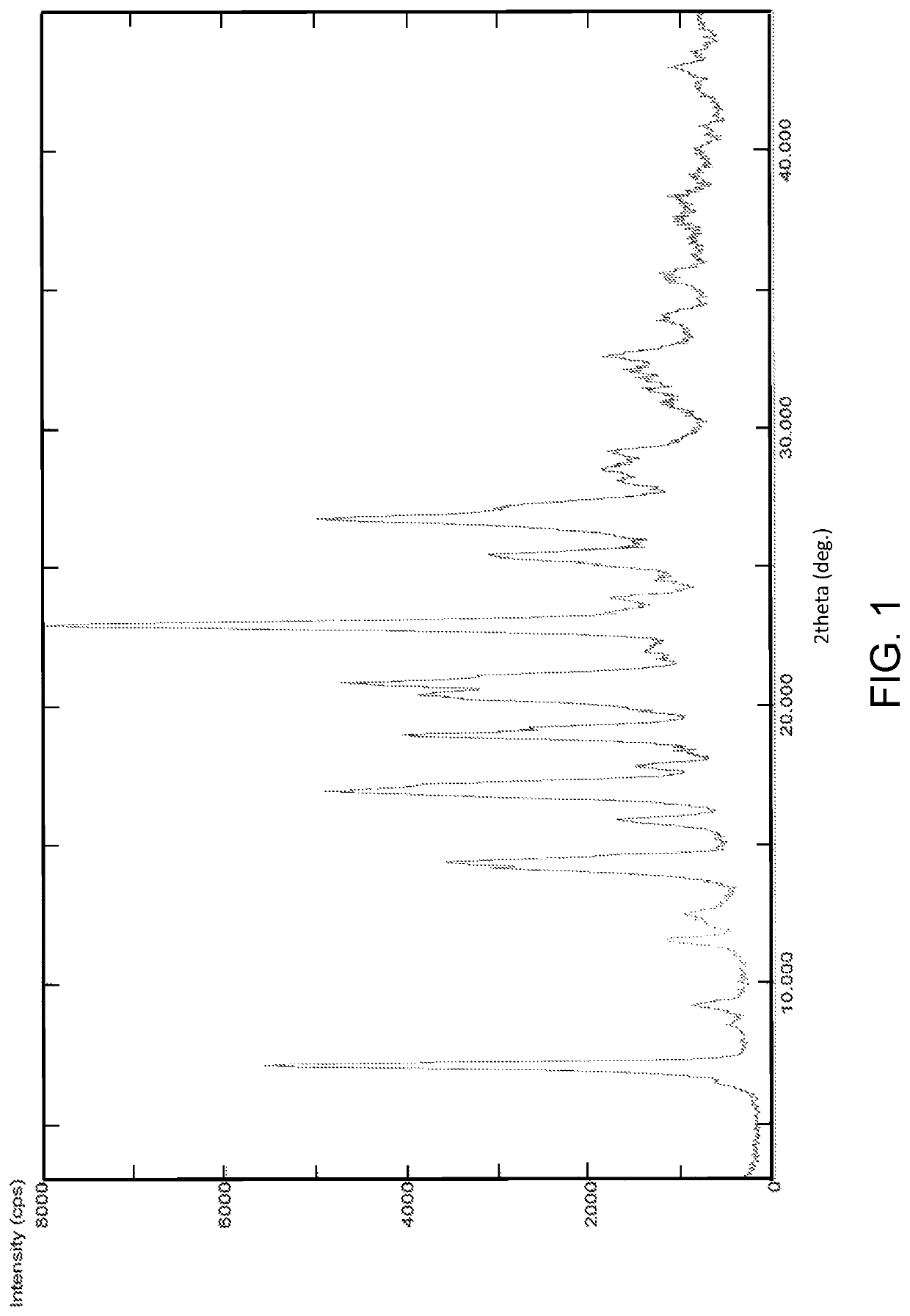
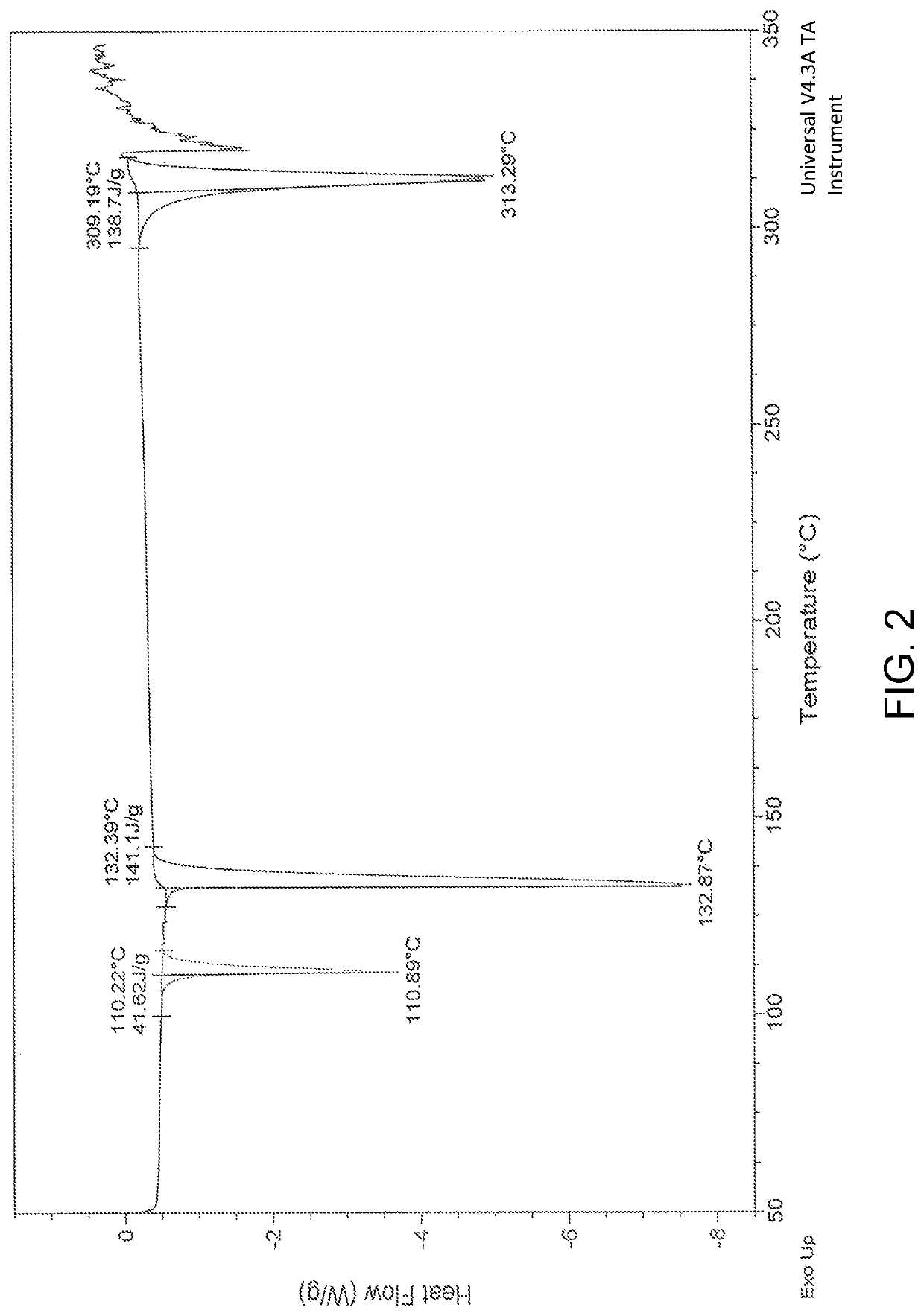
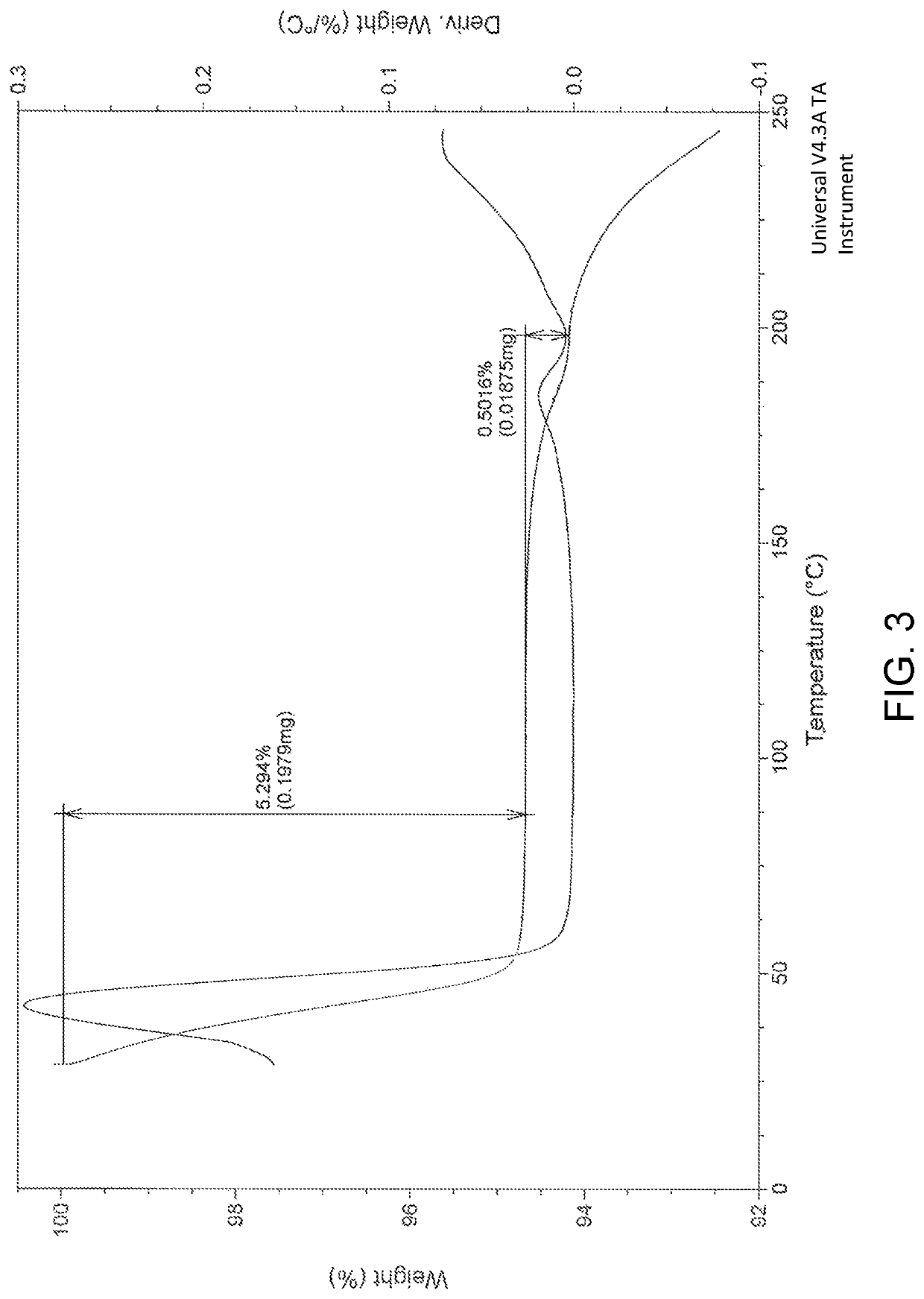
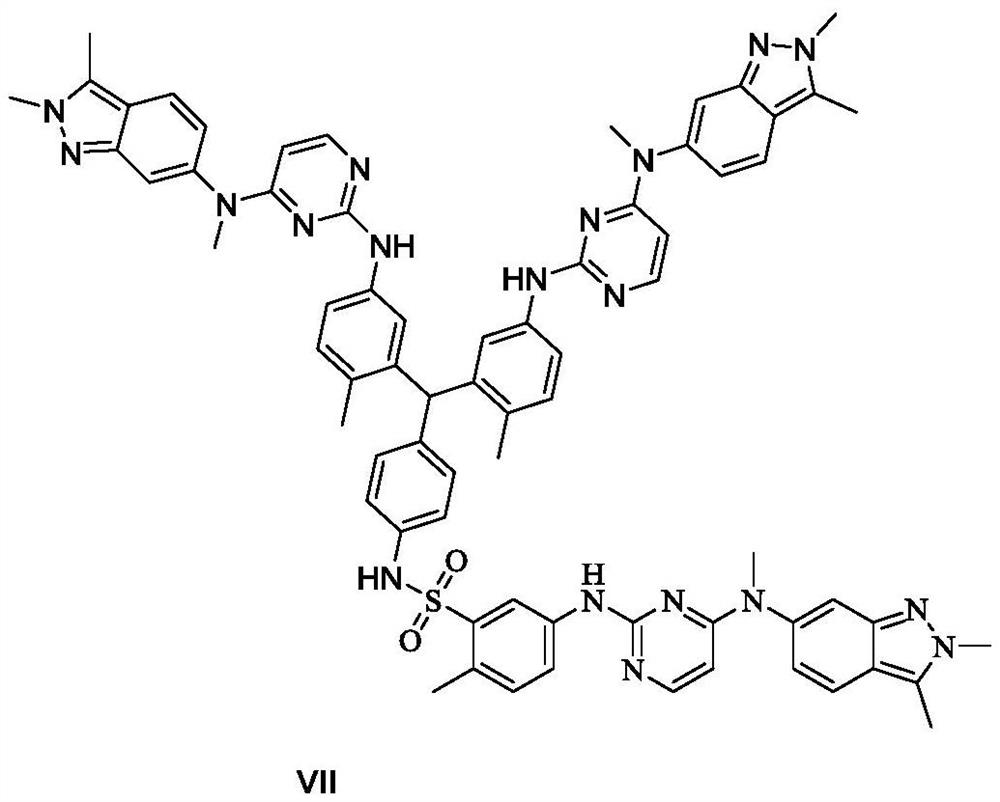
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Pazopanib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
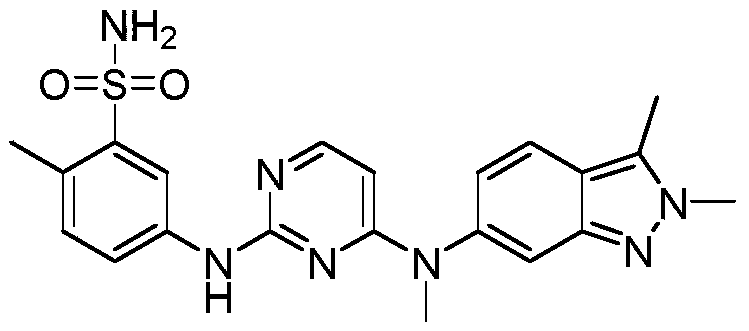
Pazopanib is used to treat kidney cancer. It may also be used to treat certain other types of cancer (soft tissue sarcoma).
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Treatment method
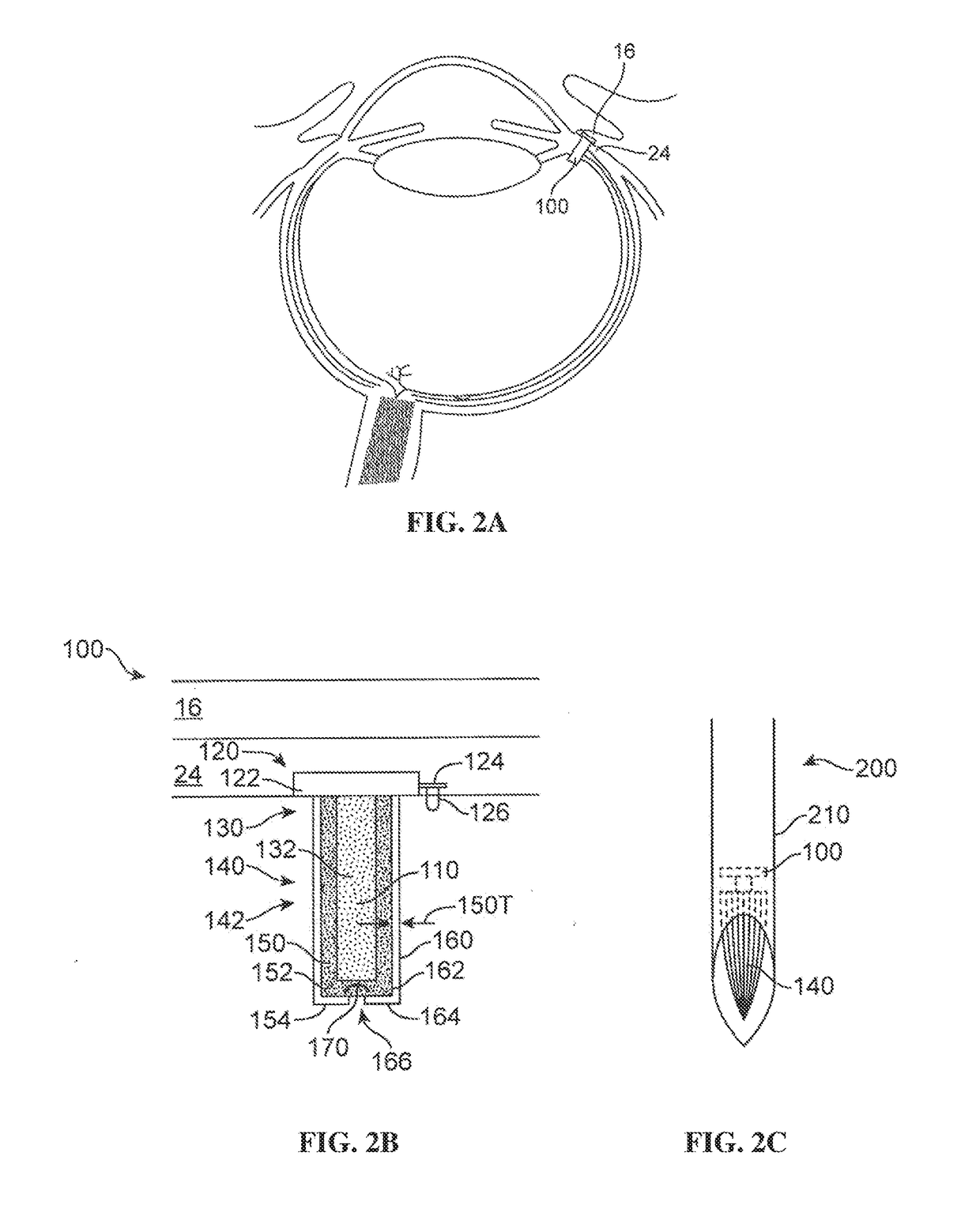
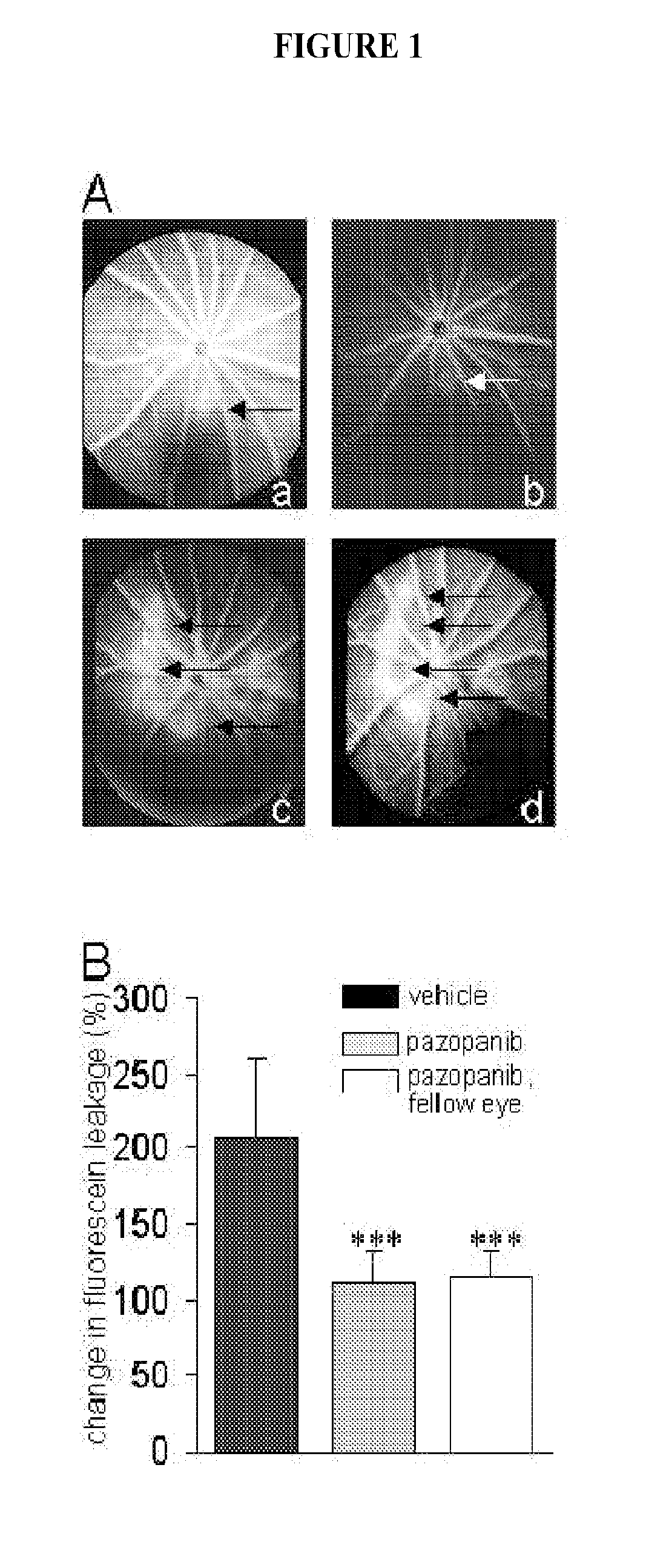
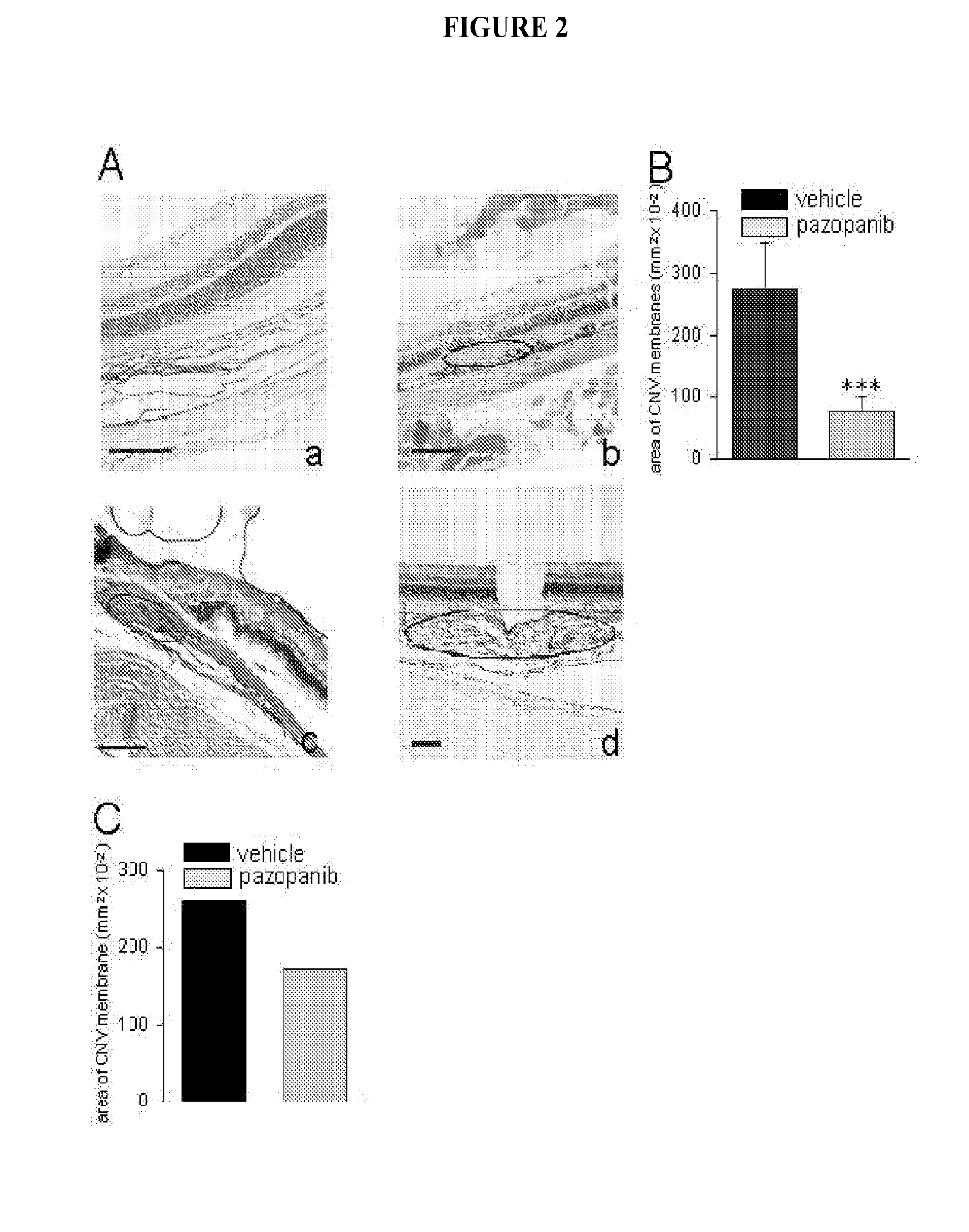
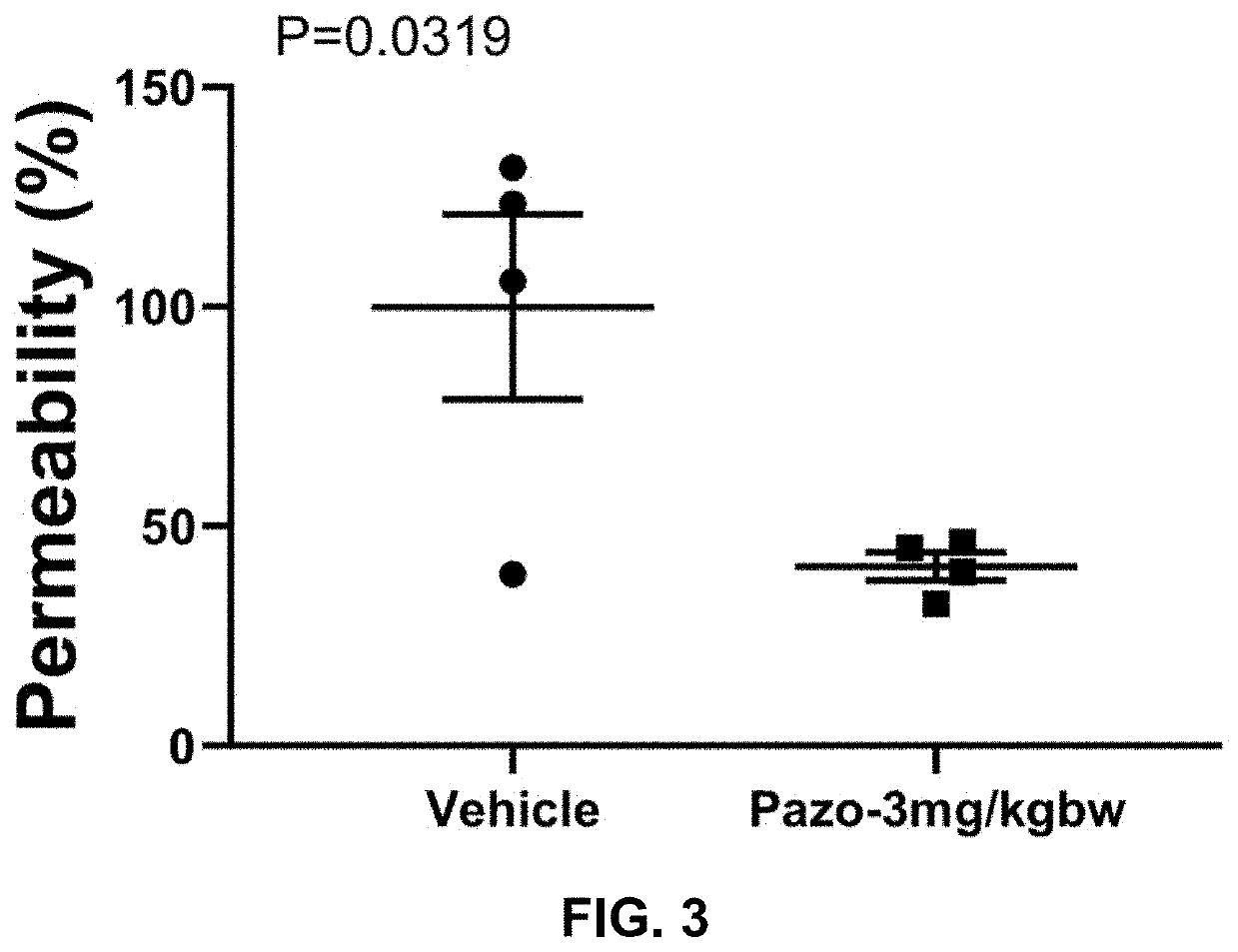
The present invention is directed to methods of treating disorders of ocular angiogenesis or vascular leakage in a patient by administration of suitable inhibitors, including pazopanib or pharmaceutically acceptable salts or hydrates thereof.
Owner:KING ANDREW G +3
Stable and Soluble Formulations of Receptor Tyrosine Kinase Inhibitors, and Methods of Preparation Thereof
ActiveUS20160038488A1Low water solubilityImprove stabilityBiocideOrganic active ingredientsReceptor tyrosine kinase inhibitorActive agent
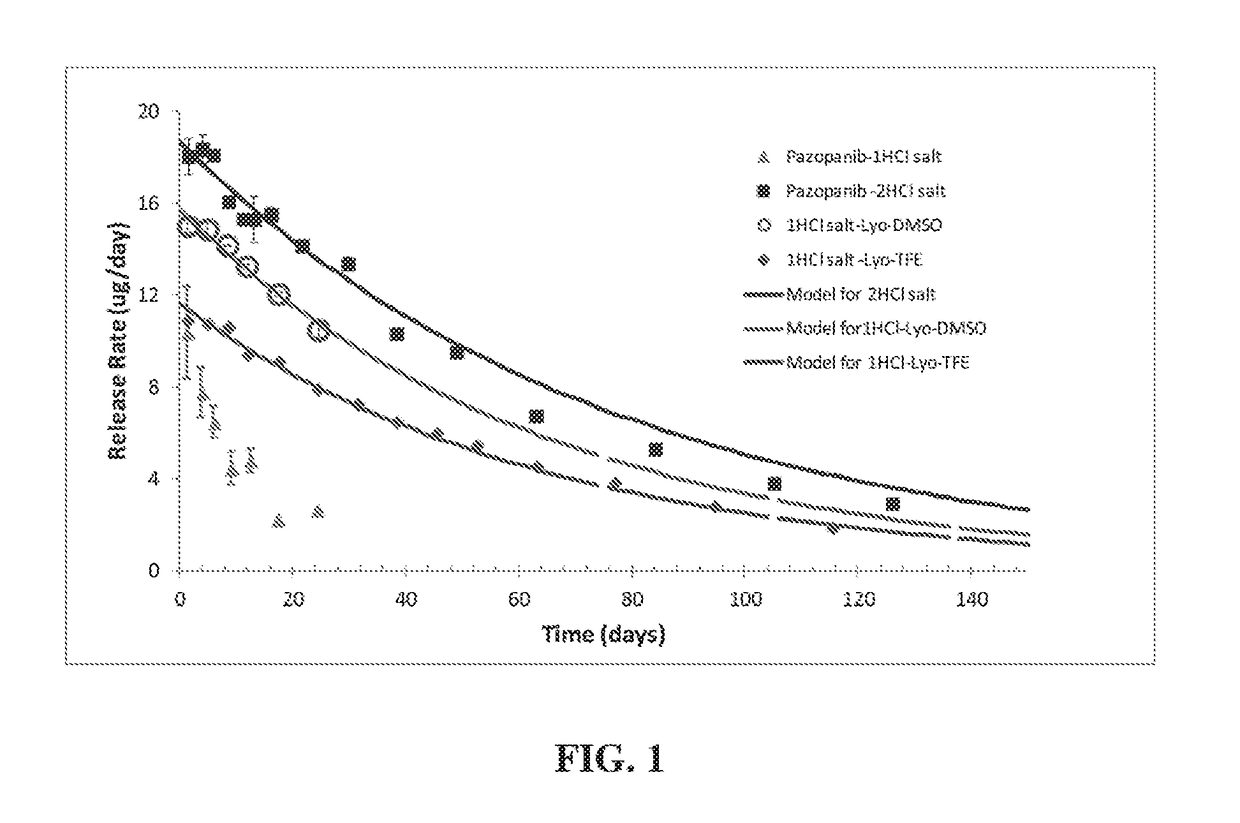
The present disclosure relates to stable formulations of receptor tyrosine kinase inhibitors (TKI), e.g., pazopanib; methods of preparation thereof; and use of the disclosed formulations in sustained delivery of the active agent to a target site. The disclosure further relates to methods of converting one polymorphic Form of a TKI to another polymorphic Form and / or an amorphous form.
Owner:FORSIGHT VISION5 INC
Hot-melt extrusion preparation of pazopanib hydrochloride and preparation method of hot-melt extrusion preparation
InactiveCN104586770AGuaranteed stabilityLower melting temperaturePowder deliveryOrganic active ingredientsPolyethylene glycolHot melt
The invention discloses a hot-melt extrusion preparation of pazopanib hydrochloride and a preparation method of the hot-melt extrusion preparation. The hot-melt extrusion preparation is characterized by comprising the following components in parts by weight: 1 part of pazopanib hydrochloride, 10 parts of a hydrophilic polymer material and 3 parts of polyethylene glycol.
Owner:SHANDONG BOMAIKANG PHARMA RES
An improved process for the preparation of pazopanib or a pharmaceutically acceptable salt thereof
The present invention is relates to an improved process for the preparation of pazopanib or a pharmaceutically acceptable salts thereof. The present invention also relates to novel polymorphic Forms of pazopanib hydrochloride, and its intermediates thereof.
Owner:LAURUS LABS
Preparation method and intermediate of Pazopanib
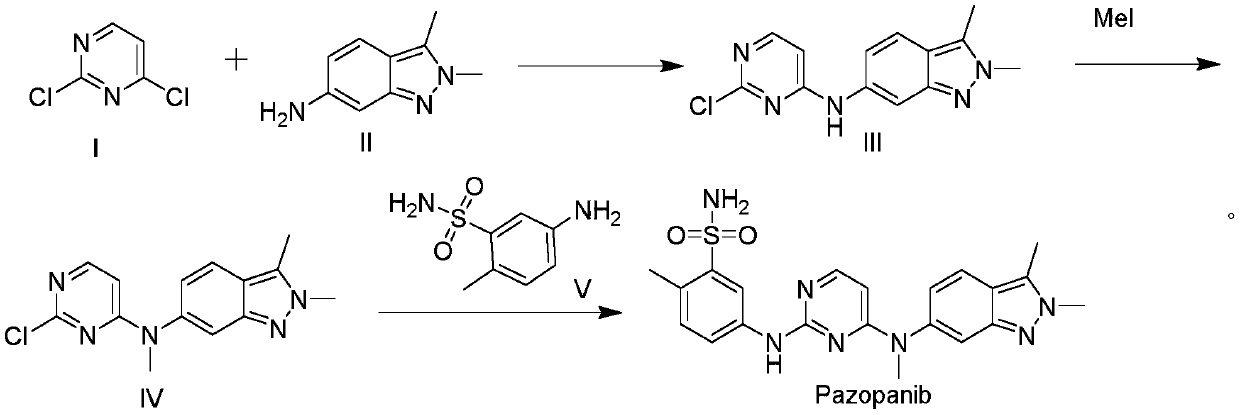
The invention discloses a preparation method and an intermediate of Pazopanib. The preparation method comprises the steps of carrying out two-step condensation reaction on 2,4-dichloro-5-nitropyrimidine so as to obtain a compound of a formula 5 (shown in the description), and carrying out reduction on the compound of the formula 5, so as to obtain Pazopanib. The preparation method has the beneficial effects that the yield of Pazopanib is increased, reaction conditions are mild, column chromatography purification is not required, and the preparation method is applicable to industrial production.
Owner:苏州东南药业股份有限公司
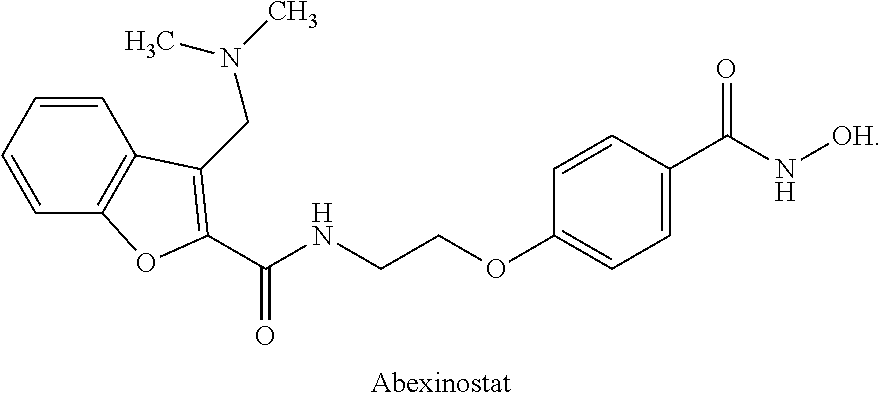
Combinations of histone deacetylase inhibitor and pazopanib and uses thereof
InactiveUS20150335609A1Improve efficiencyReduce resistanceOrganic active ingredientsBiocideDosing regimenRegimen
Dosing regimens, methods of treatment, controlled release formulations, and combination therapies that include an HDAC inhibitor, or a pharmaceutically acceptable salt thereof, and pazopanib (or a salt thereof, e.g., pazopanib HCI) are described.
Owner:PHARMACYCLICS
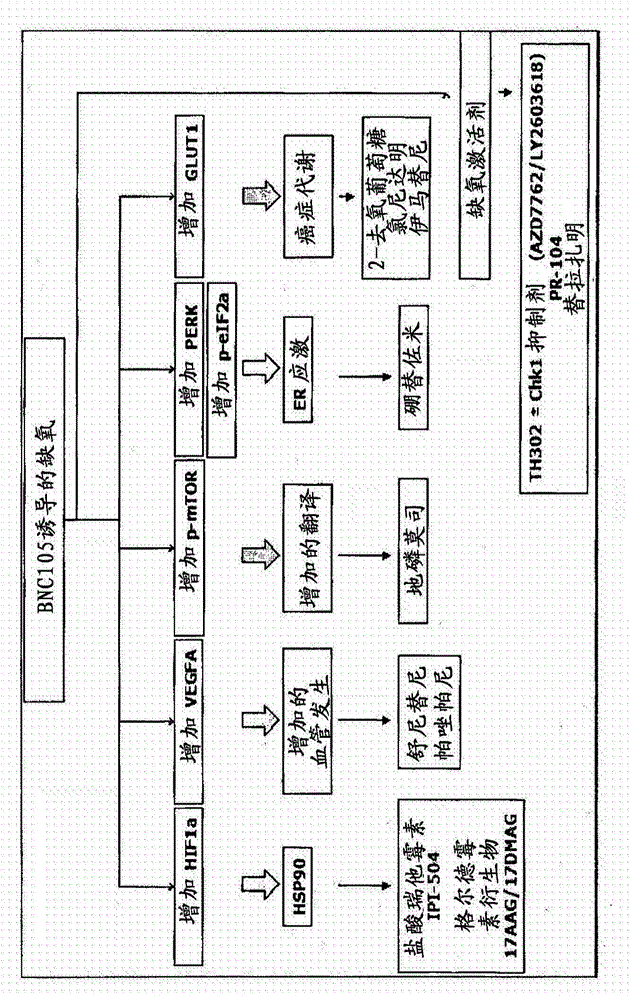
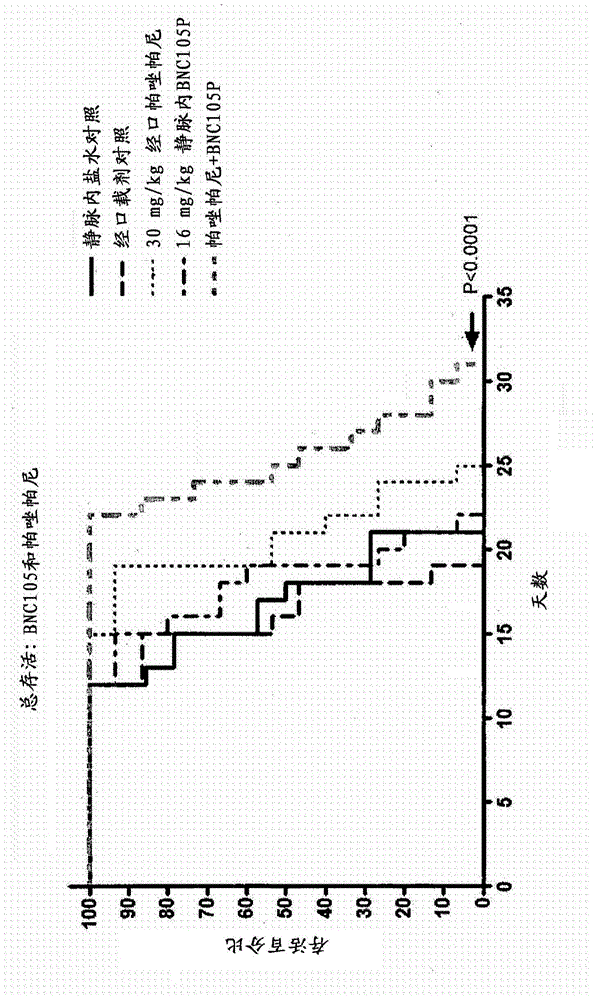
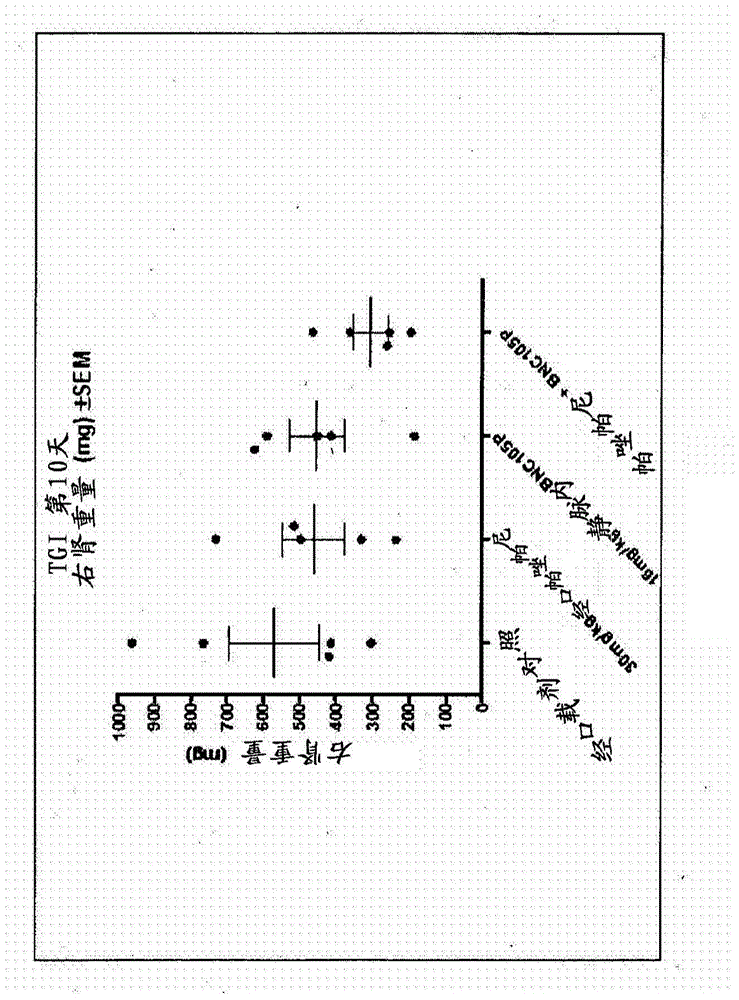
Combination therapy involving a vascular disrupting agent and an agent which targets hypoxia
InactiveCN104703595ABoron compound active ingredientsAntineoplastic agentsMedicineCombination therapy
The present invention provides a method for treating a proliferative disease in a patient. The method comprises administering to a patient in need thereof: a) a vascular disrupting agent and (b) at least one hypoxia targeting agent. Preferred combinations are BNC105 and Pazopanib and BNC 105 and Bortezomib.
Owner:BONOMICS LTD
Diagnostic and therapeutic methods for cancer
ActiveUS20190369098A1Organic active ingredientsMicrobiological testing/measurementTyrosine kinaseKidney cancer
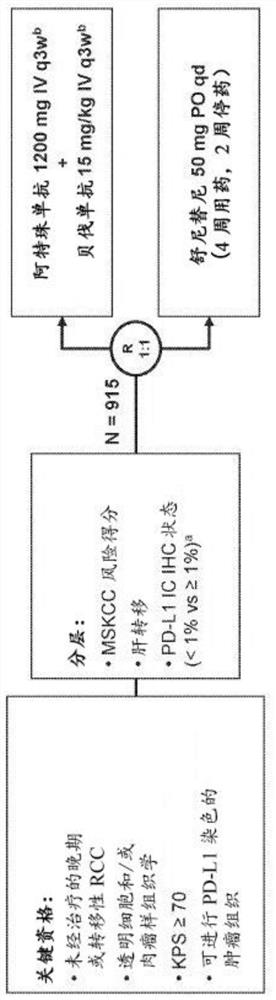
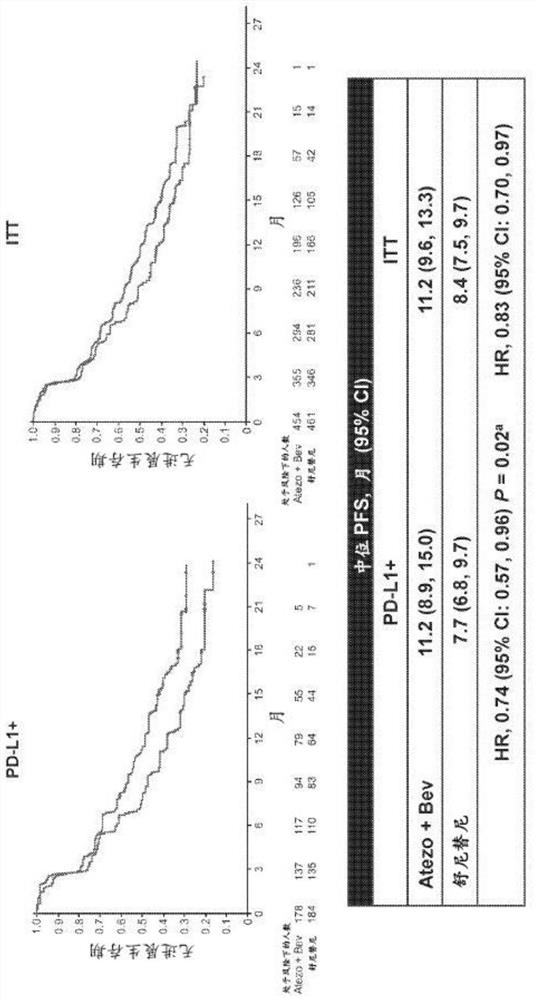
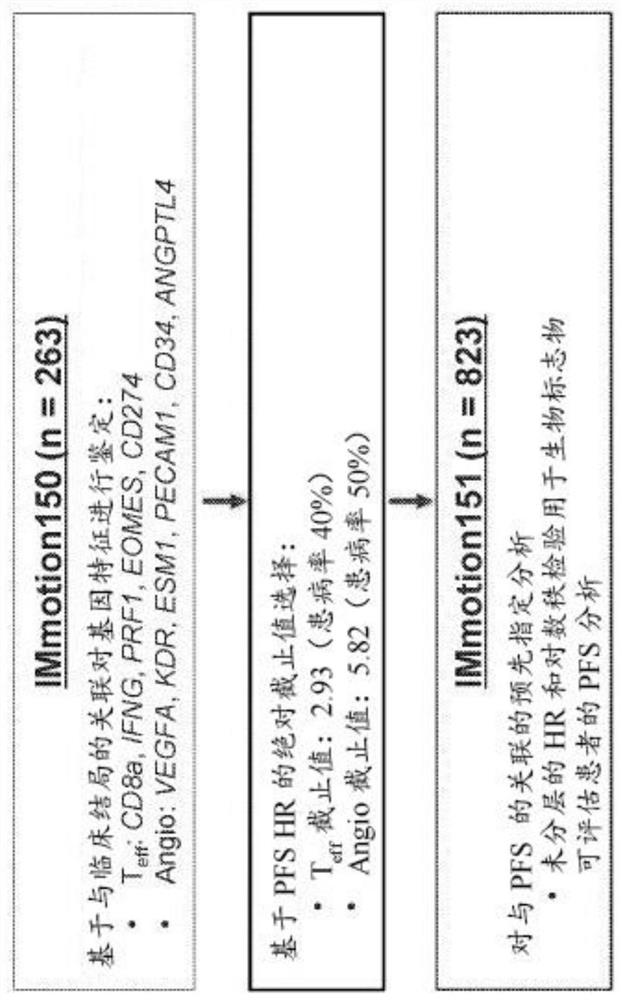
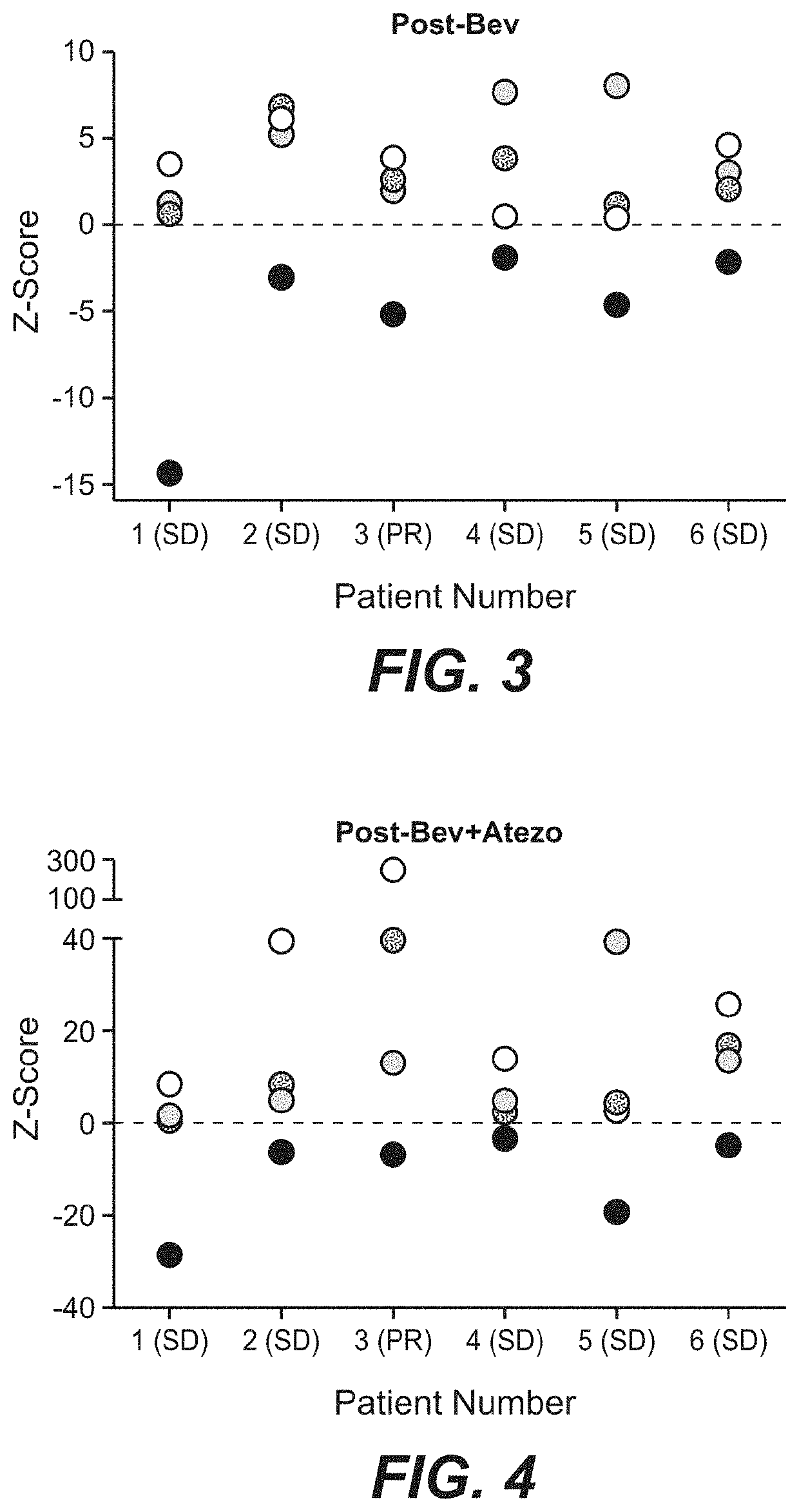
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)), lung cancer (e.g., non-small cell lung cancer (NSCLC)), bladder cancer (e.g., urothelial bladder cancer (UBC)), liver cancer (e.g., hepatocellular carcinoma (HCC)), ovarian cancer, or breast cancer (e.g., triple-negative breast cancer (TNBC))). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:GENENTECH INC
Stable and soluble formulations of receptor tyrosine kinase inhibitors, and methods of preparation thereof
ActiveUS9474756B2Low water solubilityImprove stabilityPowder deliveryOrganic active ingredientsReceptor tyrosine kinase inhibitorMedicine
The present disclosure relates to stable formulations of receptor tyrosine kinase inhibitors (TKI), e.g., pazopanib; methods of preparation thereof; and use of the disclosed formulations in sustained delivery of the active agent to a target site. The disclosure further relates to methods of converting one polymorphic Form of a TKI to another polymorphic Form and / or an amorphous form.
Owner:FORSIGHT VISION5 INC
Composition containing protein kinase inhibitor and metformin
The invention firstly provides a composition containing a protein kinase inhibitor and metformin or pharmaceutically acceptable salts thereof. The composition is characterized in that the protein kinase inhibitor is one selected from nilotinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib and regorafenib or pharmaceutically acceptable salts or solvates thereof or solvates of the pharmaceutically acceptable salts thereof, and the molar ratio of the metformin to the protein kinase inhibitor is (0.01-100):1. In-vitro bacteriostatic tests find that the composition containing the metforminand the protein kinase inhibitor can achieve a synergistic bacteriostatic effect on various bacteria such as staphylococcus aureus in the molar ratio of (0.01-100):1 (at an inhibition rate of 30%, thecombined medication index CI is smaller than 1).
Owner:黄泳华
Pharmaceutical composition for treating renal clear cell carcinoma and application thereof
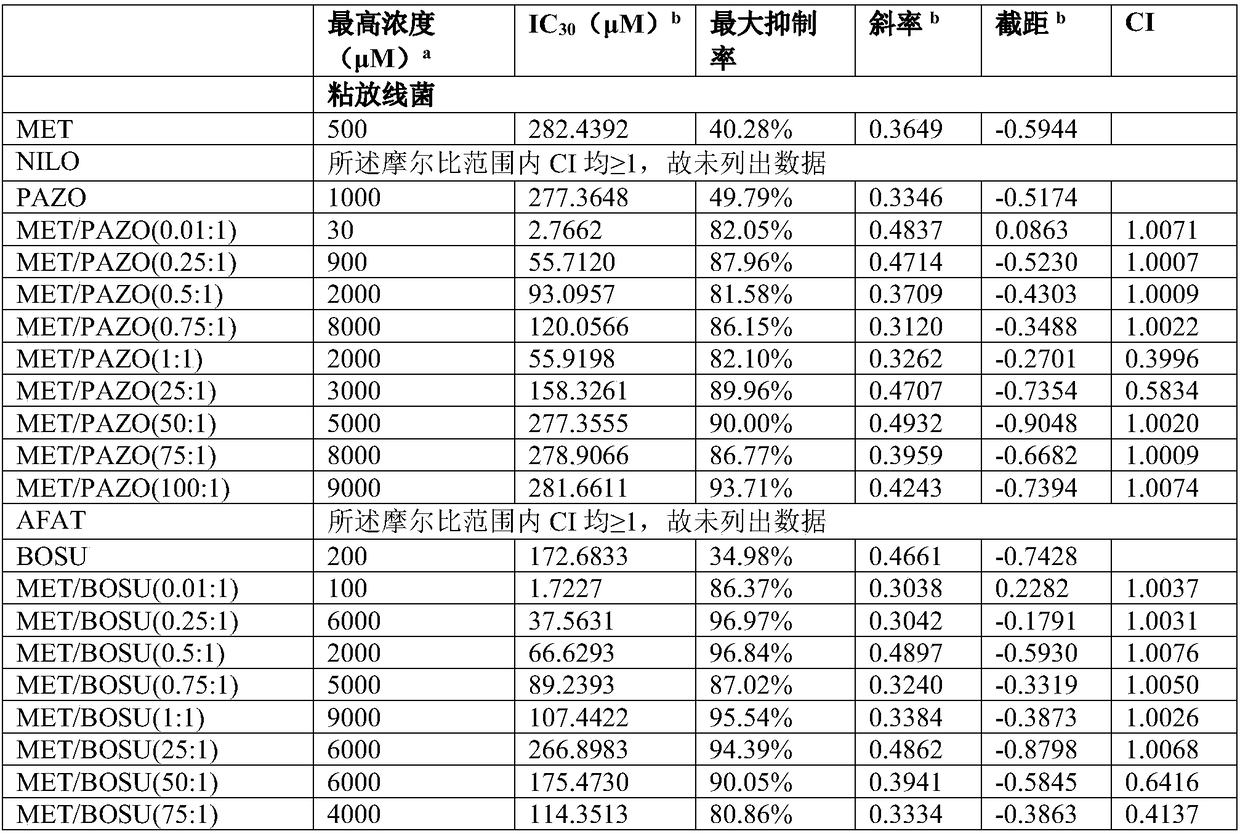
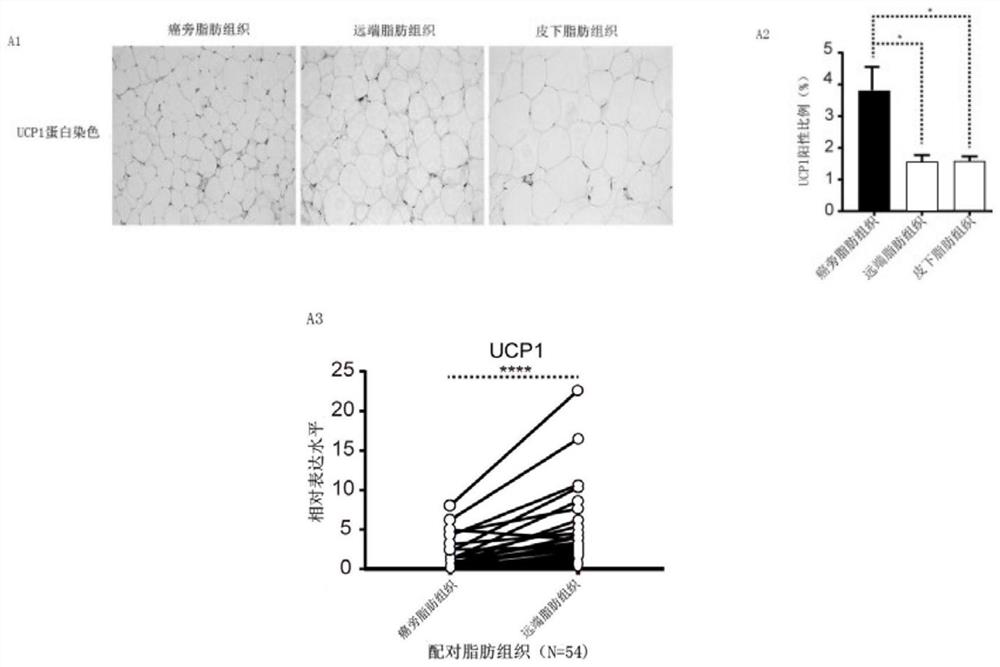
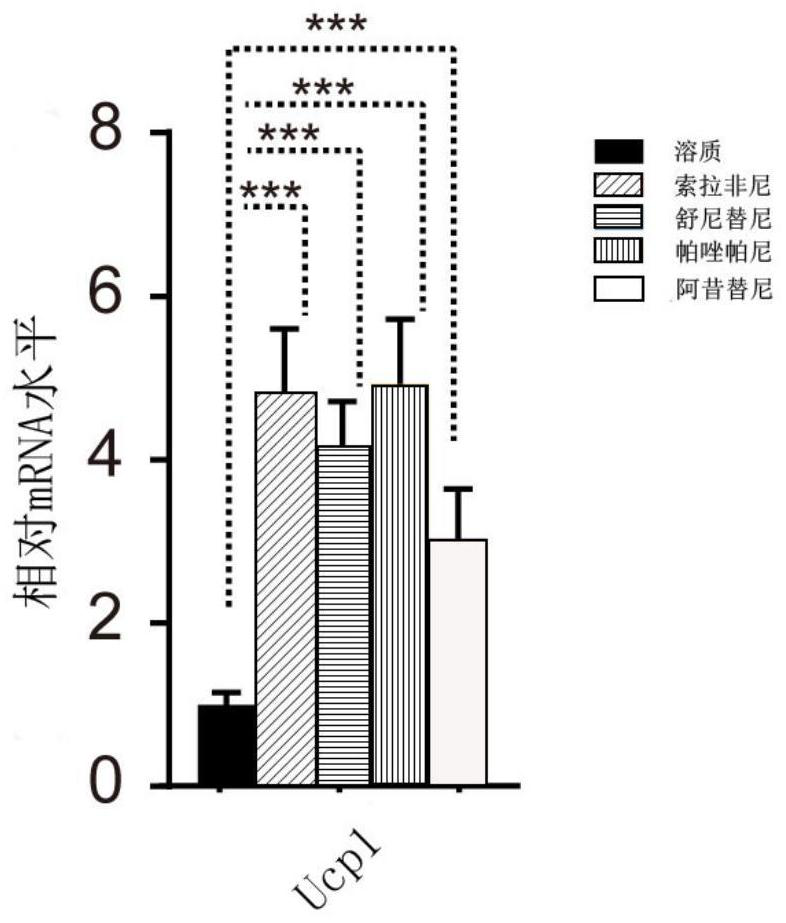
ActiveCN111789952AImprove anti-cancer effectAddressing issues that impair anticancer efficacy and lead to drug resistanceUrinary disorderAntineoplastic agentsSide effectWhite Adipocytes
The invention provides a pharmaceutical composition for treating the renal clear cell carcinoma and an application thereof. The pharmaceutical composition comprises a fat browning inhibitor and a first-line renal cancer treatment drug, wherein the first-line renal cancer treatment drug is selected from sunitinib, sorafenib, axitinib and pazopanib to form four combinations. The fat browning inhibitor in the pharmaceutical composition can improve the anti-cancer effects of the four kinds of first-line renal cancer treatment drugs, solve the problem that the four kinds of first-line renal cancertreatment drugs can cause white fat cell browning, further possibly damage the anti-cancer curative effect and cause drug resistance, reduce the treatment side effects of the drugs, and provide a newstrategy for treatment of the renal clear cell carcinoma.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Green preparation method of pazopanib hydrochloride
The invention belongs to the technical field of pharmaceutical chemistry synthesis and particularly relates to a green preparation method of pazopanib hydrochloride. The method comprises the steps ofallowing o-toluidine and N-chlorosuccinimide to give a chlorination reaction to form 2-methyl-5-chlorine-aniline, allowing 2-methyl-5-chlorine-aniline to react with a nitrous acid compound to form 6-chlorine-2H-indole hydrochloride, performing N methylation reaction to form N-methyl-6-chlorine-2H-indole, performing 3-delta carbon alkylation reaction in the presence of dimethyl sulfoxide to form 2,3-dimethyl-6-chlorine-2H-indazole, allowing 2,3-dimethyl-6-chlorine-2H-indazole to react with 2-chlorine-4-amino-pyrimidine and methyl iodide to form N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine, and at last, allowing N-(2-chloropyrimidine-4)-N-methyl-2,3-dimethyl-2H-indazole-6-amine to react with 3-sulfamate-4-methyl-aniline to form pazopanib hydrochloride. The method is lowin raw material price, simple to operate and low in operational risk, and avoids generation of waste acid; and a reaction yield and purity are high.
Owner:JINAN ASIA PHARMA TECH
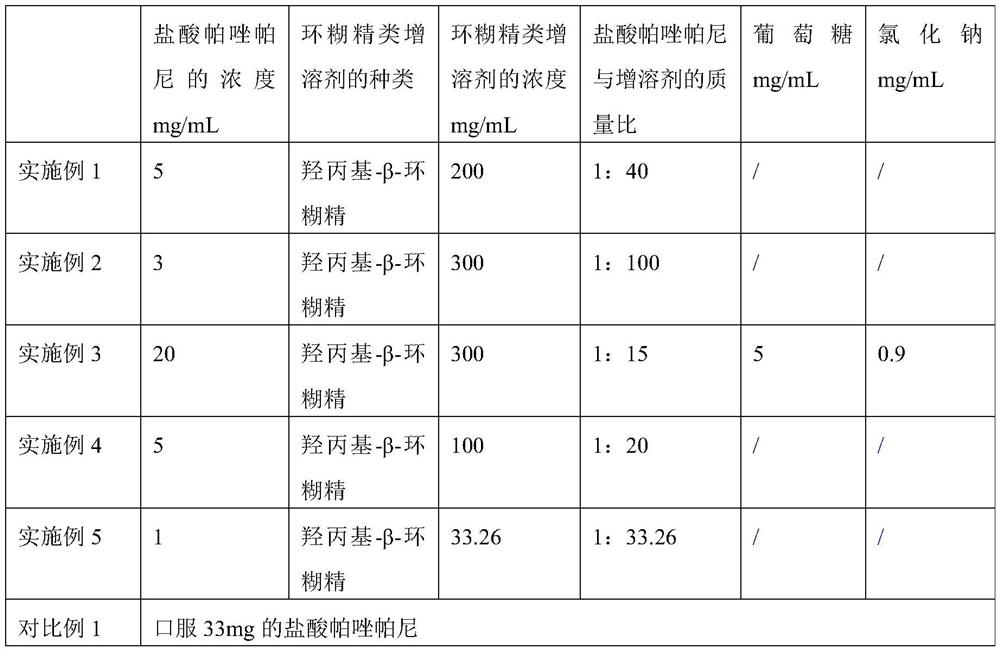
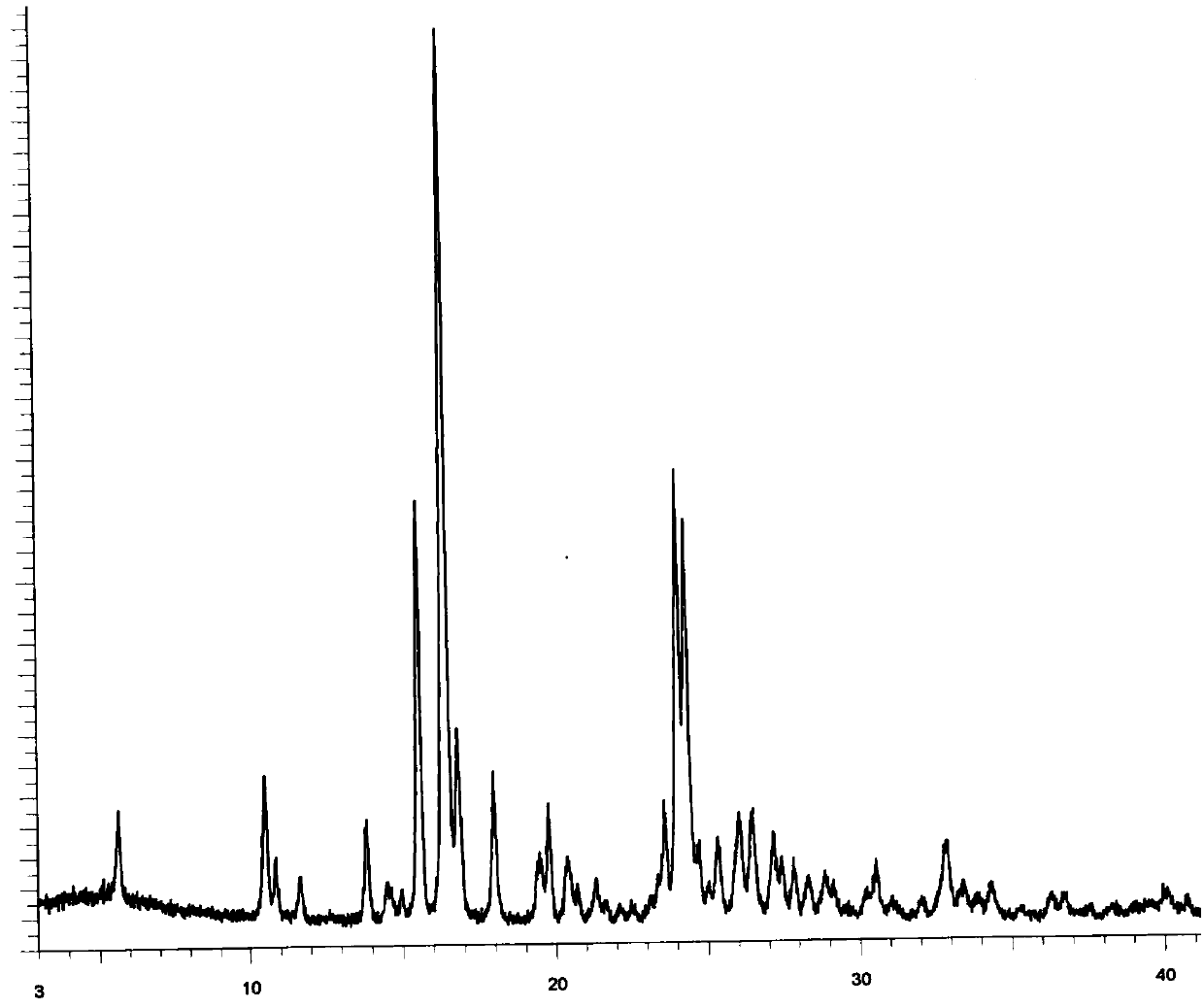
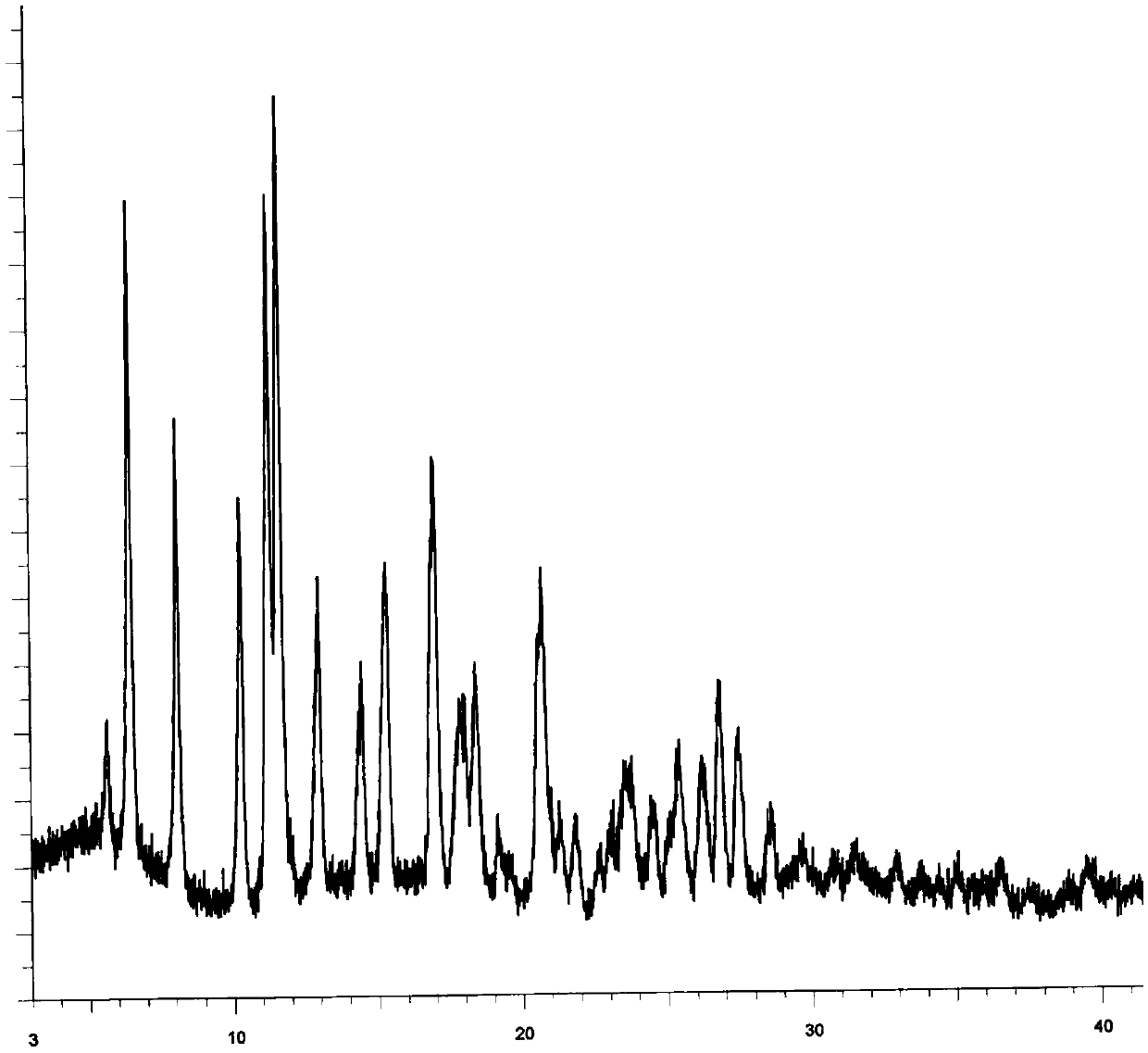
Application of pazopanib, pharmaceutical composition, injection, preparation method and application
PendingCN113350351ANo accumulationImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCyclodextrin
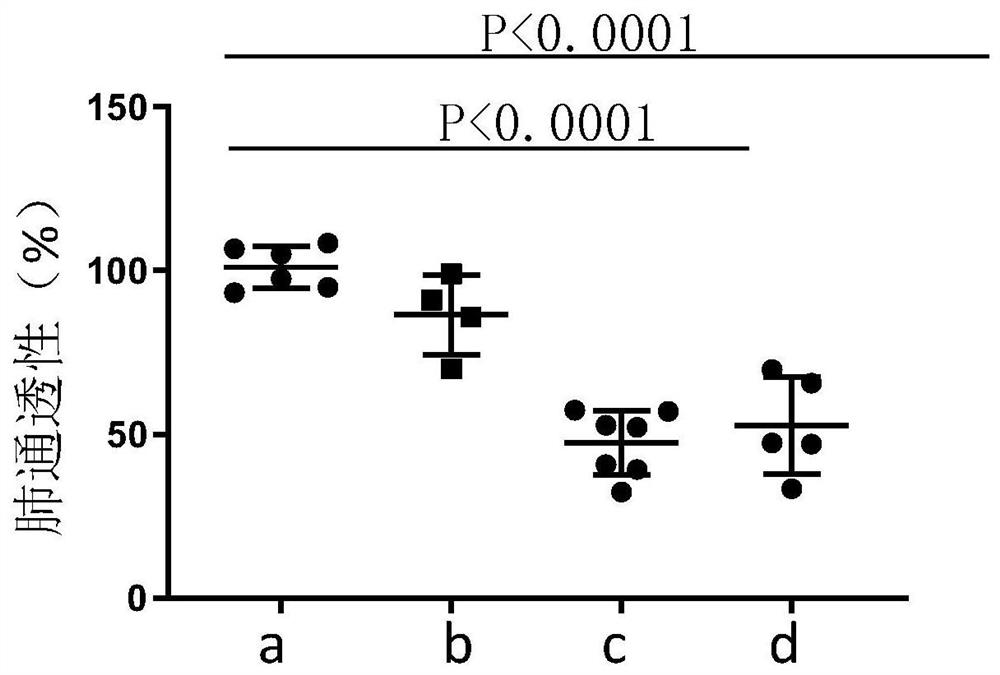
The invention discloses application of pazopanib, a pharmaceutical composition, an injection, a preparation method and application. The pharmaceutical composition comprises the following components: pazopanib, a cyclodextrin solubilizer and a solvent, wherein the mass ratio of pazopanib to the cyclodextrin solubilizer is 1: (13-100); and based on 1mL of the pharmaceutical composition, the concentration of the cyclodextrin solubilizer in the pharmaceutical composition is 6-330mg / mL. The pharmaceutical composition prepared by the invention can be used for treating diseases such as acute lung injury, pulmonary fibrosis and acute respiratory distress syndrome, and the treatment aim can be achieved by using a small amount of pazopanib; and moreover, the pharmaceutical composition is high in bioavailability, high in stability and low in impurity content, and the phenomenon of drug accumulation is avoided.
Owner:青晓制药公司
Preparation method of pazopanib hydrochloride
InactiveCN110878089AGood performance parameters of powderSmall bulk densityOrganic chemistry methodsCombinatorial chemistryPAZOPANIB HYDROCHLORIDE
The invention relates to a preparation method of pazopanib hydrochloride, and provides a preparation method of pazopanib monohydrochloride, wherein a pazopanib monohydrochloride hydrate is subjected to stirring and crystal transformation in an acetonitrile-water mixed solution, and then filtering and drying are performed to obtain pazopanib monohydrochloride. According to the invention, the preparation method has the advantages of simple process operation and high product yield, and the residual solvent of the obtained product meets the medicinal requirements.
Owner:JIANGSU HANSOH PHARMA CO LTD
HDAC and VEGFR dual-target inhibitor based on pazopanib structure and its preparation method and application
ActiveCN107619407BHigh yieldInhibit side effectsOrganic active ingredientsOrganic chemistryDiseaseStereochemistry
The present invention relates to a pazopanib-based HDAC and VEGFR double-target inhibitor, a preparation method therefor and an application thereof. Said compound has a structure as presented in formula I, II or III. The present invention also relates to a preparation method for this type of compound and the application thereof in preparing drugs for treating or preventing oncological diseases.
Owner:SHANDONG UNIV
Multikinase inhibitors and uses in prostatic hyperplasia and urinary track diseases
ActiveUS11376252B2Treating/preventing benign prostate hyperplasiaLow urinary tract symptomPharmaceutical delivery mechanismUrinary disorderDiseaseRegorafenib
A method for preventing, treating and / or improving a prostatic disease or disorder associated with epithelial hyperplasia and / or fibrosis, comprising: administering an effective amount of a multikinase inhibitor to a subject in need thereof, wherein the multikinase inhibitor has a certain spectrum of kinase inhibitory activity. The multikinase inhibitor is sunitinib, regorafenib, ponatinib, pazopanib, nintedanib and / or lenvatinib. The prostatic disease or disorder is selected from the group consisting of benign prostate hyperplasia and its associated lower urinary tract symptoms, fibrosis of ureters and renal pelvis, prostate adenoma, and prostatic intraepithelial neoplasia in animals and humans.
Owner:AIVIVA BIOPHARMA INC
Compositions and methods for treating lung injuries associated with SARS-COV-2 infections
ActiveUS11304947B2Reduced survivalPrevention and reduction of severityOrganic active ingredientsAntiviralsPTK InhibitorsALI - Acute lung injury
The present invention provides methods and compositions for treating and preventing lung injuries due to or associated with coronavirus infections that cause Severe Acute Respiratory Syndrome, including COVID-19. More specifically the present invention provides methods for treating or preventing the lung injuries associated with SARS-CoV-2 infections, such as acute lung injury (ALI), lung fibrosis, and acute respiratory distress syndrome (ARDS). The methods comprise administering a therapeutically effective amount of a pharmaceutical composition comprising a protein kinase inhibitor compound having MAP3K2 / MAP3K3 inhibition activity, such as pazopanib or nintedanib, or a pharmaceutically acceptable salt, solvate, or prodrug thereof, to a patient in need thereof. The present invention also provides devices for administering the compositions.
Owner:YALE UNIV +1
A kind of preparation method of pazopanib and its intermediate
The invention discloses a preparation method and an intermediate of Pazopanib. The preparation method comprises the steps of carrying out two-step condensation reaction on 2,4-dichloro-5-nitropyrimidine so as to obtain a compound of a formula 5 (shown in the description), and carrying out reduction on the compound of the formula 5, so as to obtain Pazopanib. The preparation method has the beneficial effects that the yield of Pazopanib is increased, reaction conditions are mild, column chromatography purification is not required, and the preparation method is applicable to industrial production.
Owner:苏州东南药业股份有限公司
Compositions and methods for treating lung injuries associated with sars-cov-2 infections
ActiveUS20210361650A1Prevention and reduction of severityReduced survivalOrganic active ingredientsAntiviralsPTK InhibitorsALI - Acute lung injury
The present invention provides methods and compositions for treating and preventing lung injuries due to or associated with coronavirus infections that cause Severe Acute Respiratory Syndrome, including COVID-19. More specifically the present invention provides methods for treating or preventing the lung injuries associated with SARS-CoV-2 infections, such as acute lung injury (ALI), lung fibrosis, and acute respiratory distress syndrome (ARDS). The methods comprise administering a therapeutically effective amount of a pharmaceutical composition comprising a protein kinase inhibitor compound having MAP3K2 / MAP3K3 inhibition activity, such as pazopanib or nintedanib, or a pharmaceutically acceptable salt, solvate, or prodrug thereof, to a patient in need thereof. The present invention also provides devices for administering the compositions.
Owner:YALE UNIV +1
Diagnostic and therapeutic methods for cancer
ActiveUS11402382B2Organic active ingredientsMicrobiological testing/measurementAntiendomysial antibodiesEfficacy
Owner:GENENTECH INC
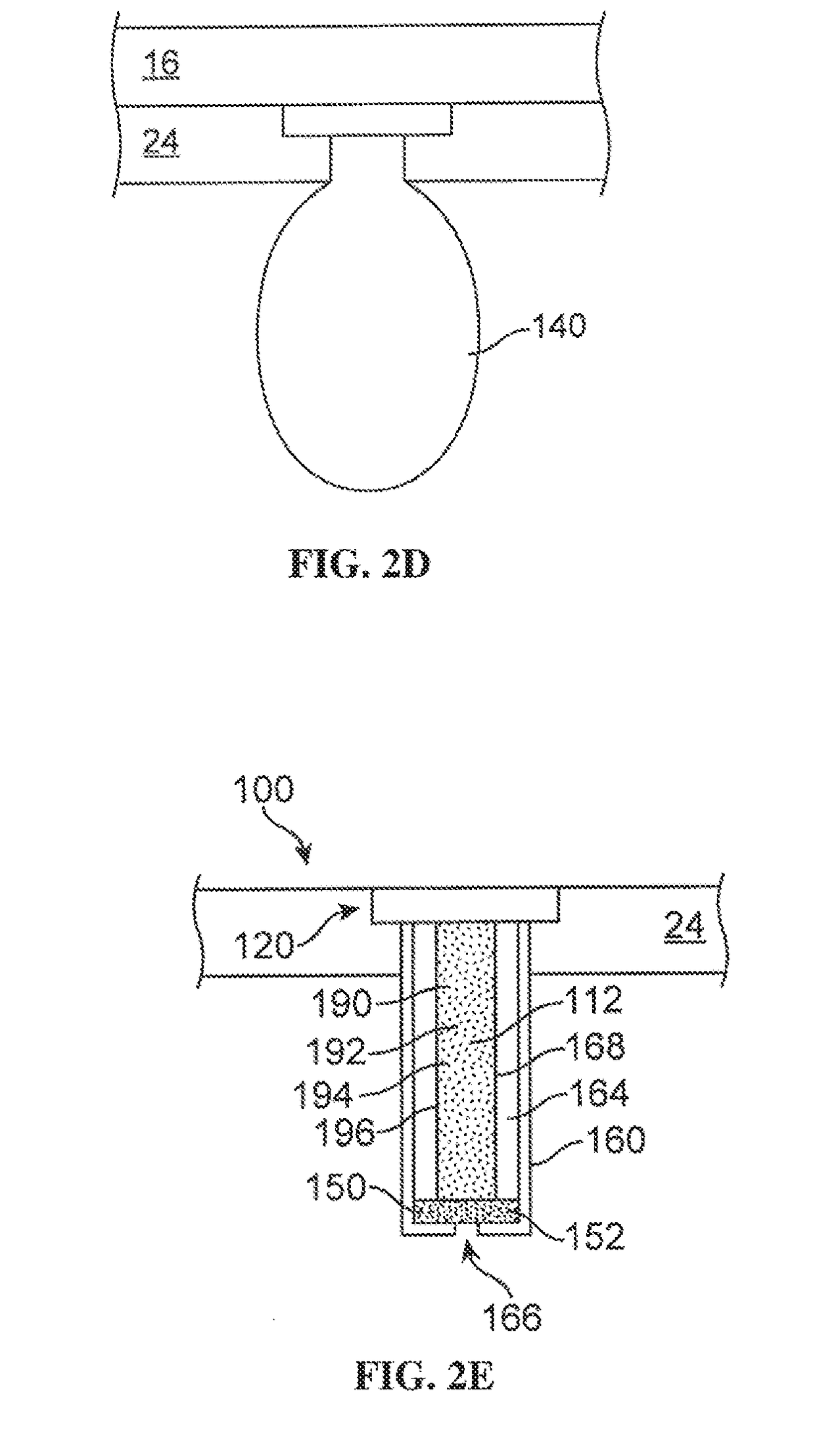
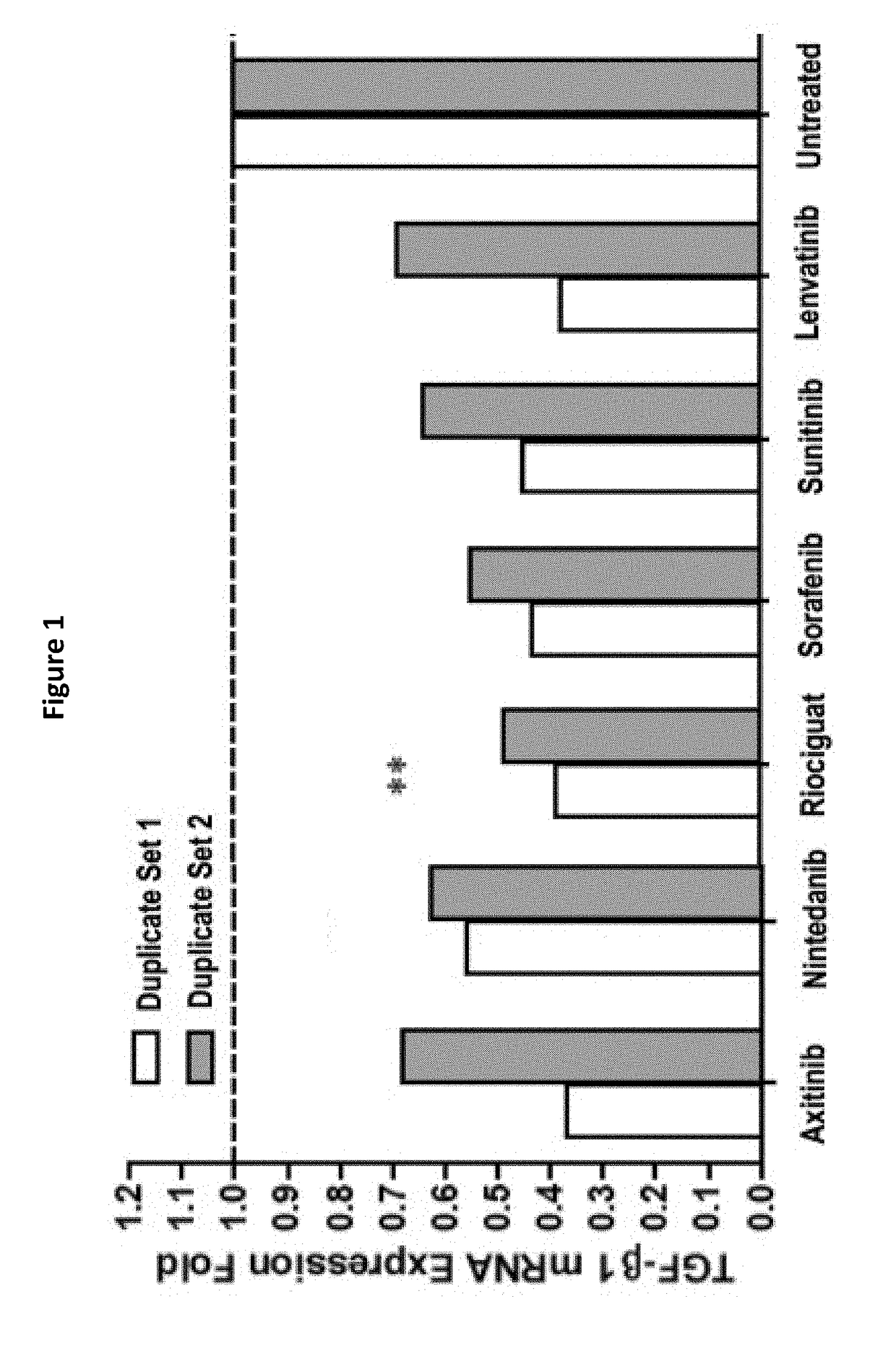
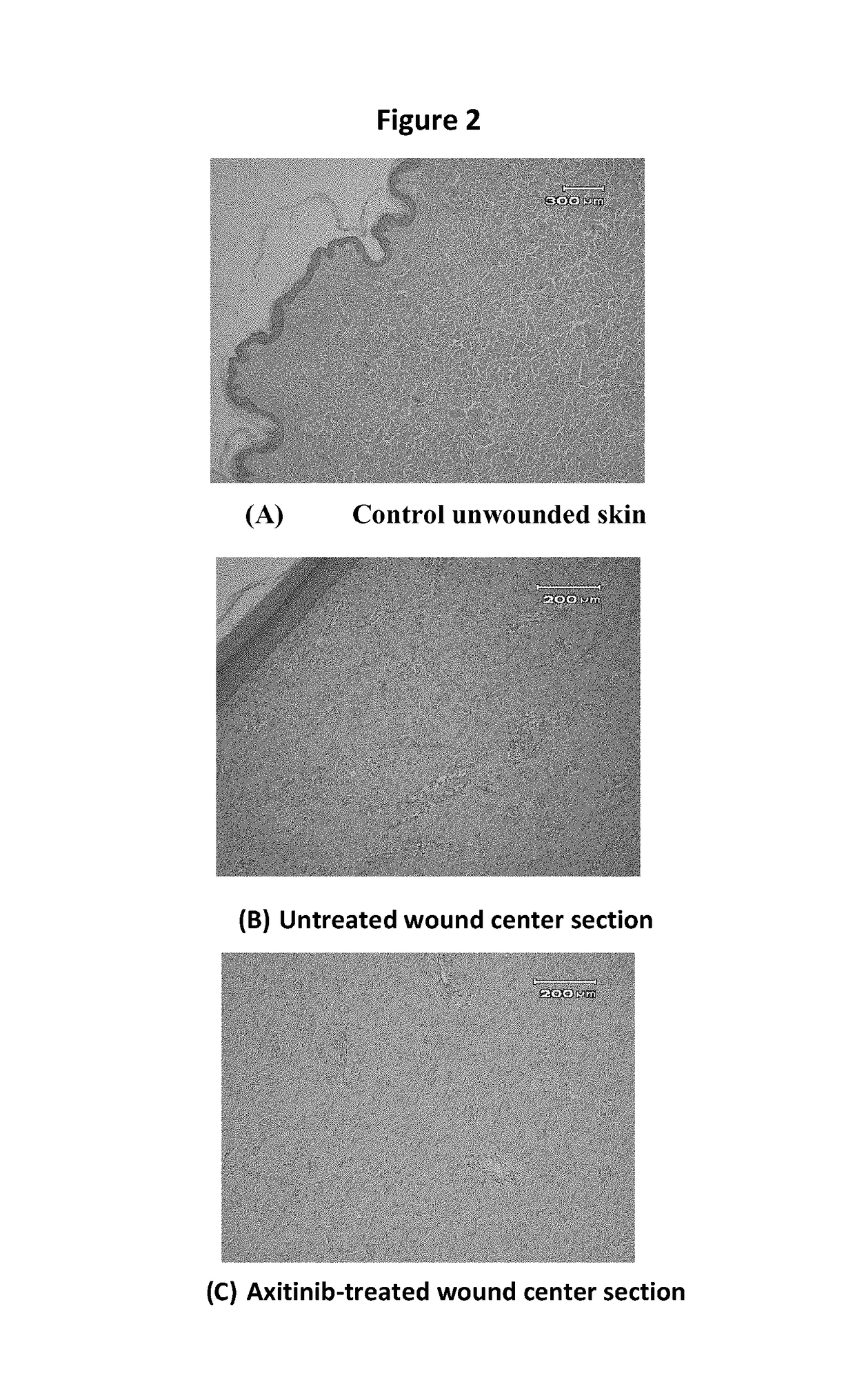
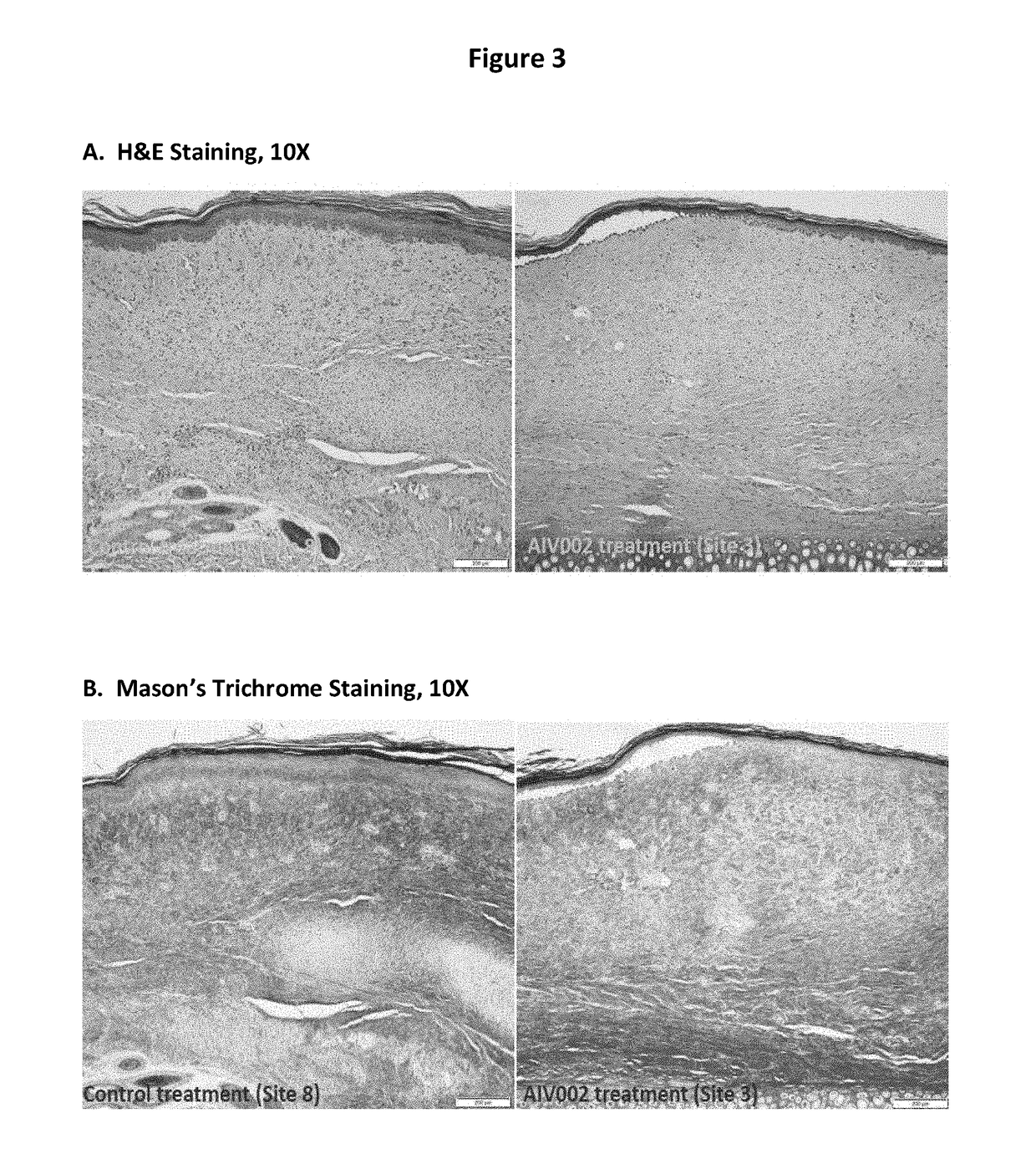
Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof
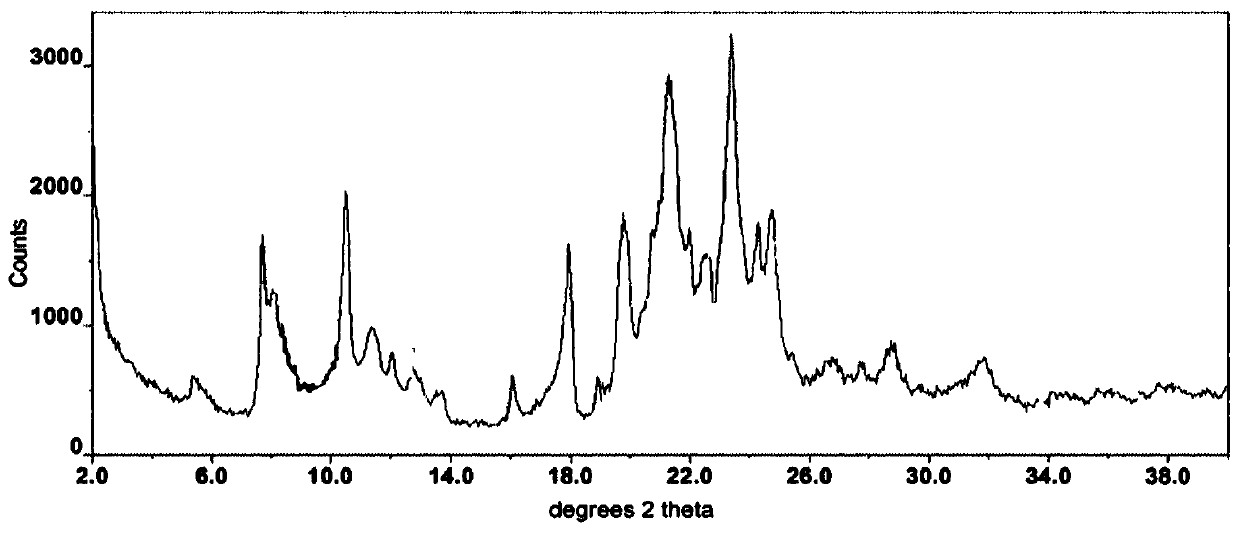
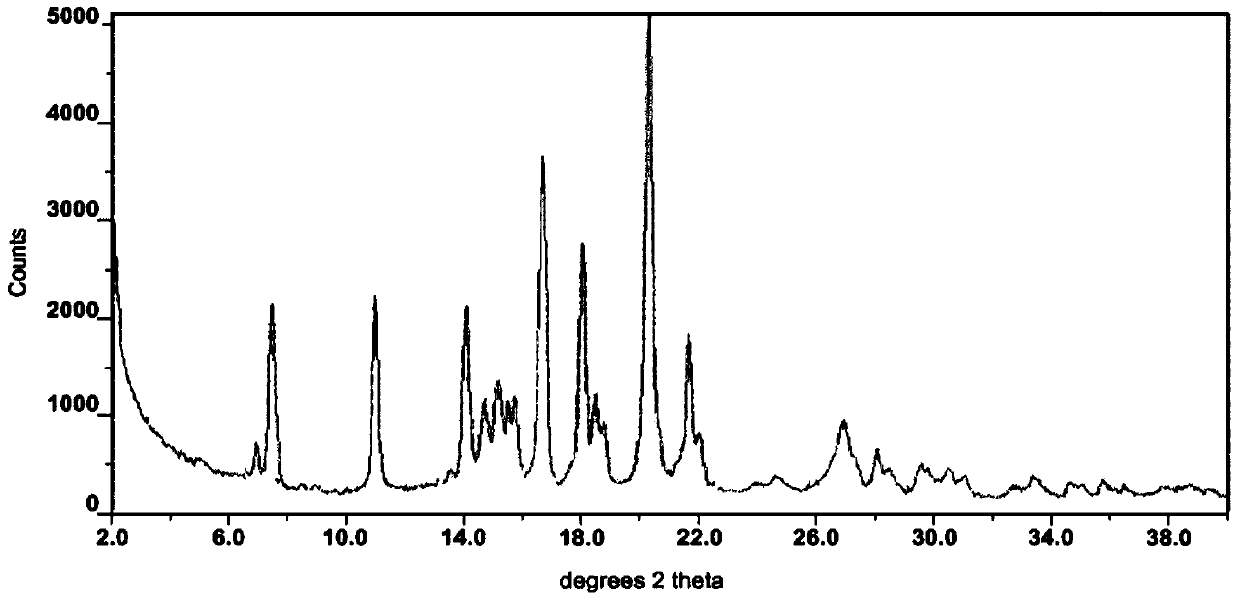
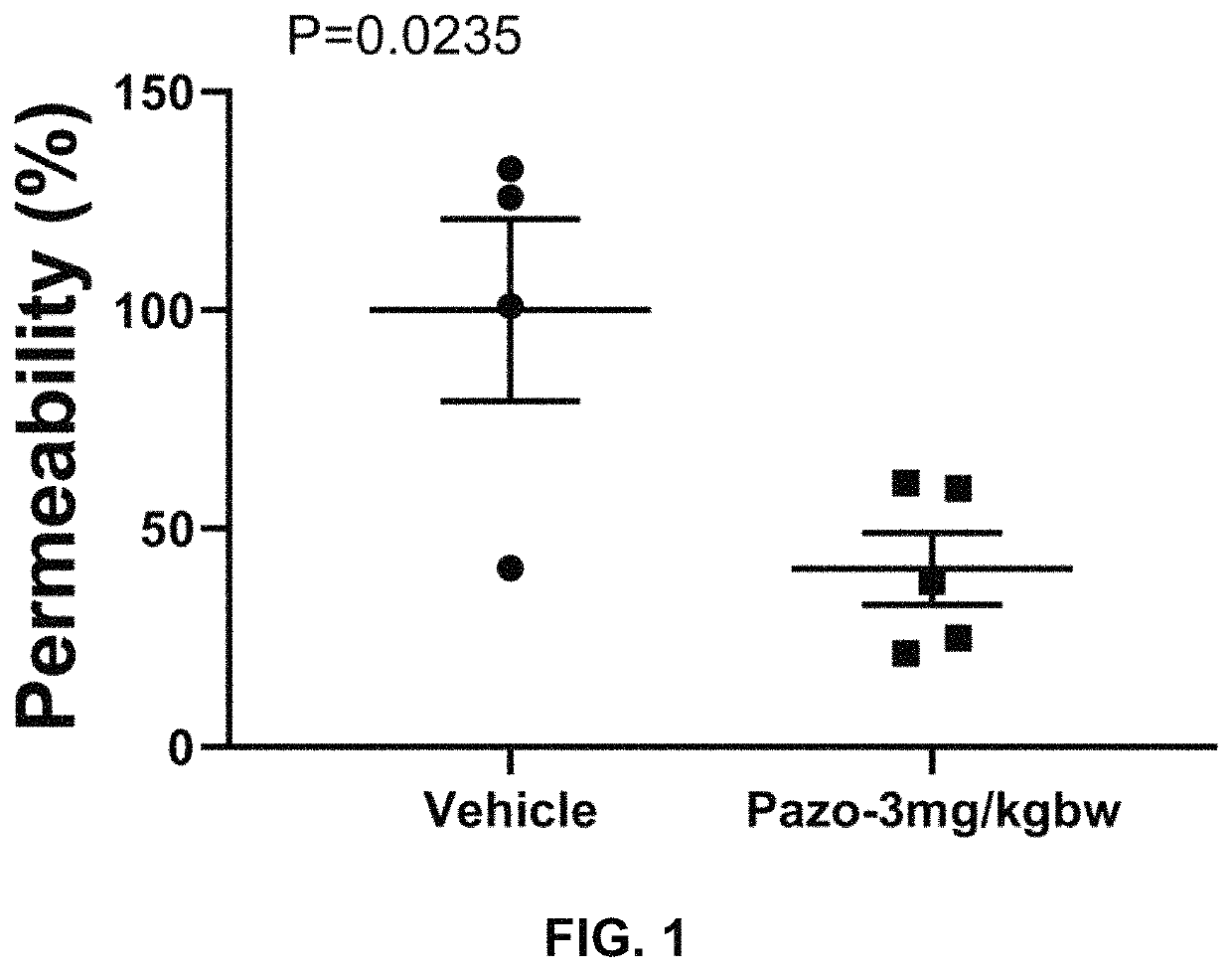
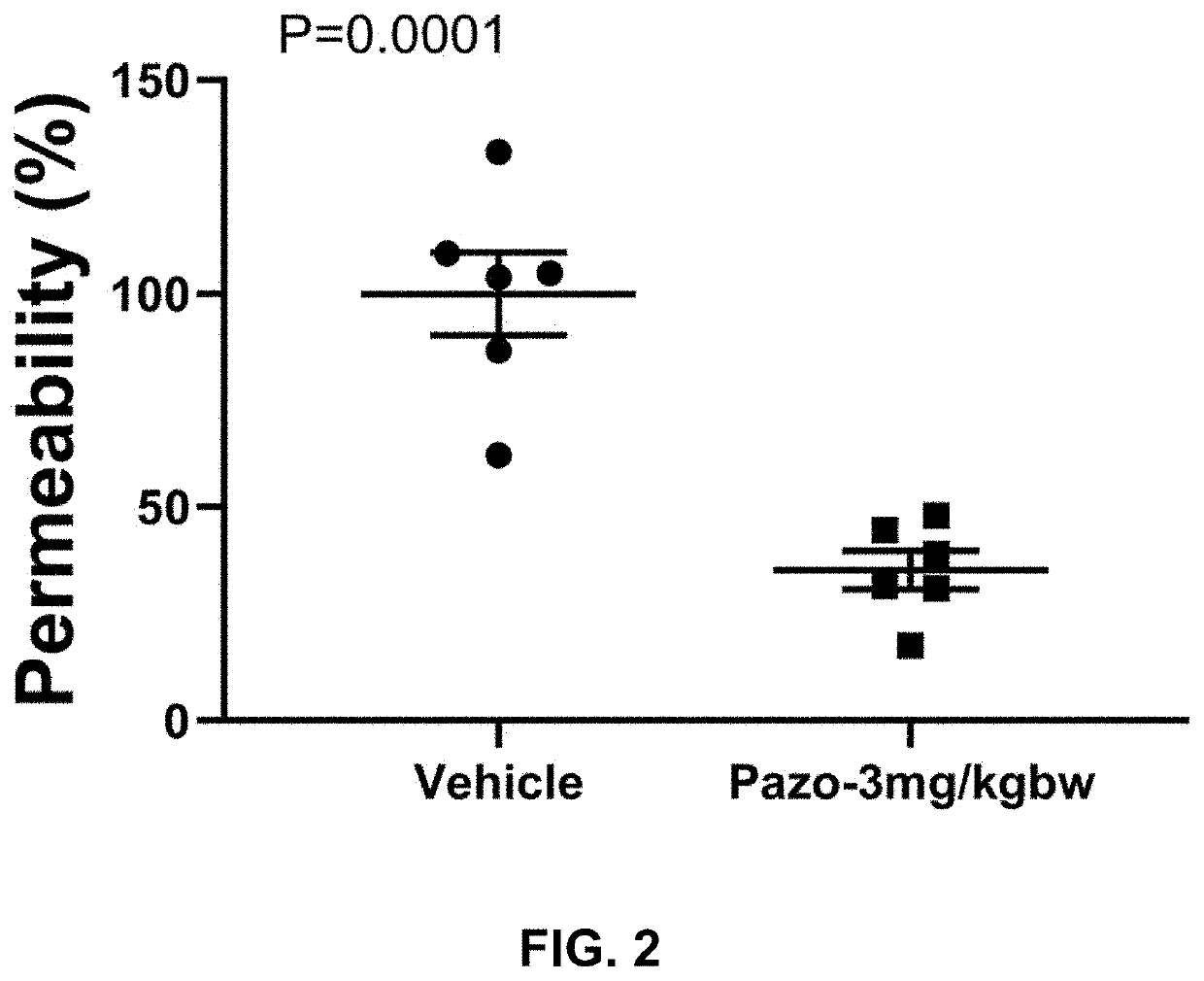
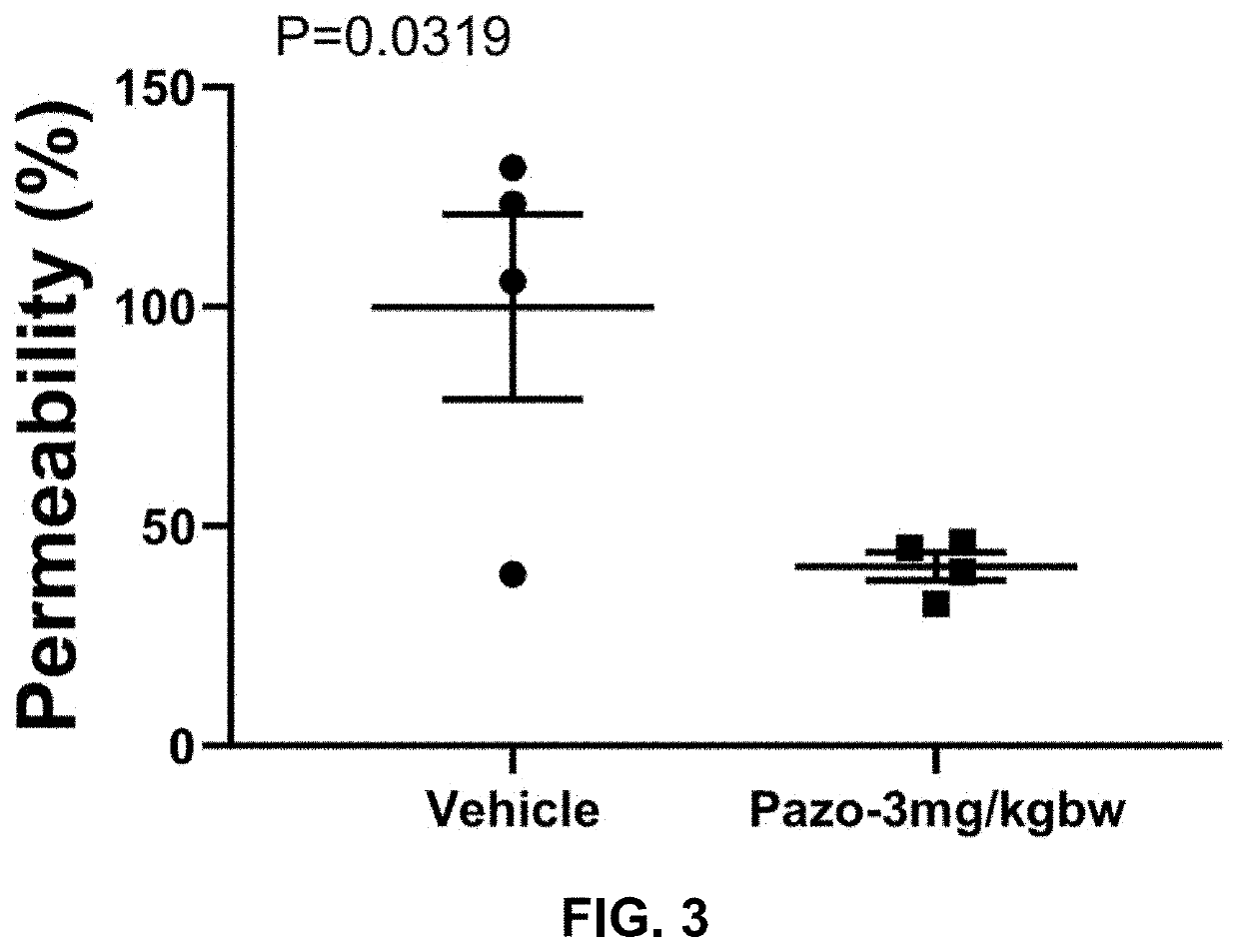
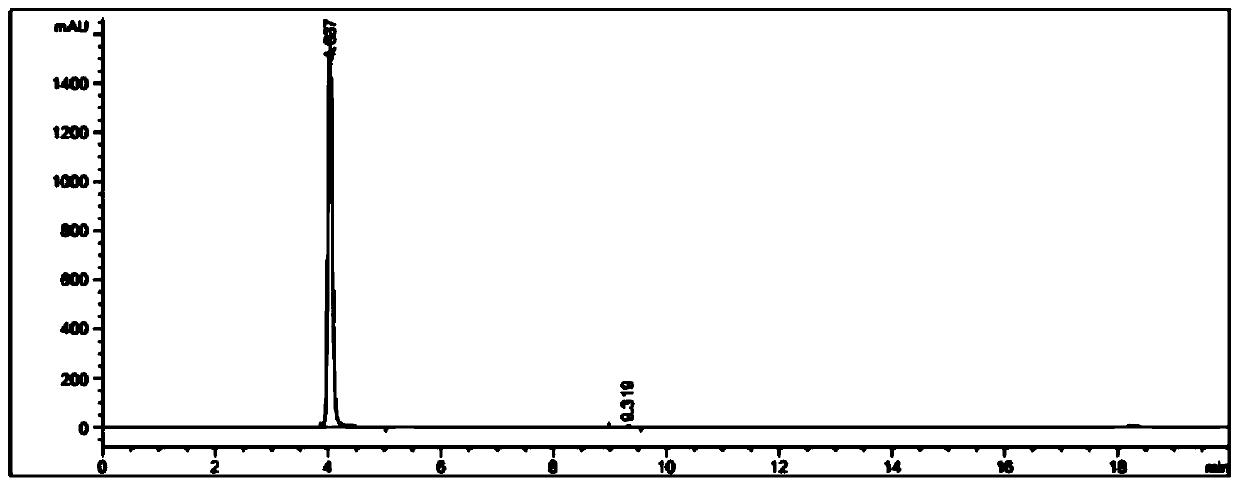
A pharmaceutical composition for prevention or treatment of a disease or disorder characterized by chronic inflammation, associated with angiogenesis and fibrosis. The pharmaceutical composition includes a multi-target inhibitor, multi-phase modulator, or multi-kinase inhibitor, such as axitinib, nintedanib, pirfenidone, riociguat, sorafenib, sunitinib, lenvatinib, regorafenib, ponatinib, or pazopanib.
Owner:AIVIVA BIOPHARMA INC
Diagnostic and therapeutic methods for sarcomatoid kidney cancer
PendingCN113196061AKidney cancer vaccineImmunoglobulins against growth factorsAntiendomysial antibodiesEfficacy
The present invention provides diagnostic methods, therapeutic methods, and compositions for the treatment of cancer (e.g., kidney cancer (e.g., renal cell carcinoma (RCC)). The invention is based, at least in part, on the discovery that expression levels of one or more biomarkers described herein in a sample from an individual having cancer can be used in methods of predicting the therapeutic efficacy of treatment with a VEGF antagonist (e.g., an anti-VEGF antibody, (e.g., bevacizumab) or a VEGFR inhibitor (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib))) and a PD-L1 axis binding antagonist (e.g., a PD-L1 binding antagonist (e.g., anti-PD-L1 antibody, e.g., atezolizumab (MPDL3280A)) or a PD-1 binding antagonist (e.g., anti-PD-1 antibody)), or with an angiogenesis inhibitor (e.g., a VEGF antagonist (e.g., a VEGFR inhibitor, (e.g., a multi-targeted tyrosine kinase inhibitor (e.g., sunitinib, axitinib, pazopanib, or cabozantinib)))).
Owner:F HOFFMANN LA ROCHE & CO AG
Combination therapy of solid cancer
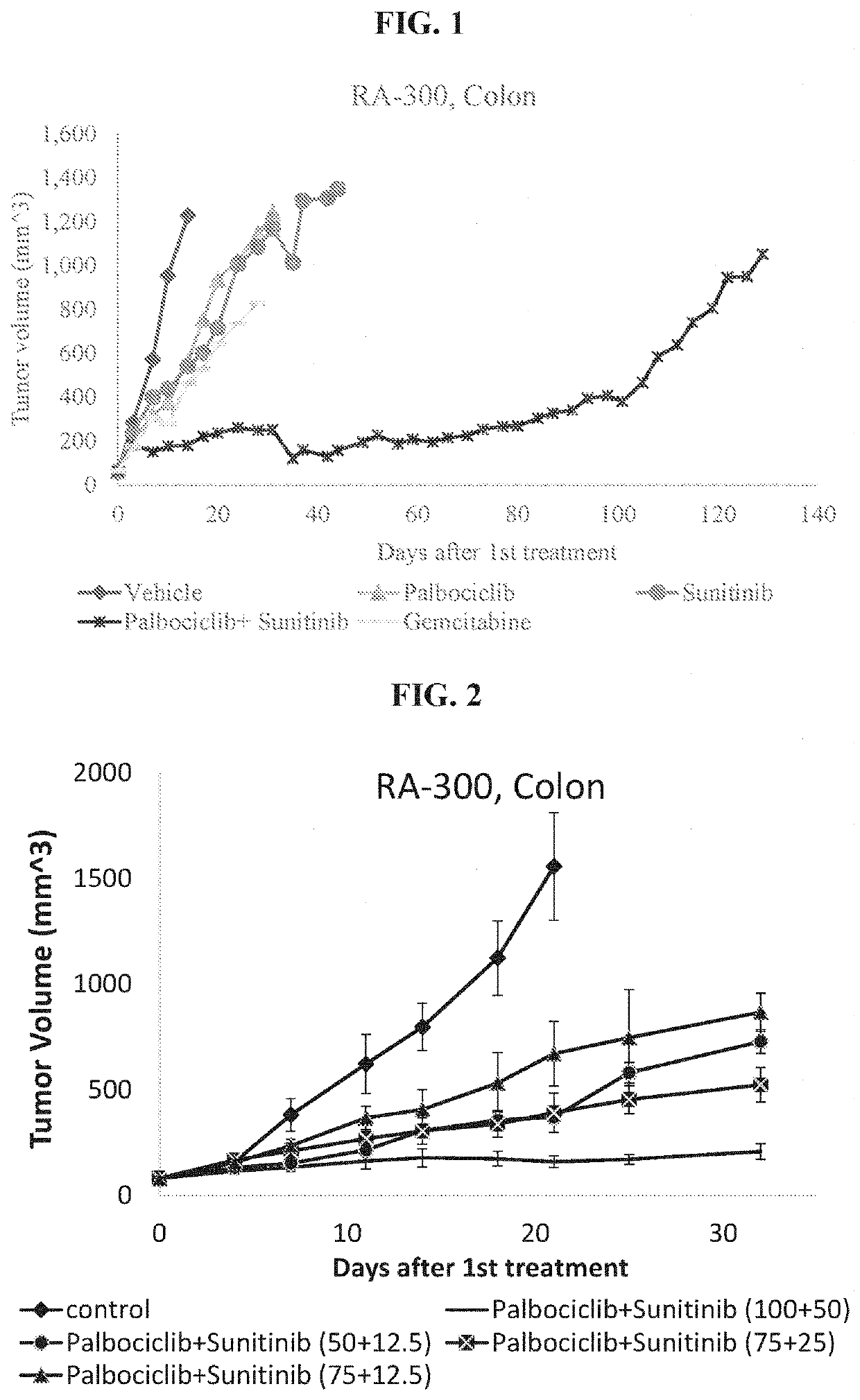
PendingUS20220047599A1Synergistic effectEffective controlAntineoplastic agentsHeterocyclic compound active ingredientsReceptor tyrosine kinase inhibitorTyrosine
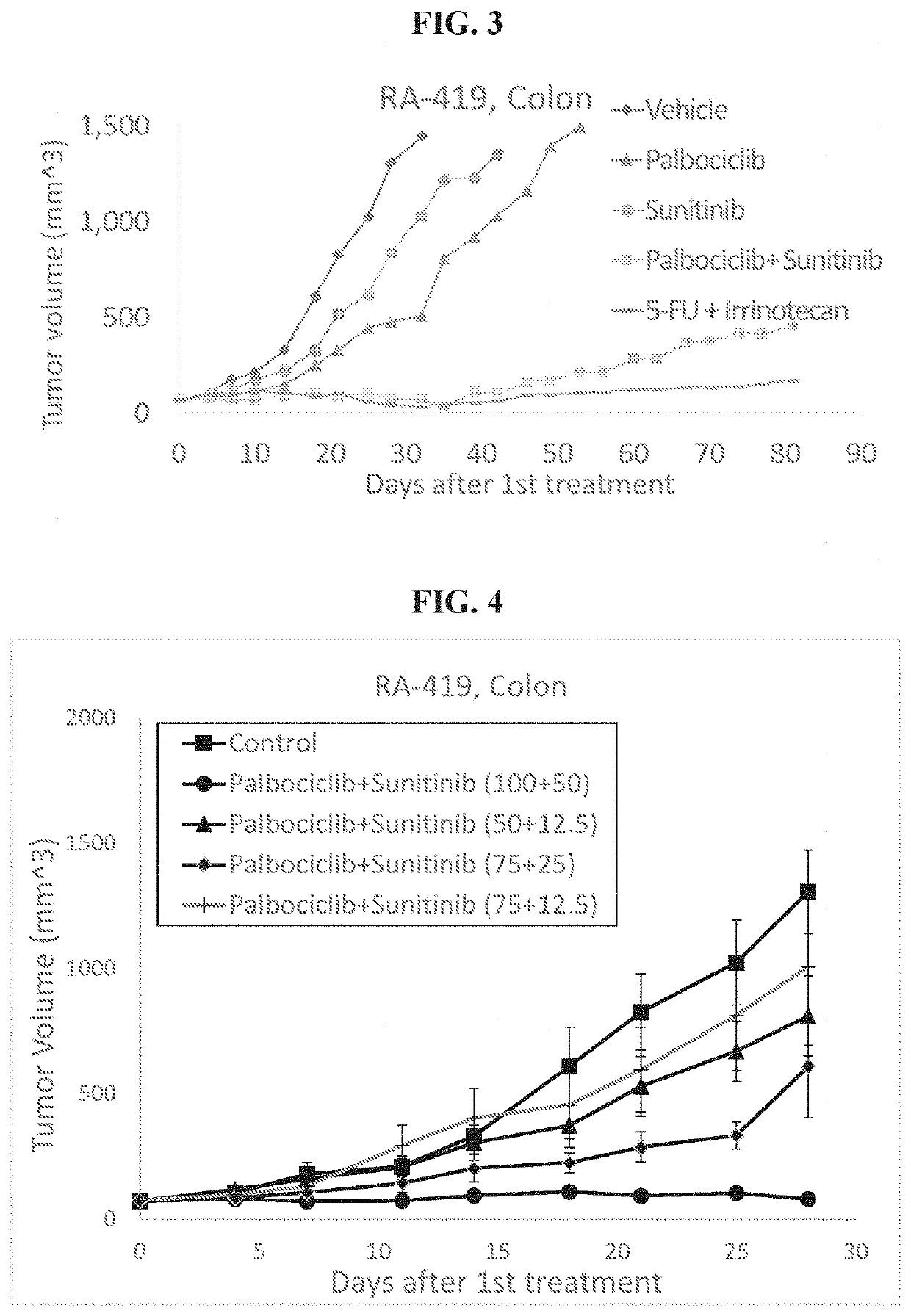
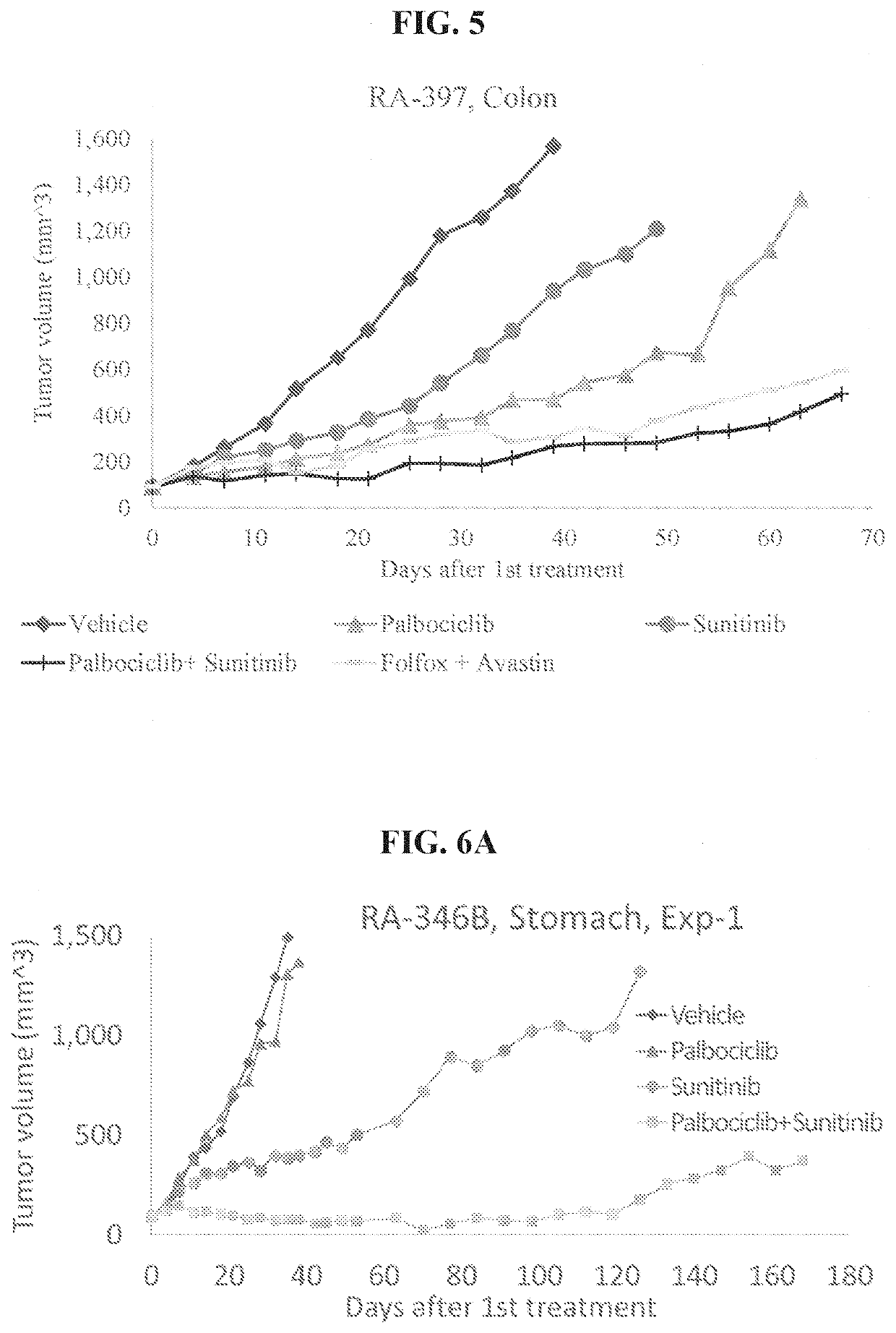
The present invention provides methods of treating solid cancer by co-administering an inhibitor of cyclin-dependent kinase 4 / 6 (CDK 4 / 6) and multi-targeted receptor tyrosine kinase inhibitor (mt RTKI). Particular examples of CDK 4 / 6 inhibitor are palbociclib, abemaciclib and ribociclib and of mt RTKI are sunitinib, sorafenib and pazopanib. Administration of the combination may confer a synergic effect in treatment solid tumors. In particular synergic combinations of palbociclib with sunitinib or sorafenib are provided that synergically inhibit progression of a plurality of solid cancer types. The invention also provides pharmaceutical compositions comprising combinations of CDK 4 / 6 inhibitors and mt RTKIs and their use in treating solid cancer.
Owner:MOR RES APPL LTD
A kind of synthetic method of pazopanib hydrochloride crude drug trimer impurity
ActiveCN110143951BRaise quality standardsHigh yieldOrganic chemistryBulk chemical productionSulfonyl chlorideNitrobenzene
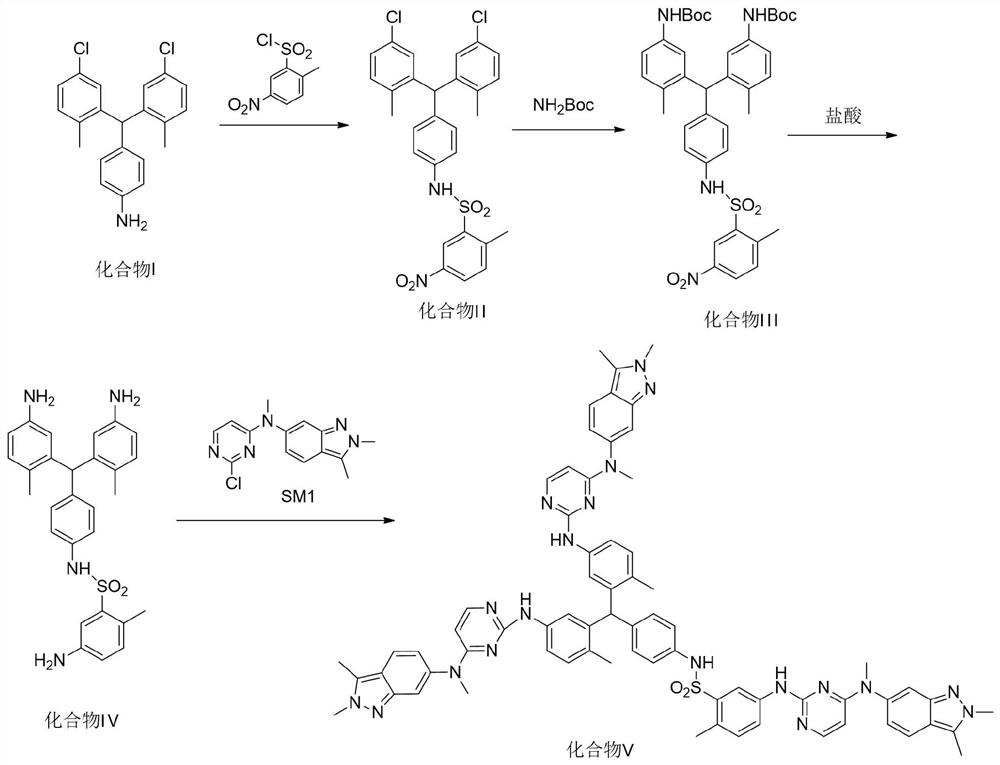
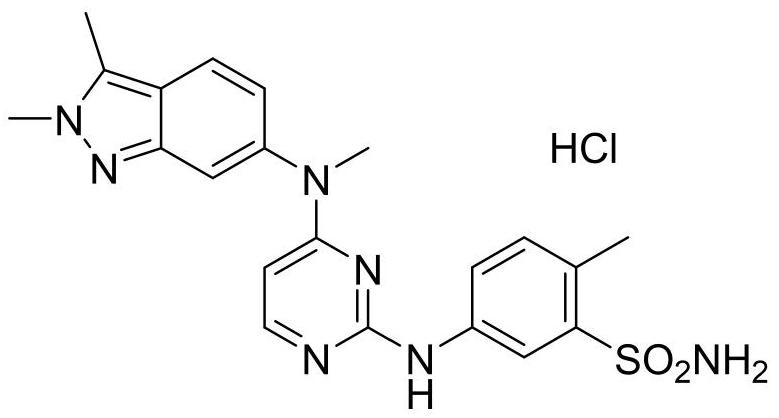
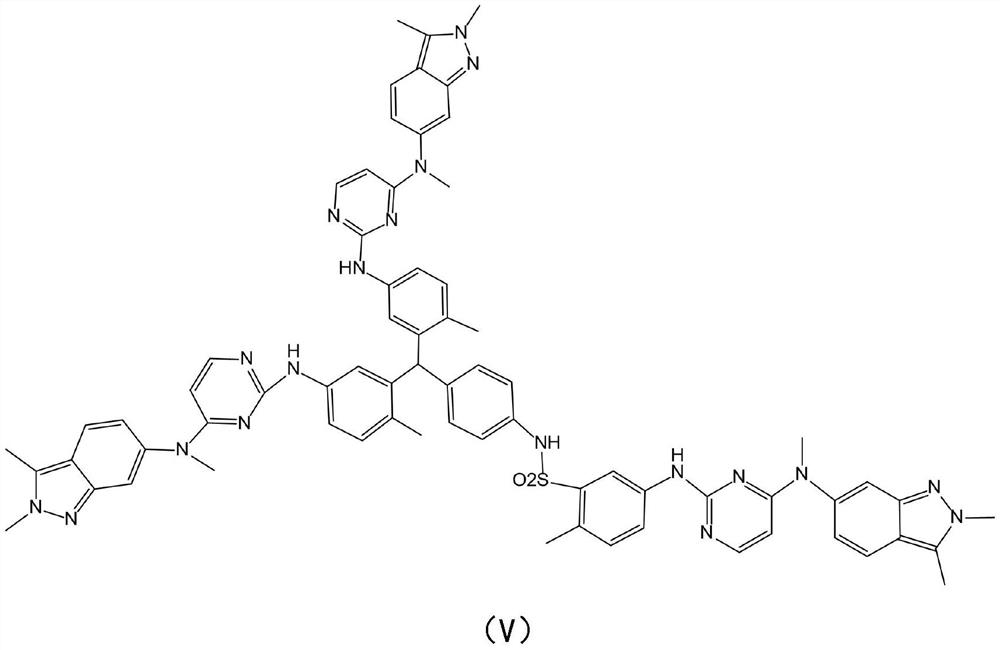
The invention provides a synthesis method of a trimer impurity of a pazopanib hydrochloride bulk drug. The synthesis method comprises following steps: taking a compound (I) as the raw material, carrying out condensation reactions between the compound (I) and 2-methyl-5-nitrobenzene sulfonyl chloride to obtain a compound (II); reacting the compound (II) with tert-butyl carbamate to generate a compound (III); reacting the compound (III) with hydrochloric acid to obtain a compound (IV); and finally carrying out condensation reactions between the compound (IV) and N-(2-chloropyrimidine-4-yl)-N-methyl-2,3-dimethyl-2H-indazole-6-amine to obtain the trimer impurity (V). The yield of the synthesis method is high; and the prepared impurity can be used as a reference substance in quality analysis ofpazopanib hydrochloride.
Owner:博泽格霖(山东)药业有限公司 +1
Method for detecting pazopanib drug concentration in human plasma by LC-MS/MS (liquid chromatography-mass spectrometry/mass spectrometry)
InactiveCN112285230AShort analysis timeImprove qualitative accuracyComponent separationBlood plasmaElectrospray
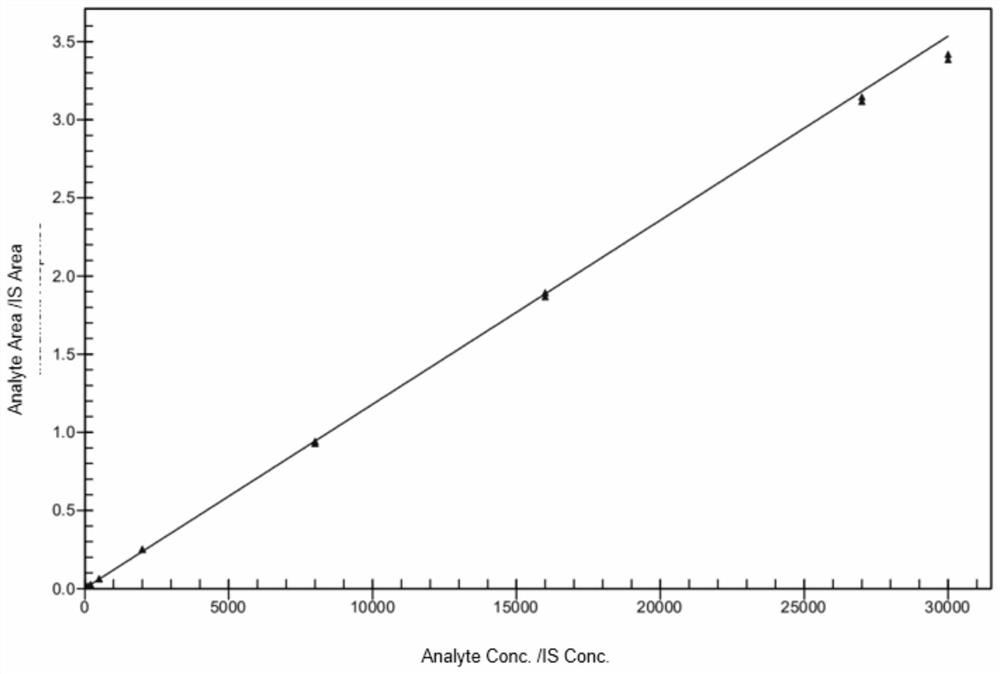
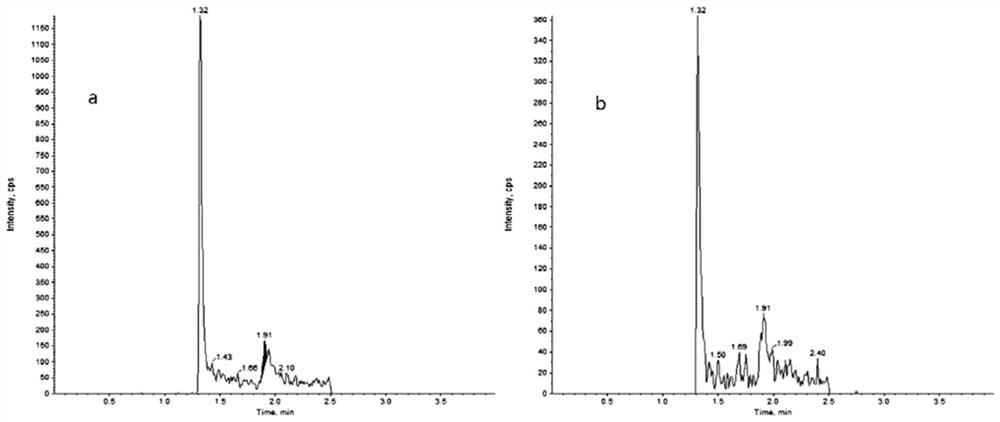
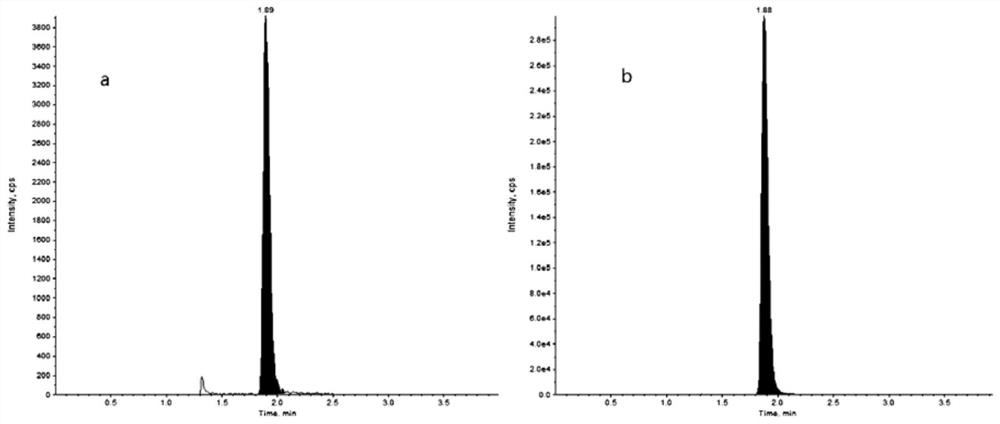
The invention discloses a method for detecting the drug concentration of pazopanib in human plasma through LC-MS / MS combination, and belongs to the technical field of drug concentration analysis. Themethod comprises the following steps: respectively mixing a series of pazopanib working solutions with human blank plasma, performing pre-treating, injecting the mixture into LC-MS / MS for analysis, adding an internal standard working solution mainly prepared from pazopanib-d6 into human plasma to be detected, performing pre-treating, injecting the mixture into LC-MS / MS for analysis, drawing a standard curve by taking the chromatographic peak area ratio of pazopanib to internal standard pazopanib-d6 as an ordinate and the concentration of pazopanib in human blank plasma as an abscissa, and calculating the concentration of pazopanib in the human plasma sample to be detected according to the standard curve, wherein a protein precipitation method is adopted to pretreat a human plasma sample, an electrospray ion source is adopted as an ionization technology, and the detection method is simple and convenient to operate, high in sensitivity, good in reproducibility, high in selectivity, shortin analysis time and suitable for large-batch detection of clinical samples.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Process for the preparation of pazopanib or a pharmaceutically acceptable salt thereof
Owner:LAURUS LABS
A kind of preparation method of pazopanib related substance
ActiveCN112694468BHigh purityThe reaction is easy to operateOrganic chemistryCombinatorial chemistryBenzophenone
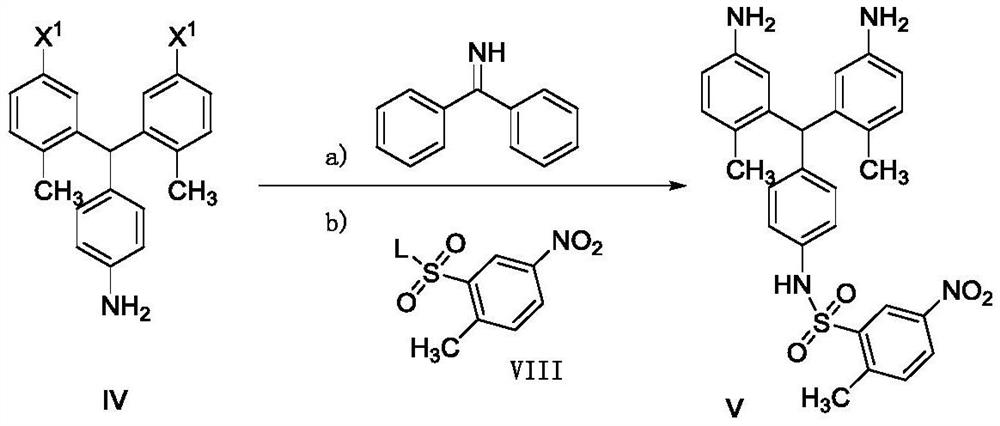
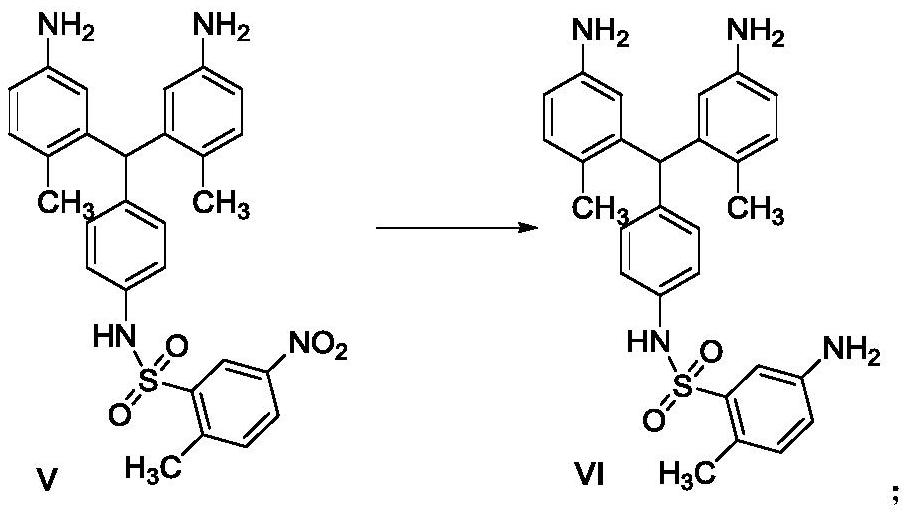
The invention belongs to the field of medicinal chemistry, and in particular relates to a preparation method of related substances of pazopanib. Using the compound shown in formula I as a raw material, reacting with the compound of formula II to prepare the compound shown in formula III, and then reacting with aniline to prepare the compound shown in formula IV or its acid addition salt, the compound shown in formula IV and benzophenone imine reaction, and then react with the compound shown in formula VIII to prepare the compound shown in formula V, and then prepare the compound shown in formula VII, the related substance of pazopanib, through nitro reduction and condensation reaction.
Owner:NANJING CHIA TAI TIANQING PHARMA +1
Stable and soluble formulations of receptor tyrosine kinase inhibitors, and methods of preparation thereof
ActiveUS20170071938A1Low water solubilityImprove stabilityOrganic active ingredientsSenses disorderReceptor tyrosine kinase inhibitorActive agent
Owner:FORSIGHT VISION5 INC
Compositions and methods of treating dermal fibrotic disorders
ActiveUS20190070160A1Preventing and modulating formationPreventing and alleviating aberrant fibrotic tissue formationDermatological disorderHeterocyclic compound active ingredientsDiseaseRegorafenib
A method for preventing and / or modulating formation of a dermal fibrotic disorder includes administering a therapeutically effective amount of a multi-phase modulator to a subject in need thereof. The multi-phase modulator is selected from the group consisting of axitinib, nintedanib, sorafenib, sunitinib, lenvatinib, panatinib, pazopanib, regorafenib, and riociguat. The dermal fibrotic disorder is acne scars, skin scars such as keloids and hypertrophic scars, wrinkles, cellulite and dermal neoplastic fibrosis, scarring alopecia, various vasculopathy, vasculitis, burn wound healing, diabetic foot syndrome, scleroderma, arthrofibrosis, peyronie's disease, dupuytren's contracture, or adhesive capsulitis
Owner:AIVIVA BIOPHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


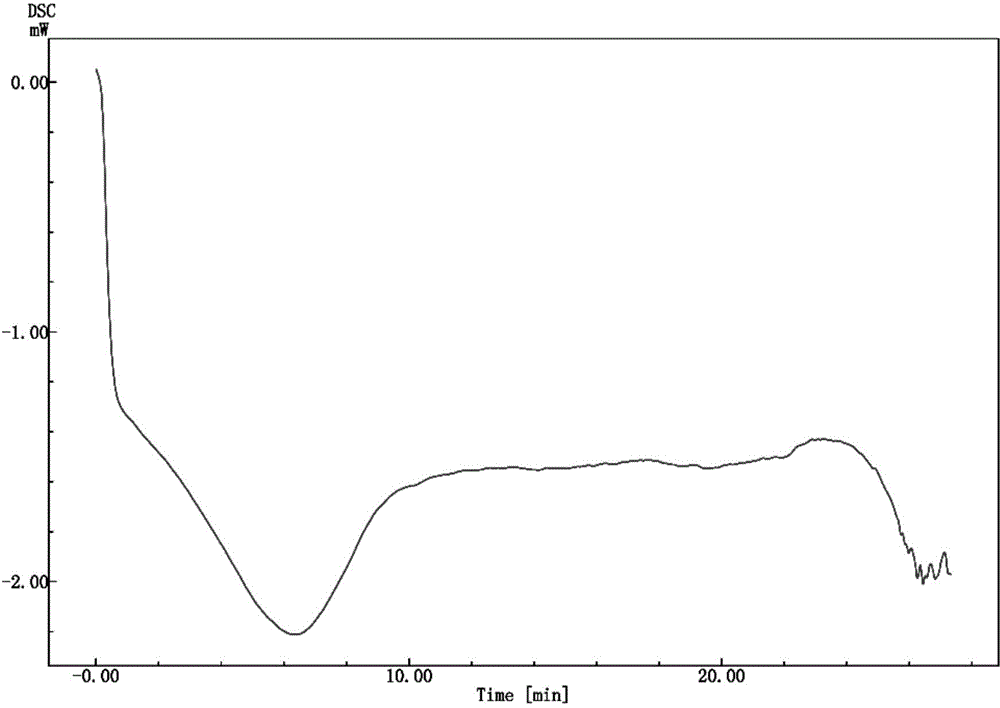
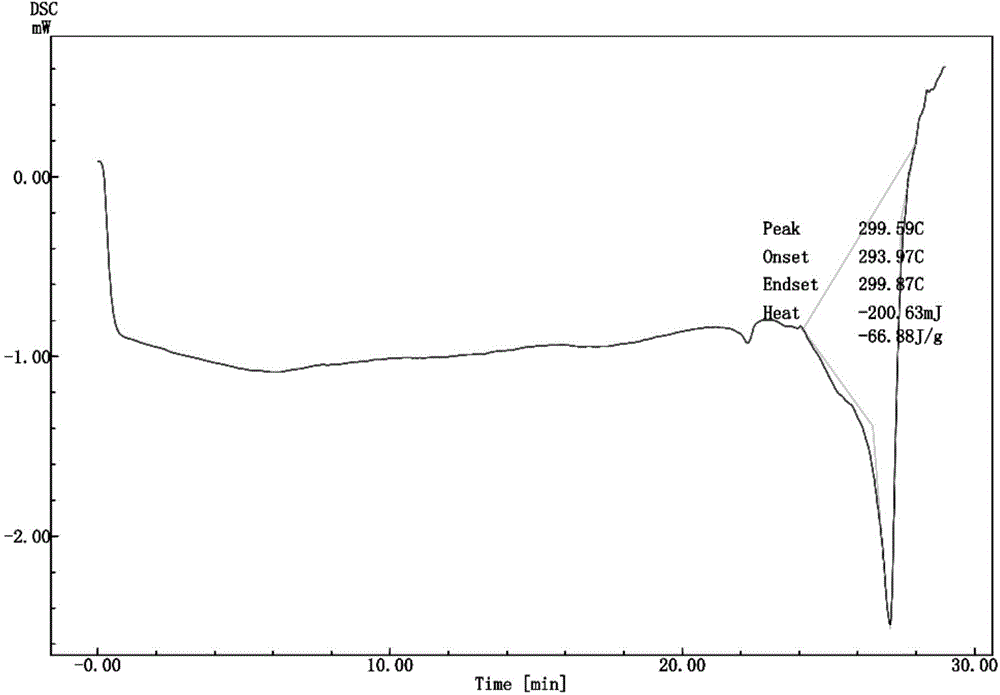
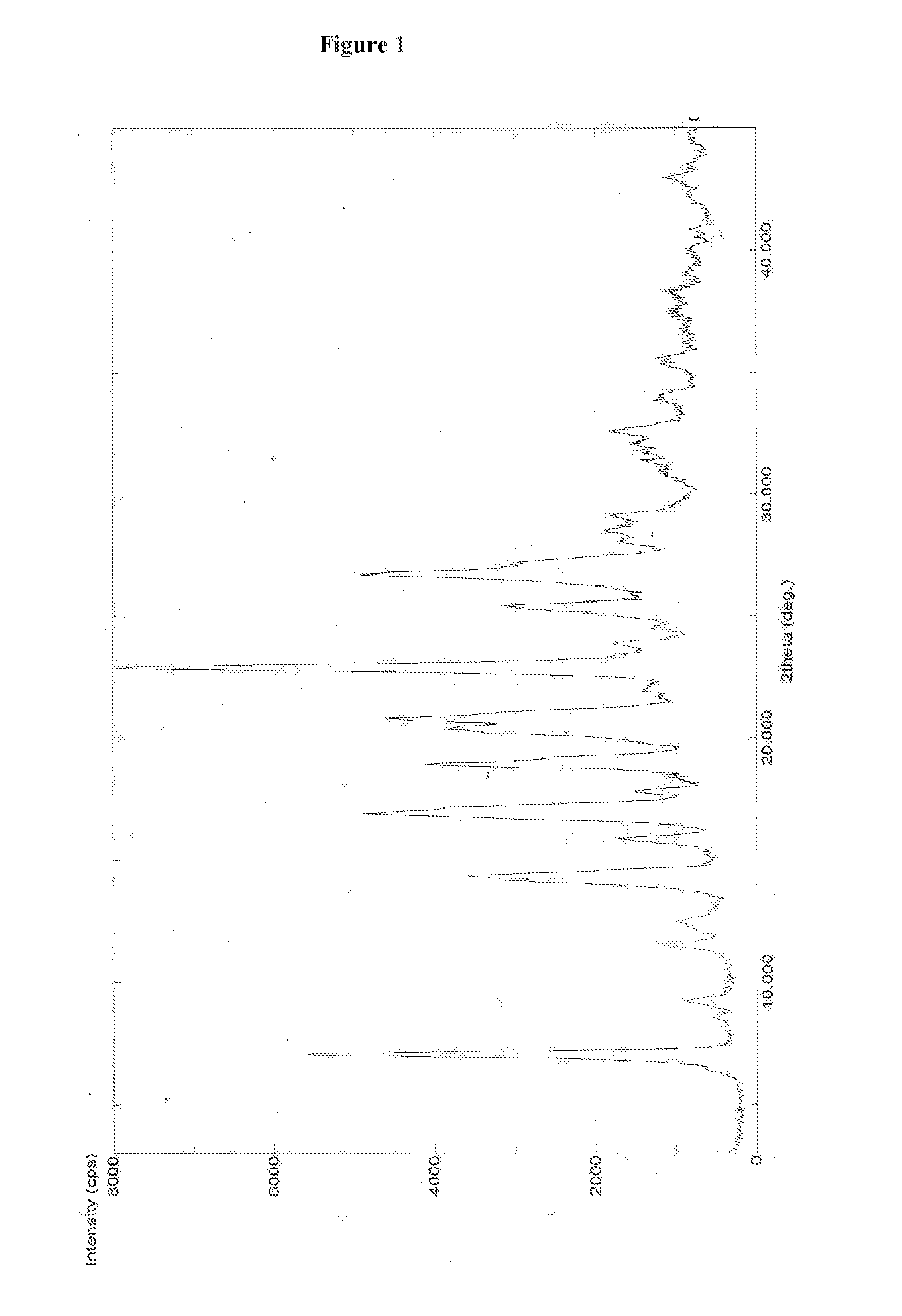
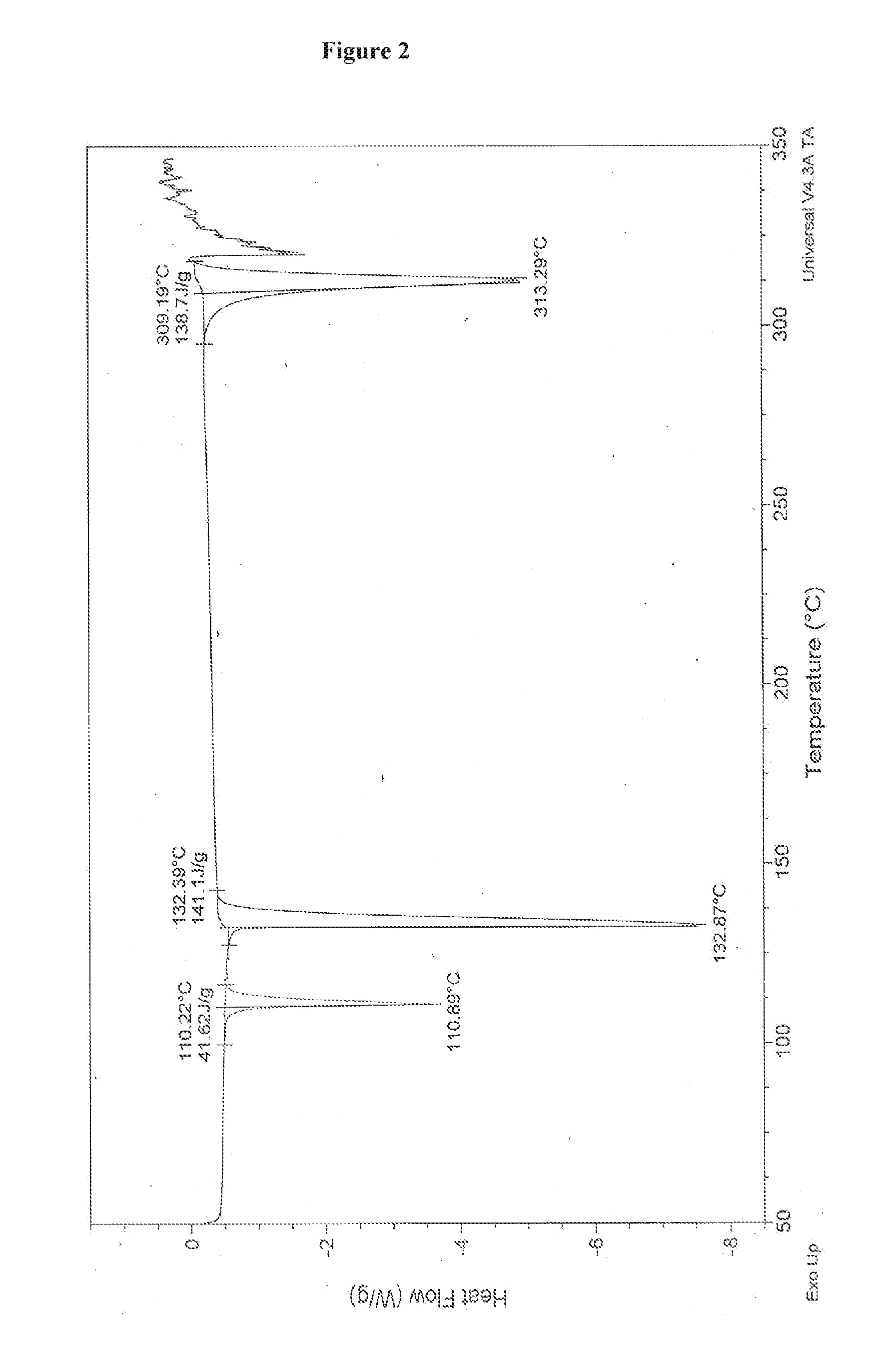
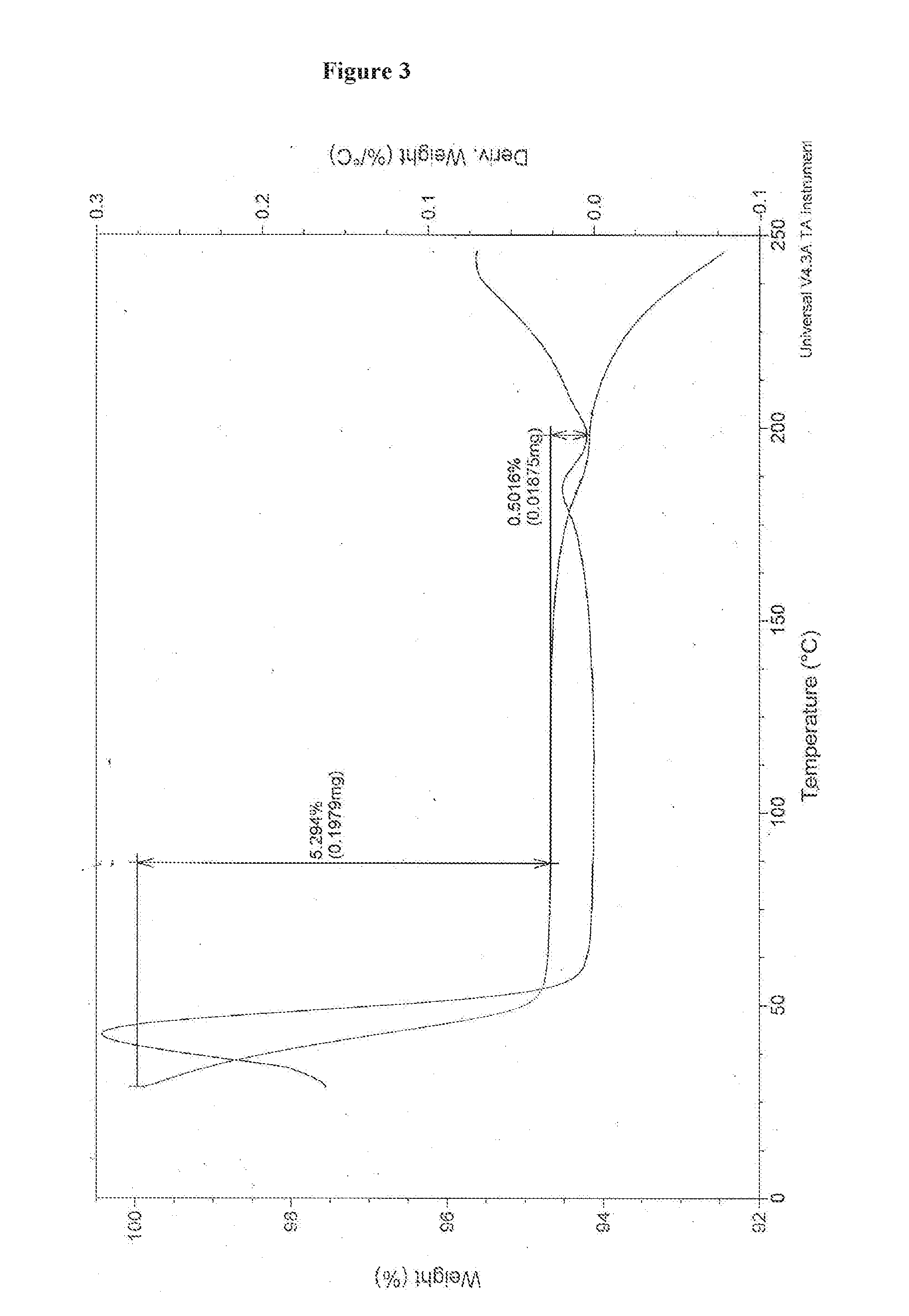
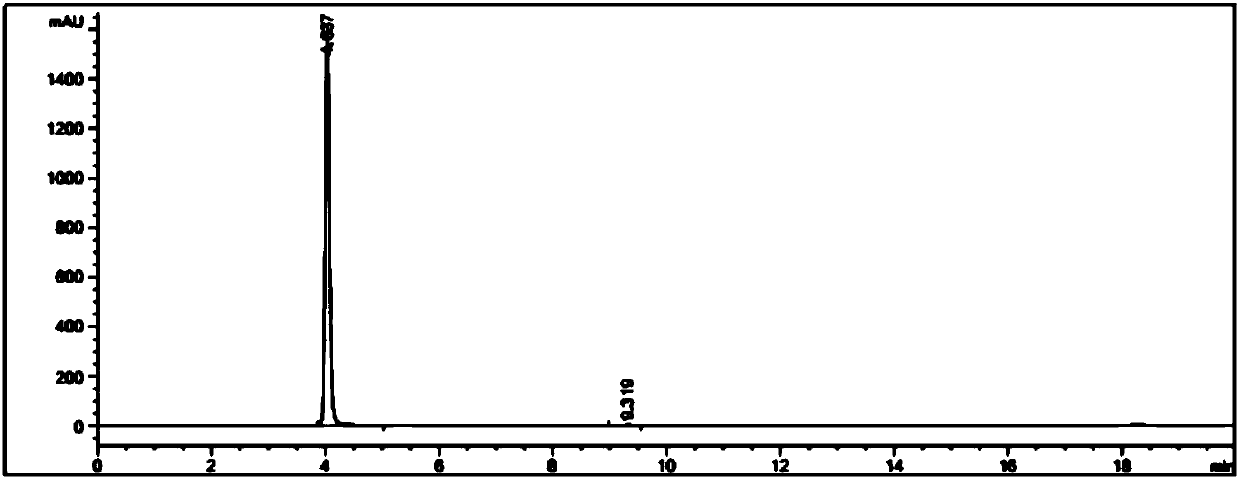





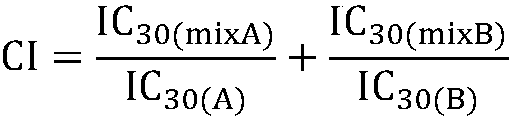
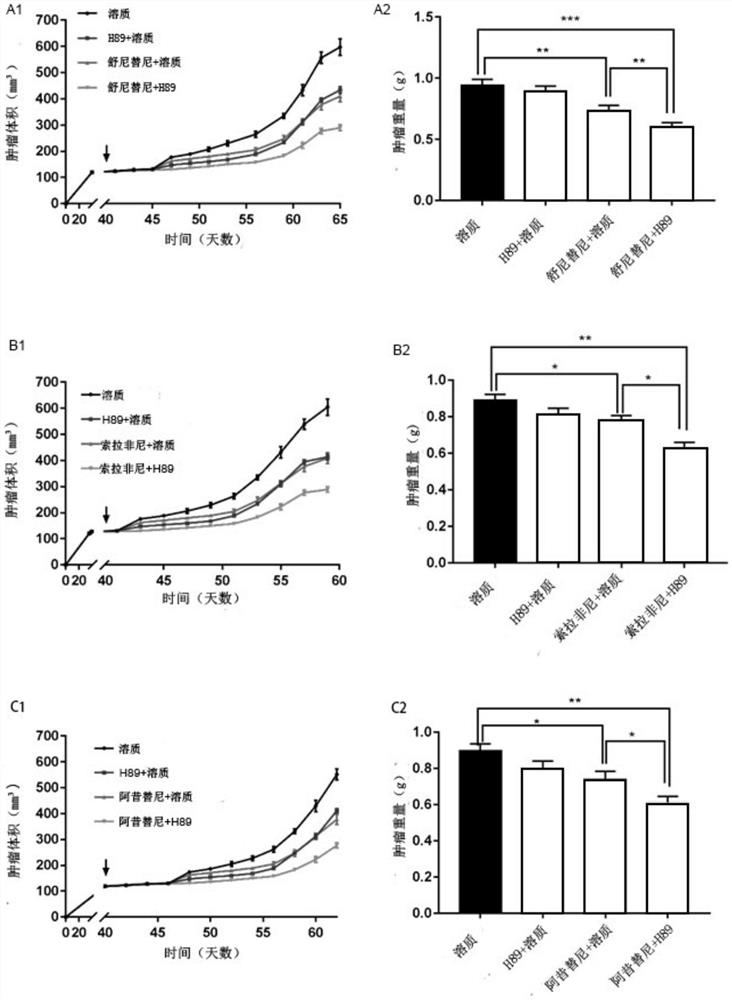
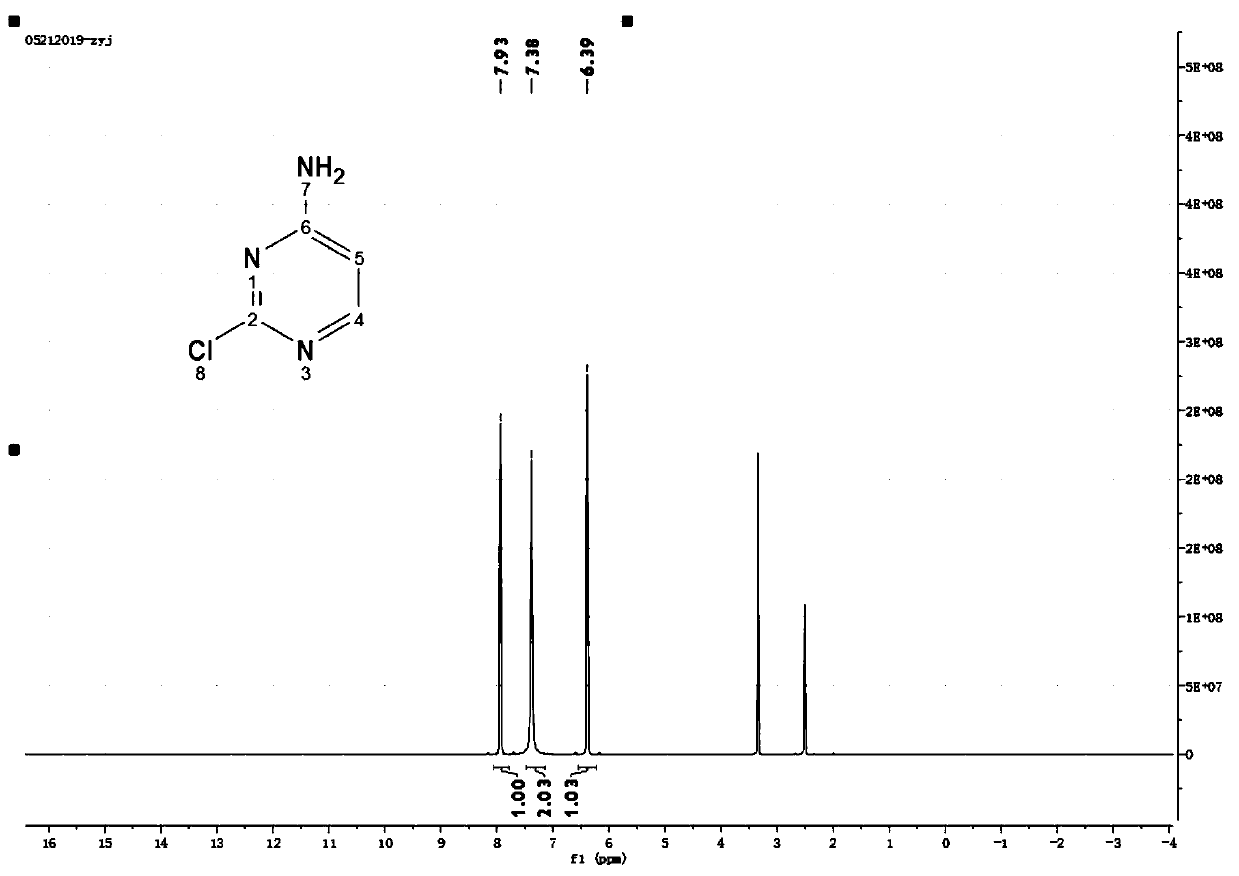
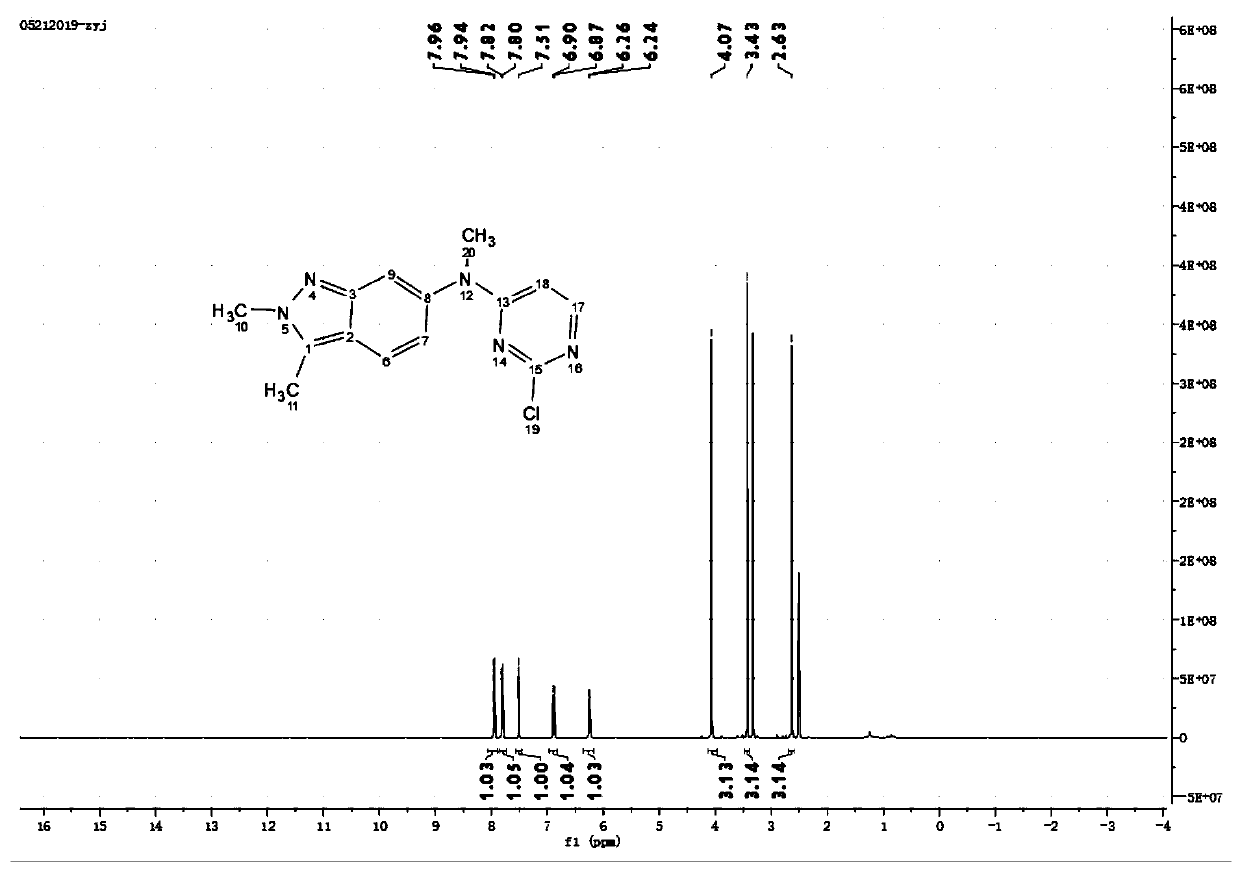






![Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68bec8f5-3bee-4ff8-b6f7-167894d17c16/HDA0003020068450000011.png)
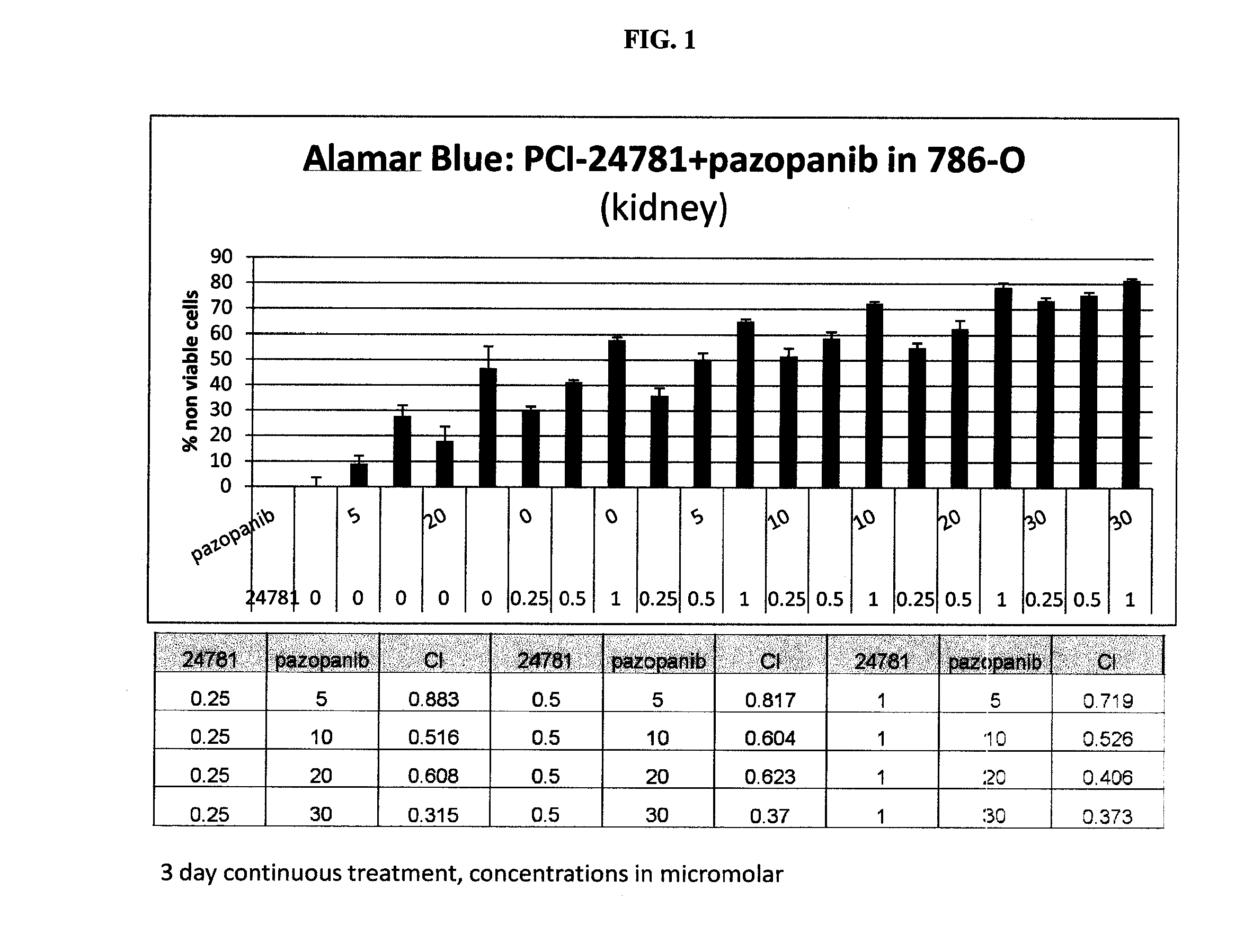
![Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68bec8f5-3bee-4ff8-b6f7-167894d17c16/HDA0003020068450000021.png)
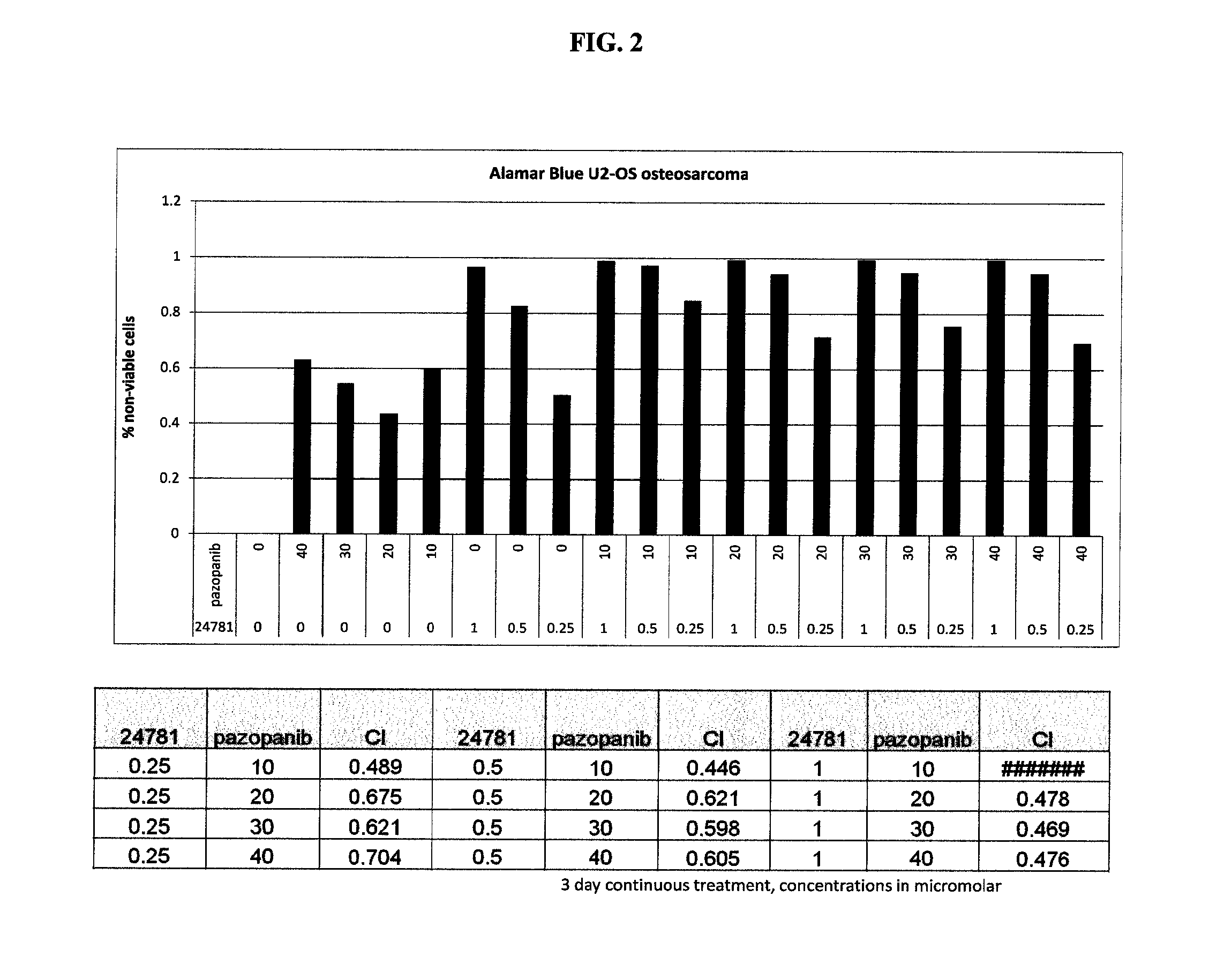
![Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof Multi-kinase inhibitors of VEGF and tgf[beta] and uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/68bec8f5-3bee-4ff8-b6f7-167894d17c16/HDA0003020068450000031.png)