Method for preparing pyrazole derivative
A kind of technology of compound and organic solvent, applied in the field of preparation of pyrazole derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0025] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0026] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0027] In the present invention, g means gram, and mL means milliliter.
Embodiment 1
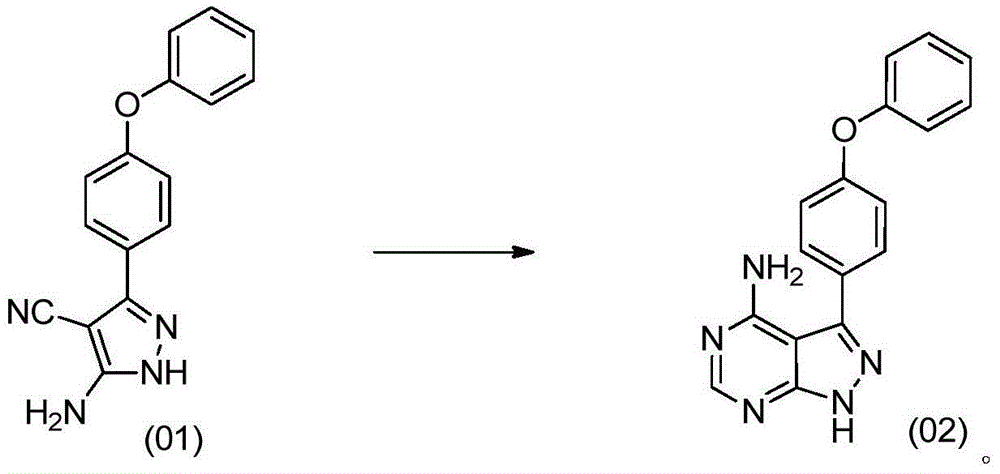
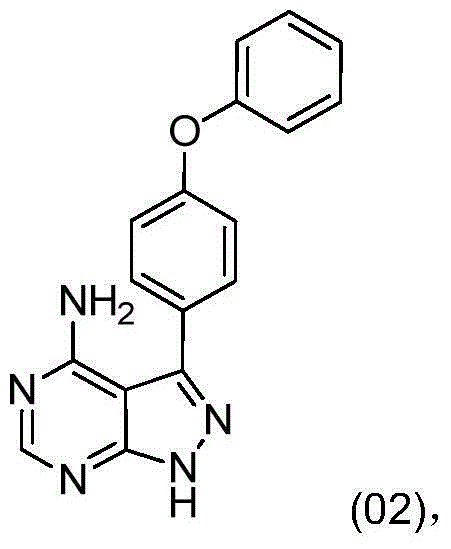
[0029] Into the reactor, add 10.00 g of 3-amino-5-(4-phenoxyphenyl)-4-cyano-1H-pyrazole, 7.61 g of formamidine acetate and 100 mL of n-butanol, and stir evenly at room temperature. Heat up to 110°C and react at 110°C for 15 hours. Then the reaction mixture was cooled to 20°C-30°C, filtered, and the filter cake was rinsed with 20mL of n-butanol, and the obtained solid was vacuum-dried to dryness at 60°C to obtain 10.21g of solid, which was confirmed by mass spectrometry and nuclear magnetic spectrum. -(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, purity: 99.43%.
[0030] Mass spectrum MS: 304.90;
[0031] NMR 1 H NMR (600MHz, DMSO) δ13.55(s, 1H), 8.22(s, 1H), 7.67(d, J=8.5Hz, 2H), 7.44(t, J=7.8Hz, 2H), 7.16(ddd , J=21.5, 13.8, 7.7Hz, 5H).
Embodiment 2
[0033] Into the reactor, add 10.00 g of 3-amino-5-(4-phenoxyphenyl)-4-cyano-1H-pyrazole, 6.74 g of formamidine acetate and 80 mL of n-butanol, and stir evenly at room temperature. Heat up to 100°C-110°C, and react at 100°C-110°C for 16 hours. Then the reaction mixture was cooled to 10°C-20°C, filtered, and the filter cake was rinsed with 20mL of n-butanol, and the obtained solid was vacuum-dried to dryness at 70°C to obtain 10.28g of solid, purity: 99.21%, confirmed by mass spectrometry as 3 -(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com