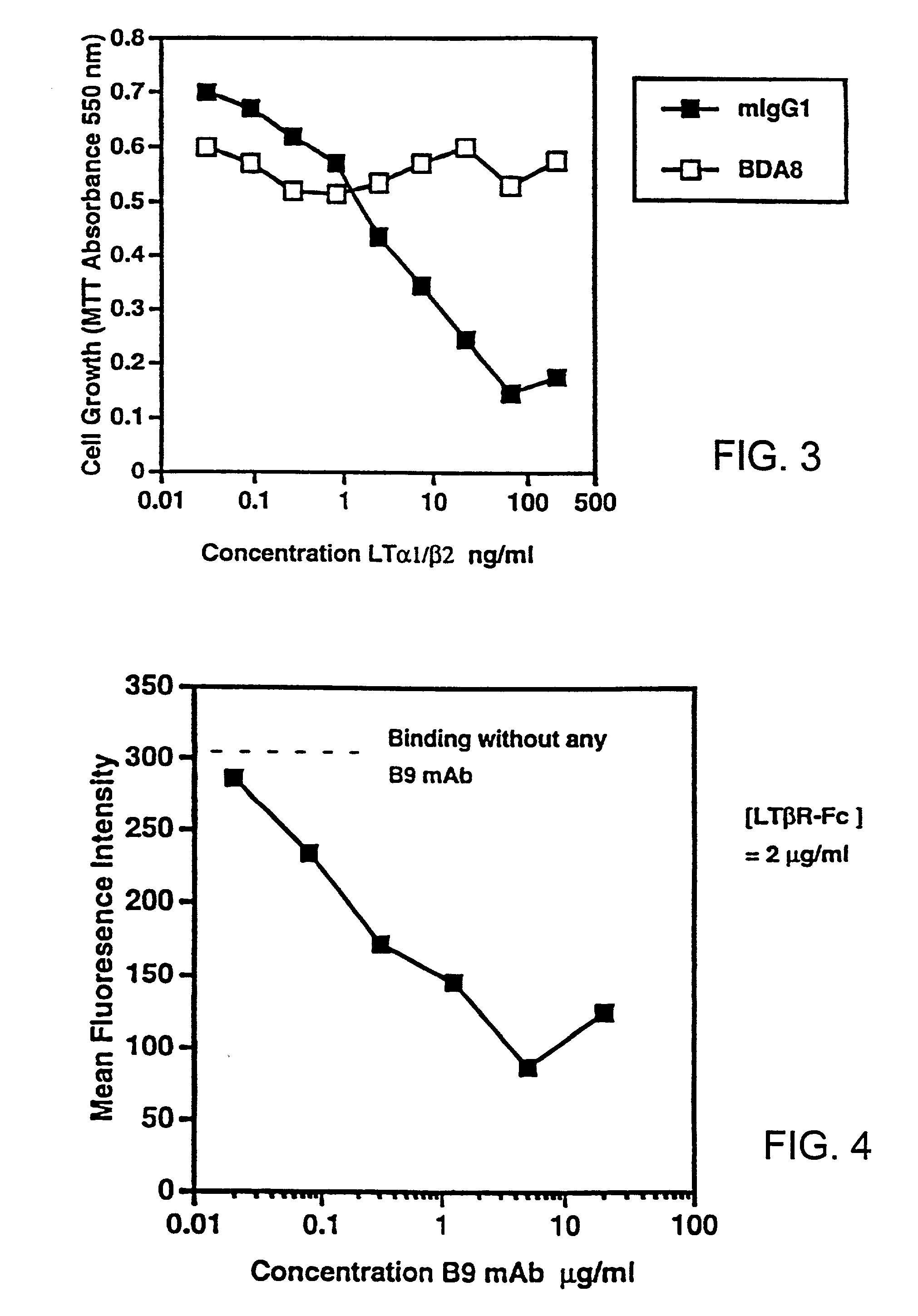
Methods for inhibiting lymphotoxin beta receptor signalling
This invention relates to compositions and methods comprising “lymphotoxin-β receptor blocking agents”, which block lymphotoxin-β receptor signalling. Lymphotoxin-β receptor blocking agents are useful for treating lymphocyte-mediated immunological diseases, and more particularly, for inhibiting Th1 cell-mediated immune responses. This invention relates to soluble forms of the lymphotoxin-β receptor extracellular domain that act as lymphotoxin-β receptor blocking agents. This invention also relates to the use of antibodies directed against either the lymphotoxin-β receptor or its ligand, surface lymphotoxin, that act as lymphotoxin-β receptor blocking agents. A novel screening method for selecting soluble receptors, antibodies and other agents that block LT-β receptor signalling is provided.
Owner:BIOGEN MA INC
Soluble pd-1 variants, fusion constructs, and uses thereof
ActiveUS20120121634A1Enhance host humoralEnhance cell-mediated immunityVirusesPeptide/protein ingredientsProtective immunityCell mediated immunity
The subject invention provides novel soluble PD-1 (sPD-1) proteins, nucleic acids, and fusion constructs thereof, for enhancing humoral and cell-mediated immunity of a subject. Also provided are therapeutic compositions comprising the sPD-1 proteins, nucleic acids, and fusion constructs of the subject invention. In a preferred embodiment, the therapeutic composition is formulated as a vaccine composition. Advantageously, the sPD-1 proteins, nucleic acids, and therapeutic compositions provide protective immunity against pathogenic infection including HIV infection. In additon, the subject invention can be used in the prevention and / or treatment of tumor or cancer.
Owner:VERSITECH LTD
Adjuvant combinations of liposomes and mycobacterial lipids for immunization compositions and vaccines
ActiveUS8241610B2Good auxiliary effectEnhance immune responseAntibacterial agentsBiocideLiposomeMycobacterium
The present invention provides a vaccine adjuvant consisting of a combination of a surfactant i.e. dimethyldeoctadecylammonium-bromide / chloride (DDA) and a lipid extract from Mycobacterium bovis BCG. The total lipid extract contains both apolar lipids, polar lipids, and lipids of intermediate polarity of which the apolar lipids were found to induce the most powerful immune responses. The total lipids may be extracted with chloroform / methanol and re-dissolved in water before the addition of surfactant. This preparation may be used to induce prominent cell-mediated immune responses in a mammal in order to combat pathogens, or as a treatment for cancer.
Owner:STATENS SERUM INST
Synthetic human papillomavirus genes
InactiveUS7001995B1Easy to insertEasy to removeBiocideGenetic material ingredientsPolynucleotide VaccinesHuman papillomavirus
Synthetic DNA molecules encoding papillomavirus proteins are provided. The codons of the synthetic molecules are codons preferred by the projected host cell. The synthetic molecules may be used as a polynucleotide vaccine which provides effective immunoprophylaxis against papillomavirus infection through stimulation of neutralizing antibody and cell-mediated immunity.
Owner:MERCK SHARP & DOHME CORP
Soluble PD-1 variants, fusion constructs, and uses thereof
The subject invention provides novel soluble PD-1 (sPD-1) proteins, nucleic acids, and fusion constructs thereof, for enhancing humoral and cell-mediated immunity of a subject. Also provided are therapeutic compositions comprising the sPD-1 proteins, nucleic acids, and fusion constructs of the subject invention. In a preferred embodiment, the therapeutic composition is formulated as a vaccine composition. Advantageously, the sPD-1 proteins, nucleic acids, and therapeutic compositions provide protective immunity against pathogenic infection including HIV infection. In addition, the subject invention can be used in the prevention and / or treatment of tumor or cancer.
Owner:VERSITECH LTD
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably Carbopol, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polypropylene block copolymer such as Pluronic®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another emodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
Nanofraction immune modulators, preparations and compositions including the same, and associated methods
ActiveUS20080081076A1Small sizeMicrobiological testing/measurementBiological material analysisCancer cellT cell
Compositions that include extracts from sources of immune modulators that include nanofraction immune modulator molecules (i.e., molecules having molecular weights of about 3,000 Da and less) are disclosed. These compositions may also include other immune modulators, such as transfer factor. Administration of compositions with extracts that include nanofraction immune modulator molecules modulates the cell-mediated immunity (e.g., down-regulates undesired T cell activity) of a subject to which such compositions are administered. When administered with transfer factor, the combination of nanofraction immune modulator molecules and transfer factor down-regulates undesired T cell activity while increasing, or up-regulating, T cell activity against pathogens and other undesirable entities, such as cancer cells and other aberrant or mutated cells. Assays and assay techniques for evaluating the immune modulation capabilities of various substances are also disclosed.
Owner:4LIFE PATENTS
Regulation of T cell-mediated immunity by D isomers of inhibitors of indoleamine-2,3-dioxygenase
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
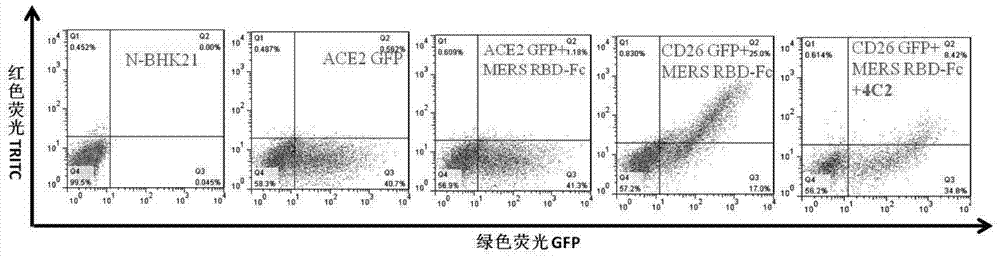
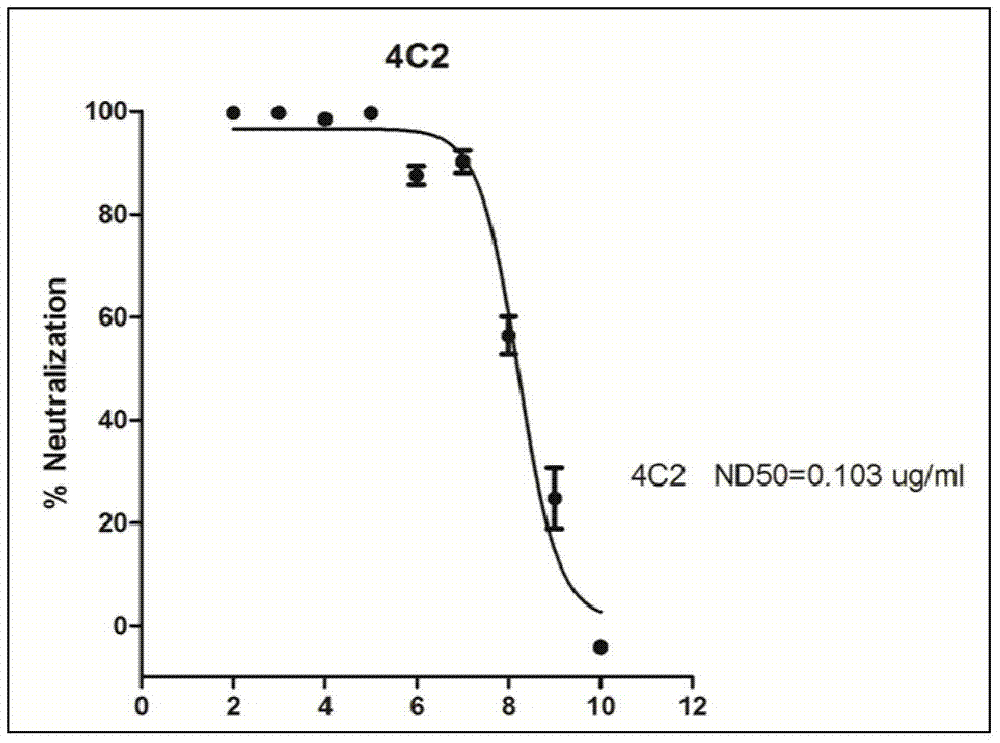
Middle east and respiratory syndrome coronavirus antibody and preparation method thereof
ActiveCN103864924APrevent intrusionHigh affinityImmunoglobulins against virusesAntibody ingredientsMiddle East respiratory syndromeBaculovirus expression vector system
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Cells expressing an alphagala nucleic acid and methods of xenotransplantation
The present invention relates to methods and compositions for the reduction of xenotransplantation rejection. Specifically, the present invention relates, first, to transgenic cells, tissues, organs and animals containing transgenic nucleic acid molecules that direct the expression of gene products, including, but not limited to enzymes, capable of modifying, either directly or indirectly, cell surface carbohydrate epitopes such that the carbohydrate epitopes are no longer recognized by natural human antibodies or by the human cell-mediated immune response, thereby reducing the human immune system response elicited by the presence of such carbohydrate epitopes. In a preferred embodiment, the transgenic cells, tissues, organs and animals express nucleic acid molecules encoding functional recombinant alpha-Galactosidase A (alphaGalA) enzyme which modifies the carbohydrate epitope Galalpha(1,3)Gal. In a more preferred embodiment, the transgenic cells, tissues, organs and animals expressing the functional recombinant alphaGalA are transgenic pig cells, organs, tissues and / or animals. Second, the present invention relates to methods for xenotransplantation comprising introducing the transgenic cells, tissues and / or organs into human recipients so that a lower level of hyperacute rejection (HAR) is observed in the human recipients relative to the level of HAR observed in human recipients having received non-transgenic cells, tissues and / or organs.
Owner:THE AUSTIN RES INST +1
Methods for the treatment and prevention of inflammatory diseases
The invention includes processes mainly for the treatment of a inflammatory diseases, such as inflammatory bowel disease, arthritis, atherosclerosis, asthma, allergy, inflammatory kidney disease, circulatory shock, multiple sclerosis, chronic obstructive pulmonary disease, skin inflammation, periodontal disease, psoriasis and T cell-mediated diseases of immunity, including allergic encephalomyelitis, allergic neuritis, transplant allograft rejection, graft versus host disease, myocarditis, thyroiditis, nephritis, systemic lupus erthematosus, and insulin-dependent diabetes mellitus. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:APOGEE BIOTECHNOLOGY CORP
Methods of reducing immunogenicity against factor viii in individuals undergoing factor viii therapy
ActiveUS20140370035A1Improve immune toleranceReduce morbidityFactor VIINervous disorderFactor iiImmune tolerance
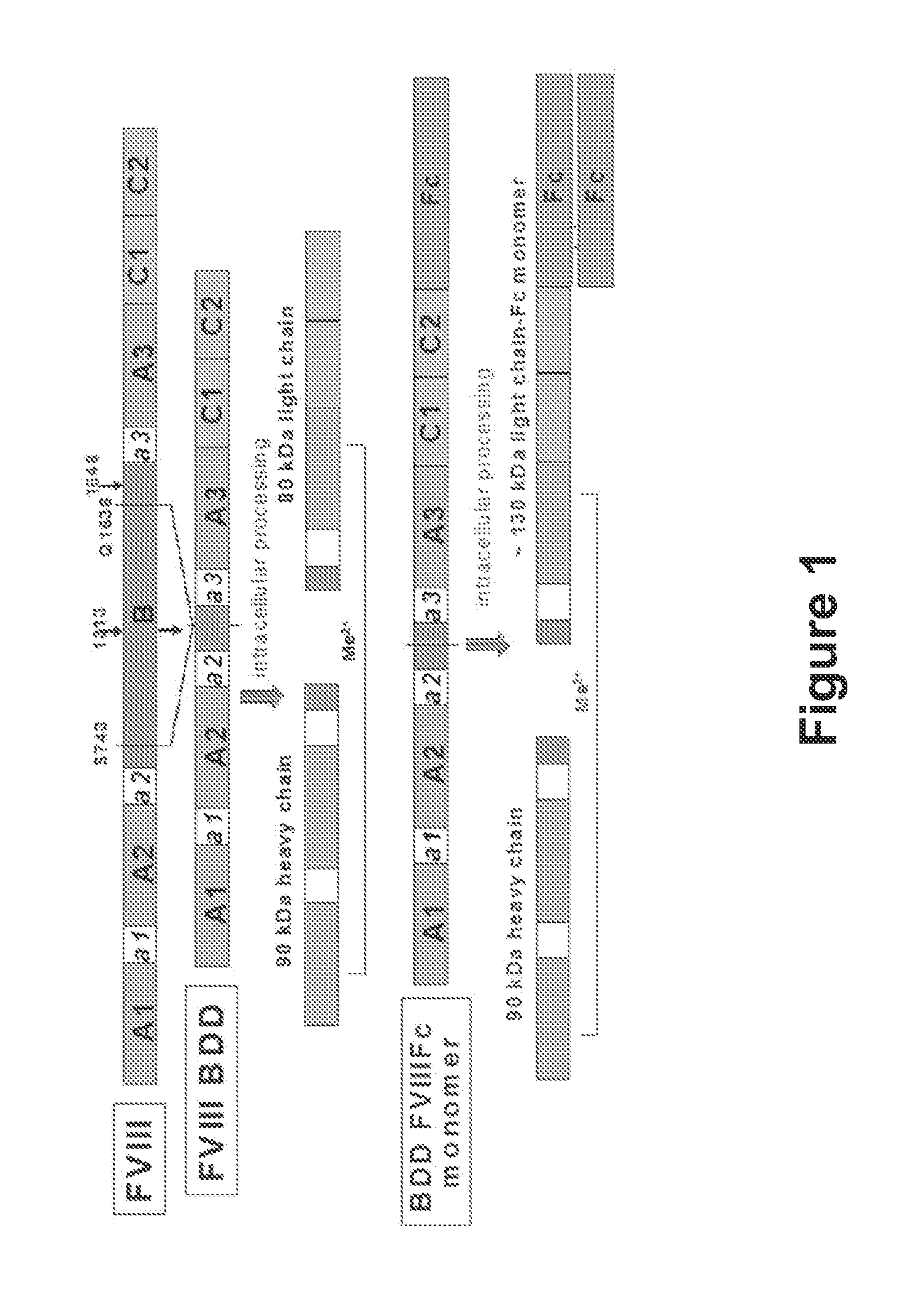
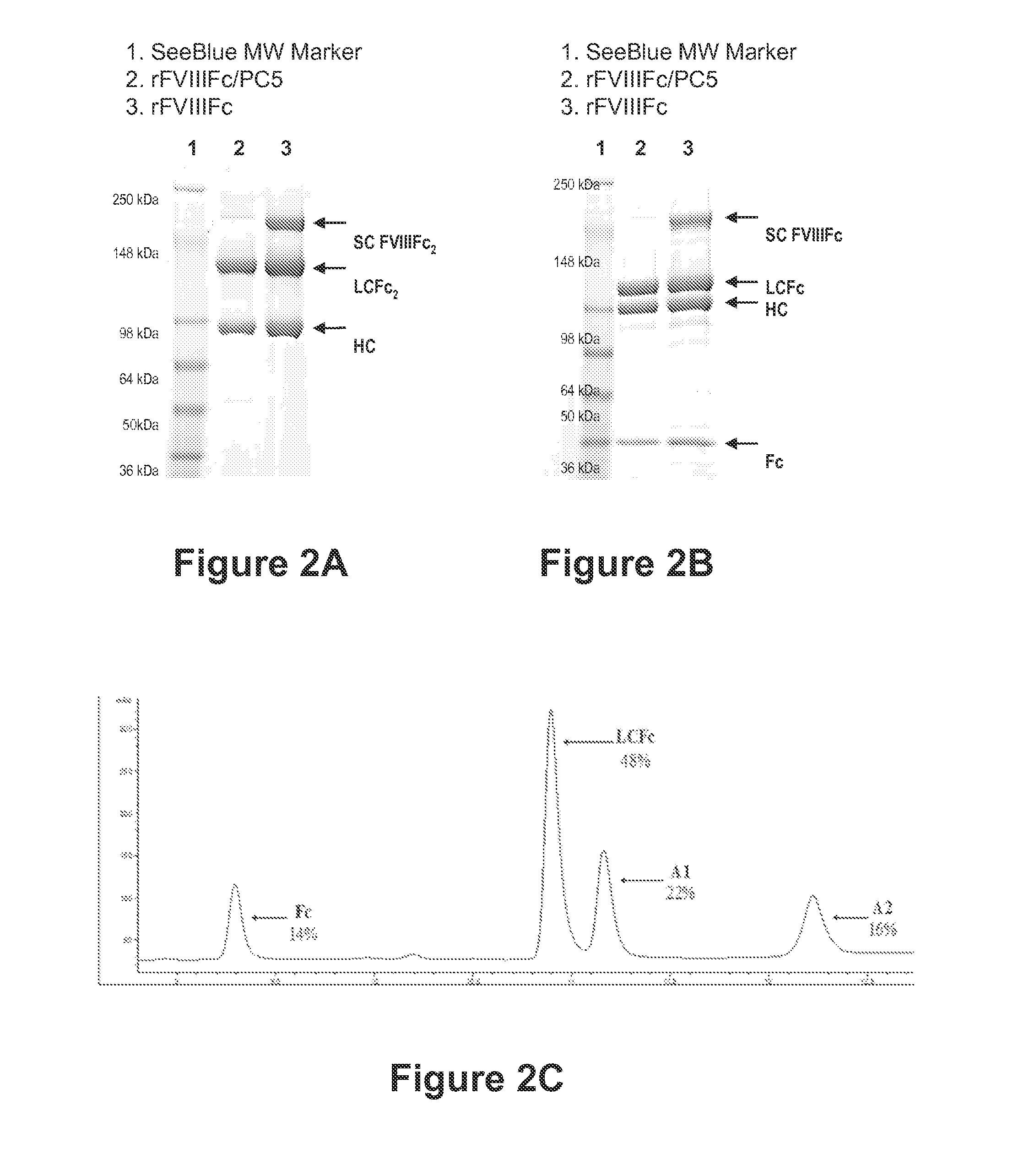
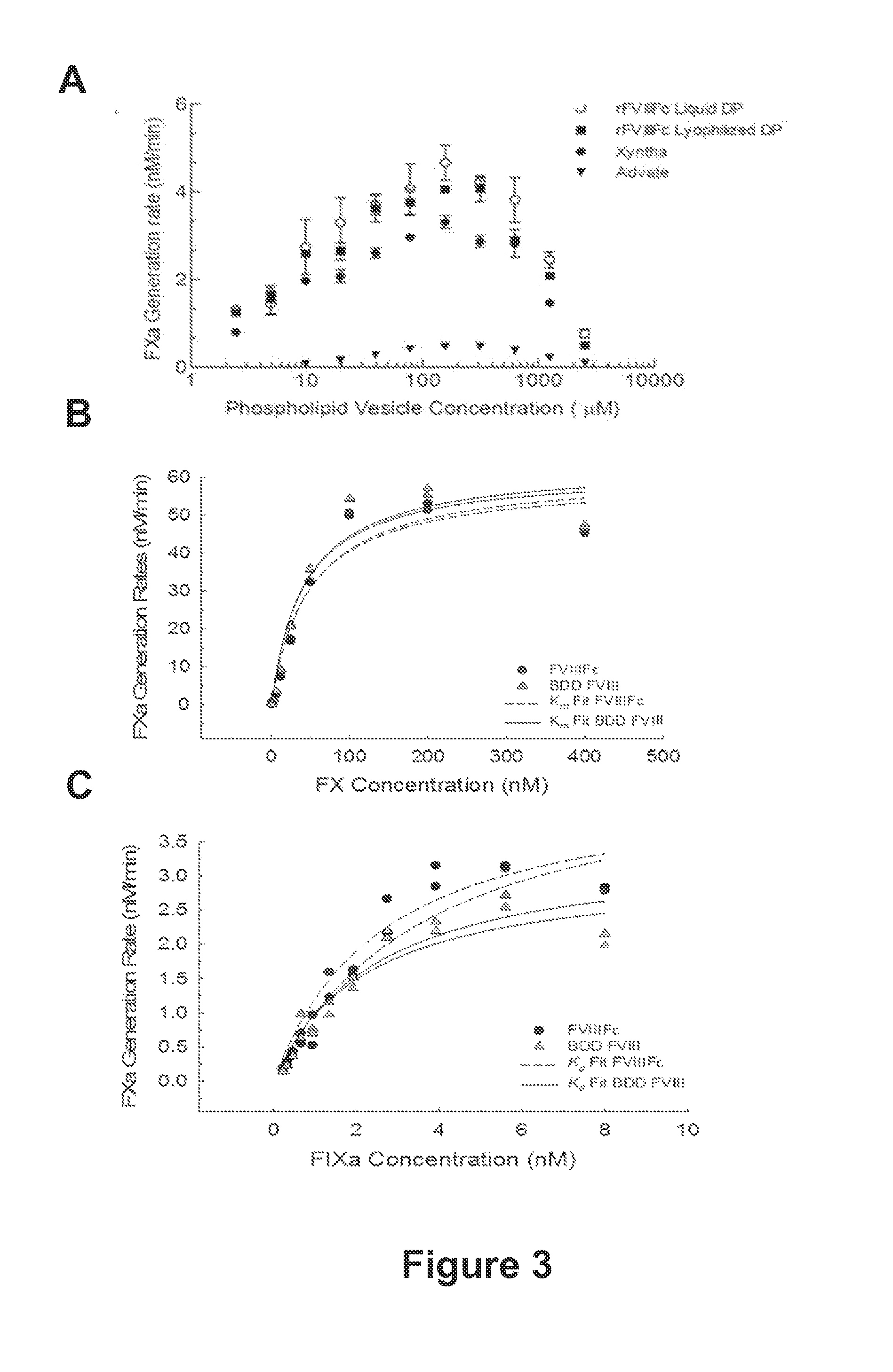
The present disclosure provides methods of administering chimeric and hybrid Factor VIII (FVIII) polypeptides comprising FVIII and Fc to subjects at risk of developing inhibitory FVIII immune responses, including anti-FVIII antibodies and / or cell-mediated immunity. The administration is sufficient to promote coagulation and to induce immune tolerance to FVIII. The chimeric polypeptide can comprise full-length FVIII or a FVIII polypeptide containing a deletion, e.g., a full or partial deletion of the B domain.
Owner:PUGET SOUND BLOOD CENT +1
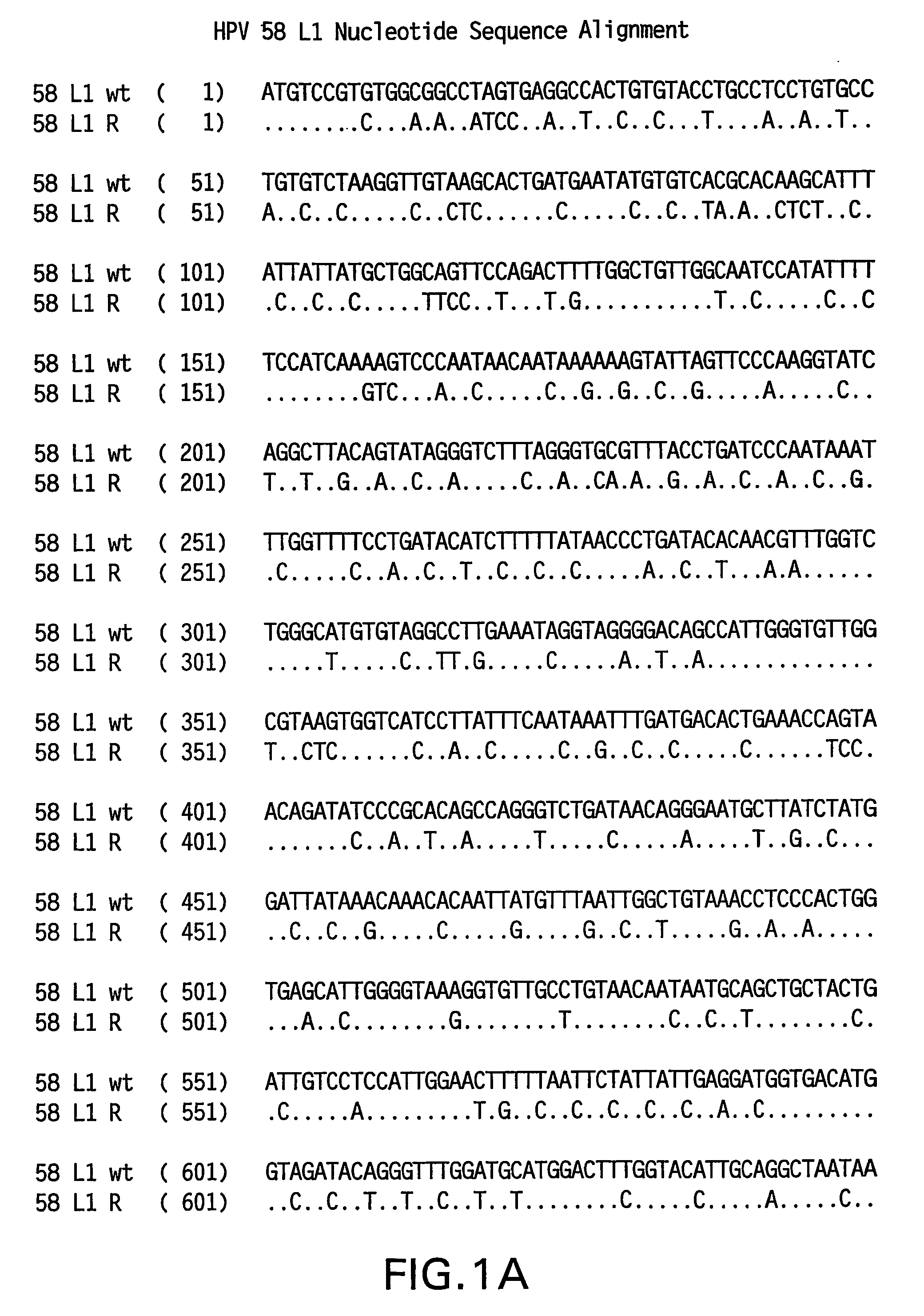
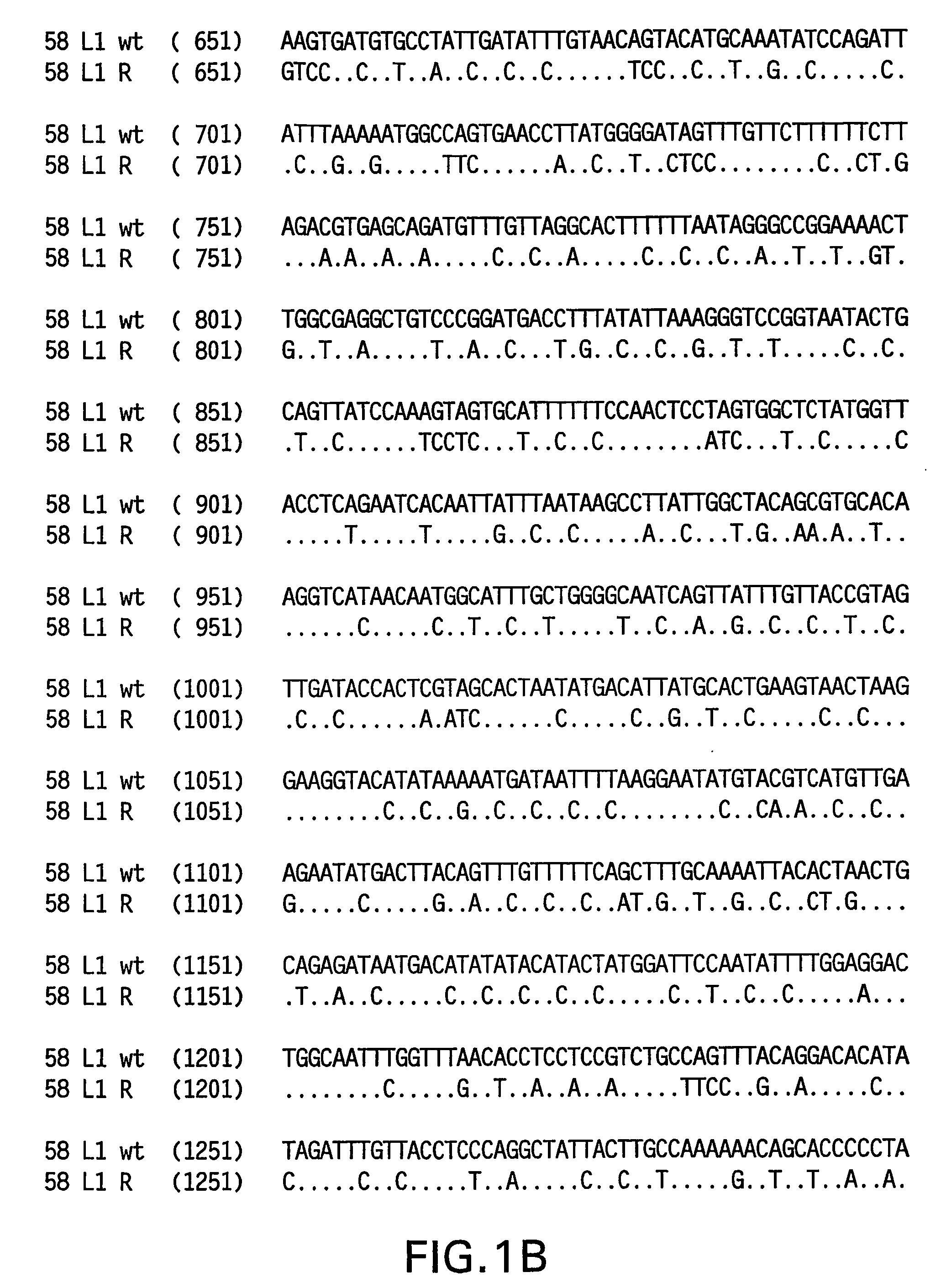
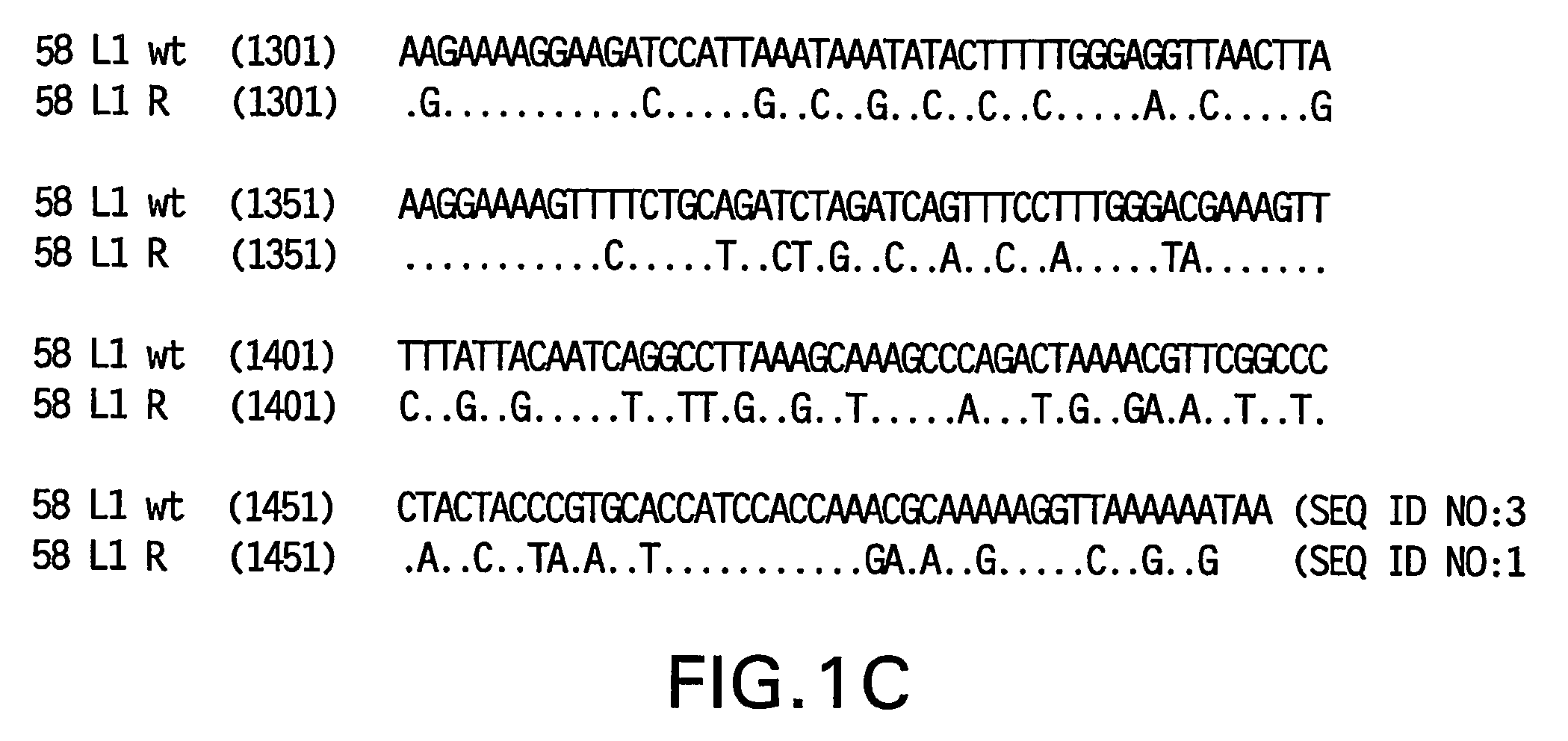
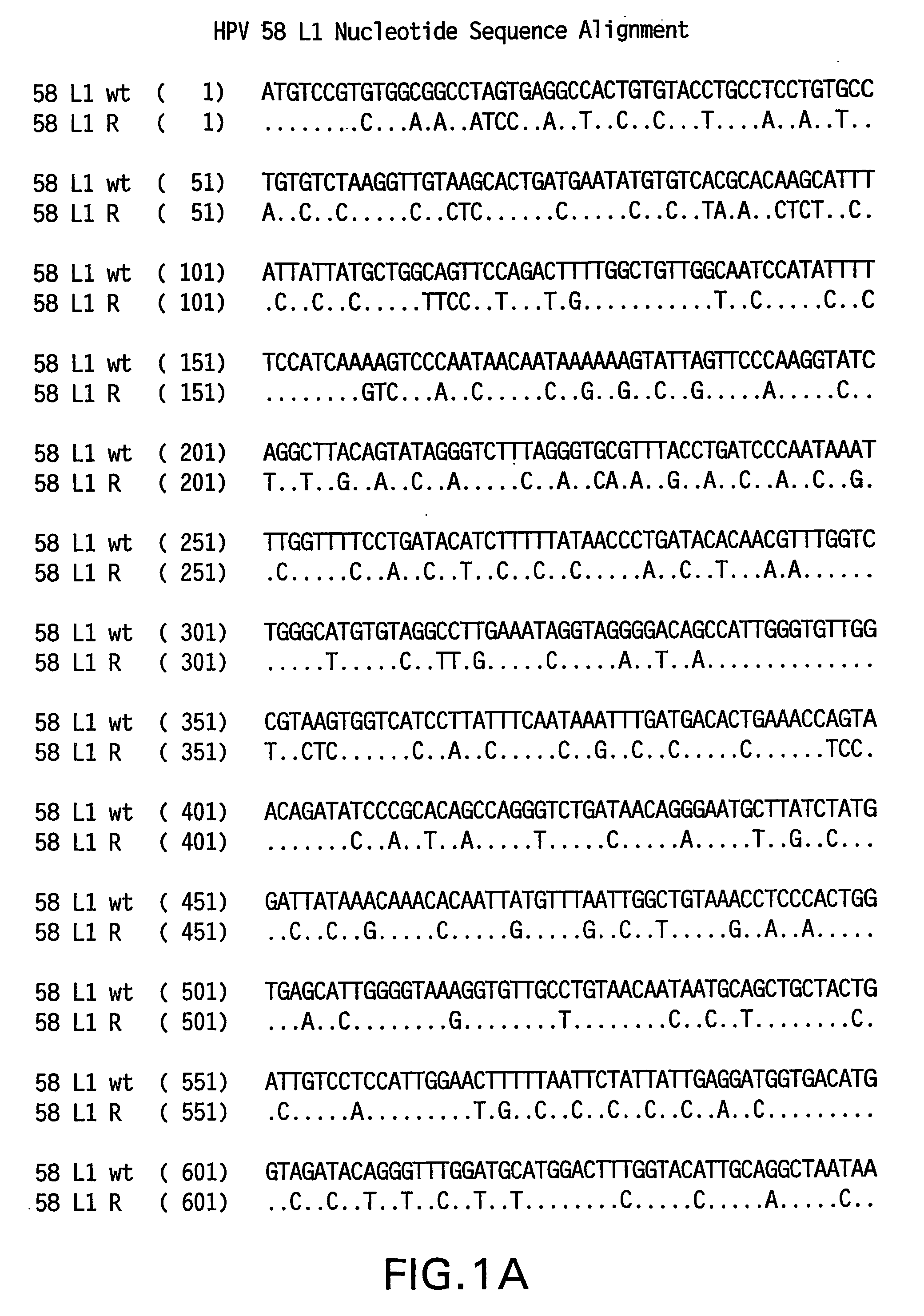
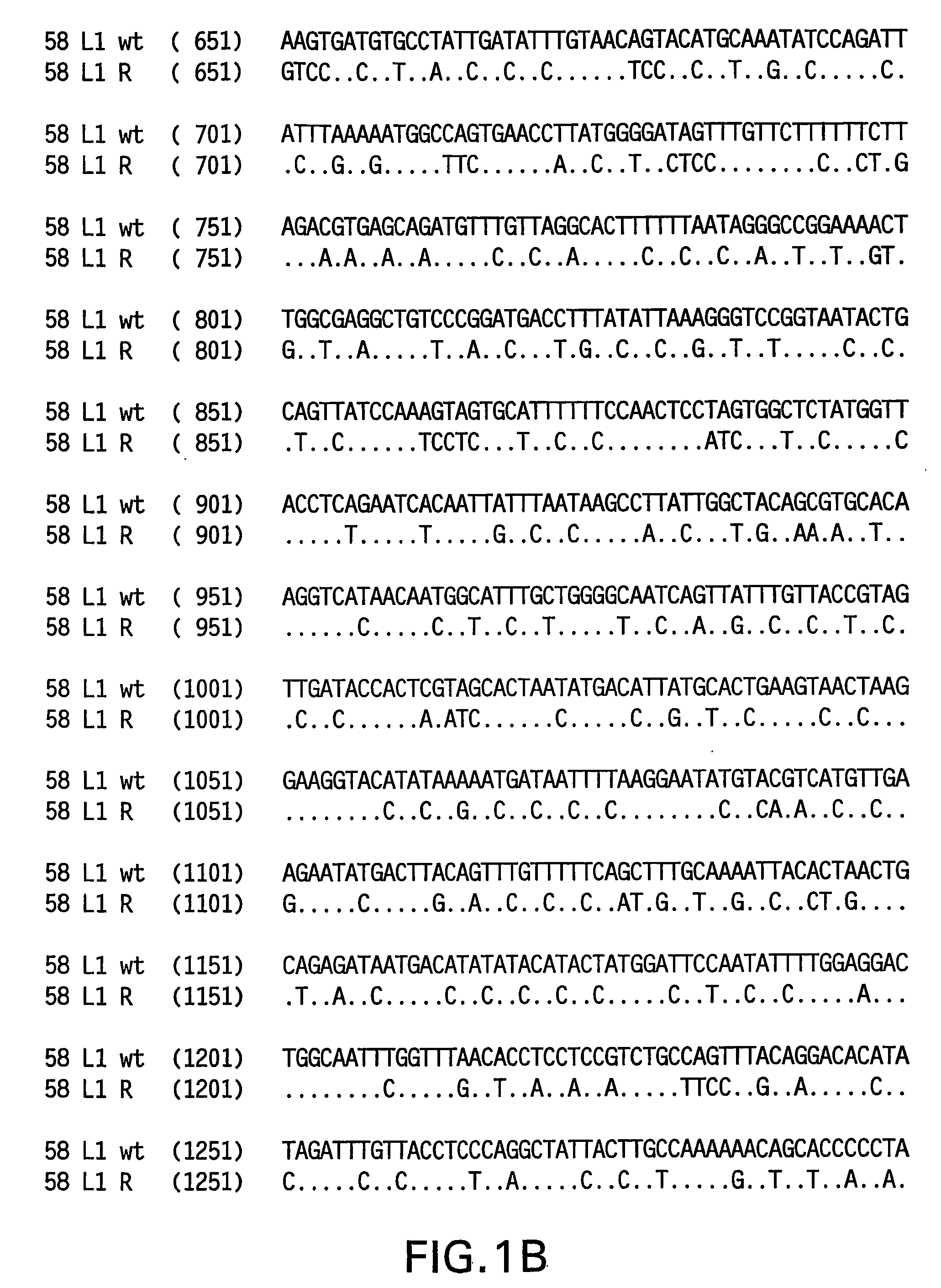
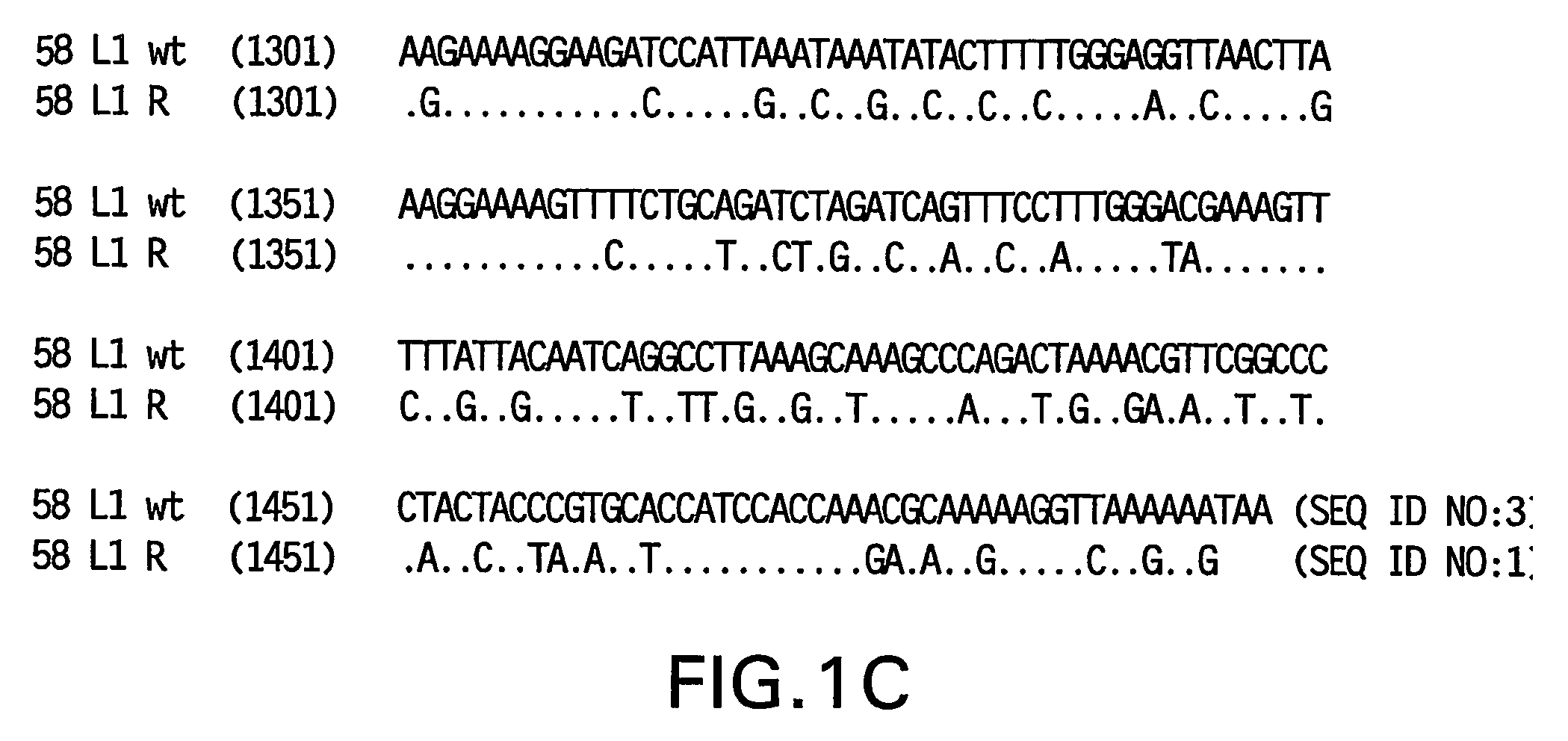
Optimized expression of HPV 58 L1 in yeast
Synthetic DNA molecules encoding the HPV58 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV58 L1 protein, wherein said polynucleotides are codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV58 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV58 VLPs. The vaccines of the present invention provide effective imnunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity and are also useful for treatment of existing HPV infections.
Owner:MERCK SHARP & DOHME LLC
Anti-neovasculature preparations for cancer
InactiveUS7252824B2Peptide/protein ingredientsMammal material medical ingredientsResearch modelTumor vasculature
Owner:MANNKIND CORP
Optimized expression of HPV 45 L1 in yeast
Owner:MERCK SHARP & DOHME LLC
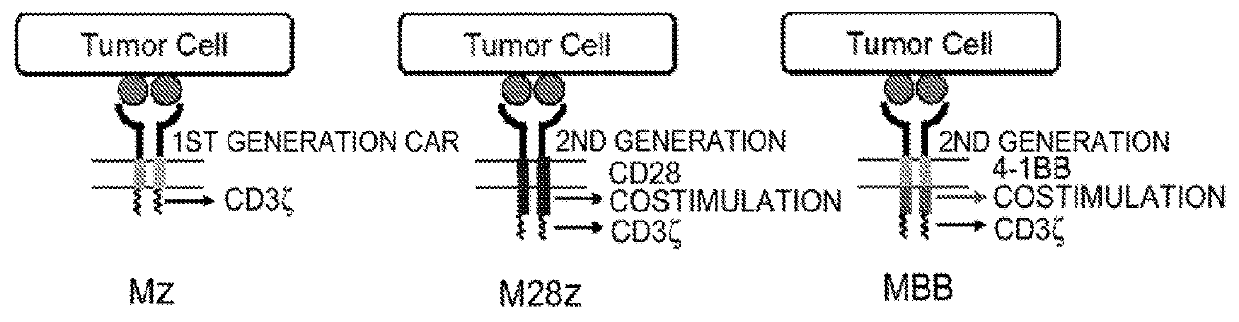
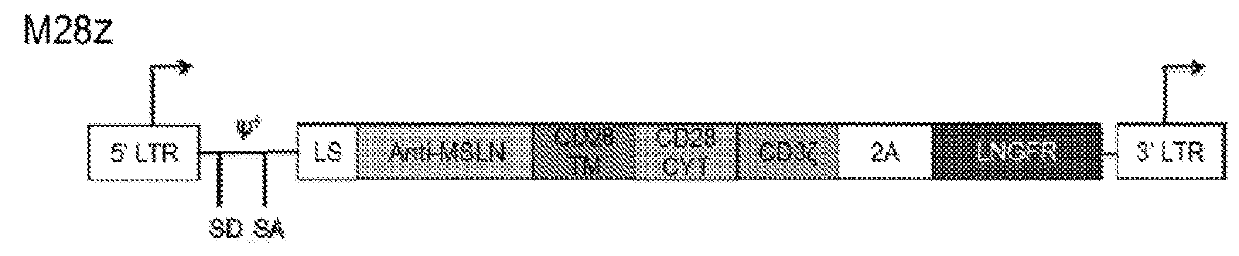
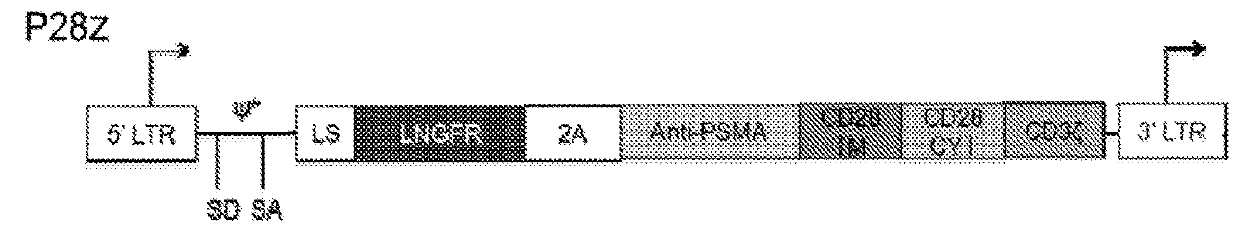
Immune cell compositions and methods of use
ActiveUS20180273601A1Restores effector functionEnhances tumor burden controlPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorT cell mediated immunity
Disclosed herein are cells that are immune cells or precursor cells thereof, which cells recombinantly express a chimeric antigen receptor (CAR), and a dominant negative form of an inhibitor of a cell-mediated immune response of the immune cell, wherein the CAR binds to a cancer antigen. Also disclosed herein are T cells that recognize and are sensitized to a cancer antigen, which T cells recombinantly express a dominant negative form of an inhibitor of a T cell-mediated immune response. Additionally provided are methods of using such cells to treat cancer in a subject in need thereof.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Canine vaccine for protection against ehrlichiosis
ActiveUS20060188524A1Antibacterial agentsBacterial antigen ingredientsAdjuvantCell mediated immunity
The present invention provides a safe and effective vaccine composition which comprises: an effective immunizing amount of an inactivated Ehrlichia canis bacterin; a pharmacologically acceptable carrier; and an immunogenically stimulating amount of an adjuvant system consisting essentially of an antibody response inducing agent and a cell-mediated immunity response inducing agent. The present invention also provides a method for the prevention or amelioration of canine ehrlichiosis in dogs.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
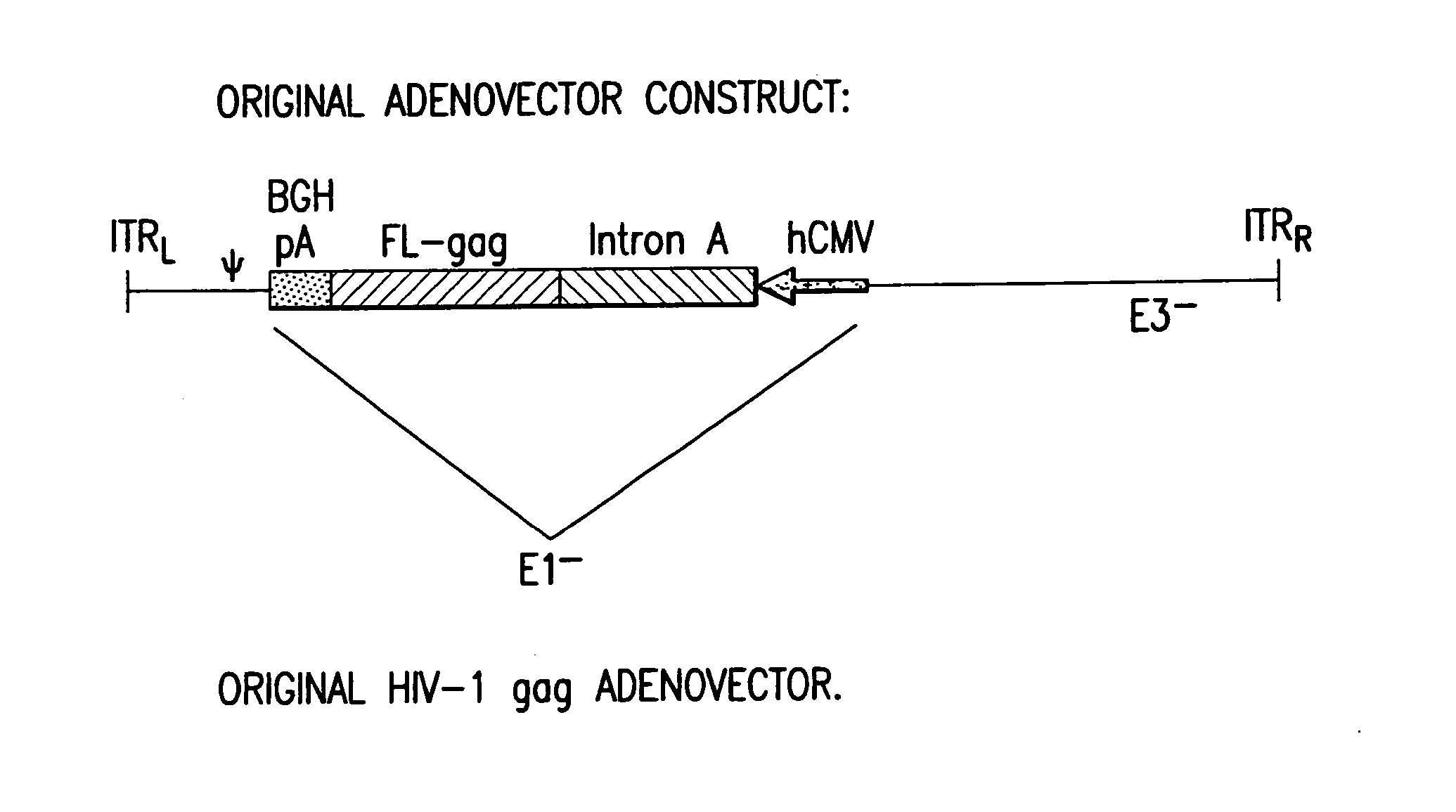
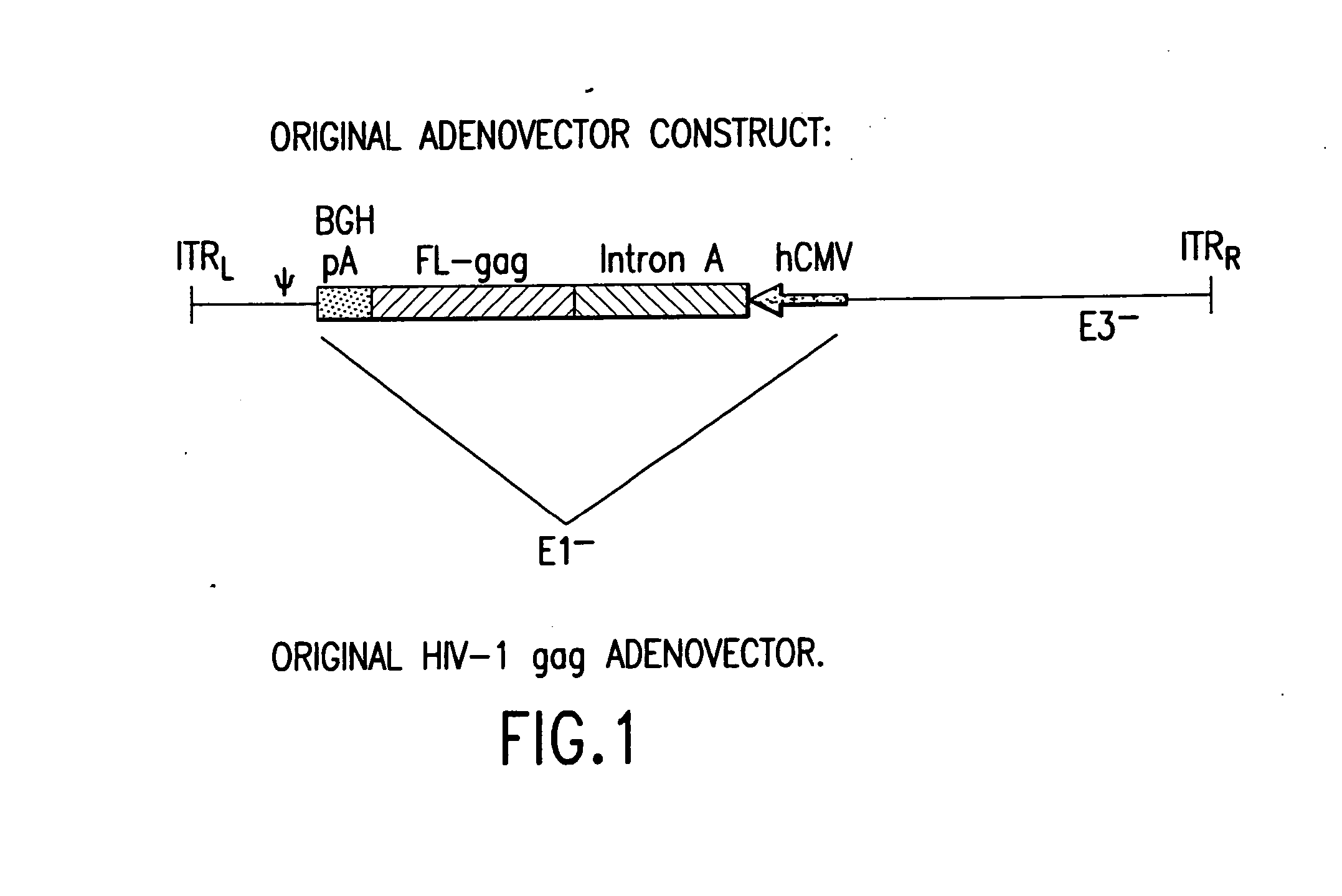
Enhanced first generation adenovirus vaccines expressing codon optimized HIV1-Gag, Pol, Nef and modifications
InactiveUS20070054395A1Genetically stableImprove featuresVectorsViral antigen ingredientsMammalReverse transcriptase
First generation adenoviral vectors and associated recombinant adenovirus-based HIV vaccines which show enhanced stability and growth properties and greater cellular-mediated immunity are described within this specification. These adenoviral vectors are utilized to generate and produce through cell culture various adenoviral-based HIV-1 vaccines which contain HIV-1 gag, HIV-1 pol and / or HIV-1 nef polynucleotide pharmaceutical products, and biologically relevant modifications thereof. These adenovirus vaccines, when directly introduced into living vertebrate tissue, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV1-Gag, Pol and / or Nef protein or biologically modification thereof, inducing a cellular immune response which specifically recognizes HIV-1. The exemplified polynucleotides of the present invention are synthetic DNA molecules encoding HIV-1 Gag, encoding codon optimized HIV-1 Pol, derivatives of optimized HIV-1 Pol (including constructs wherein protease, reverse transcriptase, RNAse H and integrase activity of HIV-1 Pol is inactivated), HIV-1 Nef and derivatives of optimized HIV-1 Nef, including nef mutants which effect wild type characteristics of Nef, such as myristylation and down regulation of host CD4. The adenoviral vaccines of the present invention, when administered alone or in a combined modality regime, will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO A +7
Optimized expression of hpv 58 l1 in yeast
ActiveUS20070036824A1High level expressionImprove immunityFungiVirus peptidesYeastHigh level expression
Synthetic DNA molecules encoding the HPV58 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV58 L1 protein, wherein said polynucleotides are codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV58 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV58 VLPs. The vaccines of the present invention provide effective imnunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunity and are also useful for treatment of existing HPV infections.
Owner:MERCK SHARP & DOHME LLC
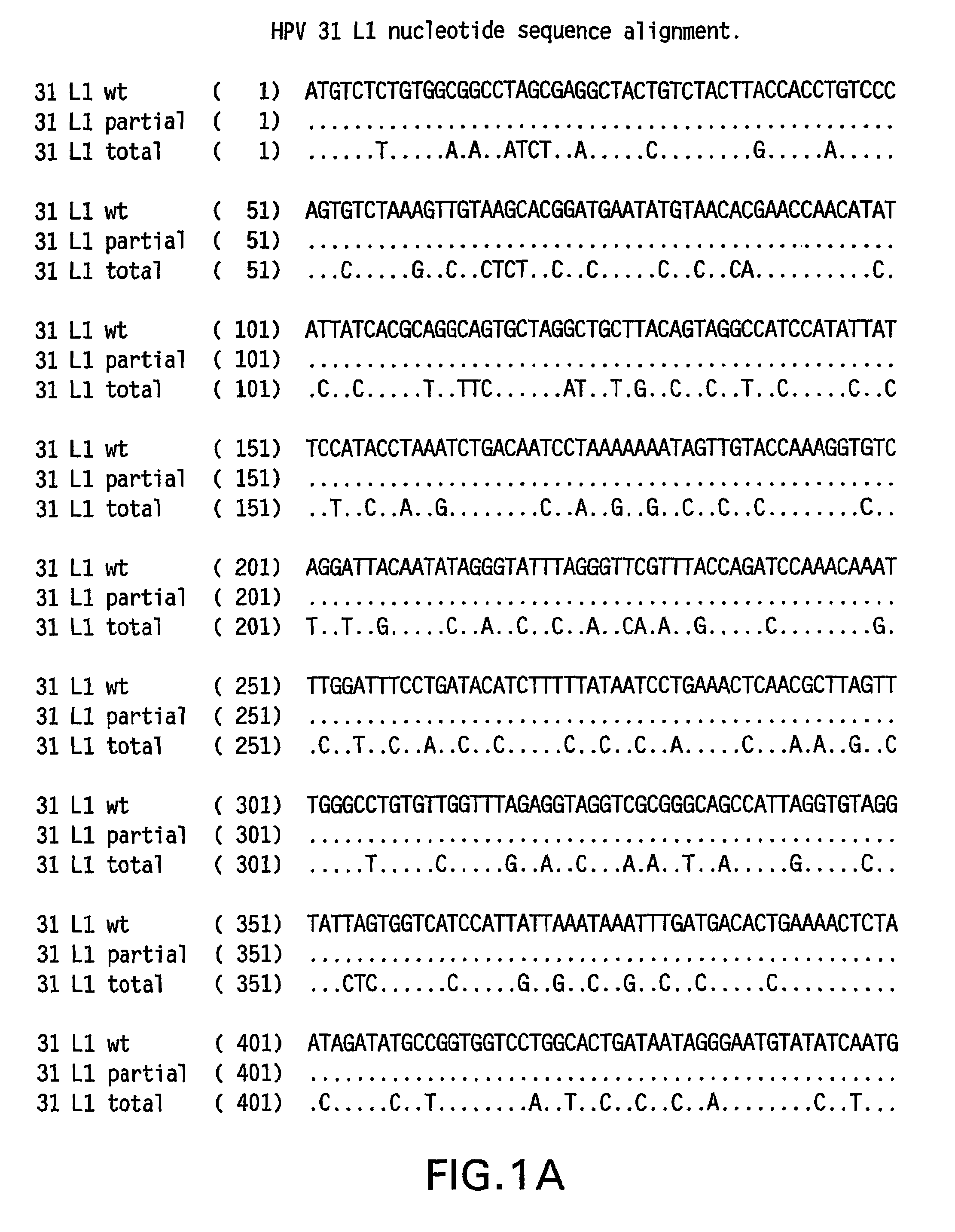
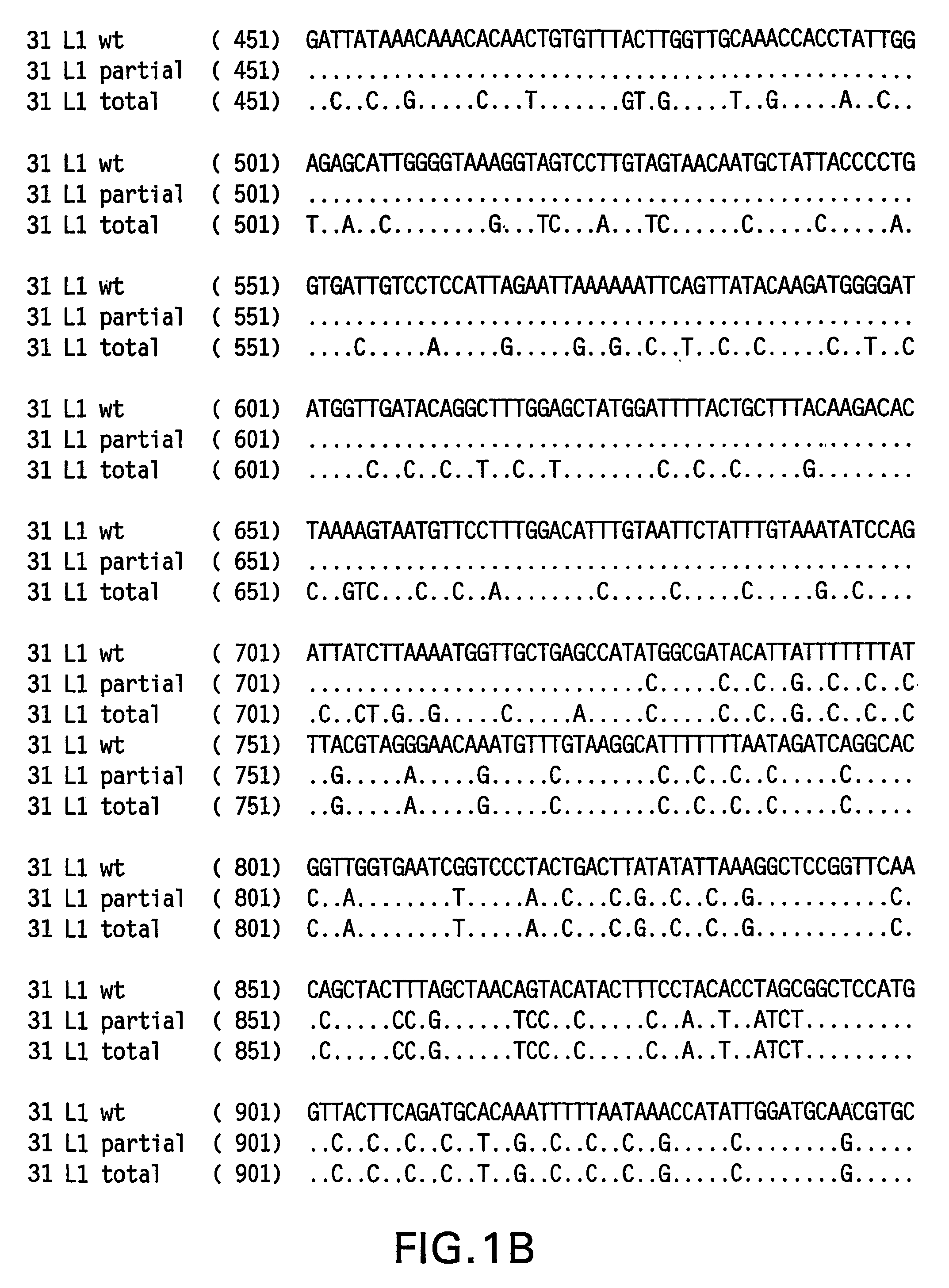
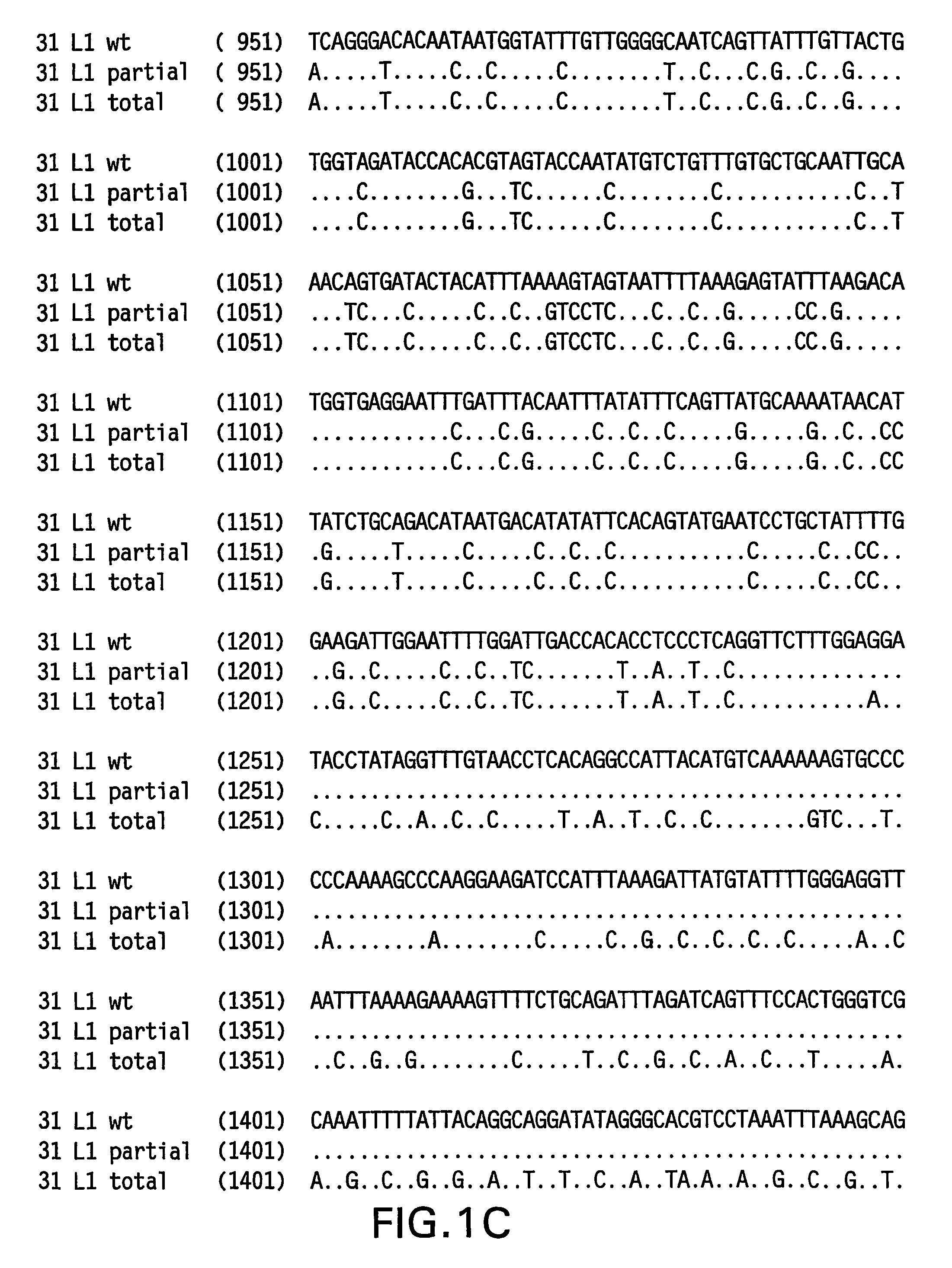
Optimized expression of HPV31 L1 in yeast
Synthetic DNA molecules encoding the HPV31 L1 protein are provided. Specifically, the present invention provides polynucleotides encoding HPV31 L1 protein, wherein said polynucleotides are free from internal transcription termination signals that are recognized by yeast. Also provided are synthetic polynucleotides encoding HPV31 L1 wherein the polynucleotides have been codon-optimized for high level expression in a yeast cell. The synthetic molecules may be used to produce HPV31 virus-like particles (VLPs), and to produce vaccines and pharmaceutical compositions comprising the HPV31 VLPs. The vaccines of the present invention provide effective immunoprophylaxis against papillomavirus infection through neutralizing antibody and cell-mediated immunityHPV31 L1 total rebuild nucleotide and aminoacid sequence: M S L W R P S 1 ATGTCTTTGT GGAGACCATC E A T V Y L P P V PTGAAGCTACC GTCTACTTGC CACCAGTCCC V S K V V S T 51 AGTCTCTAAG GTCGTCTCTA D E Y V T R T N I YCCGACGAATA CGTCACCAGA ACCAACATCT Y H A G S A 101 ACTACCACGC TGGTTCTGCTR L L T V G H P Y YAGATTGTTGA CCGTCGGTCA CCCATACTAC S I P K S D N 151 TCTATCCCAA AGTCTGACAA P K K I V V P K V SCCCAAAGAAG ATCGTCGTCC CAAAGGTCTC G L Q Y R V F 201 TGGTTTGCAA TACAGAGTCT R V R L P D P N K FTCAGAGTCAG ATTGCCAGAC CCAAACAAGT G F P D T S 251 TCGGTTTCCC AGACACCTCTF Y N P E T Q R L VTTCTACAACC CAGAAACCCA AAGATTGGTC W A C V G L E 301 TGGGCTTGTG TCGGTTTGGA V G R G Q P L G V GAGTCGGTAGA GGTCAACCAT TGGGTGTCGG I S G H P L L 351 TATCTCTGGT CACCCATTGT N K F D D T E N S NTGAACAAGTT CGACGACACC GAAAACTCTA R Y A G G P 401 ACAGATACGC TGGTGGTCCAG T D N R E C I S MGGTACCGACA ACAGAGAATG TATCTCTATG D Y K Q T Q L 451 GACTACAAGC AAACCCAATT C L L G C K P P I GGTGTTTGTTG GGTTGTAAGC CACCAATCGG E H W G K G S 501 TGAACACTGG GGTAAGGGTT P C S N N A I T P GCTCCATGTTC TAACAACGCT ATCACCCCAG D C P P L E 551 GTGACTGTCC ACCATTGGAAL K N S V I Q D G DTTGAAGAACT CTGTCATCCA AGACGGTGAC N V D T G F G 601 ATGGTCGACA CCGGTTTCGG A N D F T A L Q D TTGCTATGGAC TTCACCGCTT TGCAAGACAC K S W V P L D 651 CAAGTCTAAC GTCCCATTGG I C N S I C K Y P DACATCTGTAA CTCTATCTGT AAGTACCCAG Y L K M V A 701 ACTACTTGAA GATGGTCGCTE P Y G D T L F F YGAACCATACG GCGACACCTT GTTCTTCTAC L R R E Q M F 751 TTGCGTAGAG AACAGATGTT V R H F F N R S G TCGTAAGGCAC TTCTTCAACA GATCCGGCAC V G E S V P T 801 CGTAGGTGAA TCTGTCCCAA D L Y I K G S G S TCCGACCTGTA CATCAAGGGC TCCGGTTCCA A T L A N S 851 CCGCTACCCT GGCTAACTCCT Y F P T P S G S NACCTACTTCC CAACTCCATC TGGCTCCATG V T S D A Q I 901 GTCACCTCCG ACGCTCAGAT F N K P Y W M Q R ACTTCAACAAG CCATACTGGA TGCAGCGTGC Q G H N N G I 951 ACAGGGTCAC AACAACGGTA C W G N Q L F V T VTCTGTTGGGG TAACCAGCTG TTCGTGACTG V D T T R S1001 TGGTCGATAC CACGCGTTCTT N N S V C A A I AACCAACATGT CTGTCTGTGC TGCAATCGCT N S D T T F K1051 AACTCTGACA CTACCTTCAA S S N F K E Y L R HGTCCTCTAAC TTCAAGGAGT ACCTGAGACA G E E F D L Q1101 TGGTGAGGAA TTCGATCTGC F I F Q L C K I T LAATTCATCTT CCAGTTGTGC AAGATCACCC S A D I N T1151 TGTCTGCTGA CATCATGACCY I H S M N P A I LTACATCCACA GTATGAACCC TGCCATCCTG E D W N F G L1201 GAGGACTGGA ACTTCGGTCT T T P P S G S L E DGACCACTCCA CCTTCCGGTT CTTTGGAAGA.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com