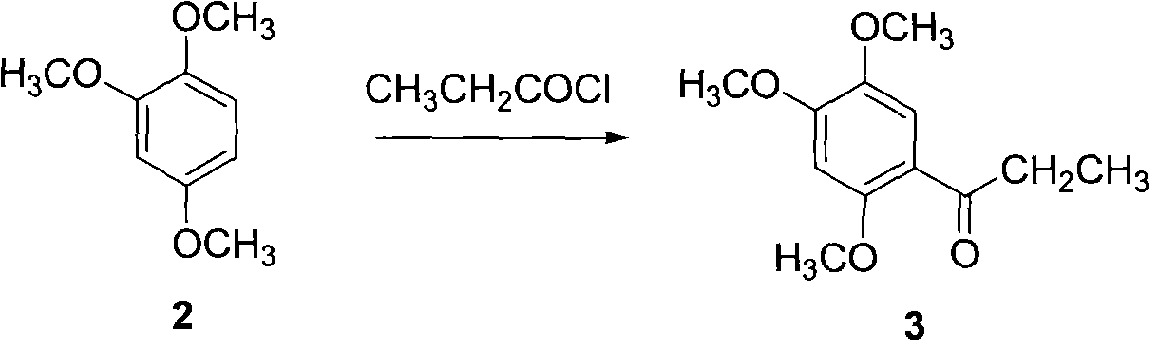Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
182 results about "Propionyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Propionyl chloride is expected to volatilize from dry soil surfaces based upon its vapor pressure. If released to water, propionyl chloride reacts vigorously with water to form propionic acid and hydrochloric acid. This decomposition reaction will be the dominant fate process in water. Occupational exposure to propionyl chloride may occur ...
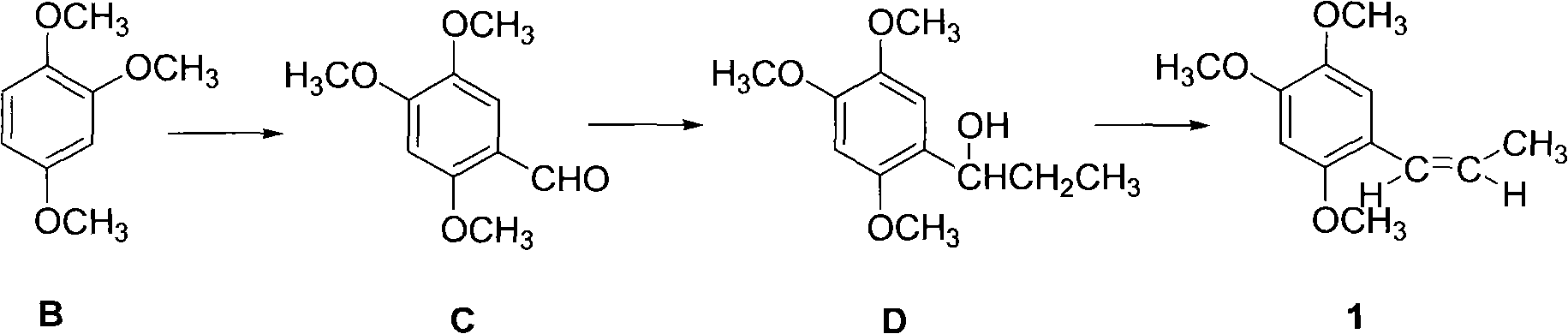
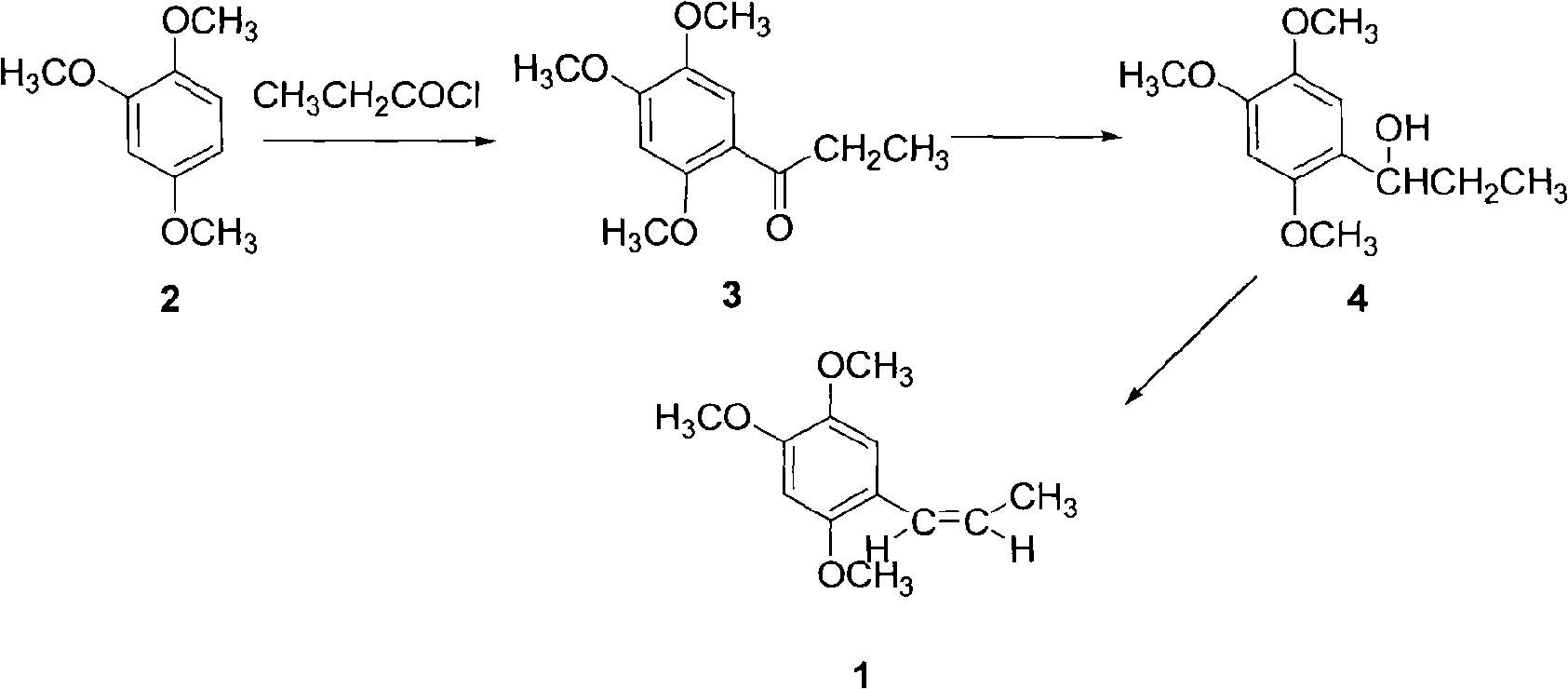
Synthetic method of anethole
InactiveCN103058835AReduce lossesLow costOrganic chemistryOrganic compound preparationSolventSide reaction
The invention discloses a synthetic method of anethole. Anisole and propionyl chloride serve as starting raw materials, are subjected to a friedel-crafts acylation reaction, then are restored through sodium borohydride, and then are catalyzed and dehydrated through organic acid and acid inorganic salt, and finally the anethole is manufactured through rectification. Side reaction is low through the adoption of acylation and reduction, a middle body is not needed to be purified and can be directly put into the next reaction, purification loss and purification cost of the middle body are reduced, yield coefficient is improved, and energy consumption is reduced. A chemical reduction method is utilized to replace a catalytic hydrogenation method, selectivity is high, response speed is fast, side reactions are small in number, and reduction yield coefficient can reach above 98%. Double catalysts are adopted to carry out ordinary pressure low temperature dehydration in dissolvent with a low boiling point, and compared with a negative pressure continuous dehydration method, dehydration time can be shortened by 90%, and the energy consumption is greatly reduced. The total yield coefficient of the method can reach 75%. A product quality index is higher than that of a natural product standard.
Owner:HUAIAN WAN BANG SPICE IND CO LTD +1
Industrial process for preparing beta-thymidine
InactiveCN1634959AShort synthetic routeSimple production processSugar derivativesPropionyl chlorideSynthesis methods
The invention belongs to medical intermediate field, and relates to a four-step synthesis method for industrial preparation of a beta thymidine. The invention is characterized in that a D-ribose is used as initial raw material. The D-ribose is transformed into the beta thymidine after direct condensation with thymine, halogenation-acylation reaction with propionyl chloride, catalytic hydrogenation reduction and catalyzed alcoholysis with total yield greater than 65%.
Owner:SHAXING CHEM TAIZHOU CITY
Method for synthesizing dendritic phenolic antioxidant
InactiveCN101704948AStructural symmetryOvercome deficienciesBulk chemical productionEthylenediamineDistillation
The invention relates to a method for synthesizing a dendritic phenolic antioxidant, which comprises the following steps: firstly, using ethylenediamine and methyl acrylate as raw materials; secondly, synthesizing frameworks of 0.5 generation polyamide-amine and 1.0 generation polyamide-amine through two steps including Michael addition reaction and amidation condensation reaction; thirdly, using the 1.0 generation polyamide-amine and beta-(3,5-di-tertiary-butyl-4-hydroxyphenyl) propionyl chloride as raw materials, and using triethylamine as an acid-binding agent and trichloromethane as a solvent to perform the amidation condensation reaction; and finally, performing reduced pressure distillation, extraction and filtering to obtain the dendritic phenolic antioxidant. The dendritic phenolic antioxidant has readily available reaction raw materials, mild reaction conditions, easy product purification and high product yield; and the reactions belongs to basic reaction types, so the dendritic phenolic antioxidant can achieve industrialization very easily.
Owner:DAQING GASOLINEEUM INST
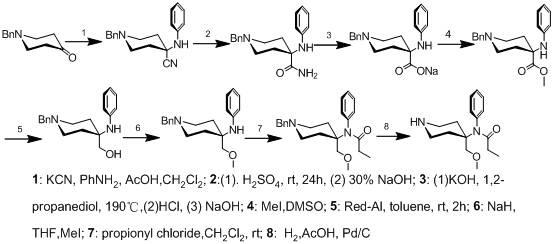
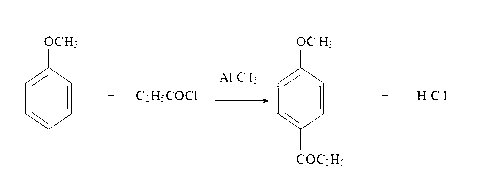
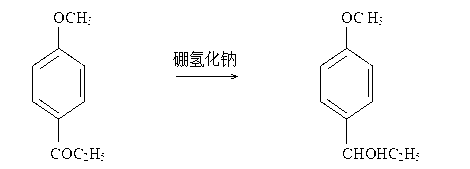
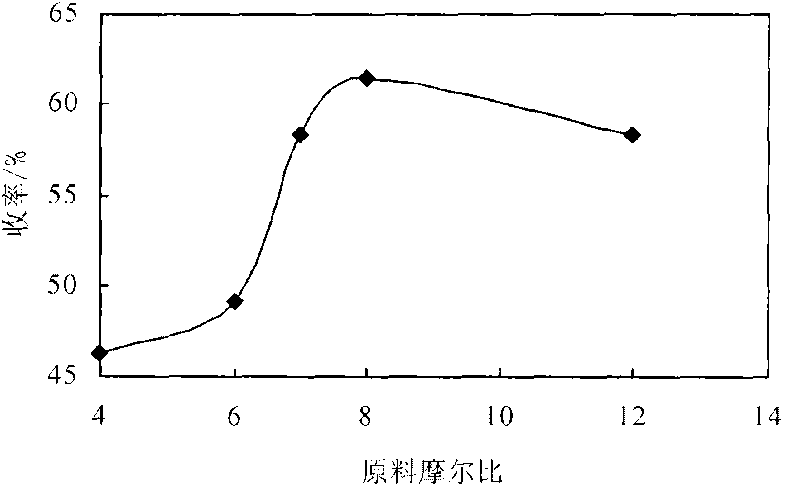
2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method
InactiveCN1310869CAtom utilization is highThree wastes lessOrganic compound preparationAmino compound preparationNitroalkaneEthyl group
The invention provides a preparation method for (I) 2- amido -2-[2-(4- alkyl phenyl)ethyl]-1,3- propanediol, which comprises: using alkyl benzene (II) as initial material, with Lewis acid existing, to take Friedel-Crafts acylating reaction with 3- halogenated propionyl chloride and generate beta- halogenated alkylpropiophenone (IV); reducing IV by hydride to obtain 3- nitro -1-(4- alkyl phenyl) propanol (V); preparing 2- nitro -2- methylol -4-(4- alkyl phenyl)-1,4butanediol (VI) by hydroxymethylation; reducing nitro and removing benzalcoholhydroxy to VI and obtaining the objective product.
Owner:江苏吴中苏药医药开发有限责任公司 +1
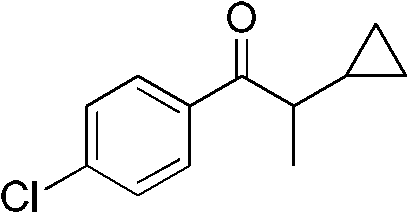
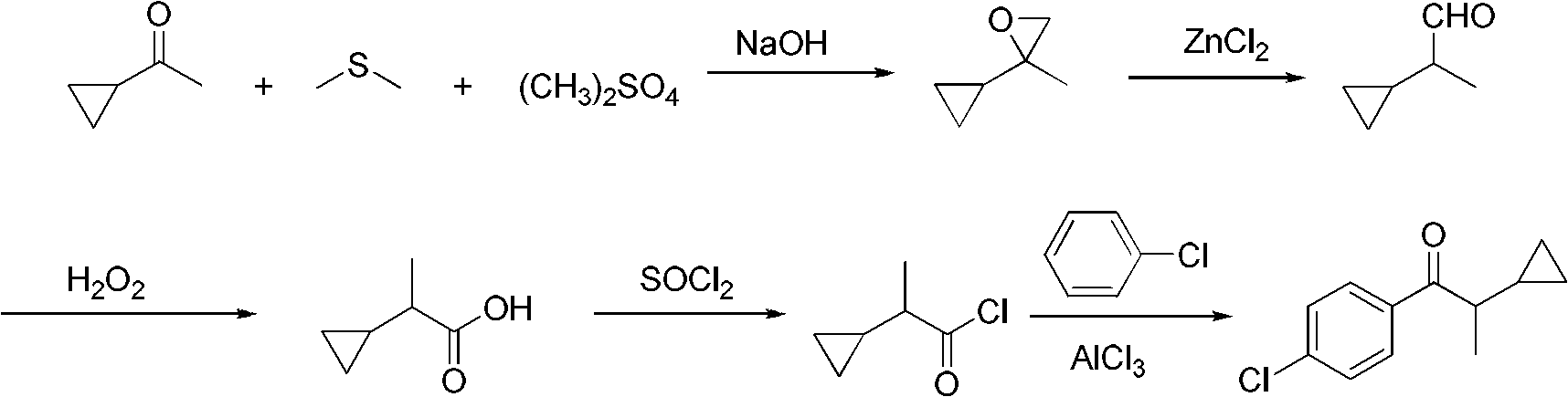
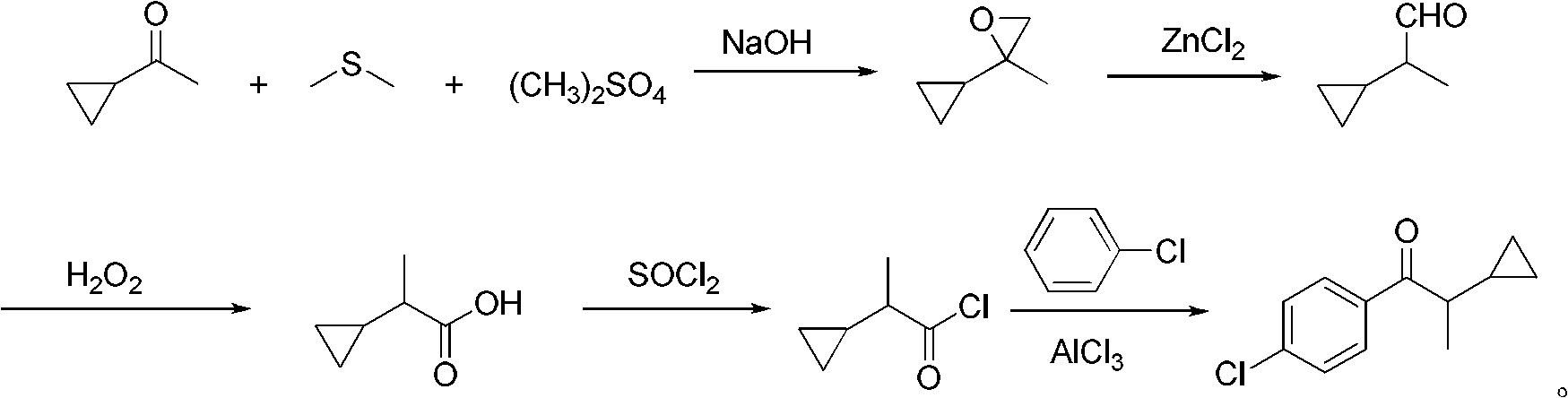
Preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
ActiveCN102584558AHigh product contentHigh yieldCarbonyl compound preparation by condensationPhenyl groupSodium hydroxide
The invention discloses a preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone. The preparation method comprises the steps that: (1) cyclopropyl methyl ketone, dimethyl sulfate, dimethyl sulfide and sodium hydroxide take epoxidation reaction to obtain 2-cyclopropyl-2-methyl propylene oxide; (2) the 2-cyclopropyl-2-methyl propylene oxide is catalyzed and re-ranged by anhydrous zinc chloride at the room temperature to obtain 2-cyclopropyl propionaldehyde; (3) the 2-cyclopropyl propionaldehyde is oxidized by 40 percent oxydol at the room temperature to obtain 2-cyclopropyl monoprop; (4) the 2-cyclopropyl monoprop and thionyl chloride take reaction to obtain 2-cyclopropyl propionyl chloride; (5) the 2-cyclopropyl propionyl chloride takes reaction with chlorobenzene and anhydrous aluminum trichloride to obtain the 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone with the content being 98.0 percent to 98.7 percent, and the total yield is 80.0 percent to 83.1 percent. The preparation method has the advantages that the steps are simple, raw materials are cheap and are easy to obtain, easy-self-ignition compounds are not used, organic solvents are not used in the steps (2) and (3), three wastes are few, and the preparation method is suitable for industrialization.
Owner:HUNAN HAILI HIGH TECH IND GRP
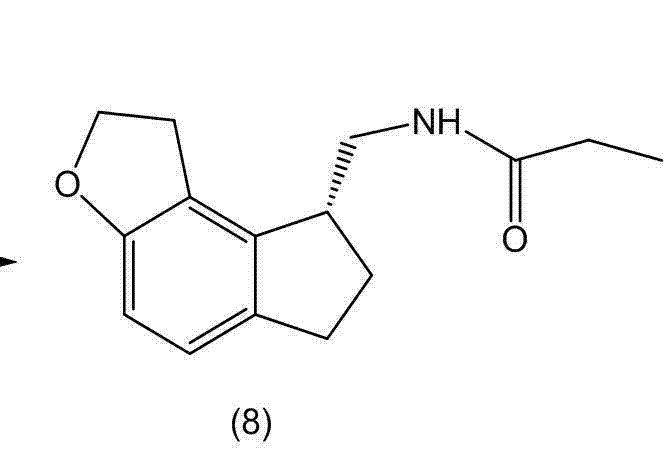
Preparation method and intermediate of ramelteon
InactiveCN102924410AReduce dosageRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionPropionyl chlorideOrganic solvent
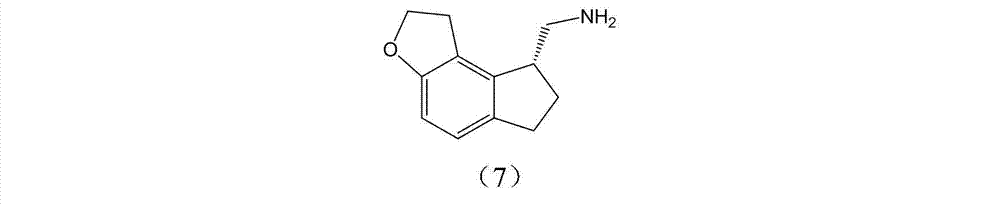
The invention discloses a preparation method and an intermediate of ramelteon. The preparation method comprises the following steps: 1. deprotecting a compound (5) in an organic solvent to obtain a compound (6); 2. after condensing the compound (6) and a chiral resolution reagent, crystallizing to obtain an S type condensate crystal, and hydrolyzing under alkaline conditions to obtain an S type optical isomer compound (7) of the compound (6); and 3. reacting the compound (7) with propionyl chloride under alkaline conditions to generate the ramelteon (8).
Owner:CHINA RESOURCES SAIKE PHARMA
Process for producing asarin
InactiveCN101492351ALow priceReduce manufacturing costOrganic chemistryOrganic compound preparationPropionyl chloridePropionic anhydride
As during the process of preparing asarin from 1, 2, 4-trimethoxybenzene, the raw materials of propionic anhydride, propionyl chloride, anhydrous aluminum chloride, anhydrous zinc chloride, dichloromethane, sodium borohydride, sodium propionate and the like with low prices are used, the production cost is lowered greatly. At the same time, the post treatment of the invention is convenient; products of each step are all solids which are convenient for detecting; the reaction yield of each step is high; and the production process generates few three wastes. In conclusion, in the invention, the 1, 2, 4-trimethoxybenzene which is obtained easily in China is used as a raw material to prepare the asarin by the reactions such as acidylation and the like; the invention has low cost, safe and simple operation, high reaction yield and less environmental pollution; and compared with the existing reported method, the invention is fit for the industrialized production better.
Owner:亚邦化工集团有限公司
Synthetic method of cyhalofop-butyl active compound
ActiveCN102584627AAvoid it happening againHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidN-Butyl Alcohol
The invention discloses a synthetic method of a cyhalofop-butyl active compound. The method comprises the following steps of: undergoing a condensation reaction on (R)-4-hydroxyphenoxypropanoic acid and 3,4-difluorobenzonilyile serving as raw materials in an organic solvent under an alkaline catalysis condition to generate an intermediate, i.e., (R)-2-[4-(2-fluoro-4-nitrile)-phenoxyl]-propanoic acid; undergoing a photochemical reaction on the intermediate, i.e., (R)-2-[4-(2-fluoro-4-nitrile)-phenoxyl]-propanoic acid to generate an intermediate, i.e., (R)-2-[4-(2-fluoro-4-nitrile)-propionyl chloride; and undergoing a third step esterification reaction on the intermediate, i.e., (R)-2-[4-(2-fluoro-4-nitrile)-propionyl chloride and n-butyl alcohol to generate the cyhalofop-butyl active compound. The method has the advantages of simple process, low production cost, high chemical content, high optical purity and the like.
Owner:JIANGSU KUAIDA AGROCHEM
Synthetic method of clethodim
ActiveCN105418470AEasy to operateLess solventSulfide preparationPropionyl chloride2 cyclohexene 1 one
Provided is a synthetic method of clethodim. The invention relates to cyclohexenone herbicide clethodim, namely, 2-(1-(((3-chloro-2-propenyl)oxy)imino)propyl)-5-(2-(ethylthio)propyl)-3-hydroxy-2-cyclohexene-1-one. A chain reaction is conducted on ethanethiol, crotonaldehyde, ethyl acetoacetate, ethylsuleenyl heptenone and propionyl chloride which serve as main raw materials and a trace catalyst to generate the clethodim. The synthetic method of the clethodim is green, environmentally friendly and high in economic benefit.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
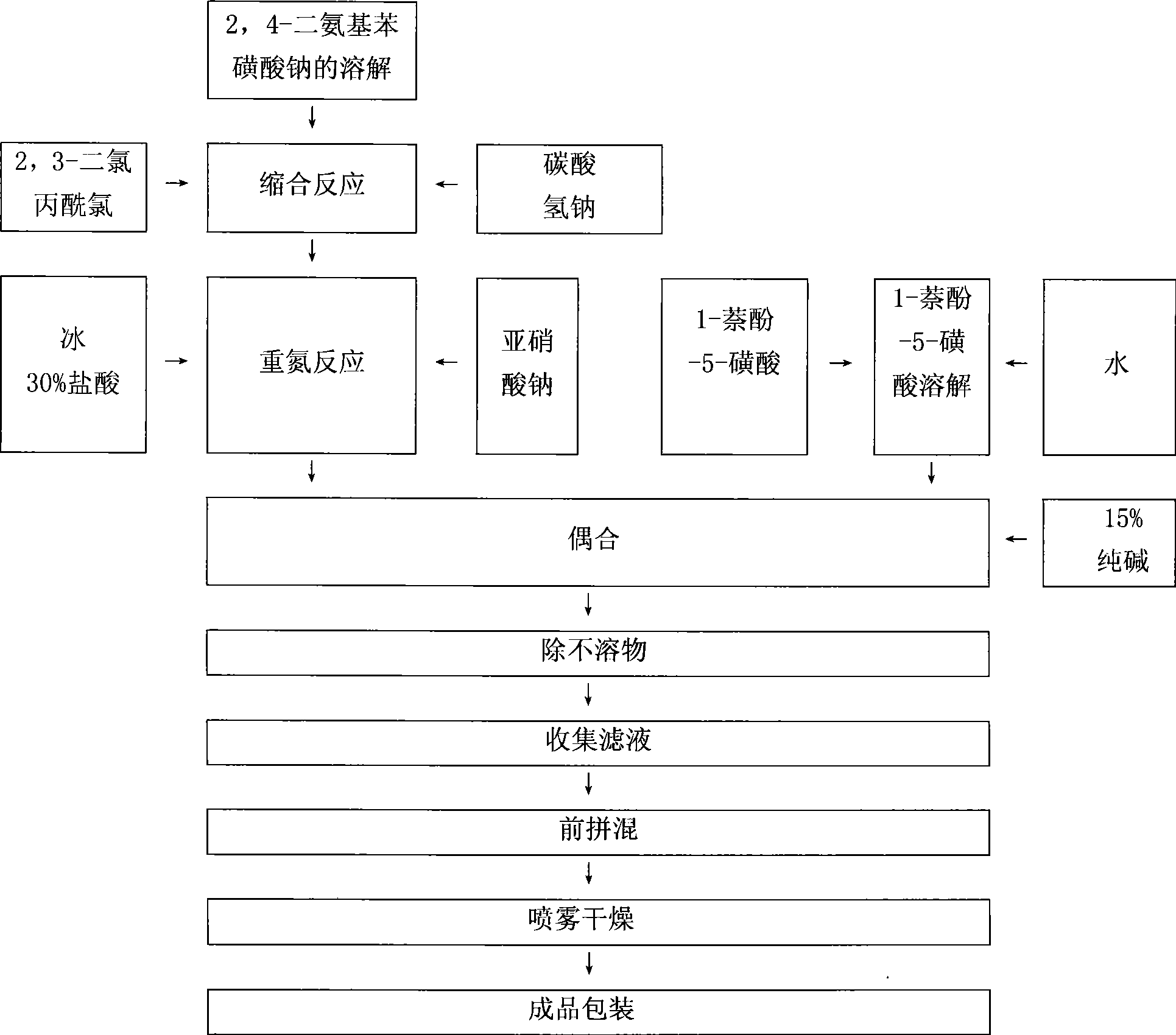
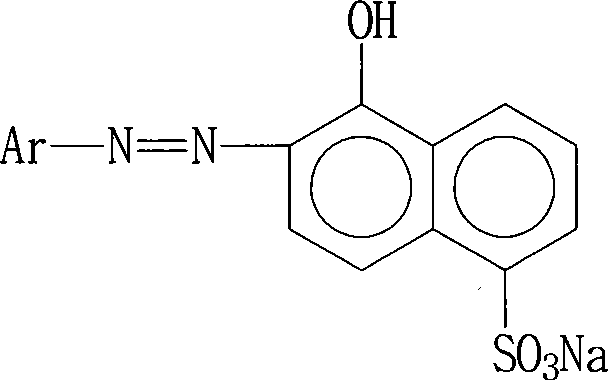
Red reactive dye for fur and preparation thereof
InactiveCN101481523AImprove responseImprove solubilityReactive dyesDyeing processSolubilitySulfonate
The invention discloses a red reactive dye used for fur and a preparation method thereof. The red reactive dye used for fur takes 2, 4-diaminobenzene sodium sulfonate, 1-naphthol-5-sulfoacid, 2, 3-dichloro propionyl chloride as main raw materials. The red reactive dye of the invention is prepared by condensation, diazotization, coincidence, chromatic light adjustment, intensity adjustment, drying and packaging. The red reactive dye used for fur in the invention has relatively high responsiveness, good solubility and bright-colored and beautiful chromatic light; in addition, the light fastness property is relatively good, the exhaustion rate and the color fixing rate are very high. The pre-blending technology and the virgin stock spraying technology are adopted after film processing. Therefore, waste water and waste residue are not generated, greatly contributing to the environmental protection.
Owner:TIANJIN DEK CHEM
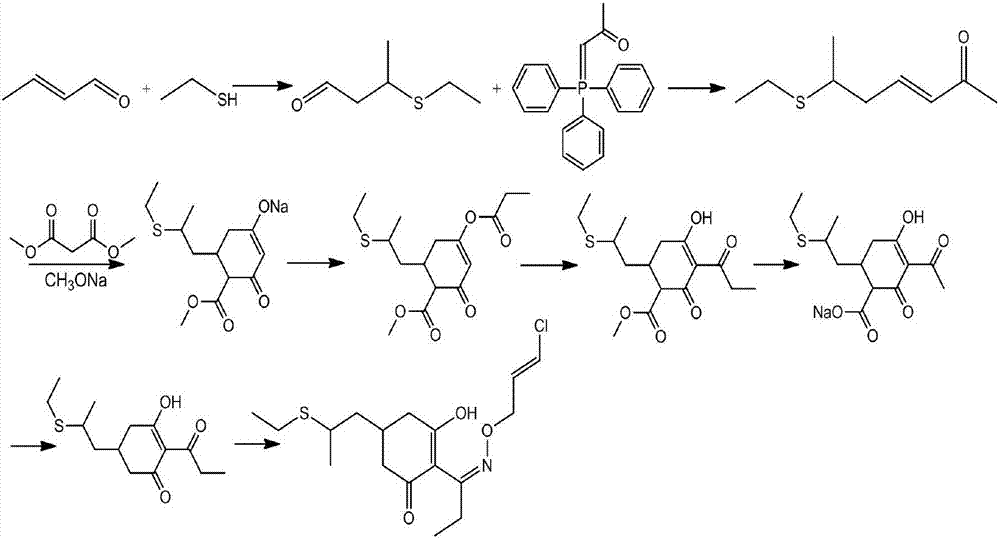
Method for synthesizing clethodim
InactiveCN107162945AHigh yieldImprove product qualitySulfide preparationPropionyl chlorideEthanethiol
The present invention proposes a method for synthesizing clethodim, comprising 3,5-heptadiene-2-one (formula II) and ethanethiol for nucleophilic addition to prepare clethodim intermediate 6-ethylthio ‑3‑heptene‑2‑ketone (formula III); formula III reacts with dimethyl malonate and propionyl chloride to prepare (±)‑3‑[propionyloxy]‑5‑[ 2-(Ethylthio)propyl]-6-[methoxyformyl]cyclohex-2-enone (Formula V); Formula V is then rearranged, hydrolyzed and decarboxylated to obtain (±)-2- [Propionyl]-3-[hydroxyl]-5-[2-(ethylthio) propyl]cyclohex-2-enone (formula VIII); formula VIII reacts with chloroallyloxyamine to obtain alkene Clethodim (formula I), the method for preparing clethodim by using intermediate 3,5-heptadiene-2-one (formula II) in the present invention is proposed for the first time, and the clethodim (formula I) prepared by this method is generally The yield is high, the product quality is good, and the method has low production cost and little environmental pollution, and is suitable for large-scale production.
Owner:江苏威格瑞斯化工有限公司
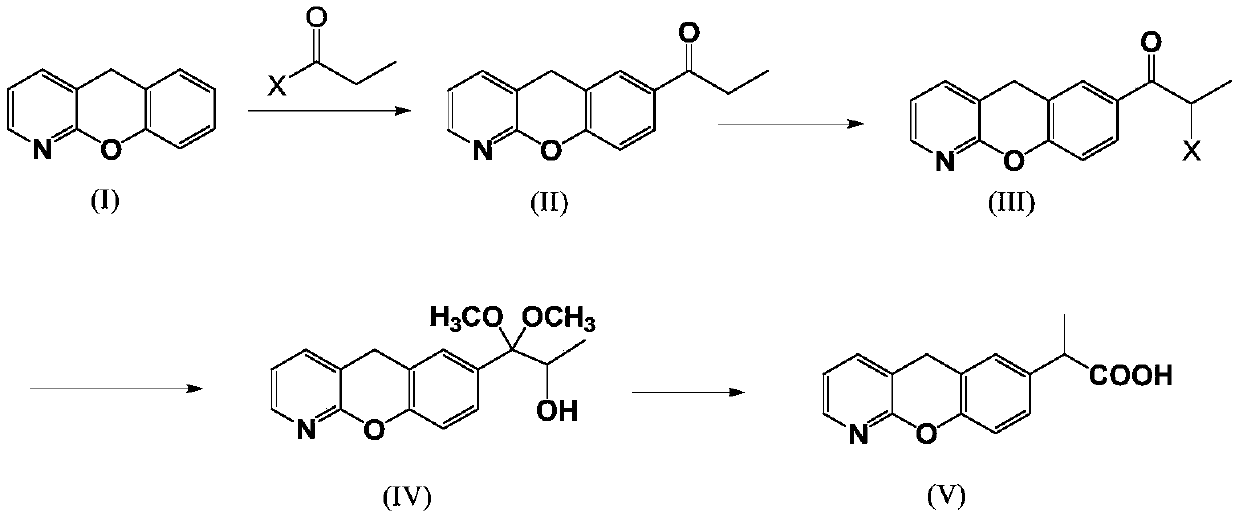
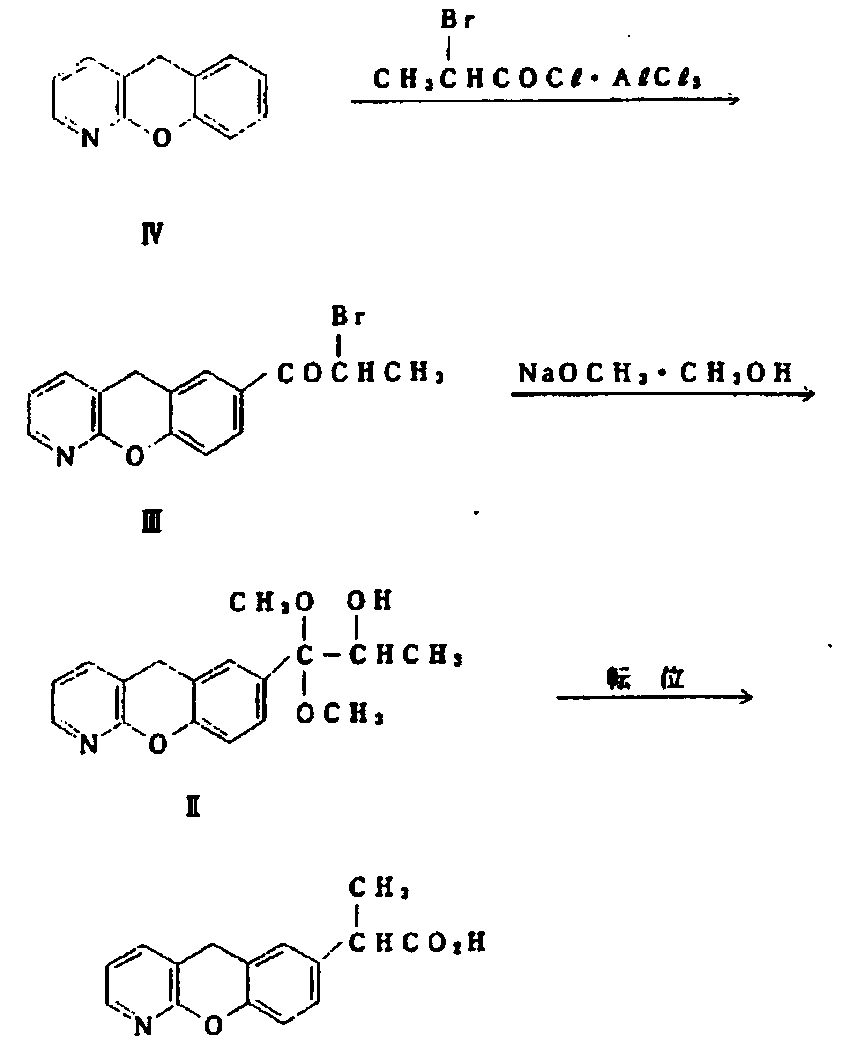
Novel preparation method of pranoprofen
The invention discloses a novel preparation method of pranoprofen. The novel preparation method of the pranoprofen has the advantages of being high in yield, having less impurities, and being safe andenvironmentally friendly. The novel preparation method of the pranoprofen comprises the following steps that step 1, 5H-[1]-benzopyran[2,3-b]pyridine and propionyl chloride are directly acylated; step 2, bromination is carried out; step 3, potassium tert-butoxide or sodium tert-butoxide is used for hydrolysis, and finally, rearrangement is carried out to obtain the pranoprofen.
Owner:GUANGDONG XIANQIANG PHARMA +2
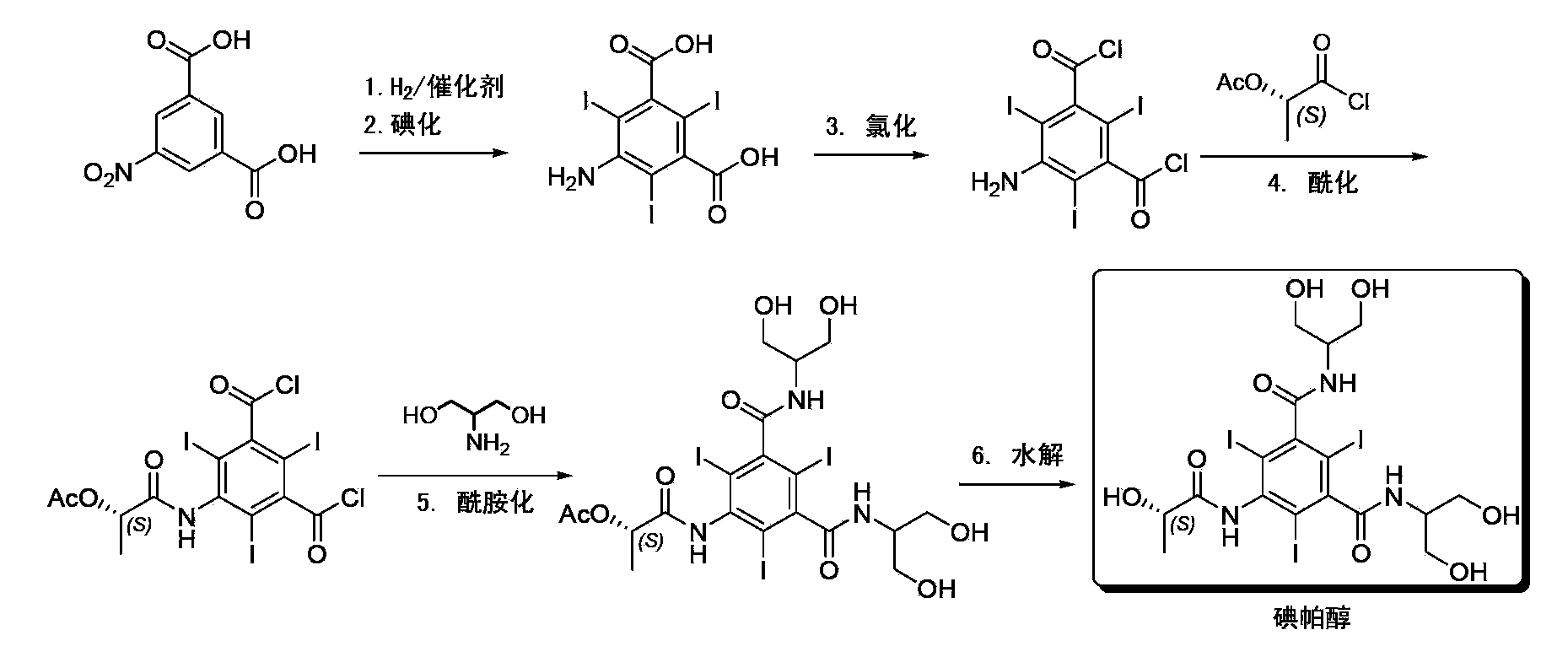
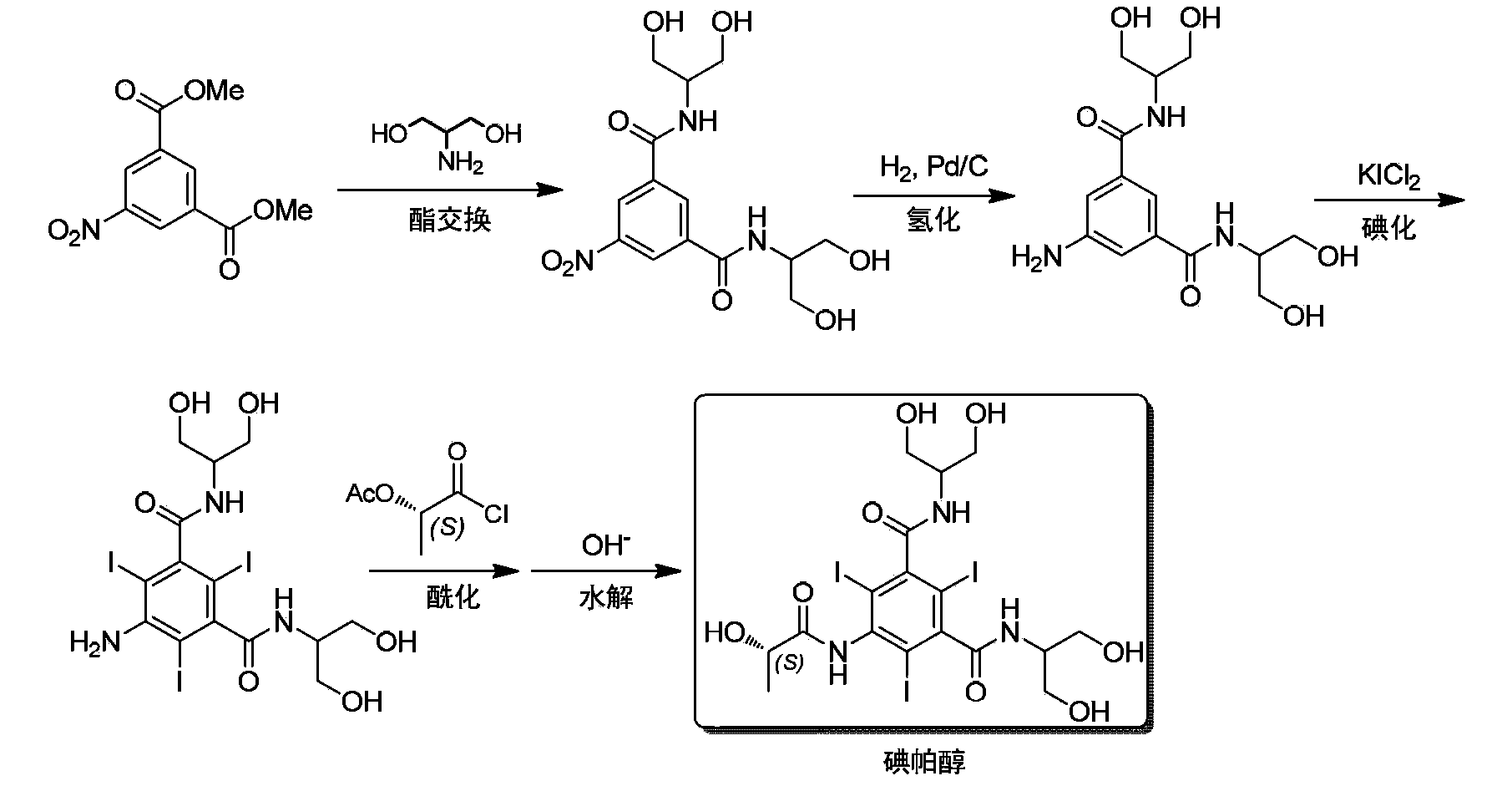
Synthesis of iopamidol and preparation of iopamidol synthesis intermediate
ActiveCN103382160AEasy to separateReduce difficultyOrganic compound preparationCarboxylic acid amides preparationPropionyl chlorideIodine
The invention discloses a method for preparation of (S)-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxopropyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide (iopamidol) shown in the formula I from 5-amino-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide shown in the formula II. The method comprises the following steps that a, the compound shown in the formula II and a mixed anhydride as an appropriate protective agent undergo a reaction to produce a mixed ester shown in the formula III; b, the mixed ester shown in the formula III and (S)-2-(acetoxy)propionyl chloride undergo a reaction so that an amino group at the 5th site is acylated and a compound shown in the formula IV is obtained; and c, the compound shown in the formula IV undergoes a hydrolysis reaction under acidic or alkaline conditions or undergoes an alcoholysis reaction so that all acyl groups of the compound shown in the formula IV are removed and the iopamidol shown in the formula III is obtained. The invention relates to a synthesis intermediate of the iopamidol shown in the formula III.
Owner:ZHEJIANG HISYN PHARMA
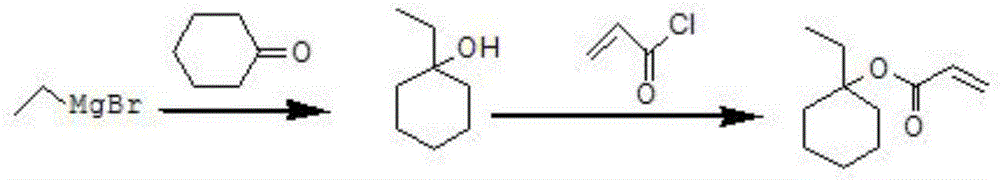
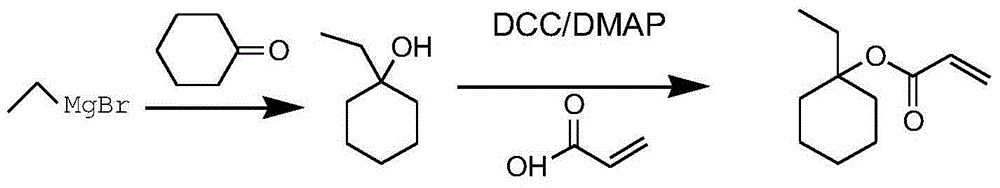
Method for preparing 1-ethylcyclohexyl acrylate
ActiveCN104910012AHigh purityEasy to operatePreparation from carboxylic acid halidesOrganic compound preparationCyclohexanoneGrignard reagent
The invention relates to a method for preparing 1-ethylcyclohexyl acrylate. The method comprises the following steps: magnesium turnings are fetched, and a mixed liquid of bromoethane and a reaction solvent is dropped into the magnesium turnings; bromoethane is subjected to a reaction with the magnesium turnings, such that a Grignard reagent is prepared; cyclohexanone is dissolved in toluene, and the solution is dropped into the Grignard reagent; when dropping is finished, a reaction is continued under a maintained temperature; when the Grignard reaction is finished, the temperature of the reaction system is reduced to 0-40 DEG C, and an acid-binding agent is added; a dichloromethane solution of propionyl chloride is dropped in, and an esterification reaction is carried out; when dropping is finished, the reaction is continued for 1-5h under a temperature maintained at 10-70 DEG C, such that a reaction liquid is obtained; the reaction liquid is poured into water for quenching; liquid separation is carried out; a water phase is extracted with dichloromethane; organic phases are combined; washing and drying are carried out, and the solvent is removed, such that a crude product is obtained; a polymerization inhibitor is added into a distillation flask, and the crude product is subjected to reduced-pressure distillation; and a distillate at a temperature of 98-100 DEG C under a pressure of 60Pa is collected, such that 1-ethylcyclohexyl acrylate is obtained. The method provided by the invention has the advantages of high yield, low cost, simple preparation, and suitability for expanded production. With the method, a GC purity can reach 99.5%. The method has a good application prospect.
Owner:VALIANT CO LTD
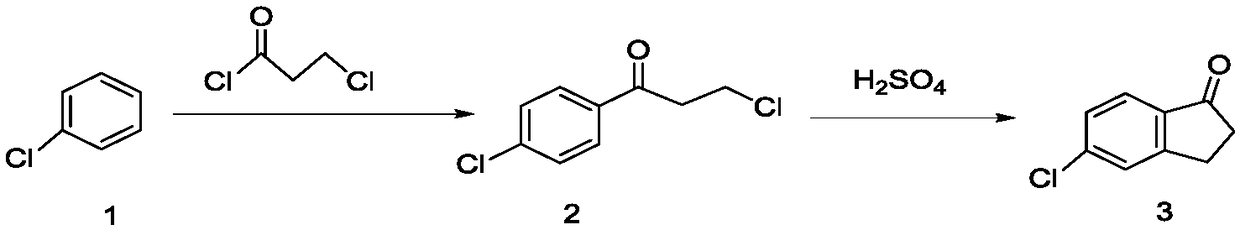
5-chloro-2,3-dihydro-1-indanone preparation method
ActiveCN109293488ASolve the problem of sublimation before meltingOvercoming Pollution DisadvantagesCarbonyl compound preparation by condensationAluminium chlorideChlorobenzene
The invention provides a 5-chloro-2,3-dihydro-1-indanone preparation method, which comprises: carrying out a reaction by using chlorobenzene and 3-chloro-propionyl chloride as raw materials and usinga mixed molten salt as catalyst and a solvent to obtain 5-chloro-2,3-dihydro-1-indanone, wherein the mixed molten salt is a mixture comprising any two or three materials selected from potassium chloride, sodium chloride and aluminum chloride. According to the present invention, the mixed molten salt potassium chloride-sodium chloride-aluminum chloride is used as the catalyst, and becomes liquid ata temperature of about 100 DEG C, such that the problem that aluminum chloride is sublimated before melting is solved; and the mixed molten salt potassium chloride-sodium chloride-aluminum chloride is simultaneously used as the catalyst and the solvent in the reaction of chlorobenzene and 3-chloro-propionyl chloride, such that the conversion rate of the reaction can achieve 98% at the temperatureof 120-130 DEG C, and the yield of 5-chloro indanone can achieve 75%.
Owner:四平市精细化学品有限公司
Method for preparing 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride
InactiveCN102127007AShort production processImprove securityOrganic chemistrySilanesPotassium cyanide
The invention discloses a method for preparing 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride, which belongs to the technical field of preparation of medicinal intermediates. The 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride is synthesized by using 1-phenyl-4-piperidone and aniline as initiative raw materials and by seven reactions. In raw materials used in the invention, trimethyl cyanato silane is used in place of potassium cyanide or sodium cyanide which is a highly toxic raw material to make the operation and management more convenient; potassium tert-butoxide and other alkalis are used in place of sodium hydride which is a dangerous and flammable reagent to improve the safety of scale-up production; propionic anhydride is used in place of highly irritant propionyl chloride to improve the safety, purity and yield of experiments; dimethyl sulfate is used in place of expensive methyl iodide to further control the production cost; and the high-pressure liquid chromatography (HPLC) purity of the obtained 4-(N-phenylpropionamide)-4-methoxymethyl-piperidine hydrochloride product can reach 99.7 percent, the single impurity content is less than 0.1 percent, the total yield can reach 23 percent, and the product is a hydrochloride which is favorable for storage and transport.
Owner:ZHEJIANG LANGHUA PHARMA
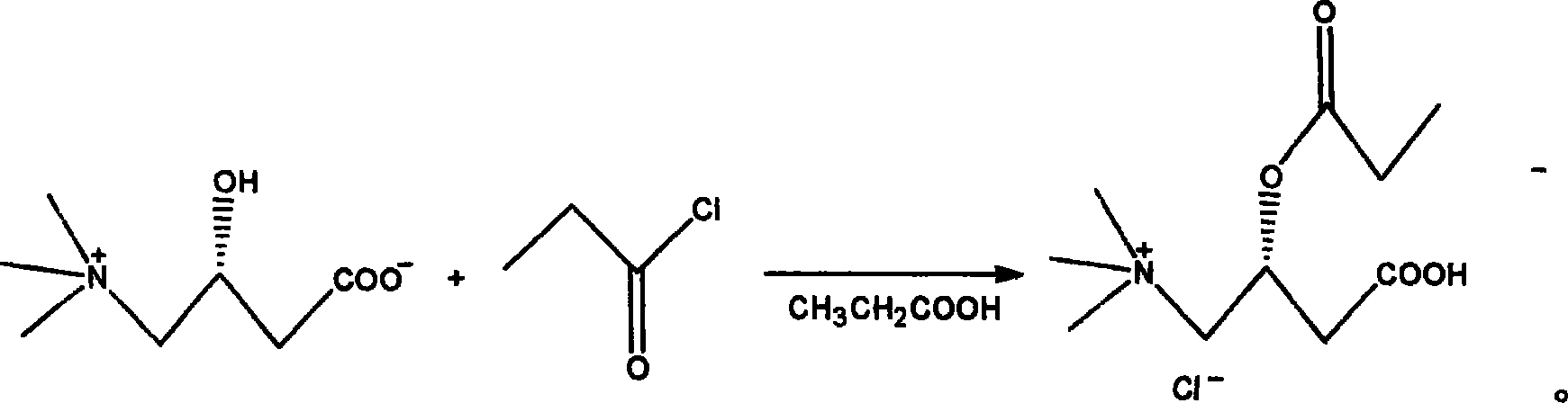
Method for synthesizing propionyl levo-carnitine hydrochlorate
ActiveCN1995010AEasy to purifyShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationPropionyl chlorideAlcohol
The invention discloses a new synthesizing method of propionyl left-handed carnitine hydrochlorate, which comprises the following steps: 1. adopting inner salt of left-handed carnitine as raw material and propionic acid as solvent; adding propionyl chloride; heating to react; decompressing to evaporate propionic acid; adding acetone; stirring to disperse; cooling to evolve; sucking; drying to obtain rough product; 2. heating to dissolve rough product through alcohol or carbinol; sucking the filtrate; decompressing; distilling to condense; evaporating partial alcohol or carbinol; cooling; adding acetone to stir evenly; cooling to evolve; filtering; drying; obtaining the pure product.
Owner:リャオニンコンセプヌトラシーオーエルティーディー
Propionyl-L-carnitine synthesis technology and detection method of related substance and its content
InactiveCN1651402ALow costOperational securityOrganic compound preparationComponent separationPropanoic acidSolvent
A process for synthesizing the propionyl-L-carnitine features the acylation reaction between L-carnitine and propionyl chloride in propionic acid as solvent under the catalysis of p-toluenesulfonic acid. The method for measuring the contents of propionyl-L-carnitine and associated substances features that the efficient liquid-phase chromatography is used to measure the content of propionyl-L-carnitine and the thin-layer chromatography is used to detect associated substances.
Owner:SUZHOU LANXITE BIOTECH
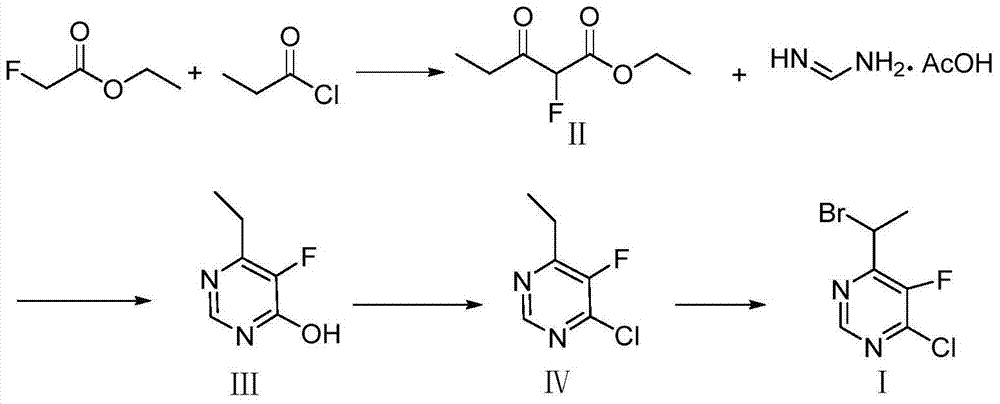
Method for synthesizing 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine
ActiveCN103896855AMild reaction conditionsEasy to operateOrganic chemistryFormamidine acetateSynthesis methods
The invention discloses a method for synthesizing 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine. The synthesis method comprises the steps of reacting 2-fluoro-ethyl acetate which is inexpensive and readily available and is used as an initial material with propionyl chloride under basic conditions in a solvent to synthesize an intermediate product 2-fluoro propionyl ethyl acetate; then carrying out cyclization on 2-fluoro propionyl ethyl acetate and formamidine acetate as well as a base in a solvent to obtain a cyclized product; then chlorinating the cyclized product with a chlorinating reagent; and finally adding a brominating reagent and brominating the chlorinated product in the presence of an initiator to obtain 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine. The synthesis method disclosed by the invention has the advantages of simple process, available raw materials, high yield, safety and environmental protection, and is convenient to industrialize.
Owner:JUHUA GROUP TECH CENT
Novel synthesis method for 3-(alkoxy methyl phosphoryl)propionate
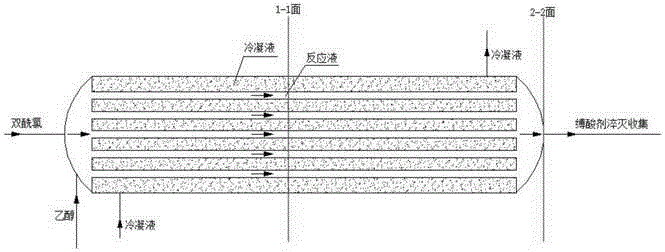
ActiveCN106565778AImprove heat exchange capacityEasy to operateGroup 5/15 element organic compoundsChemical/physical/physico-chemical processesSynthesis methodsReaction tube
The invention discloses a novel synthesis method for 3-(alkoxy methyl phosphoryl)propionate (short for "propionate") by carrying out esterification reaction on 3-(methyl phosphoryl chloride)propionyl chloride (short for "bis(acyl chloride) ") serving as a raw material. The method in which a novel array tubular reaction device is mainly adopted comprises the steps: feeding raw materials including bis(acyl chloride) and a hydroxyl compound from one side, then, releasing heat by reaction in glass tubes, enabling a condensed fluid to be in cross flow contact with the glass tube on the outer layer of a reaction tube, carrying out strong heat exchange, carrying out quenching treatment by using an acid binding agent after discharging as an esterification product, and then, collecting a product, so that continuous esterification synthesis reaction and sufficient heat exchange with a reaction solution can be realized, and an HCl gas serving as a byproduct has no corrosion to equipment. The method is simple in operation, high in heat exchange capability, precise in temperature control, environment-friendly and wide in large-scale application prospect.
Owner:ANHUI COSTAR BIOCHEM CO LTD
Red reactive dye for fur and preparation thereof
InactiveCN101481528AImprove responseImprove solubilityReactive dyesDyeing processSulfonateSolubility
Owner:TIANJIN DEK CHEM
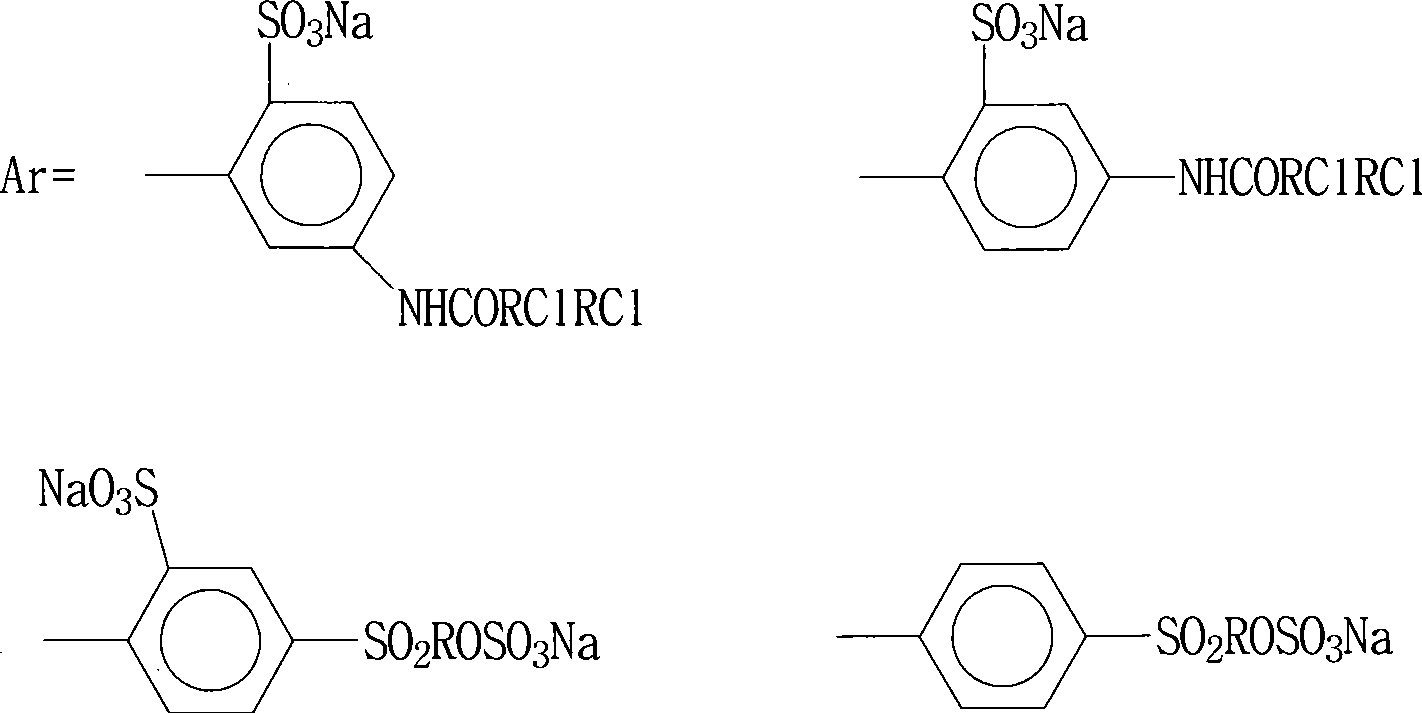
Bright yellow reactive dye for fur and preparation thereof
InactiveCN101481521AImprove responseImprove solubilityReactive dyesDyeing processSolubilityPropionyl chloride
The invention discloses a bright yellow reactive dye used for fur and a preparation method thereof. 2, 4-diaminobenzene sulfonic acid potassium, 2, 3-dichloro propionyl chloride and 3-formamido group-4-methyl-6-hydroxide radical-N-ethide pyridine are taken as main raw materials. The reactive dye of the invention is prepared by condensation, diazotization, coincidence, hydrolyzation, refining, chromatic light adjustment, intensity adjustment, drying and packaging. The bright yellow reactive dye used for fur in the invention has relatively high responsiveness, good solubility and bright-colored and beautiful chromatic light; in addition, the light fastness property is relatively good and the exhaustion rate and the color fixing rate are very high. A spraying method adopting the pre-blending technology is applied in the invention. Therefore, dust is not generated, greatly contributing to environmental protection.
Owner:TIANJIN DEK CHEM
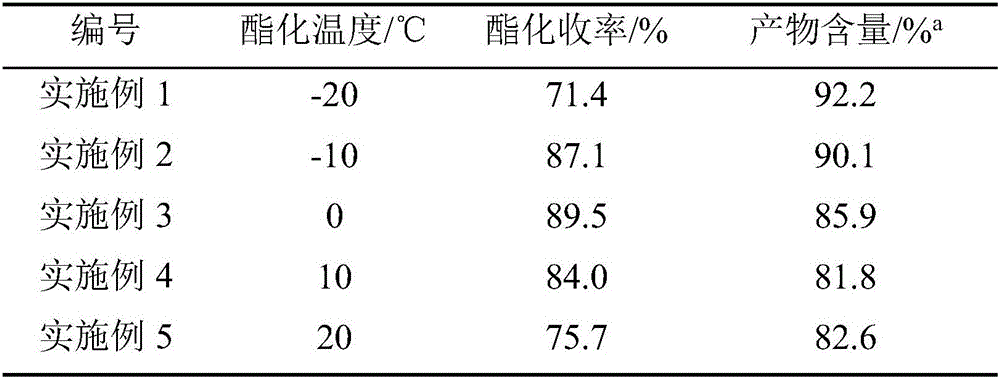
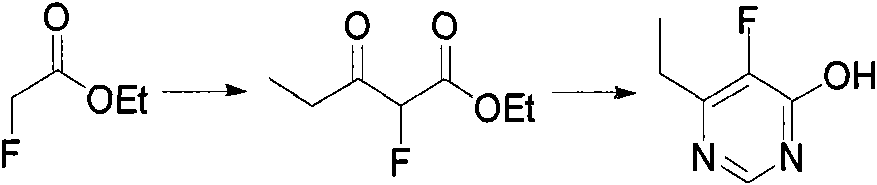
Method for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine and intermediate thereof
InactiveCN102060784AReduce pollutionRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid esters preparationPropionyl chlorideFormamidine acetate
The invention relates to a new concise and efficient method for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine and an intermediate 2-fluoro-3-oxo ethyl valerate thereof. 6-ethyl-5-fluoro-4-hydroxy pyrimidine is an important intermediate for synthesizing a broad-spectrum antifungal drug voriconazole. At present, the methods for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine have the defects of more reaction steps, complex processes, more three wastes and high cost and are not favourable for industrial production. To solve the above problems, the method provided by the invention comprises the following reaction steps (see the drawing 1): using ethyl fluoroacetate and propionyl chloride as raw materials under the reaction temperature to synthesize 2-fluoro-3-oxo ethyl valerate under appropriate alkali action; and using 2-fluoro-3-oxo ethyl valerate and formamidine acetate as raw materials under the reaction temperature to synthesize 6-ethyl-5-fluoro-4-hydroxy pyrimidine under appropriate reaction system. The method provided by the invention is safe, environment-friendly, concise and efficient.
Owner:NANTONG FINC PHARMA CHEM
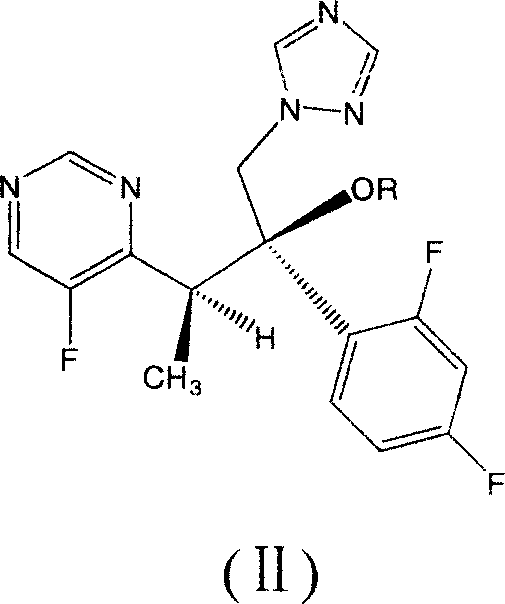
Voriconazole derivate and preparation process thereof
InactiveCN100999518ATo achieve asymmetric synthesisHigh yieldOrganic active ingredientsAntimycoticsPropanoic acidEnantiomer
This invention involves Voriconazole derivatives. The invention also involves Voriconazole derivatives preparation methods, including the following steps : 2 - (5-fluoro-4-yl) acetate and ethanol for esterification, generate 2 -(5-fluoro-4-yl) ethyl acetate, in alkaline conditions takes reaction with methylation agent, generate 2 - (5-fluoro-4-yl) ethyl propionate; hydrolysis of 2 - (5-fluoro-4-yl) propionic acid, through split gain S-2 - (5-fluoro-4-yl) propionic acid; for chlorination to gain S-2 - (5-fluoro-4-yl) propionyl chloride; occurred Friedel-Crafts reaction, generating S-1 - 2, 4-difluoro-2 - (5-fluoro-4-yl) acetone; with 1-methyl - 1-H-1 ,2,4 - triazol under alkali conditions to take reaction to gain voriconazole and the series of voriconazole derivatives. The methods described in this invention has short routes, only use of a pair of enantiomers separation, the overall yield has been greatly improved, is a simple and easy method for synthesis of voriconazole and its derivatives.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Aryloxyphenoxies propionate compound and its preparation method and application
InactiveCN107176950AHas herbicidal activityOrganic chemistryHerbicides and algicidesPropionatePropionyl chloride
The invention discloses a piperonyl-containing aryloxyphenoxies propionate compound, which has the following structure: the formula is shown in the specification. The specific preparation method includes steps of taking catechol as raw material, and compounding heliotropin through two-step reaction; performing reduction reaction with NaBH4 to prepare 3, 4- methylenedioxy phemethylol; reacting 2-(4-aryloxyphenoxies) propionyl chloride with prepare 3, 4- methylenedioxy phemethylol to prepare two piperonyl-containing aryloxyphenoxies propionate compounds. The compound has good weeding activity; especially, the weeding activity of monocotyledon weed is better than that of dicotyledonous weed; the preparation method is simple to operate, and the yield is high.
Owner:赣州市正畅塑胶有限公司
Preparation method of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride
InactiveCN103539677AEase of industrial implementationOrganic compound preparationAmino compound preparationIndacaterolN-butyl nitrite
The invention belongs to the technical field of synthesis of a medical immediate, and particularly relates to a synthesis method of a key intermediate 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride of indacaterol. The method comprises the following steps: by taking ethylbenzene as a raw material, preparing a compound I by propionyl chloride; preparing a compound II from the compound I by cyclization reaction; preparing a compound III by reaction of the compound II and butyl nitrite; preparing a compound IV from the compound III by palladium hydrogen reduction; finally preparing a compound V from the compound IV under protection of trifluoroacetyl; preparing a compound VI from the compound V by amino acetylation reaction; preparing a compound VII from the compound VI by reduction; carrying out deprotection, hydrolysis and acidification on the compound VII, so as to obtain the final product compound VIII, namely the 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride. The method disclosed by the invention is simple and convenient to operate, reasonable in reaction flow, low in cost, good in product quality, free of pollution to environment, and applicable to industrial production; the content is greater than 99%.
Owner:湖北万知化工医药股份有限公司
Preparation method of articaine hydrochloride
ActiveCN102060840AHigh yieldRaw materials are easy to getOrganic chemistryArticaine HydrochloridePropionyl chloride
The invention discloses a preparation method of articaine hydrochloride. The method comprises the following steps: carrying out amidation reaction on 4-methyl-3-aminothiophene-2-methyl formate utilized as a raw material and 2-chloro propionyl chloride; then carrying out the ammoniation reaction with propylamine; and carrying out salification reaction with concentrated hydrochloric acid, thus obtaining the articaine hydrochloride. The invention has the following advantages: the process route of the preparation method is suitable for industrial production, the rigorous reaction conditions are avoided, raw materials are easy to obtain, the operation is simple, and the yield coefficient is high.
Owner:BENGBU BBCA MEDICINE SCI DEV
Preparation method for dihydrosafrole
InactiveCN102070596AHigh yieldThe operation process is simple and convenientOrganic chemistryPotassium hydroxidePolyethylene glycol
Owner:ZHEJIANG UNIV
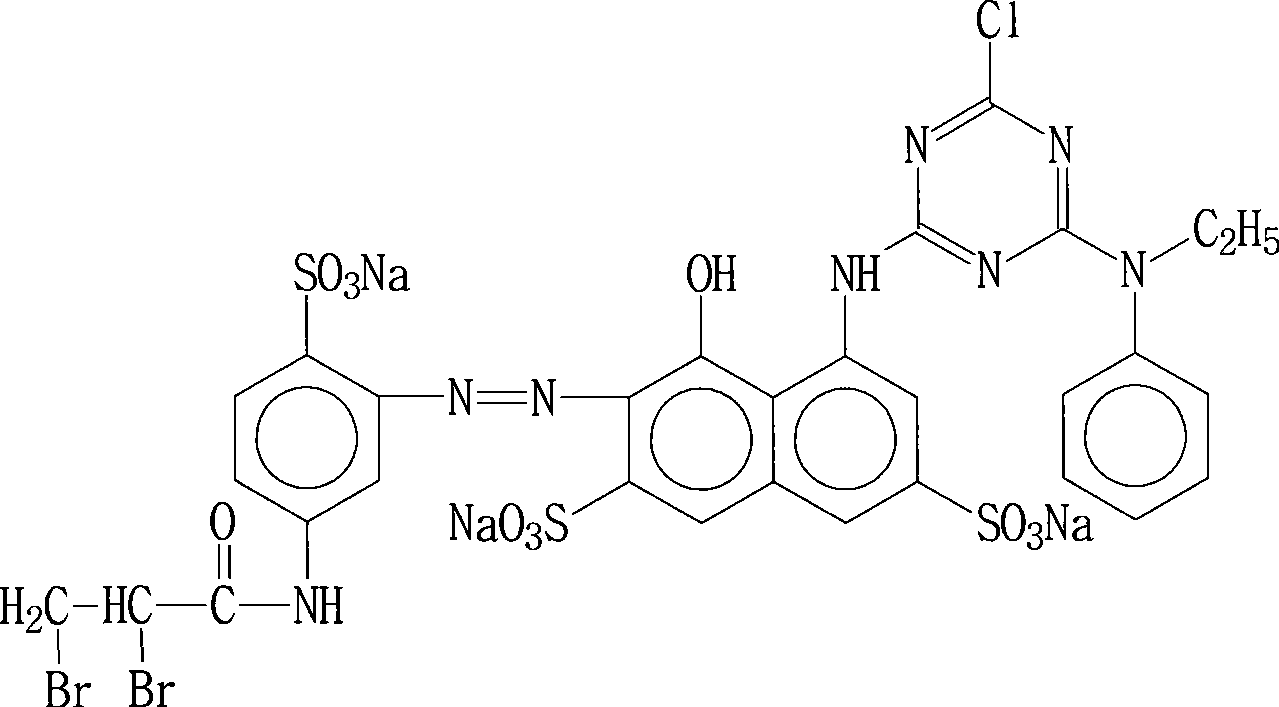
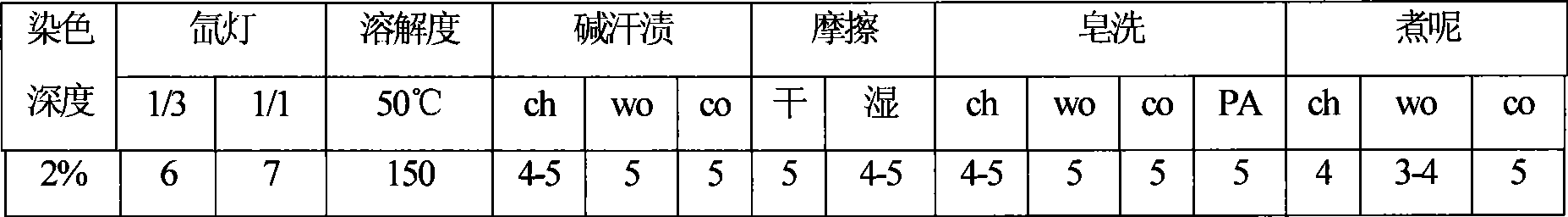
Preparation of red reactive dye for fur
The invention provides a method for preparing vital red GN, comprising the following steps: firstly, H acid solution is added to cyanuric chloride solution for reaction, after the reaction is over, N-hexyl aniline is added for reaction, thus obtaining condensation solution; then 2, 3-dibromo propionyl chloride is added to 2, 4-diaminobenzene sulfonic acid sodium for reaction, after the reaction is over, diazo reaction is carried out by adding sodium nitrite solution under acidic condition, thus obtaining diazonium solution; finally, coincidence reaction is carried out by adding the diazonium solution into the condensation solution, thus obtaining the vital red GN of the invention. The vital red GN for fur prepared by the invention has relatively high responsiveness, good solubility and bright-colored and beautiful chromatic light; in addition, the light fastness property is very good, the wet fastness property is excellent and the exhaustion rate and the color fixing rate are very high. The vital red GN of the invention can be applicable for dyeing wool, pashm and non-shrinkable wool.
Owner:TIANJIN DEK CHEM
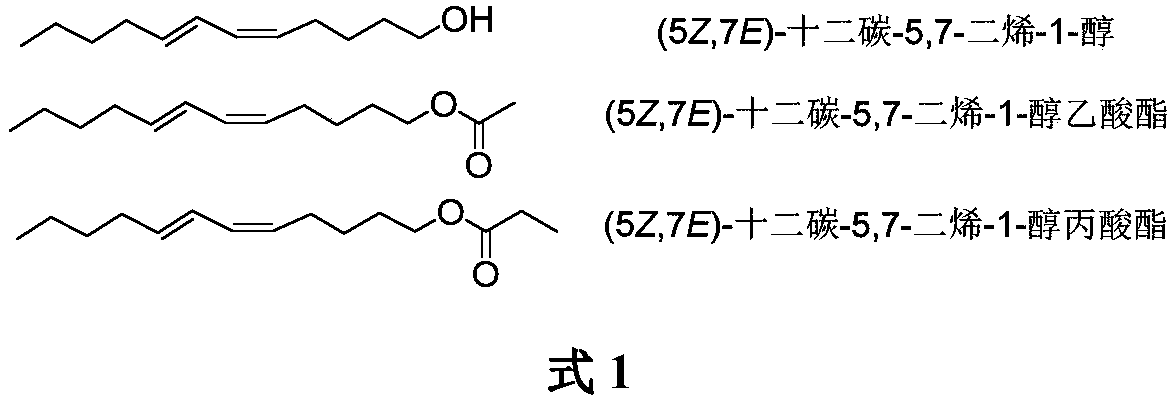
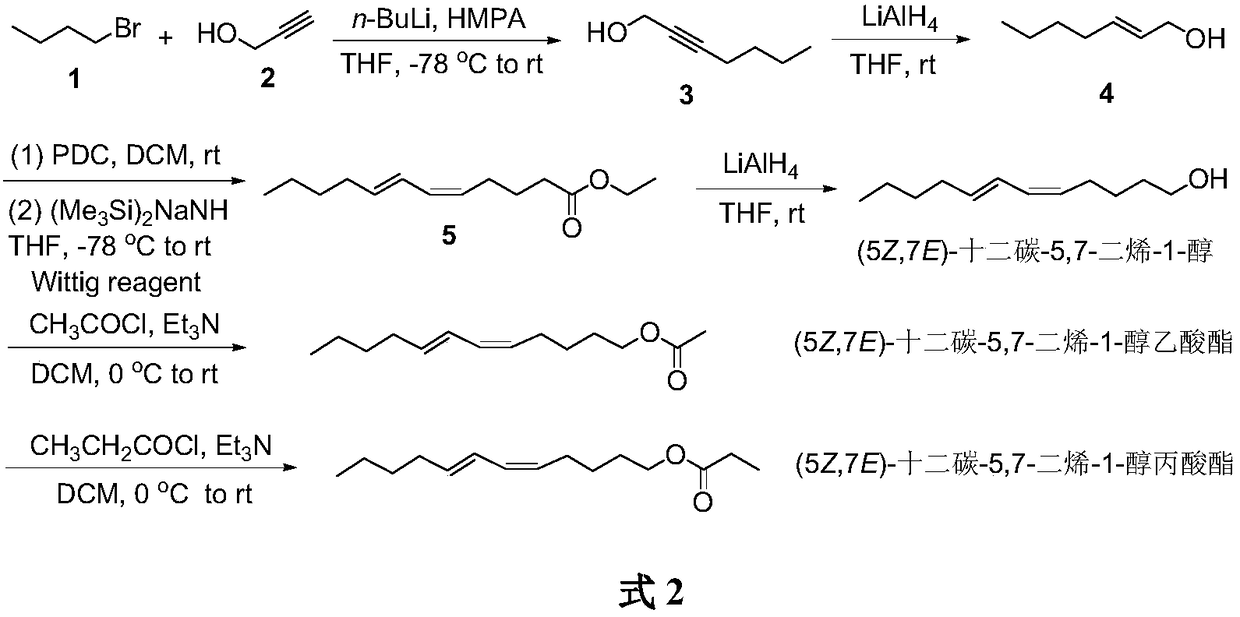
Synthesis of (5Z,7E)-dodecane-5,7-diene-1-alcohol as well as acetate and propionate thereof
ActiveCN109456182AOrganic compound preparationPreparation by hydrogenationTriphenyl phosphoniumEthyl Chloride
The invention belongs to the technical field of insect pheromone synthesis, and discloses a new method for synthesizing (5Z,7E)-dodecane-5,7-diene-1-alcohol as well as acetate and propionate thereof.The method takes propynol as an initial raw material to be subjected to coupling with 1-bromobutane to generate 2-heptyne-1-alcohol, triple bond is reduced to be E-type double bond through LiAlH4, 2-heptyne-1-alcohol is oxidized to be olefine aldehyde through PDC, olefine aldehyde reacts with a Wittig reagent (5-ethyoxyl-5-oxopentyl)triphenyl phosphonium bromide to produce (5Z,7E)-dodecane-5,7-dienoic acid ethyl ester which is reduced by LiAlH4 to obtain (5Z,7E)-dodecane-5,7-diene-1-alcohol, (5Z,7E)-dodecane-5,7-diene-1-alcohol reacts with acetyl chloride and propionyl chloride finally to obtain (5Z,7E)-dodecane-5,7-diene-1-alcohol acetate and (5Z,7E)-dodecane-5,7-diene-1-alcohol propionate. The method utilizes LiAlH4 to reduce the triple bond to be the E-type double bond, and utilizes theWittig reaction of the Wittig reagent with the tail end provided with ester group and aldehyde to directly build the Z-type double bond, the synthesis route is simple, convenient and efficient, and the reaction condition is moderate and environmentally friendly.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




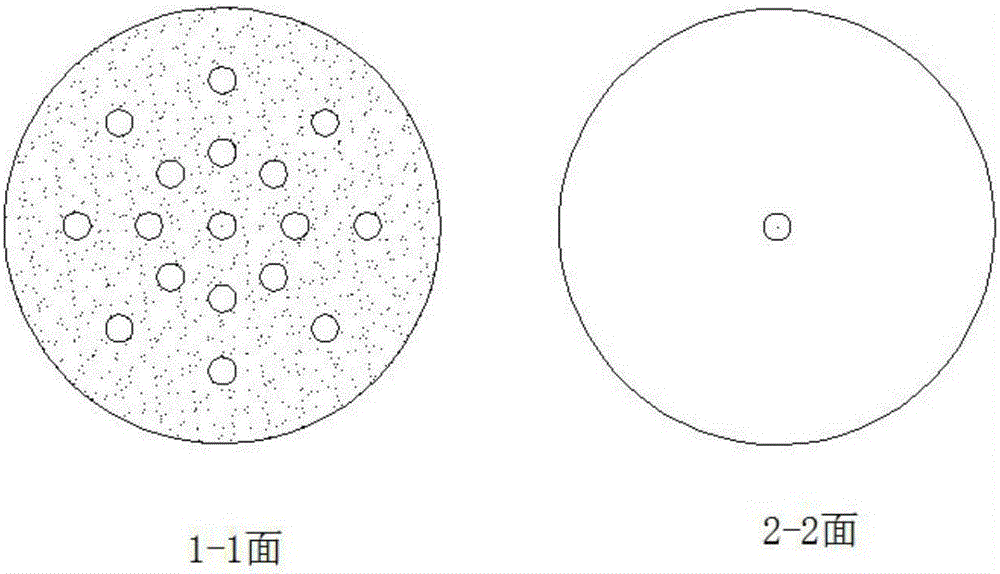
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C1.PNG)
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C2.PNG)
![2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method 2-amido-2-[2-(4-alkylphenyl)ethyl]-1,3-methyl glycol preparation method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6951d141-6d8e-4d5b-b5a8-4293d3c0a10a/C2005100957660002C3.PNG)