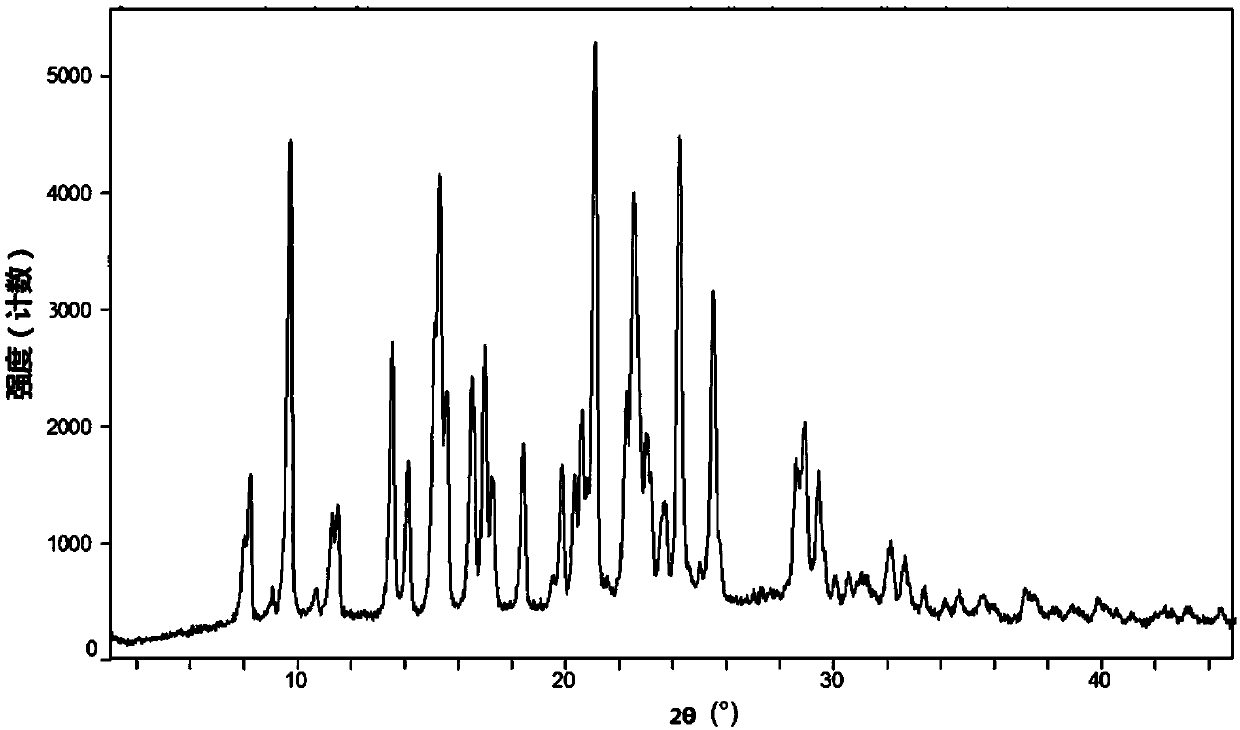
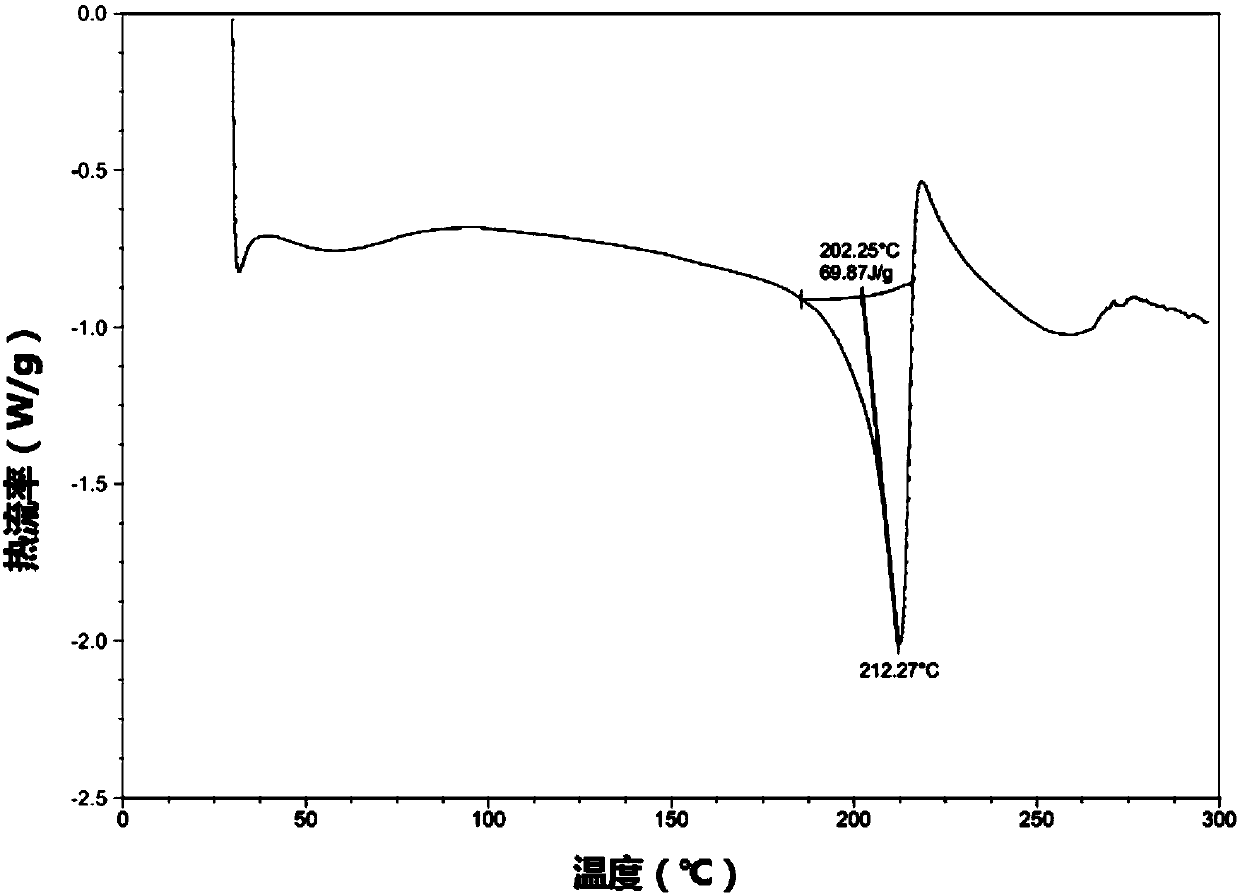
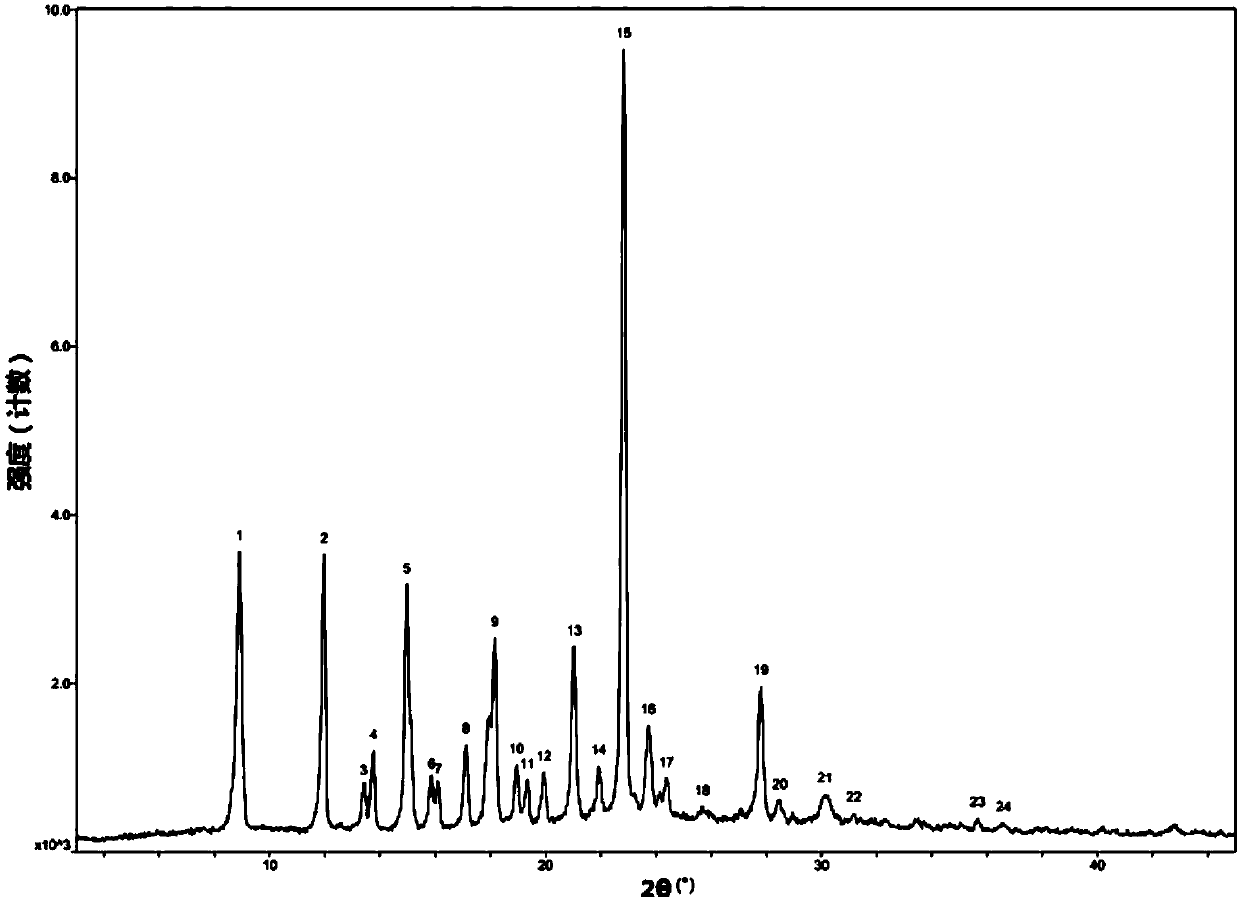
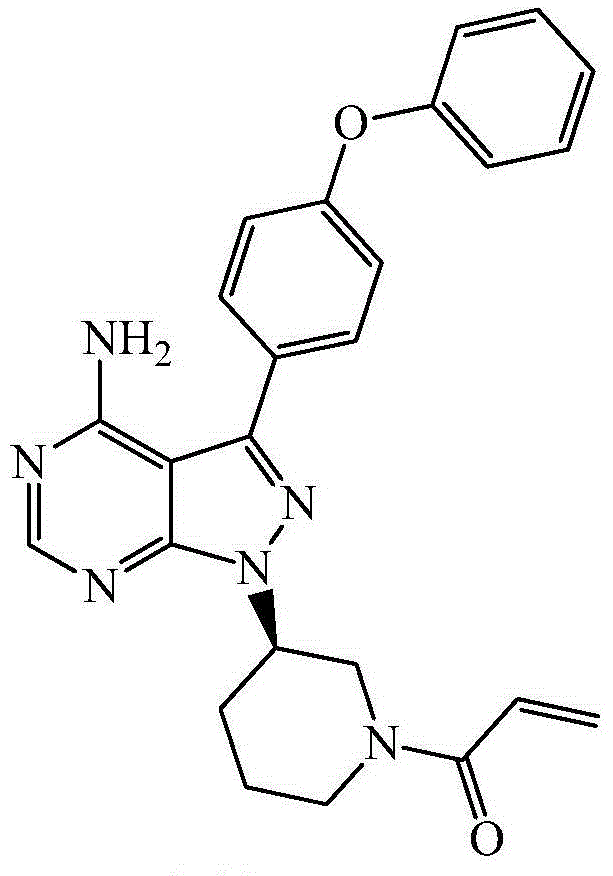
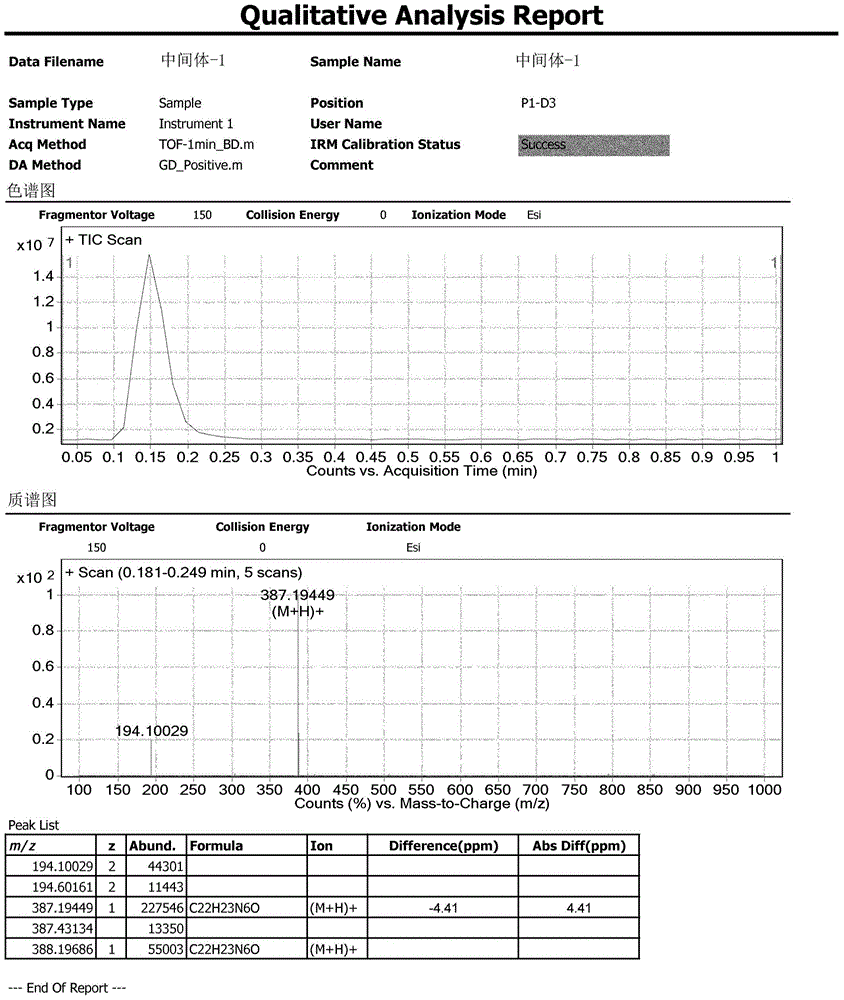
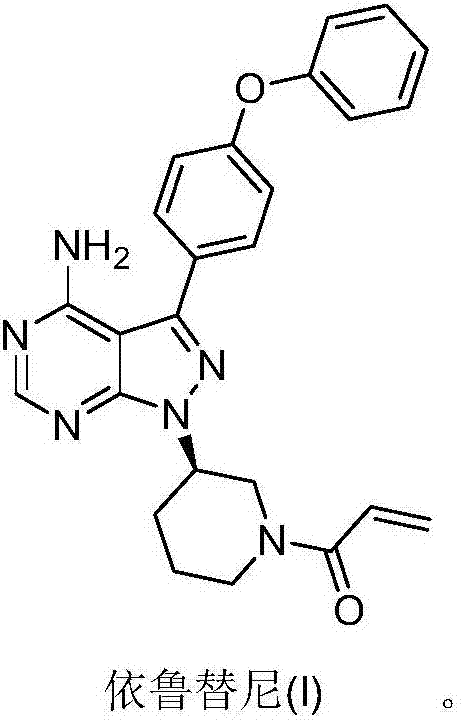
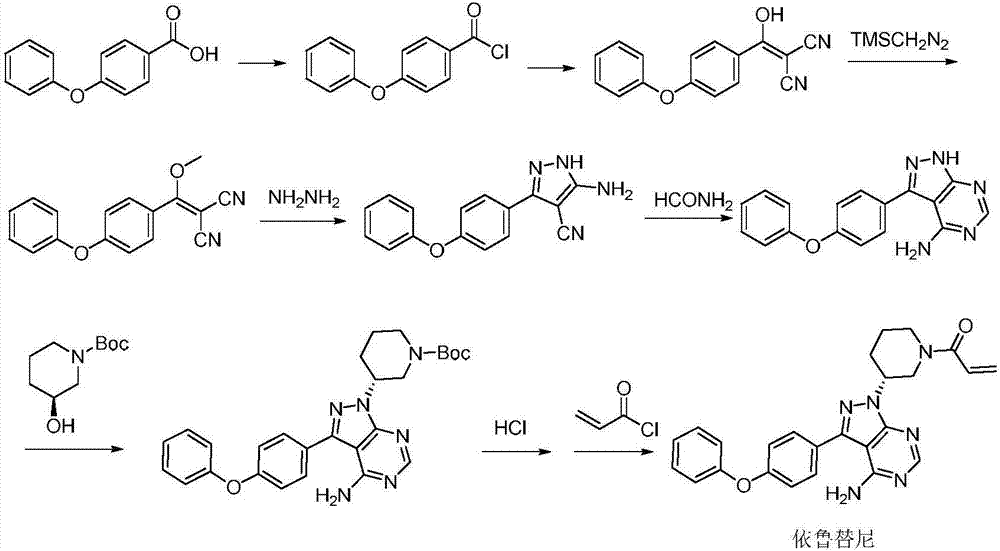
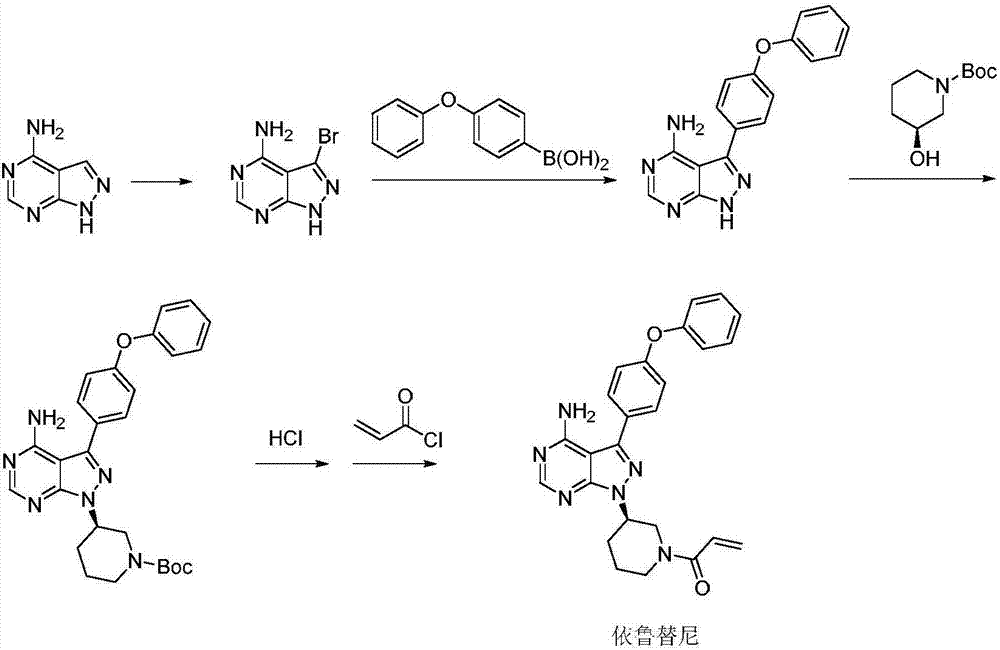
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Ibrutinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
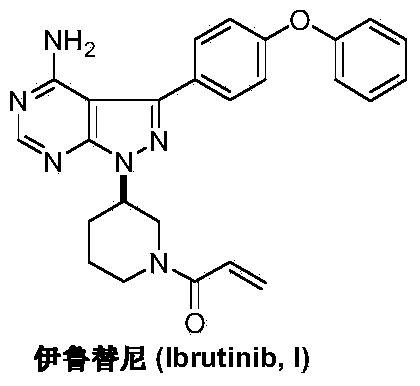
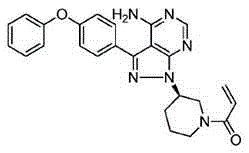
This medication is used to treat certain cancers (such as mantle cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, Waldenstrom's macroglobulinemia).
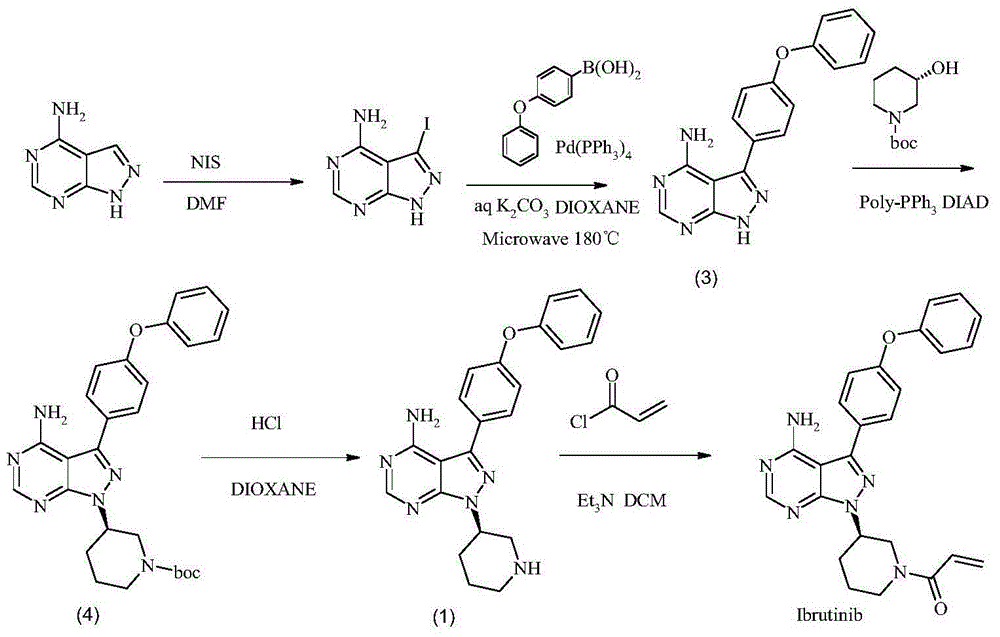
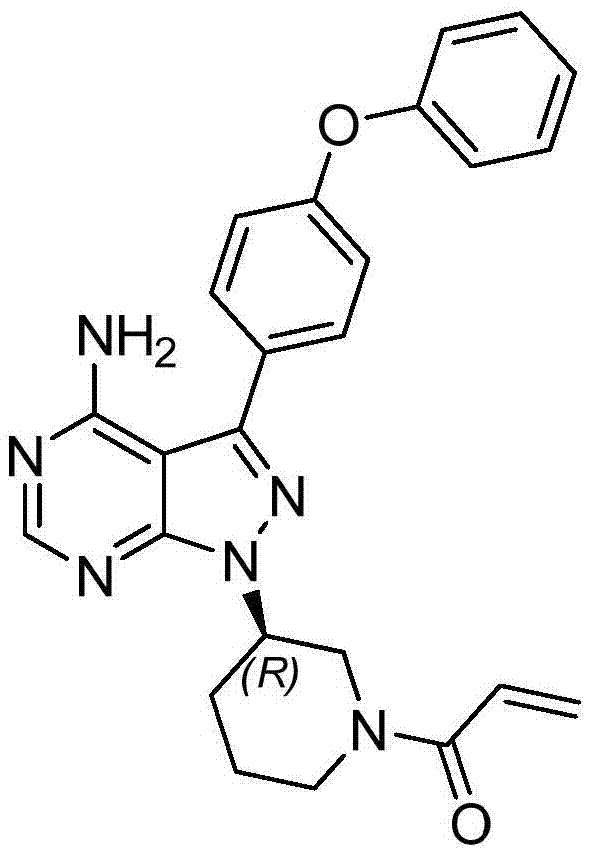
Preparation method of Ibrutinib
ActiveCN103626774AEase of industrial productionRaw materials are easy to getOrganic chemistryCyanideBenzoyl chloride
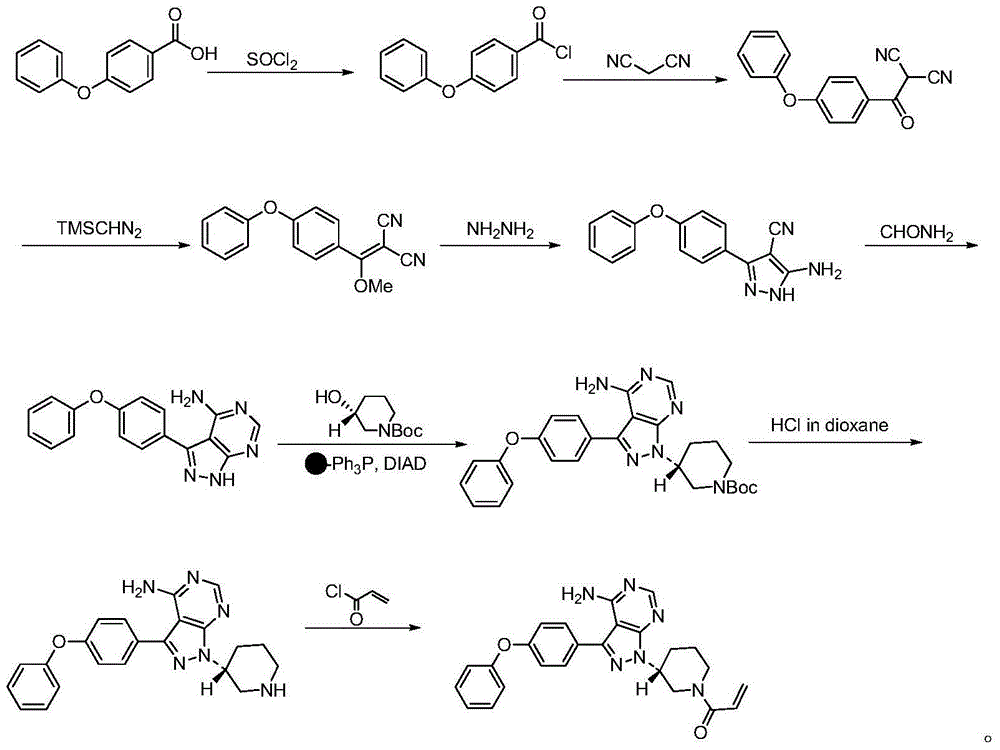
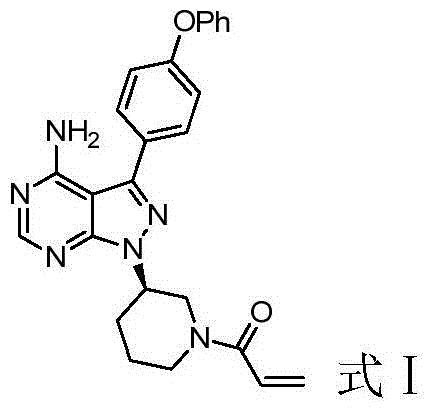
The invention discloses a preparation method of Ibrutinib (I). The preparation method comprises the following steps: performing condensation and methyl oxidization reaction on 4-phenoxyl benzoyl chloride (II) serving as a raw material and methylene cyanide and dimethyl sulfate to generate 4-phenoxyl phenyl (methoxyl) ethenylidene dicyan methane (III); performing pyrazol cyclization reaction between the intermediate (III) and 1-(3R-diazanyl-1-piperidyl)-2-propylene-1-ketone(IV) to obtain 1-[(3R)-[3-(4-phenoxyl phenyl)-4-nitrile-5-amino-1H-pyrazolyl]-1-piperidyl]-2-propylene-1-ketone(V). The pyrimidine cyclization reaction between the intermediate (V) and a cyclization agent is performed to prepare the Ibrutinib (I). The preparation method has easily-available raw materials, and is simple in process, economical and environment-friendly, and suitable for industrial production.
Owner:TONGLING WANGYANTANG BIOTECHNOLOGY CO LTD
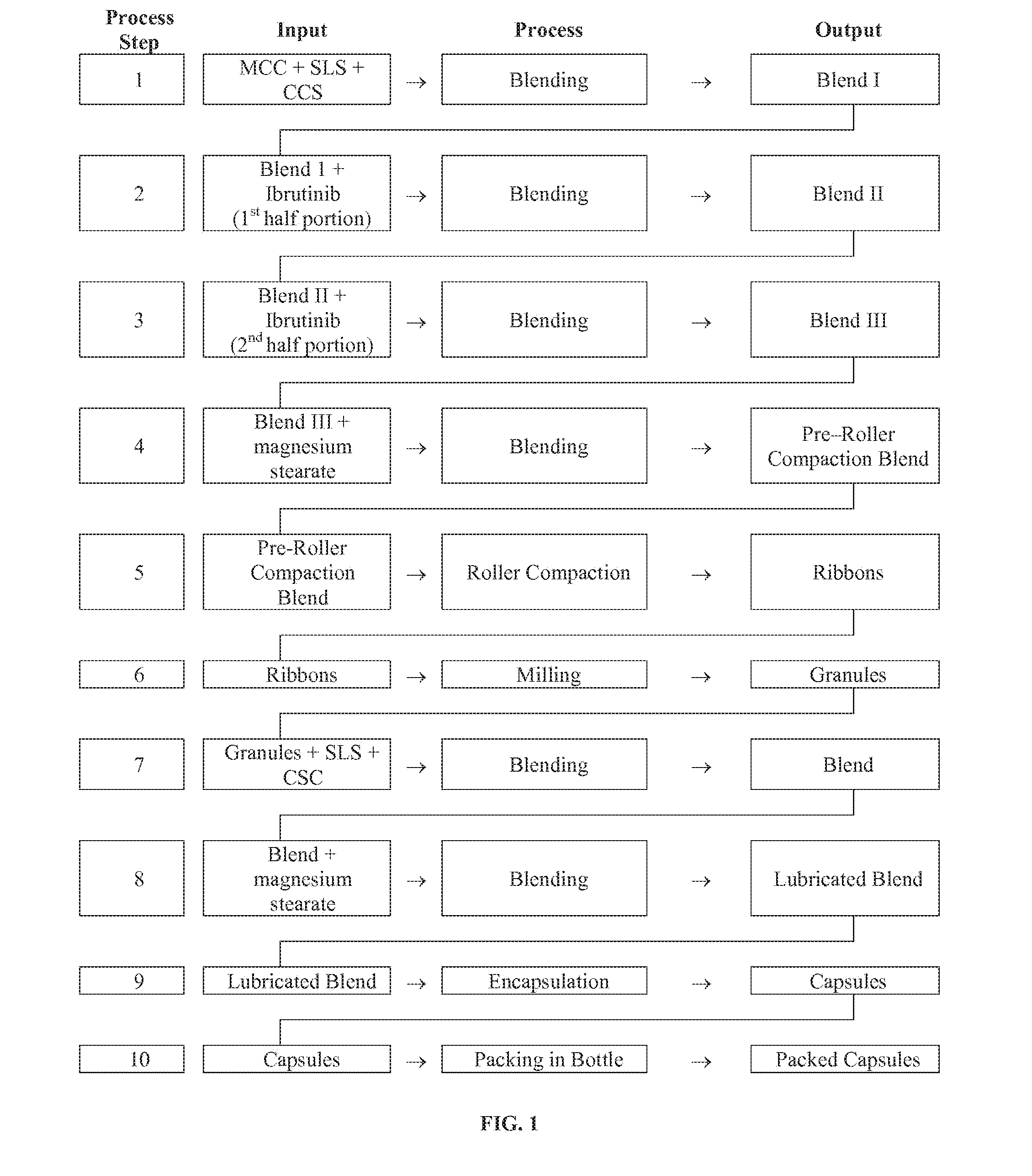
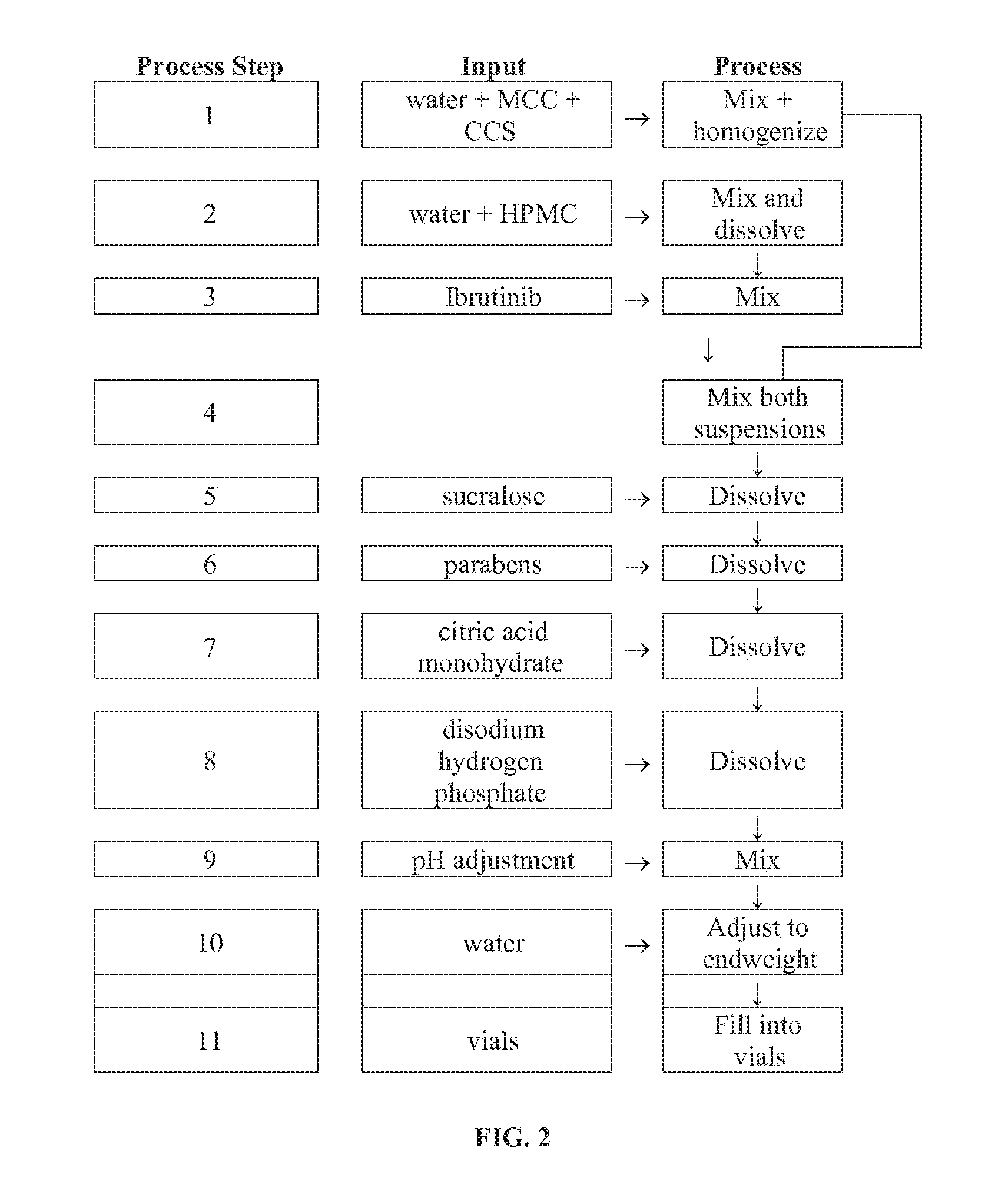
Compositions Containing Ibrutinib
InactiveUS20160287594A1Easy to sprinkleOrganic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
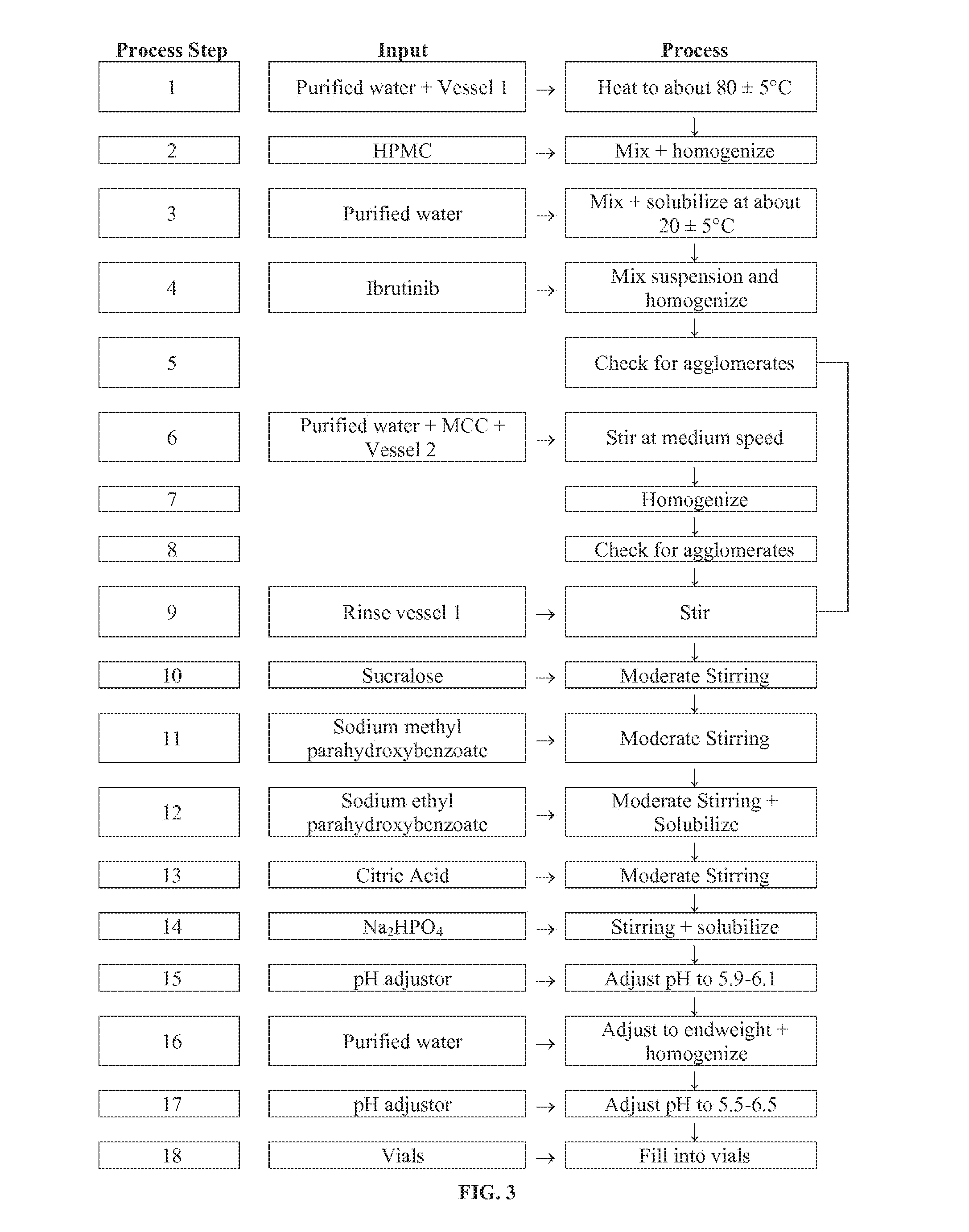
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
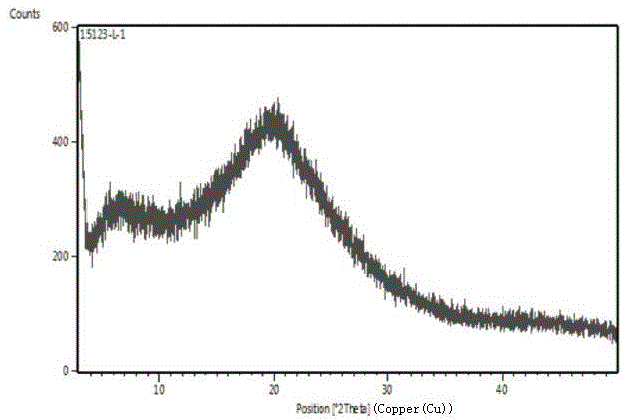
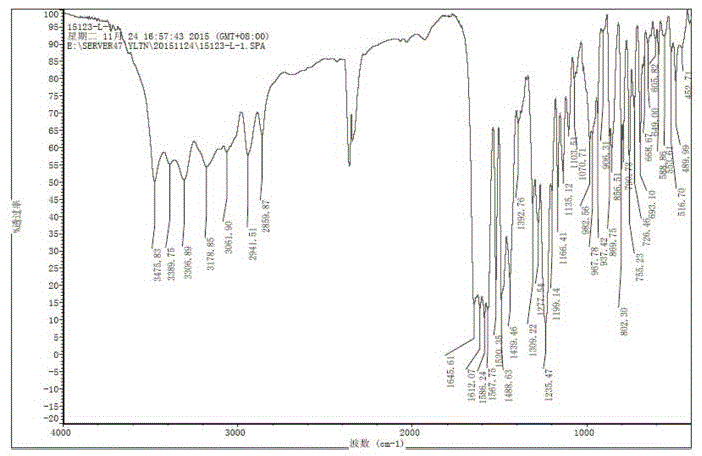
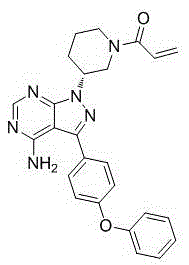
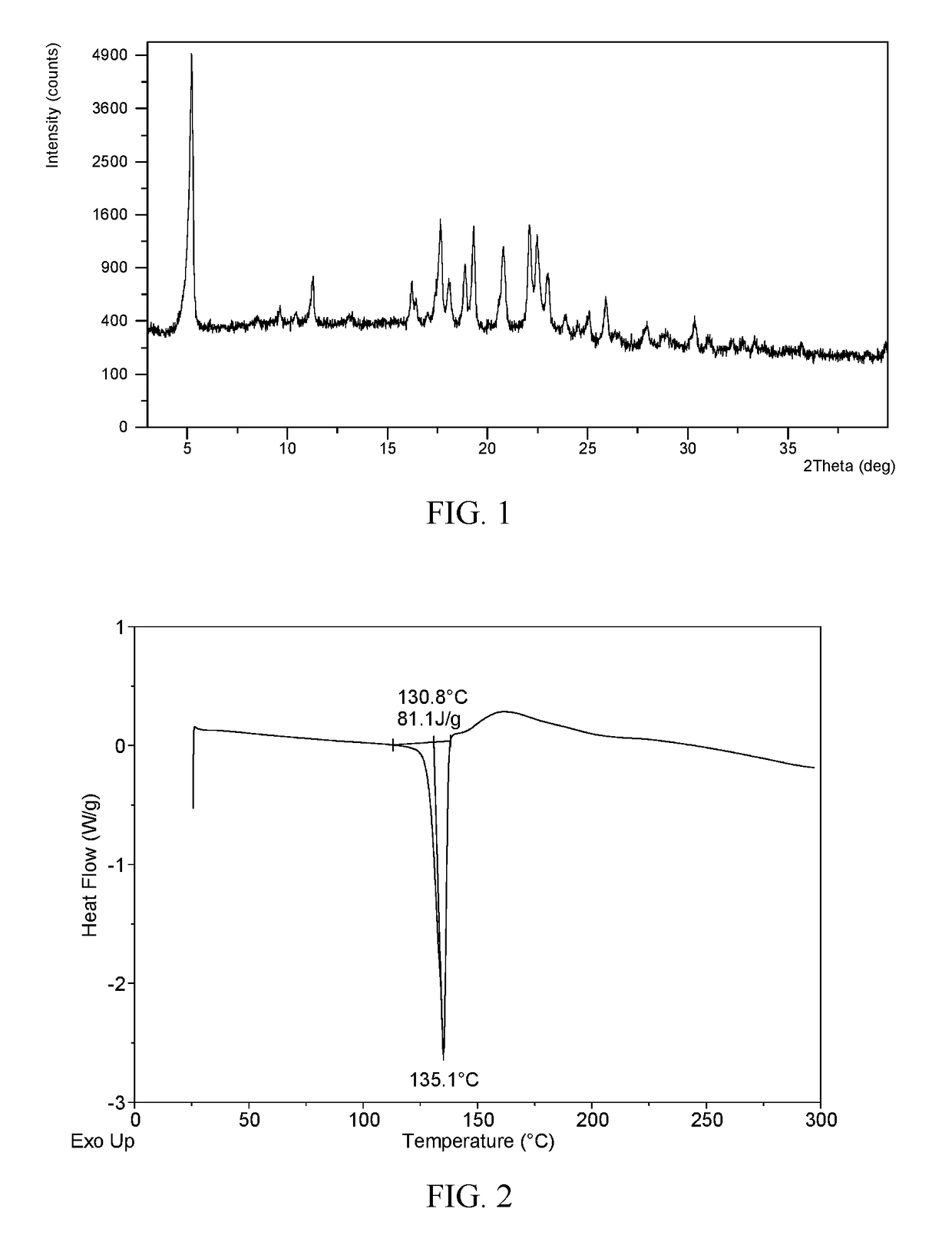
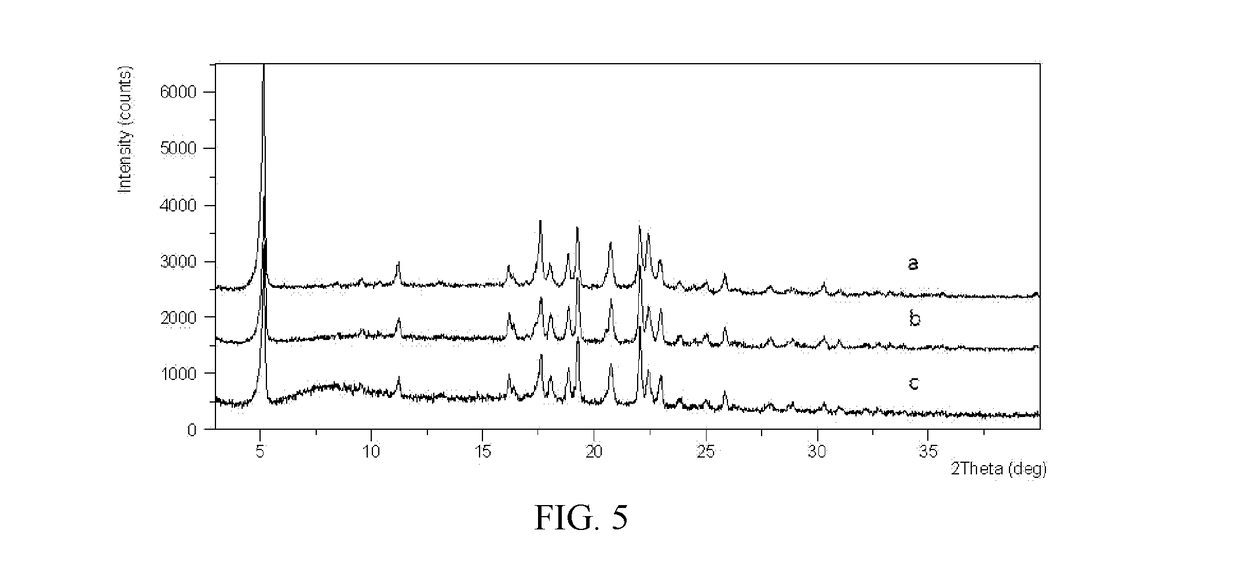
Novel crystal forms of ibrutinib and preparation method thereof
InactiveCN105294696AImprove thermal stabilityOrganic active ingredientsOrganic chemistry methodsKetoneCombinatorial chemistry
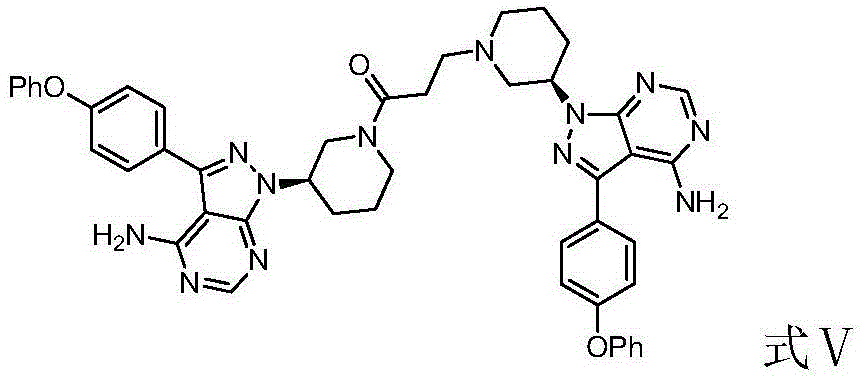
The invention provides two crystal forms of ibrutinib, a preparation method and applications thereof. Specifically, the invention provides crystal forms of 1-[(3R)-3-[4-amino-3-(4-phenoxylphenyl)-1H-pyrazolo[3,4-d]pyrimidine-1-yl]-1-piperidyl]-2-propylene-1-one, a preparation method and applications thereof.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +1
Novel crystal form of ibrutinib and preparation method thereof
InactiveCN105085529AOrganic active ingredientsOrganic chemistryCombinatorial chemistryPharmaceutical technology
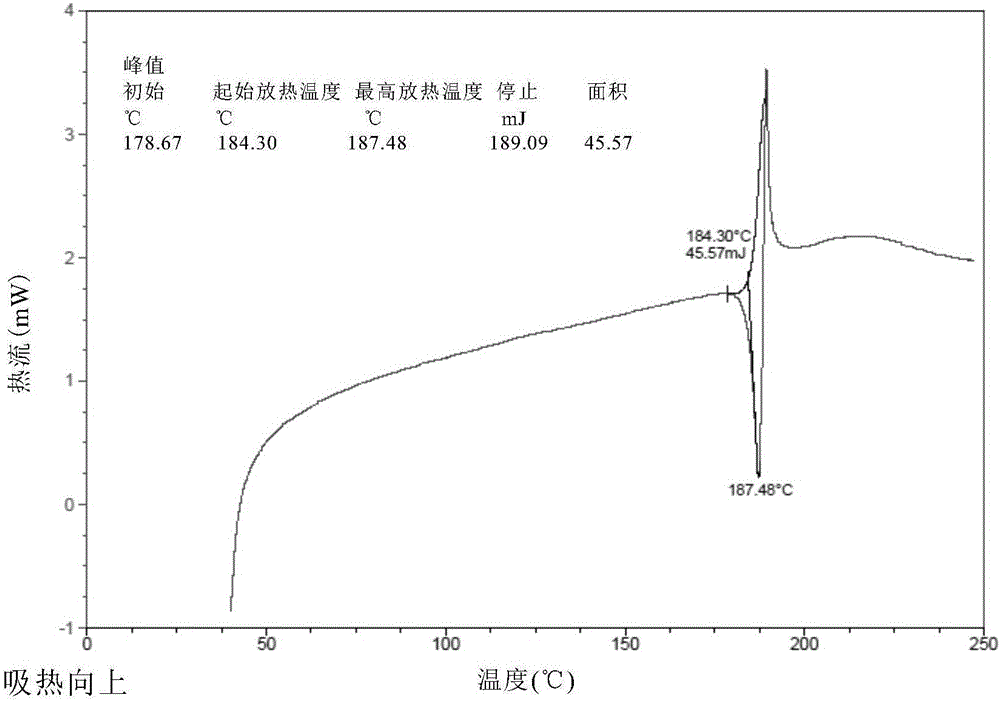
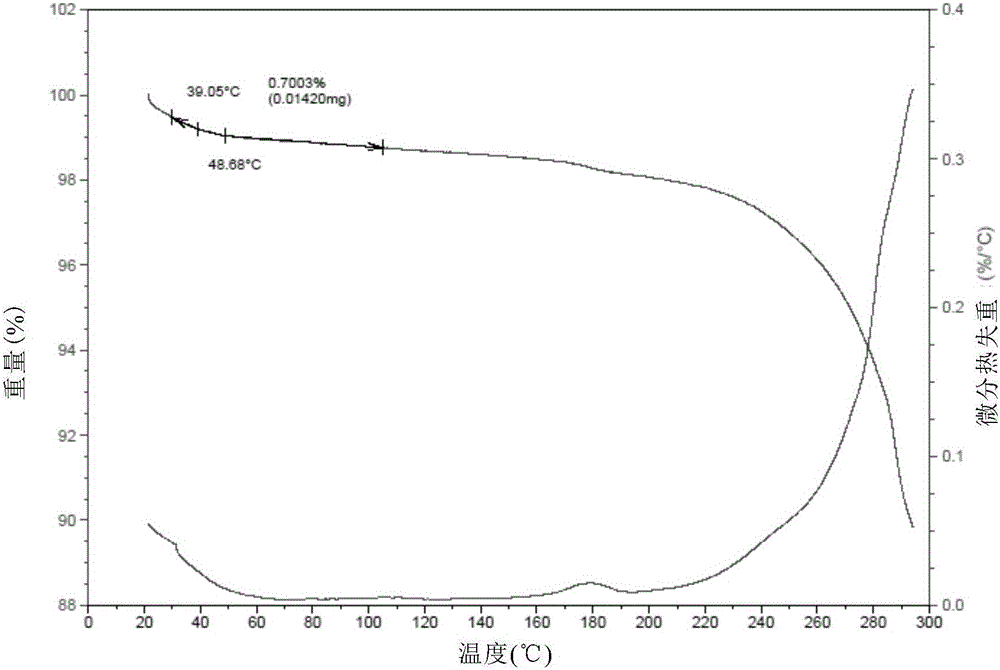
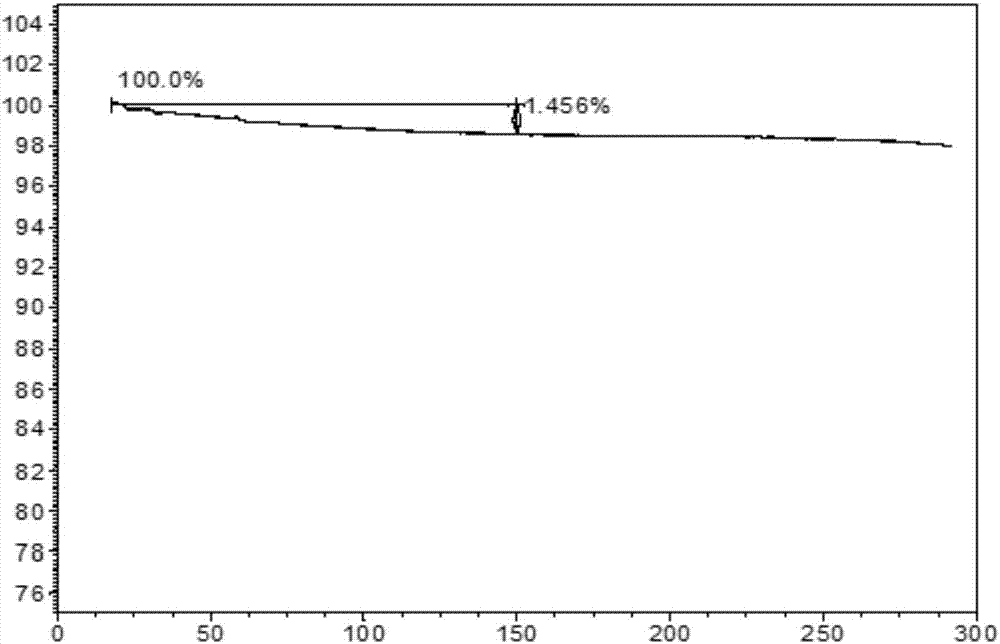
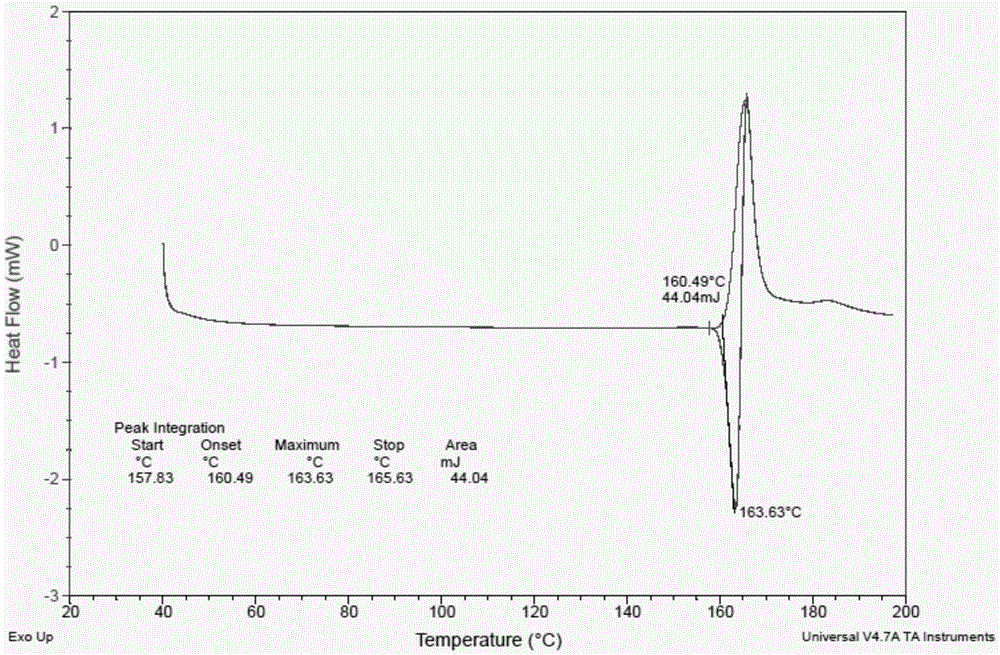
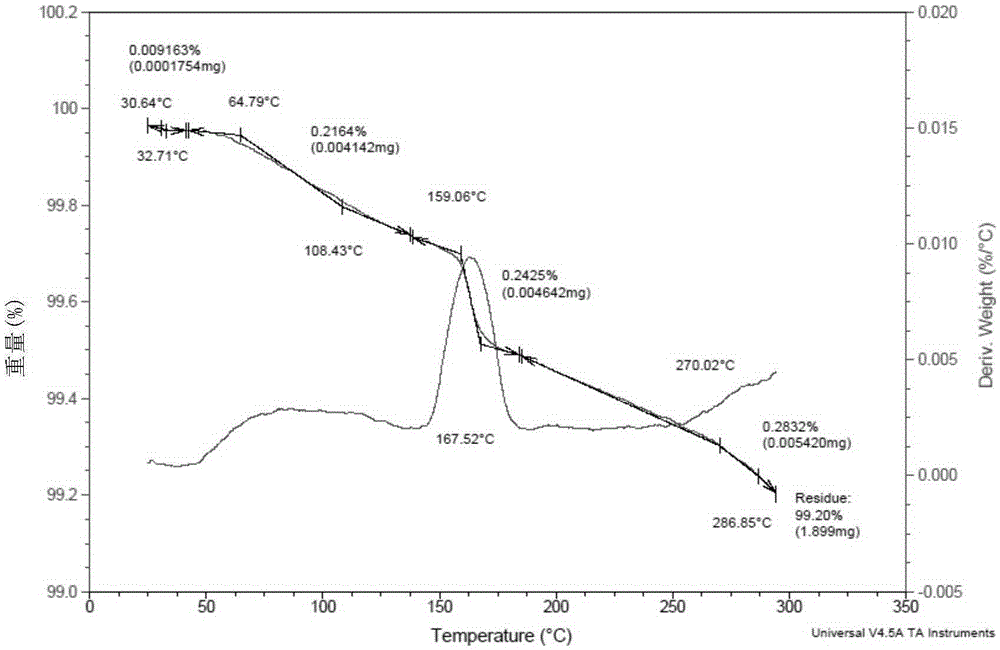
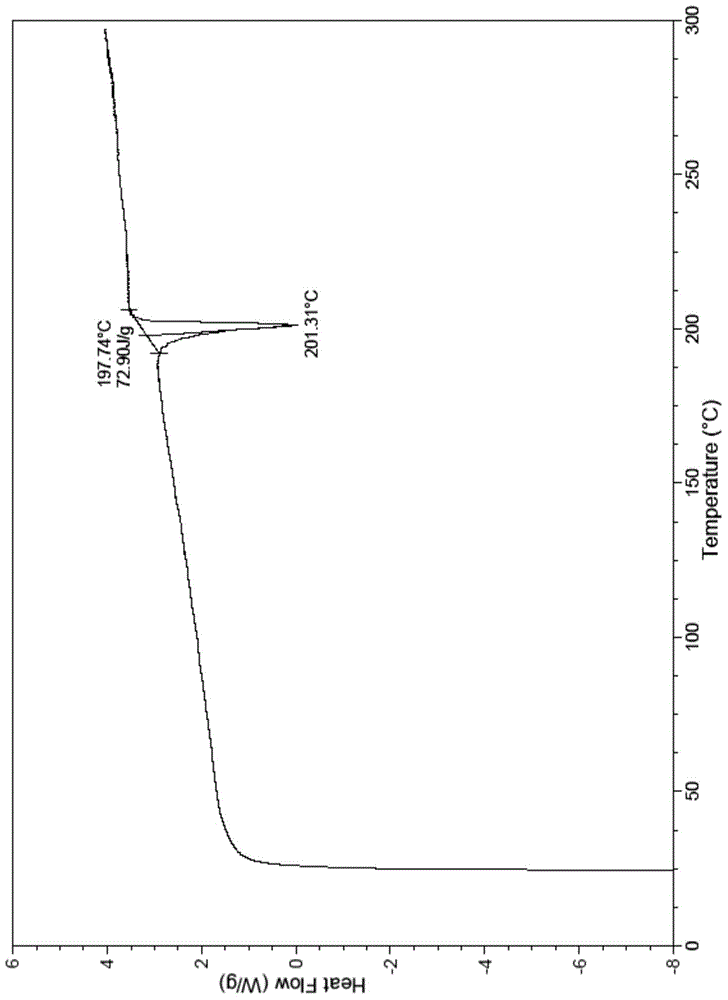
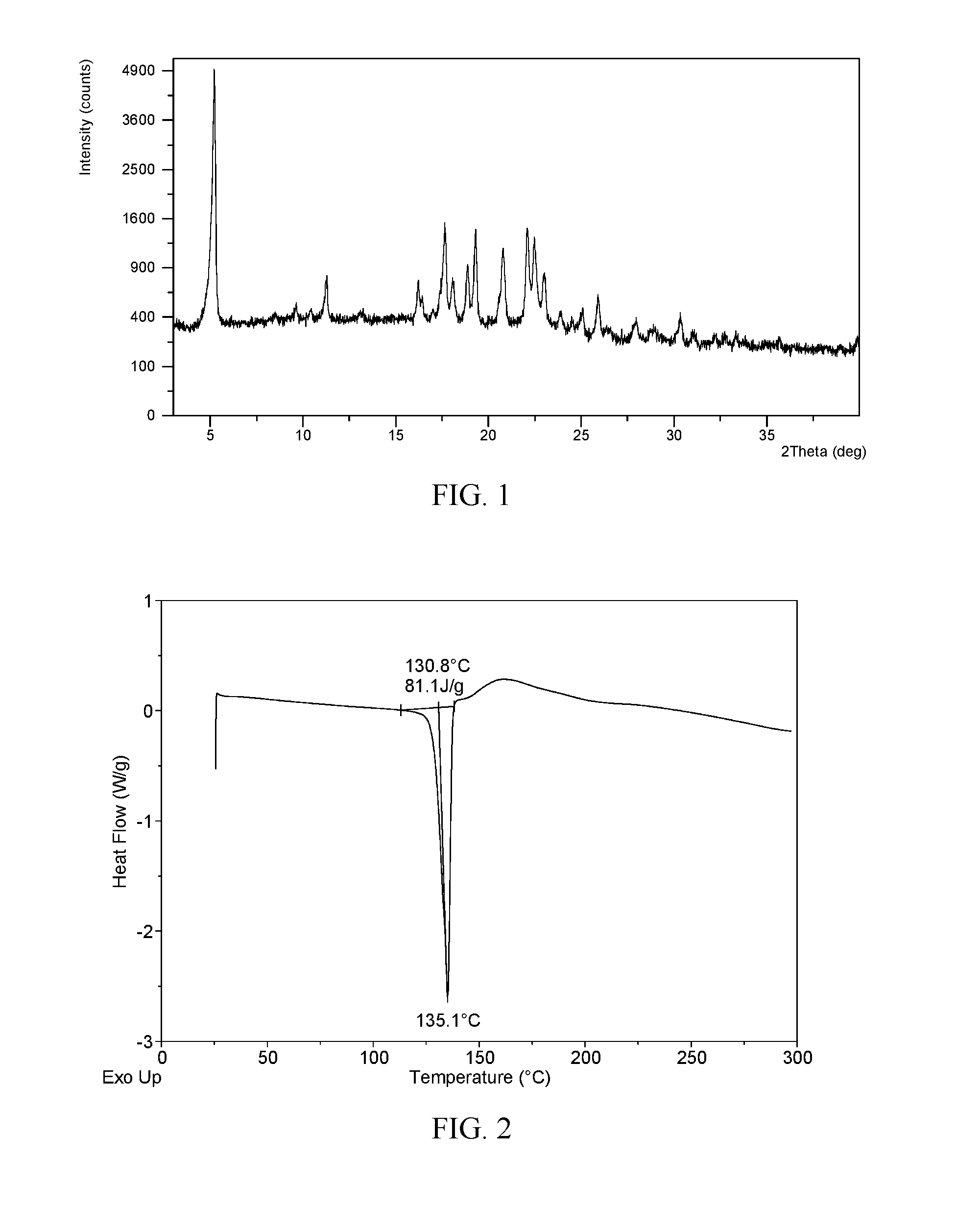
The present invention relates to a novel crystal form of ibrutinib and a preparation method thereof, and belongs to the technical field of pharmacy. The differential scanning calorimetry curve of the novel crystal form has endothermic peak at 194-204 DEG C; and the novel crystal form has good physical and chemical properties, is conducive to the production operation, and can be used in the preparation of pharmaceutical preparations.
Owner:SUNSHINE LAKE PHARM CO LTD
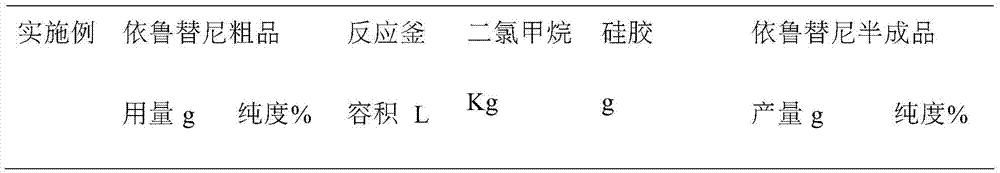
Ibrutinib purification method
InactiveCN105440040AEfficient removalSimple and fast operationOrganic chemistryOrganic solventPurification methods
The invention discloses an ibrutinib purification method. The ibrutinib purification method comprises the following steps: 1) dissolving a crude product of ibrutinib in a moderately polar organic solvent, adding silica gel used for column chromatography, mixing the materials, carrying out stirring and filtering and concentrating obtained filtrate until dryness, thus obtaining a semi-finished product of ibrutinib; carrying out impurity separation with a simple silica gel adsorption method and obtaining the semi-finished product of ibrutinib with purity of 99.5% after purification; 2) then carrying out separation by recrystallization, thus obtaining a finished product of ibrutinib with purity over 99.8%. The ibrutinib purification method can achieve the effect of effectively removing the impurities in the crude product of ibrutinib, is simple in purification operation and high in yield and can be applied to industrial production.
Owner:ZHEJIANG JINGXIN PHARMA
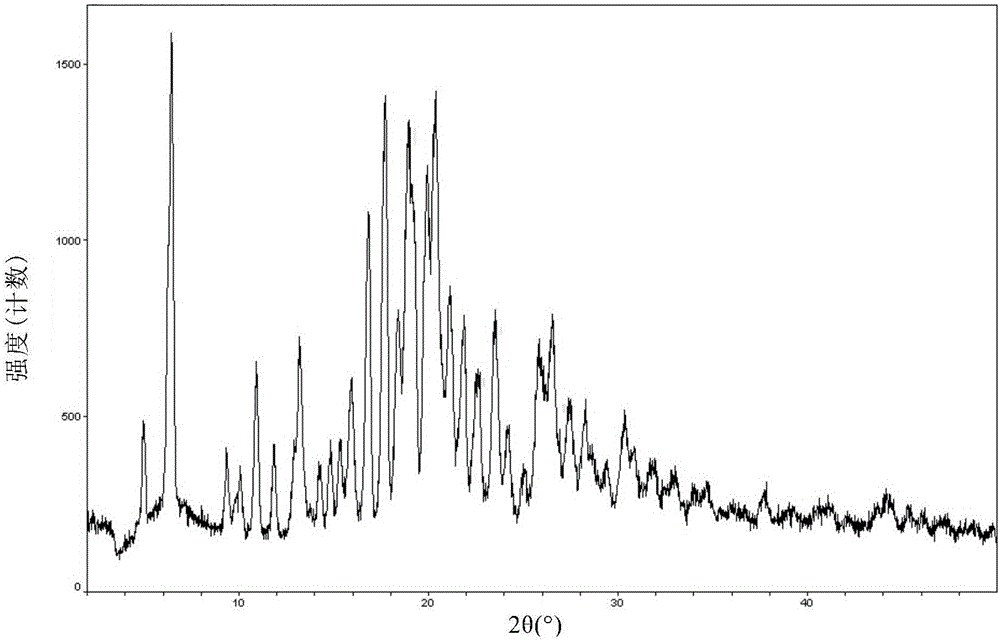
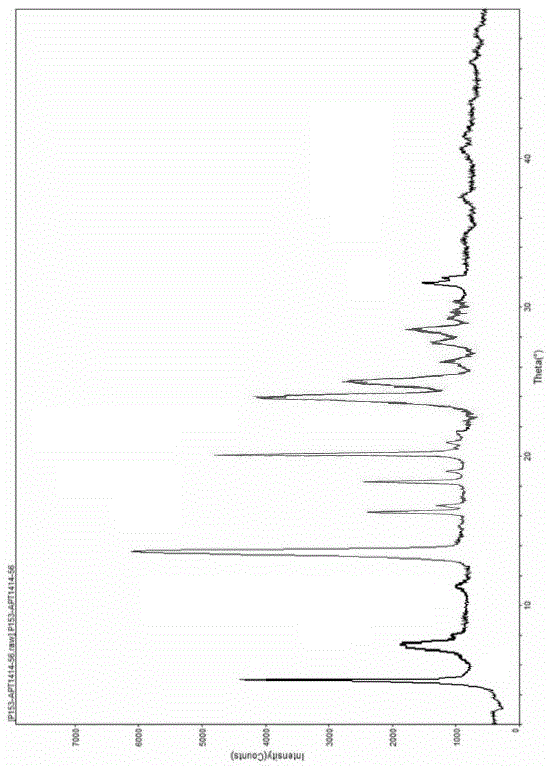
Crystal form F of ibrutinib and preparation method
InactiveCN105646498AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistry methodsSolubilitySolvent
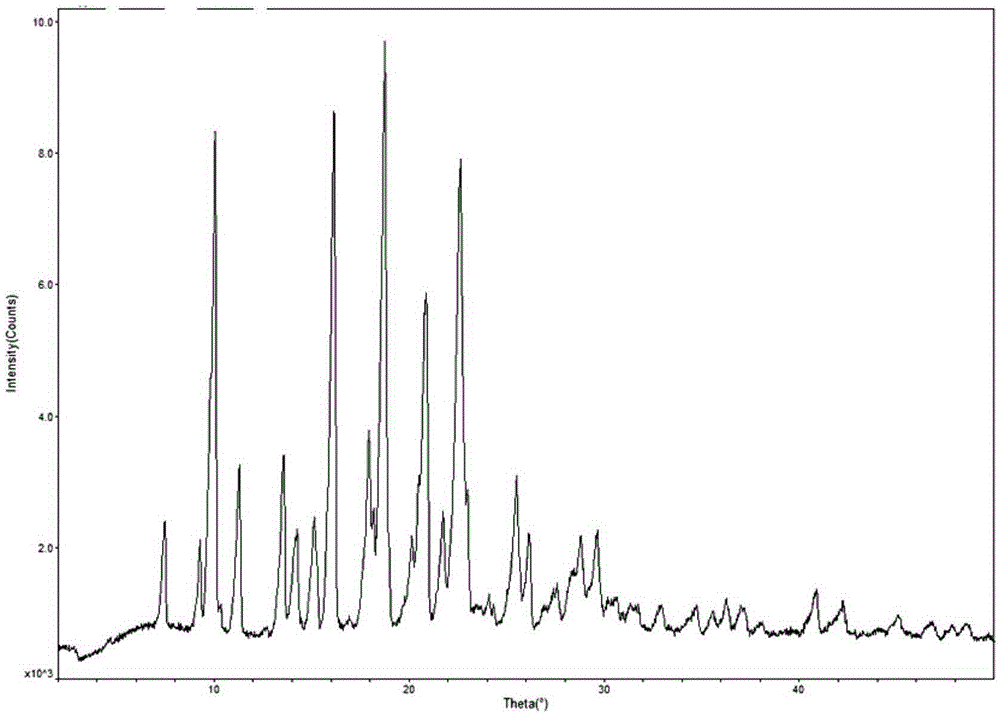
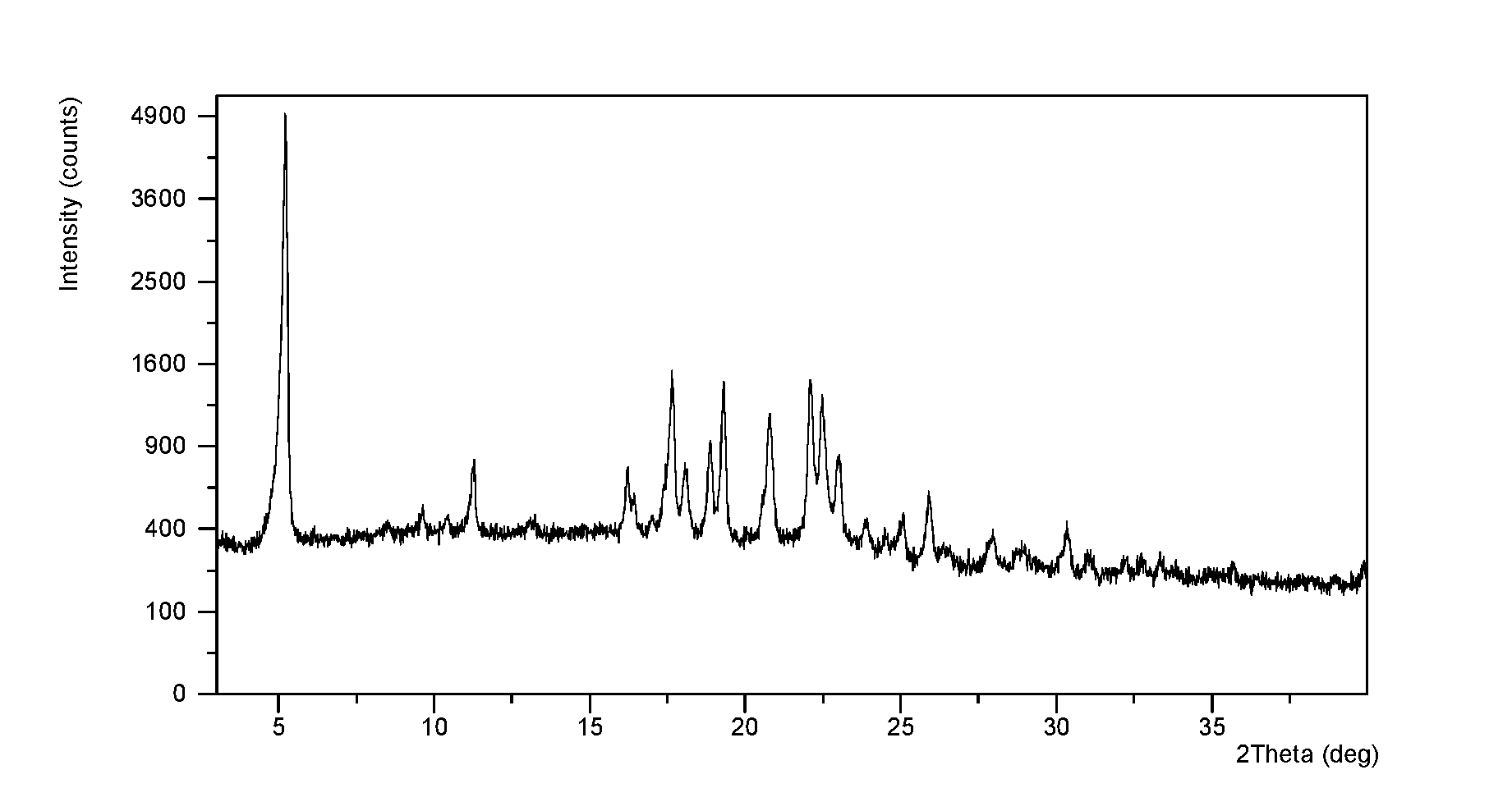
The invention discloses a crystal form F of ibrutinib. The crystal form F is characterized in that X-ray powder diffraction (X-RPD) which adopts Cu-Kalpha radiation and is represented with a 2theta angle has diffraction peaks in positions at angles of 3.7 degrees plus or minus 0.2 degrees, 6.7 degrees plus or minus 0.2 degrees, 13.2 degrees plus or minus 0.2 degrees, 16.1 degrees plus or minus 0.2 degrees, 19.1 degrees plus or minus 0.2 degrees, 20.0 degrees plus or minus 0.2 degrees, 23.8 degrees plus or minus 0.2 degrees and 24.6 degrees plus or minus 0.2 degrees. Related solvents in a preparation process of the crystal form F are cheap, the conditions are mild, the operation is simple, good controllability and reproducibility are realized, further, the prepared crystal form has great stability, the HPLC (high performance liquid chromatography) purity is higher than 99%, and the phenomenon of crystal transformation can be avoided; besides, the solubility is high, the dissolubility is good, and the bioavailability is high.
Owner:孙霖
Method for preparing ibrutinib
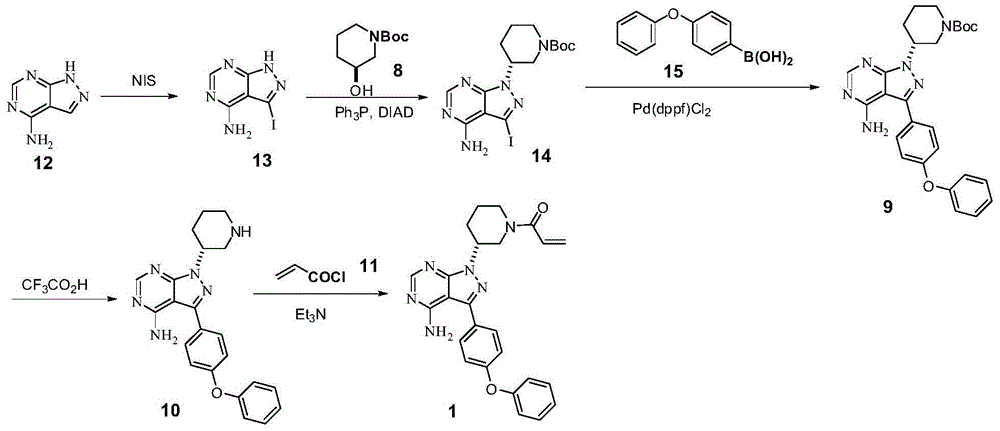
The invention discloses a method for preparing ibrutinib, and relates to an intermediate compound used for preparing the ibrutinib, wherein the intermediate compound has a structural formula shown in a formula II. The invention also discloses a method for preparing an intermediate, which is characterized in that a compound in a formula III and an acyl chloride derivative are subjected to a reaction, and then the ibrutinib is obtained through a further reaction. The impurity content of the ibrutinib prepared by the method is obviously reduced.
Owner:SHANGYU JINGXIN PHARMA
Synthesis method of ibrutinib
The invention discloses a synthesis method of ibrutinib. The method uses Suzuki coupling reaction and Kumada coupling reaction, does not need to separate the intermediate, obtains the intermediate (9) at high yield by a one-pot process, and uses mixed anhydrides instead of acryloyl chloride. The technique utilizes cheap 4-halodianisole as the initial raw material, adopts Suzuki coupling reaction and Kumada coupling reaction, and uses the one-pot process. The whole route is disclosed in the specification. The method can obtain the intermediate (9) (the chemical purity and optical purity are greater than or equal to 99%) at high yield without separating and purifying the intermediate, and avoids microwave, high temperature / high pressure and other specific reaction conditions; and the acrylic acid and mixed anhydrides generated by acyl chloride and sulfonyl chloride are used instead of the acryloyl chloride in the last step to avoid the amidation reaction of the intermediate (10) in multiple sites and reduce the generation of the byproduct, thereby obtaining the high-purity ibrutinib at high yield. The method has the advantages of short process route and lower cost, and is beneficial to the environment and suitable for industrialized scale-up production.
Owner:ARROMAX PHARMATECH
Crystalline form i of ibrutinib
ActiveUS20170002009A1Improve stabilityReduce moisture absorption performanceOrganic active ingredientsOrganic chemistry methodsDiseaseBruton's tyrosine kinase
Owner:CRYSTAL PHARMATECH CO LTD +1
Preparation method for ibrutinib
ActiveCN105859728AHigh purityHigh yieldGroup 4/14 element organic compoundsFormamidine acetateDrugs synthesis
The invention discloses a preparation method for ibrutinib and belongs to the technical field of drug synthesis. The preparation method specifically includes the steps that 3-amino-4-cyano pyrazol and formamidine acetate serve as initial raw materials, and ibrutinib is obtained through a cyclization reaction, a halogenating reaction, a nucleophilic substitution reaction, a Mitsunobu reaction and an amidation reaction. According to the method, the raw materials are easy to obtain, conditions are mild, the process operability and controllability are high, cost is low, the yield is high, fewer side products are generated, purification is easy, and the high-quality product is obtained.
Owner:南京红太阳医药研究院有限公司
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
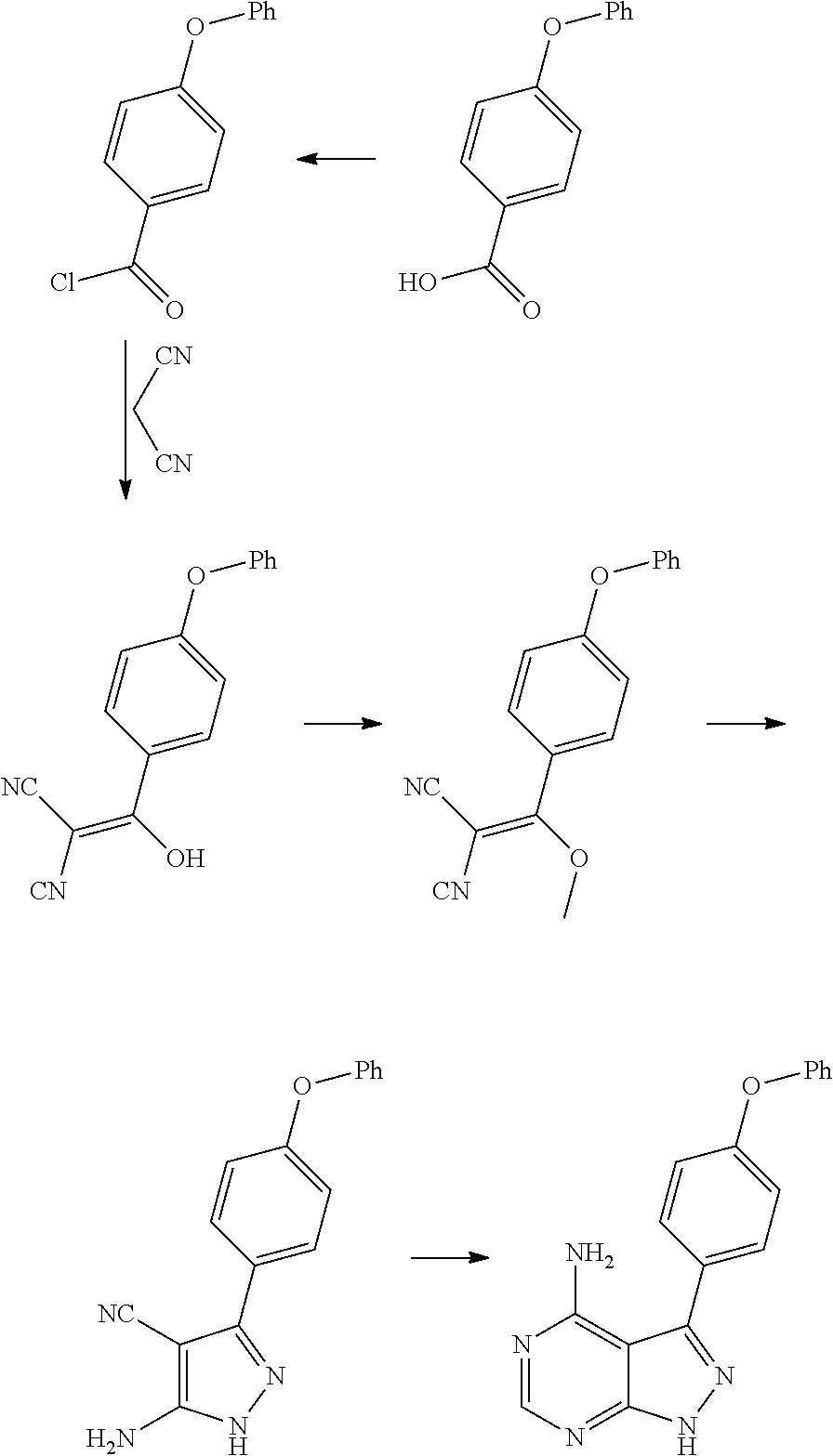
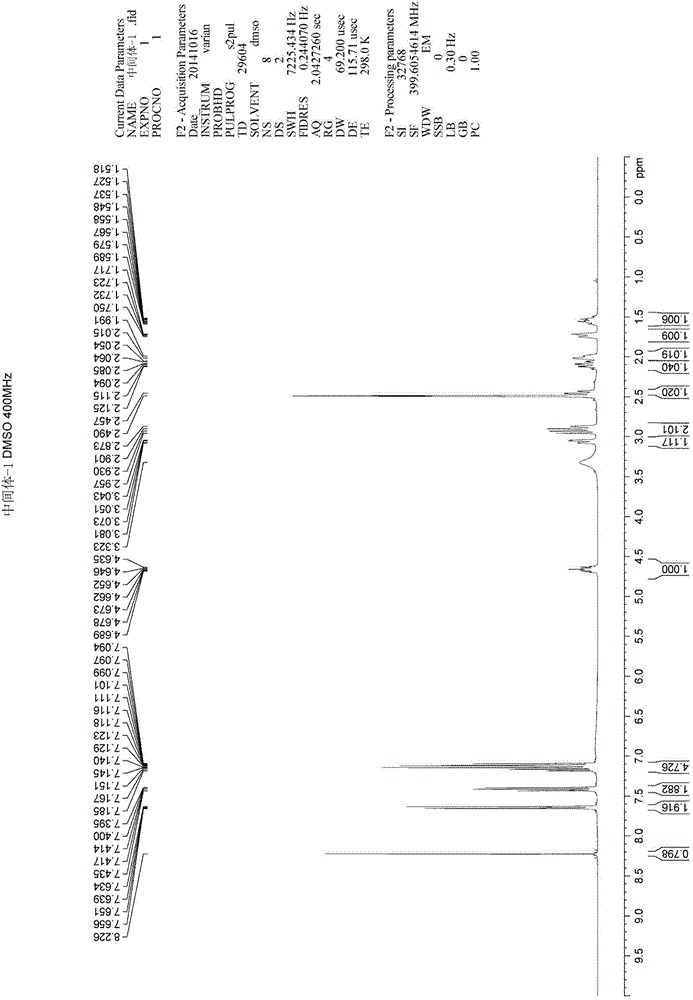
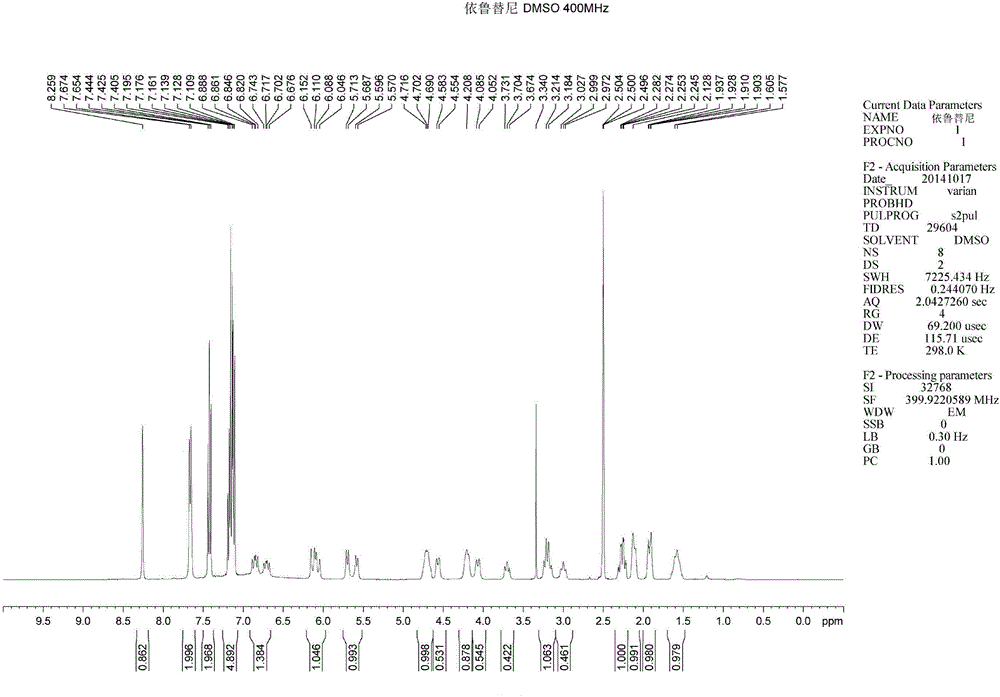
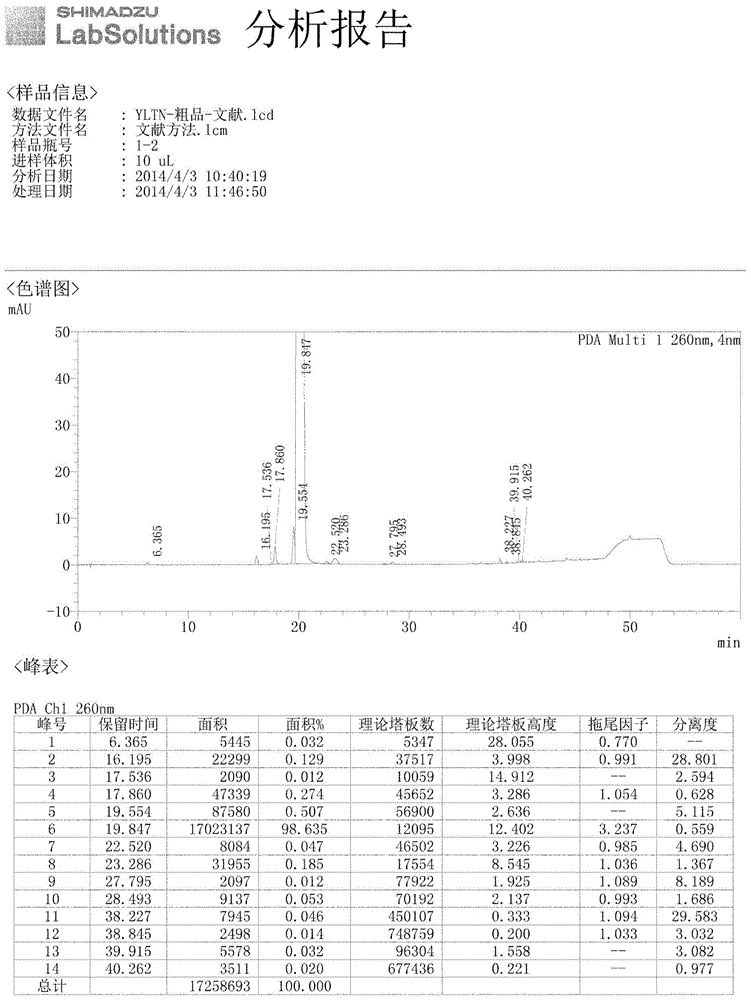
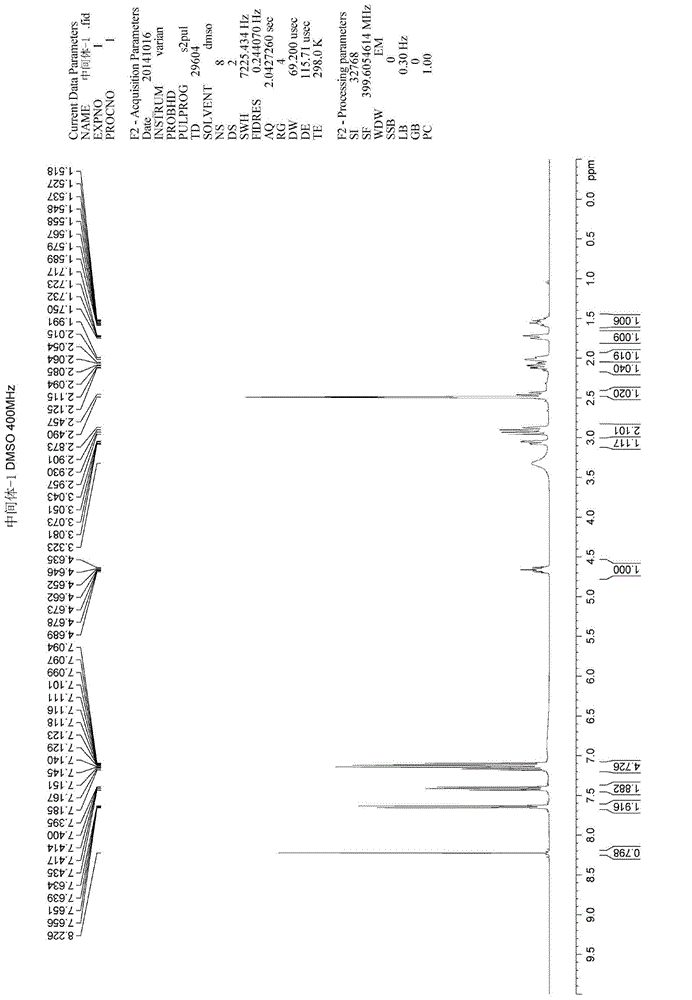
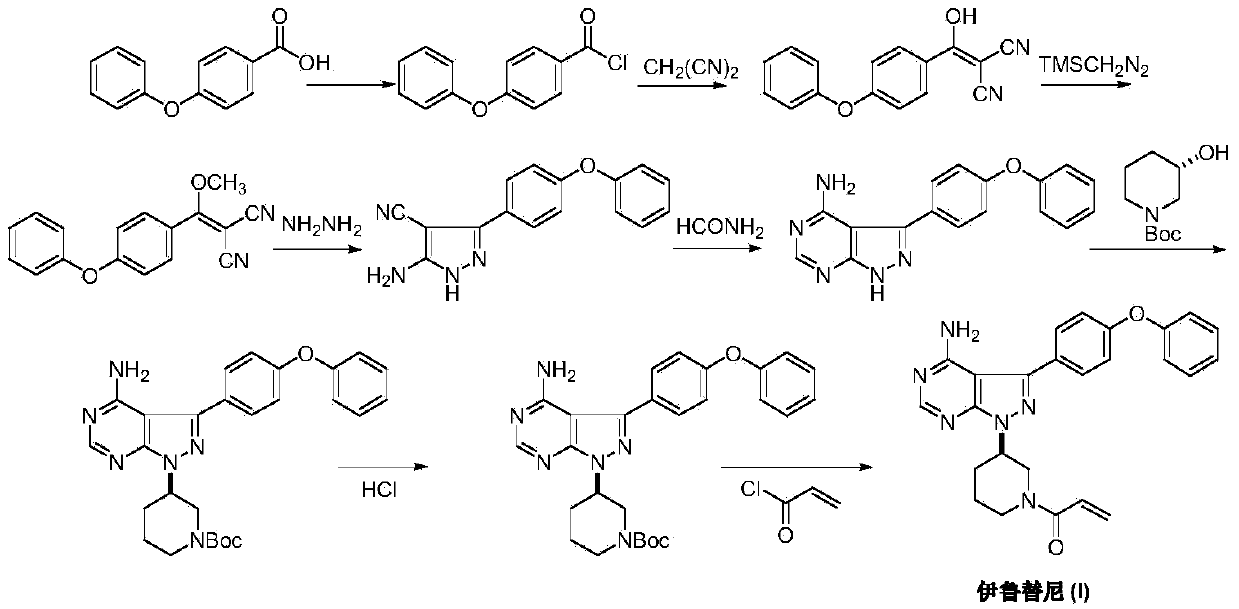
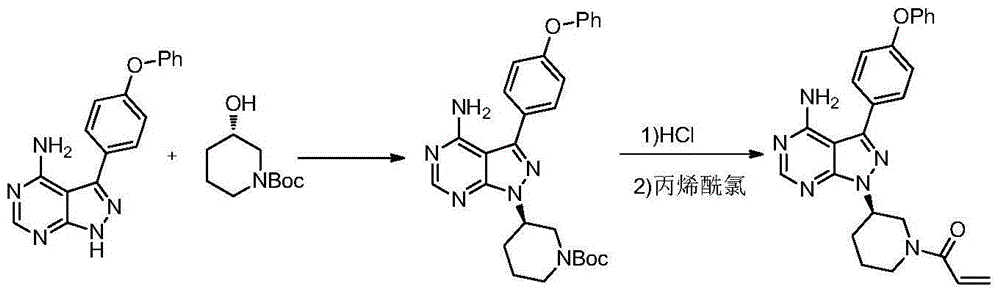
Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib
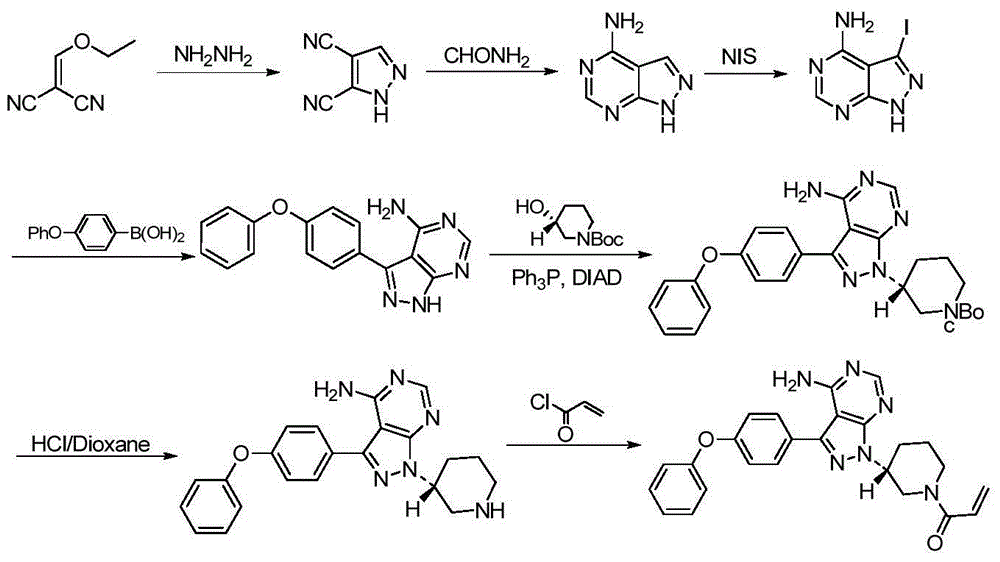
The invention discloses a method for synthesizing an important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib. The method comprises the following steps: step one, condensing malononitrile and triethyl orthoformate and then carrying out a one-pot reaction with hydrazine hydrate to obtain 5-amino-4-cyano-pyrazole; step two, condensing the 5-amino-4-cyano-pyrazole with formamide to prepare a compound of formula (II) 4-amino-1H-pyrazolo[3,4-d]pyrimidine; step three, carrying out a bromination reaction of the compound of formula (II) and brominating agent to obtain a compound of formula (III) 4-amino-3-bromine-1H-pyrazolo[3,4-d]pyrimidine; step four, carrying out a Stille reaction on the compound of formula (III) and trimethyl p-phenoxy phenyltin under the catalyst effect of metal palladium to prepare a compound of formula (I) 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine. According to the method for synthesizing the important intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib provided by the invention, raw materials are low in price and easily obtained, the reaction conditions are moderate, the operation is simple and convenient, the method is suitable for industrial production, and a new way is provided for preparing Ibrutinib and intermediate.
Owner:HUAIHAI INST OF TECH
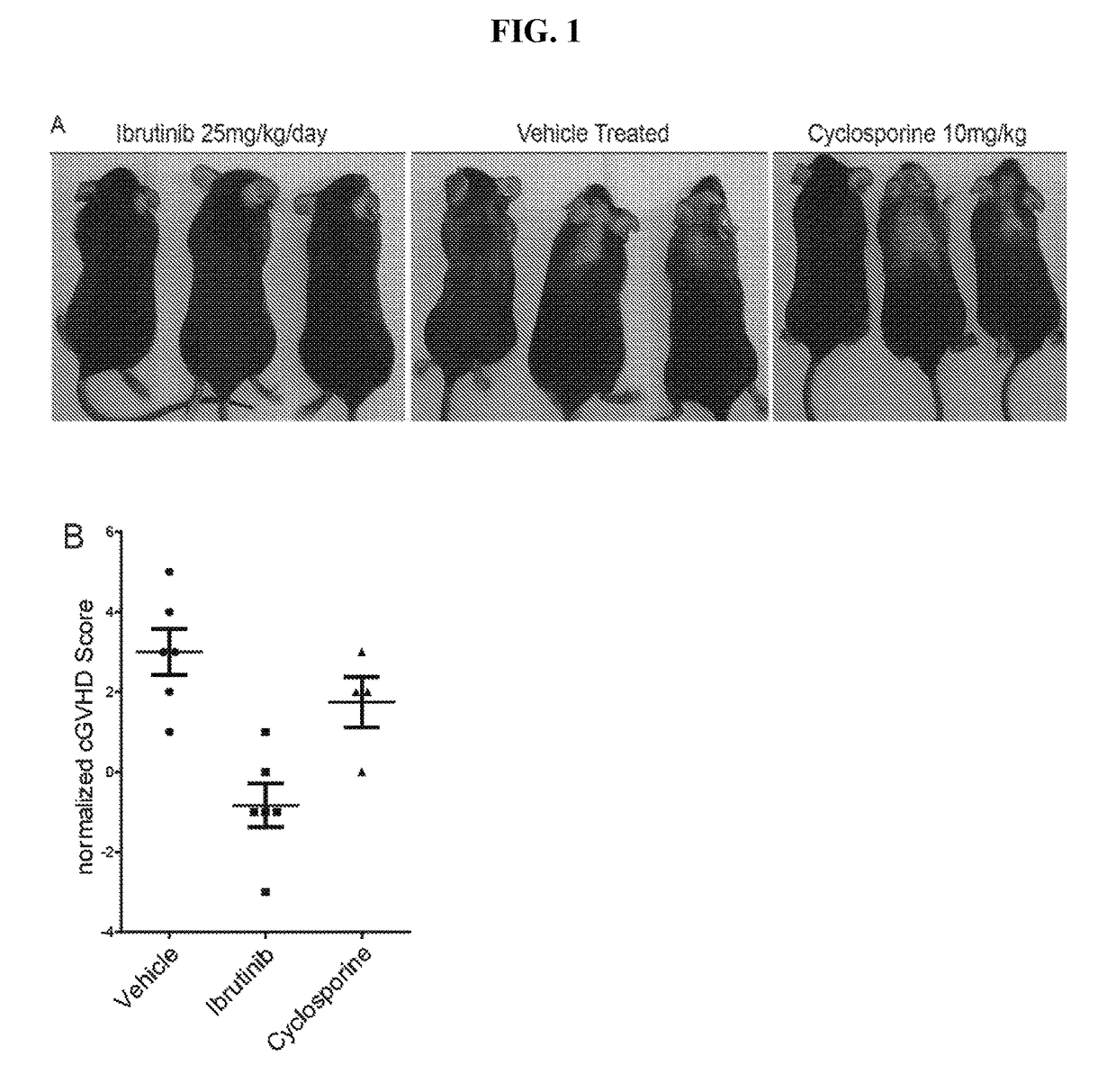
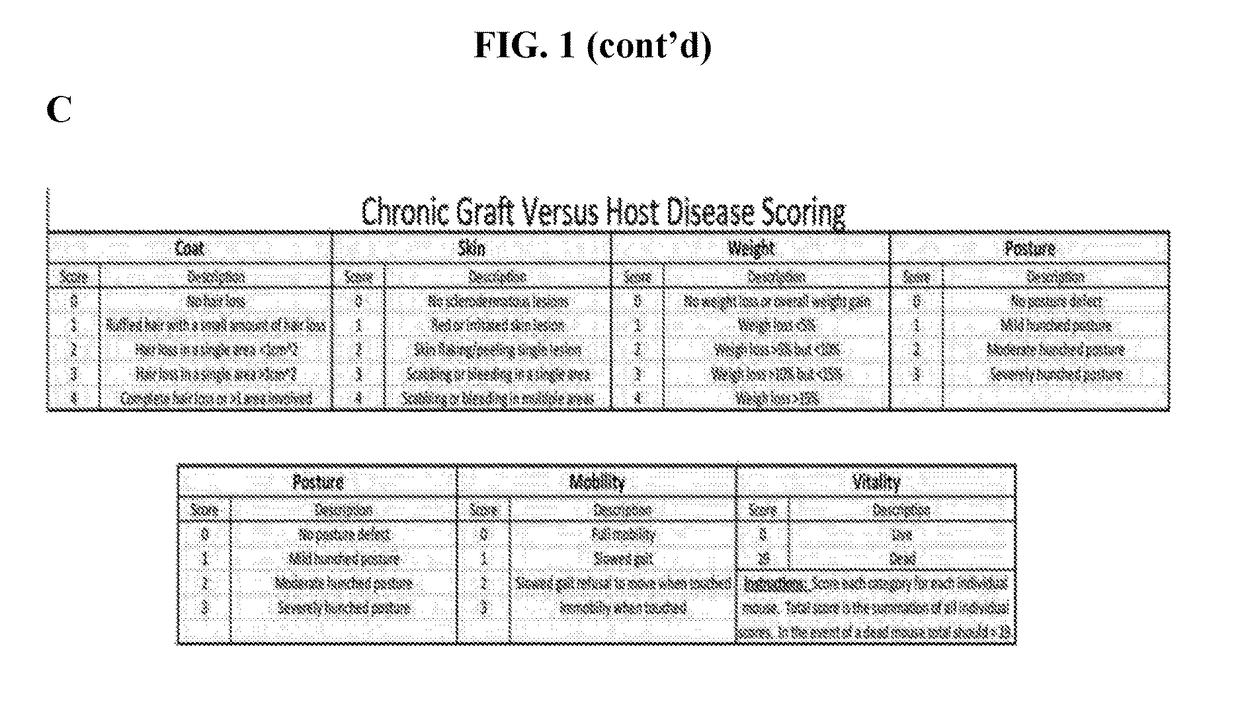
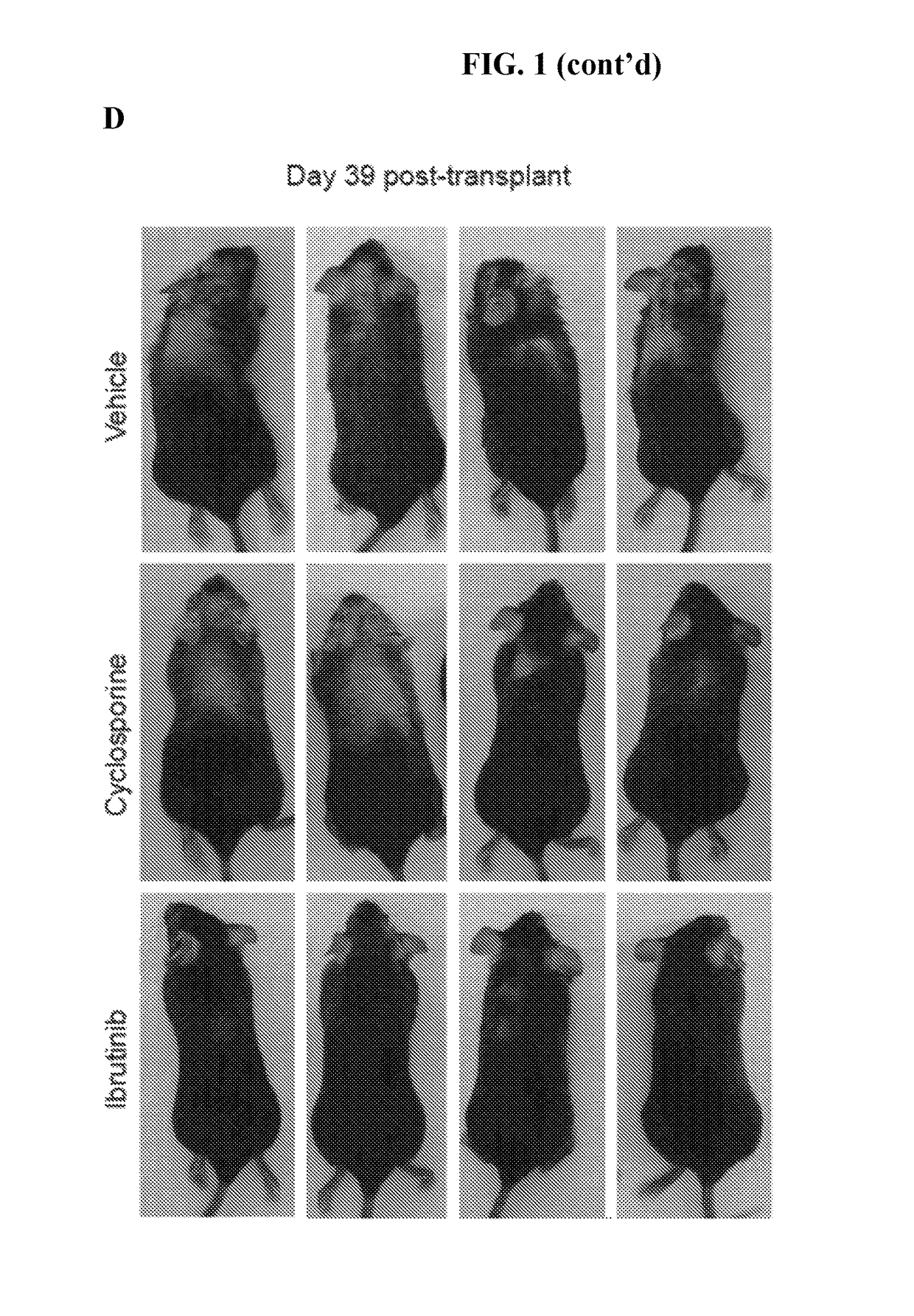
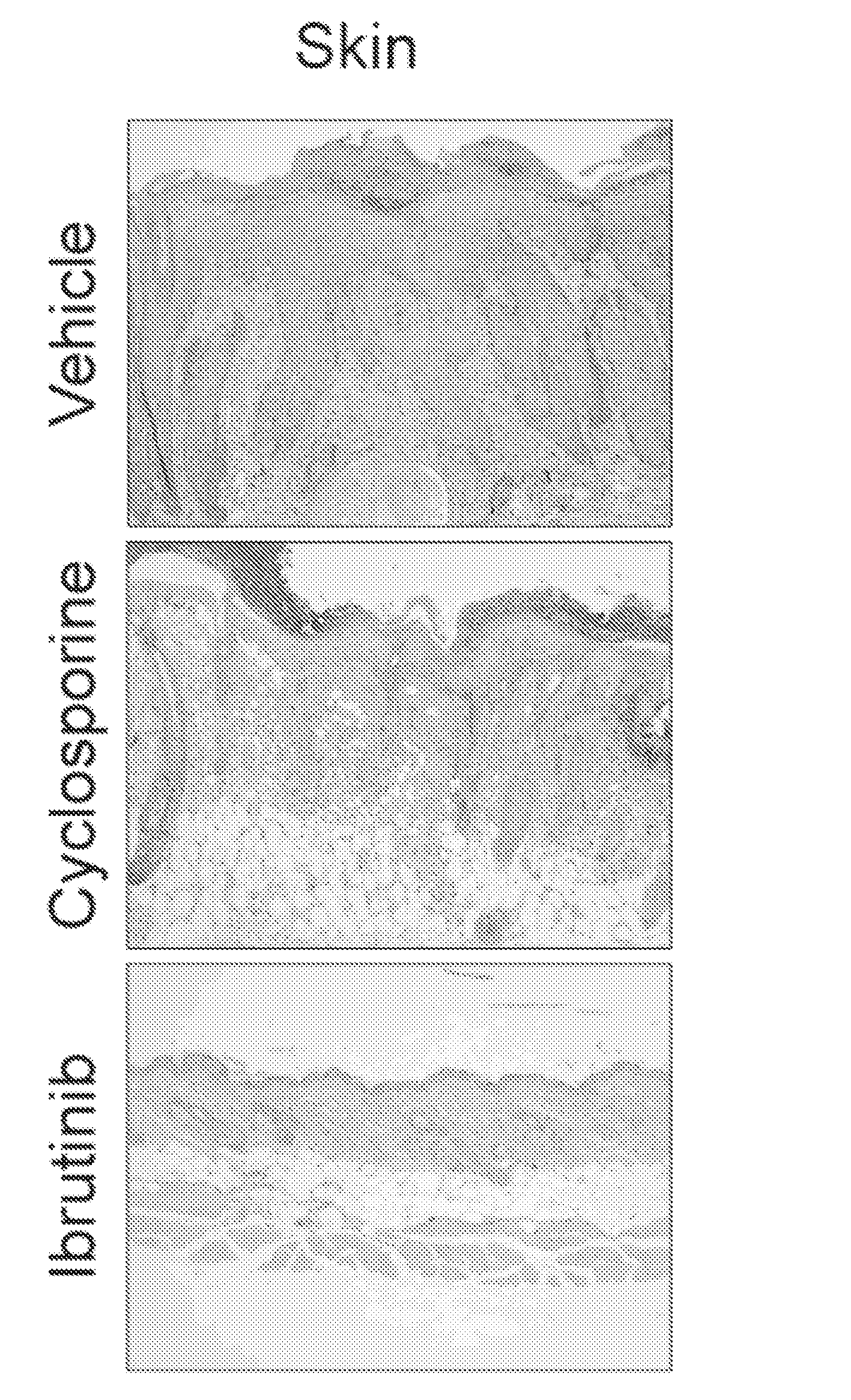
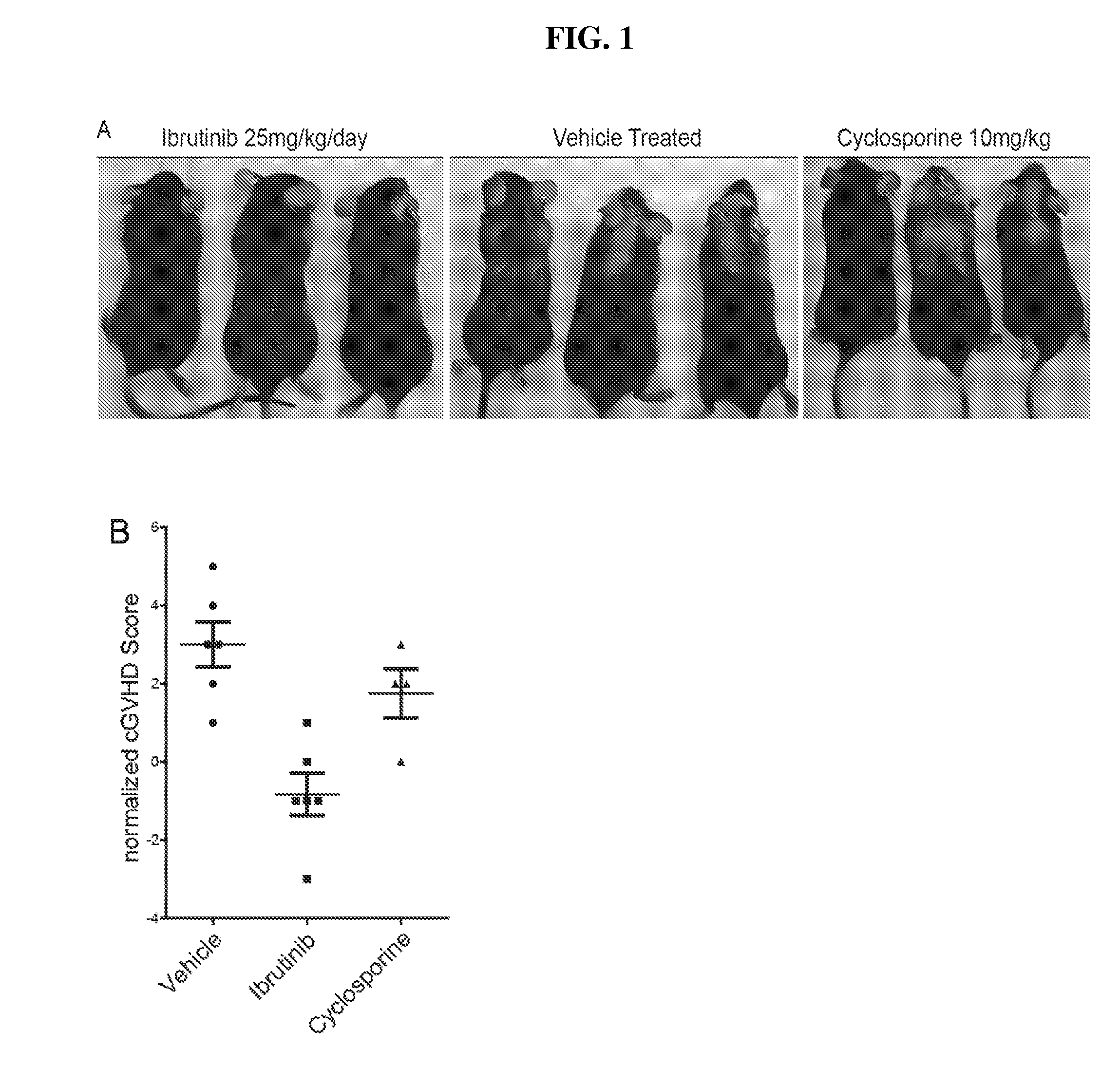
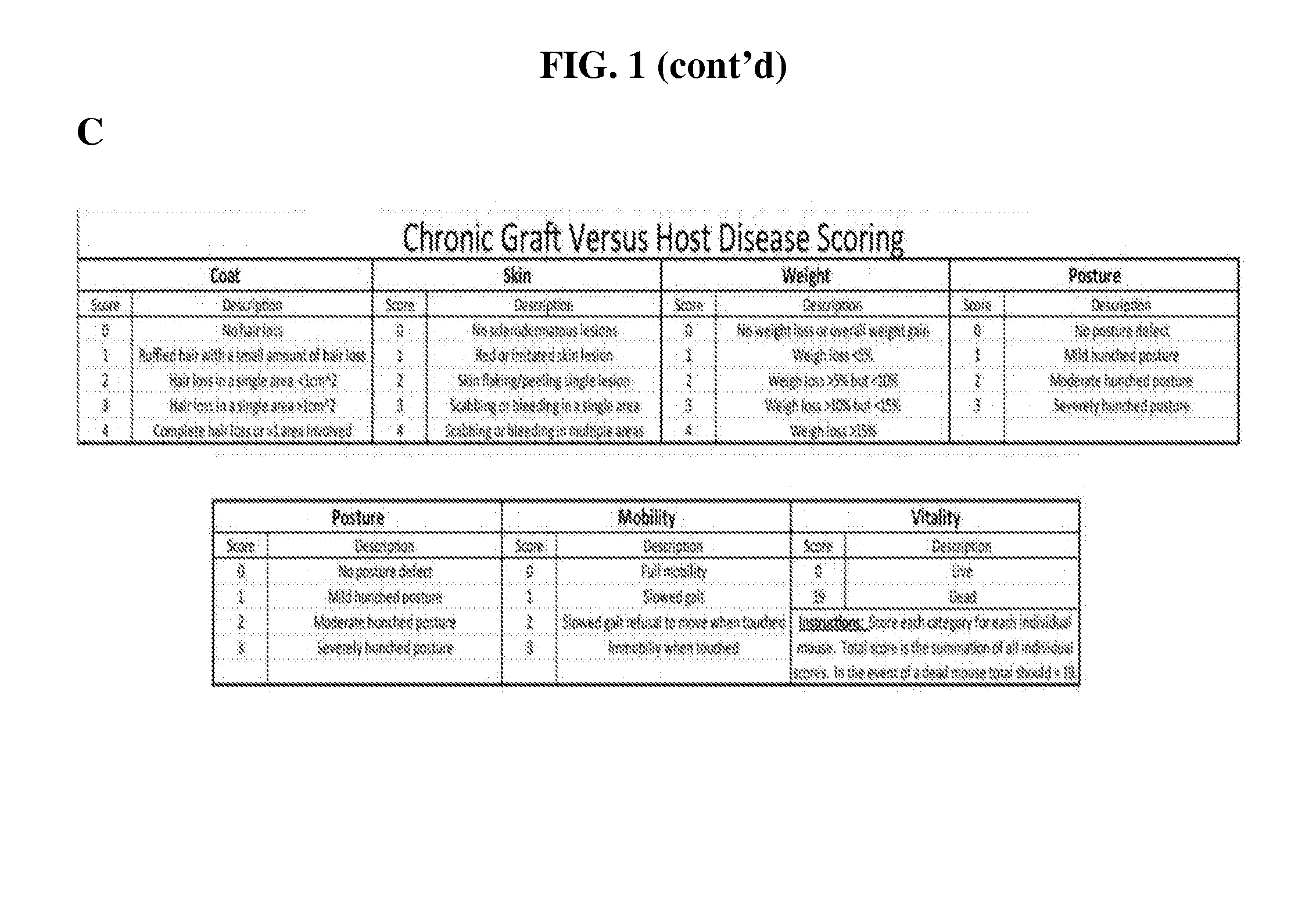
Methods of treating and preventing graft versus host disease
ActiveUS9795604B2Reduce severityAvoid it happening againOrganic active ingredientsDigestive systemIbrutinibInternal medicine
Described herein are methods for treating and preventing graft versus host disease using ACK inhibitors. The methods include administering to an individual in need thereof an ACK inhibitor such as ibrutinib for treating and preventing graft versus host disease.
Owner:PHARMACYCLICS
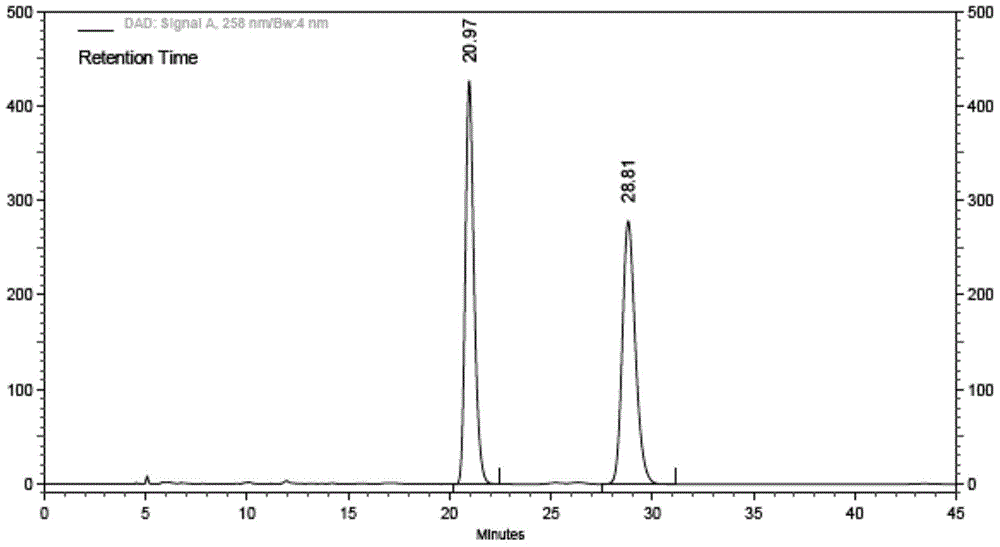
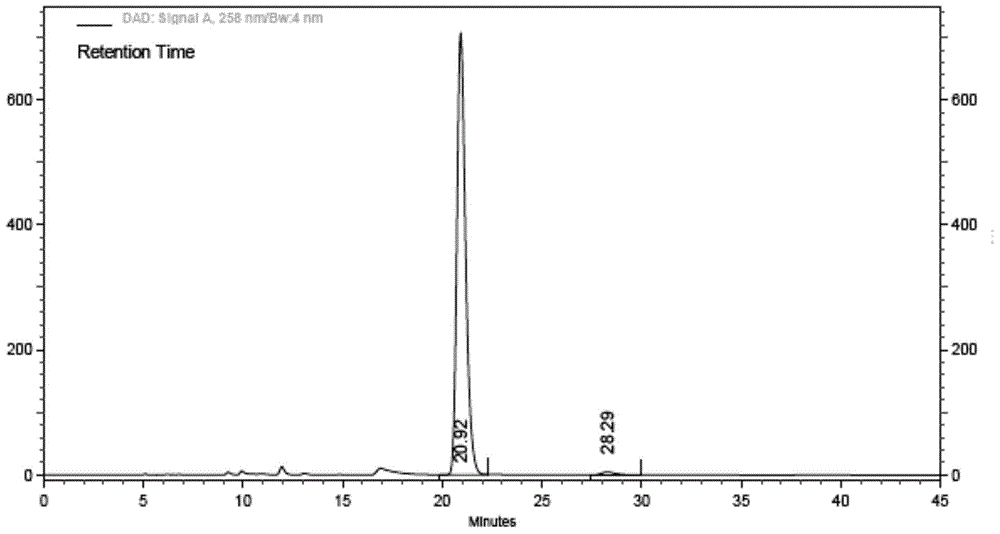
Ibrutinib and test method of isomer of ibrutinib
The invention relates to ibrutinib and a liquid-phase separation test method of a corresponding isomer impurity of ibrutinib and belongs to the technical field of separation testing. The test method comprises the steps of taking a CHIRALPAK AD-H, 4.6mm*250mm, 5-micrometer chiral chromatographic column as a separation chromatographic column, and taking a mixed solvent of an alkene solvent and an alcohol solvent at a volume ratio of 35:65 as a mobile phase. The method can effectively separate and test ibrutinib and the corresponding isomer impurity of ibrutinib, and be used for production, and a separation degree can reach above 5.
Owner:RUYUAN HEC PHARM
Ibrutinib preparation method, ibrutinib intermediate, and ibrutinib intermediate preparation method
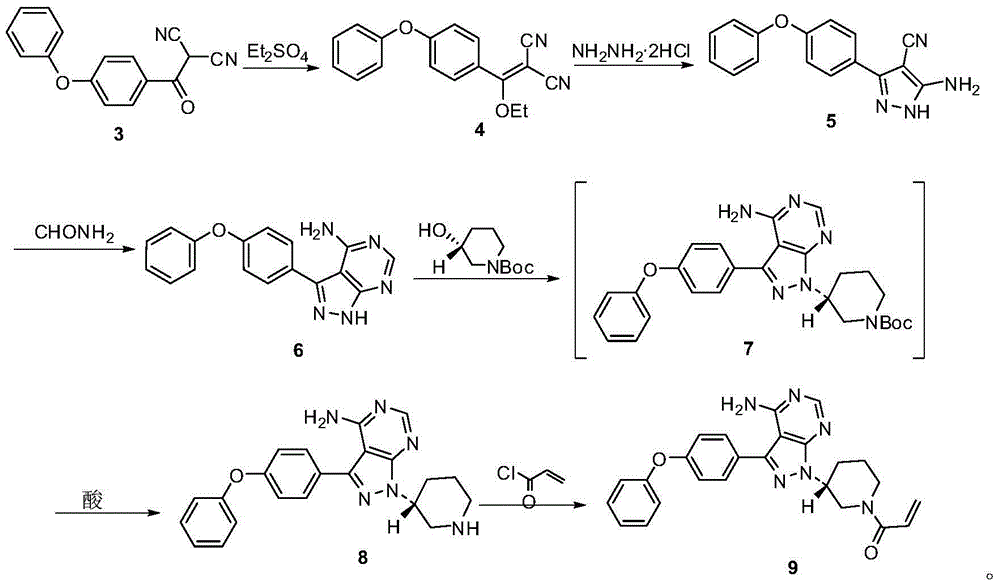
InactiveCN106188062AEasy to separate and purifyReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationHydrazine dihydrochlorideFormamide
The present invention provides an ibrutinib preparation method, which comprises that (a) a compound (3) reacts with diethyl sulfate to obtain a compound (4); (b) the compound (4) reacts with a hydrazine dihydrochloride to obtain a compound (5); (c) the compound (5) reacts with formamide to obtain a compound (6); (d) after the compound (6) reacts with (R)-1-Boc-3-hydroxypiperidine, an acid is added to make a compound (7) be subjected to a deprotection reaction to obtain a compound (8); and (e) the compound (8) reacts with acryloyl chloride to obtain a compound (9) ibrutinib. The present invention further provides an ibrutinib intermediate represented by a formula (4) and a preparation method of the intermediate compound (8). According to the present invention, the method has advantages of low cost, good safety and high yield, and is suitable for large-scale production. The reaction route is defined in the specification.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
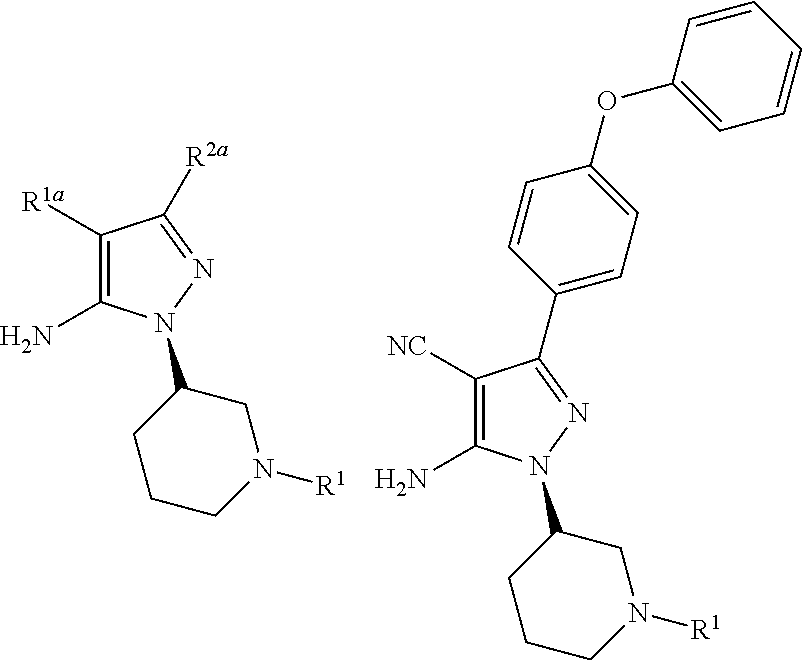
Processes and intermediates for preparing a medicament
Disclosed is a process for the preparation of the following compounds:where R1, R1a and R2a have the definitions in the description, as well as a process to prepare other intermediates that may be useful to synthesize downstream products, especially compounds that are useful as medicaments, for instance Bruton's tyrosine kinase (Btk) inhibitors such as ibrutinib. Also disclosed are other processes, other intermediates and compounds per se.
Owner:JANSSEN PHARMA NV
Ibrutinib amorpphis and preparation method thereof
The invention discloses a method for preparing ibrutinib amorpphis. The method comprises the following steps: (1) adding ibrutinib into a high-quality organic solvent, and heating to completely dissolve the ibrutinib; (2) adding the solution into an anti-solvent, and performing stirring and curing, wherein in the adding process, the temperature of the anti-solvent system is maintained below 5 DEG C; (3) filtering, decompressing and drying solids to obtain the ibrutinib amorpphis. The ibrutinib amorpphis prepared by the method is stable and suitable for preparation production.
Owner:CHONGQING PHARMA RES INST
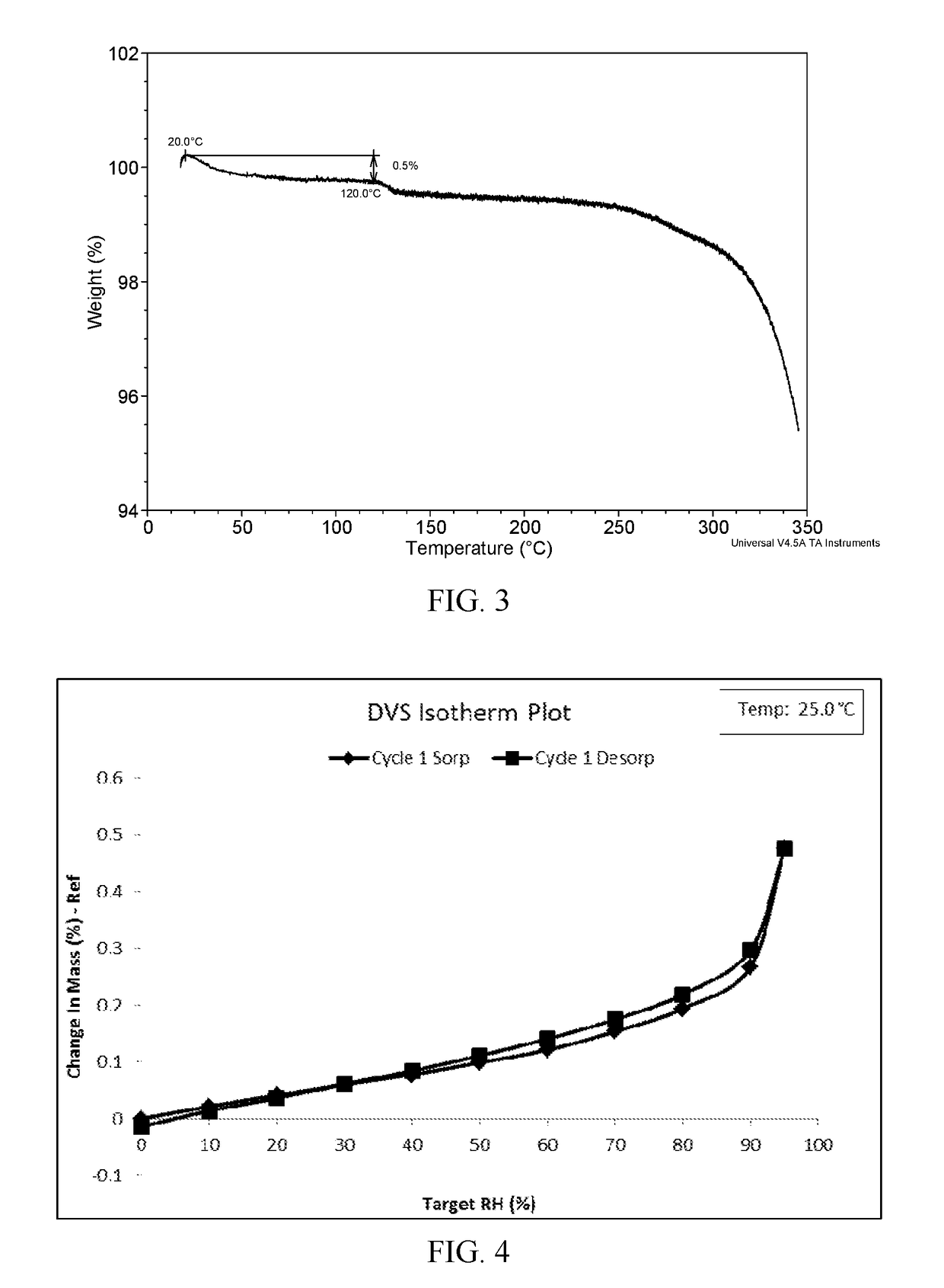
New ibrutinib crystal form and preparation method thereof
InactiveCN106117214AImprove thermal stabilityLow hygroscopicityOrganic active ingredientsOrganic chemistry methodsHeat stabilityCombinatorial chemistry
The invention provides a new ibrutinib crystal form and a preparation method thereof. More specifically, the invention provides an ibrutinib crystal form III and a preparation method thereof. The ibrutinib crystal form III prepared by the preparation method has good heat stability, and is stably stored under the conventional conditions, thus being suitable for industrial application.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP
Preparation method of ibrutinib
InactiveCN104557946ARaw materials are easy to getSimple processOrganic chemistryDiisopropyl azodicarboxylateTriphenylphosphine
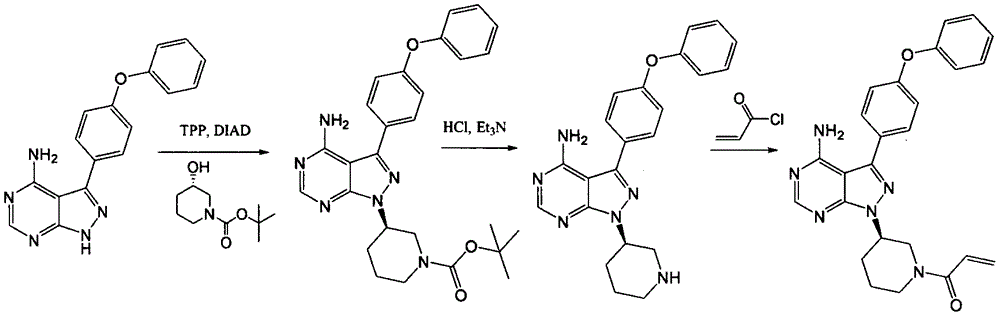
The invention relates to a preparation method of ibrutinib. The preparation method comprises the following steps: allowing 4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo [3, 4-d] pyrimidine, 1-[3(S)-hydroxy-1-piperidinyl]-2-propen-1-one, and triphenylphosphine to be dissolved in tetrahydrofuran as a reaction solvent at a temperature of -20 to 100 DEG C, and then slowly adding diisopropyl azodicarboxylate, and stirring until the reaction is finished to obtain the ibrutinib. The preparation method provided by the invention has the advantages of easy availability of raw materials, mild conditions, simple process, economy and environmental protection, and is suitable for industrial production.
Owner:王立强 +1
Novel crystal form of ibrutinib and preparation method of novel crystal form
InactiveCN107286163AImprove solubilityImprove oral bioavailabilityOrganic chemistry methodsX-rayBioavailability
The invention provides a novel crystal form of ibrutinib. The novel crystal form is named as a crystal form 1; the 2-theta value of the X-ray powder diffraction pattern of the novel crystal form has characteristic peaks at 5.8 degrees + / - 0.2 degree, 10.8 degrees + / - 0.2 degree, 19.2 degrees + / - 0.2 degree, and 21.6 degrees + / - 0.2 degree; the novel crystal form has a heat absorption peak when being heated to about 150.3 DEG C; the novel crystal form is an anhydrous substance and is non-hygroscopic. The crystal form I of ibrutinib provided by the invention is good in solubleness, beneficial to improvement of oral bioavailability of medicines and relatively good in clinical application values. The crystal form I of ibrutinib is good in stability and almost has no hygroscopicity, so that stability of the medicines in the storage and transportation process and the safety in clinical application are ensured. The crystal form I of ibrutinib provided by the invention is simple in preparation method operation, low in cost, free of special requirement on production equipment, easy in industrial production and relatively great in application value.
Owner:SHANGHAI SUNTECH PHARMA
Preparation method of ibrutinib
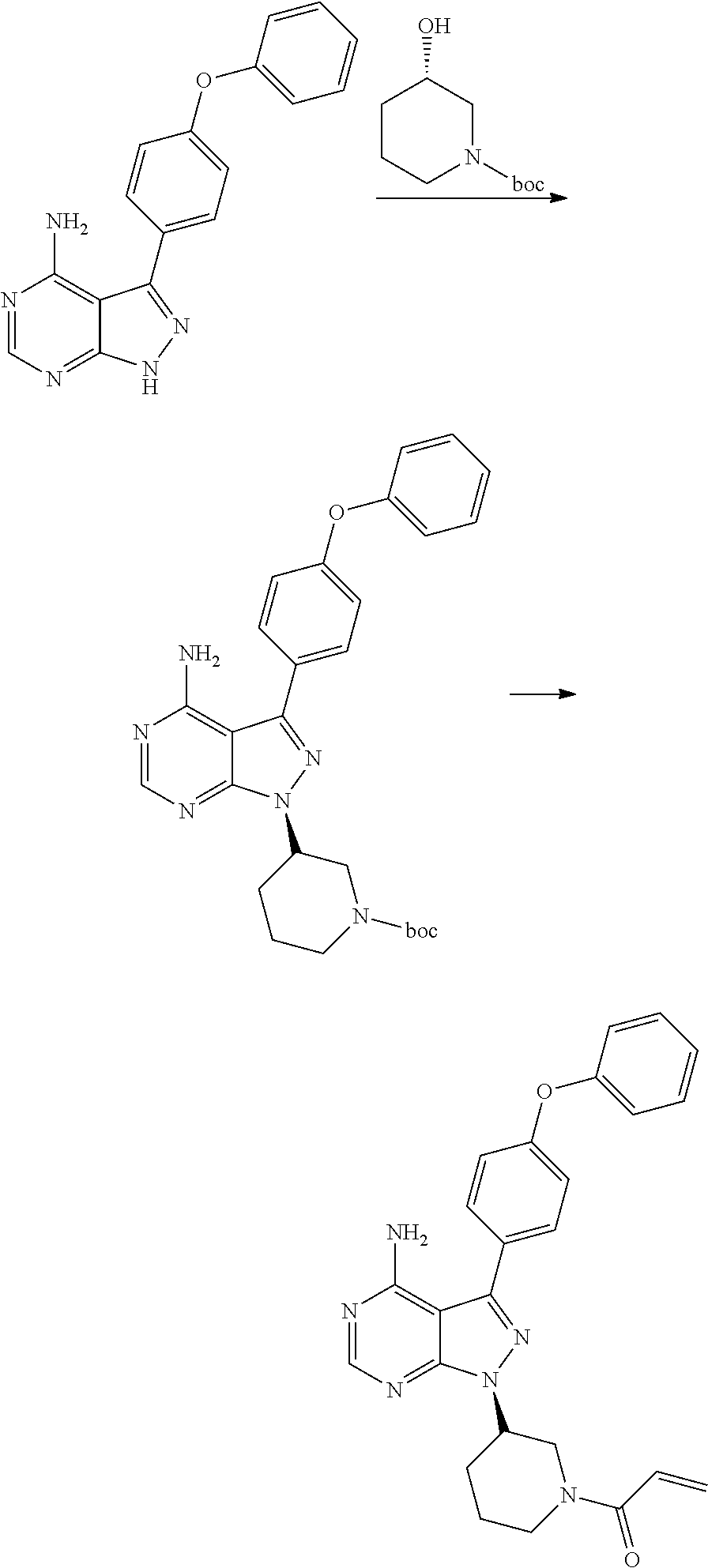
ActiveCN106008526AHigh yieldQuality improvementOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupMitsunobu reaction
The invention relates to the technical field of medicine, in particular to a preparation method of ibrutinib. The preparation method of ibrutinib comprises the following steps that 4-amino-3-(4-phenoxy phenyl)-1H-pyrazol[3,4-d]pyrimidine and S-1-tert-butyloxycarbonyl-3-hydroxyl piperidine are prepared into a compound shown in the formula IV (please see the formula in the description) through a Mitsunobu reaction, and a Boc protecting group of the compound shown in the formula IV is removed to prepare a compound shown in the formula V (please see the formula in the description); the compound shown in the formula V and acrylic ester are prepared into a compound shown in the formula I (please see the formula in the description) in the presence of a catalyst and an activating agent. According to the preparation method, the reaction process is mild in condition, few reaction steps are needed, high temperature and copious cooling are not needed, no high-toxicity reagent is adopted, and the whole synthesizing process is stable and controllable; ibrutinib prepared through the method is high in yield and quality and has the advantages of being good in stability, high in purity, convenient to store and the like.
Owner:BIOCOMPOUNDS PHARMACEUTICAL INC +1
Ibrutinib salts, crystals, preparation method, pharmaceutical compositions and applications thereof
InactiveCN109776543AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistryBenzoic acidSolubility
The invention discloses ibrutinib salts, crystals, a preparation method, pharmaceutical compositions and the application thereof. The crystals of the ibrutinib salts have a structure as shown in formula (I), wherein X is HCl, HBr or benzoic acid. The preparation method comprises the following steps: mixing ibrutinib with X in an organic solvent, heating to 40-130 DEG C, completely dissolving, cooling, and crystallizing. The crystals of the three ibrutinib salts are all of new crystal forms, have excellent solubility and thermal stability, greatly improve the hygroscopic property, and greatly improve the solubility and bioavailability of ibrutinib. The preparation method of the crystals is simple, is suitable for large-scale industrial production, and has a good industrial application prospect.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
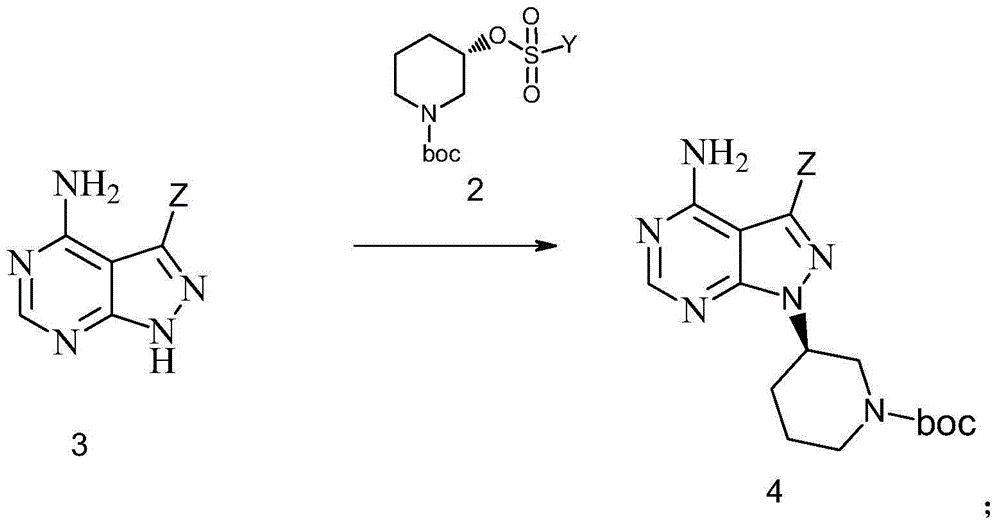
Preparation method of Ibrutinib intermediate
The invention discloses a preparation method of an Ibrutinib intermediate shown as a formula 4. The method comprises the following steps: in a organic solvent, under effect of an acid-binding agent, a compound shown as a formula 2 and a compound shown as a formula 3 are subjected to a nucleophilic substitution reaction, and the Ibrutinib intermediate shown as the formula 4 is prepared. The acid-binding agent is one or more of sodium carbonate, potash and sodium hydride; wherein, Y is C1-C6 alkyl, phenyl or C1-C6 alkyl-substituted phenyl; and Z is halogen, or 4-phenoxyl-phenyl. The preparation method has the advantages of simple operation, low cost, less reagent amount, less three wastes, environmental pollution, simple post-treatment, high yield, and high optical purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Methods of treating and preventing graft versus host disease
ActiveUS20150118209A1Reduce severityPrevents and reduces GVHDBiocideOrganic active ingredientsIbrutinibInternal medicine
Described herein are methods for treating and preventing graft versus host disease using ACK inhibitors. The methods include administering to an individual in need thereof an ACK inhibitor such as ibrutinib for treating and preventing graft versus host disease.
Owner:PHARMACYCLICS
Crystalline form I of ibrutinib
ActiveUS9751889B2Improve stabilityReduce moisture absorption performanceOrganic active ingredientsBiocideDiseaseBruton's tyrosine kinase
Owner:CRYSTAL PHARMATECH CO LTD +1
Separation and purification method of ibrutinib intermediate
InactiveCN106967071AImprove separation and purification efficiencyOrganic chemistryPurification methodsChloride
The invention discloses a separation and purification method of an ibrutinib intermediate-(3R)-4-amino-3-(4-phenoxyphenyl)-1-(1-tert-butoxycarbonylpiperidine-3-group)-1-1H-pyrazole [3, 4-d] pyrimidine. The separation and purification method includes: after Mitsunobu reaction for preparing the intermediate stops, adding magnesium chloride into a mixture; cooling after back-flowing; filtering to remove compound precipitate of magnesium chloride and triphenyl phosphine oxide to obtain the intermediate high in purity. Column chromatography is omitted in the process, so that the separation and purification method is high in efficiency and low in cost.
Owner:FUJIAN INST OF MICROBIOLOGY
Preparation method of Ibrutinib drug impurity
The invention belongs to the field of pharmaceutical synthesis, and relates to an impurity in the bulk pharmaceutical chemical production process and a preparation method of the impurity, in particular to a process impurity of Ibrutinib 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)-1Hpyrazole [3,4-d]pyrimidine-1-yl]-1-piperidyl]-2-propylene-1-ketone and a preparation method of the process impurity.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
HPLC method for analyzing ibrutinib and ibrutinib-preparation-related substances and usage of impurities as reference standard
The invention belongs to the field of medicine synthesis, and particularly relates to impurities appearing in the preparing process of ibrutinib (1-[(3R)-3-[4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine-1-butyl]-1-piperidyl]) and a method for analyzing an ibrutinib preparation. A method for detecting the impurities includes the following steps that 1, the ibrutinib or medicine with the ibrutinib is dissolved into a solvent to prepare a sample solution; 2, samples of one or more of compounds A to H are dissolved into a solvent to prepare a reference standard solution or a reference substance solution; 3, the chromatographic technique is carried out on the sample solution and the reference standard solution; 4, existence of any one or more of the compounds A to H in the ibrutinib or the medicine with the ibrutinib is detected.
Owner:BEIJING CREATRON INST OF PHARMA RES CO LTD
High-efficiency preparation method for ibrutinib
ActiveCN107383017AMild reaction conditionsEasy to operateOrganic chemistryBulk chemical productionOrganic synthesisIbrutinib
The invention discloses a high-efficiency preparation method for ibrutinib, which belongs to the technical field of organic synthesis. A concrete synthetic route is shown in a specification. The method has the advantages of mild reaction condition, simple operation, low cost, convenient purifying, friendly environment, and high product optical purity, and is suitable for industrial production.
Owner:HENAN NORMAL UNIV
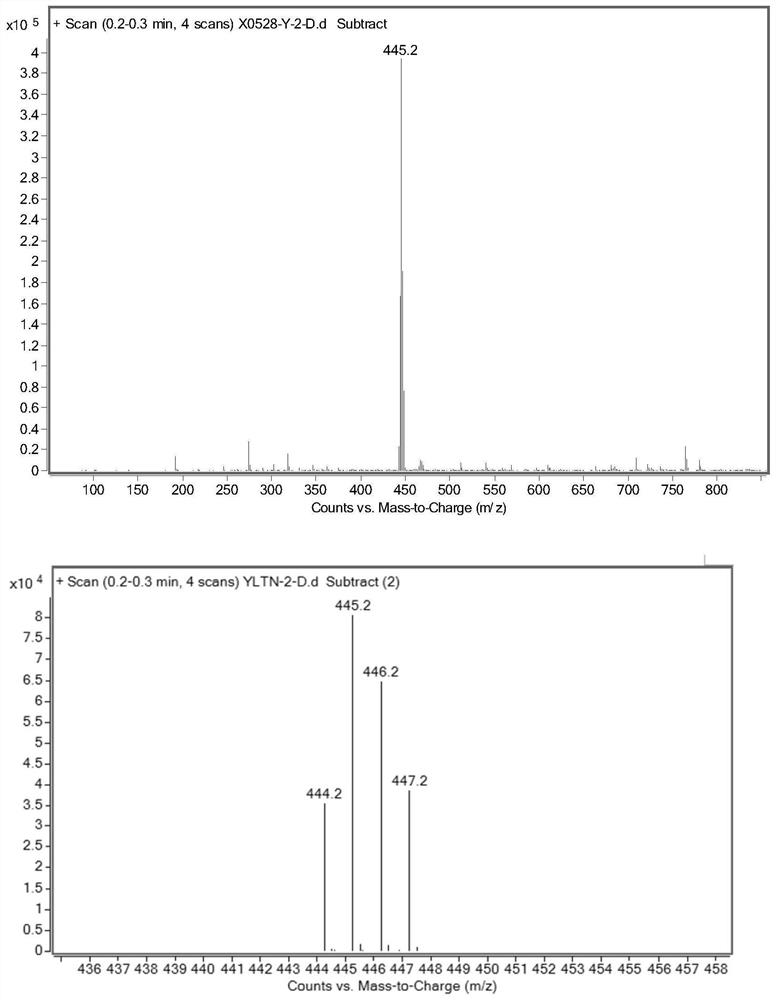
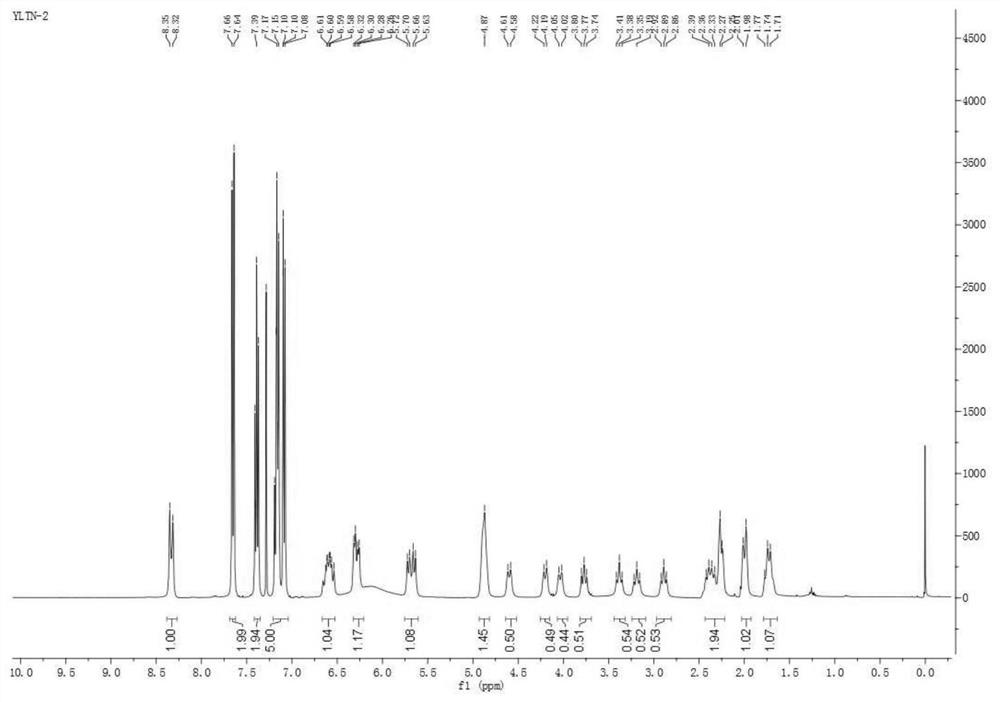
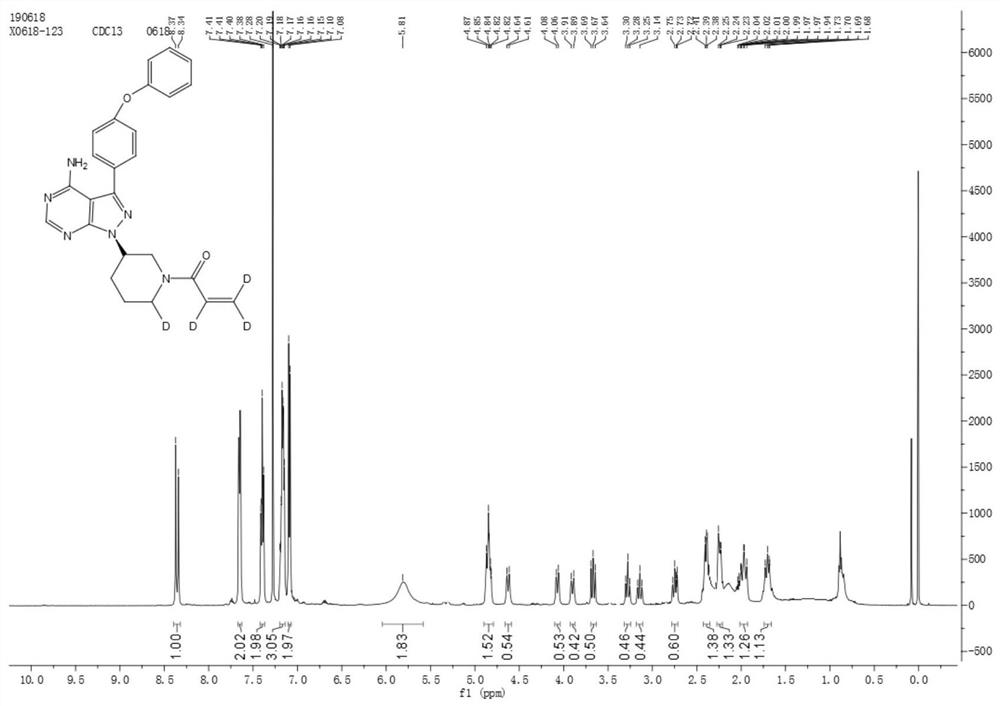
Preparation method of deuterated ibrutinib
ActiveCN112876484AImprove absorptionSimple methodOrganic chemistry methodsAntineoplastic agentsPharmacyPhotosens
The invention belongs to the field of chemical pharmacy, and particularly relates to a preparation method of deuterated ibrutinib. The preparation method of the deuterated ibrutinib provided by the invention comprises the following step of: synthesizing the positioned deuterated ibrutinib from the raw material ibrutinib under the illumination condition under the catalysis of a photosensitizer. The method provided by the invention has the characteristics of simple operation, mild conditions, high yield, good selectivity, environmental protection and the like, and the synthesized deuterated ibrutinib significantly increases the absorption degree of the drug, thereby providing a new process route for the synthesis of selective deuterated ibrutinib.
Owner:绍兴舜邦医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000011.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000012.PNG)
![Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib Method for synthesizing intermediate 4-amino-3-(4-phenoxy-phenyl)-1H-pyrazolo[3,4-d]pyrimidine of Ibrutinib](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2815104a-272d-435c-ad87-5493807452c7/BSA0000103894400000021.PNG)