Detection method of pranoprofen eye drop related substances
A detection method and related substance technology, applied in the field of chemical analysis, can solve the problems of small amount of impurities, instability of pranoprofen to light, poor specificity, etc., and achieve good product quality, good durability, and good specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
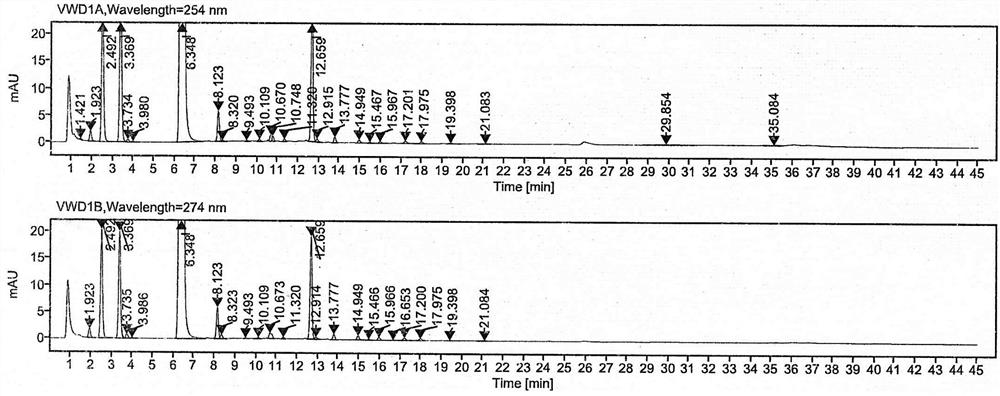
[0045] Embodiment 1: detection of related substances of pranoprofen eye drops
[0046] (1) High performance liquid chromatography conditions:
[0047] Chromatographic column: Agilent infinitylab proshell 120 EC-C18, (3.0×100mm, 2.7µm)
[0048] Detection wavelength: 254nm&274nm
[0049] Injection volume: 20µL
[0050] Column temperature: 25°C
[0051] Sample concentration: 0.2mg / mL
[0052] Flow rate: 0.5mL / min
[0053] Mobile phase: mobile phase A: 0.10mol / L ammonium acetate buffer (adjust the pH to 4.50±0.02 with glacial acetic acid); mobile phase B: acetonitrile.
[0054] Gradient elution time and flow ratios are as follows:
[0055]
[0056] (2) Sample and solution preparation:
[0057] Blank excipient mixture: weigh 1.6g of boric acid, 0.8g of borax, 10mg of edetate disodium hydrate, 7mg of benzalkonium chloride, 0.15g of polysorbate 80, add 80mL of water for injection and stir until dissolved, then add water for injection to volume To 100mL, shake well, that is...
Embodiment 2
[0070] Example 2: Methodological Validation
[0071] 1. Precision test
[0072] Instrument and chromatographic conditions are all with embodiment 1.
[0073] repeatability
[0074] Precisely measure 1 mL of pranoprofen eye drops, put it in a 5 mL measuring bottle, dilute to the mark with diluent, and shake well. Configure 6 copies in parallel. Take 6 parts of blank solution, blank auxiliary material solution, and repeatability test solution respectively, inject samples using the chromatographic conditions of Example 1, record the chromatogram, and calculate according to the area normalization method. The test results at a wavelength of 274nm are shown in Table 2.
[0075] Table 2 Summary of repeatability test results
[0076]
[0077] The test results of the precision test show that the RSD% of the impurities in the 6 samples of the analysis method of the present invention is less than 5%, and the method has good repeatability.
[0078] 2. Forced degradation test
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com