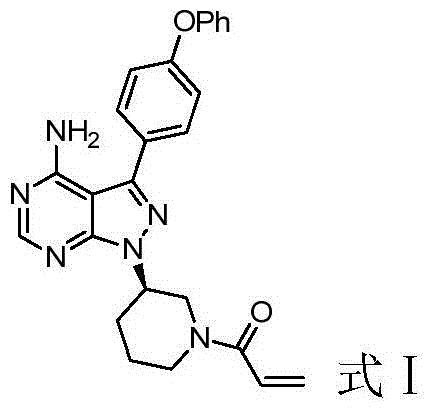
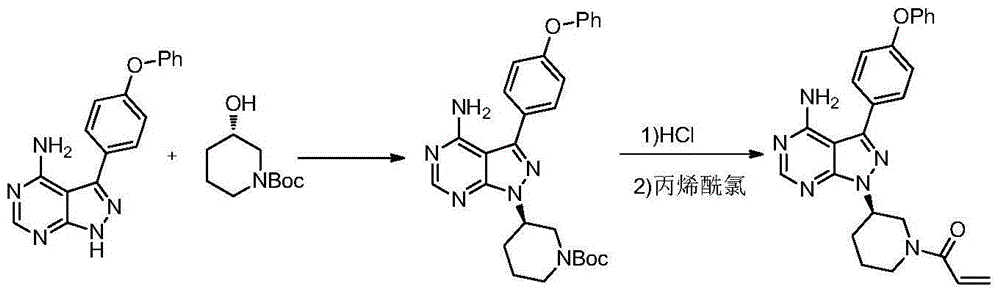
Method for preparing ibrutinib
A technology of ibrutinib and compounds, applied in the field of drug synthesis, can solve problems such as difficult separation, strong irritation, and difficulty in obtaining raw materials, and achieve the effect of simplifying the process and reducing the production of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1-(3-(4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)-3- Chloro-propan-1-one
[0036]
[0037] Formula III piperidine compound (8.0g, 20.7mmol) was added in dichloromethane (100ml), diisopropylethylamine (2.95g, 22.8mmol) was added, and under ice-water bath cooling, 3-chloro A solution of propionyl chloride (2.5g, 19.7mmol) in 20ml of dichloromethane (argon protection), after dropping, stirred for 15 minutes, added 50ml of water, stirred until the two phases were clear, separated the water phase, and added the organic phase Wash once with 20 ml of 1N hydrochloric acid, separate the organic phase and spin dry. Column chromatography, eluent (dichloromethane:methanol=30:1), collected the product to obtain 9.6 g of the title compound with a yield of 97%.
[0038] 1 H NMR (400M Hz, CDCl 3 ),δ8.36(s,1H),7.67-7.64(m,2H),7.43-7.38(m,2H),7.29-7.15(m,3H),7.10(d,J=4.0Hz,2H), 5.9(brs,2H),4.88-4.83(m,2H),3.88-3.72(m,3H),3.35-3.33(m,2H),
[0039]...
Embodiment 2
[0042] 1-(3-(4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)-3- Bromo-propan-1-one
[0043]
[0044] The piperidine compound of formula III (2.0g, 5.18mmol) was added in dichloromethane (20ml, insoluble), diisopropylethylamine (1.47g, 11.4mmol) was added, and under cooling in an ice-water bath, 3 -A solution of bromopropionyl chloride (0.84g, 4.92mmol,) in 10 ml of dichloromethane (argon protection), after dropping, stir for 5 minutes, add 20 ml of water, stir until the two phases are clear, and separate the upper aqueous phase , the organic phase was washed once with 20 ml of 1N hydrochloric acid, the organic phase was separated and spin-dried, column chromatography, eluent (dichloromethane: methanol = 30:1), collected to obtain 2.6 g of the title compound, yield 96.4% .
[0045] 1 H NMR (400M Hz, CDCl 3 ),δ8.36(s,1H),7.60-7.56(m,2H),7.47-7.36(m,2H),7.29-7.15(m,3H),7.08(d,J=3.6Hz,2H), 5.8(brs,2H),4.88-4.83(m,2H),3.76-3.58(m,3H),3.35-3.33(m...
Embodiment 3
[0049] 1-(3-(4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)propan-2 -en-1-one
[0050]
[0051] Formula III piperidine compound (4.0g, 10.35mmol) was added in dichloromethane (40ml), diisopropylethylamine (1.47g, 11.4mmol) was added, and under ice-water bath cooling, 3-chloro A solution of propionyl chloride (1.25g, 9.83mmol) in 10ml of dichloromethane (argon protection), after dropping, stirred for 5 minutes, added 20ml of water, stirred until the two phases were clear, separated the upper aqueous phase, and the organic phase Add 20 ml of 1N hydrochloric acid to wash once, separate the organic phase and spin dry, add 40 ml of acetone, 20 ml of 2.5N sodium hydroxide solution, stir at room temperature for 30 minutes, add 40 ml of ethyl acetate for extraction, and use anhydrous sulfuric acid for the organic phase Sodium-dried and spin-dried to obtain 4.2 g of the title compound, the yield was 92%, and the impurity content of the dimer of formula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com