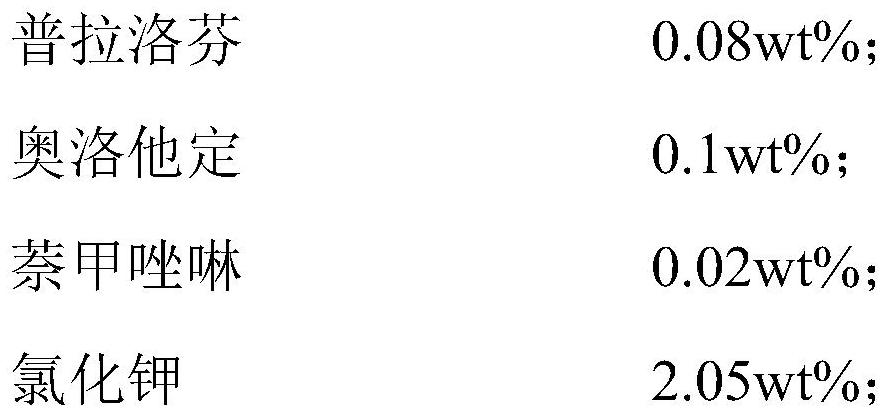Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Pranoprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
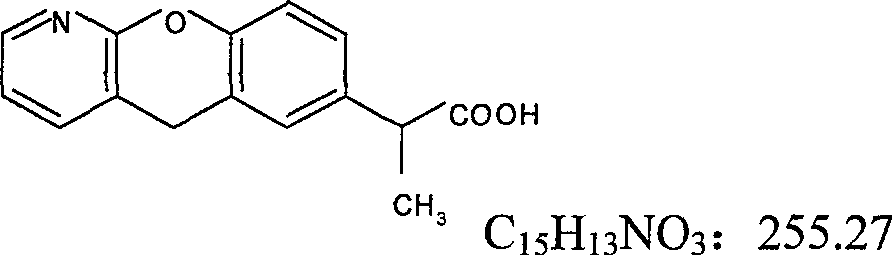
Pranoprofen (INN) is a nonsteroidal anti-inflammatory drug (NSAID) used in ophthalmology.
Novel preparation method of pranoprofen
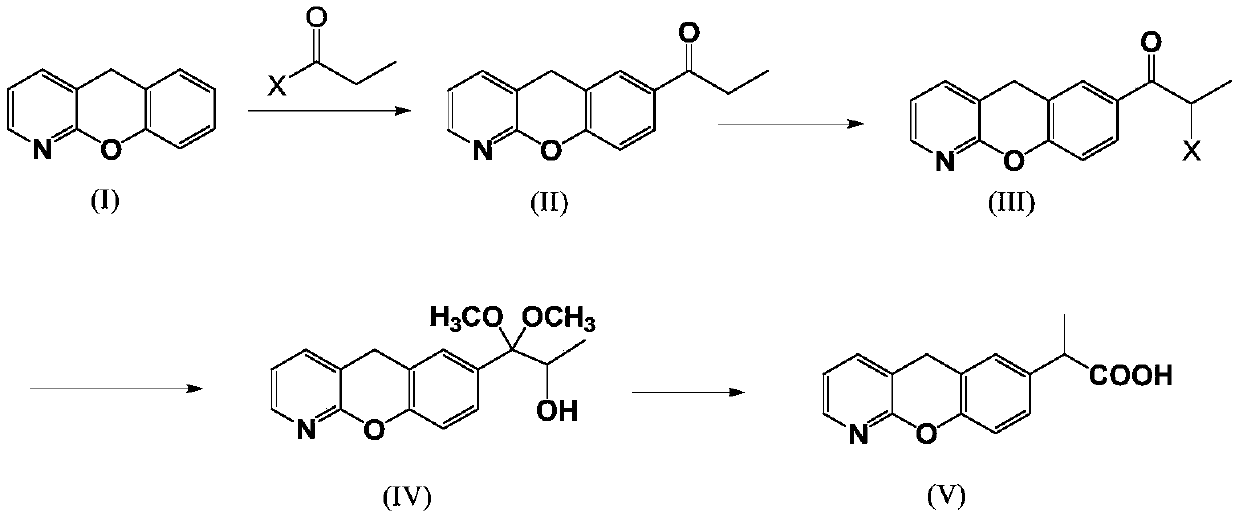
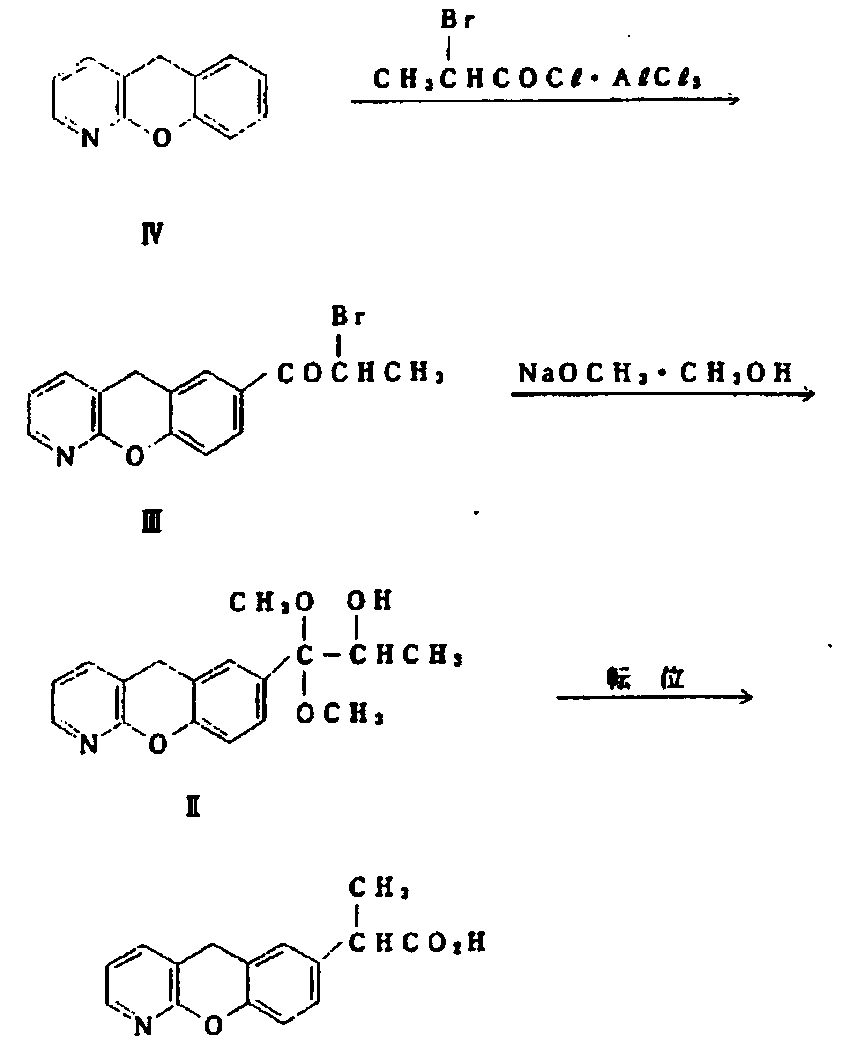
The invention discloses a novel preparation method of pranoprofen. The novel preparation method of the pranoprofen has the advantages of being high in yield, having less impurities, and being safe andenvironmentally friendly. The novel preparation method of the pranoprofen comprises the following steps that step 1, 5H-[1]-benzopyran[2,3-b]pyridine and propionyl chloride are directly acylated; step 2, bromination is carried out; step 3, potassium tert-butoxide or sodium tert-butoxide is used for hydrolysis, and finally, rearrangement is carried out to obtain the pranoprofen.
Owner:GUANGDONG XIANQIANG PHARMA +2
Method for preparing novel Pranoprofen key intermediates
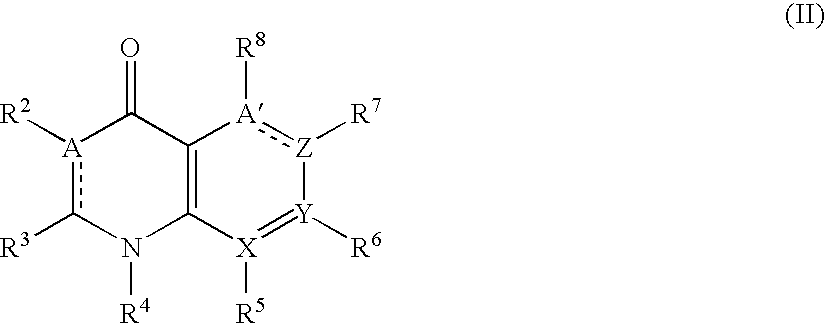
The present invention discloses a preparation method to produce a compound with formula (I) 9-oxa-1-aza athrone, in which a compound with formula (II) reacts with a cyclization reagent under high temperature to obtain the compound with the formula (I), which is an important medium substance in preparation process of Pranoprofen.
Owner:BEIJING D VENTUREPHARM TECH DEV
Compound non-carrier antibacterial eye drops containing practofren and its production
An antibacterial eye medicine containing pranoprofen contains non-steroid antiphlogistic medicine, aminoglycoside-type antibacterial medicine, polyvinyl pyrrolidone, and pharmacologically acceptable carrier. Its preparing process is also disclosed.
Owner:SHANGHAI SINE PHARMA LAB
New pranoprofen eye drops and its preparation method
ActiveCN1823772AQuality improvementStable and reliable storageOrganic active ingredientsSenses disorderClinical efficacyCurative effect
A kind of eye-drops of pranoprofen with long stay time in eye and no bitter taste is prepared through dissolving pranoprofen and relative additives in water, adding the aqueous solution of sodium hyaluronate, stirring, adding water, filtering and filling it in containers.
Owner:JIANGSU JIBEIER PHARMA
Synthetic method for pranoprofen
ActiveCN103864804AIncrease profitFew synthetic stepsOrganic chemistryEthyl groupCombinatorial chemistry
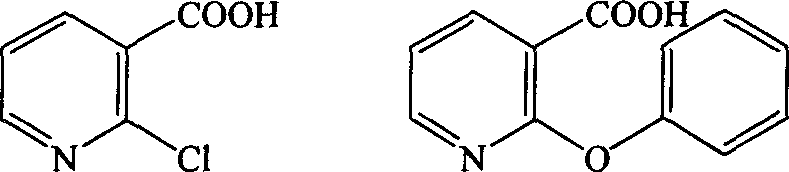
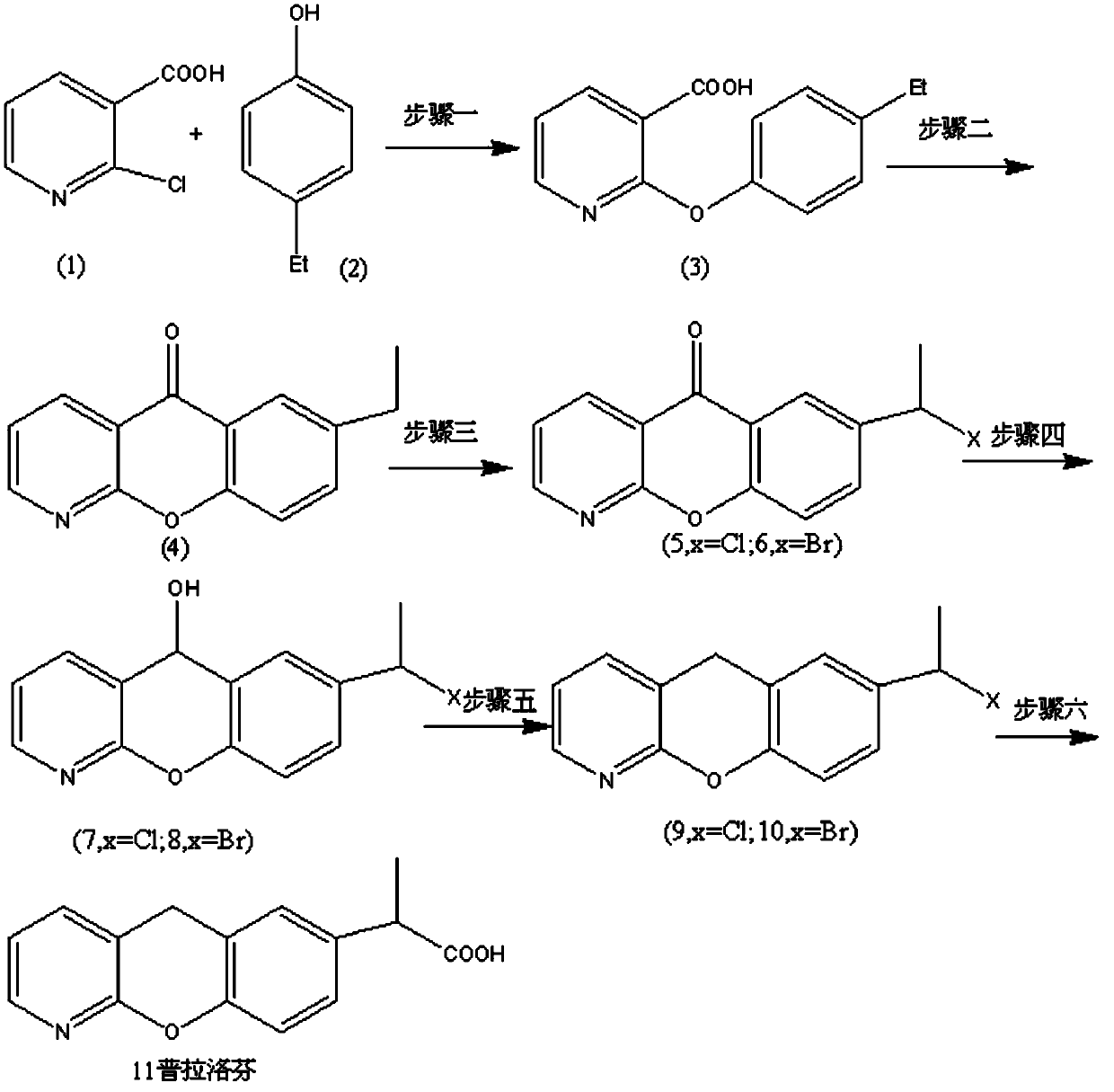
The invention provides a synthetic method for pranoprofen and a novel compound structure which can be used for preparation of pranoprofen. More specifically, 2-chloronicotinic acid and paraethyl phenol are used as raw materials, and nucleophilic substitution, ring closure, halogenation, carbonyl group reduction, hydroxyl group elimination, a Grignard reaction and CO2 carbonyl group insertion are carried out so as to prepare pranoprofen.
Owner:TIANJIN JINYAO GRP
Positively charged water-soluble prodrugs of aryl- and heteroarylpropionic acids with very fast skin penetration rate
ActiveCN101506161AGood absorption rateImprove solubilitySenses disorderNervous disorderSolubilityPhosphate
Owner:TECHFIELDS BIOCHEM CO LTD
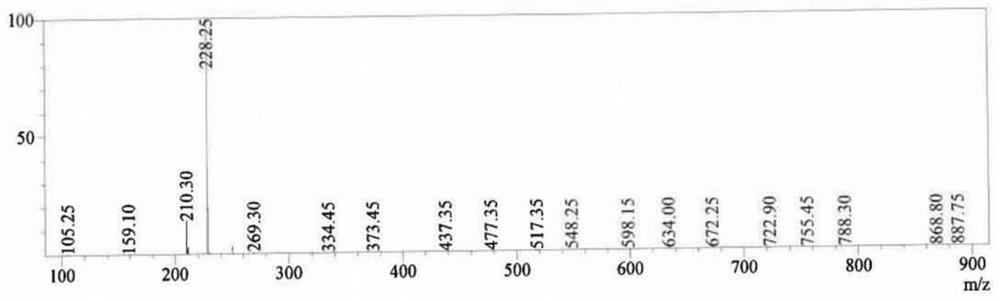
Compound A1, preparation method thereof and application of compound A1 as pranoprofen impurity
PendingCN113214276AHigh purityImprove qualityOrganic chemistryComponent separationUse medicationCombinatorial chemistry
The invention discloses a compound A1, a preparation method thereof and an application of the compound A1 as a pranoprofen impurity. A compound B reacts with a strong alkali solution in a reaction solvent under the protection of inert gas to obtain the compound A1. The prepared compound A1 is high in purity after being subjected to structure confirmation and HPLC analysis, is applied to quality research of pranoprofen bulk drugs as the pranoprofen impurity, and provides important guiding significance for safe medication.
Owner:SHANDONG RUIAN PHARMA CO LTD
Purification method of pranoprofen
The invention discloses a purification method of pranoprofen, and relates to the field of preparation of nonsteroidal anti-inflammatory drugs. The problem that a simple, practicable and low-cost purification method of the pranoprofen does not exist in the prior art, and consequently the prepared pranoprofen cannot reach the expected quality requirements is solved. According to the technical key point, the purification method comprises the following steps that (1) beating is conducted, specifically, pranoprofen powder is added in a methanol aqueous solution, the temperature is risen to 50-60 DEG C, stirring is conducted for 3-5 hours under the temperature, and serous liquid is obtained; (2) the serous liquid is filtered; and (3) the pranoprofen obtained through filtering is mixed with the methanol aqueous solution and is dissolved, filtering, devitrification and drying are conducted while the solution is hot, and the f pranoprofen finished product is obtained. According to the purification method of the pranoprofen, operation is easy and convenient, and the prepared pranoprofen is high in purity, low in impurity content and high in yield.
Owner:GUANGZHOU HANPU PHARM CO LTD
Pranoprofen in-situ gelling eye drop and preparation method thereof
InactiveCN102885768AFix stability issuesResolution timeOrganic active ingredientsSenses disorderMass compositionPatient compliance
A pranoprofen in-situ gelling eye drop is disclosed. The pranoprofen in-situ gelling eye drop comprises the components by mass percent: 0.1% of pranoprofen, 1%-40% of thickening agent, 0.03%-1.0% of pH adjusting agent, 0.01%-0.03% of bacteria inhibitor, 0.005%-0.2% of antioxidant, 0.01%-0.05% of chelating agent, and 0.1%-4.0% of solubilizing agent. The pranoprofen in-situ gelling eye drop disclosed by the invention has the characteristics that a material (e.g., poloxamer 407 and sodium alginate) with good biocompatibility and having the feature of temperature sensitivity, pH sensitivity or ion sensitivity is used as a matrix, the eye drop is administered in vitro under a solution state, phase change occurs immediately at the administered site in vivo in accordance with different environments inside and outside human body, so as to form in-situ gel; and the eye drop is convenient to use, high in bioavailability and long in residence time and results in good compliance of patients. The invention further discloses a preparation method of the pranoprofen in-situ gelling eye drop.
Owner:JIANGSU JIBEIER PHARMA
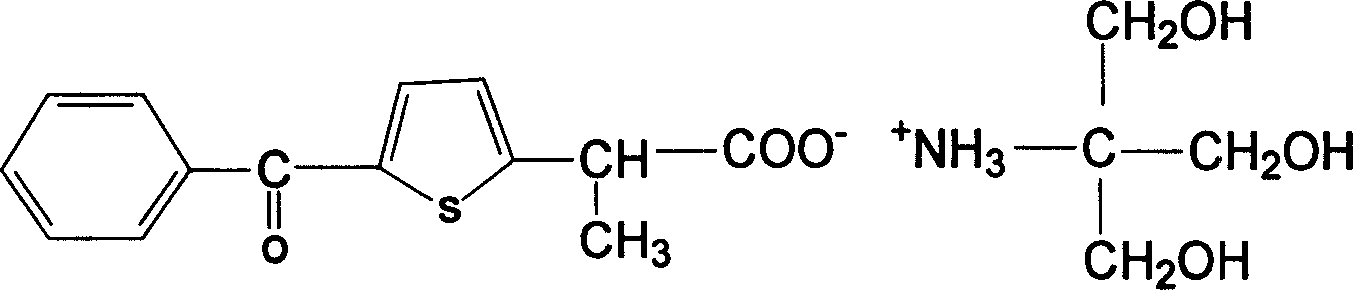
Carboxyl contained NSAIDS (nonsteroidal anti-inflammatory drugs) salt
Disclosed are non-steroidal analgesic and analgesic anti-inflammatory agents containing carboxyl including sodium, calcium, zinc, magnesium, N-n-octylgucamine, Arginine, Lycine or Trometamol salts of Loxoprofen, Ketoprofen, Pranoprofen, Tiaprofenic acid, Butibufen, Omolofen, epoxy indene acid, Lobuprofen, Clofenamic acid, Clonixin, Fenoprofen, Benorilate, Flurbiprofen, Alminoprofen, Bucloxic acid, Sulindac, Zidometacin, Acemetacin, Ketorolac, Risedronic acid, Sulindac, Lonaprofen, aspirin, Florfenicol, tiaprofenic acid, overall evaluation shows that trometamol salts are the best choice in terms of physicochemical properties, solvability, stability, local irritation, blood vessel irritation, and bioavailability for oral administration.
Owner:陈文展
Ophthalmic solution
InactiveCN1438886AEliminate or relieve congestionSenses disorderOrganic chemistryConjunctivaBlood vessel
The present invention provides an ophthalmic solution containing pranoprofen or a pharmacologically acceptable salt thereof, and a vasoconstrictor, which is effective for ameliorating congestion in outer ocular areas, particularly conjunctiva and pericorneal area.
Owner:SENJU PHARMA CO LTD
New pranoprofen eye drops and its preparation method
ActiveCN100522162CQuality improvementStable and reliable storageOrganic active ingredientsSenses disorderDrugs solutionClinical efficacy
The invention discloses a new pranoprofen eye drop and a preparation method thereof. The preparation method of the new pranoprofen eye drops of the present invention is: after pranoprofen and related adjuvant are dissolved in water, the aqueous solution that has swelled sodium hyaluronate is added in the medicinal liquid, stir evenly, add water to enough Measure, filter, sub-package, and get it. The invention provides a new pranoprofen eye drop and a preparation method thereof, which basically overcomes the defects of the existing pranoprofen eye drop. Sodium hyaluronate prolongs the life of the drug through physical thickening and moisturizing and lubricating effects. During the residence time on the ocular surface, the medicine is not easy to flow into the mouth and nasal cavity. First, the loss of the medicine is reduced. Second, the bitter taste of the pranoprofen eye drops is overcome, the compliance of the patient is improved, and the clinical efficacy is ensured.
Owner:JIANGSU JIBEIER PHARMA
Eye drops containing pranoprofen and preparation method of eye drops
InactiveCN112587479AImprove stabilityRelief the painOrganic active ingredientsSenses disorderDiseaseIrritation
The invention discloses eye drops containing pranoprofen and a preparation method of the eye drops, and relates to the technical field of eye drops. The eye drops is prepared from the following raw materials in parts by weight of 0.1-0.5 part of pranoprofen, 0.1-0.7 part of sodium hyaluronate, 0.2-0.6 part of sulfobutyl ether-beta-cyclodextrin, 0.2-0.4 part of a stabilizer, 0.1-0.3 part of a pH regulator, 0.3-1.0 part of a bacteriostatic agent, 0.3-0.8 part of an osmotic pressure regulator and 85-96 parts of water for injection. According to the eye drops prepared by the invention, the sulfobutyl ether-beta-cyclodextrin and the sodium hyaluronate are added, and the sodium hyaluronate can obviously relieve clinical symptoms such as pain, itching, burning sensation, foreign body sensation ofxerophthalmia and the like, so that hyperemia caused by diseases such as blepharitis, conjunctivitis and the like can be well relieved, the effect is remarkable, and the eye drops have a wide marketprospect; and the eye drops are good in stability and free of preservatives, the dosage of anti-inflammatory drugs is reduced, and meanwhile, the anti-inflammatory effect is enhanced, and irritation to eyes is reduced.
Owner:苏州工业园区天龙制药有限公司
Method for stabilizing dibutylhydroxytoluene
ActiveCN106068123AImprove thermal stabilityGuaranteed contentOrganic active ingredientsSenses disorderPolytetramethylene terephthalateInterior space
The objective of the present invention is to provide a technique for increasing the thermal stability of dibutylhydroxytoluene and suppressing a decrease in the content thereof over time in a liquid drug that includes dibutylhydroxytoluene and pranoprofen and / or a salt thereof. By mixing, into the liquid drug that includes dibutylhydroxytoluene and pranoprofen and / or a salt thereof, at least one item selected from the group consisting of a chondroitin sulfate ester, aspartic acid, alginic acid, an alginic acid derivative, pharmaceutically acceptable salts of these, cyanocobalamin, panthenol, a tocopherol, and a tocopherol derivative, and by employing a resin including polybutylene terephthalate as a resin that constitutes an inner wall surface (such as the wall surface of an interior space in a spout portion and / or a lid wall surface facing a spout opening in the spout portion) of an accommodating container, the thermal stability of the dibutylhydroxytoluene in the liquid drug can be increased, adsorption of the dibutylhydroxytoluene to the container can be suppressed, and a decrease in the dibutylhydroxytoluene content over time can be suppressed.
Owner:SENJU PHARMA CO LTD
A kind of refining purification method of pranoprofen
ActiveCN110372713BLow costHigh Quality Standard RequirementsOrganic chemistryNon steroid anti inflammatory drugAntiinflammatory drug
Owner:GUANGZHOU HANPU PHARM CO LTD
Formulation composition and preparation method of pranoprofen-containing suspension
ActiveCN105853352AGood suspensionImprove stabilityOrganic active ingredientsAntipyreticCarboxymethyl cellulosePolyvinyl alcohol
The invention discloses a formulation composition and a preparation method of a pranoprofen-containing suspension. The formulation composition of the pranoprofen-containing suspension comprises the following components: pranoprofen, polyvinyl alcohol, sodium microcrystalline cellulose-carboxymethyl cellulose and water, wherein polyvinyl alcohol accounts for 0.5%-2.5% by mass of the formulation composition of pranoprofen-containing suspension; sodium microcrystalline cellulose-carboxymethyl cellulose accounts for 0.5%-1% by mass of the formulation composition of pranoprofen-containing suspension. According to the invention, polyvinyl alcohol and sodium microcrystalline cellulose-carboxymethyl cellulose are conventionally mixed to achieve excellent suspension effect, while the formulation composition significantly improves the stability of products during storage.
Owner:WUHAN LEADPHARM TECH CO LTD
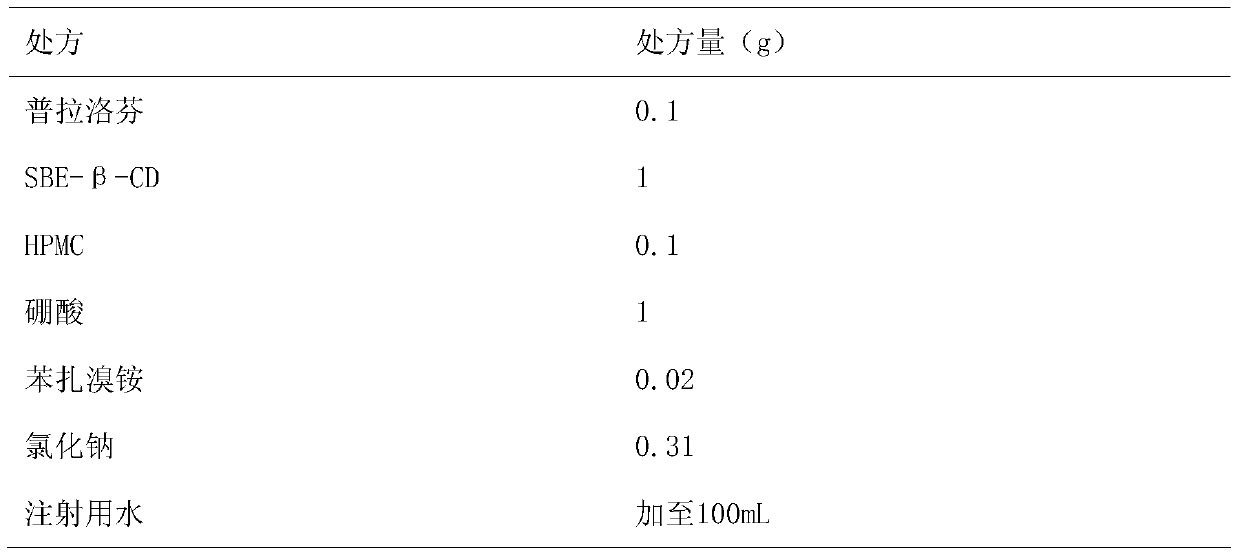
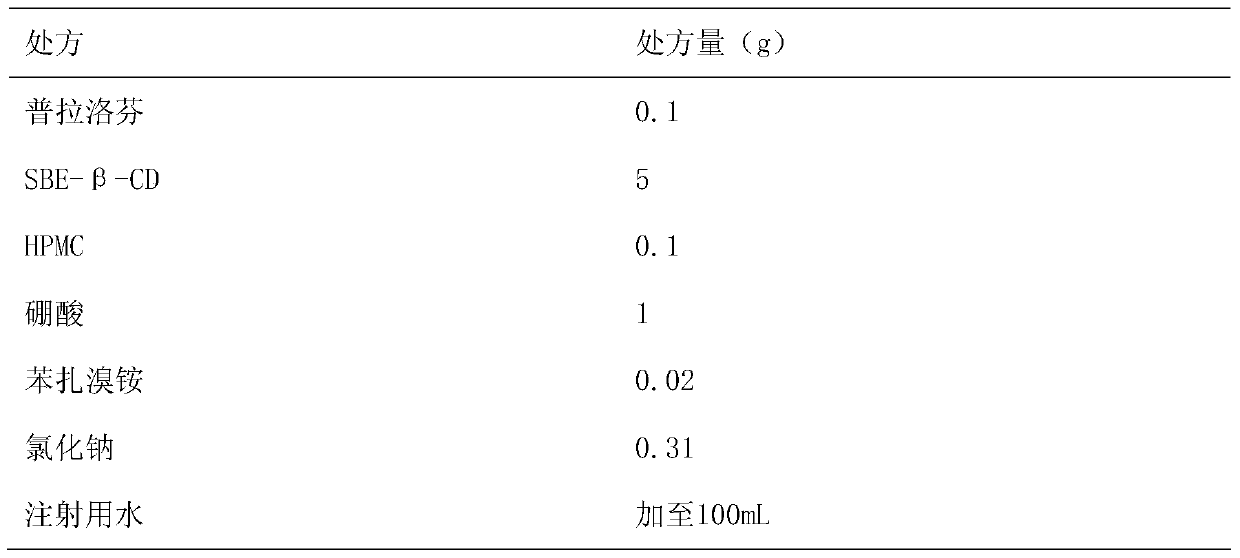
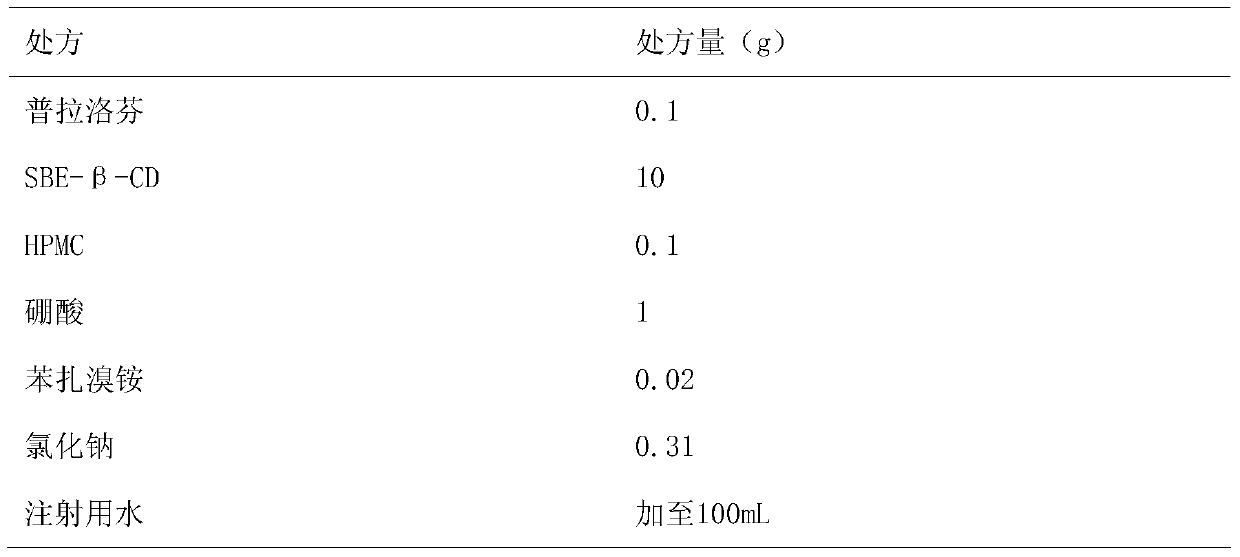
Pranoprofen eye drops containing sulfobutyl ether-beta-cyclodextrin and preparation method thereof
ActiveCN104997729AImprove stabilityOrganic active ingredientsSenses disorderMicropore FilterIrritation
The invention belongs to the technical field of medicine, and discloses pranoprofen eye drops containing sulfobutyl ether-beta-cyclodextrin. The pranoprofen eye drops comprise active component pranoprofen, the solubilizer sulfobutyl ether-beta-cyclodextrin, a stabilizer, a pH conditioning agent, a bacteriostatic agent and an osmotic pressure conditioning agent. The stabilizer is one or the mixture of a high-molecular polymer polyethylene glycol, polyving akohol, povidone, polyvinylpyrrolidone, sodium hyaluronate, poloxamer and hydroxypropyl methylcellulose. According to the preparation method, SBE-beta-CD is dissolved into water for injection to be heated at the temperature of 60 DEG C, the pranoprofen is added so as to be dissolved completely, the other auxiliary materials are added in sequence, stirring is performed until thorough dissolving, then, the mixture is cooled to the room temperature, the pH value is conditioned, water for injection is replenished, and the mixture is filtered through a 0.22-micrometer micropore filter membrane to obtain the pranoprofen eye drops. According to the pranoprofen eye drops containing the sulfobutyl ether-beta-cyclodextrin, using irritation to the eyes is lowered, and the stability is remarkably improved.
Owner:SHENYANG PHARMA UNIVERSITY
Compound A2, preparation method thereof and application of compound A2 as pranoprofen impurity
InactiveCN113234082AHigh purityImprove qualityOrganic chemistryUse medicationCombinatorial chemistry
The invention discloses a compound A2, a preparation method thereof and an application of the compound A2 as a pranoprofen impurity. The method comprises the steps: dropwise adding a solution formed by bromine and carbon tetrachloride into the compound B in a solvent carbon tetrachloride and an initiator, and then adding a sodium hydroxide solution to obtain a compound A2. The prepared compound A2 is high in purity after being subjected to structure confirmation and HPLC analysis, is applied to quality research of pranoprofen bulk drugs as a pranoprofen impurity, and provides important guiding significance for safe medication.
Owner:SHANDONG RUIAN PHARMA CO LTD
Method for suppressing decrease in viscosity of aqueous liquid
ActiveCN103796653BLow viscosityGuaranteed viscosityOrganic active ingredientsSenses disorderHyaluronic acidChemistry
Disclosed is a novel means for suppressing decrease over time in the viscosity of an aqueous liquid agent which contains hyaluronic acid or a pharmaceutically acceptable salt thereof. The means includes a method for suppressing decrease in the viscosity of an aqueous liquid agent which contains hyaluronic acid or a pharmaceutically acceptable salt thereof, and this method is characterized by blending pranoprofen or a pharmaceutically acceptable salt thereof into the aqueous liquid agent.
Owner:SENJU PHARMA CO LTD
The synthetic method of pranoprofen
ActiveCN103864804BIncrease profitFew synthetic stepsOrganic chemistryCompound structureGrignard reaction
The invention provides a synthetic method for pranoprofen and a novel compound structure which can be used for preparation of pranoprofen. More specifically, 2-chloronicotinic acid and paraethyl phenol are used as raw materials, and nucleophilic substitution, ring closure, halogenation, carbonyl group reduction, hydroxyl group elimination, a Grignard reaction and CO2 carbonyl group insertion are carried out so as to prepare pranoprofen.
Owner:TIANJIN JINYAO GRP
Eye drop capable of assisting treatment of conjunctivitis
InactiveCN110870847AImprove immunityReduce biosynthesisSenses disorderInorganic non-active ingredientsMannitolEthyl acetate
The invention discloses an eye drop capable of assisting the treatment of conjunctivitis, and belongs to the field of pharmaceutical chemicals. The eye drop contains the following components in partsby weight: 100-120 parts of water, 50-60 parts of an isothiocyanate, 40-55 parts of pranoprofen, 30-40 parts of sodium nitrate, 20-30 parts of citric acid, 20-28 parts of sodium citrate, 20-26 parts of potassium nitrate, 20-25 parts of disodium hydrogen phosphate, 20-25 parts of sodium sulfate, 20-23 parts of sodium hyaluronate, 18-21 parts of ethyl acetate, 15-19 parts of soy phospholipid, 13-16parts of curcumin, 10-13 parts of a polyphenol flavonoid, 6-10 parts of povidone, 6-8 parts of mannitol, 3-6 parts of glucose, and 1-3 parts of a preservative. The eye drop solves the problem a traditional eye drops can only have a moisturizing effect on eyes and cannot assist in the treatment or relief of the conjunctivitis.
Owner:李达欣
Aqueous liquid preparation
InactiveCN1210028CStabilization methods for photostabilizationOrganic active ingredientsSenses disorderPharmaceutical drugSulfanilamide
The present invention provides an aqueous solution containing pranoprofen or a pharmacologically acceptable salt thereof, and a sulfa drug, and a method for making pranoprofen or a pharmacologically acceptable salt thereof stable to light in an aqueous solution, which includes adding a sulfa drug to the aqueous solution containing pranoprofen or a pharmacologically acceptable salt thereof.
Owner:SENJU PHARMA CO LTD
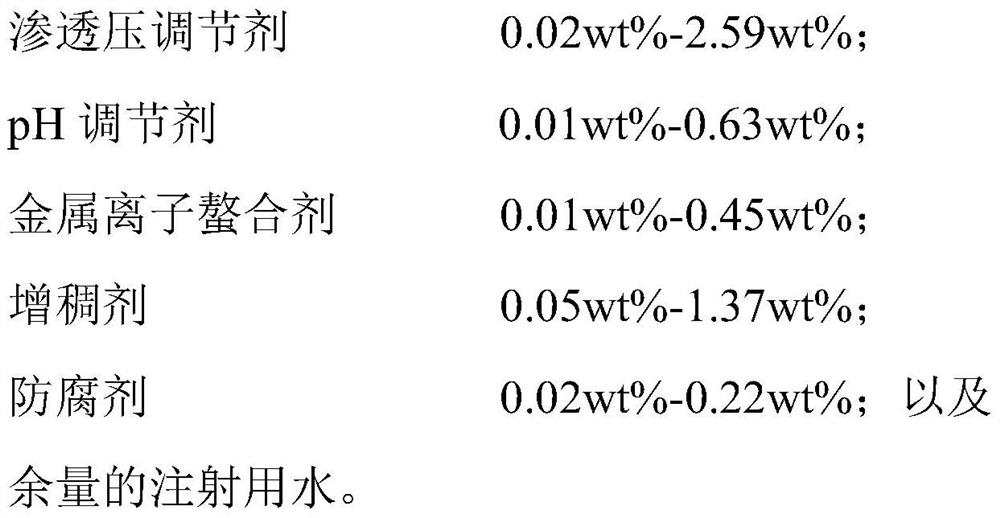
Ophthalmic composition as well as preparation method and application thereof
PendingCN114099505ASuppress generationGood treatment effectAntibacterial agentsOrganic active ingredientsConjunctivaOcular inflammation
The present invention relates to an ophthalmic composition comprising pranoprofen and olopatadine and / or naphazoline. The invention also relates to a preparation method of the ophthalmic composition, which comprises the following steps: mixing the pranoprofen, the olopatadine and / or the naphazoline, the osmotic pressure regulator, the pH regulator, the metal ion chelating agent, the thickening agent, the preservative and the water for injection to obtain the ophthalmic composition. The invention also relates to an application of the ophthalmic composition in preparation of a medicine for treating ocular inflammation, and the ocular inflammation comprises external eye and / or anterior segment inflammation caused by gram-positive bacterium and / or gram-negative bacterium infection. When the ophthalmic composition provided by the invention is used as eye drops, the treatment effect on eye inflammation of a patient can be improved, and adverse effects of eye stimulation, conjunctival congestion, edema and the like after medication can be avoided, so that the medication compliance of the patient is improved, and the recovery time of the patient is shortened.
Owner:湖北远大天天明制药有限公司
Pranoprofen eye drops containing sulfobutyl ether-β-cyclodextrin and preparation method thereof
ActiveCN104997729BImprove stabilityOrganic active ingredientsSenses disorderIrritationPolyvinyl alcohol
The invention belongs to the technical field of medicine, and discloses a pranoprofen eye drop containing sulfobutyl ether-β-cyclodextrin, which comprises active ingredient pranoprofen, solubilizer sulfobutyl ether-β-cyclodextrin Essence, stabilizer, pH regulator, bacteriostatic agent and osmotic pressure regulator; The stabilizer is macromolecular polymer polyethylene glycol, polyvinyl alcohol, povidone, polyvinylpyrrolidone, sodium hyaluronate, povidone One of loxamer, hypromellose or a mixture thereof. The preparation method of the present invention is to dissolve SBE-β-CD in water for injection, add pranoprofen under heating at 60°C, and after it is completely dissolved, add other auxiliary materials in turn, stir until completely dissolved, and cool to room temperature. Adjust the pH value, add water for injection to the full amount, and filter through a 0.22 μm microporous membrane to obtain the product. The pranoprofen eye drops containing sulfobutyl ether-β-cyclodextrin reduces the irritation to the eyes during use, and significantly improves the stability of the pranoprofen eye drops.
Owner:SHENYANG PHARMA UNIVERSITY
Stabilization method of dibutyl hydroxytoluene
ActiveCN106068123BImprove thermal stabilityGuaranteed contentOrganic active ingredientsSenses disorderPolytetramethylene terephthalatePolybutylene terephthalate
Owner:SENJU PHARMA CO LTD
Novel pranoprofen eye drop and preparation method thereof
The invention discloses a novel pranoprofen eye drop and a preparation method thereof. The preparation method of the eye drop disclosed by the invention comprises the steps of: dissolving pranoprofen and relevant accessories in water; adding a swelling carbomer aqueous solution to the liquid medicine and then uniformly stirring; adding a sufficient amount of water; and finally, filtering and dispensing to obtain the eye drop. According to the novel pranoprofen eye drop and the preparation method thereof, the defects of the current pranoprofen eye drop are overcome; the residence time of the medicine on ocular surface is prolonged by the physical thickening and moisturizing lubrication effects of carbomer; and furthermore, the medicine seldom flows into mouth and nasal cavity so as to reduce medicine loss and avoid bitterness from the pranoprofen eye drop, and therefore, compliance of patients is increased and clinical curative effect is ensured.
Owner:JIANGSU JIBEIER PHARMA
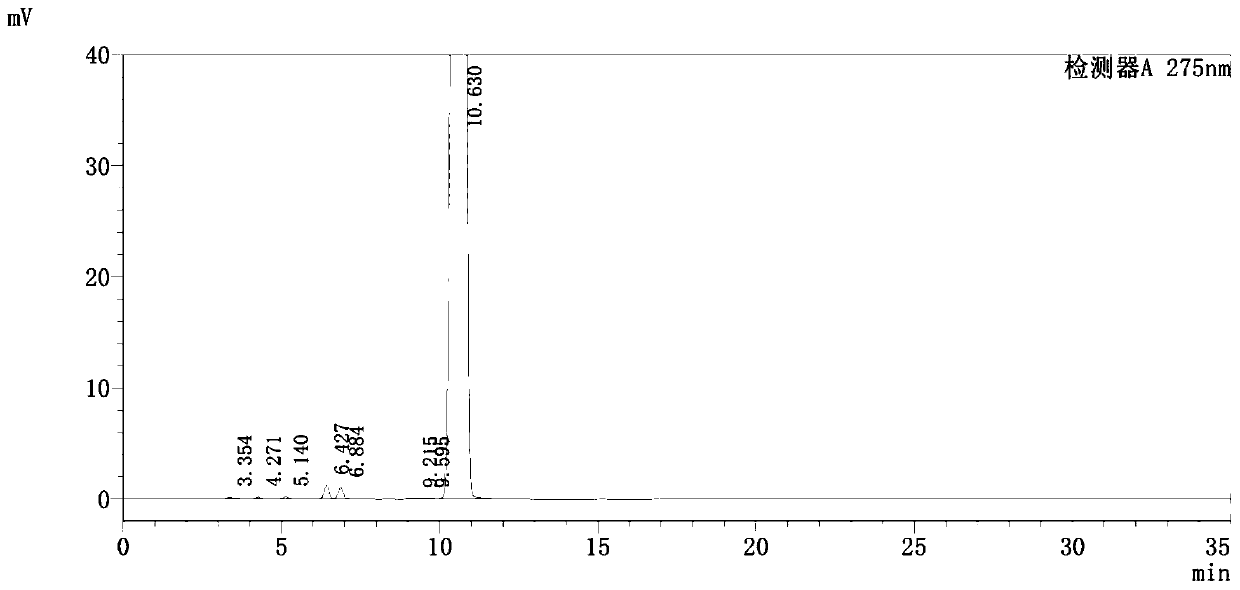
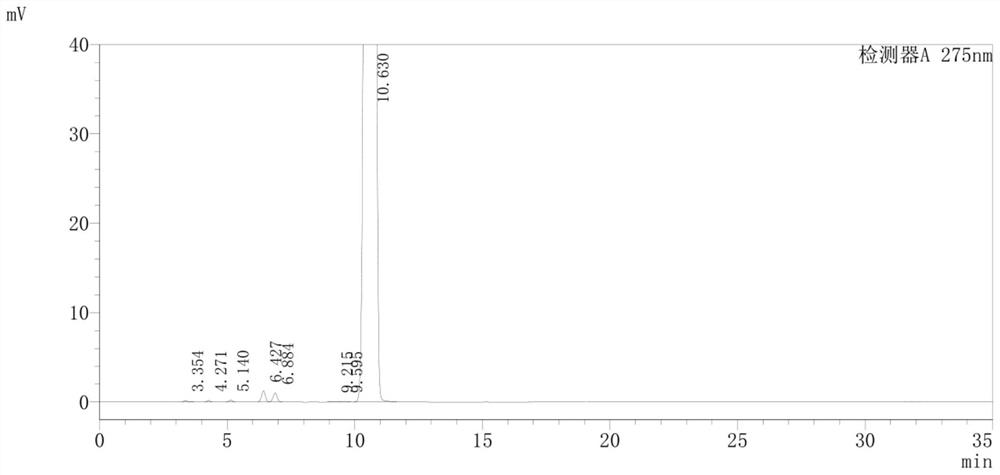
Detection method of pranoprofen eye drop related substances
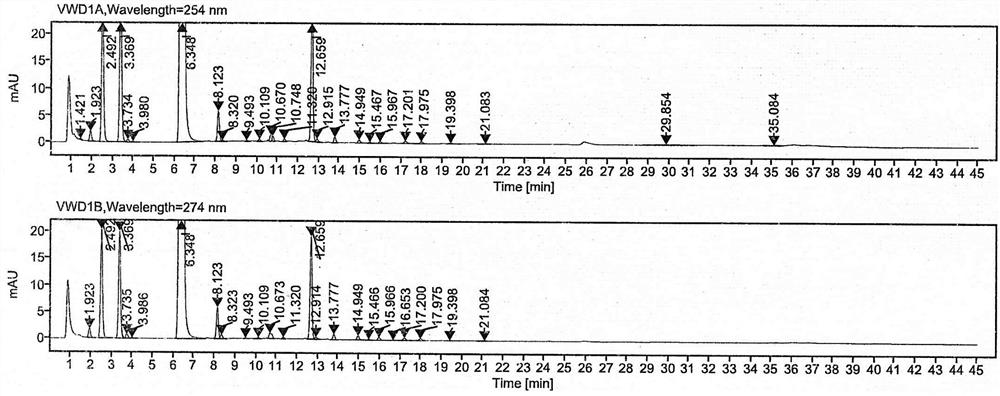
PendingCN114544817AAvoid product qualityAvoid interferenceComponent separationSilanesGradient elution
The invention discloses a high performance liquid chromatography method for separating and detecting related substances in pranoprofen eye drops, which adopts a chromatographic column with octadecylsilane chemically bonded silica as a filler and acetate buffer solution-acetonitrile as a mobile phase for gradient elution, and can quickly and effectively separate the related substances in the pranoprofen eye drops. Comprise process impurities, illumination degradation impurities, oxidative degradation impurities and the like. Compared with the conventional high performance liquid chromatography, the method has the advantages that the analysis time is obviously shortened, the specificity is high, the sensitivity is high, the durability is good, and the operation is simple and convenient.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Method for stabilizing arylcarboxylic acid, stabilizer thereof and aqueous solution containing stabilized arylcarboxylic acid
InactiveUS7320985B2Successful stabilizationSalicyclic acid active ingredientsBiocideCarboxylic acidAryl radical
A method for stabilizing an arylcarboxylic acid, which comprises adding a heterocyclic base to the arylcarboxylic acid or a pharmacologically acceptable salt thereof, a stabilizer thereof and an aqueous solution containing a stabilized arylcarboxylic acid. According to the stabilization method of the present invention, arylcarboxylic acid and pharmacologically acceptable salts thereof, particularly pranoprofen, can be stabilized at every temperature range, particularly at lower temperatures, thereby making the production of an aqueous solution to be used as an eye drop, nasal drop, ear drop and the like possible.
Owner:SENJU PHARMA CO LTD
Aqueous liquid preparation
InactiveCN1438887AImprove photostabilityOrganic active ingredientsSenses disorderSulfanilamideAqueous solution
The present invention provides an aqueous solution containing pranoprofen or a pharmacologically acceptable salt thereof, and a sulfa drug, and a method for making pranoprofen or a pharmacologically acceptable salt thereof stable to light in an aqueous solution, which includes adding a sulfa drug to the aqueous solution containing pranoprofen or a pharmacologically acceptable salt thereof.
Owner:SENJU PHARMA CO LTD
Compound non-carrier antibacterial eye drops containing practofren and its production
An antibacterial eye medicine containing pranoprofen contains non-steroid antiphlogistic medicine, aminoglycoside-type antibacterial medicine, polyvinyl pyrrolidone, and pharmacologically acceptable carrier. Its preparing process is also disclosed.
Owner:SHANGHAI SINE PHARMA LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com