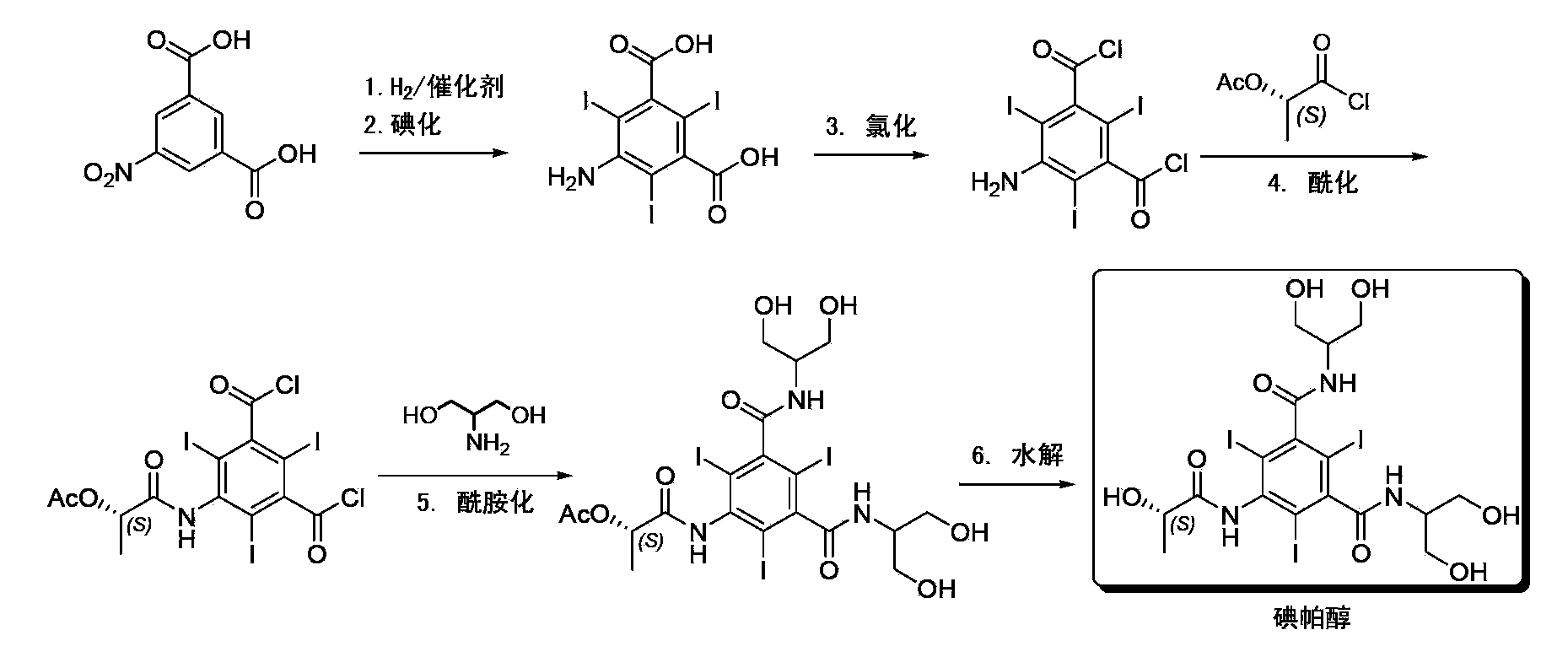
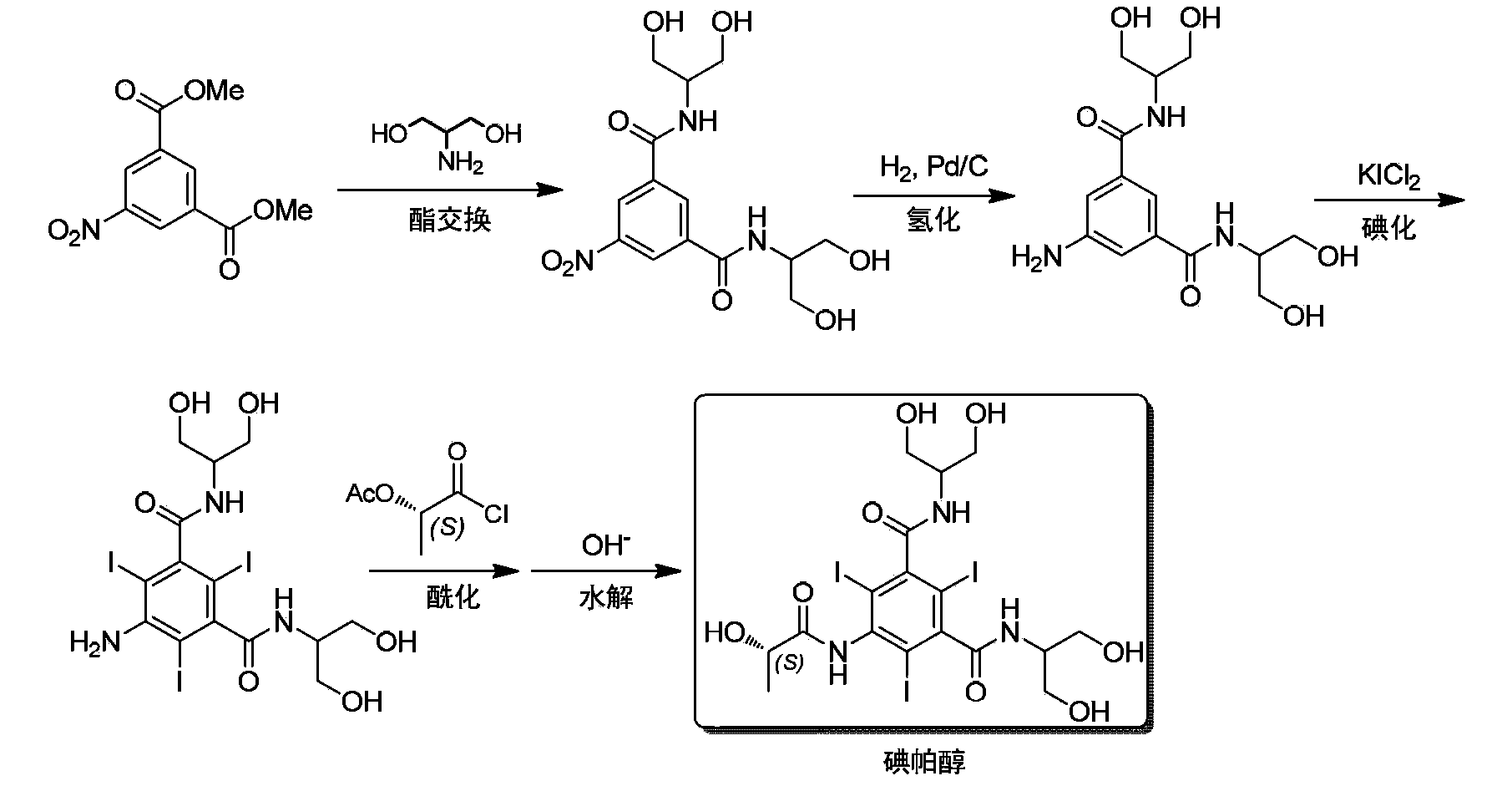
Synthesis of iopamidol and preparation of iopamidol synthesis intermediate
A compound and mixed anhydride technology, applied in the field of new intermediates, can solve the problem of waste of expensive chiral reactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The reaction of step a) for the preparation of compounds of formula (III) is preferably in which there is a slightly stoichiometric excess of a suitably selected "mixed anhydride" relative to the compound of formula (II) and optionally a catalytic amount (generally 0.01 to 0.1 molar / 1 mole of compound of formula (II)) in N,N-dimethylacetamide solution of 4-(dimethylamino)pyridine of compound of formula (II). Optional 4-(dimethylamino)pyridine can be added to the reaction mixture as such or supported on the resin.
[0050] Four moles of "mixed anhydride" must be used per mole of starting compound (II). But in general, there is a slight excess, such as about 10%, 20% or even 25%, preferably a molar excess of up to about 50%; it is preferred to use five moles of "mixed anhydride" per mole of starting compound (II).
[0051] The reaction of step a) is generally carried out at room temperature, but can also be carried out at lower or higher temperatures, for example, a tem...
Embodiment 1
[0077] Preparation of N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-nitro-1,3-benzenedicarboxamide
[0078]Add 5-nitro-isophthalic acid dimethyl ester (120g, 0.50mol), 2-amino-1,3-propanediol (136g, 1.50mol), methanol (960mL) in a 2000mL three-necked flask, stir and heat Return to reflux for 24 hours; cool down to 20°C, filter, wash the filter cake twice with methanol (20mL×2), and dry to obtain 178.4g of the product, with a yield of 99.5%.
[0079] 1 H-NMR, 13 C-NMR, IR and MS were consistent with the shown structure.
Embodiment 2
[0081] N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-amino-2,4,6-triiodo-1,3-benzenedicarboxamide (compound of formula (II)) preparation of
[0082] Suspend the N,N'-bis[2-hydroxyl-1-(hydroxymethyl)ethyl]-5-nitro-1,3-benzenedicarboxamide (120g, 0.34mol) prepared in Example 1 in 90 ℃ water (1200mL), stirring, acetic acid to adjust the pH to below 5; under the protection of nitrogen, add 30g of Raney nickel (wet weight), replace the nitrogen with hydrogen and pressurize to 1.4MPa, keep at 90 ℃ for more than 6 hours. After the reaction is complete, filter.
[0083] The filtrate was warmed up to 50°C, and potassium iodide dichloride (KICl 2 , 263g, 1.11mol) aqueous solution, after the dropwise addition, continue to keep warm at 90°C and stir the reaction for more than 3 hours; after the reaction is completed, recover excess iodine by sublimation method.
[0084] The reaction solution was cooled to 20°C, filtered, and the filter cake was washed with 50 mL×4 water, and dried to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com