Preparation method of amide
An amide, selected technology, applied in the field of amide preparation, can solve problems such as complexity, long reaction steps, and reduced economy, and achieve the effects of reducing reaction steps, improving utilization rate, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
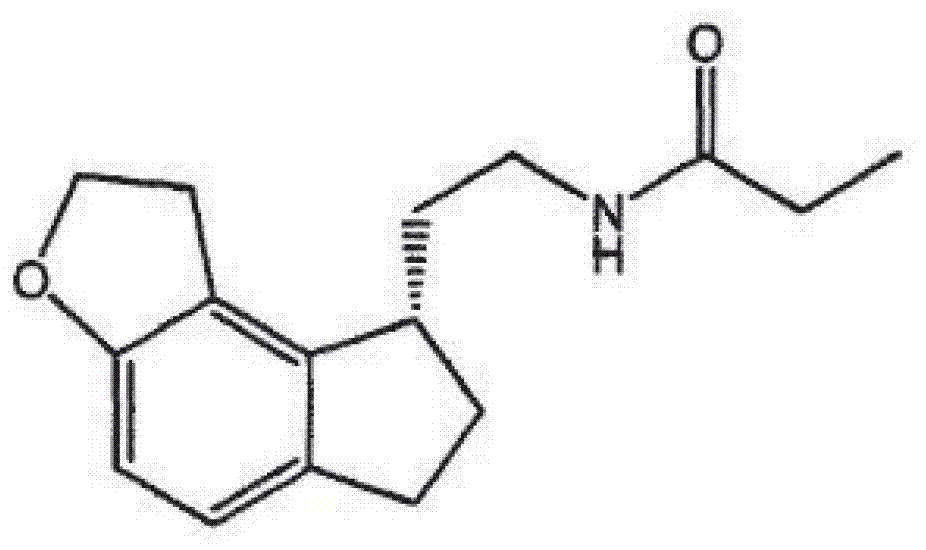
[0044] Example 1: Preparation of compound 1 (tasimelteon)
[0045] (compound 1)
[0046](1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropylcarboxamide ( ), (the starting reactant can be prepared according to the method disclosed in Chinese patent application CN102675268A), 1.9g of sodium borohydride is added in 40ml of anhydrous tetrahydrofuran, heated to reflux, wherein (1R,2R)-2-(2,3 The mol ratio of -dihydrobenzofuran-4-yl)cyclopropylcarboxamide and sodium borohydride is about 1:5, slowly add dropwise the mixed solution of 12.5g propionic acid and 20ml anhydrous tetrahydrofuran (sodium borohydride and propionic acid The molar ratio is about 1:3.3), the addition is completed in 12 hours, and then the reaction is continued at 70°C for about 6 hours, down to room temperature, the material is slowly poured into 100ml of ice water, and about 20g of hydrochloric acid with a mass concentration of 10% is added dropwise while stirring , adjust the pH value to about 2. The obtain...
Embodiment 2
[0048] Example 2: Preparation of Compound 2 (Rimeteon)
[0049] (Compound 2)
[0050] 3.2g of the compound As the starting reactant (the starting reactant can be prepared according to the preparation method described in the patent JP11080106), 1.9 g of sodium borohydride is added to 40 ml of anhydrous tetrahydrofuran, and heated to reflux, wherein the mole of the starting reactant and sodium borohydride The ratio is about 1:5, slowly add a mixed solution of 12.5g propionic acid and 20ml anhydrous tetrahydrofuran (the molar ratio of sodium borohydride and propionic acid is about 1:3.8), add it in 12 hours, and then continue the reaction at 70°C After about 6 hours, cool down to room temperature, slowly pour the material into 100ml of ice water, add about 20g of hydrochloric acid with a mass concentration of 10% under stirring, and adjust the pH to about 2. The obtained product was distilled off under reduced pressure to remove the solvent tetrahydrofuran, and then extracte...
Embodiment 3
[0053] Embodiment 3: Preparation of compound 3 (melatonin)
[0054] (compound 3)
[0055] 3.5g of the compound As the starting reactant (the starting reactant can be prepared according to the preparation method described in the document Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences; vol.251; (1960); p.394), 1.9 g of hydroboration Sodium was added in 40ml of anhydrous tetrahydrofuran, heated to reflux, wherein the molar ratio of the initial reactant to sodium borohydride was about 1:5, and a mixed solution of 20.9g of acetic acid and 20ml of anhydrous tetrahydrofuran (sodium borohydride and acetic acid) was slowly added dropwise. The molar ratio is about 1:3.5), and the addition was completed in 12 hours, and then the reaction was continued at 70°C for about 6 hours, and the temperature was lowered to room temperature, and the material was slowly poured into 100ml of ice water, and about 20g of hydrochloric acid with a mass concentration of 10% was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com