Method for synthesizing dendritic phenolic antioxidant
A technology of phenolic antioxidant and synthesis method, which is applied in the production of bulk chemicals, etc., can solve the problems of poor antioxidant performance and poor compatibility, and achieve the effect of easy operation and appropriate reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Preparation of 3,5-propionyl chloride: Accurately weigh 5.56g of 3,5-propionic acid and put it into a dry 250mL three-neck flask, add 50mL of chloroform to dissolve, and place in a constant temperature magnetic stirring water bath at 50°C In, slowly drop 4mL of thionyl chloride, react for 5h. Distill under reduced pressure at 62°C and 133Pa to remove the solvent chloroform and unreacted thionyl chloride to obtain β-(3,5-di-tert-butyl-4-hydroxyphenyl)propionyl chloride light yellow The crystal, referred to as 3,5-propionyl chloride, has a yield of 97.8%.
Embodiment 2
[0013] Example 2: Preparation of 1.0G polyamide-amine: Accurately weigh 9g of ethylenediamine and dissolve it in 30g of methanol, slowly add 103.2g of methyl acrylate dropwise while stirring, react at 25°C for 24h, at 50°C, 133.3Pa Distilled under reduced pressure under the conditions to obtain 0.5G polyamidoamine; then accurately weigh 12.0g 0.5G polyamidoamine, dissolve it in 60g methanol, slowly add 108.0g ethylenediamine dropwise while stirring, and react at 25°C After 24 hours, under the conditions of 72°C and 133Pa, vacuum distillation was carried out for 3 hours to obtain 1.0 g of polyamidoamine.
Embodiment 3
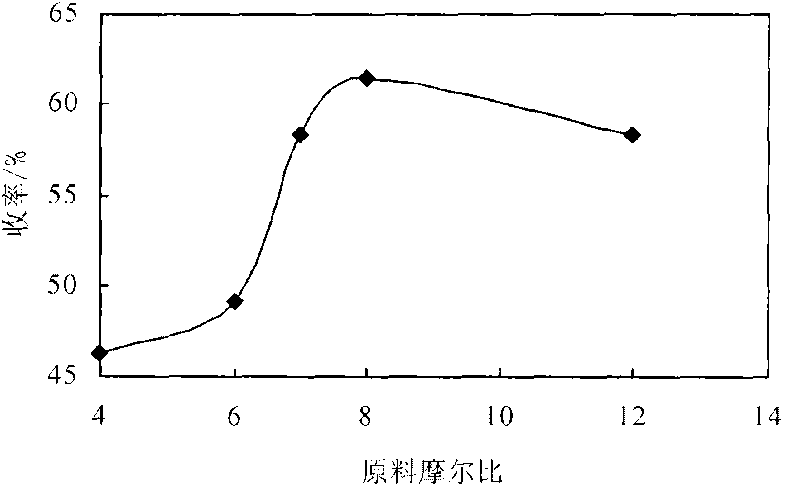
[0014] Embodiment 3: Utilize the 3,5-propionyl chloride synthesized in embodiment 1 and embodiment 2 and 1.0G polyamido-amine, 3,5-propionyl chloride is dissolved in chloroform, prepare 3,5-propionyl chloride Chloroform solution: Accurately weigh 2.0g of 1.0-generation polyamide-amine in a dry 250mL three-necked bottle, dissolve it in 50mL of chloroform, add 3mL of acid-binding agent triethylamine, and put it in a constant temperature magnetic stirring water bath at 15°C , use a constant pressure dropping funnel to add dropwise the chloroform solution of 3,5-propionyl chloride, after the addition, raise the reaction temperature to 30°C, react for 12 hours, and investigate the relationship between 3,5-propionyl chloride and 1.0-generation polyamide-amine Effect of molar ratio on the yield of dendritic antioxidants. The result is as figure 1 shown. Result shows, when 3, the mol ratio of 5-propionyl chloride and 1.0 generation polyamide-amine is 8: 1, the yield of dendritic phe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com