Pranoprofen eye drops containing sulfobutyl ether-β-cyclodextrin and preparation method thereof
A technology containing sulfobutyl ether and sulfobutyl ether, which can be used in pharmaceutical formulations, non-active ingredients of polymer compounds, sensory diseases, etc., can solve the problems of strong irritation and low bioavailability, and achieve the goal of improving stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
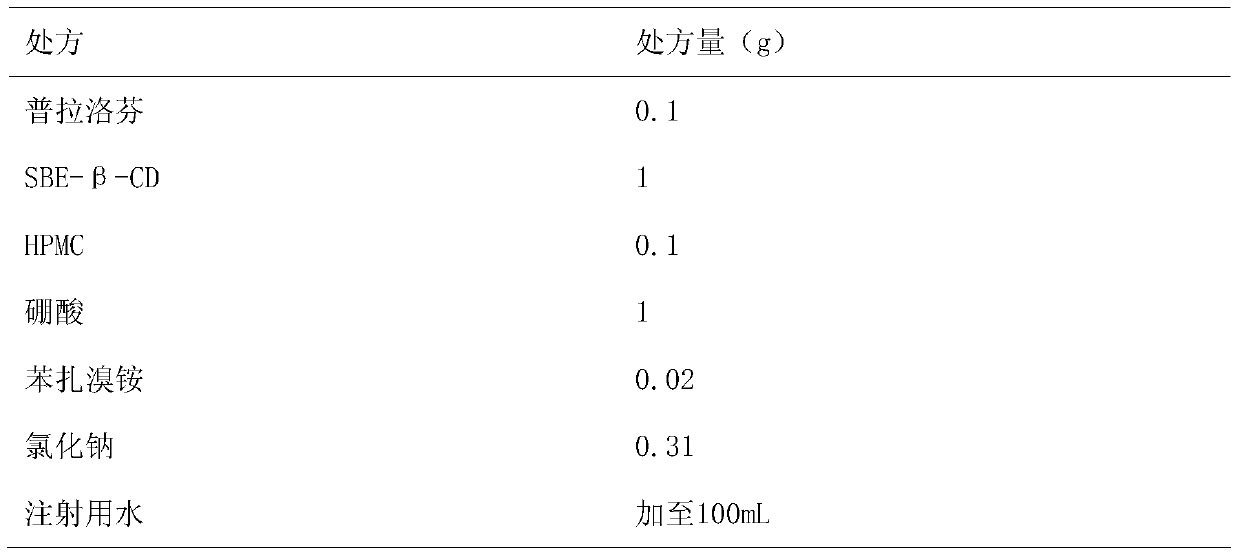
[0021] Embodiment 1: prepare 0.1% pranoprofen eye drops with 1% SBE-β-CD solution and 0.1% HPMC as solubilizer and stabilizer
[0022]
[0023] Preparation method: Dissolve the prescription amount of SBE-β-CD in 90mL of water for injection, add boric acid to dissolve, heat in a water bath to 60°C, add 0.1g of pranoprofen, stir until completely dissolved, add other excipients in turn, stir to make After it is completely dissolved, adjust the pH value to 7.5, and add water for injection to 100ml. Filter through a 0.22μm microporous membrane, fill in divided doses into ampoules, and obtain the product.
Embodiment 2
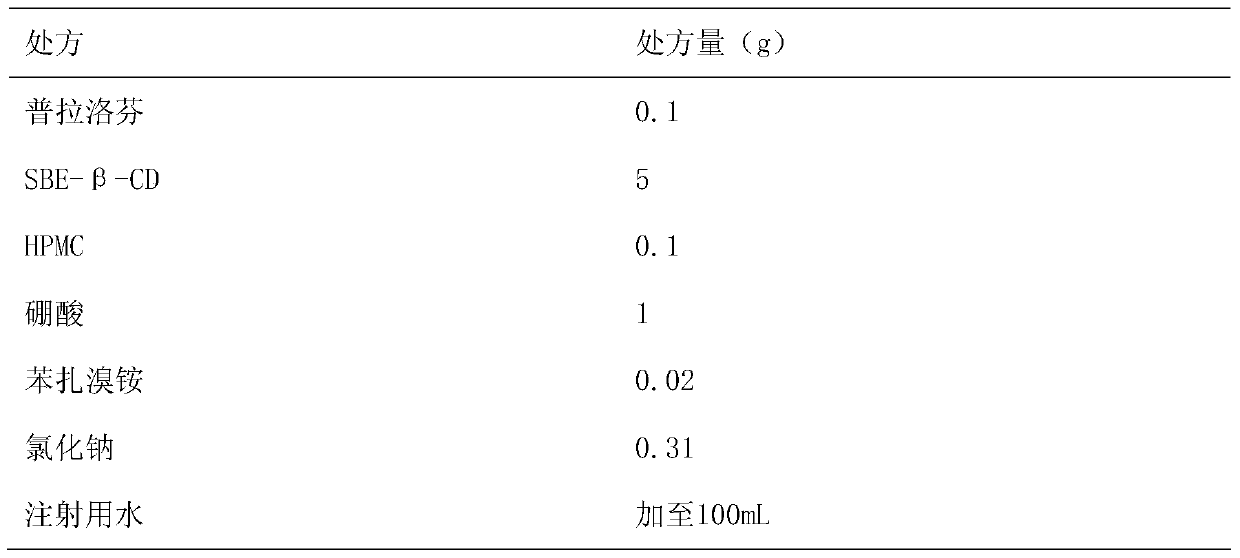
[0024] Embodiment 2: prepare 0.1% pranoprofen eye drops with 5% SBE-β-CD solution and 0.1% HPMC as solubilizer and stabilizer
[0025]
[0026] Preparation method: Dissolve the prescription amount of SBE-β-CD in 90mL of water for injection, add boric acid to dissolve, heat in a water bath to 60°C, add 0.1g of pranoprofen, stir until completely dissolved, add other excipients in turn, stir to make After it is completely dissolved, adjust the pH value to 7.5, and add water for injection to 100ml. Filter through a 0.22μm microporous membrane, fill in divided doses into ampoules, and obtain the product.
Embodiment 3
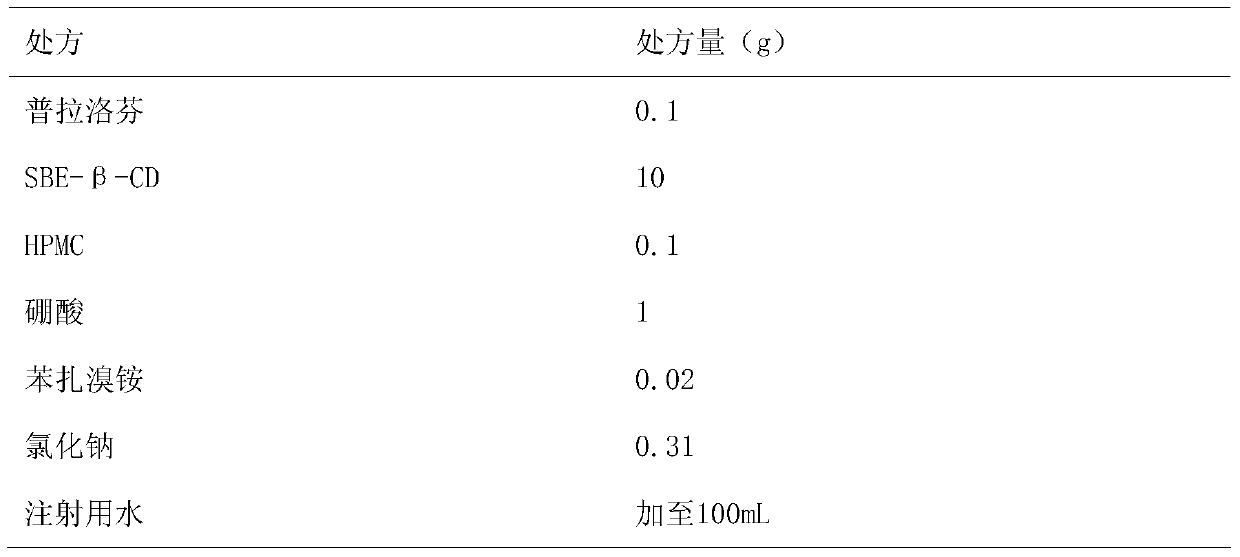
[0027] Embodiment 3: prepare 0.1% pranoprofen eye drops with 10% SBE-β-CD solution and 0.1% HPMC as solubilizer and stabilizer
[0028]
[0029] Preparation method: Dissolve the prescription amount of SBE-β-CD in 90mL of water for injection, add boric acid to dissolve, heat in a water bath to 60°C, add 0.1g of pranoprofen, stir until completely dissolved, add other excipients in turn, stir to make After it is completely dissolved, adjust the pH value to 7.5, and add water for injection to 100ml. Filter through a 0.22μm microporous membrane, fill in divided doses into ampoules, and obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com