Purification method of pranoprofen
A technology of refining and purifying methanol water solution, which is applied in the field of preparation of non-steroidal anti-inflammatory drugs, can solve the problems of long cycle, increased production cost, and ineffective separation of pranoprofen impurity C.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
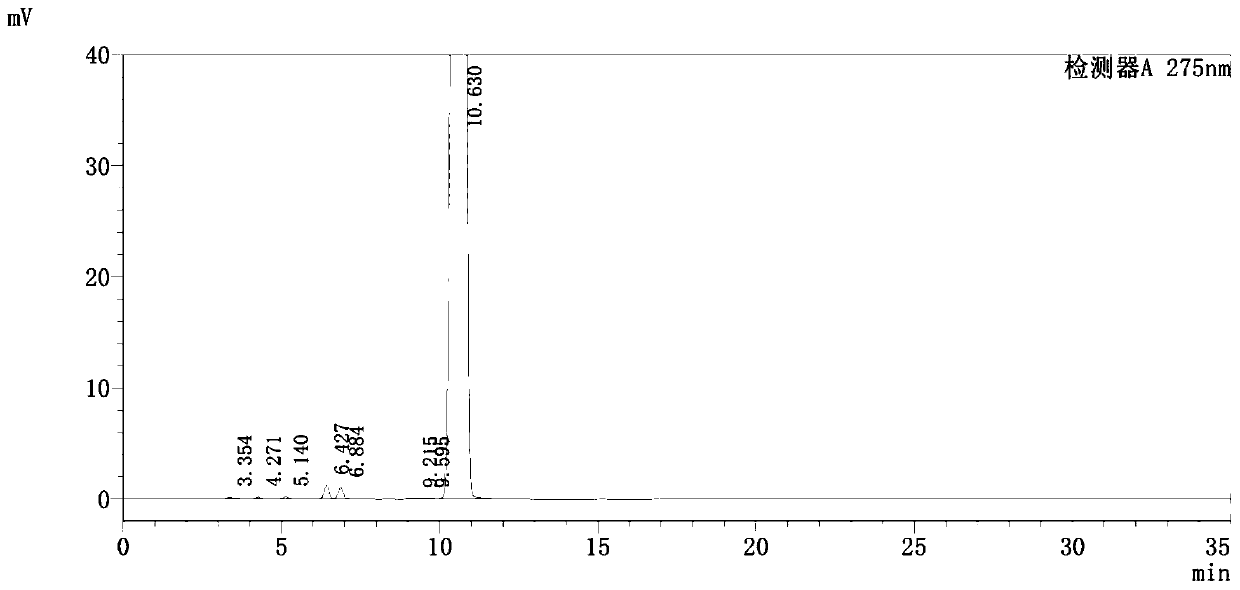
[0041]Grind the pranoprofen to be treated and pass through a 60-mesh sieve, add 10 times the amount of 30% methanol aqueous solution, heat up to 50 ° C, keep stirring for 3 hours, cool to room temperature (25 ° C), filter, and use a small amount of the same The filter cake is rinsed with concentrated methanol aqueous solution, drained, and dried at low temperature, and the beating yield is 96.5%. Take the pranoprofen that has been beaten, add 35 times the amount of 50% methanol aqueous solution and put it in the decolorization tank, heat and stir to dissolve it, reflux for 10 minutes, filter it to the crystallization tank while it is hot, cool to 20 ° C, and crystallize for 5 Hour. Filter, rinse the crystals with a small amount of recrystallization solvent (50% methanol solution), drain, and dry at 50°C to obtain the finished product of pranoprofen. The impurity chromatogram of the product is as follows figure 2 As shown, its chromatographic purity is 99.75%, wherein the im...
Embodiment 2
[0045] Grind the pranoprofen to be treated and pass through an 80 mesh sieve, add 8 times the amount of 50% aqueous methanol solution, heat up to 60°C for reflux and keep stirring for 4 hours, cool to room temperature, filter, and use a small amount of methanol of the same concentration- The filter cake was rinsed with aqueous solution, drained, and dried at low temperature. The beating yield was 94.7%. Take the pranoprofen that has been beaten, add 25 times the amount of 50% methanol aqueous solution and put it in the decolorization tank, heat and stir to dissolve it, reflux for 30 minutes, filter it to the crystallization tank while it is hot, cool to 25 ° C, and crystallize for 5 Hour. Filter, rinse the crystals with a small amount of recrystallization solvent (50% methanol solution), drain, and dry at 55°C to obtain the finished product of pranoprofen. The impurity chromatogram of the product is as follows image 3 As shown, its chromatographic purity is 99.80%, wherein ...
Embodiment 3
[0049] Grind the pranoprofen to be treated and pass through a 100 mesh sieve, add 6 times the amount of 70% methanol solution, heat up to 50-60°C, keep stirring for 3 hours, cool to room temperature, filter, and rinse with a small amount of 70% methanol solution The filter cake is drained and dried at low temperature, and the beating yield is 88.7%. Take the pranoprofen that has been beaten, add 30 times the amount of 50% methanol aqueous solution, put it in the decolorization tank, heat it while stirring to dissolve, reflux for 10-30 minutes, filter it into the crystallization tank while it is hot, and cool it to 20-25 °C , crystallization for 5 hours. Filter, rinse the crystals with a small amount of recrystallization solvent, drain them, and dry them below 55°C to obtain the finished product of pranoprofen. The impurity chromatogram of the product is as follows Figure 4 As shown, its chromatographic purity is 99.75%, wherein the content of impurity A is 0.11%, the conten...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com