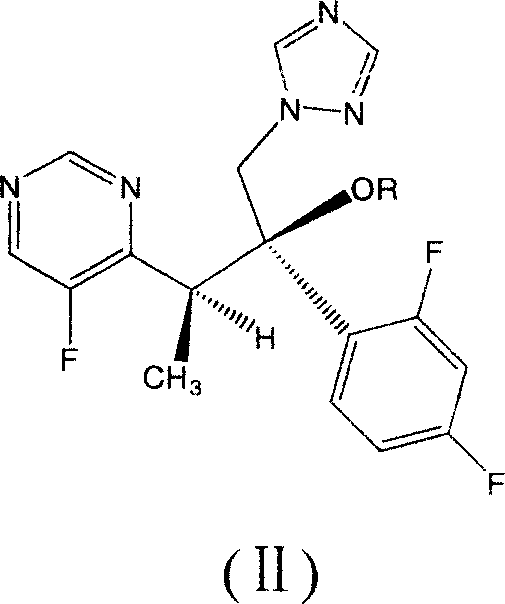
Voriconazole derivate and preparation process thereof
A technology of voriconazole and compounds, applied in the field of voriconazole derivatives and its preparation, can solve the problems of long steps and low yield, and achieve the effect of short route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 compound 2-(5-fluoro-4-yl) ethyl acetate (II):
[0040] Into a 100ml three-necked flask, add 25ml of absolute ethanol, 5g of 2-(5-fluoro-4-yl)acetic acid, carefully dropwise add 2ml of concentrated sulfuric acid, slowly heat up to reflux state after adding, keep warm for half an hour, cool to room temperature, slowly Pour into cold sodium carbonate solution, stir while pouring, keep the solution alkaline, precipitate solid, filter, wash the filter cake with a small amount of cold water, discard the filtrate, and air-dry the filter cake naturally to obtain 7.2g of white powder, melting point 77-78 °C, yield 94%.
[0041] 1 H NMR (CDCl 3 )δ: 8.90(d, J=3Hz, 1H), 8.50(d, J=3Hz, 1H), 3.50(s, 2H), 4.10(f, J=8Hz, 2H), 1.30(t, J=8Hz , 3H).
Embodiment 2
[0042] Preparation of Example 2 Compound 2-(5-fluoro-4-yl) ethyl propionate (III):
[0043] In a 100ml three-necked flask, add 25ml of anhydrous acetonitrile, 10g of anhydrous potassium carbonate, 5g of ethyl 2-(5-fluoro-4-yl)acetate, heat to reflux, add 3.8g of dimethyl sulfate dropwise, after the dropwise addition, Reflux for 6 hours, TLC detects the end point, recover the solvent, pour the residue into water, precipitate a light yellow solid, filter, wash the filter cake with a small amount of water, discard the filtrate, and recrystallize the filter cake with 95% ethanol to obtain 4.60 g of a white solid, melting point 69.5-71.5°C, yield 86%. 1 H NMR (CDCl 3)δ: 8.89(d, J=3Hz, 1H), 8.48(d, J=3Hz, 1H), 3.62(f, J=7.6Hz, 1H), 4.10(f, J=8Hz, 2H), 1.30( t, J=8Hz, 3H), 1.20 (d, J=7.5Hz, 3H).
Embodiment 3
[0044] Preparation of Example 3 Compound S-2-(5-fluoro-4-yl)propionic acid (IV):
[0045] Add the solid obtained in the second step to 25ml of dilute hydrochloric acid, heat to reflux for 2 hours, evaporate most of the water, adjust the pH to 8 with sodium bicarbonate solution, and precipitate a white solid, filter, add the filter cake to 10ml of water, add equimolar -(2-phenylethylamine), heated to dissolve, cooled to room temperature to obtain granular colorless transparent crystals, filtered, heated and dissolved the crystals in 5ml of water, adjusted the pH value to 8, cooled to precipitate 1.8g of white solid, separated After the mother liquor was racemized and resolved, another 1.6g was obtained with a melting point of 189-190°C and a yield of 86.1%. 1 H NMR (CDCl 3 )δ: 11.05(s, 1H), 8.88(d, J=2.8Hz, 1H), 8.30(d, J=2.8Hz, 1H), 3.85(q, J=7.6Hz, 1H), 1.39(d, J=7.6Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com