A kind of preparation method of isavuconazole
A technology of isavuconazole and triazole, applied in the field of medicine, can solve the problems of rising cost, reduced yield, numerous process steps, etc., and achieve the effects of reducing reaction steps, improving yield and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
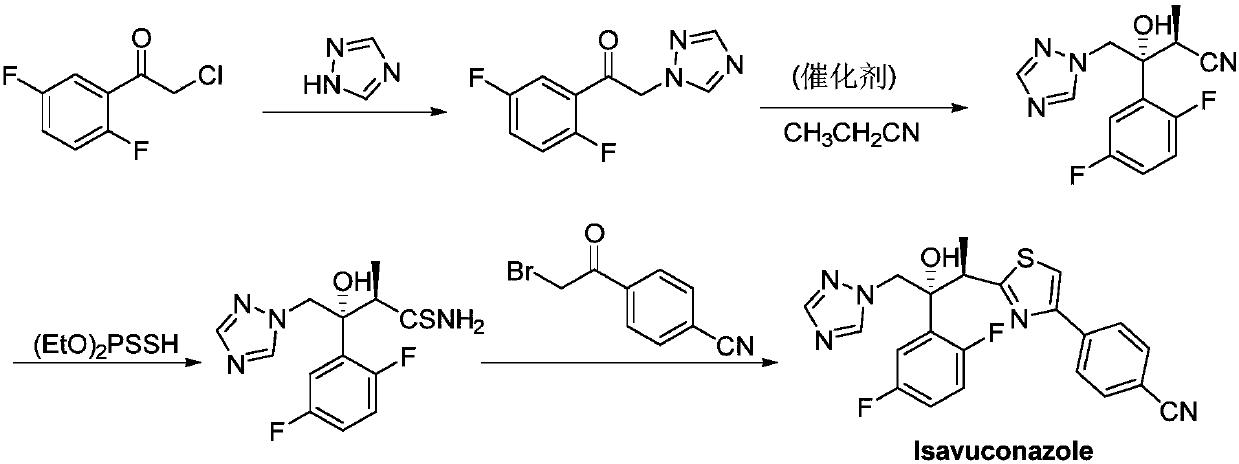
[0022] The invention provides a preparation method of isavuconazole, such as figure 1 As shown, it includes the following steps:
[0023] (a) Add 1 mol of difluorophenylacetyl chloride, 1.2 mol of triazole, 0.15 mol of CuI, 1.5 mol of potassium carbonate and 0.8 L of DMF (N,N-dimethylformamide) into the reaction kettle, and heat up to 80°C The reaction was stirred for 10 hours, and TLC (thin layer chromatography) detected that the reaction was complete; the filtrate was filtered and concentrated under reduced pressure to recover DMF, and the residue was recrystallized with ethyl acetate to obtain an off-white solid, the first product, with a yield of 73 %;
[0024] (b) In the three-necked reaction flask, add 4mol propionitrile, 0.1mol catalyst (comprising 0.05mol C-9 primary amine cinchona base, chemical formula is And 0.05mol cuprophylline, the chemical formula is ), 0.1mol benzoic acid and 0.5L N,N-dimethylacetamide (DMA), cooled to -10°C, stirred and added dropwise 0.3...
Embodiment 2
[0028] This embodiment provides a preparation method of isavuconazole, the preparation steps of which are basically the same as those in Example 1, the difference is that in step (b), no benzoic acid is added, and the yield of the final second product is 75% %, dr. is 95:5.
Embodiment 3
[0030] This embodiment provides a preparation method of isavuconazole, the preparation steps of which are basically the same as those in Example 1, except that in step (b), the C-9 primary amine cinchonaline and copper-colored tree in the catalyst The molar ratio of the base is 5:1, the yield of the final second product is 76%, and the dr. is 95:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com