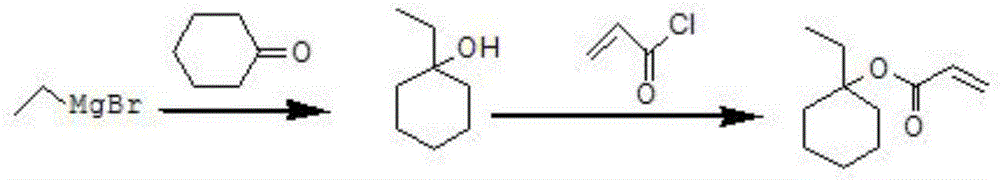
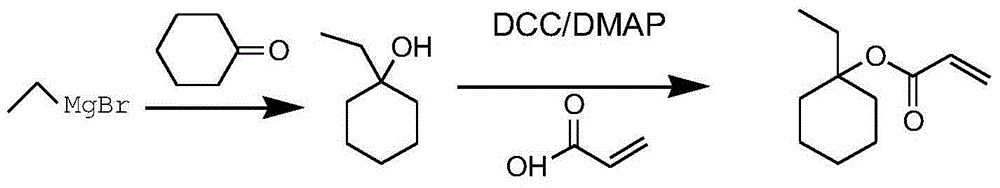
Method for preparing 1-ethylcyclohexyl acrylate
A cyclohexyl acrylate and ethyl technology, applied in the field of organic chemical synthesis, can solve the problems of complicated post-processing process, loss and the like, and achieve the effects of convenient industrial production, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 1-ethylcyclohexyl acrylate: Weigh 6.2g (0.26mol) of magnesium chips into a 500ml three-neck flask and protect it with dry nitrogen. Weigh 32.0 g (0.296 mol) of bromoethane, 46.0 g of tetrahydrofuran and 60.0 g of toluene, mix them evenly, and place them in a constant pressure dropping funnel with an internal temperature of 13°C. Add 5mL of a mixture of tetrahydrofuran, toluene and ethyl bromide to the magnesium chips. After the reaction is initiated, a large amount of heat is released, and the internal temperature rises to 35°C. The solution turns gray and white smoke comes out. When the internal temperature dropped to 15°C, the mixture of ethyl bromide, tetrahydrofuran and toluene was continuously added dropwise and the stirring was started. Control the internal temperature to <35°C, drop it for 1 hour, then transfer to room temperature (20-30°C) and stir for 1 hour to prepare the Grignard reagent.
[0030] In addition, take 19.6g (0.2mol) cyclohexanone...
Embodiment 2
[0036] Preparation of 1-ethylcyclohexyl acrylate: Weigh 6.2g (0.26mol) of magnesium chips into a 500ml three-neck flask and protect it with dry nitrogen. Weigh 32.0 g (0.296 mol) of bromoethane and 86.0 g of tetrahydrofuran, mix them uniformly and place them in a constant pressure dropping funnel with an internal temperature of 13°C. Add 5 mL of a mixture of tetrahydrofuran and ethyl bromide to the magnesium chips. After the reaction is initiated, a large amount of heat is released, and the internal temperature rises to 35 ° C. The solution turns gray and white smoke comes out. When the internal temperature dropped to 15°C, the mixture of ethyl bromide and tetrahydrofuran was continuously added dropwise and the stirring was started. Control the temperature <35°C, drop it for 1h, then transfer to room temperature (20-30°C) and stir for 1 hour, no magnesium chips remain.
[0037] Take by weighing 19.6g (0.2mol) cyclohexanone and be dissolved in 20.0g toluene and be placed in th...
Embodiment 3
[0043]Preparation of 1-ethylcyclohexyl acrylate: Weigh 6.2g (0.26mol) of magnesium chips into a 500ml three-neck flask and protect it with dry nitrogen. Weigh 32.0 g (0.296 mol) of bromoethane, 46.0 g of tetrahydrofuran and 60.0 g of toluene, mix them evenly, and place them in a constant pressure dropping funnel with an internal temperature of 13°C. Add 5mL of a mixture of tetrahydrofuran, toluene and ethyl bromide to the magnesium chips. After the reaction is initiated, a large amount of heat is released, and the internal temperature rises to 35°C. The solution turns gray and white smoke comes out. When the internal temperature dropped to 15°C, the mixture of ethyl bromide, tetrahydrofuran and toluene was continuously added dropwise and the stirring was started. Control the internal temperature to <35°C, drop it for 1 hour, then transfer to room temperature (20-30°C) and stir for 1 hour to prepare the Grignard reagent.
[0044] Take by weighing 19.6g (0.2mol) cyclohexanone a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com