The synthetic method of triptolide intermediate
A technology for intermediates and compounds, applied in the field of preparation of organic compounds, can solve the problems of low selectivity, insufficient stereoselectivity, long routes, etc., and achieve good stereoselectivity and yield, good non-corresponding selectivity, and good reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
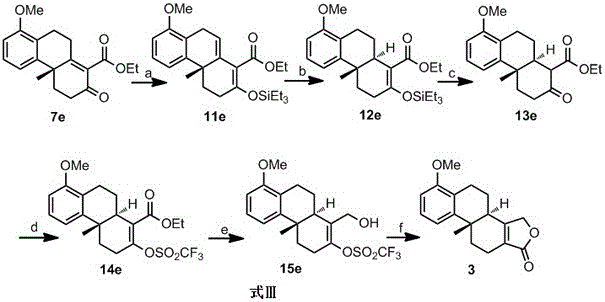
[0029] Embodiment 1: the reaction process is shown in formula 9, namely R in formula 7 1 , R 2 , R 3 and R 4 is methyl, R 5 is trifluoromethyl, that is, the base used for the conversion of compound 7a to compound 11a is triethylamine, the trialkylsilicon reagent is trimethylchlorosilane, and the catalytic hydrogenation catalyst used for the conversion of compound 11a to 12a is 5% Pd-C , the conversion of compound 12a to compound 13a is carried out under the action of tetrabutylammonium fluoride, the base used for the conversion of compound 13a to compound 14a is pyridine, the sulfonylation reagent is trifluoromethanesulfonic anhydride, and the reducing agent for reducing compound 14a It is diisobutylaluminum hydride, and the catalyst for the carbonyl insertion reaction of compound 15a is palladium acetate.
[0030] Formula 9
[0031] Preparation of compound 11a
[0032]In a 250 mL round-bottomed flask, compound 7a (6.28 g, 20 mmol) was dissolved in 100 mL of dry dic...
Embodiment 2
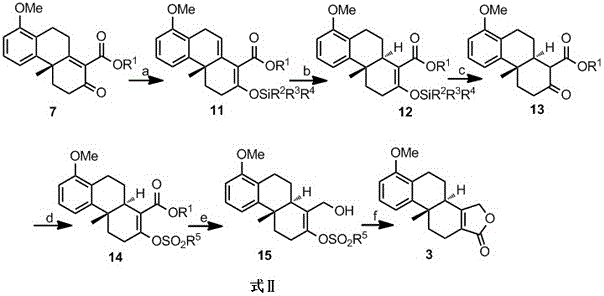
[0043] Embodiment 2: the reaction process is shown in formula 10, namely R in formula 7 1 is tert-butyl, R 2 , R 3 and R 4 is phenyl, R 5 For p-tolyl, that is, the base used to convert compound 7b to compound 11b is potassium carbonate, the trialkylsilicon reagent is triphenylchlorosilane, the catalytic hydrogenation catalyst used to convert compound 11b to 12b is Raney Ni, and compound 12b is converted to Compound 13b was carried out under the action of 2M hydrochloric acid, the base used for the conversion of compound 13b to compound 14b was triethylamine, the sulfonylation reagent was p-toluenesulfonyl chloride, the reducing agent for reducing compound 14b was sodium borohydride-iodine, and the compound The catalyst for the carbonyl insertion reaction of 15b is palladium chloride.
[0044] Formula 10
[0045] Preparation of compound 11b
[0046] In a 250 mL round-bottomed flask, compound 7b (6.5 g, 20 mmol) was dissolved in 100 mL of dry dichloromethane, potassiu...
Embodiment 3
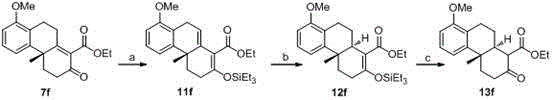
[0057] Embodiment 3: Reaction process is shown in formula 11, namely R in formula 7 1 is n-butyl, R 2 is tert-butyl, R 3 and R 4 is methyl, R 5 The base used for the conversion of the p-methyl group, i.e., compound 7c to compound 11c, was imidazole, the trialkylsilicon reagent was tert-butyldimethylsilyl chloride, and the catalytic hydrogenation catalyst used for the conversion of compound 11c to 12c was PtO 2 , the conversion of compound 12c to compound 13c is carried out under the action of p-toluenesulfonic acid, the base used for the conversion of compound 13c to compound 14c is diisopropylethylamine, the sulfonylation reagent is methanesulfonyl chloride, and the reduction of compound 14c The reagent is sodium borohydride-boron trifluoride ether, and the catalyst for the carbonyl insertion reaction of compound 15c is diacetonitrile palladium dichloride.
[0058] Formula 11
[0059] Preparation of compound 11c
[0060] In a 250 mL round-bottomed flask, compound 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com