Compositions Containing Ibrutinib
a technology of ibrutinib and ibrutinib, which is applied in the direction of drug compositions, inorganic non-active ingredients, granulation by pressing, etc., can solve the problems of interfering with the ability of cancer cells to grow, divide, repair, communicate with other cells, etc., and achieve the effect of facilitating the sprinkling of capsule contents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solid Compositions Containing Ibrutinib
[0118]Solid compositions containing Ibrutinib were prepared for inclusion in a capsule as described in the following.
[0119]A. 140 mg Capsule
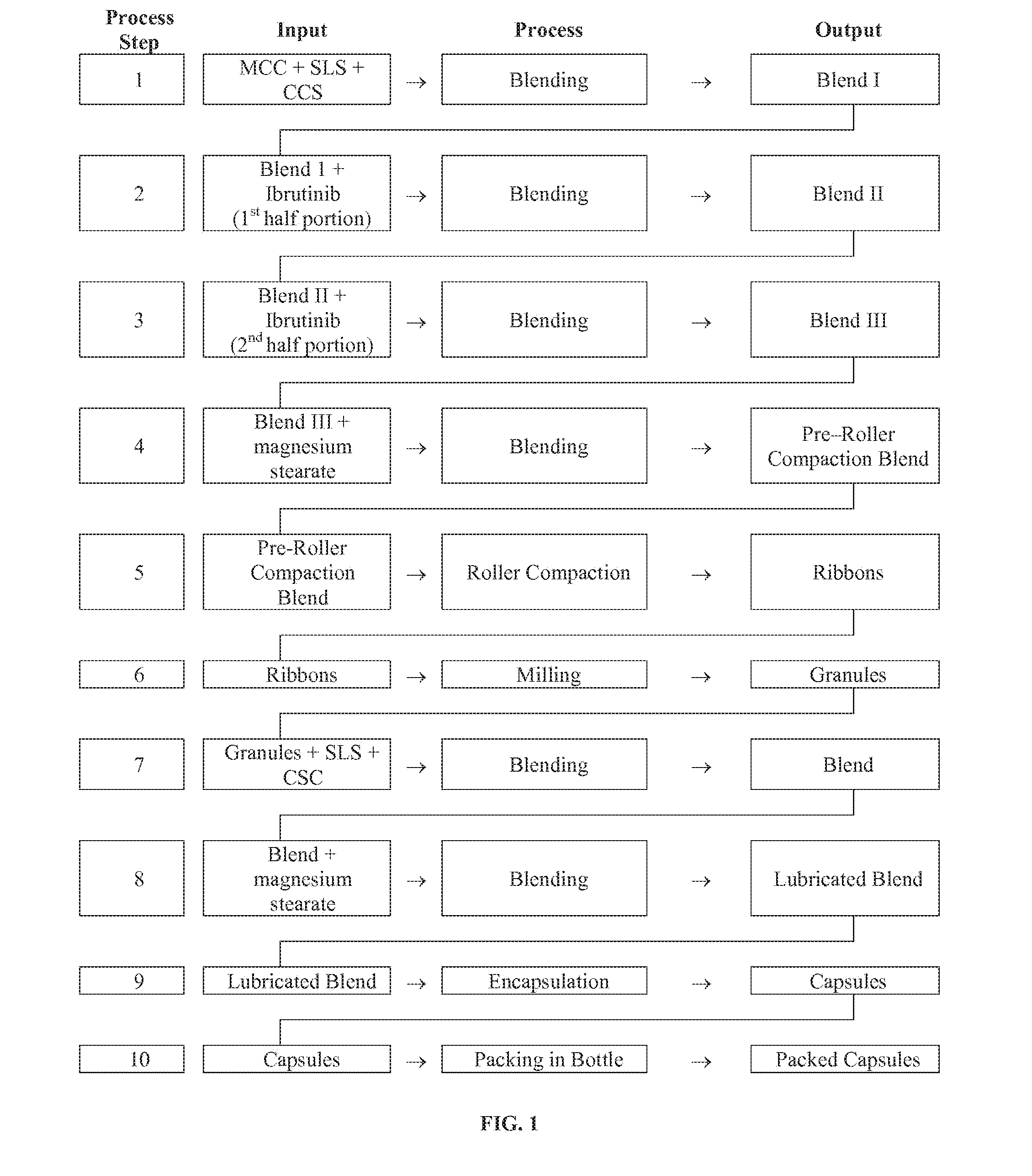
[0120]In this process, an intragranular blend was prepared by mixing MCC (151.49 mg; Avicel PH 101), SLS (9.40 mg; Kolliphor; fine), and CCS (13.10 mg; Ac-di-sol) in a vessel. This was then mixed with Ibrutinib (70 mg; micronized, Lonza, Nansha). The remaining Ibrutinib (70 mg) was then added and the composition mixed. Magnesium stearate (0.8 mg; Non-Bovine #5712) was then added to this mixture and the same blended to provide a pre-roller compaction blend. The pre-roller compaction blend was then roller compacted to form ribbons. The ribbons were then milled to provide a composition containing granules.
[0121]The granules were then blended with a second portion of SLS (4.6 mg; Kolliphor; fine) and CCS (9.9 mg; Ac-di-sol). To this blend was then added a second portion of magnesium stearate (0.8 mg; Non-Bovine...
example 2
Liquid Suspension Composition Containing Ibrutinib
[0125](i) 70 mg / mL Ibrutinib Liquid Suspension
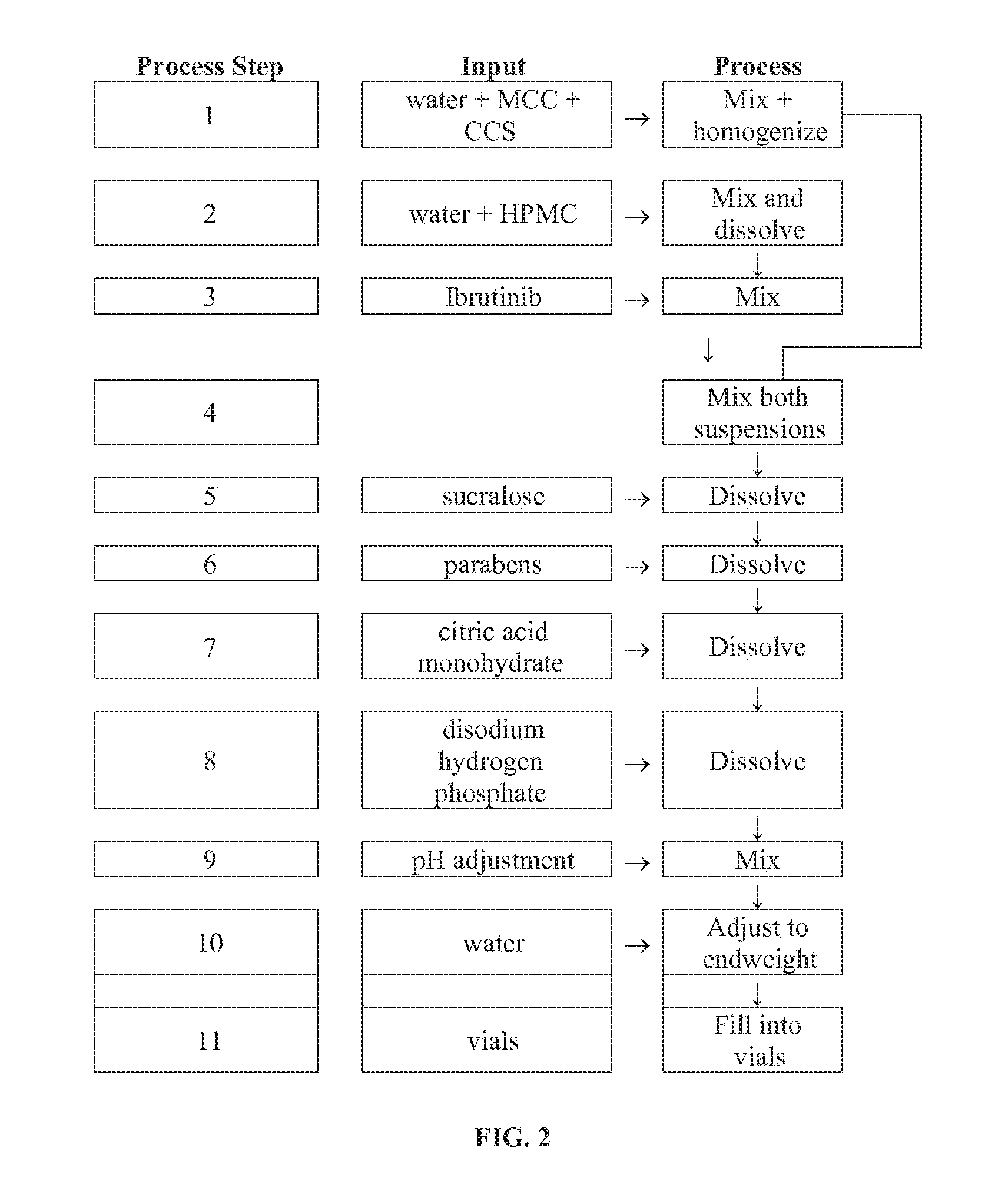
[0126]A liquid composition containing 70 mg / mL of Ibrutinib was prepared. Specifically, water (300 mL) was mixed with a composition MCC of CCS (Avicel RC591; 6.5 g) for 30 minutes. This dispersion was then homogenized for 30 seconds using a SILVERSON® homogenizer L2R at the maximal speed (7500 rpm). HPMC (2910 5 mPas; 1.25 g) was mixed with water (120 mL) until homogeneous using a magnetic stirrer. Micronized Ibrutinib (35 g, Lonza Clinical) was then added to the HPMC solution and mixed for 120 minutes. The MCC / CCS dispersion was then mixed with the Ibrutinib mixture. Sucralose (0.5 g), sodium methyl parahydroxybenzoate (0.5725 g), and sodium ethyl parahydroxybenzoate (0.2875 g) were added to the mixture. After about 10 minutes stirring, citric acid monohydrate (0.7565 g), and disodium hydrogen phosphate anhydrous parenteral (0.69 g) were then added to this mixture. The mixture was stirre...
example 3
Large Scale Preparation of a Suspension Containing Ibrutinib Preparation of 4L Batch
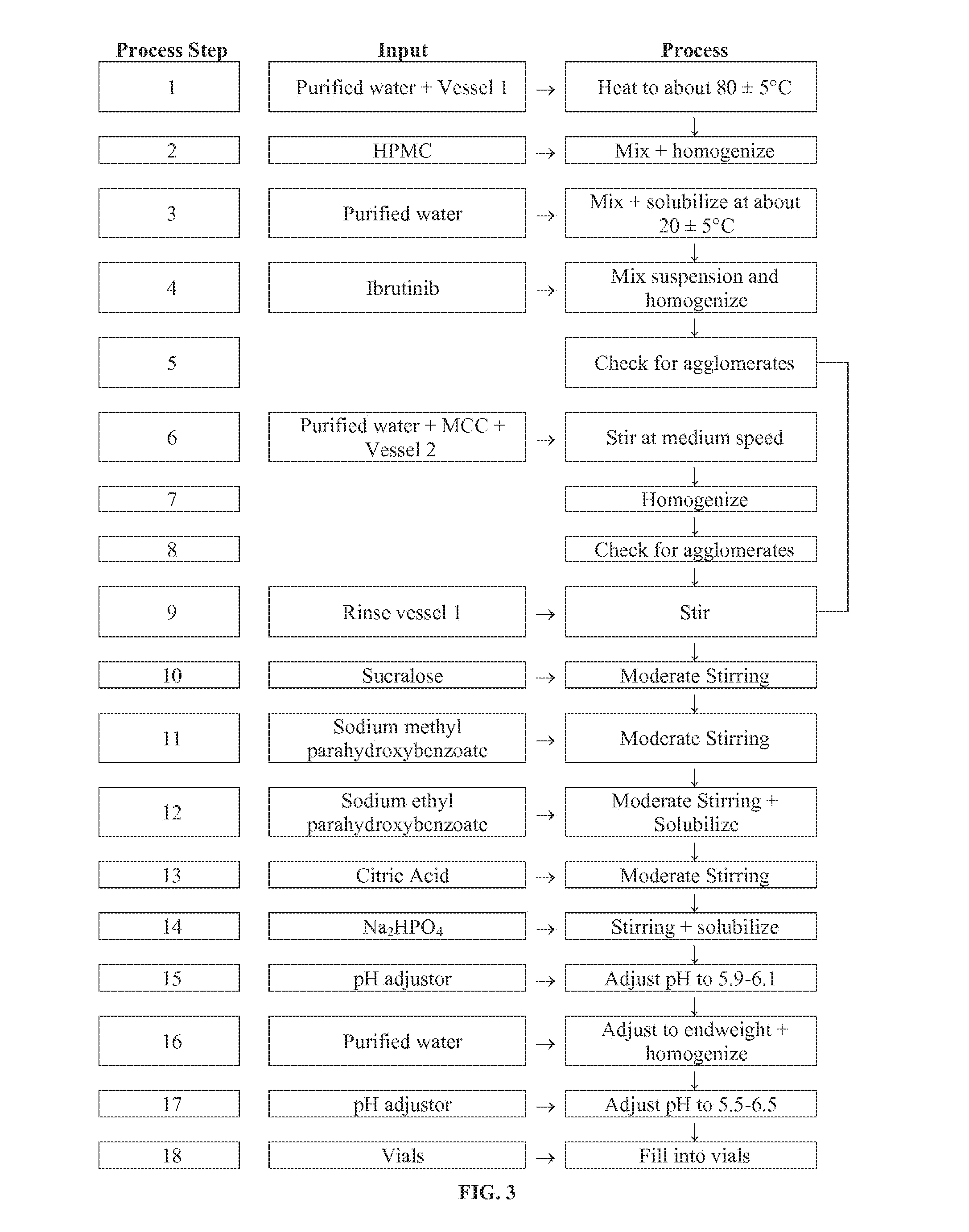
[0131]Purified water (480 g) was added to a vessel and warmed to about 83° C. at a stirring rate of about 400 rpm for about 60 minutes. HPMC (10.002 g) was slowly added to the vessel and the mixture stirred at a rate of about 7600 rpm for about 4 minutes until the mixture was homogenized. To this vessel was added purified water (480 g) and then mixture was stirred for about 5 minutes at a rate of about 500 rpm at room temperature until the mixture was solubilized. Ibrutinib (278.6 g) was added to the mixture and it was stirred at 600 rpm for about 2 h until it was homogenous. The mixture was monitored using a microscope for agglomerates.
[0132]Purified water (2400 g) was then added to a second vessel. At a rate of about 500 rpm, MCC (51.74 g; Avicel) was added to the second vessel over a period of 3 minutes, followed by stirring at a rate of about 400 rpm for about 60 minutes. This mixture was then ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com