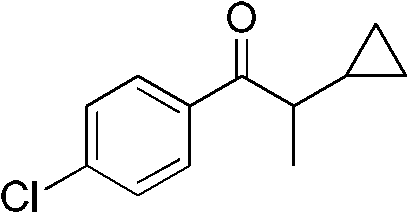
Preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A technology of cyclopropyl propionic acid and cyclopropyl methyl ketone, which is applied in the field of preparation of 1--2-cyclopropyl-1-propanone, can solve the problem of easy self-polymerization of chlorophenylacetonitrile, complex operation, and water content in the reaction system Strict efficiency requirements and other issues, to achieve the effect of reducing three wastes, high yield, cheap and easy-to-obtain raw materials and solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
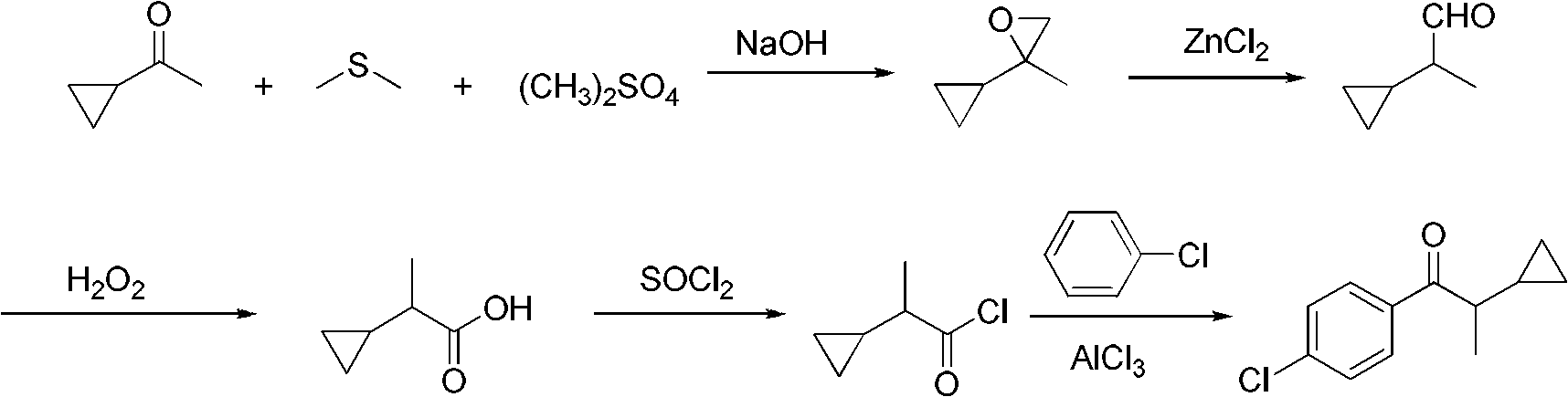
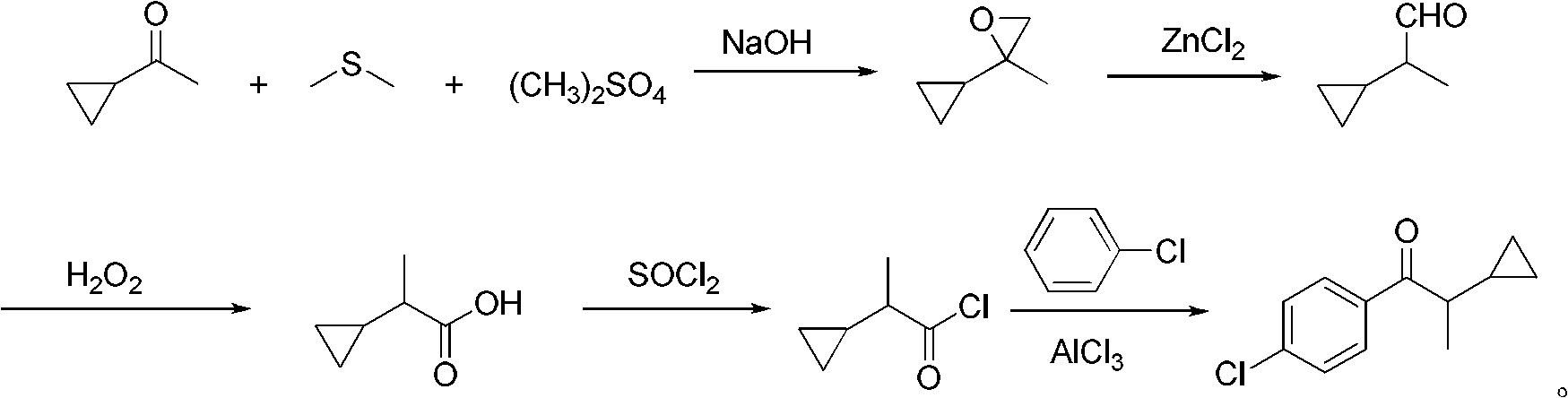
[0029] Add 28.3g (0.22mol) of dimethyl sulfate, 136g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-necked flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen Sodium oxide 17.8g (0.44mol) was added in portions within 1 hour, and the reflux reaction was continued for 2 hours. After the reaction is finished, use 10% dilute hydrochloric acid to neutralize, stand still and separate the phases, and the organic phase is distilled under normal pressure to recover dimethyl sulfide. 18.6 g of the still liquid is 2-cyclopropyl-2-methyl propylene oxide, with a content of 96.2%. (gas chromatography), yield 93.2%.
[0030] Add 18.6g of 2-cyclopropyl-2-methyl propylene oxide to a 100mL three-necked flask, add 0.6g of anhydrous zinc chloride at room temperature, stir for 4 hours, and add 16g (0.23mol) of 50% hydrogen peroxide dropwise at room temperature , After adding in 1 hour, the reaction was continued for ...
Embodiment 2
[0033] Add 28.3g (0.22mol) of dimethyl sulfate, 170g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-neck flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen 18.6 g (0.46 mol) of sodium oxide was added in portions within 1 hour, and the reflux reaction was continued for 2 hours. After the reaction is finished, neutralize with 10% dilute hydrochloric acid, stand still and separate the phases, and the organic phase is distilled under normal pressure to recover dimethyl sulfide. 18.9 g of the still liquid is 2-cyclopropyl-2-methyl propylene oxide, with a content of 96.5%. (gas chromatography), yield 95.0%.
[0034] Add 189g of 2-cyclopropyl-2-methylpropylene oxide to a 100mL three-necked flask, add 0.7g of anhydrous zinc chloride at room temperature, stir and react for 6 hours, then add 18.2g (0.26mol) of 50% hydrogen peroxide dropwise at room temperature, The addition was completed in 1 hour, and th...
Embodiment 3
[0037] Add 28.3g (0.22mol) of dimethyl sulfate, 170g of dimethyl sulfide, and 16.9g (0.20mol) of cyclopropylmethyl ketone into a 500mL three-neck flask with a stirring and reflux tube, reflux at 40°C for 1 hour, and dissolve the hydrogen Sodium oxide 19.4g (0.48mol) was added in batches within 1 hour, and the reflux reaction was continued for 3 hours. Neutralize with 10% dilute hydrochloric acid after the reaction, stand still and separate the phases, and reclaim dimethyl sulfide by atmospheric distillation of the organic phase. 19.2 g of the still liquid is 2-cycloendyl-2-methylepoxyne, with a content of 96.1 % (gas chromatography), yield 94.1%.
[0038] Add 19.2g of 2-cyclopropyl-2-methyl propylene oxide to a 100mL three-necked flask, add 0.96g of anhydrous zinc chloride at room temperature, stir and react for 6 hours, then add 19.7g (0.28mol) of 50% hydrogen peroxide dropwise at room temperature , The addition was completed in 1 hour, and the reaction was continued for 4 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com