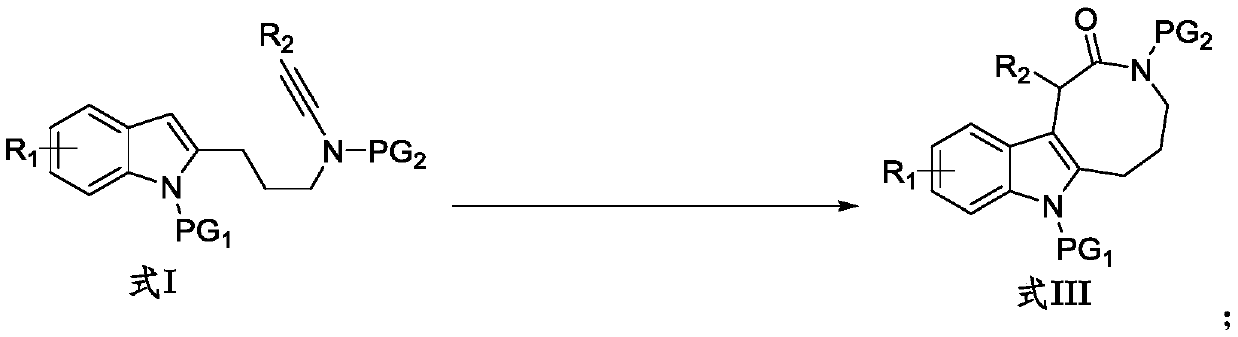
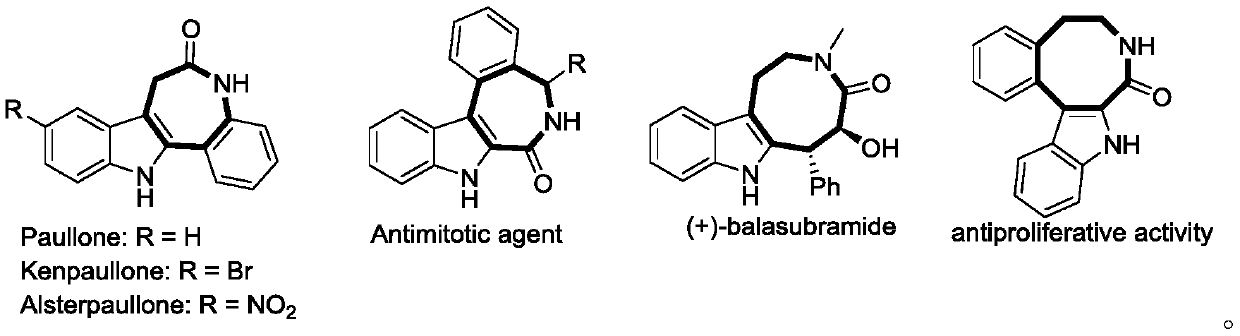
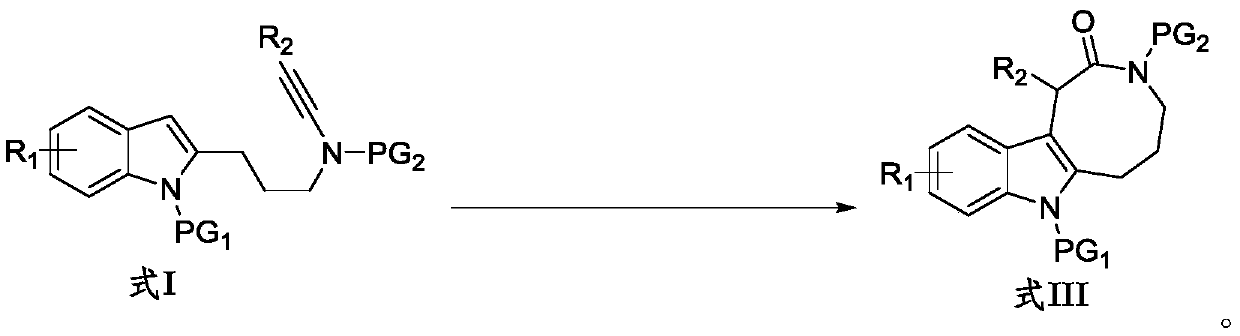
Preparation method of indole condensed octatomic lactam compounds
A technology for the fusion of lactam compounds and indole, which is applied in organic chemistry and other fields, can solve the problems of limited synthesis methods and difficulty in forming a skeleton, and achieve the effects of wide substrate adaptability, easy reaction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Into the Schlenk lock reactor, sequentially add additive NaBAr F 4 (0.04mmol, 35.6mg), the catalyst zinc trifluoromethanesulfonate (Zn(OTf) 2 ) (0.02mmol, 7.3mg), the indolyl alkyne amide compound (0.20mmol) shown in formula I-1 and the N-oxide oxidant (R=2,6-Cl 2 , 60.0mg, 0.4mmol), then add the organic solvent DCE (dichloroethane, 2mL), replace the reactor with a nitrogen atmosphere, heat and stir at 80°C for 0.5h, monitor the completion of the reaction by TLC, and concentrate the reaction solution The residue was obtained and separated by silica gel column chromatography (elution solvent: n-hexane / ethyl acetate) to obtain the indole-fused eight-membered lactam compound represented by formula III-1. Yield: 92%. White solid (mp 225-226°C). 1 H NMR (400MHz, CDCl 3)δ8.05(d,J=8.8Hz,1H),7.37–7.32(m,3H),7.31–7.26(m,1H),7.25–7.16(m,2H),7.11–7.06(m,1H) ,6.84(d,J=8.0Hz,1H),5.51(s,1H),4.11–3.98(m,1H),3.82–3.73(m,1H),3.51–3.23(m,5H),3.17(s ,3H),2.10–1.97(m,2H)...
Embodiment 2
[0036] The oxidizing agent (R=2-Br, 2equiv) shown in formula II, the reaction time is 12h, does not add auxiliary agent NaBAr F 4 , all the other conditions are the same as in Example 1, and the productive rate is 27%.
Embodiment 3
[0038] The oxidizing agent (R=2-Cl, 2equiv) shown in formula II, the reaction time is 12h, no additive NaBAr is added F 4 , all the other conditions are the same as in Example 1, and the productive rate is 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com