Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Formamidine acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formamidine acetate salt 99% Synonym: Formamidine acetic acid salt CAS Number 3473-63-0. Linear Formula HN=CHNH 2 · CH 3 COOH . Molecular Weight 104.11 . EC Number 222-442-5. MDL number MFCD00012866. PubChem Substance ID 24894786
Preparation method for ibrutinib
ActiveCN105859728AHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateDrugs synthesis
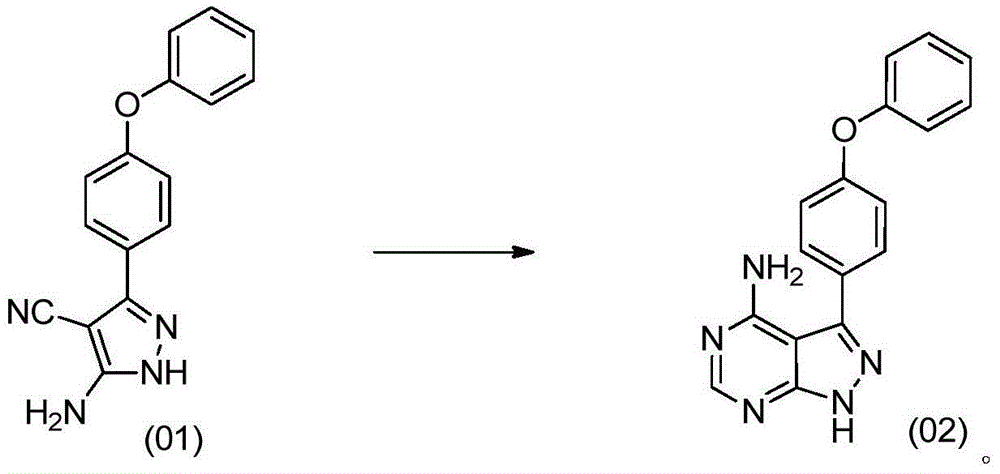
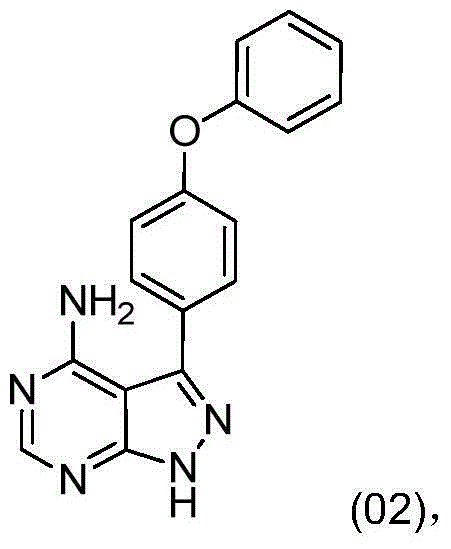
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN104860950AHigh puritySolve the problem of complicated and refined synthesisOrganic chemistryAcetic acidAlcohol
The invention discloses a novel method used for preparing high-purity 4-chloropyrrolo[2,3-d]pyrimidine in industrialization scale. According to the method, ethyl cyanoacetate, bromo-acetal, formamidine acetate, and sodium alcoholate are taken as raw materials; N,N-dimethylformamide, ethyl alcohol, and phosphorus oxychloride are taken as solvents; and 4-chloropyrrolo[2,3-d]pyrimidine is obtained via three-step approach. The process route of the method is simple; time is short; next step feed can be carried out directly without complex purification process of intermediates; no toxic agent is used; the method is safe and reliable, and is low in cost; total yield is higher than 30%; and the purity of obtained products is as high as 99.2%.
Owner:CHONGQING PHARMA RES INST
Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine
InactiveCN105622616AIncrease productivityImprove product qualityOrganic chemistryCyanoacetic acidFormamidine acetate
The invention relates to a preparation method of 4-chloropyrrolo[2,3-d]pyrimidine.The method includes the following steps of obtaining 2-cyano-3-(1,3-dioxolan)ethyl propionate after 2-bromomethyl-1,3-dioxolane and ethyl cyanoacetate which are used as the raw materials react with alkaline matter as the catalyst; conducting cyclization on obtained 2-cyano-3-(1,3-dioxolan)ethyl propionate and formamidine acetate with alkaline matter as the catalyst, and adding hydrochloric acid for hydrolysis cyclization to obtain pyrrolo[2,3-d]pyrimidin-4-ol; making obtained pyrrolo[2,3-d]pyrimidin-4-ol react with phosphorus oxychloride to obtain 4-chloropyrrolo[2,3-d]pyrimidine.The method for preparing 4-chloropyrrolo[2,3-d]pyrimidine is simple in technological process, the requirement for production conditions is low, the product is easy to purify and high in yield, and the production efficiency and product quality of 4-chloropyrrolo[2,3-d]pyrimidine are remarkably improved.
Owner:ABA CHEM SHANGHAI
Novel compound L-4-terazine-phenylalanine, preparation method and application thereof
InactiveCN102627615AGood medicineHigh yieldPeptide preparation methodsOrganic additionFormamidine acetateProtein molecules
The invention discloses a novel amino acid derivative L-4-terazine-phenylalanine (3-(4-(1,2,4,5-tetrazin-3-yl) phenyl)-2-aminopropanoic acid). L-4-cyan-phenylalanine is used as an initial reactant and reacts with formamidine acetate and anhydrous hydrazine under catalysis of sulfur; then the reactants are oxidized by sodium nitrite to generate L-4-1,2,4,5-terazine-phenylalanine. The L-4-1, 2, 4, 5-terazine-phenylalanine is integrated into the biologically active peptide and protein molecules as phenylalanine / tyrosine analogue, and can be applied to on biological orthogonal field based on inverse electronic Diels-Alder reaction as a biomarker; meanwhile, the L-4-1,2,4,5-terazine-phenylalaninethe can be introduced into biologically active peptide as phenylalanine / tyrosine analogue through solid phase polypeptide synthesis method and conduct pharmacological evaluation, so as to improve drug property of certain biologically active peptide.
Owner:LANZHOU UNIVERSITY
Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine
The invention belongs to the technical field of chemical synthesis, and particularly relates to a preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine, which comprises the followingsteps: 1) synthesizing an intermediate I, namely 1-Boc-1-aminopyrrole, by taking tert-Butyl carbazate and 2, 5-dimethoxytetrahydrofuran as raw materials; 2) enabling the intermediate I to react withan isocyanate methanesulfonate to synthesize an intermediate II, namely 1-Boc-1-amino-(9ci)-1H-pyrrole-2-carbonitrile; 3) performing deamination protection on the intermediate II under an acidic condition to obtain an intermediate III, namely 1-amino-(9ci)-1H-pyrrole-2-carbonitrile hydrochloride; 4) performing a ring closing reaction on the intermediate III and formamidine acetate to obtain an intermediate IV, namely 4-aminopyrrolo [2, 1-f] [1, 2, 4] triazine; and 5) reacting the intermediate IV with a bromination reagent to obtain a final product, namely 7-bromopyrrolo [2, 1-f] [1, 2, 4] thiazine-4-amine. The preparation method disclosed by the invention has the advantages of cheap and easily available raw materials, few reaction steps, high yield, mild reaction conditions and capabilityof realizing large-scale production.
Owner:CHENGDU AF BIOCHEM
Room-temperature A-site doping method for APbX3 perovskite quantum dots
InactiveCN108774157AImprove performanceMaterial nanotechnologyAmino preparation from aminesQuantum yieldFormamidine acetate
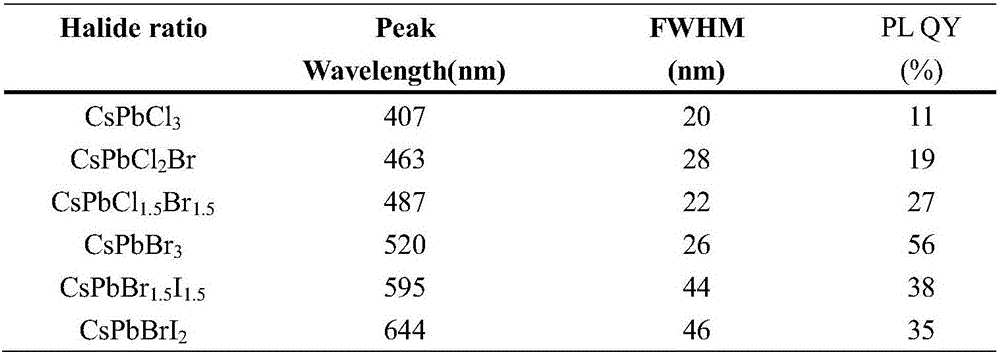
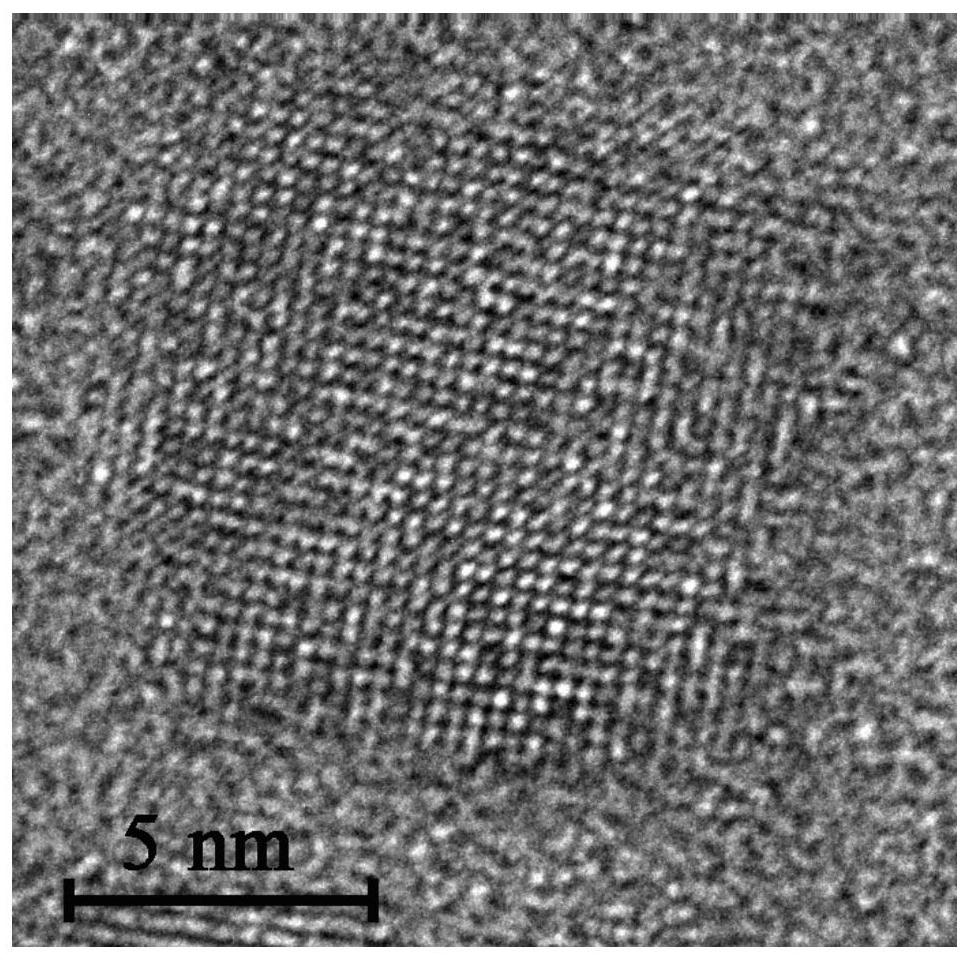
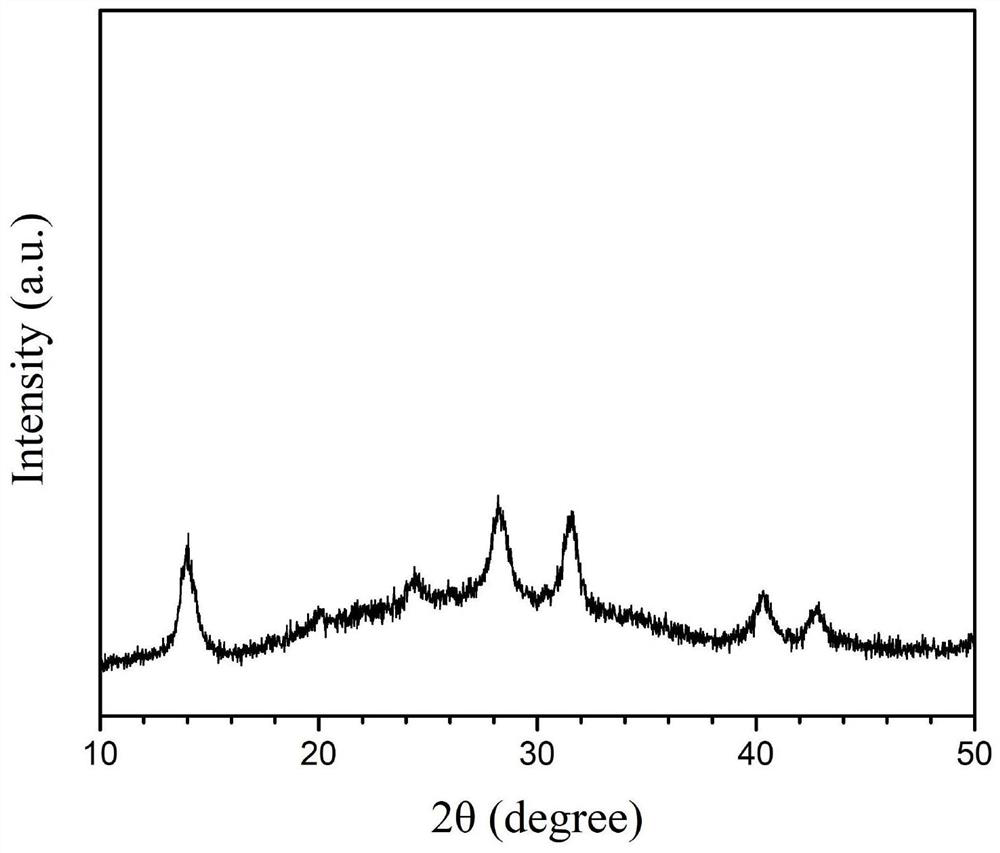
The invention discloses a room-temperature A-site doping method for APbX3 perovskite quantum dots. The method comprises the following steps: 1) dissolving lead bromide and an organic ligand in toluene, performing stirring to dissolve the lead bromide and the organic ligand in order to obtain a lead precursor, and dissolving cesium carbonate, and formamidine acetate, methylamine acetate or guanidine carbonate in a long alkyl chain organic acid to obtain a doped A-site precursor, wherein the doped A-site precursor includes a doped A-site precursor salt; 2) injecting the doped A-site precursor obtained in step 1) into the lead precursor obtained in the step 1), and carrying out a full reaction to obtain a quantum dot stock solution; and 3), centrifuging and purifying the quantum dot stock solution obtained in step 2) to obtain A-site doped APbX3 perovskite quantum dots. The room-temperature A-site doping method for APbX3 perovskite quantum dots improves the performances of perovskite QLEDdevices by doping the A-site Cs matrix of the CsPbX3 with formamidine, methylamine or guanidine. The fluorescence quantum yield PLQY of the doped APbX3 quantum dots reaches 90% or above, and the efficiency (EQE) of the QLEDs of the doped APbX3 quantum dots reaches 10 or more.
Owner:NANJING UNIV OF SCI & TECH
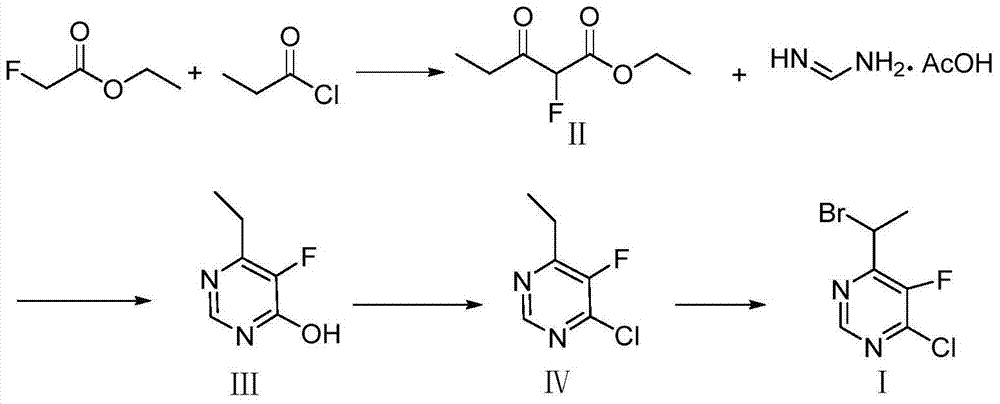
Method for synthesizing 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine
ActiveCN103896855AMild reaction conditionsEasy to operateOrganic chemistryFormamidine acetateSynthesis methods
The invention discloses a method for synthesizing 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine. The synthesis method comprises the steps of reacting 2-fluoro-ethyl acetate which is inexpensive and readily available and is used as an initial material with propionyl chloride under basic conditions in a solvent to synthesize an intermediate product 2-fluoro propionyl ethyl acetate; then carrying out cyclization on 2-fluoro propionyl ethyl acetate and formamidine acetate as well as a base in a solvent to obtain a cyclized product; then chlorinating the cyclized product with a chlorinating reagent; and finally adding a brominating reagent and brominating the chlorinated product in the presence of an initiator to obtain 4-(1-bromoethyl) -5-fluoro-6-chloropyrimidine. The synthesis method disclosed by the invention has the advantages of simple process, available raw materials, high yield, safety and environmental protection, and is convenient to industrialize.
Owner:JUHUA GROUP TECH CENT
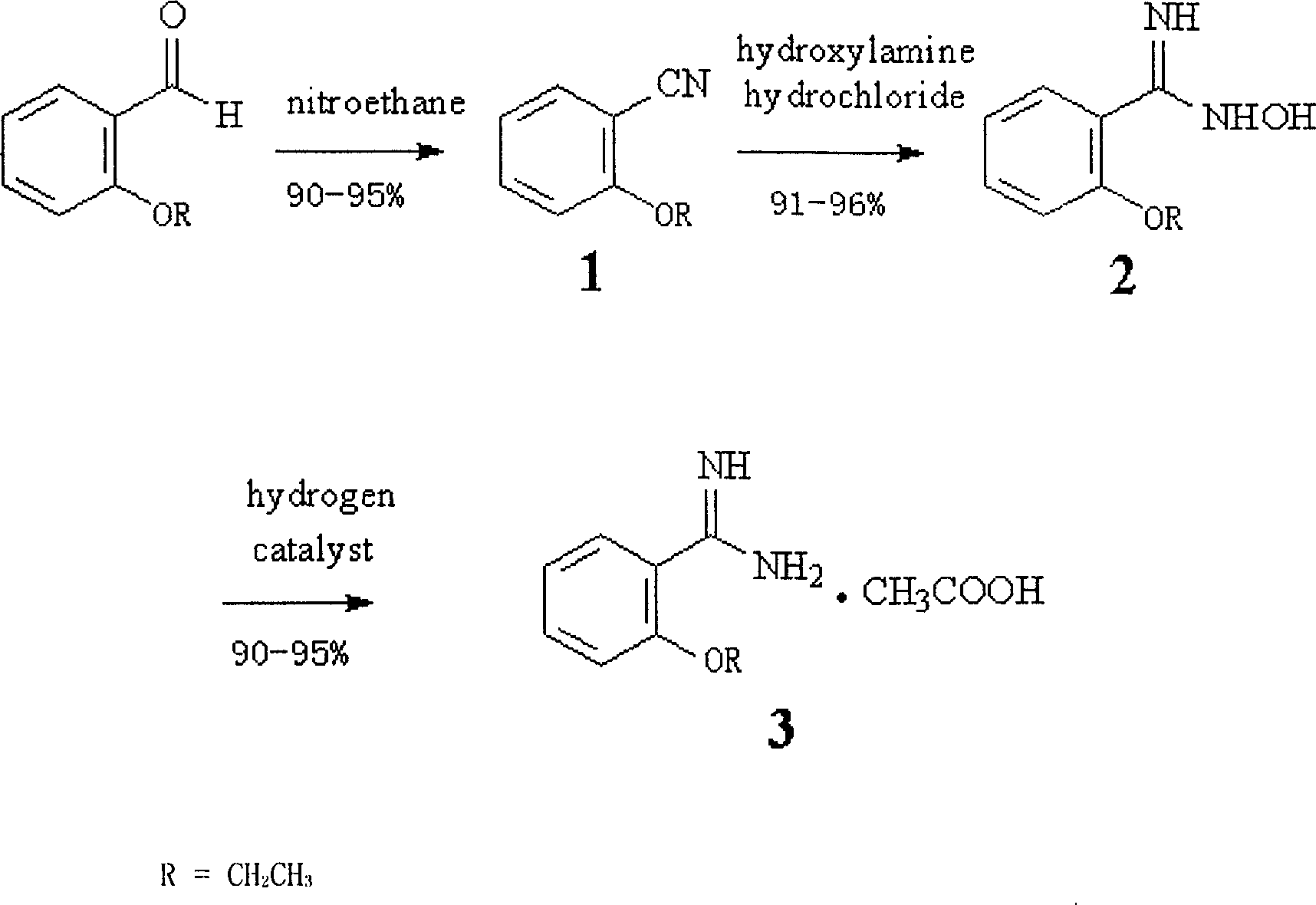
Method for synthesizing O-ethoxy phenyl formamidine acetate
InactiveCN1830955ASimple processLow equipment requirementsOrganic chemistryNitroethaneFormamidine acetate
A process for preparing o-ethoxybenzamidine acetate includes such steps as reflux reaction between o-ethoxy benzaldehyde and nitroethane to obtain o-ethoxy benzonitrile, reflux reacting on hydroxyammonium hydrochloride to obtain o-ethoxy benzamidoxime, and catalytic hydrogenating while stirring.
Owner:SHANDONG NORMAL UNIV
Method for preparing gefitinib intermediate
The invention relates to a method for preparing a gefitinib intermediate, in particular to a method for preparing 4-chlorine-6-(3-chlorine propoxy)-7-methoxy quinazoline. A formula II compound 6-(3-hydroxy propoxy)-7-methoxy quinazoline-4 (3H)-ketone and thionyl chloride are reacted to obtain the gefitinib intermediate. The formula II compound uses 5-hydroxy-4-methoxy group-2-nitrobenzene methyl formate as a raw material to be condensed with halohydrin shown in a formula VI, nitro is reduced, and formamidine acetate cyclization and thionyl chloride chlorination are reacted to obtain the gefitinib intermediate. The synthesis scheme provided by the invention reduces the generation of by-products by introduction of hydroxyl, the production cost is reduced, meanwhile, the environment pollution is reduced, the quality of products is increased, and the purity of the prepared 4-chlorine-6-(3-chlorine propoxy)-7-methoxy quinazoline is over 98 percent. Compared with the prior art, the method is greatly increased and improved, wherein, X is halogen.
Owner:浙江瑞博制药有限公司
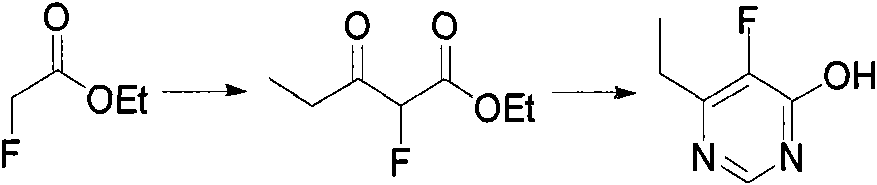
Method for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine and intermediate thereof
InactiveCN102060784AReduce pollutionRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid esters preparationPropionyl chlorideFormamidine acetate
The invention relates to a new concise and efficient method for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine and an intermediate 2-fluoro-3-oxo ethyl valerate thereof. 6-ethyl-5-fluoro-4-hydroxy pyrimidine is an important intermediate for synthesizing a broad-spectrum antifungal drug voriconazole. At present, the methods for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine have the defects of more reaction steps, complex processes, more three wastes and high cost and are not favourable for industrial production. To solve the above problems, the method provided by the invention comprises the following reaction steps (see the drawing 1): using ethyl fluoroacetate and propionyl chloride as raw materials under the reaction temperature to synthesize 2-fluoro-3-oxo ethyl valerate under appropriate alkali action; and using 2-fluoro-3-oxo ethyl valerate and formamidine acetate as raw materials under the reaction temperature to synthesize 6-ethyl-5-fluoro-4-hydroxy pyrimidine under appropriate reaction system. The method provided by the invention is safe, environment-friendly, concise and efficient.
Owner:NANTONG FINC PHARMA CHEM
Synthesis method of 4,6-dichloro-5-fluoropyrimidine compound
ActiveCN101445485ANo pollution in the processShort reaction timeOrganic chemistrySodium methoxideFormamidine acetate
The invention discloses a method for preparing a 4,6-dichloro-5-fluoropyrimidine compound, which comprises the following steps: preparing 4,6-dihydroxy-5-fluoropyrimidine from 2-diethyl fluoropropionate and formamidine acetate in the presence of sodium methoxide; and reacting 4,6-dihydroxy-5-fluoropyrimidine with a solvent and a chlorinating agent in the presence of tertiary amine catalyst to obtain 4,6-dichloro-5-fluoropyrimidine. The invention has the advantages of simple process, low cost, environment friendly, high yield and high purity of the product.
Owner:上海旭东海普南通药业有限公司
Method for preparing 4,6-dihydroxy-pyrimidine from byproduct hydrocyanic acid of acrylonitrile
ActiveCN102442954AReduce unit consumptionReduce generationOrganic chemistryOrganic synthesisFormamidine acetate
The invention relates to a synthetic method of 4,6-dihydroxy-pyrimidin, belonging to the field of organic synthesis. In the synthetic method, fatty alcohol, chlorine hydridem, hydrocyanic acid, ammonium acetate and ammonia gas react first. The synthetic method is characterized in that the generated formamidine acetate and alkoxide and malonic ester undergo a ring-closure reaction to generate the 4,6-dihydroxy-pyrimidi. The byproduct hydrocyanic acid is used an initial raw material to prepare the formamidine acetate; and the formamidine acetate and the malonic ester are synthesized to form the 4,6-dihydroxy-pyrimidin. The synthetic method has the advantages of low unit consumption of the raw materials, high yield, low cost of products, fewer three wastes and less pollution to environment.
Owner:YINGKOU YINGXIN CHEM TECH CO LTD
Preparation method of voriconazole
InactiveCN104744441AEasy to prepareReduce manufacturing costOrganic chemistrySodium acetateFormamidine acetate
The invention relates to a preparation method of voriconazole, wherein the preparation method comprises the following specific steps: adding alpha-fluoropropionyl ethyl acetate and a methanol solution of formamidine acetate into a methanol solution of sodium methylate, carrying out cyclization to obtain 6-ethyl-5-fluoropyrimidine-4(3H)-one, chloridizing to obtain 4-chloro-6-ethyl-5-fluoropyrimidine, dissolving 4-chloro-6-ethyl-5-fluoropyrimidine in tetrahydrofuran, dropwise adding into a tetrahydrofuran solution of lithium diisopropylamide, adding a tetrahydrofuran solution of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole-1-yl)ethyl ketone, carrying out a reaction to obtain 3-(4-chloro-5-fluoropyrimidine-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1-yl)butyl-2-ol, dissolving in ethanol, adding 10% palladium-carbon and sodium acetate, and then splitting to obtain voriconazole. According to the preparation method of voriconazole, the preparation method is simple, and the production cost is low.
Owner:李磊
A kind of preparation method of ibrutinib
ActiveCN105859728BHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateMitsunobu reaction
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
Preparation method of Afatinib
InactiveCN104478863ARaw materials are cheap and easy to getOrganic chemistryPhenylboronic acidFormamidine acetate
The invention discloses a preparation method of Afatinib. According to the method, 4-fluorobenzonitrile is taken as the raw material, and an intermediate 3-[4-(N,N-dimethylamino)-1-oxo-2- butene-1-yl] amino-4-[(S)-(tetrahydrofuran-3-yl) oxy]-6-aminobenzonitrile (IX) is obtained after nitrification, reduction, acylation, nitrification and reduction; the intermediate (IX) and formamidine acetate are subjected to ring closure to generate 4-amino-6-[[4-(N,N-dimethylamino)-1-oxo-2- butene-1-yl] amino]-7-[(S)-tetrahydrofuran-3-yl) oxy]quinazoline (X); the intermediate (X) and 3-chloro-4-phenylboronic acid have a Chan-Lam reaction to generateAfatinib (I). Cheap 4-fluorobenzonitrilewhich is easy to obtain is taken as the starting raw material, a preparation method of the intermediate (X) is not reported, and the preparation method of Afatinib (I) is not reported in literature.
Owner:KANGBOLAI TIANJIN DRUG RES & DEVCO
Preparation method of formamidinium bromide perovskite quantum dots with controllable size
ActiveCN112694418ALow priceEasy to makeMaterial nanotechnologyOrganic chemistryFormamidine acetateFormamidinium
The invention relates to a preparation method of formamidinium bromide perovskite quantum dots with controllable size. The preparation method comprises the following steps: 1) completely dissolving a certain amount of formamidine acetate, lead bromide, hydrogen bromide, oleic acid and oleylamine in dimethylformamide to prepare a perovskite precursor; 2) injecting the precursor and different amounts of hydrogen bromide solutions into toluene to obtain a mixed solution; 3) centrifuging the mixed solution in a centrifuge tube to remove impurities to obtain formamidinium bromide perovskite quantum dots; and 4) dispersing the product in toluene to obtain the formamidinium bromide perovskite quantum dots with controllable size. The method has the advantages that the raw materials are cheap and easy to obtain, required equipment is simple, popularization is easy, and the size of the quantum dots can be adjusted by adding the amount of HBr. The quantum dots are suitable for the technical field of quantum dot material controllable preparation.
Owner:NORTHWEST UNIV
Method for preparing pyrazole derivative
The invention relates to a method for preparing a pyrazole derivative for preparing ibrutinib, and belongs to the technical field of pharmacy. The method comprises the following steps: reacting raw materials with formamidine acetate in an organic solvent at 80-130 DEG C, cooling after the reaction is completed, and collecting solids to obtain a product, wherein the organic solvent is one or more of N,N-dimethyl formamide, N,N-dimethylacetamide, ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, glycol dimethyl ether, n-butyl alcohol, isobutanol, n-amyl alcohol and isoamyl alcohol. The method can be used for solving the problem of long-time high temperature reaction in the prior art, is simple and convenient, is low in cost, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
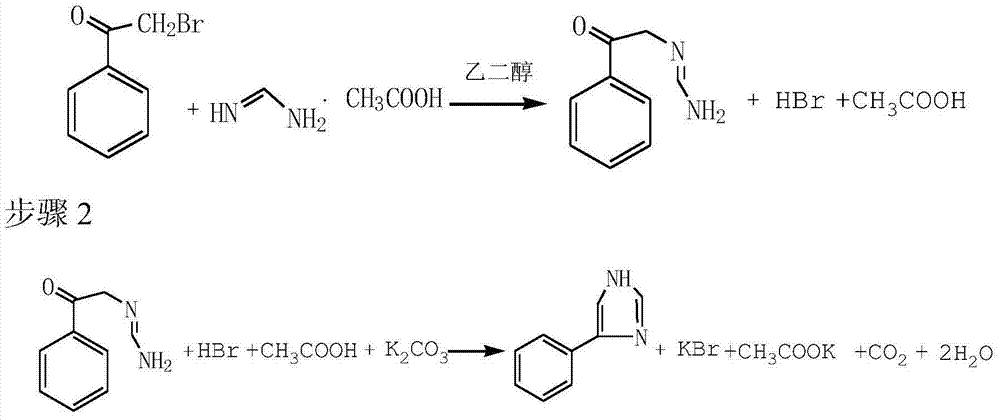
Preparation method of 4-phenylimidazole
The invention discloses a preparation method of 4-phenylimidazole. The method comprises the following steps: 1) substitution, namely, dissolving alpha-bromoacetophenone into formamidine acetate, and reacting in ethylene glycol, so as to obtain a nucleophilic replace intermediate; 2) cyclizing, namely, adding potassium carbonate as an acid-binding agent, and controlling the temperature to carry out a cyclization reaction; and 3) post-treatment, namely obtaining the 4-phenylimidazole by post-treatment after the reaction is ended. According to the technical scheme disclosed by the invention, the 4-phenylimidazole is synthetized from conventional chemical raw materials; the preparation method is simple in process, less in reaction steps, and convenient to purify; an industrial operation is facilitated; the target product is white in color and luster; the content of the final product can be up to over 99%; the yield is greater than 45%.
Owner:ZHANGJIAGANG XINYI CHEM
Method for preparing formamidine acetate
InactiveCN101717351AReduce pollutionThe reaction process is simple to operateOrganic chemistryCarbamateOrthoformic acid
The invention relates to a method for preparing formamidine acetate. The method comprises the following steps of: according to a molar ratio of 1.0:(0.9-1.5):(0.9-1.5):(1.0-2.0):(2.0-5.0) of formonitrile to anhydrous fatty alcohol to anhydrous hydrogen chloride to ammonium acetate to ammonia, generating a hydrochloride of alkyl carbamate by the formonitrile, the anhydrous fatty alcohol and the anhydrous hydrogen chloride; generating ammonium chloride and an acetate of the alkyl carbamate by exchanging with the ammonium acetate without generating orthoformic acid trialkyl ester through alcoholysis; and continuously ammoniating the acetate of the alkyl carbamate to generate the formamidine acetate. In the reaction process of the method, the raw materials do not contain the orthoformic acid trialkyl ester, thus the reaction process has simple operation, low cost, less environmental pollution and high yield.
Owner:YINGKOU YINGXIN CHEM TECH CO LTD
One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine
ActiveCN111533747ASolve pollutionSettlement yieldOrganic chemistryChemical synthesisChlorosulfuric acid
The invention discloses a one-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine, and belongs to the field of organic chemical synthesis. According to the method, a one-pot method is adopted, pyrrole is used as a raw material, chlorosulfonyl isocyanate is used as a cyanation reagent, O-[4-nitro-2-(trifluoromethyl)phenyl] hydroxylamine is used as an amination reagent, formamidine acetateis used as a cyclization reagent, and the pyrrolo[2,1-F][1,2,4]triazin-4-amine with a high yield and high purity is prepared. The method disclosed by the invention is simple to operate, high in product yield, high in purity, mild in reaction condition, less in energy consumption, less in pollution and very suitable for industrial production.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Preparation method of targeted drug AZD3759 intermediate
ActiveCN110590683AThe process steps are simpleEasy to operateOrganic chemistryActivated carbonAcetyl chloride
The invention discloses a preparation method of a targeted drug AZD3759 intermediate, which comprises the following steps of: firstly, 6-nitro veratric acid is hydrolyzed under alkaline conditions toobtain 2-nitro-4-methoxy-5-hydroxybenzoic acid, the 2-nitro-4-methoxy-5-hydroxybenzoic acid is then reduced by hydrazine hydrate under the action of catalyst ferric chloride hexahydrate activated carbon mixture to obtain 2-amino-4-methoxy-5-hydroxybenzoic acid, 2-amino-4-methoxy-5-hydroxybenzoic acid is reacted with formamidine acetate to obtain 4,6-dihydroxy-7-methoxyquinazoline, 4,6-dihydroxy-7-methoxyquinazoline is reacted with acetyl chloride under alkaline conditions to obtain 4-hydroxyl-6-acetoxy-7-methoxyquinazoline, finally 4-hydroxyl-6-acetoxy-7-methoxyquinazoline is reacted with 3-chlorine-2-fluoroaniline by Mitsunobu reaction under the action of triphenylphosphine and azo reagent to obtain 4-[(3-chloro-2-fluorophenyl)amino]-6-acetoxy-7-methoxyquinazoline. The invention reduces the synthesis steps, reduces the use of harmful compounds, reduces the production cost and optimizes the production operation.
Owner:山东四环药业股份有限公司
High-yield halogen perovskite/silica microsphere composite phosphor one-step synthesis method
InactiveCN107523296AImprove luminosityEasy to operateOrganic chemistryLuminescent compositionsIce waterSynthesis methods
The invention provides a high-yield halogen perovskite / silica microsphere composite phosphor one-step synthesis method, comprising the following steps of placing aminated silica microspheres, lead halide or tin halide, cesium carbonate or methylamine bromide or formamidine acetate and octadecene in a flask, and adding a surfactant thereto, wherein the surfactant is oleic acid or a mixture of the same volume of oleylamine and oleic acid, placing the surfactant at 150 to 300 DEG C, and stirring at 1200r / min for 5 to 10 minutes, and then cooling with ice water bath for 30s. The composite phosphor prepared by the method has the advantages of excellent luminous performance, simple operation and large-scale production.
Owner:NANJING UNIV OF SCI & TECH
High-quality amidino perovskite FAPbI3 colloidal quantum dot and preparation method thereof
InactiveCN112159425APrecisely control the proportional relationshipUniform shapeGroup 4/14 organic compounds without C-metal linkagesNanoopticsFormamidine acetateIodide
The invention discloses a high-quality amidino perovskite FAPbI3 colloidal quantum dot and a preparation method thereof. The preparation method comprises the following steps: by taking formamidine acetate, lead acetylacetonate and 9-octadecenyl amine iodide (oleylamine iodine) as three precursors and taking classic oleylamine and oleic acid as surface ligands, firstly, mixing a solution of formamidine acetate, lead acetylacetonate and 1-octadecene and oleic acid, and carrying out heating and stirring under the protection of inert gas to obtain a clear and transparent solution, taking a properamount of oleylamine iodine dissolved in anhydrous toluene, injecting the oleylamine iodine into the clarified solution, rapidly cooling the reaction solution by using an ice-water bath, and finally carrying out centrifugal purification to obtain the high-quality FAPbI3 colloidal quantum dot. The method is simple and easy to implement, short in process, easy to control in operation and suitable for application and popularization, and the obtained nano material is uniform in morphology and has excellent optical characteristics and thermal stability.
Owner:YANGZHOU UNIV
Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine
ActiveCN104086553AFew reaction stepsEasy to operateOrganic chemistryN dimethylformamideN-Butyllithium
The invention discloses a method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine. According to the invention, a compound IX is adopted as a a raw material, and is subjected to a reaction with a compound X under the effect of alkali, such that a compound XI is obtained; the compound XI is subjected to a ring-closing reaction with formamidine acetate, such that a compound XII is obtained; and the compound XII is subjected to a reaction with a bromination reagent, such that the compound 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine is obtained. The compound IX can be obtained through the following reactions: a compound XIII is subjected to a reaction with RCl under an alkali condition, such that a compound XIV is obtained; the compound XIV is subjected to a reaction with n-butyllithium, N,N-dimethylformamide, ammonia water and iodine, such that a compound XV is obtained; and deprotection is carried out under an acidic condition, such that the compound IX is obtained. The preparation method provided by the invention has the advantages of less reaction steps, simple operation, high yield, and mild reaction conditions. A total yield can reach 84.2%. The process route of the method is suitable for large-scale preparations.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine
InactiveCN104311567ASave raw materialsEasy to operateOrganic chemistryOrganic compound preparationSodium acetateFormamidine acetate
The invention discloses a method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine. The method comprises the following steps: using nitroacetophenone as a raw material, reacting the nitroacetophenone and N-bromo-succinimide to generate 2-bromo-1-(4-nitrophenyl)aceton by using para-toluenesulfonic acid as a catalyst, further reacting the 2-bromo-1-(4-nitrophenyl)aceton, sodium acetate and tetrabutylammonium bromide to generate 2-acetoxyl-1-(4-nitrophenyl)aceton, through reducing nitro into amino, reacting the 2-bromo-1-(4-nitrophenyl)aceton and hydrochloric acid, and reducing acetoxyl group into hydroxy to obtain 1-(4-aminophenyl)-2-hydroxy-aceton; then reacting the 1-(4-aminophenyl)-2-hydroxy-aceton, malononitrile and diethylamine to obtain 2-amino-4-(4-aminophenyl)-3-furan nitrile; finally reacting the 2-amino-4-(4-aminophenyl)-3-furan nitrile and formamidine acetate to obtain the 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine. The method has the advantages that the operation is simple and the total recovery is high.
Owner:SHANGHAI INST OF TECH
Process for the preparation of erlotinib
Owner:CERBIOS PHARMA
Chemical modification method for laccase and application of modified laccase
ActiveCN108823177AGuaranteed chemical modification effectAvoid influenceOxidoreductasesCellulose treatment using microorganisms/enzymesFiberCross-link
The invention discloses a chemical modification method for laccase and application of modified laccase. The chemical modification method for laccase comprises the following steps: successively addinga laccase inhibitor, a carboxyl activator and an amino group-containing chemical modifier into an aqueous laccase solution, and carrying out dialysis on a reaction system after a reaction is completedso as to obtain chemically-modified laccase, wherein the amino group-containing chemical modifier is formamidine acetate or L-tryptophan methyl ester hydrochloride, and the carboxyl activator is 2-ethyl-5-phenylisoxazolium-3'-sulfonate. According to the chemical modification method for laccase, the laccase inhibitor is added in the process of a cross-linking reaction so as to protect the active center site of laccase from influence, so the active group, i.e., the carboxyl group, on a laccase chain is protected by forming a peptide bond through the cross-linking reaction of activated carboxylgroups and amino groups, which enables the chemical modification effect of the laccase to be effectively ensured. The chemically-modified laccase prepared in the invention can be widely applied to fiber modification for improvement of the paper making performance of fibers.
Owner:SOUTH CHINA AGRI UNIV
A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine
ActiveCN111533747BImprove pollutionReduce typesOrganic chemistryChemical synthesisChlorosulfuric acid
The invention discloses a one-pot method for preparing pyrrolo[2,1-F][1,2,4]triazine-4-amine, which belongs to the field of organic chemical synthesis. The inventive method adopts a one-pot method, uses pyrrole as a raw material, uses chlorosulfonic acid isocyanate as a cyanation reagent, O-[4-nitro-2-(trifluoromethyl)phenyl] hydroxylamine is an amination reagent, and acetic acid Formamidine is a cyclization reagent, and pyrrolo[2,1-F][1,2,4]triazine-4-amine with high yield and high purity is prepared. The method of the invention has the advantages of simple operation, high product yield, high purity, mild reaction conditions, less energy consumption and less pollution, and is very suitable for industrialized production.
Owner:SHANGHAI LINKCHEM TECH CO LTD
Method for preparing 4,6-dihydroxy-pyrimidine from byproduct hydrocyanic acid of acrylonitrile
ActiveCN102442954BReduce unit consumptionReduce generationOrganic chemistryOrganic synthesisFormamidine acetate
The invention relates to a synthetic method of 4,6-dihydroxy-pyrimidin, belonging to the field of organic synthesis. In the synthetic method, fatty alcohol, chlorine hydridem, hydrocyanic acid, ammonium acetate and ammonia gas react first. The synthetic method is characterized in that the generated formamidine acetate and alkoxide and malonic ester undergo a ring-closure reaction to generate the 4,6-dihydroxy-pyrimidi. The byproduct hydrocyanic acid is used an initial raw material to prepare the formamidine acetate; and the formamidine acetate and the malonic ester are synthesized to form the 4,6-dihydroxy-pyrimidin. The synthetic method has the advantages of low unit consumption of the raw materials, high yield, low cost of products, fewer three wastes and less pollution to environment.
Owner:YINGKOU YINGXIN CHEM TECH CO LTD
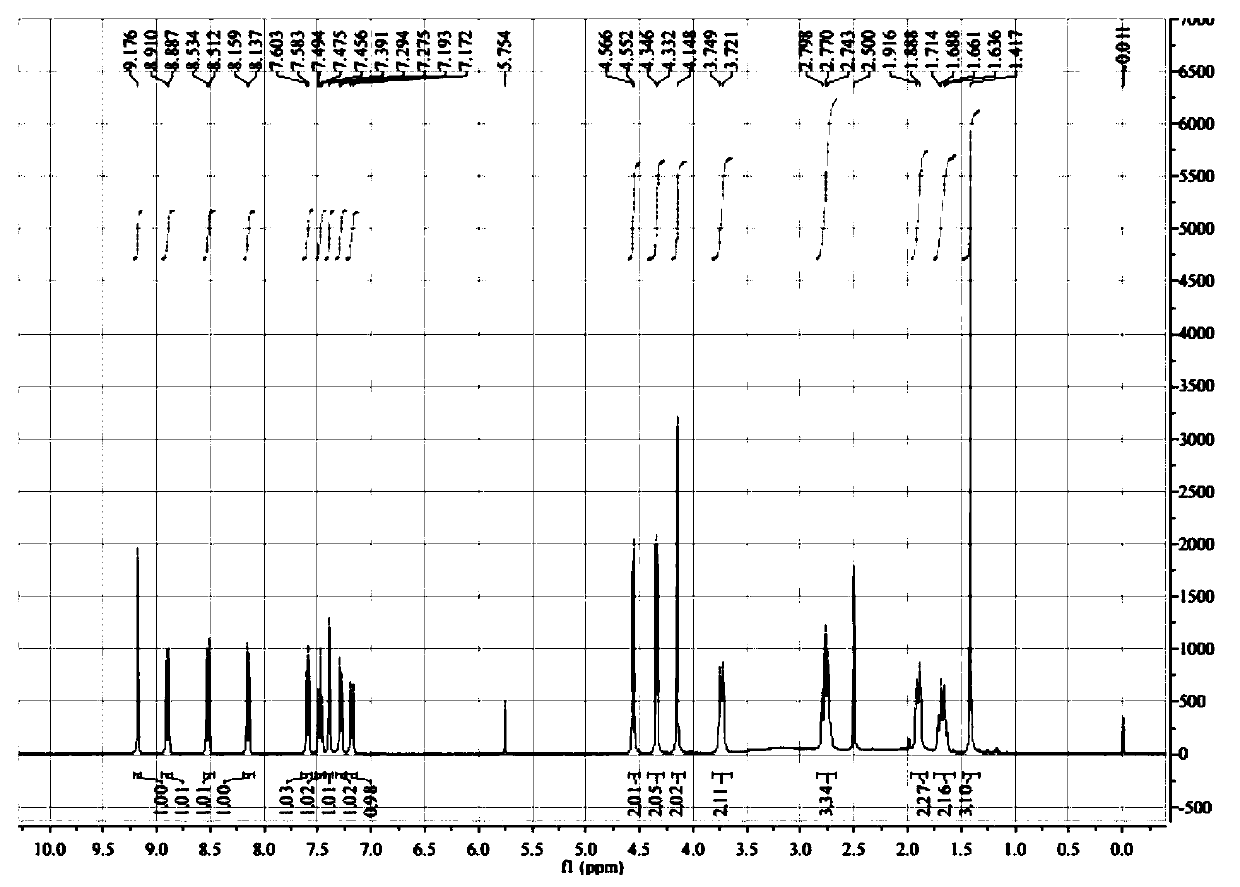
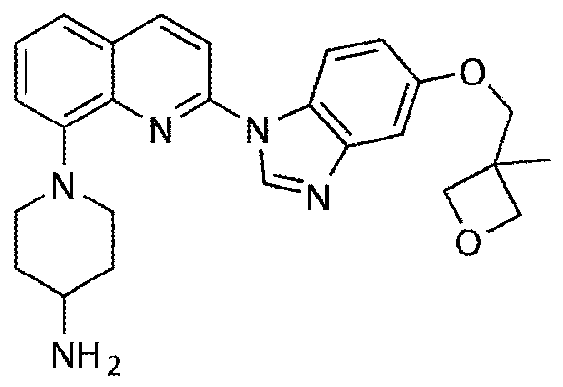
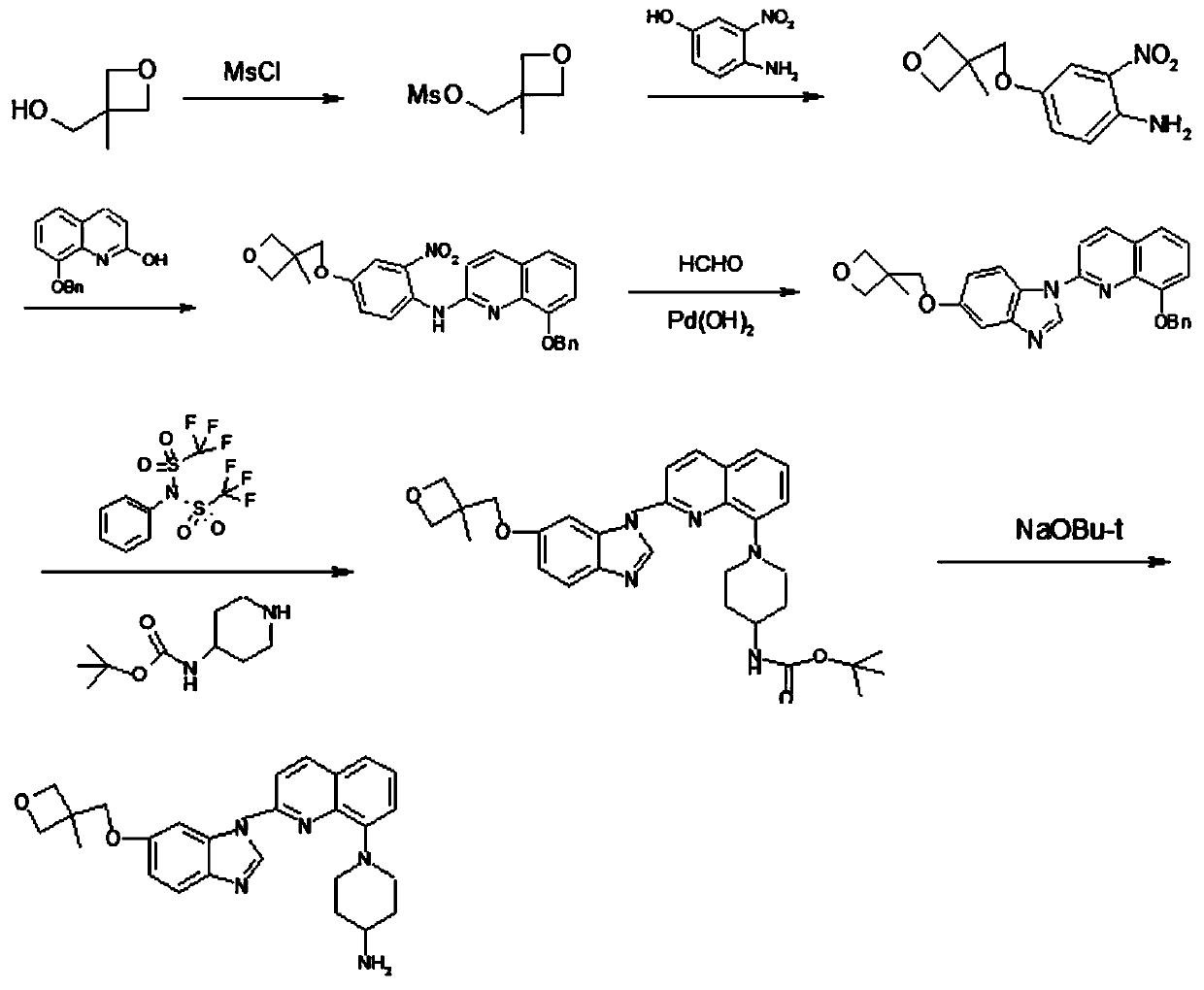
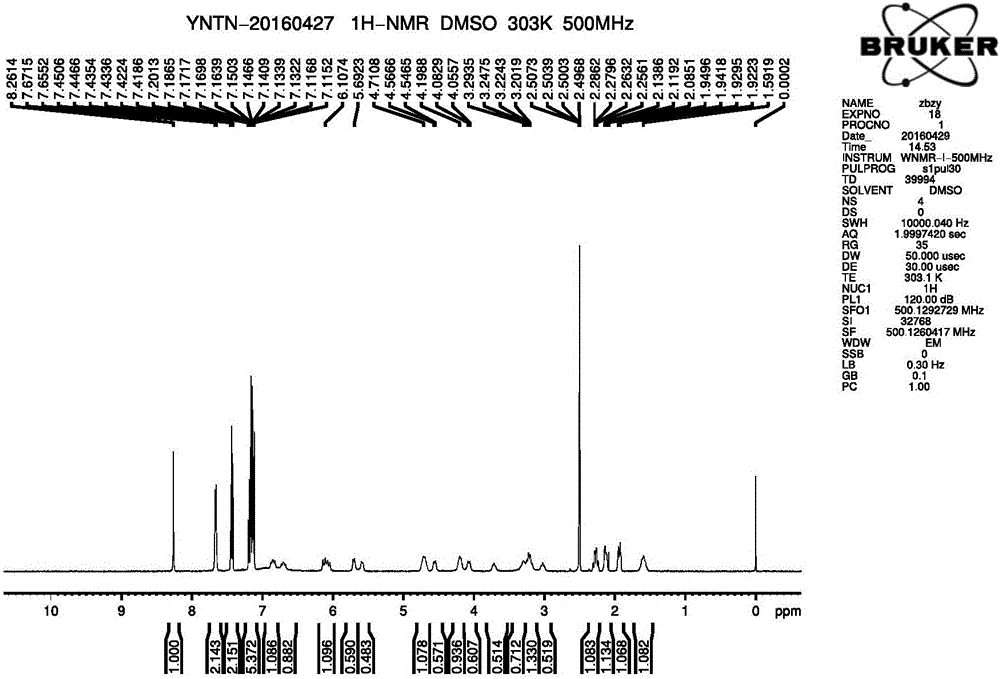
A kind of synthesis method of medicine for treating leukemia
The invention relates to a synthesis method of a drug for treating leukemia. The synthesis method comprises that 1, a compound 3-methyl-3-oxetanemethanol and sodium metal undergo a reaction to produce 3-sodium methoxide-3-methyl-3-oxetane, 2, 4-amino-3-nitrochlorobenzene is added into the product obtained by the step 1 so that reddish-orange solids (A1) are obtained, a compound 8-benzyloxyquinolin-2-chlorine and A1 undergo a reaction to produce orange solids A2, 3, the compounds A2, 13g of palladium / carbon and formic acid undergo a reaction, formamidine acetate is added into the reaction product, and the mixture is subjected to reflux and drying so that white-like (or yellow) solids A3 are obtained, and 4, the compounds A3 and benzenesulfonyl chloride undergo a reaction, the product is dried to form maize-yellow solids A4, BINAP, A4, tert-butyl piperidin-4-yl-carbamate and potassium carbonate undergo a reaction, the reaction product is dried to form A5, and the A5 is refined through the step 5 so that a pure product is obtained. In the steps 1 and 2, a one-pot method is used.
Owner:扬州市三药制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine Method used for preparing 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/314a8975-9605-4db5-b49d-381a5f6f289a/2014100615097100002DEST_PATH_IMAGE001.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000011.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000012.PNG)
![Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine Preparation method of 4-chloropyrrolo[2,3-d]pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cee64228-9d48-4822-950c-dfcfcacc3615/BDA0000929222940000021.PNG)



![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000021.png)
![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000031.png)
![Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine Preparation method of 7-bromopyrrolo [2, 1-f][1, 2, 4] thiazine-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/686d6434-d716-4732-89d1-53ce0fc7652e/BDA0002287786710000041.png)










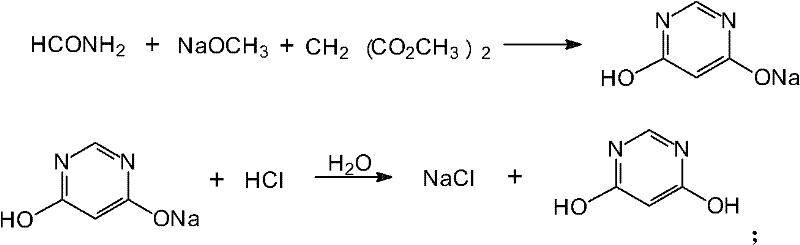
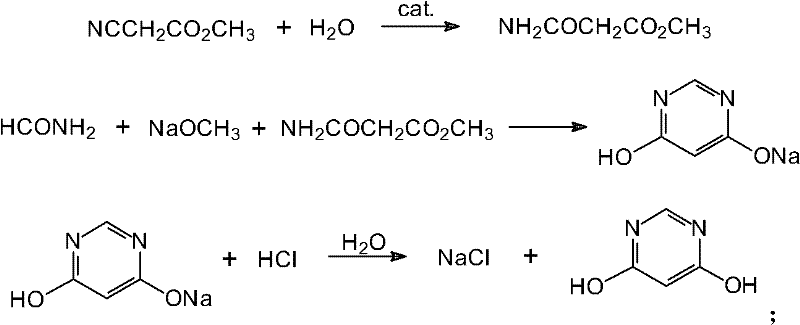







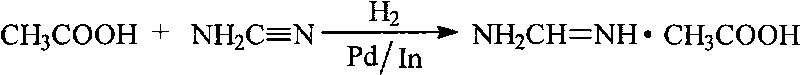
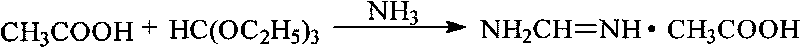

![One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b70eddf8-79b0-4927-b81e-dcc9747cc695/HDA0002511275080000011.png)
![One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b70eddf8-79b0-4927-b81e-dcc9747cc695/HDA0002511275080000021.png)
![One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine One-pot method for preparing pyrrolo[2,1-F][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b70eddf8-79b0-4927-b81e-dcc9747cc695/BDA0002511275070000011.png)



![Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ae0ce247-20ec-4003-9563-847d412ef469/BDA0000520492180000011.PNG)
![Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ae0ce247-20ec-4003-9563-847d412ef469/BDA0000520492180000021.PNG)
![Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine Method for preparing 7-bromoimidazo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ae0ce247-20ec-4003-9563-847d412ef469/BDA0000520492180000022.PNG)
![Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b373d252-044a-4b4d-adef-c9229574fa23/DEST_PATH_IMAGE002A.PNG)
![Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b373d252-044a-4b4d-adef-c9229574fa23/DEST_PATH_IMAGE004A.PNG)
![Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine Method for preparing 4-amino-3-(4-aminobenzene)furo[2,3-d] pyrimidine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b373d252-044a-4b4d-adef-c9229574fa23/DEST_PATH_IMAGE006A.PNG)



![A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0fc3c13f-d82e-46f4-abd1-f60f21b69746/HDA0002511275080000011.png)
![A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0fc3c13f-d82e-46f4-abd1-f60f21b69746/HDA0002511275080000021.png)
![A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine A kind of one pot method prepares the method for pyrrolo[2,1-f][1,2,4]triazin-4-amine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0fc3c13f-d82e-46f4-abd1-f60f21b69746/BDA0002511275070000011.png)