Method for preparing novel Pranoprofen key intermediates
The technology of a compound, azanthone, is applied in the field of preparation of new important intermediates of pranoprofen, which can solve the problems of phosphorus oxychloride post-processing difficulties, environmental pollution, and difficulty in industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
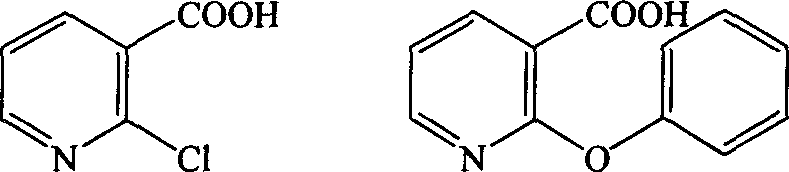
[0024] Embodiment 1: Preparation of 2-phenoxynicotinic acid
[0025] Into a 10L three-necked flask, sequentially add 4064ml of anhydrous methanol, 1096g of sodium methoxide, 3088g of phenol and 1600.0g of 2-chloronicotinic acid, and heat to reflux. The solvent was recovered until the temperature of the reaction liquid reached 175-180°C, and the stirring was continued for 2 hours. After cooling, add 6.4L of water and adjust the pH value to 4-5 with concentrated hydrochloric acid. Then adjust the pH to weak alkaline with 10% sodium bicarbonate solution, and extract with ethyl acetate. The aqueous phase was separated, and the pH value was adjusted to 1-2 with concentrated hydrochloric acid, and a large amount of white solid was precipitated. After suction filtration, the filter cake was dried at 60° C. for 24 hours to obtain 2084 g of white solid with a yield of 95.36%. Melting point: 177.0-179.0°C.
Embodiment 2
[0026] Embodiment 2: Preparation of 9-oxa-1-azanthone
[0027] Add 4086ml of polyphosphoric acid and 1224g of 2-phenoxynicotinic acid into a 10L three-necked flask, raise the temperature to 135-140°C and stir for 10 hours. After cooling, 12L of water was added dropwise in an ice bath, and then 3.0L of 33.3% NaOH solution was added dropwise, a large amount of off-white solid precipitated, and was suction filtered. The filter cake was washed with 10% NaOH solution until alkaline. Then wash with water until neutral, and filter with suction. The filter cake was dried at 60°C for 24 hours to obtain 1029 g of white solid. The yield was 91.2%. Melting point: 182-183°C.
Embodiment 3
[0028] Embodiment 3: Preparation of 9-oxa-1-azanthone
[0029] Add 4500ml of polyphosphoric acid and 1224g of 2-phenoxynicotinic acid into a 10L three-necked flask, heat up to 135-140°C and stir for 8 hours. After cooling, 12L of water was added dropwise in an ice bath, and then 3.2L of 33.3% NaOH solution was added dropwise, a large amount of off-white solid precipitated, and was suction filtered. The filter cake was washed with 10% NaOH solution until alkaline. Then wash with water until neutral, and filter with suction. The filter cake was dried at 60°C for 24 hours to obtain 1020 g of white solid. The yield is 90%. Melting point: 182-183°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com