Novel synthesis method for 3-(alkoxy methyl phosphoryl)propionate
An alkoxymethylphosphoryl and propionate technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, chemical/physics/physicochemical processes, etc., can solve the problem of acid binding agents Selectivity difference temperature control and other issues, to achieve the effect of environmental friendliness, simple operation, broad scale application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
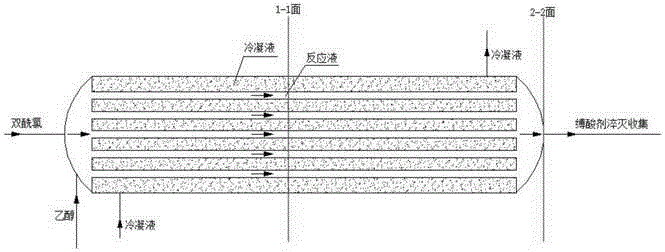
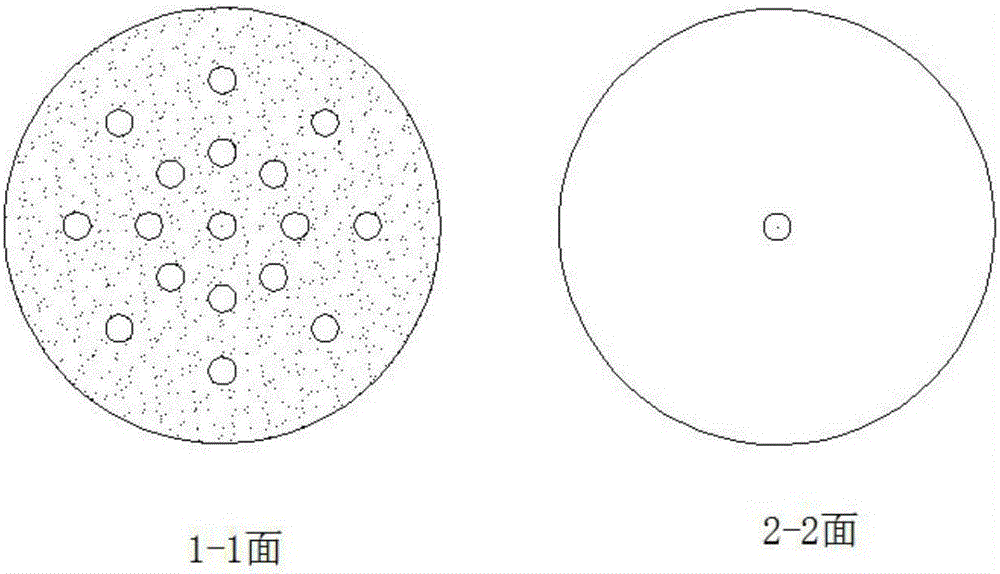
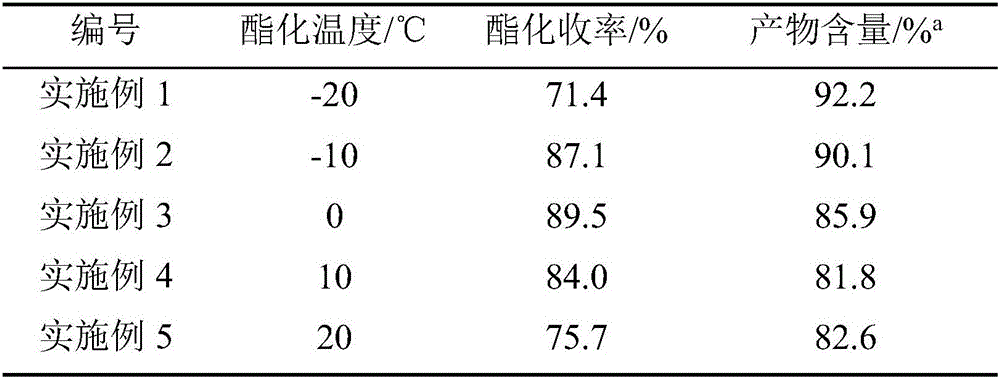
[0026] Weigh 37.8g of bisacyl chloride and 34g of methylene chloride and seal them in bottle A for later use, and weigh 36.8g of ethanol in bottle B. After the cooling circulation system was turned on to control different esterification temperatures, the bisacyl chloride solution and ethanol were pumped into the new array tube reaction device in proportion to carry out the esterification reaction by using micro-injection pumps respectively. The reaction solution is quenched with an acid-binding agent to collect the product, and the incoming and outgoing product is weighed. After the product was evaporated to dryness, it was washed and filtered with ethyl acetate, the solid was weighed, and the filtrate was weighed by rotary evaporation to dryness, and the sample was analyzed by GC and the yield was calculated. The data are shown in the table below:
[0027]
[0028] a Esterification conditions: n (双酰氯) :n (乙醇) =1:4; Bisacyl chloride solution feed rate: 5g / min.
Embodiment 6-11
[0030] Weigh 37.8 g of bisacyl chloride and 34 g of dichloromethane and seal them in bottle A for later use, and weigh ethanol in bottle B in different molar ratios. After turning on the cooling circulation system to the required temperature, the bisacyl chloride solution and ethanol were pumped into the new array tubular reaction device in proportion to carry out the esterification reaction by using a micro-injection pump respectively. The reaction solution is quenched with an acid-binding agent to collect the product, and the incoming and outgoing product is weighed. After the product was evaporated to dryness, it was washed and filtered with ethyl acetate, the solid was weighed, and the filtrate was weighed by rotary evaporation to dryness, and the sample was analyzed by GC and the yield was calculated. The data are shown in the table below:
[0031]
[0032]
[0033] b Esterification conditions: esterification temperature: -10°C; feeding rate of bisacyl chloride sol...
Embodiment 12-16
[0035] Weigh 37.8g of bisacyl chloride and 34g of methylene chloride and seal them in bottle A for later use, and weigh 36.8g of ethanol in bottle B. After turning on the cooling circulation system to the required temperature, the bisacyl chloride solution and ethanol were pumped into the new array tubular reaction device in proportion to carry out the esterification reaction using a micro-injection pump, and the feeding speed of different bisacyl chloride solutions was controlled. The reaction solution is quenched with an acid-binding agent to collect the product, and the incoming and outgoing product is weighed. After the product was evaporated to dryness, it was washed and filtered with ethyl acetate, the solid was weighed, and the filtrate was weighed by rotary evaporation to dryness, and the sample was analyzed by GC and the yield was calculated. The data are shown in the table below:
[0036]
[0037] c Esterification conditions: n (双酰氯) :n (乙醇) =1:4; Esterificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com