Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Macroglobulinemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macroglobulinemia is the presence of increased levels of macroglobulins in the circulating blood. It is a plasma cell dyscrasia, resembling leukemia, with cells of lymphocytic, plasmacytic, or intermediate morphology, which secrete a monoclonal immunoglobulin M component. There is diffuse infiltration by the malignant cells of the bone marrow and also, in many cases, of the spleen, liver, or lymph nodes. The circulating macroglobulin can produce symptoms of hyperviscosity syndrome: weakness, fatigue, bleeding disorders, and visual disturbances. Peak incidence of macroglobulinemia is in the sixth and seventh decades of life. (Dorland, 28th ed)
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS7642238B2Prevent and reverse bone lossPrevent bone lossPeptide/protein ingredientsMicrobiological testing/measurementNormal boneNewly diagnosed
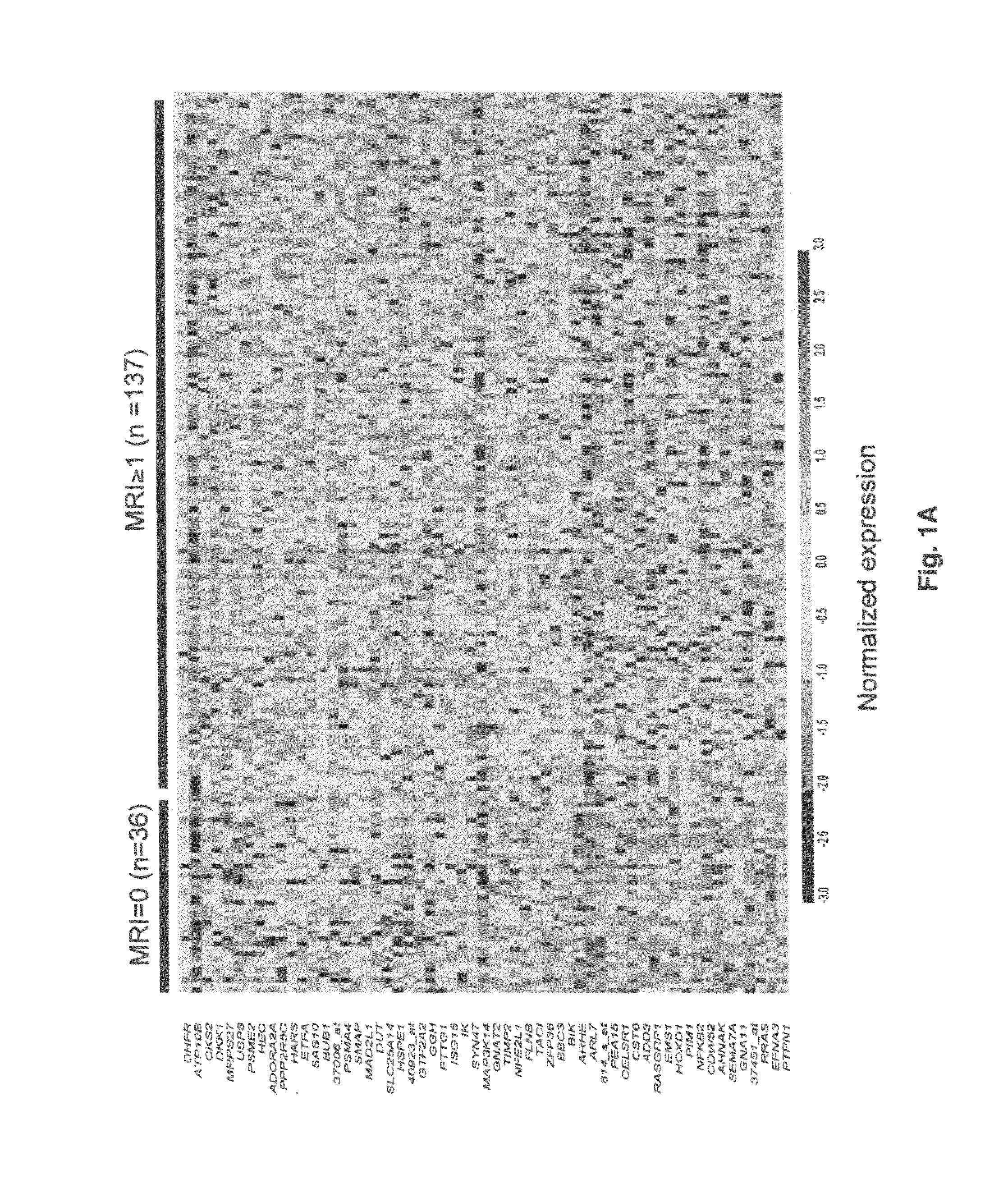
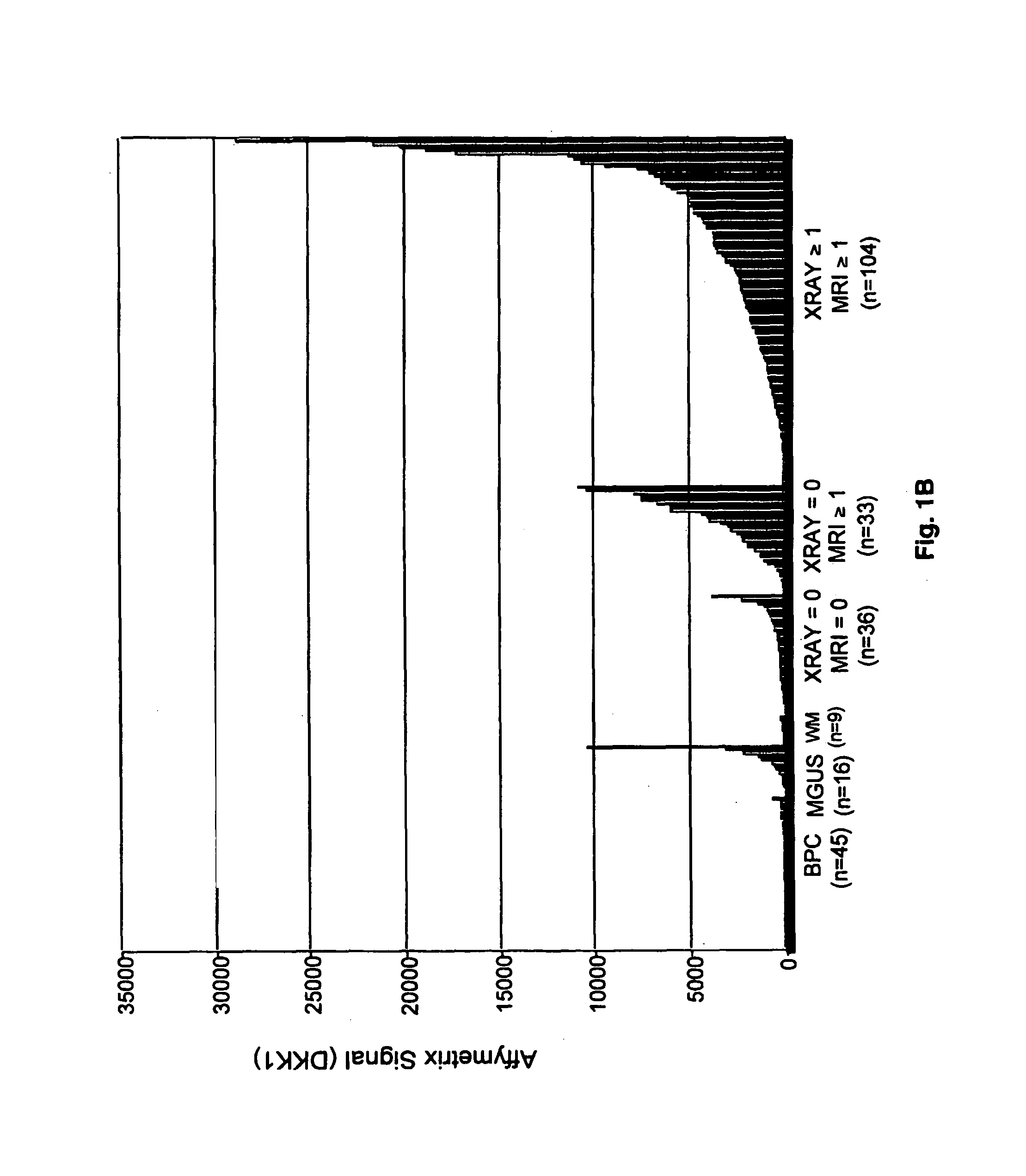
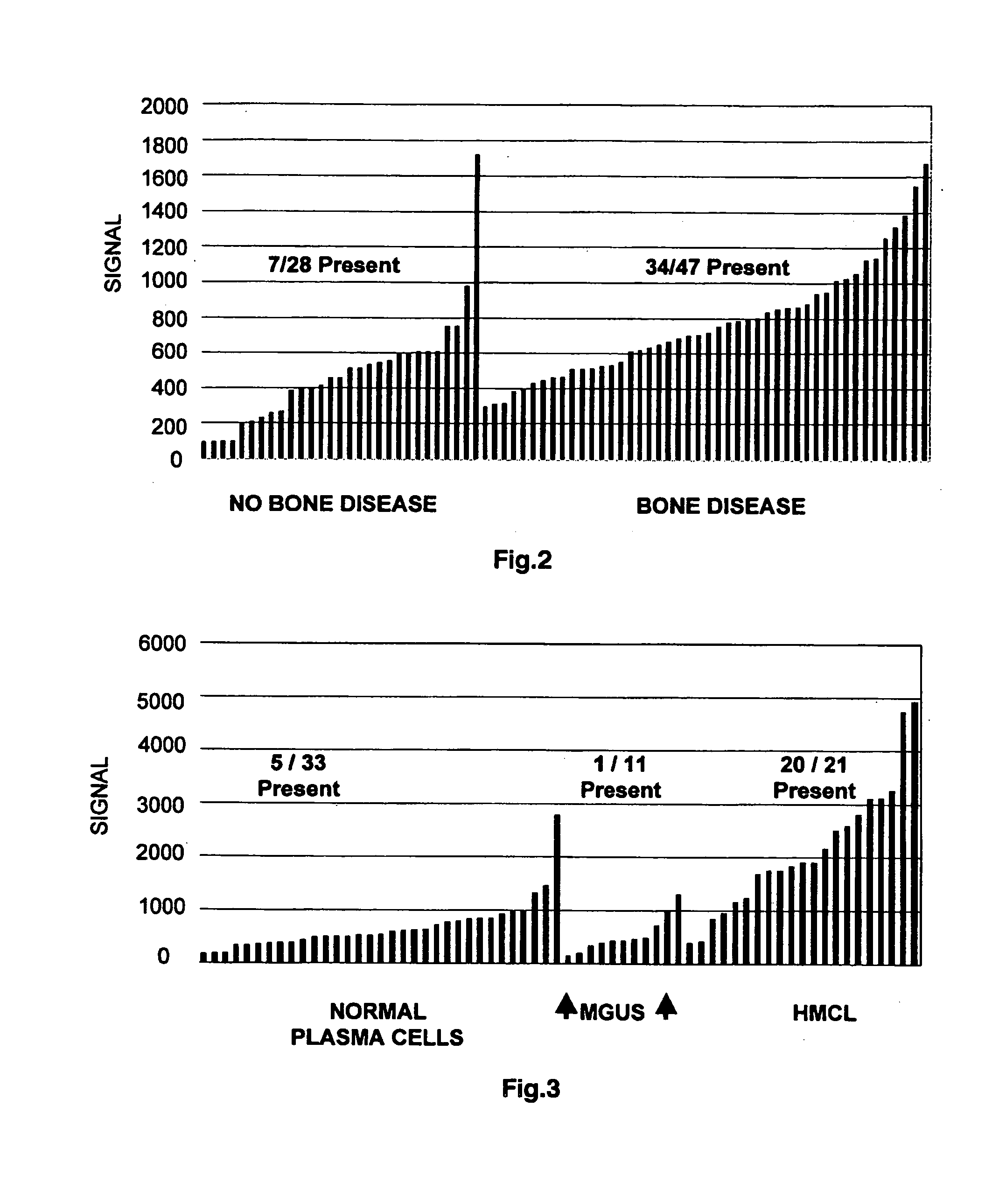
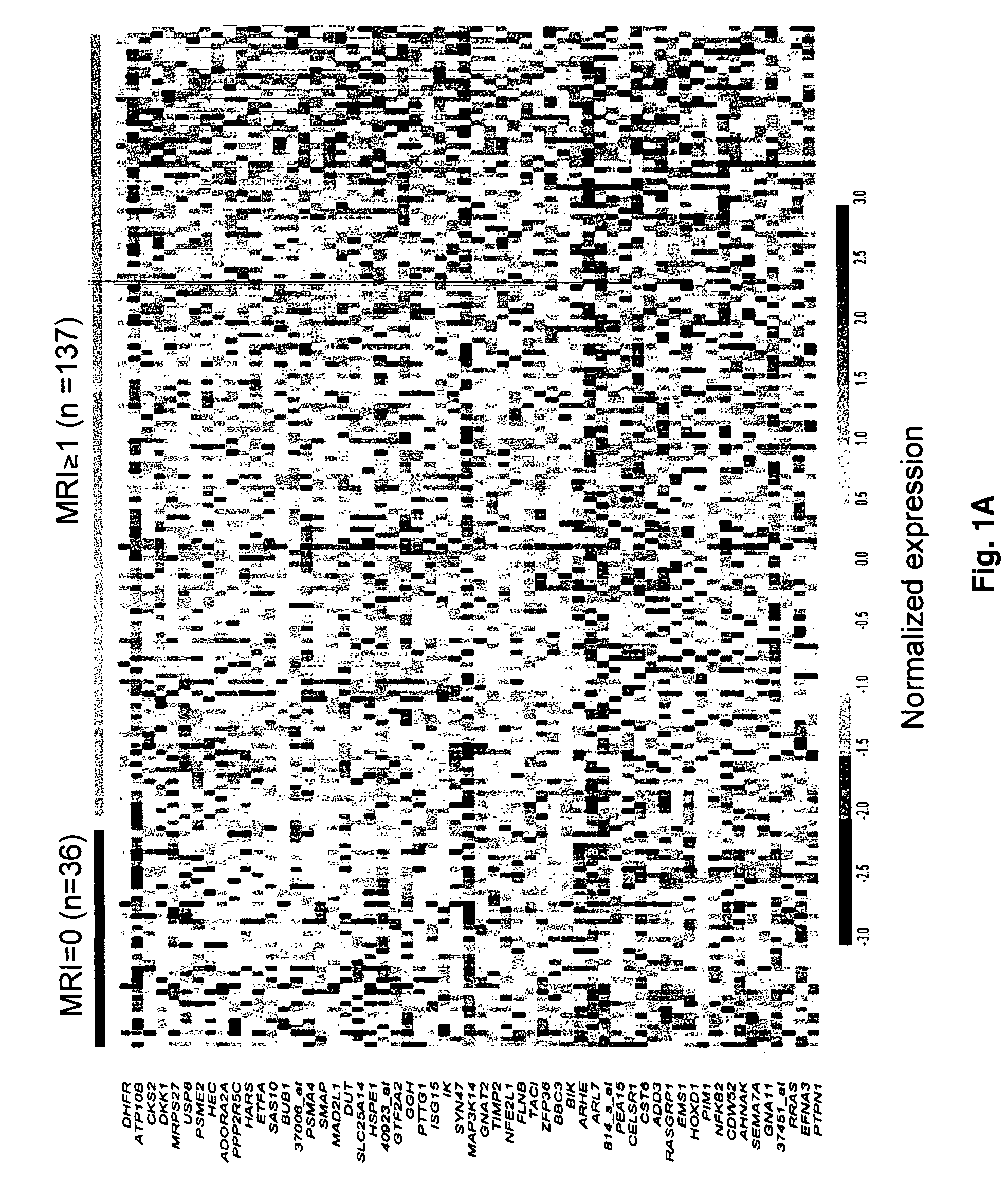
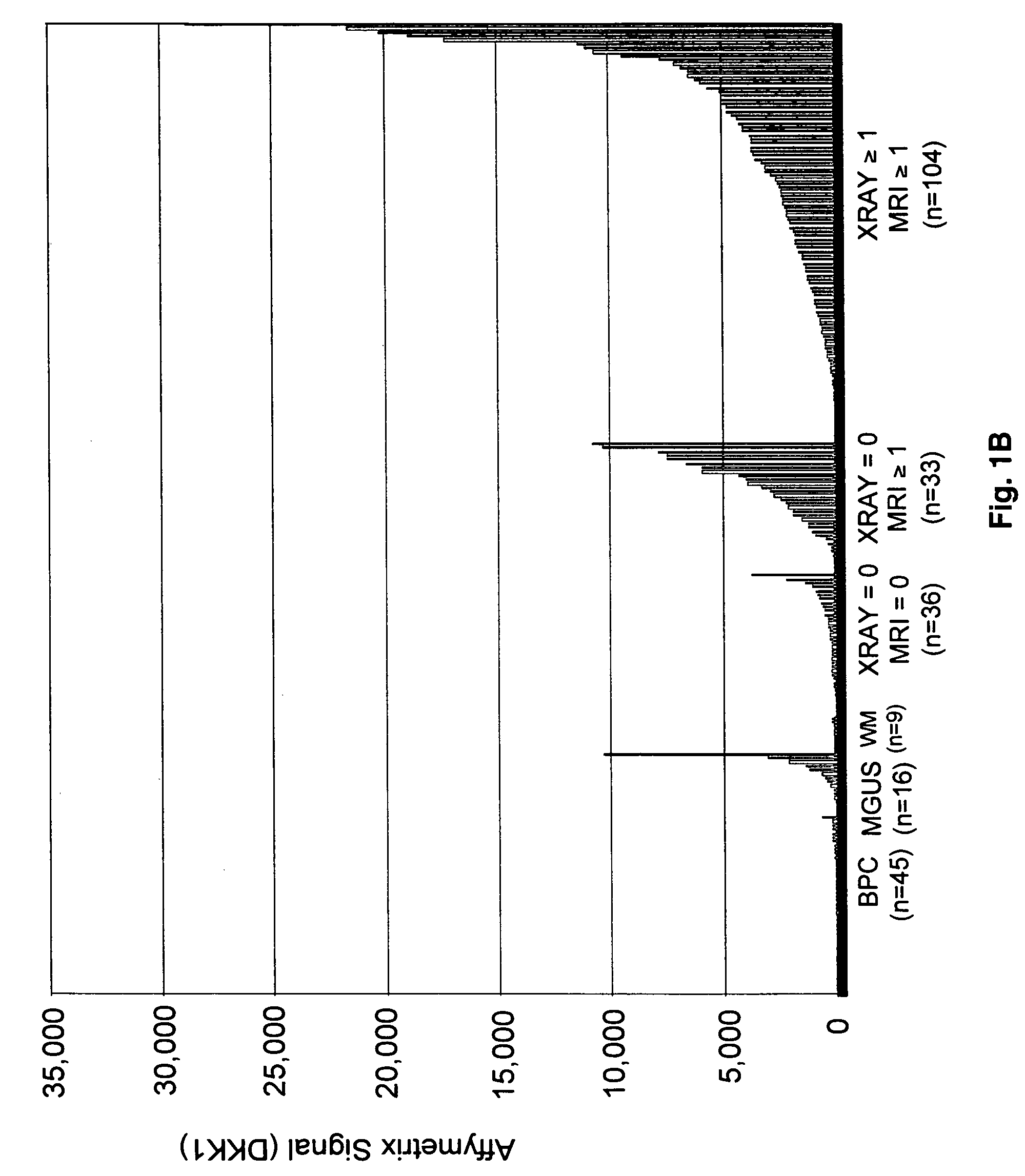
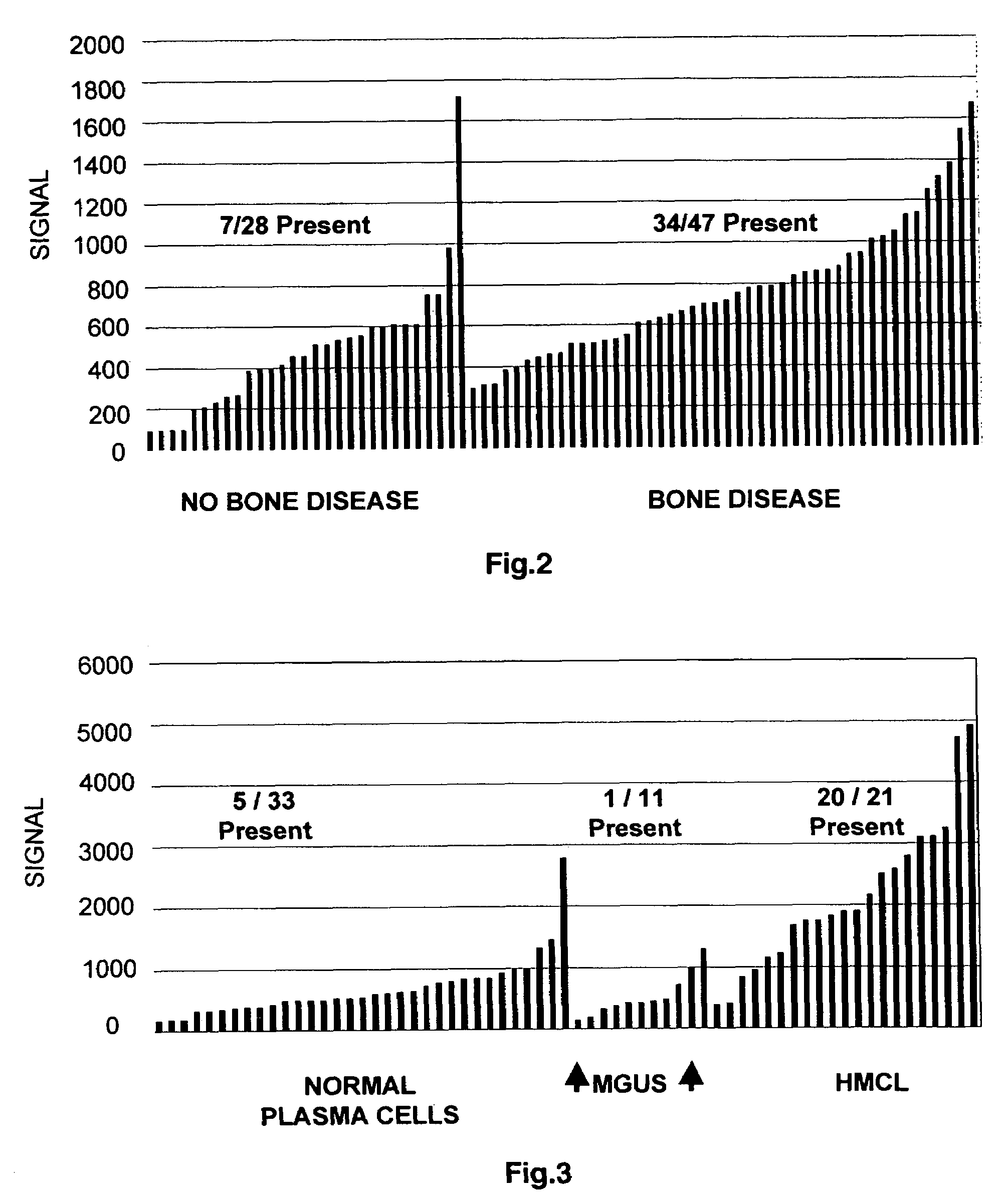
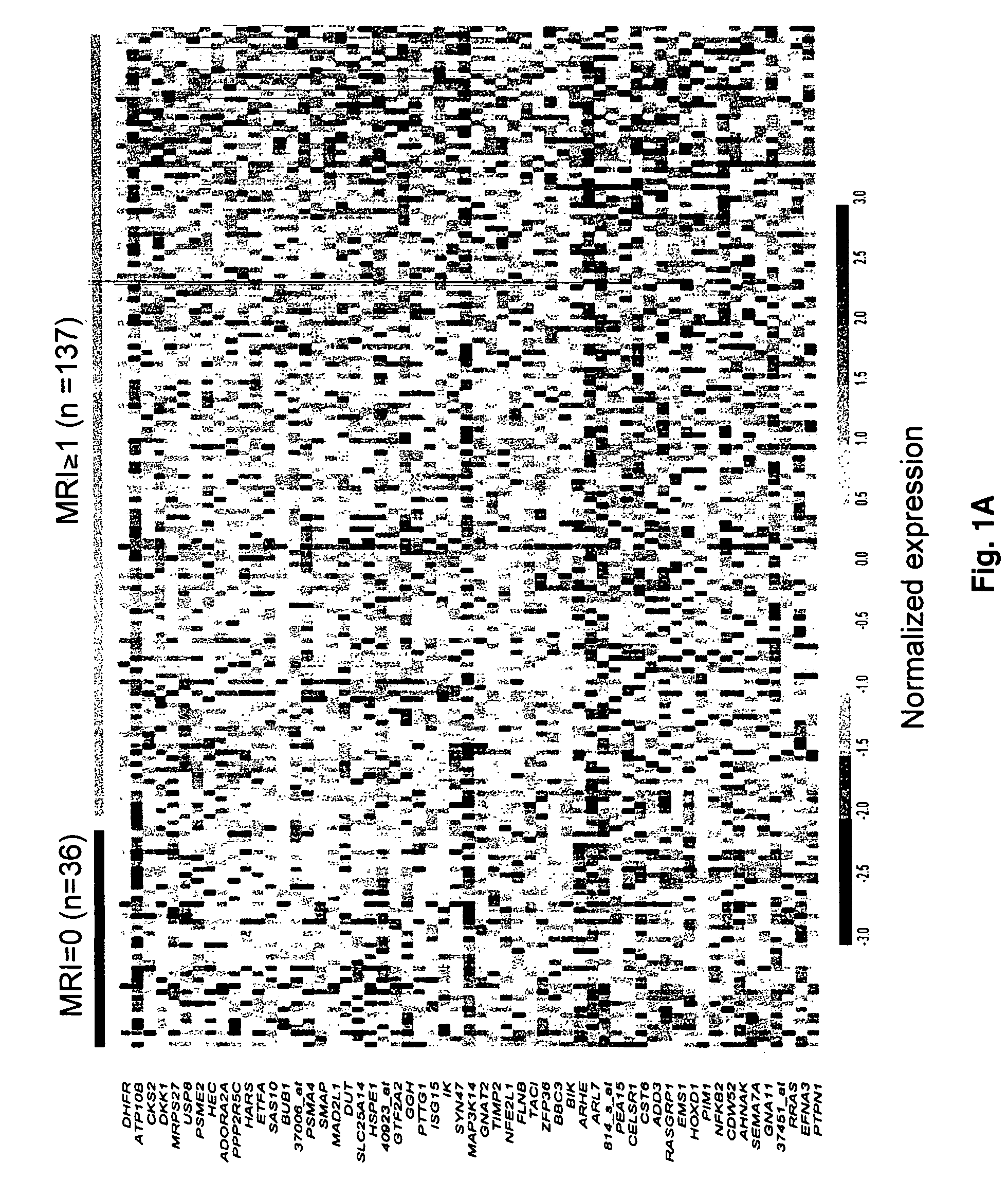
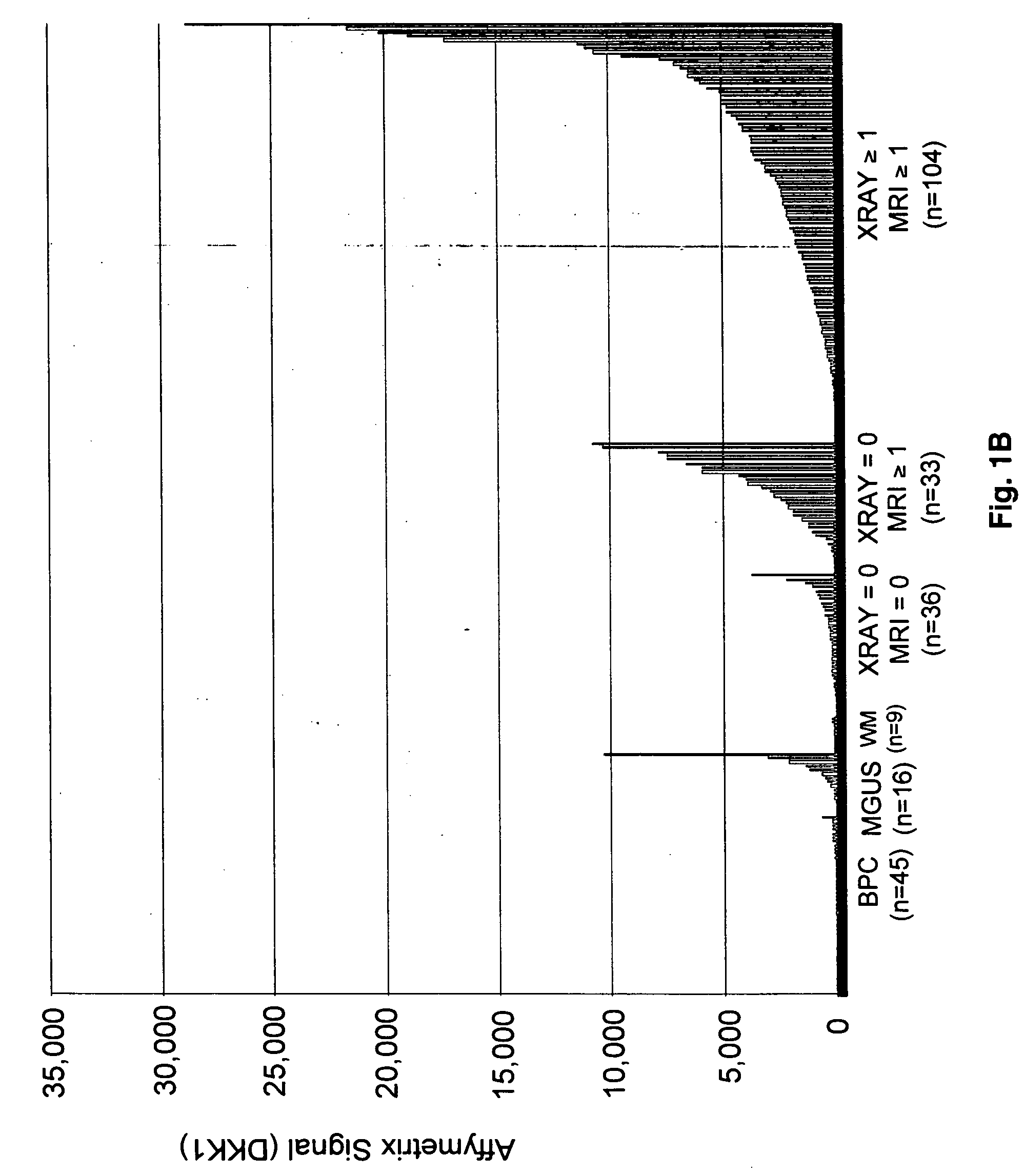
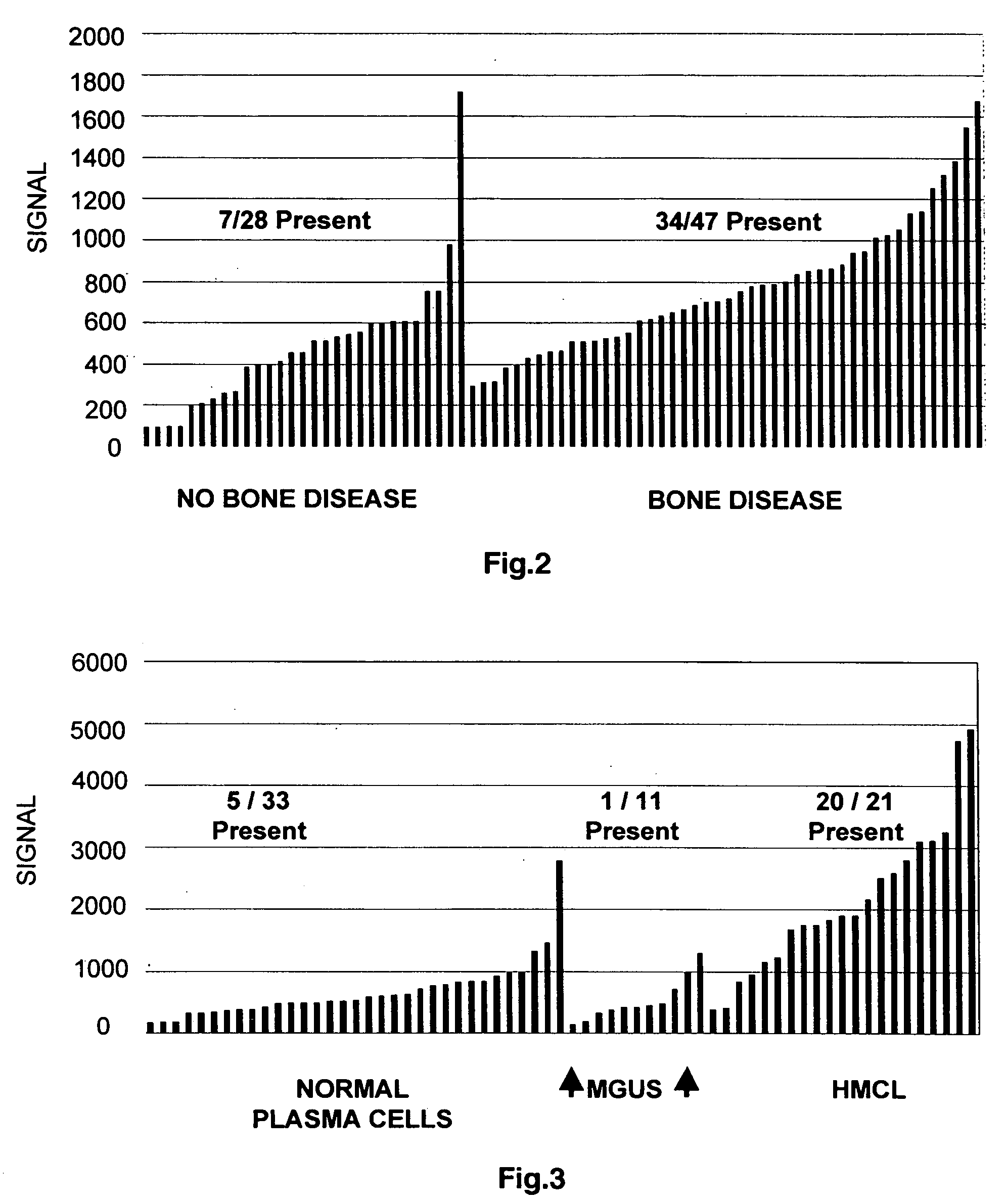
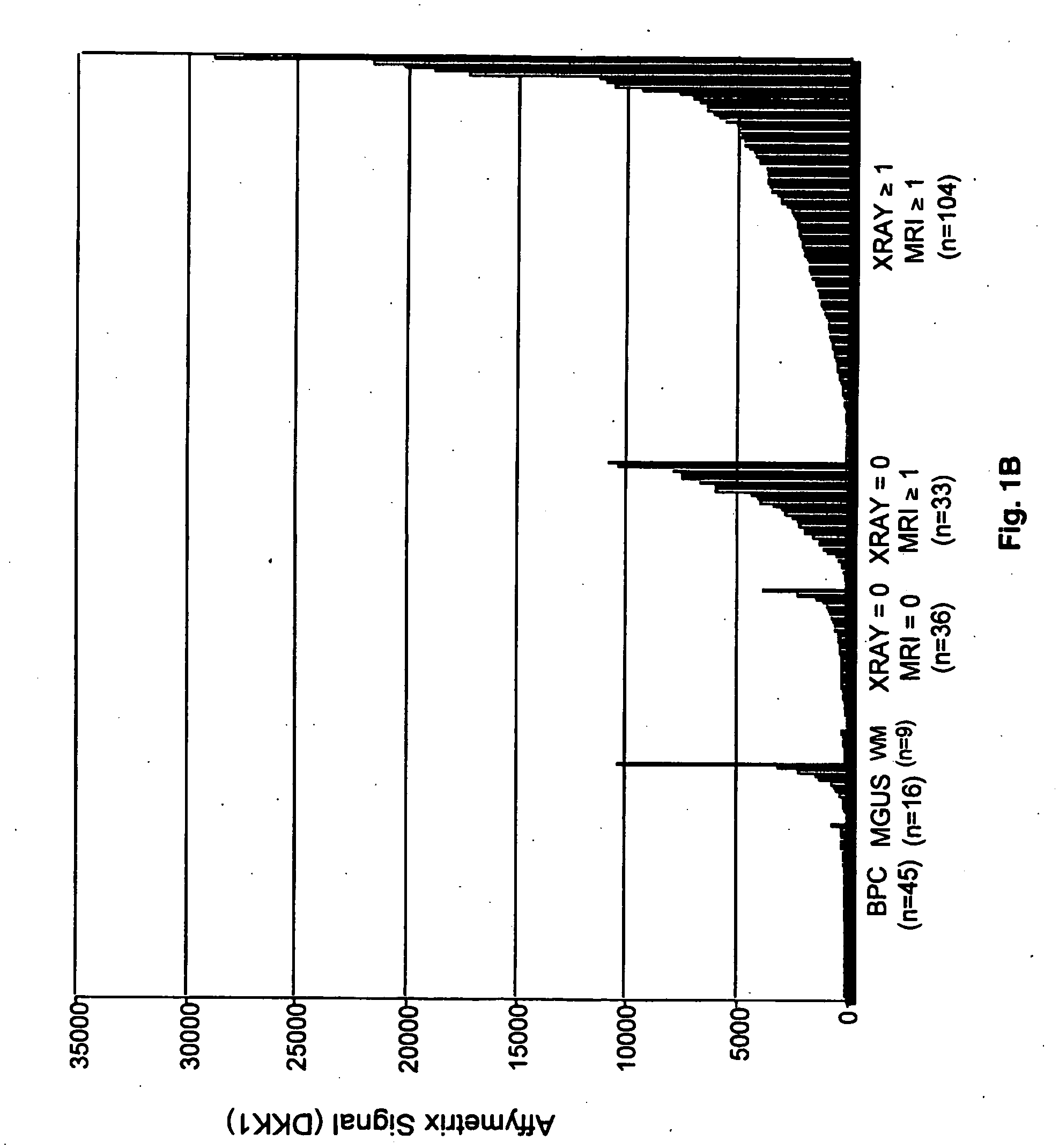
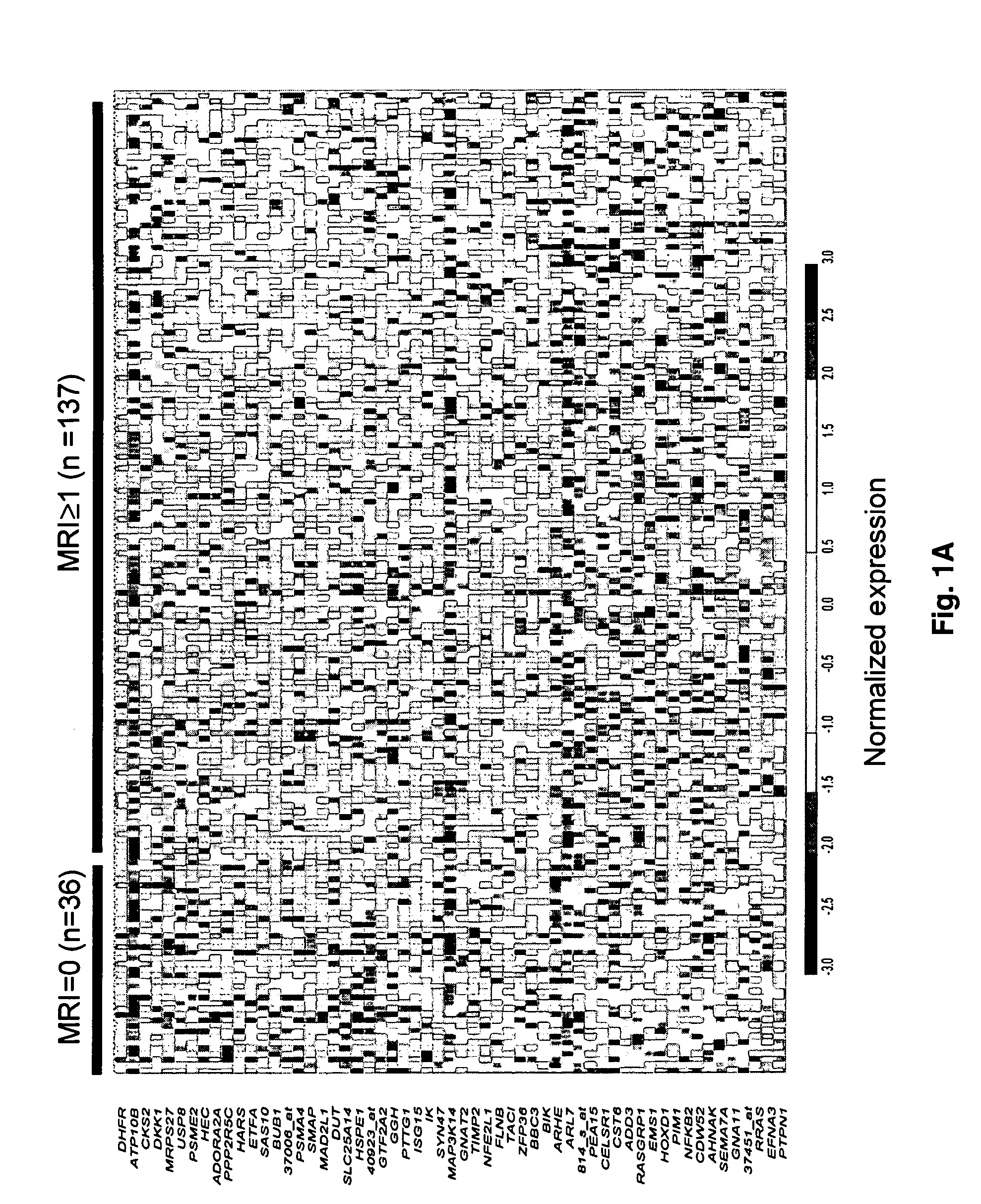
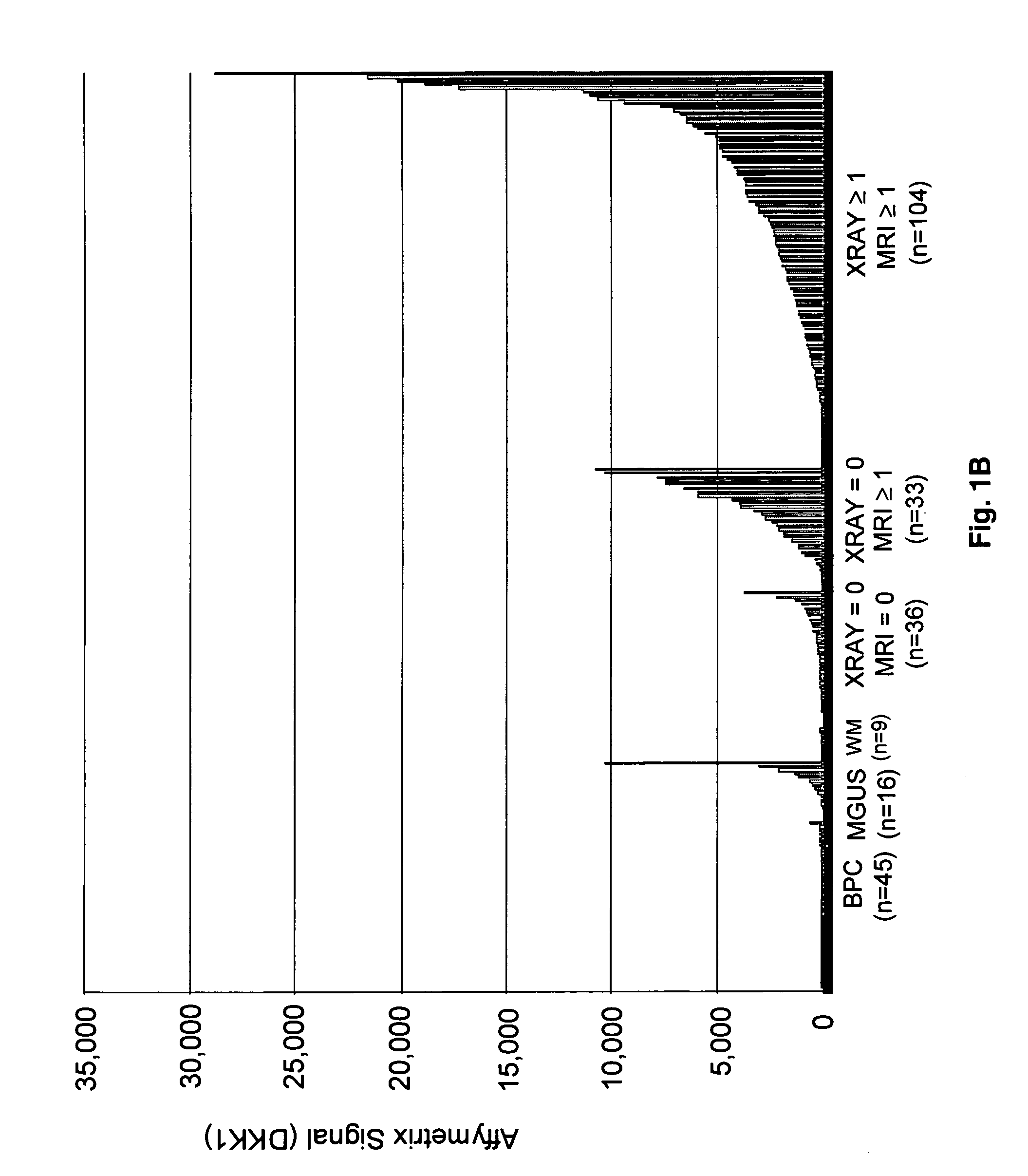
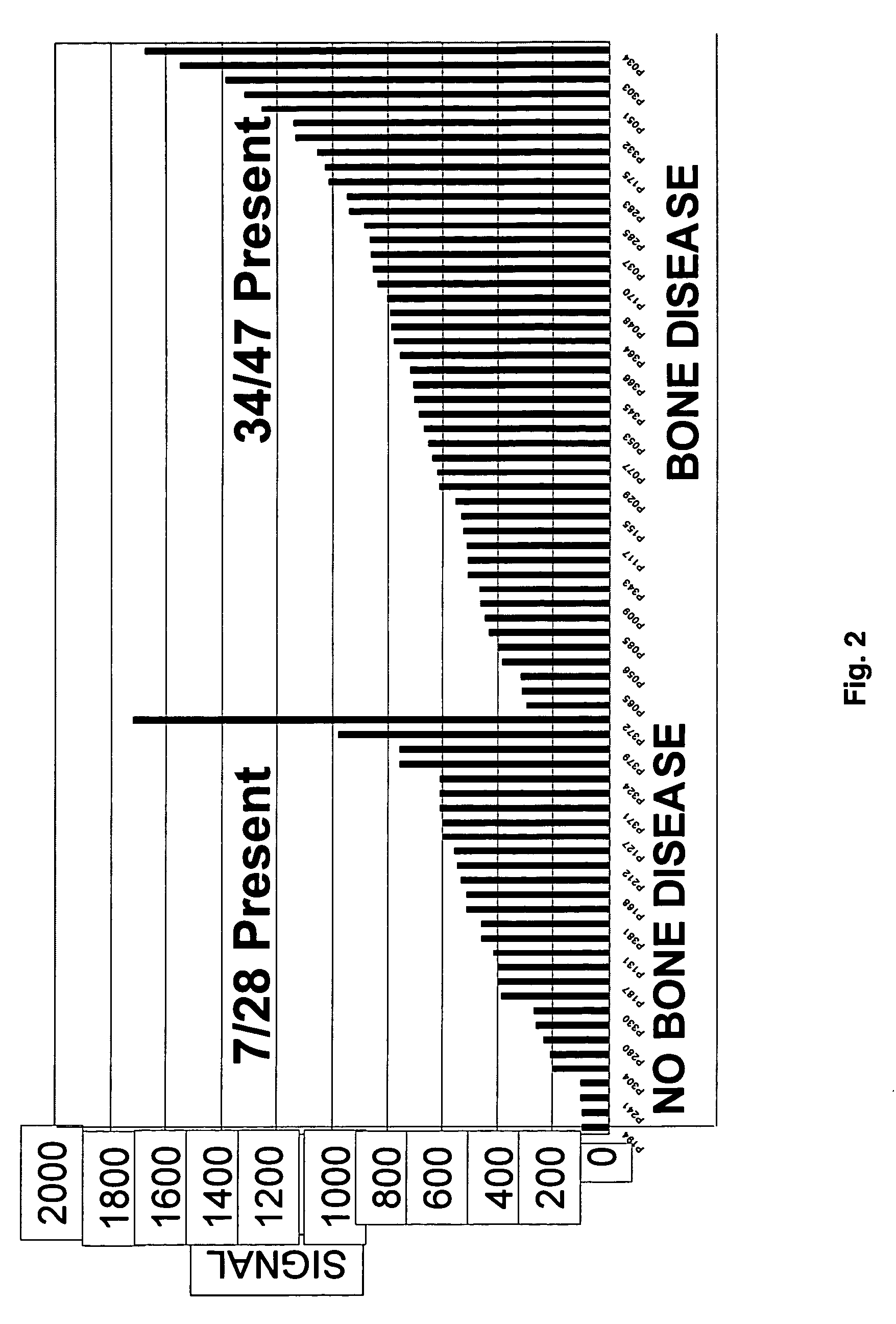
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
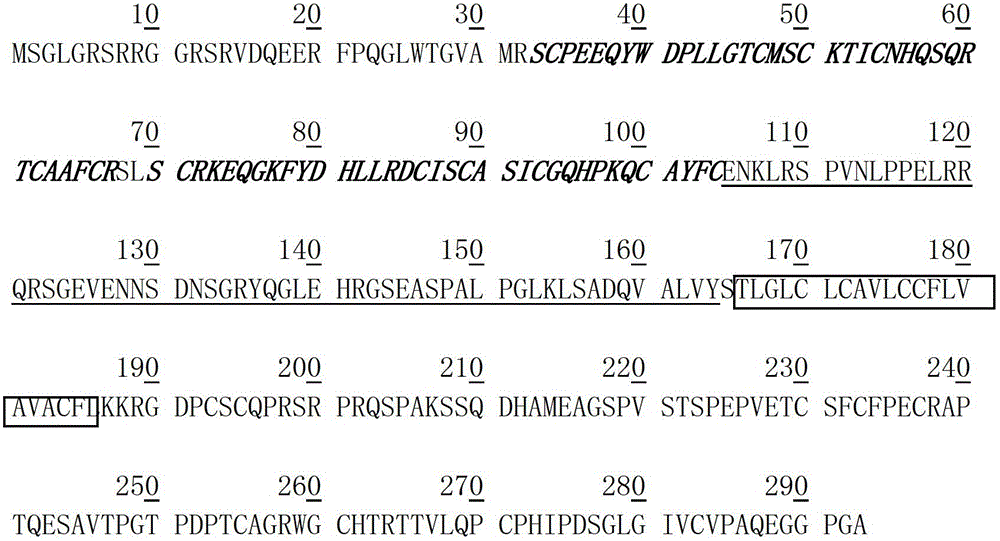
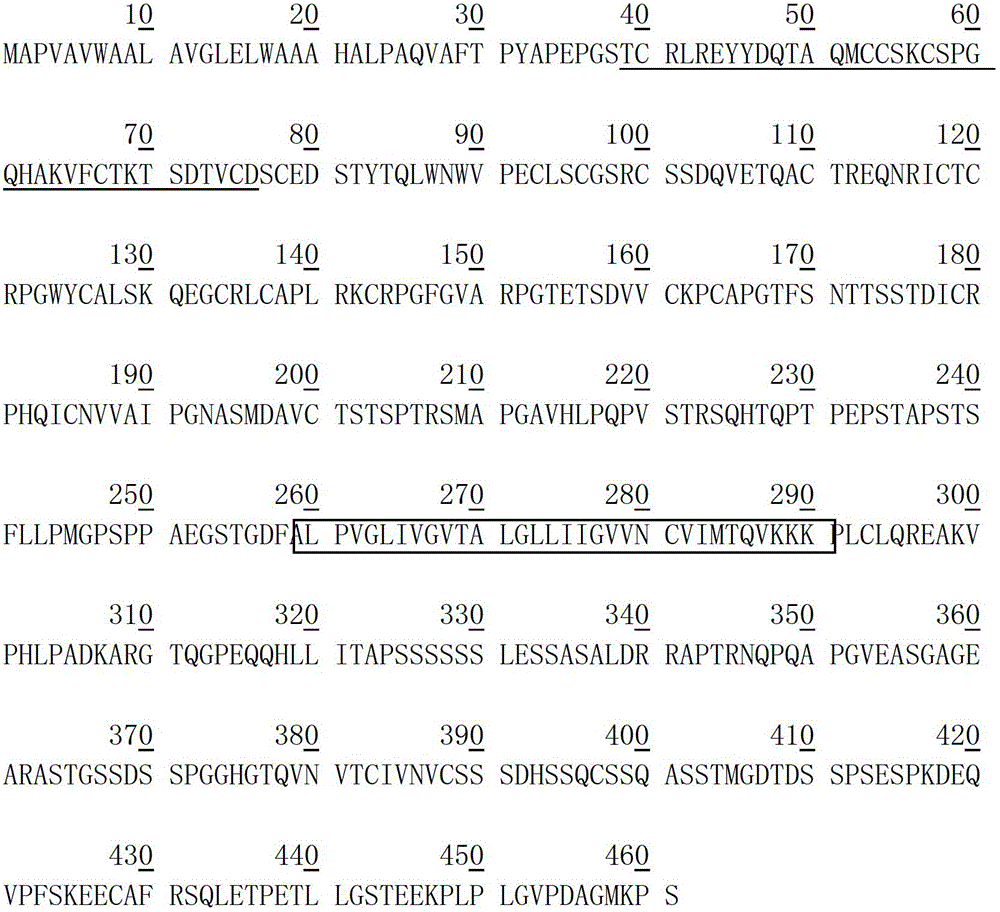
Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia
Disclosed are methods of treating Waldenstrom's Macroglobulinemia comprising administering to the animal, a therapeutically effective amount of a heterocyclic compound of Formula I.
Owner:TRIPHASE RES & DEV I
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS20060019895A1Increase bone massBone lossPeptide/protein ingredientsMicrobiological testing/measurementFrzbNewly diagnosed
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Molecular determinants of myeloma bone disease and use thereof
InactiveUS20070066558A1Increase bone massBone lossGenetic material ingredientsMicrobiological testing/measurementNewly diagnosedNormal bone
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease reduce tumor burden in multiple myeloma and to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS7459437B2Block alkaline phosphatase productionReduce decreasePeptide/protein ingredientsMicrobiological testing/measurementWaldenstrom macroglobulinemiaNormal bone
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:UNIV OF ARKANSAS FOR MEDICAL SCI THE
Methods for evaluating and treating waldenstrom's macroglobulinemia
ActiveUS20160222465A1ResilienceSuccessful treatmentOrganic active ingredientsMicrobiological testing/measurementMacroglobulinemiaMacroglobulin
Owner:DANA FARBER CANCER INST INC
Fusion protein inhibiting taci-baff complex formation and preparation method therefor and use thereof
ActiveUS20170081387A1Reduce concentrationReduce weightPeptide/protein ingredientsAntibody mimetics/scaffoldsBALB/cAutoimmune disease
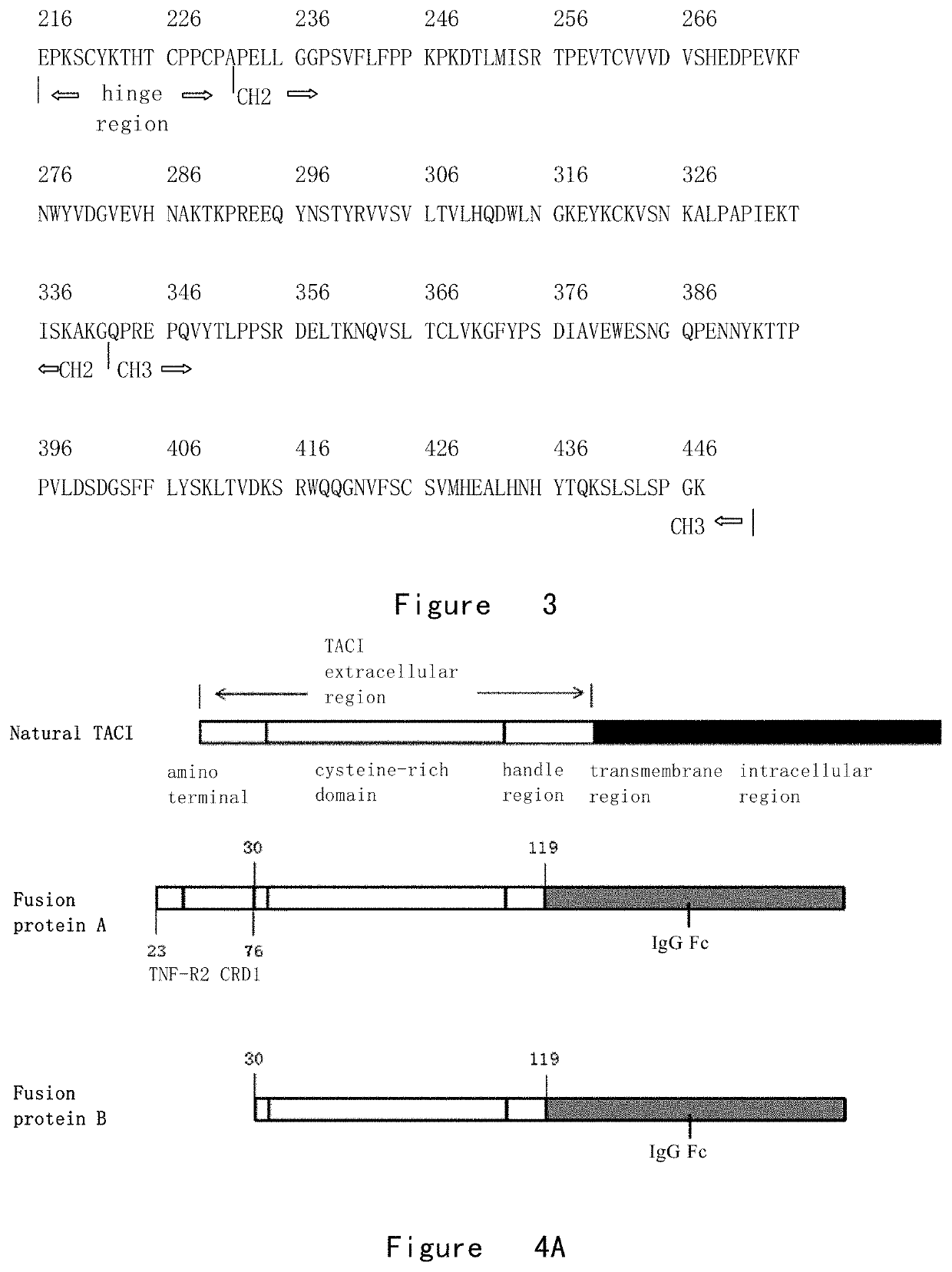
The present invention provides a fusion protein inhibiting TACI-BAFF complex formation and preparation method therefor and use thereof. Specifically, the fusion protein of the present invention is of high biological activity for blocking BAFF / APPRIL, and may significantly lower the serum IgM concentration in normal Balb / c mice as well as the serum IgM and IgE concentration in C57 / B6 mice with asthma. The TACI-Fc fusion protein of the present invention may be used in the treatment of autoimmune diseases, including asthma, systemic lupus eythematosus and rheumatoid arthritis, etc., and may also be used in the treatment of B cell enrichment-related diseases, including multiple myeloma, chronic lymphocytic leukemia, macroglobulinemia and plasmacytic leukemia, etc.
Owner:SHANGHAI KANDA BIOTECHNOLOGY CO LTD +1
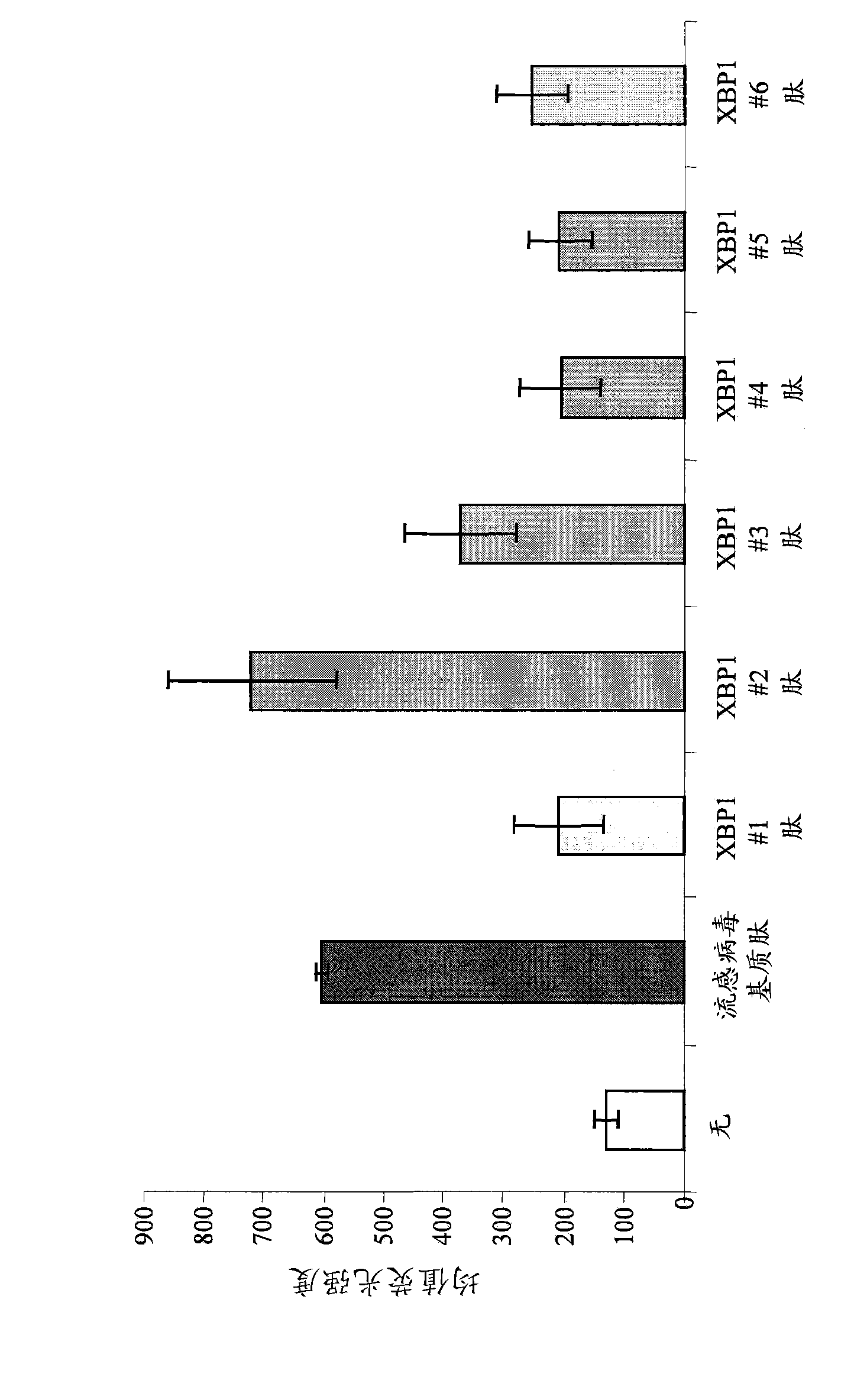
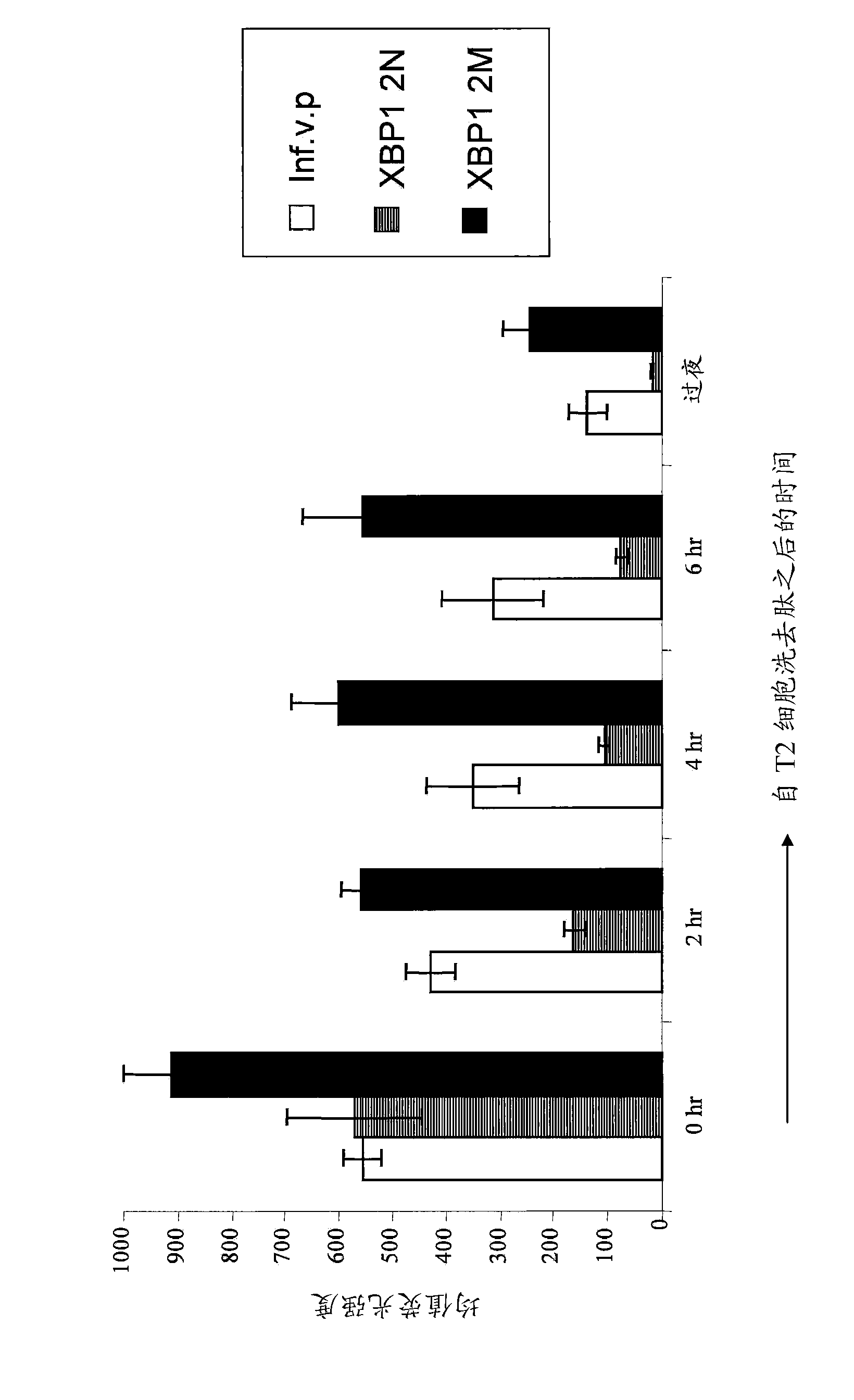
XBP1, CD138, and CS1 peptides
The invention discloses features, inter alia, immunogenic XBP1-, CD138-, and CS1-derived peptides (and pharmaceutical compositions thereof). The peptides can be used in a variety of methods such as methods for inducing an immune response, methods for producing an antibody, and methods for treating a cancer (e.g., a plasma cell disorder such as multiple myeloma or Waldenstrom's macroglobulinemia). The peptides can also be included in MHC molecule multimer compositions and used in, e.g., methods for detecting a T cell in a population of cells.
Owner:DANA FARBER CANCER INST INC
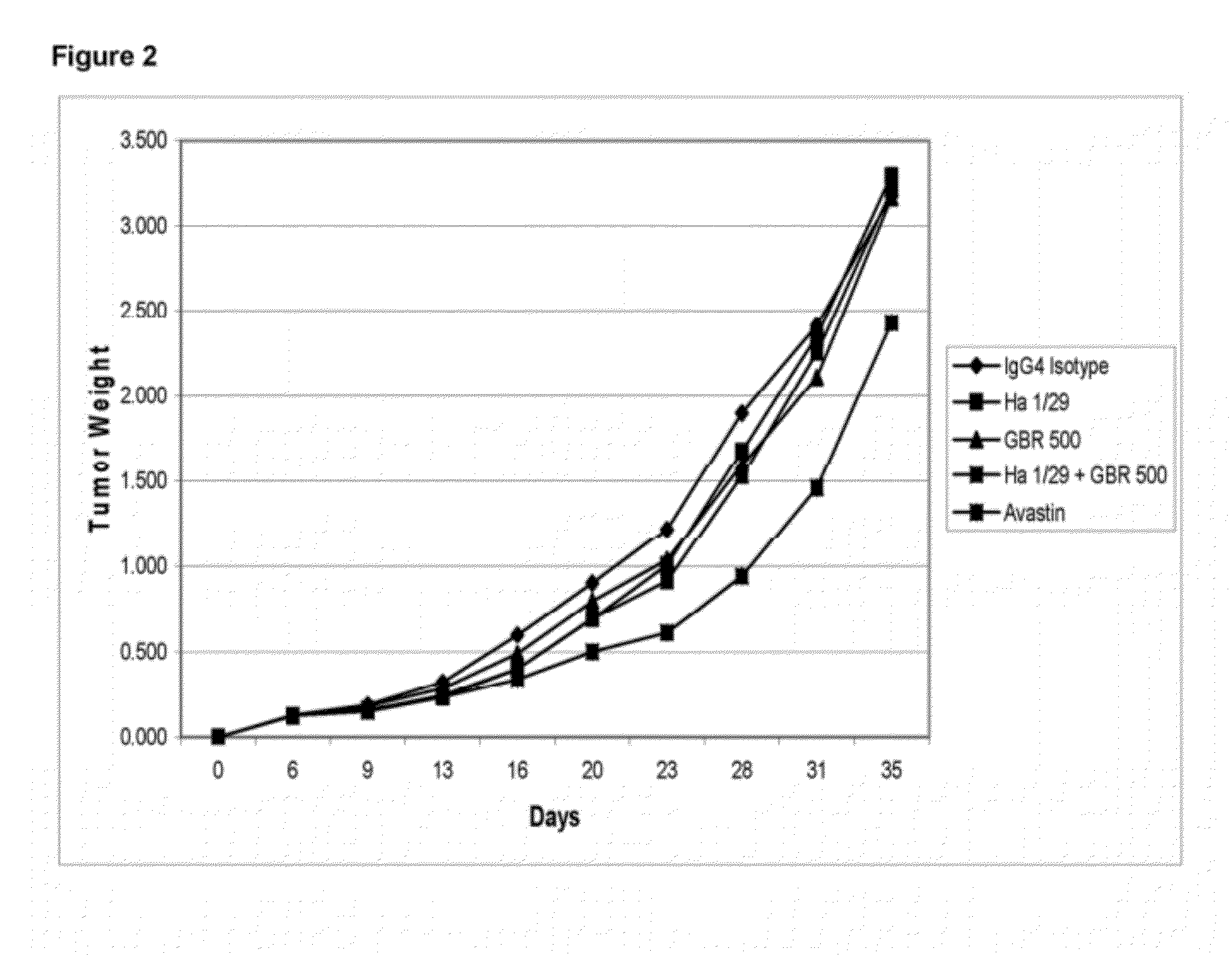
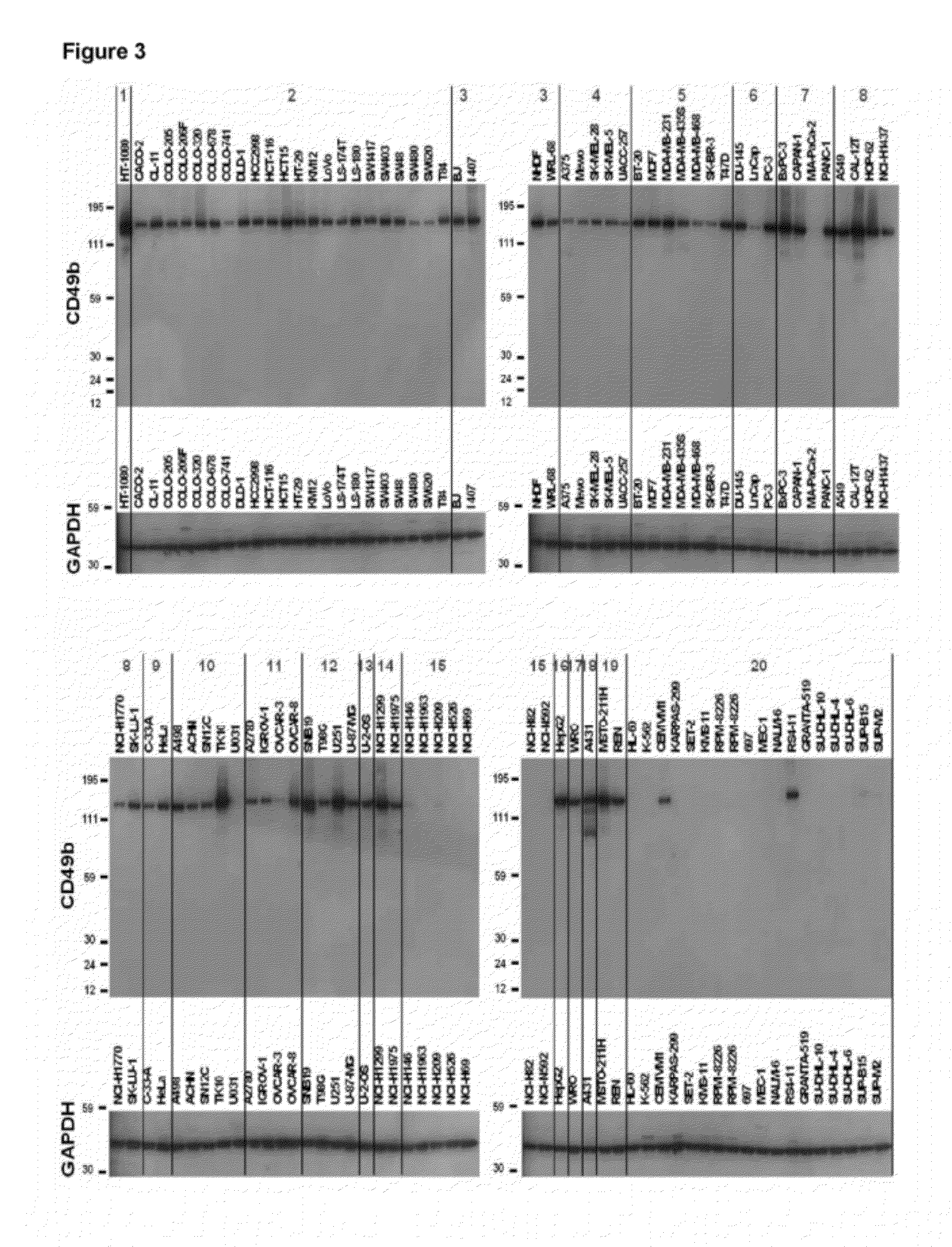
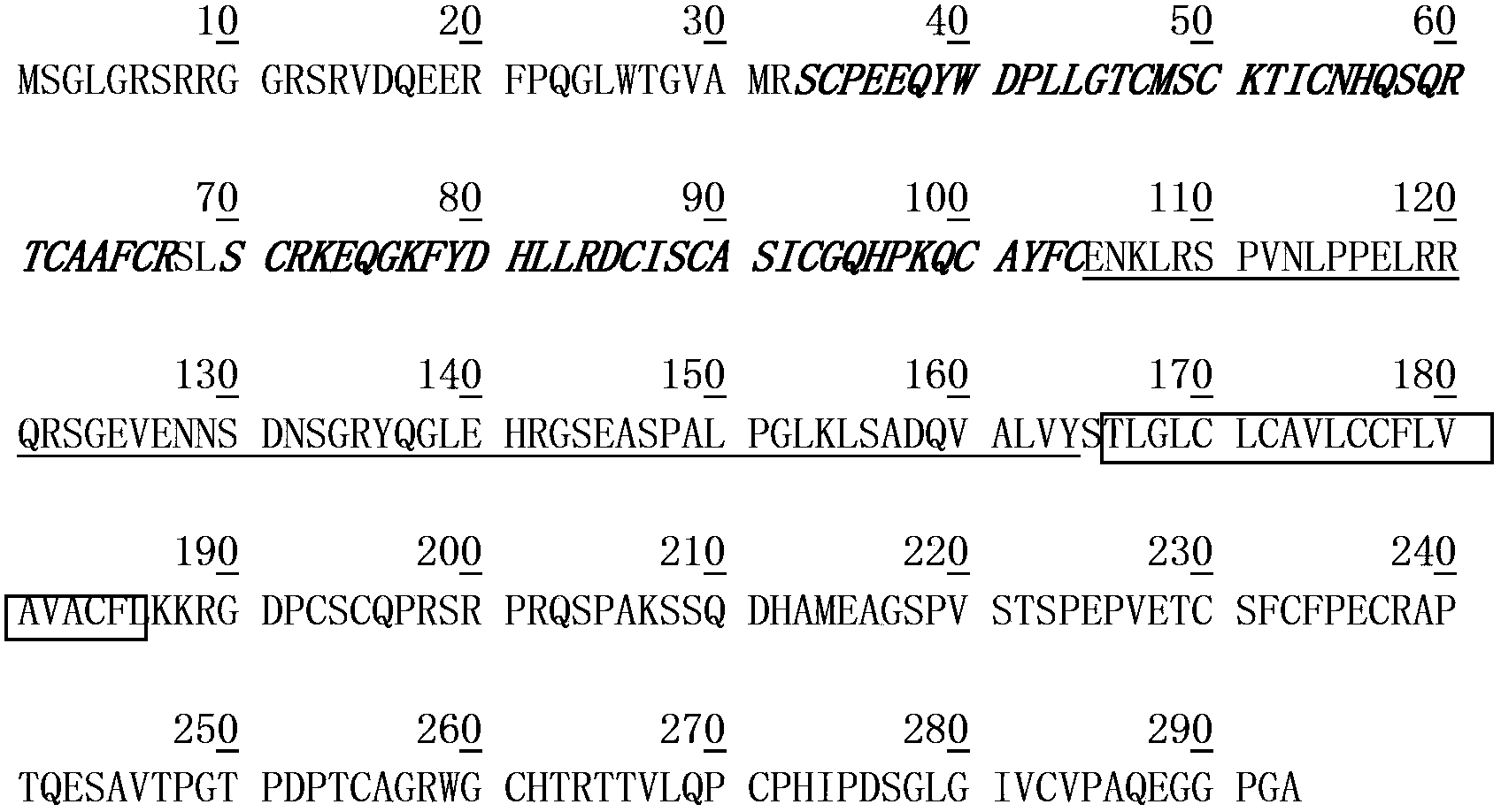
Treatment with anti-alpha2 integrin antibodies
The invention relates to treatment of cancer. More specifically the invention relates to methods of treating cancer selected from the group consisting of squamous cell cancer, lung cancer including small-cell lung cancer, non-small cell lung cancer, adenocarcinoma of the lung, and squamous carcinoma of the lung, cancer of the peritoneum, hepatocellular cancer, gastric or stomach cancer including gastrointestinal cancer, pancreatic cancer, glioblastoma, cervical cancer, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal cancer, liver cancer, prostate cancer, vulval cancer, thyroid cancer, hepatic carcinoma and various types of head and neck cancer, as well as B-cell lymphoma including low grade / follicular non-Hodgkin's lymphoma (NHL); small lymphocytic (SL) NHL; intermediate grade / follicular NHL; intermediate grade diffuse NHL; high grade immunoblastic NHL; high grade lymphoblastic NHL; high grade small non-cleaved cell NHL; bulky disease NHL; mantle cell lymphoma; AIDS-related lymphoma; and Waldenstrom's Macroglobulinemia; chronic lymphocytic leukemia (CLL); acute lymphoblastic leukemia (ALL); Hairy cell leukemia; chronic myeloblastic leukemia; and post-transplant lymphoproliferative disorder (PTLD), as well as abnormal vascular proliferation associated with phakomatoses, edema such as that associated with brain tumors, Meigs' syndrome, melanoma, mesothelioma, multiple myeloma, fibrosarcoma, osteosarcoma and epidermoid carcinoma, by administering antibodies directed to α2β1 integrin.
Owner:ICHNOS SCI SA
Fusion protein for inhibiting formation of TACI-BAFF complex and preparation method and application thereof
ActiveCN103833856AExtended half-lifeLower serum concentrationFungiBacteriaImmunologic disordersDisease
The invention provides a fusion protein for inhibiting formation of a TACI-BAFF complex and a preparation method and application thereof. Specifically, the fusion protein provided by the invention has high biological activity for enclosing BAFF / APPRIL, can significantly reduce serum IgM concentration of the normal Balb / c mice, and serum IgM and IgE concentration of C57 / B6 of asthmatic mice. The TACI-Fc fusion protein provided by the invention can be used for the treatment of autoimmune diseases, including asthma, systemic lupus erythematosus and rheumatoid arthritis, also can be used for the treatment of diseases related to B cell increase, such as multiple myeloma, chronic lymphocytic leukemia, macroglobulinemia and plasma cell leukemia.
Owner:SHANGHAI KANDA BIOTECHNOLOGY CO LTD
Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia
ActiveUS8394816B2BiocideHeavy metal active ingredientsWaldenstrom macroglobulinemiaMacroglobulinemia
Owner:TRIPHASE RES & DEV I
Thiazolyl-containing compounds for treating proliferative diseases
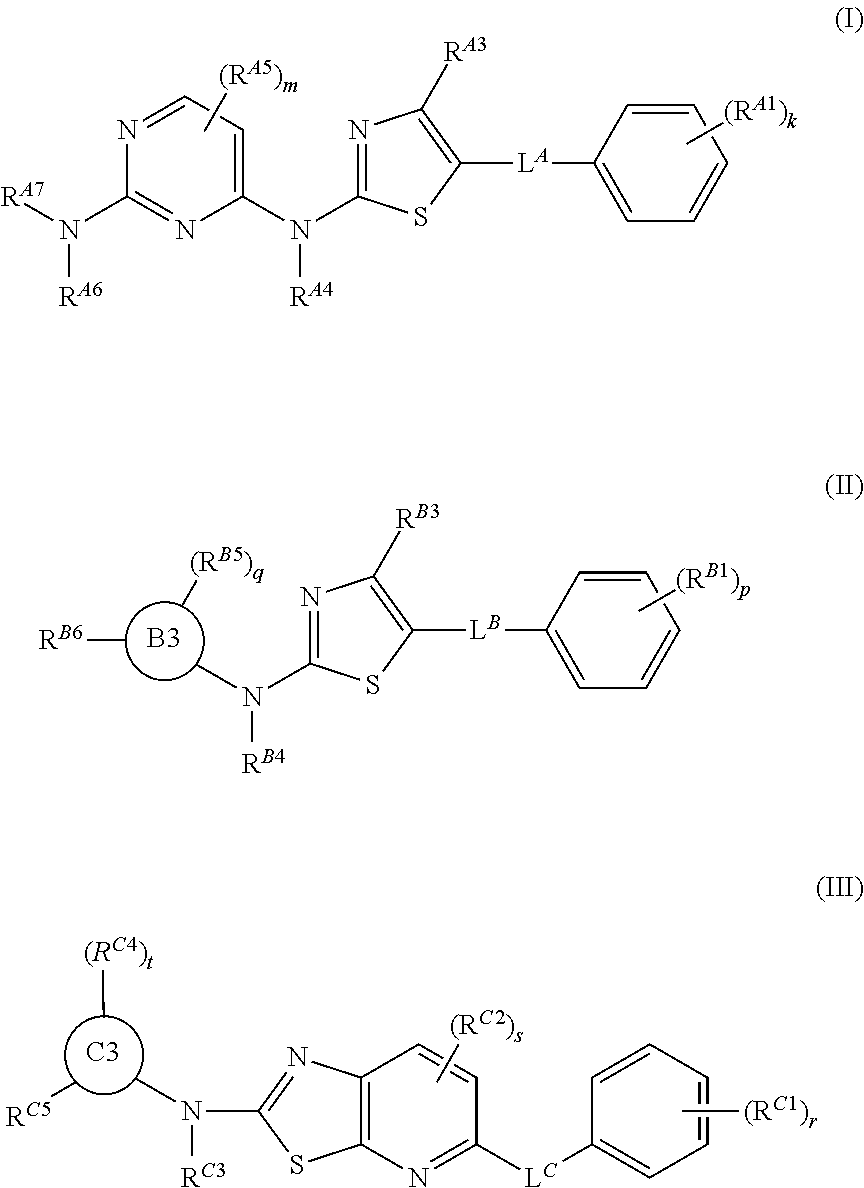
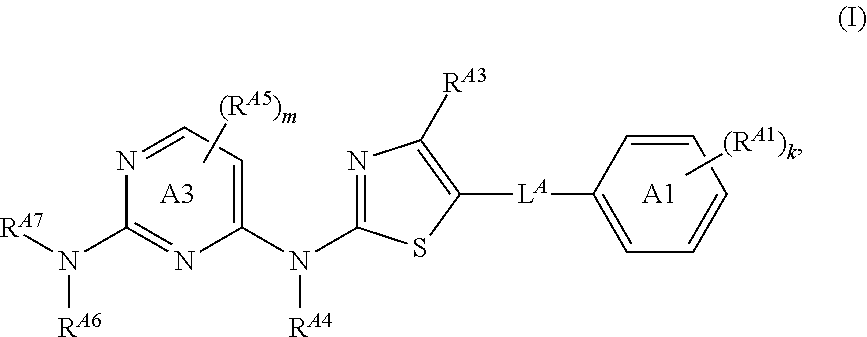
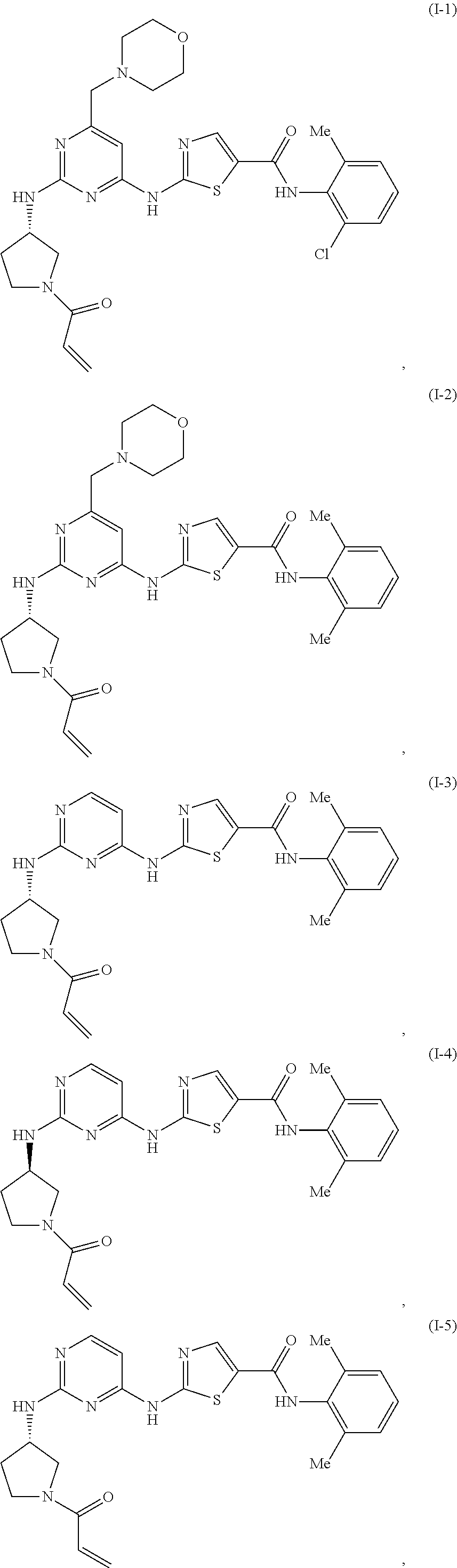
ActiveUS20170233411A1Convenient treatmentReduces and avoids symptomOrganic chemistryAntineoplastic agentsBruton's tyrosine kinaseSrc family kinase
The present disclosure provides thiazolyl-containing compounds of Formula (I), (II), or (III). The compounds described herein may be able to inhibit protein kinases (e.g., Src family kinases (e.g., hemopoietic cell kinase (HCK)), Bruton's tyrosine kinase (BTK)) and may be useful in treating and / or preventing proliferative diseases (e.g., myelodysplasia, leukemia, lymphoma (e.g., Waldenstrom's macroglobulinemia)) and in inducing apoptosis in a cell (e.g., malignant blood cell). Also provided in the present disclosure are pharmaceutical compositions, kits, methods, and uses including or using a compound described herein.
Owner:DANA FARBER CANCER INST INC
Methodologies and methods for measuring higher molecular weight transthyretin or equivalents as a clinical biomarker
InactiveUS20210223266A1Multiple applicationsCost-effectiveDisease diagnosisBiological testingWaldenstrom macroglobulinemiaGeneral practioner
This invention provides methodologies and methods for measuring concentrations of higher molecular weight (HMW) transthyretin (TTR) species, or functional equivalents, in a biological sample that are useful as a clinically applicable biomarker (diagnostic, prognostic, predictive, treatment response, safety) for multiple diseases and conditions. Our discovery is immediately applicable and generally available for the general practitioner for use in diseases where currently few to no biomarkers available like light-chain amyloidosis (AL), a B-cell / plasma cell cancer / dyscrasia that is also associated with multiple myeloma, monoclonal gammopathy of undetermined significance (MUGS), B-cell lymphomas, and Waldenstrom macroglobulinemia. Methodologies and methods are disclosed.
Owner:TTR THERAPEUTICS
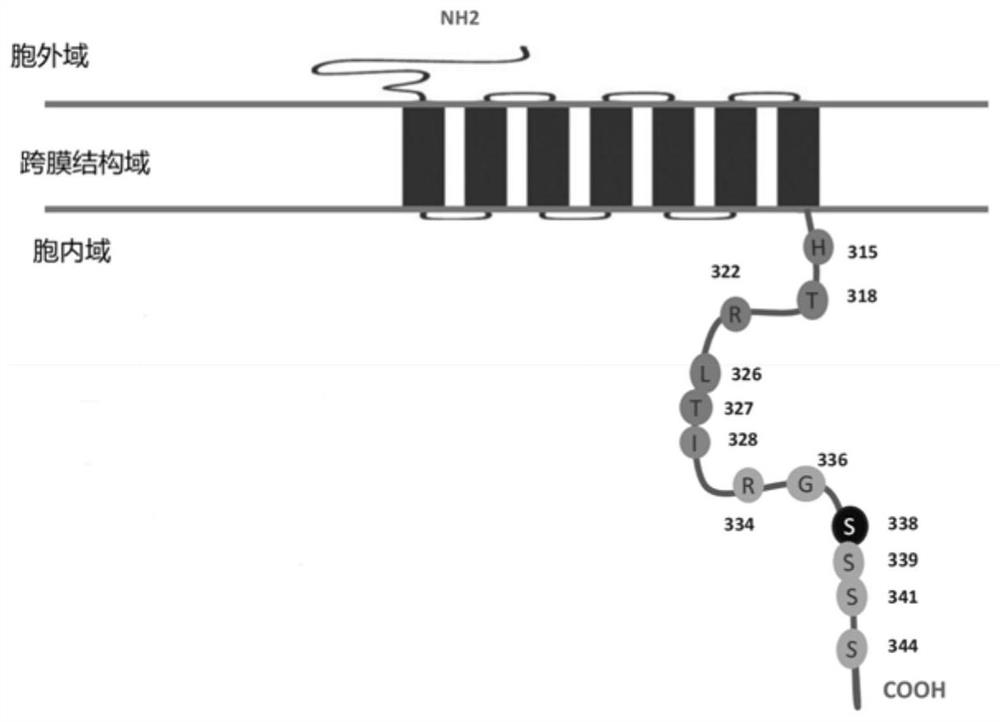
Treatment of C1013G/CXCR4-associated waldenstroöm's macroglobulinemia with an anti-CXCR4 antibody
ActiveUS10233248B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsCXCR4Macroglobulinemia
The present disclosure provides a method for treating a subject afflicted with Waldenstrm's macroglobulinemia (WM) comprising administering to the subject a therapeutically effective amount of an antibody or an antigen-binding portion thereof that specifically binds to a CXCR4 receptor expressed on the surface of a WM cell. The disclosure also provides a therapeutic regimen for treating a patient afflicted with C1013G / CXCR4-associated WM.
Owner:BRISTOL MYERS SQUIBB CO
Molecular determinants of myeloma bone disease and use thereof
InactiveUS7811750B2Prevent and reverse bone lossPrevent bone lossMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsWaldenstrom macroglobulinemiaNewly diagnosed
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to reduce tumor burden in multiple myeloma and to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Compositions Containing Ibrutinib
InactiveUS20170252344A1Organic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Treatment of c1013g/cxcr4-associated waldenström's macroglobulinemia with an Anti-cxcr4 antibody
ActiveUS20160272715A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsWaldenstrom macroglobulinemiaCXCR4
The present disclosure provides a method for treating a subject afflicted with Waldenstrm's macroglobulinemia (WM) comprising administering to the subject a therapeutically effective amount of an antibody or an antigen-binding portion thereof that specifically binds to a CXCR4 receptor expressed on the surface of a WM cell. The disclosure also provides a therapeutic regimen for treating a patient afflicted with C1013G / CXCR4-associated WM.
Owner:BRISTOL MYERS SQUIBB CO
Fusion protein inhibiting TACI-BAFF complex formation and preparation method therefor and use thereof
ActiveUS10562954B2Reduce concentrationReduce weightPeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides a fusion protein inhibiting TACI-BAFF complex formation and preparation method therefor and use thereof. Specifically, the fusion protein of the present invention is of high biological activity for blocking BAFF / APPRIL, and may significantly lower the serum IgM concentration in normal Balb / c mice as well as the serum IgM and IgE concentration in C57 / B6 mice with asthma. The TACI-Fc fusion protein of the present invention may be used in the treatment of autoimmune diseases, including asthma, systemic lupus eythematosus and rheumatoid arthritis, etc., and may also be used in the treatment of B cell enrichment-related diseases, including multiple myeloma, chronic lymphocytic leukemia, macroglobulinemia and plasmacytic leukemia, etc.
Owner:SHANGHAI KANDA BIOTECHNOLOGY CO LTD +1
Treatment with Anti-alpha2 integrin antibodies
ActiveUS20100158904A1Inhibit bindingAvoid stickingNervous disorderMuscular disorderUterine carcinomaLymphoid Tumor
The invention relates to treatment of cancer. More specifically the invention relates to methods of treating cancer selected from the group consisting of squamous cell cancer, lung cancer including small-cell lung cancer, non-small cell lung cancer, adenocarcinoma of the lung, and squamous carcinoma of the lung, cancer of the peritoneum, hepatocellular cancer, gastric or stomach cancer including gastrointestinal cancer, pancreatic cancer, glioblastoma, cervical cancer, ovarian cancer, liver cancer, bladder cancer, hepatoma, breast cancer, colon cancer, colorectal cancer, endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal cancer, liver cancer, prostate cancer, vulval cancer, thyroid cancer, hepatic carcinoma and various types of head and neck cancer, as well as B-cell lymphoma including low grade / follicular non-Hodgkin's lymphoma (NHL); small lymphocytic (SL) NHL; intermediate grade / follicular NHL; intermediate grade diffuse NHL; high grade immunoblastic NHL; high grade lymphoblastic NHL; high grade small non-cleaved cell NHL; bulky disease NHL; mantle cell lymphoma; AIDS-related lymphoma; and Waldenstrom's Macroglobulinemia; chronic lymphocytic leukemia (CLL); acute lymphoblastic leukemia (ALL); Hairy cell leukemia; chronic myeloblastic leukemia; and post-transplant lymphoproliferative disorder (PTLD), as well as abnormal vascular proliferation associated with phakomatoses, edema such as that associated with brain tumors, Meigs' syndrome, melanoma, mesothelioma, multiple myeloma, fibrosarcoma, osteosarcoma and epidermoid carcinoma, by administering antibodies directed to α2β1 integrin.
Owner:ICHNOS SCI SA
Inhibitory Oligonucleotides for Treating Tumors
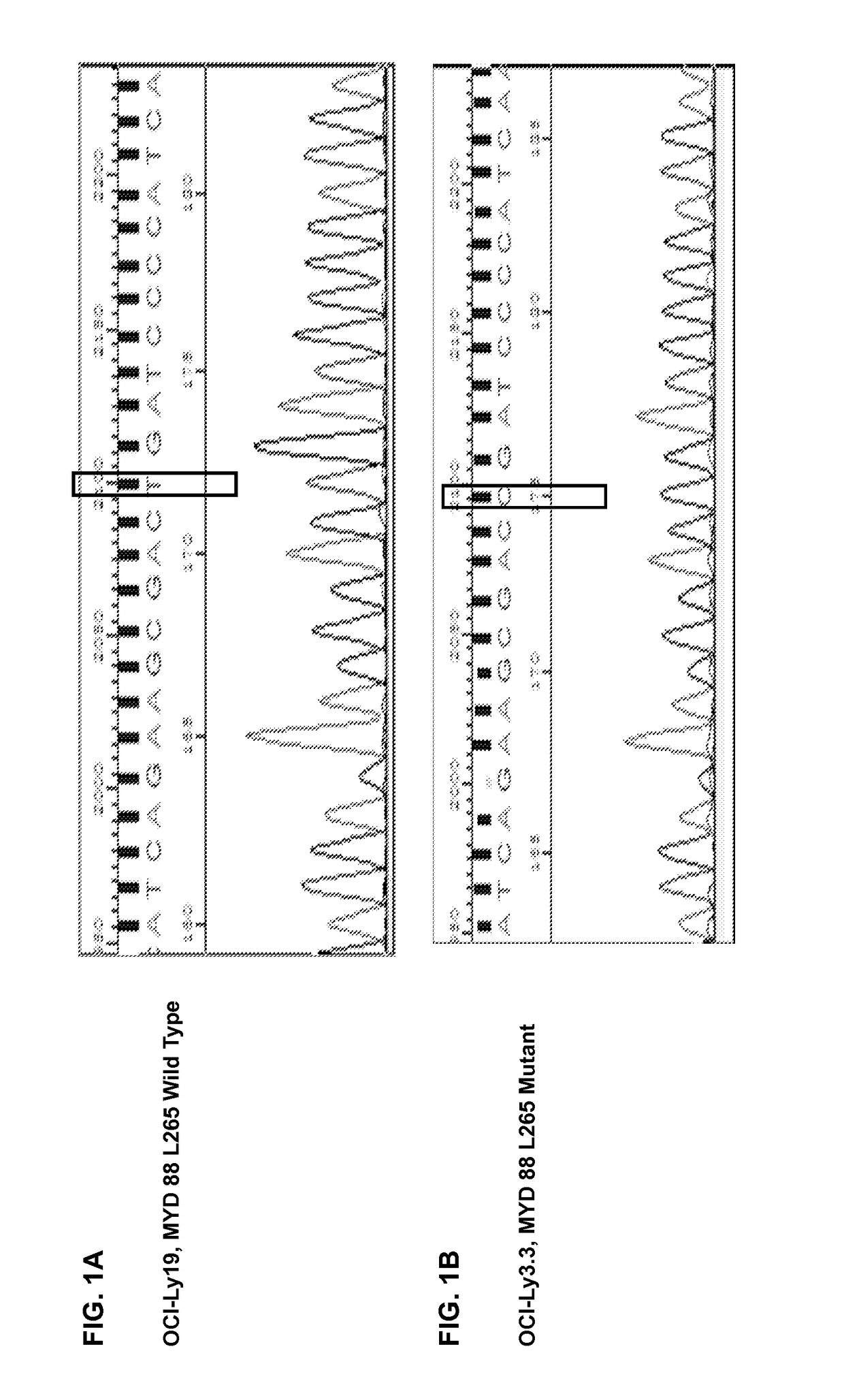
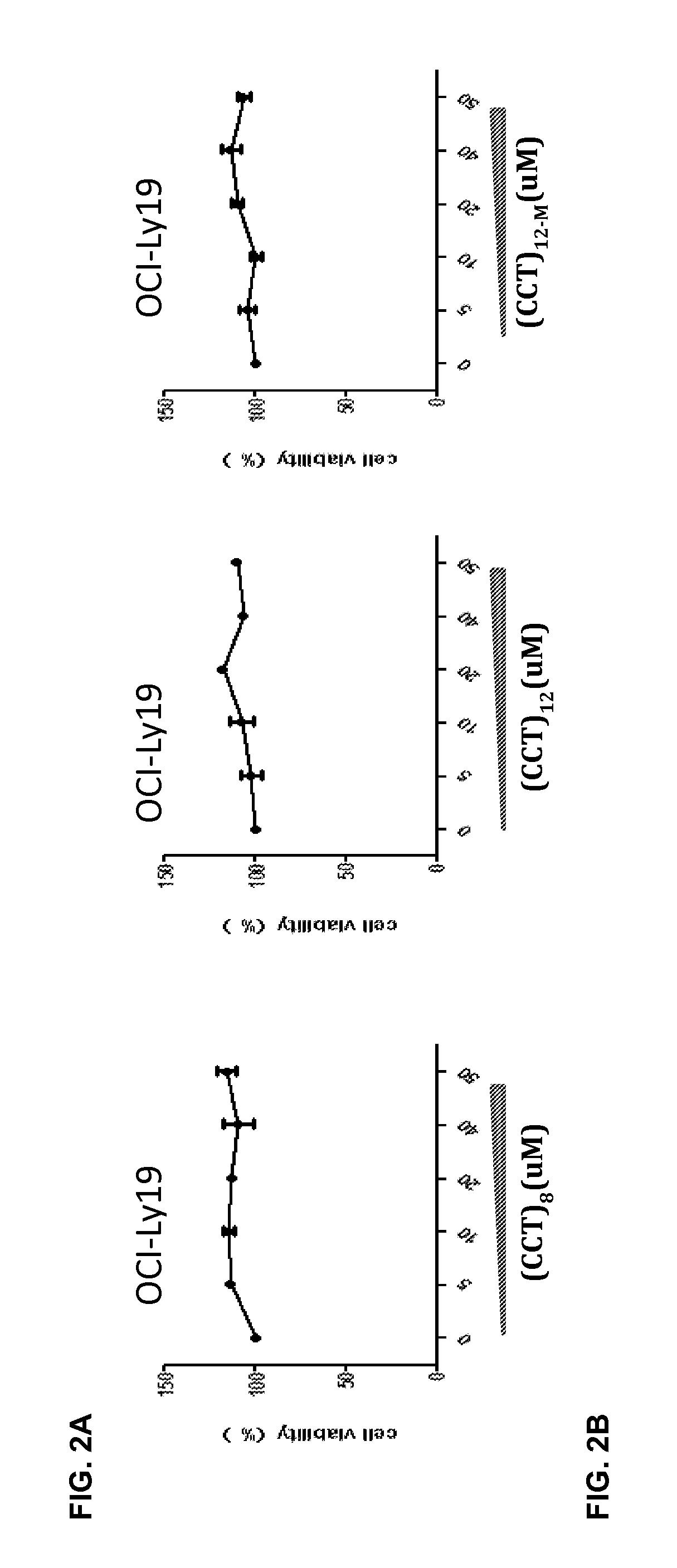
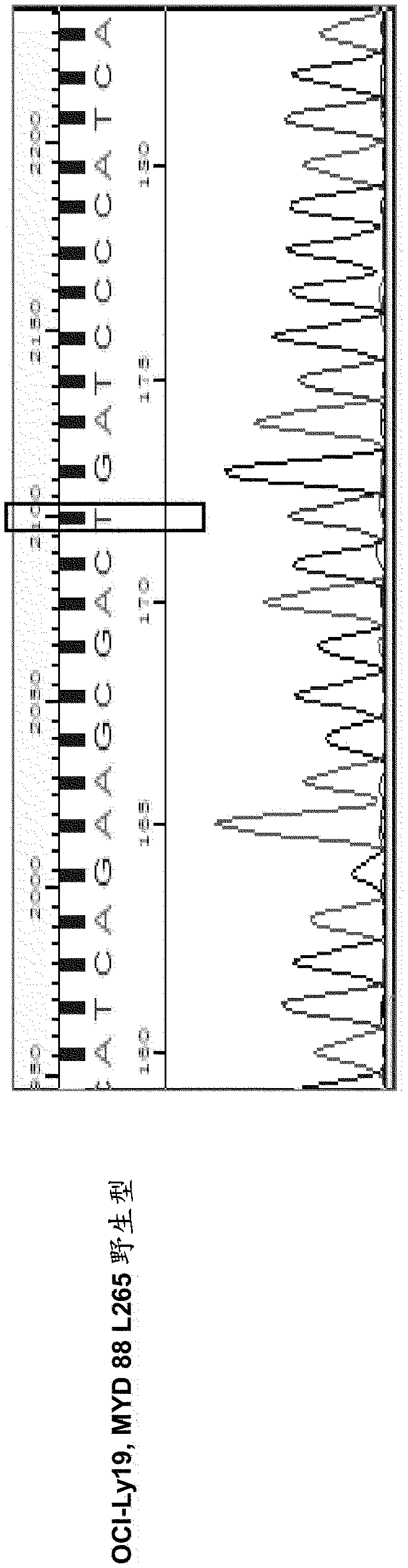
A method for treating B-cell lymphoma in a subject that has been diagnosed as having a B-cell lymphoma characterized by a mutation in MYD88 and is in need of such treatment is presented. The lymphoma is treated with an oligonucleotide having a sequence 5′-(CCT)n-3′. The B-cell lymphoma may be activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) or Waldenstrom's macroglobulinemia (WM).
Owner:SBI BIOTECH CO LTD
Methods for evaluating and treating Waldenstrom's macroglobulinemia
ActiveUS10526660B2ResilienceSuccessful treatmentOrganic active ingredientsMicrobiological testing/measurementMacroglobulinemiaMacroglobulin
Owner:DANA FARBER CANCER INST INC
Methods for the treatment and prevention of abnormal cell proliferation using TACI-fusion molecules
InactiveUS8808696B2Peptide/protein ingredientsAntibody mimetics/scaffoldsMacroglobulinemiaAbnormal cells
The present invention provides methods and compositions for treatment of hyperproliferative disorders and cancers, including multiple myeloma and Waldenström's macroglobulinemia, comprising administering to a patient in need of the treatment a TACI-Ig fusion molecule in amount sufficient to suppress proliferation-inducing functions of BlyS and APRIL.
Owner:ARES TRADING SA +1
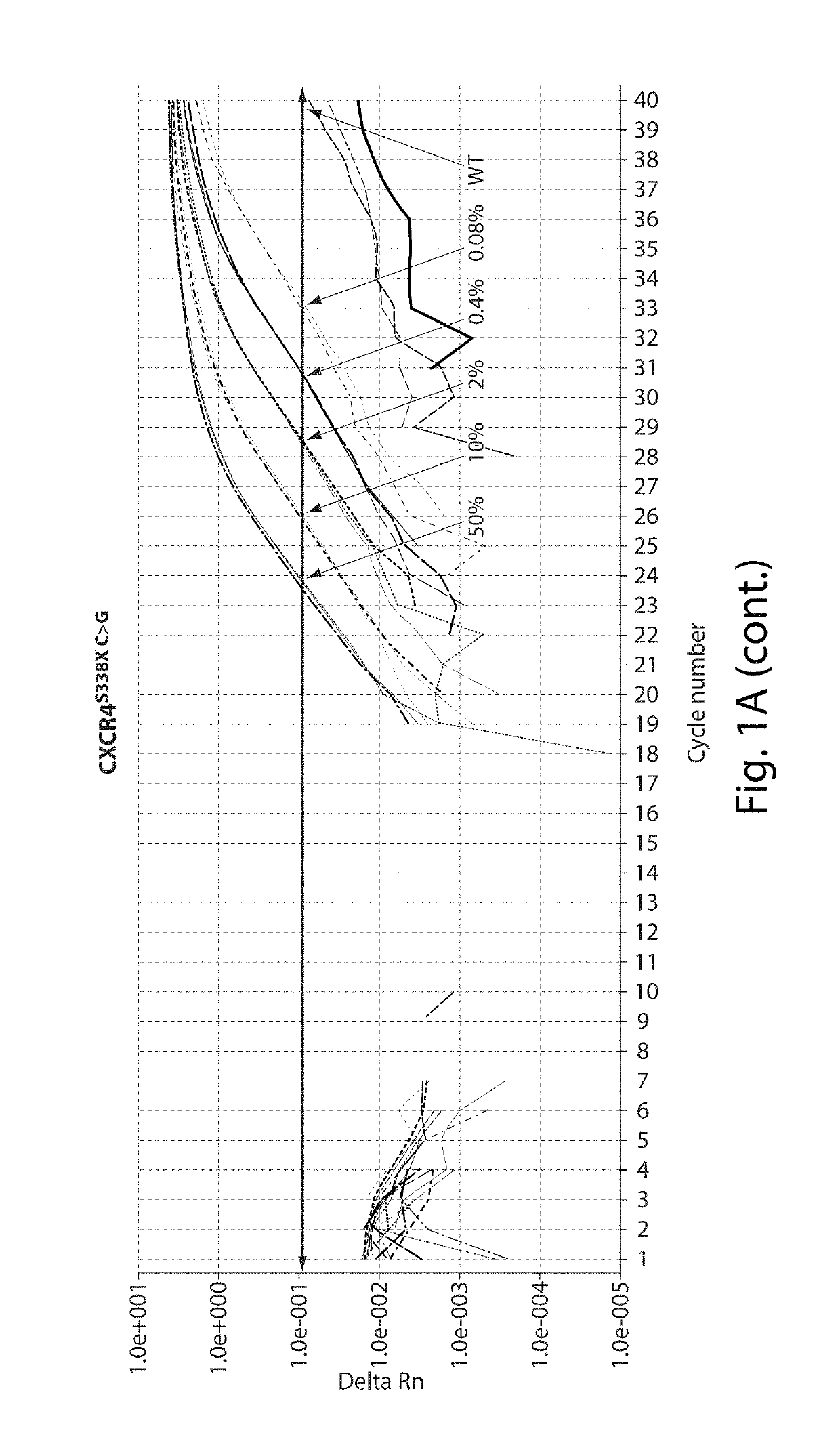
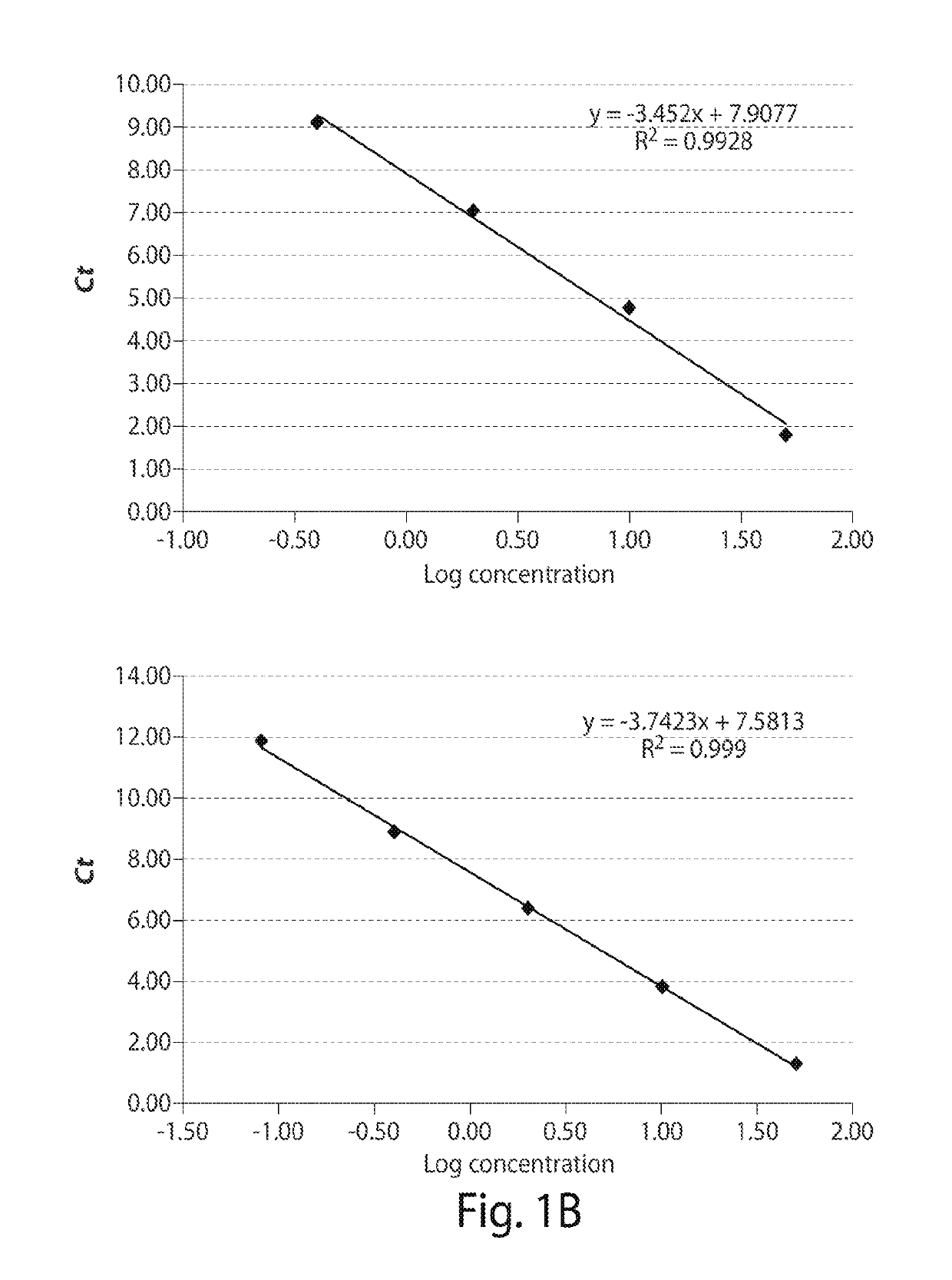
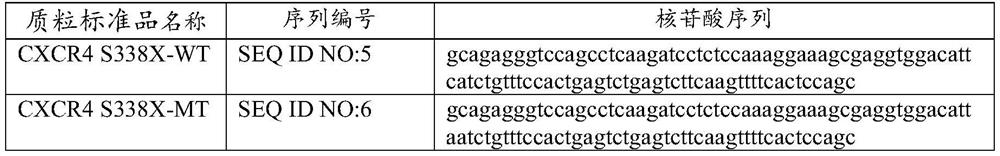
Digital PCR kit, primer and probe for detecting Waldenstrom macroglobulinemia related gene mutation
ActiveCN113736880AMicrobiological testing/measurementAgainst vector-borne diseasesWaldenstrom macroglobulinemiaGenes mutation
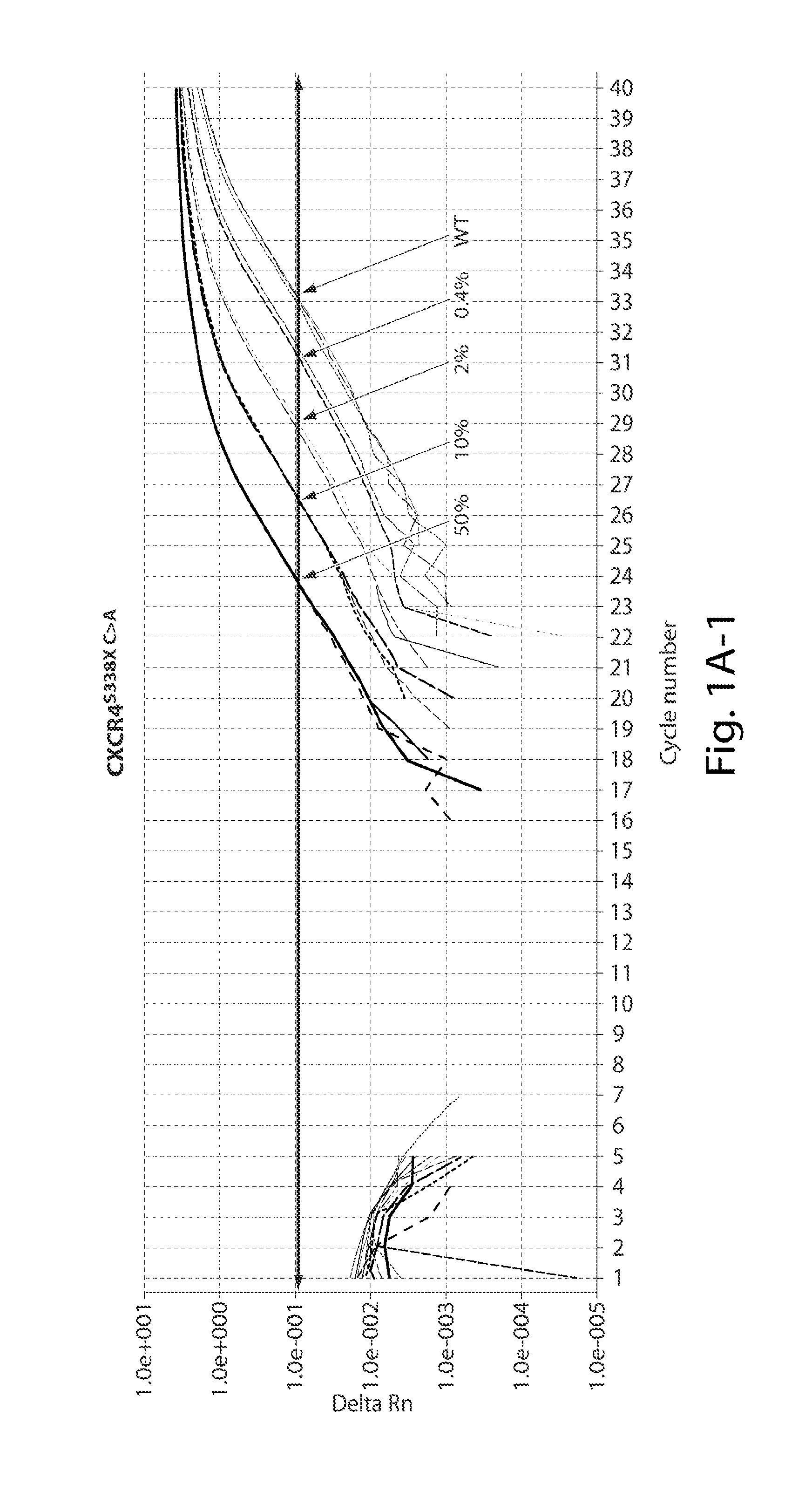
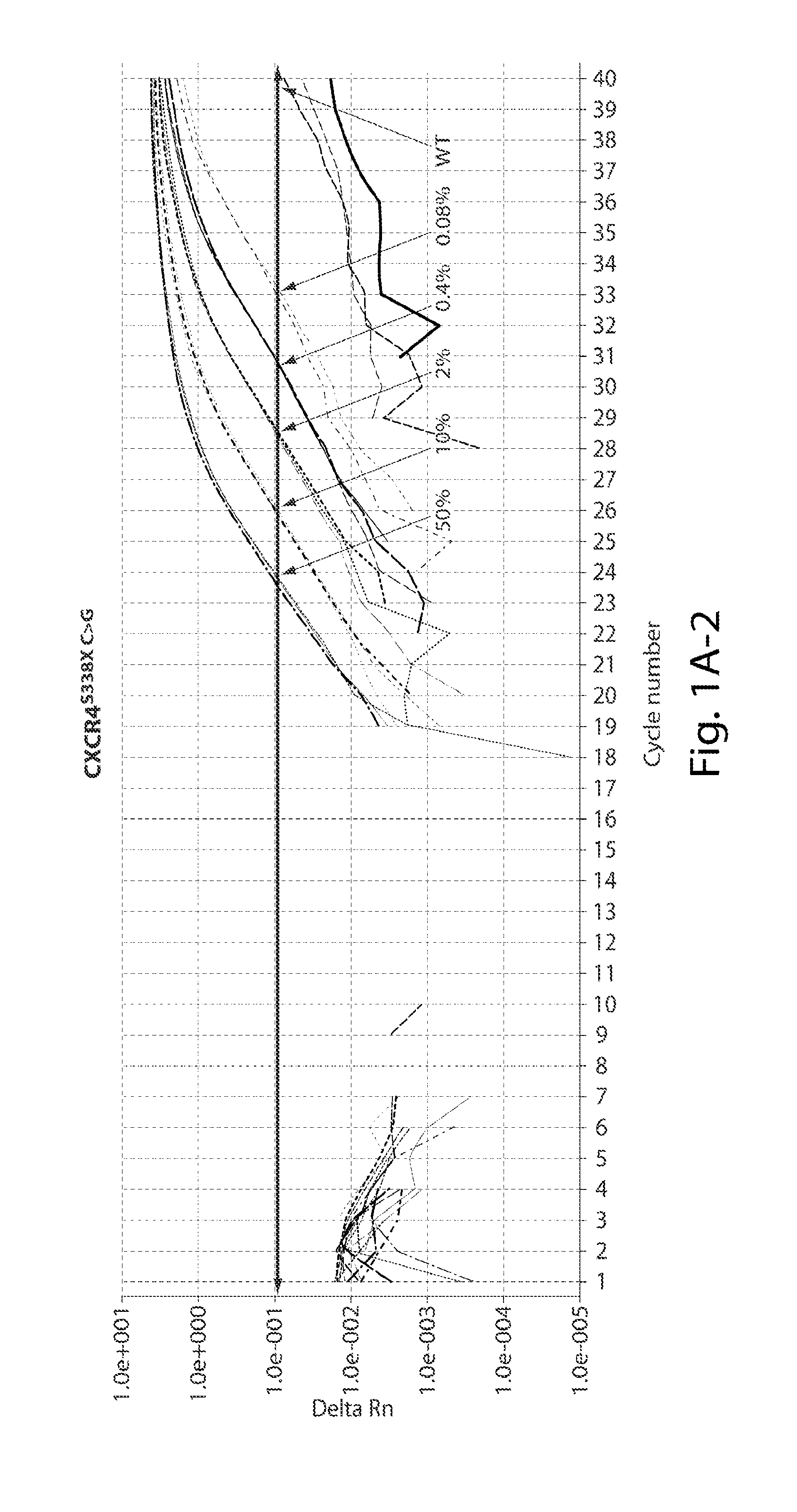
The invention relates to the field of biological detection, in particular to a digital PCR kit, a primer and probe for detecting WM (macroglobulinemia) related gene mutation. The kit contains the primer and the probe which are used for detecting the mutation of the CXCR4S338X site. According to the kit, a digital PCR technology is adopted, the human CXCR4S338X mutation site is quantitatively detected, and reference is provided for curative effect monitoring and clinical medication of clinical doctors on targeted medication of WM patients.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV +1
XBP1, CD138, and CS1 peptides
Various features are disclosed herein, including immunogenic XBP1-, CD138-, and CS1-derived peptides (and pharmaceutical compositions thereof), among others. The peptides can be used in a variety of methods, such as for inducing an immune response, for generating antibodies, and for treating cancer (e.g. plasma cell disorders such as multiple myeloma or Waldenstrom's macroglobulinemia) approach. The peptides can also be included in MHC molecule multimer compositions, and can also be used, for example, in methods for detecting T cells in a population of cells.
Owner:DANA FARBER CANCER INST INC
Fusion protein inhibiting the formation of taci-baff complex and its preparation method and use
ActiveCN103833856BExtended half-lifeLower serum concentrationFungiBacteriaBALB/cImmunologic disorders
The invention provides a fusion protein for inhibiting formation of a TACI-BAFF complex and a preparation method and application thereof. Specifically, the fusion protein provided by the invention has high biological activity for enclosing BAFF / APPRIL, can significantly reduce serum IgM concentration of the normal Balb / c mice, and serum IgM and IgE concentration of C57 / B6 of asthmatic mice. The TACI-Fc fusion protein provided by the invention can be used for the treatment of autoimmune diseases, including asthma, systemic lupus erythematosus and rheumatoid arthritis, also can be used for the treatment of diseases related to B cell increase, such as multiple myeloma, chronic lymphocytic leukemia, macroglobulinemia and plasma cell leukemia.
Owner:SHANGHAI KANDA BIOTECHNOLOGY CO LTD
Inhibitory oligonucleotides for treating tumors
A method for treating B-cell lymphoma in a subject that has been diagnosed as having a B-cell lymphoma characterized by a mutation in MYD88 and is in need of such treatment is presented. The lymphoma is treated with an oligonucleotide having a sequence 5'-(CCT)n-3'. The B-cell lymphoma may be activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) or Waldenstrom's macroglobulinemia (WM).
Owner:SBI BIOTECH CO LTD
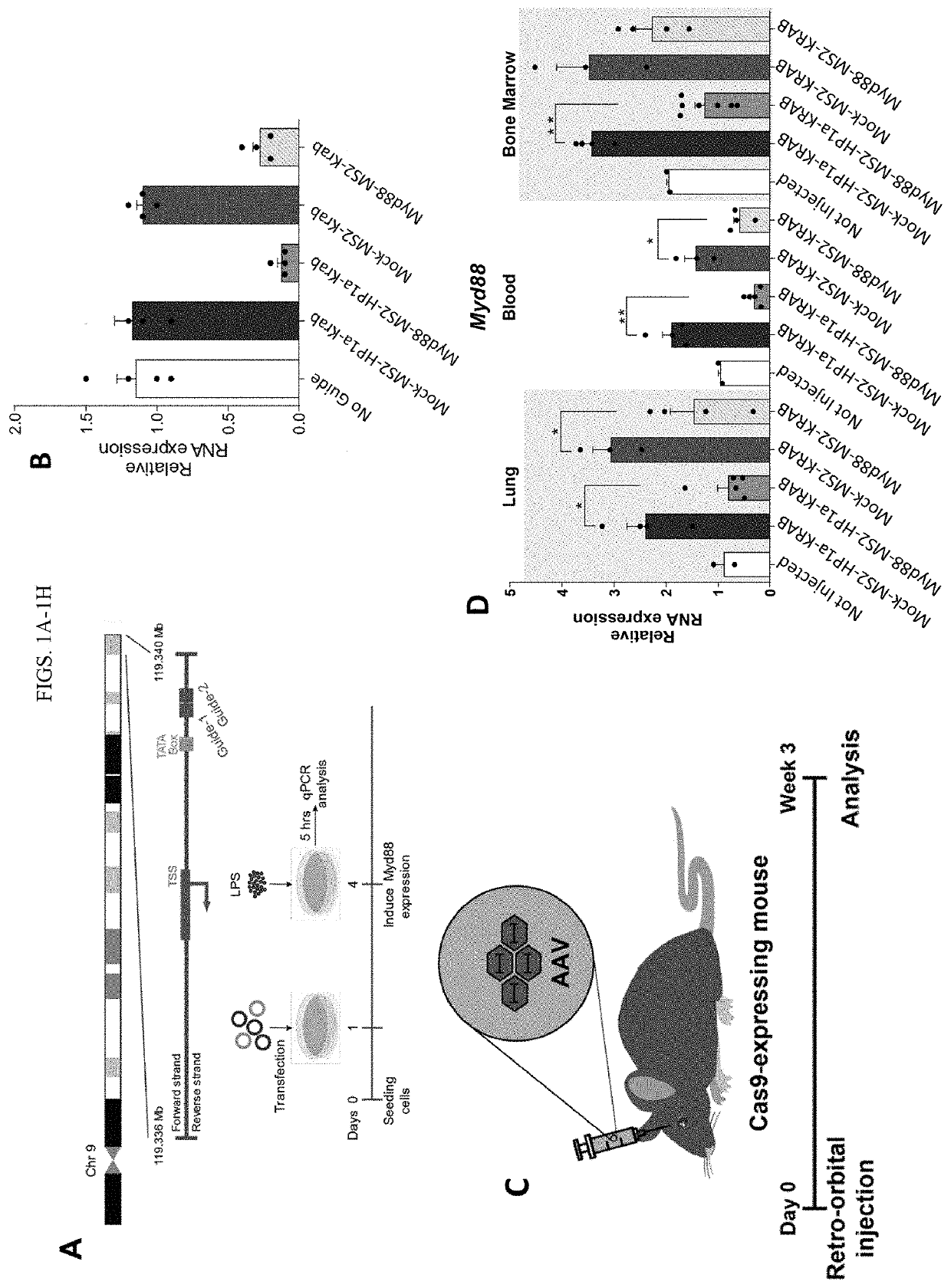
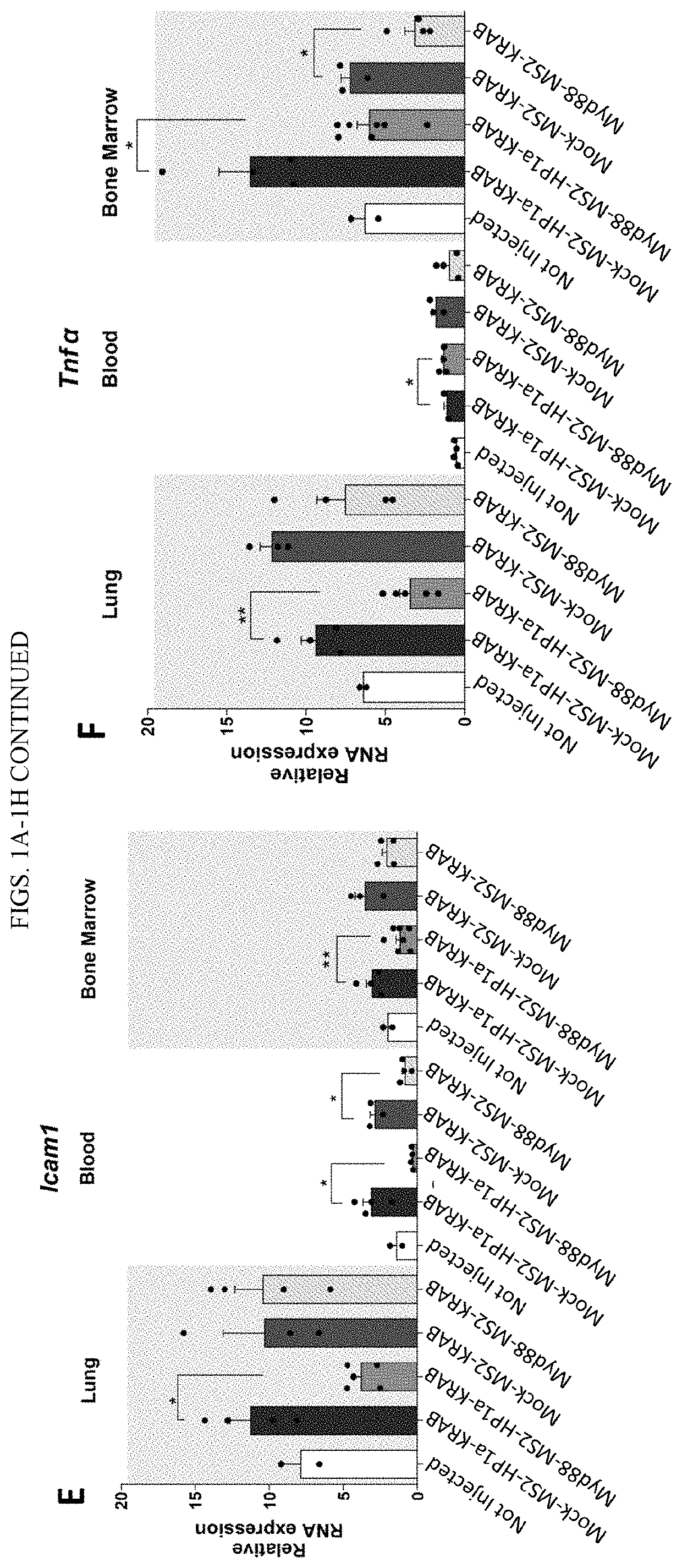
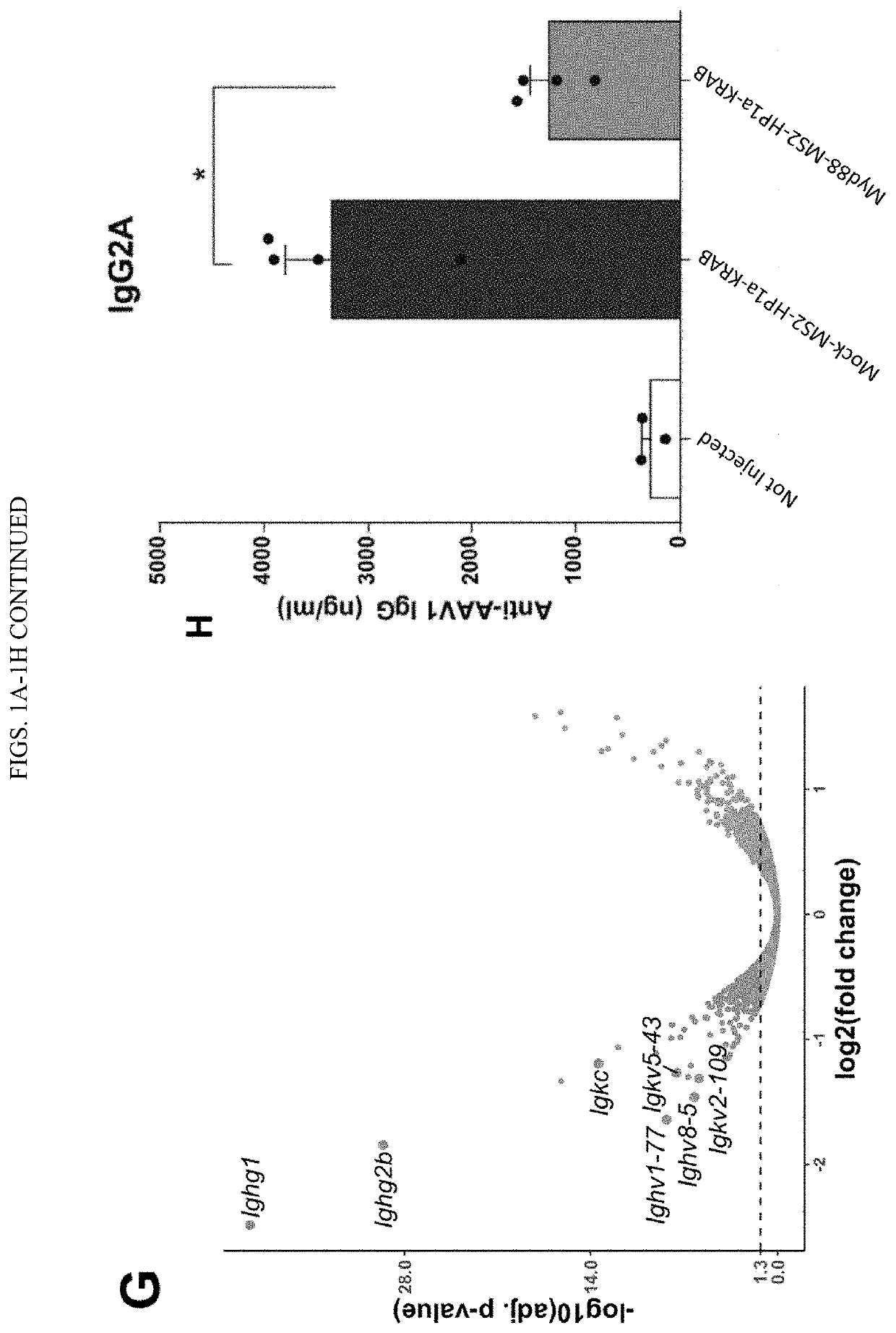
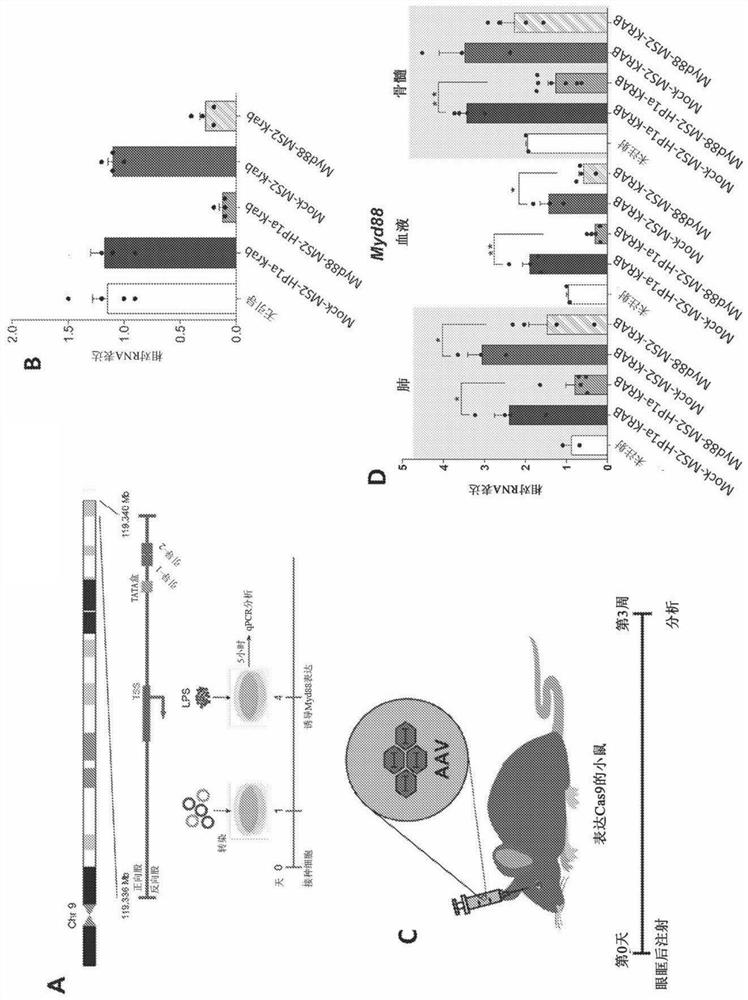
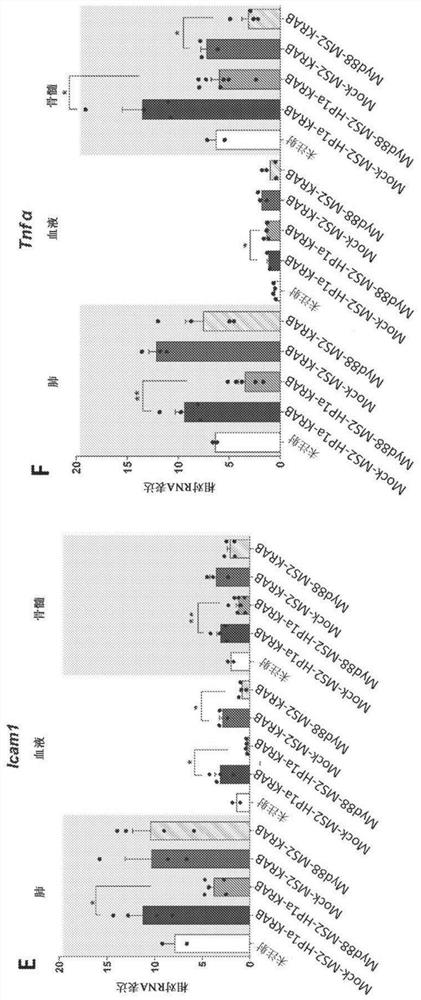
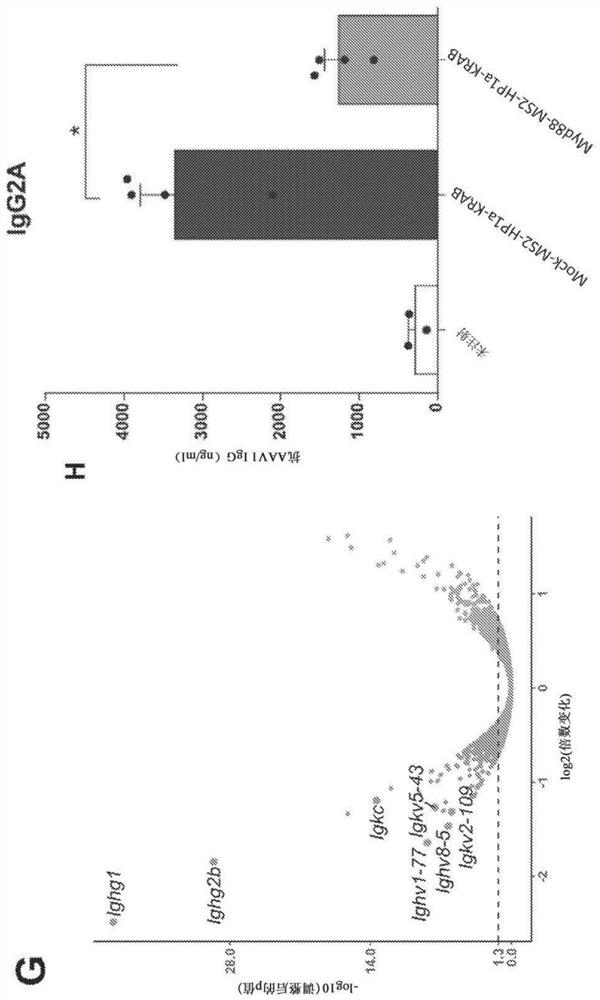
Synthetic immunomodulation with a crispr super-repressor in vivo
PendingUS20210380990A1Lower Level RequirementsFusion with RNA-binding domainVectorsMacroglobulinemiaImmunomodulations
Provided herein are CRISPR-based synthetic repression systems as well as methods and compositions using the synthetic repression systems to treat septicemia, an adverse immune response in a subject and Waldanstrom macroglobulinemia.
Owner:ARIZONA STATE UNIVERSITY
Synthetic immunomodulation with a crispr super-repressor in vivo
Provided herein are CRISPR-based synthetic repression systems as well as methods and compositions using the synthetic repression systems to treat septicemia, an adverse immune response in a subject and Waldanstrom macroglobulinemia.
Owner:ARIZONA STATE UNIVERSITY
Method for separating and purifying α2-macroglobulin from Cohn Fraction IV precipitation
ActiveUS11066441B2Good alkali resistanceEfficient use ofSolid sorbent liquid separationPeptide preparation methodsUltrafiltrationBlood plasma
The present invention discloses a method for separating and purifying α2-macroglobulin from Cohn Fraction IV precipitation, in which Cohn Fraction IV precipitation is treated by ammonium sulfate precipitation, zinc ion affinity chromatography, gel filtration, and ultrafiltration and concentration sequentially and thereby α2-macroglobulin is obtained finally. With the method provided in the present invention, purified α2-macroglobulin plasma protein that has a clinical application value is obtained, the Cohn Fraction IV precipitation is changed from a discarded material into a valuable material, and plasma is utilized comprehensively. In addition, the method is easy and simple to use, easy to scale up, and suitable for separation and purification of α2-macroglobulin at a large scale.
Owner:ACADEMY OF MILITARY MEDICAL SCI
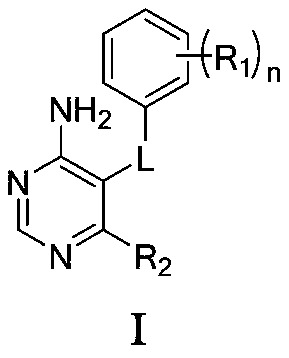
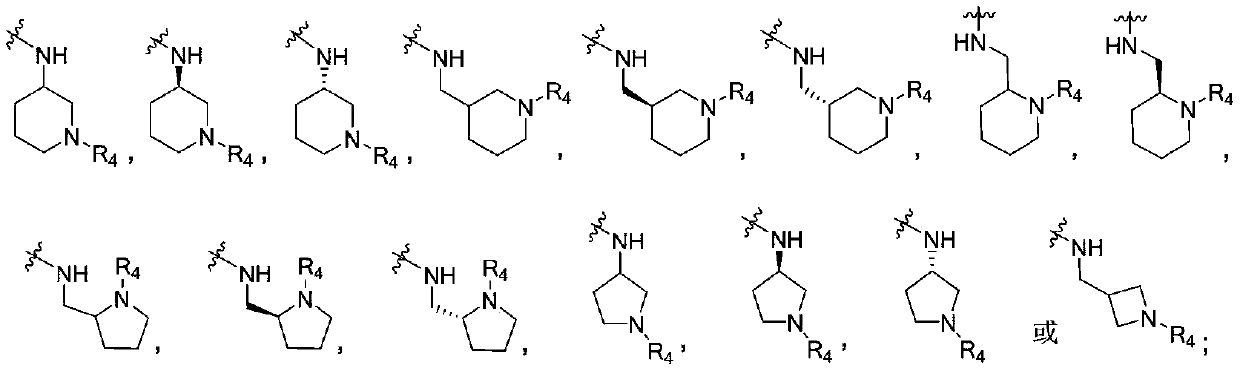
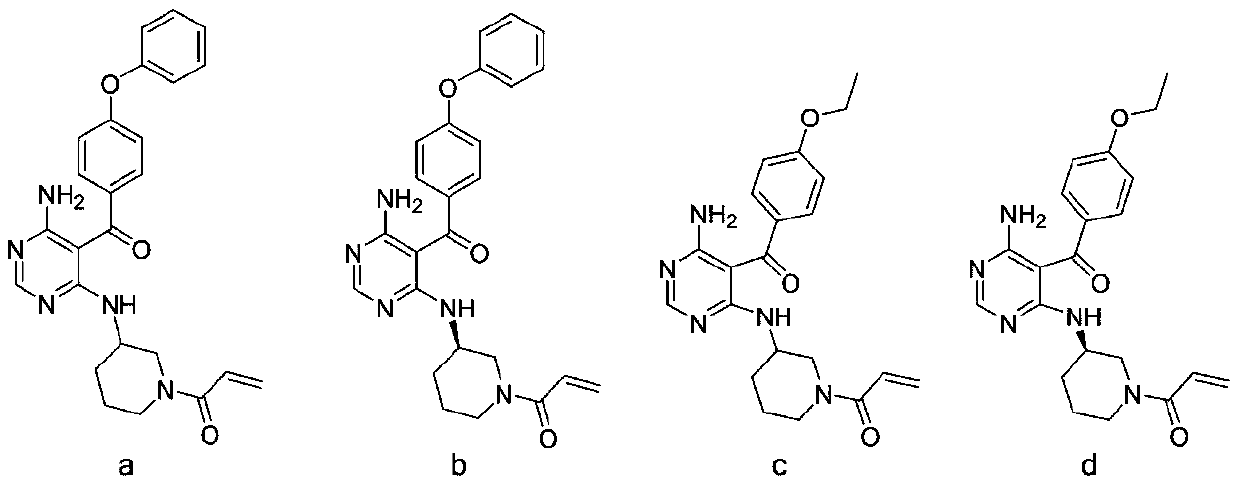
4-aminopyrimidine compound, its preparation method and medical application
ActiveCN107043366BOrganic active ingredientsOrganic chemistryWaldenstrom macroglobulinemiaAutoimmune condition
The present invention relates to the field of medicines, and specifically relates to a 4-aminopyrimidine compound having a structural feature represented by formula (I), or pharmaceutically acceptable salts thereof, a preparation method for the compound, and a use of the compound and the salts as a Bruton tyrosine kinase (BTK) inhibitor. A result of experiments shows that the compound in the present invention has a significant inhibition effect on the BTK, and can be used for treating thromboembolism, inflammatory disorders, autoimmune diseases, Waldenstrom macroglobulinemia, B cell lymphomas, and other diseases.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a40b2f8b-c388-4e82-bceb-bf0003f6dbb6/US20090156469A1-20090618-D00000.png)
![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a40b2f8b-c388-4e82-bceb-bf0003f6dbb6/US20090156469A1-20090618-D00001.png)
![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom's macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a40b2f8b-c388-4e82-bceb-bf0003f6dbb6/US20090156469A1-20090618-D00002.png)












![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/502a4628-d6b5-4b09-960d-7ac3cfc49552/US08394816-20130312-D00001.png)
![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/502a4628-d6b5-4b09-960d-7ac3cfc49552/US08394816-20130312-D00002.png)
![Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating Waldenstrom's Macroglobulinemia](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/502a4628-d6b5-4b09-960d-7ac3cfc49552/US08394816-20130312-D00003.png)