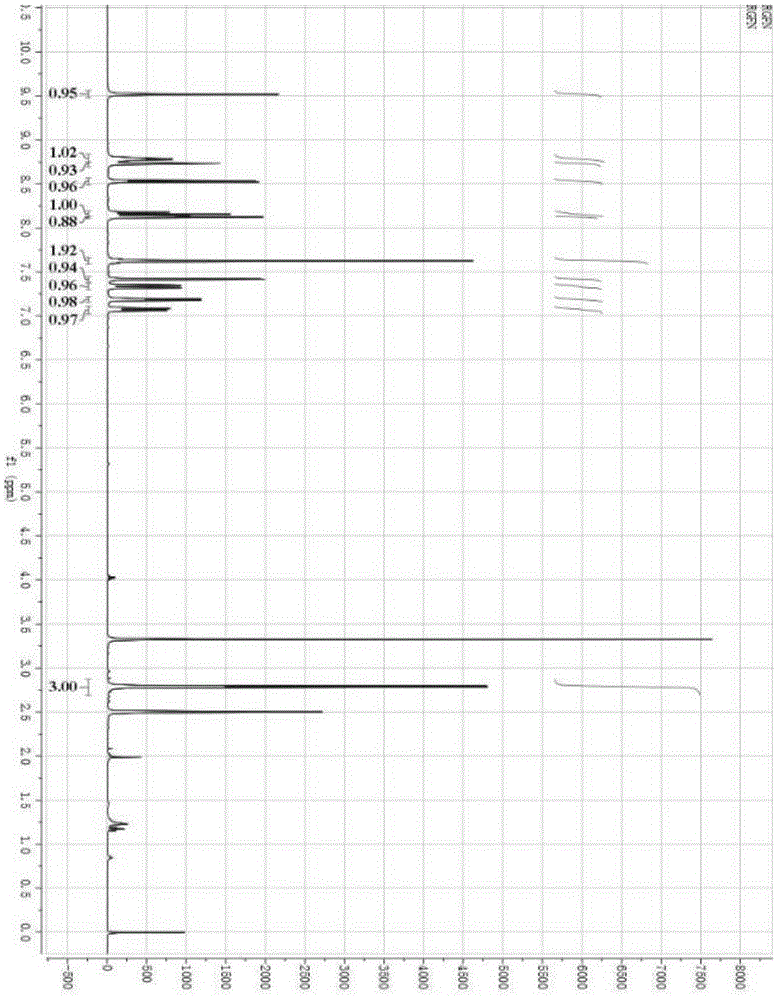
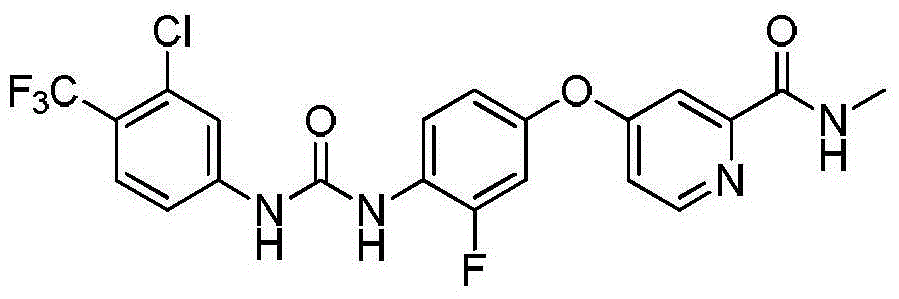
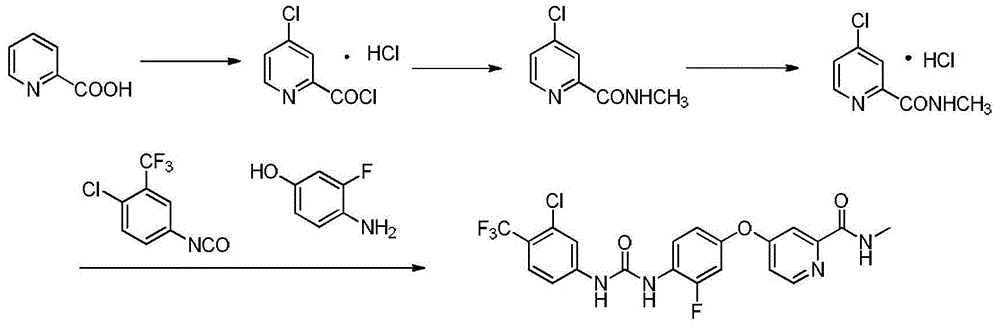
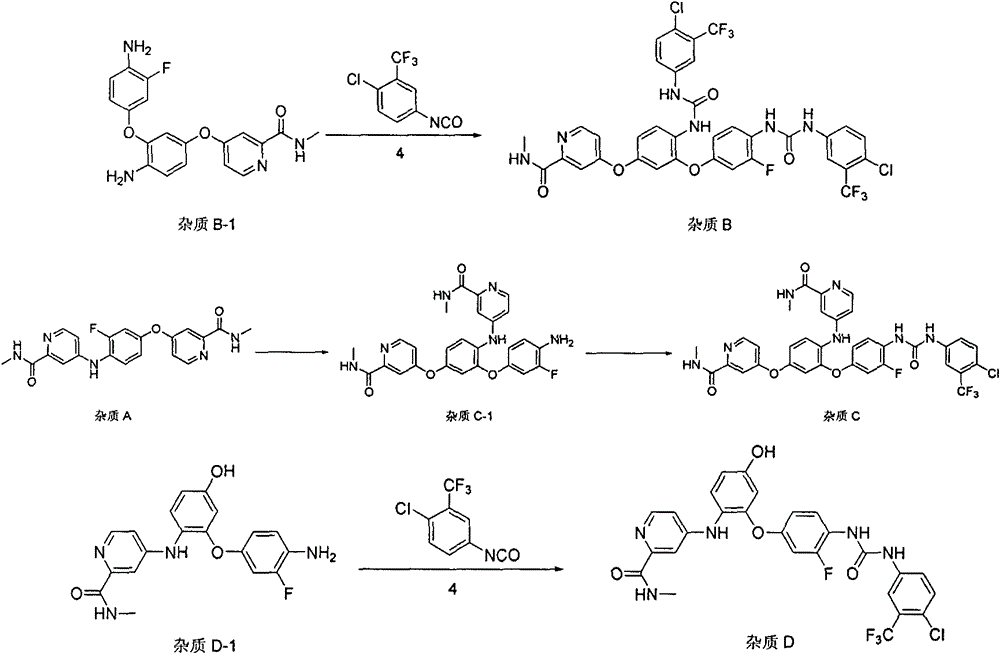
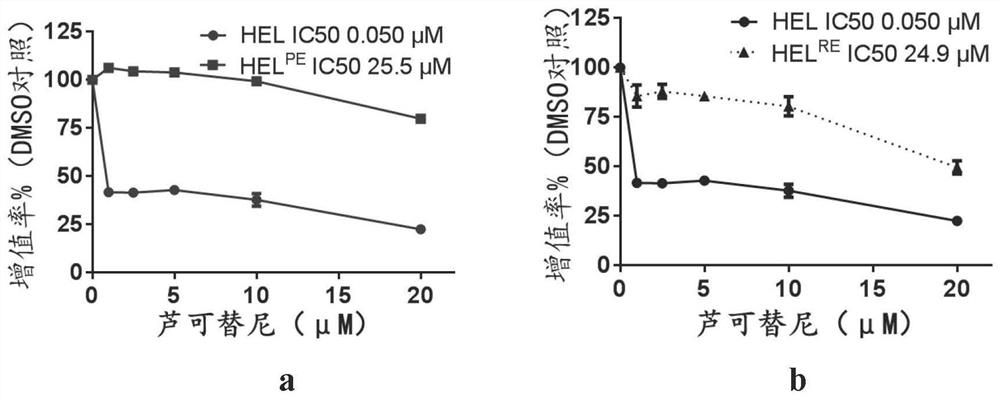
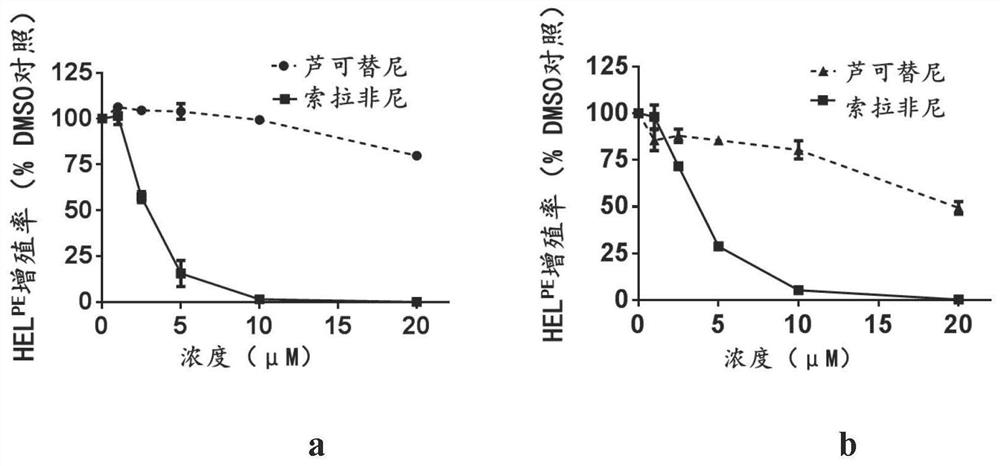
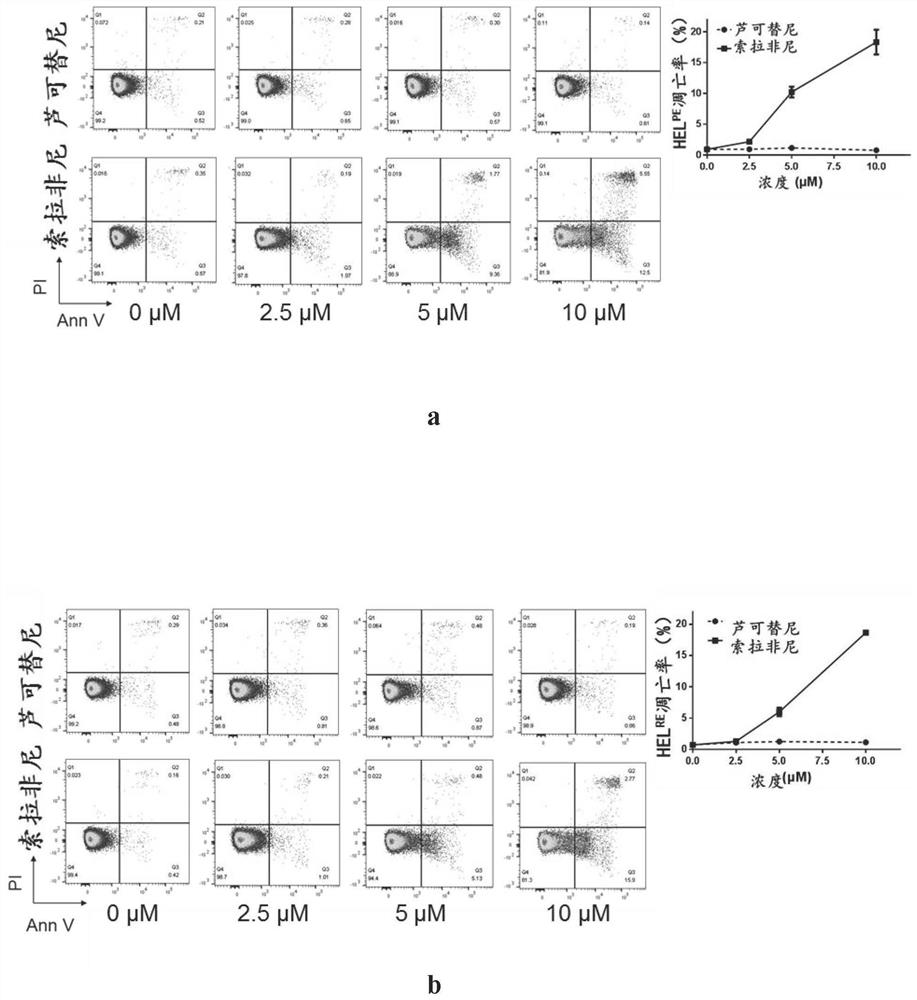
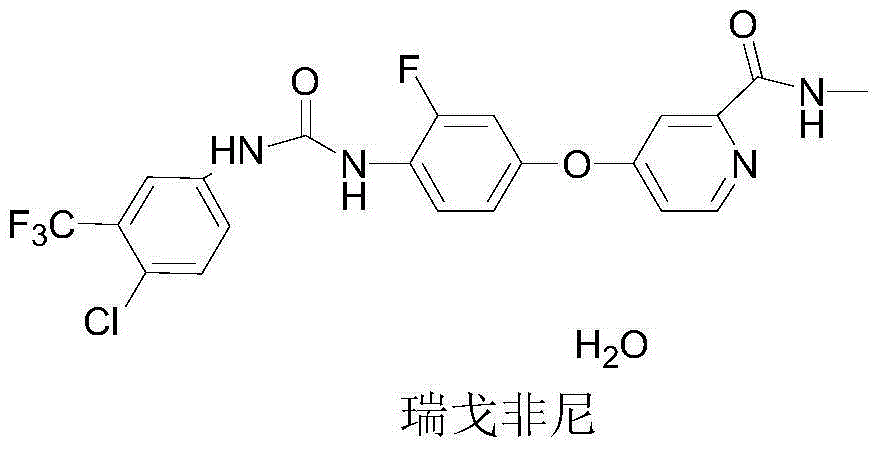
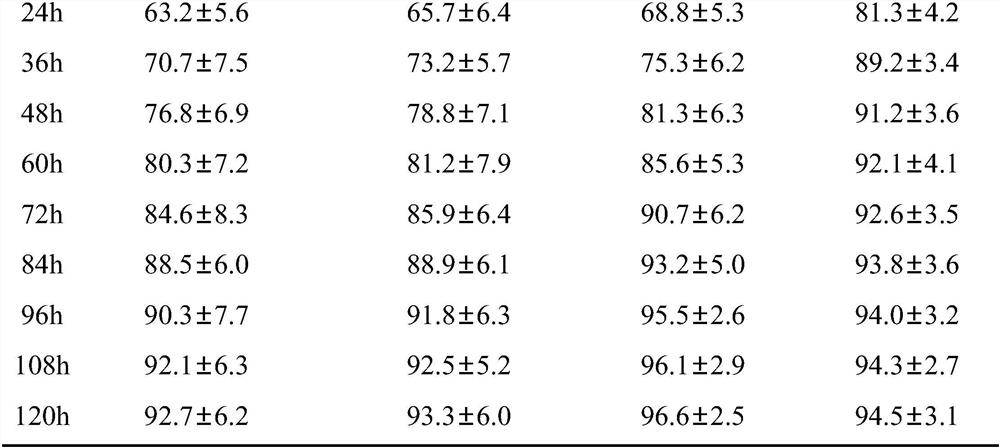
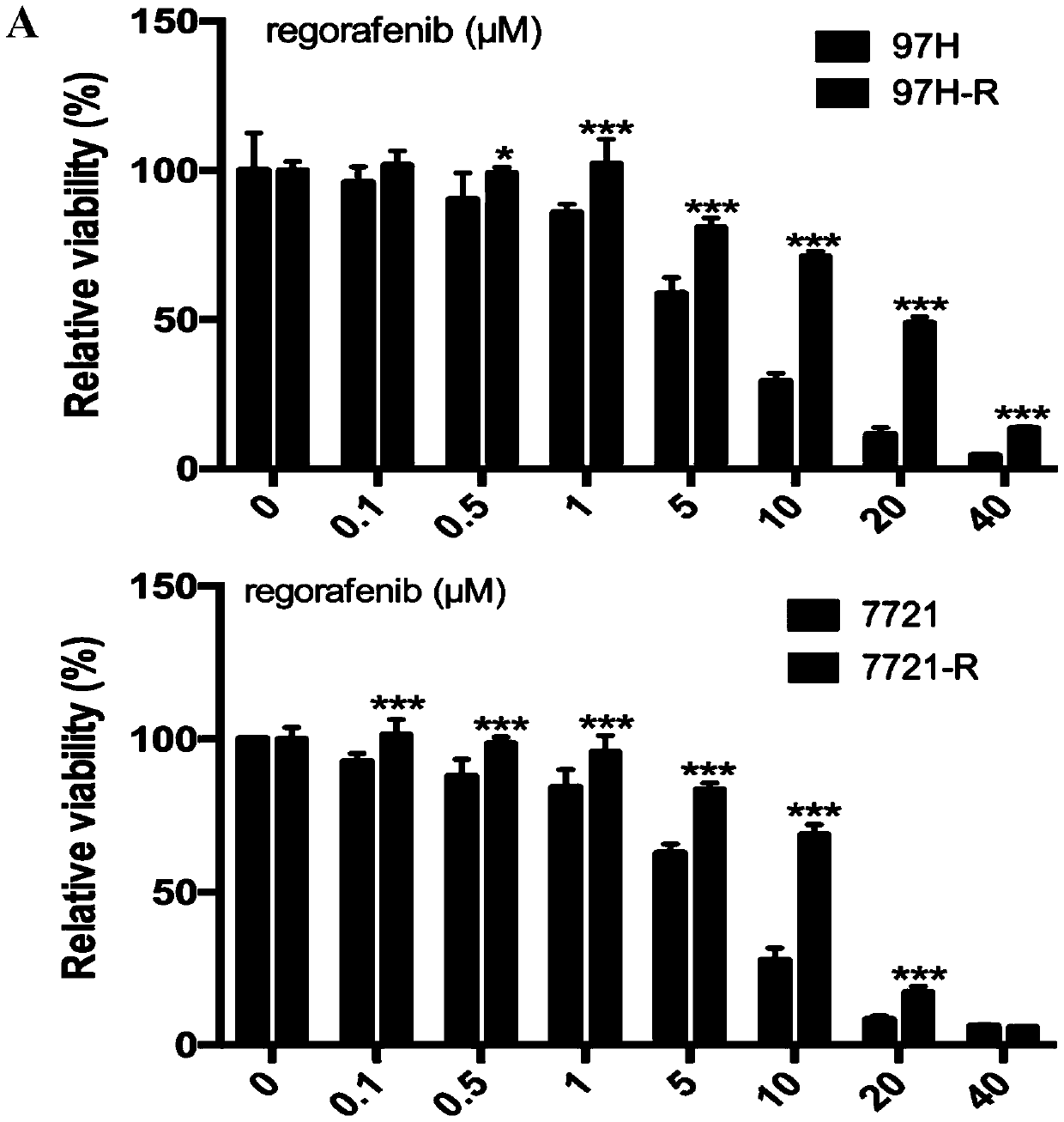
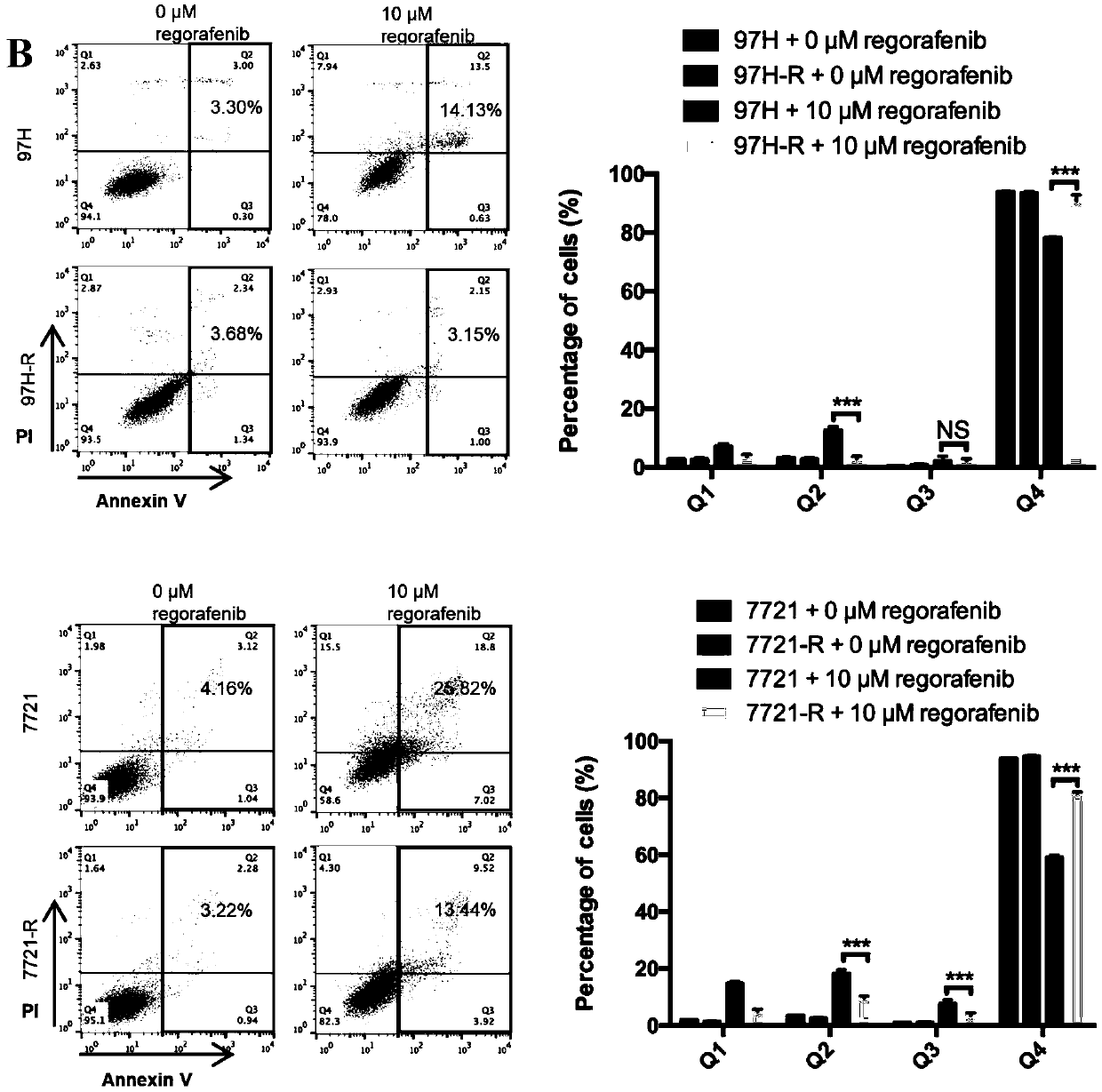
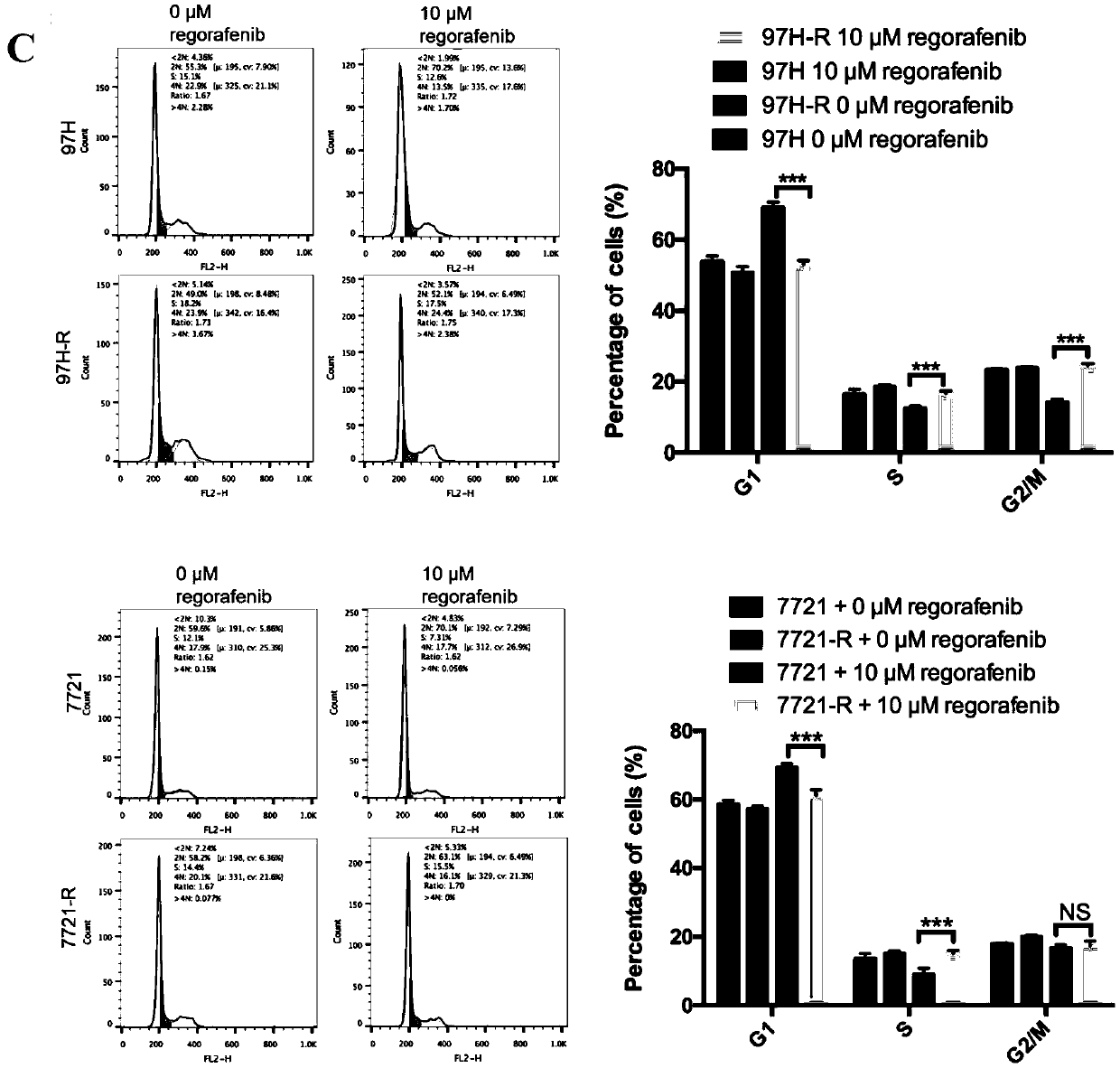
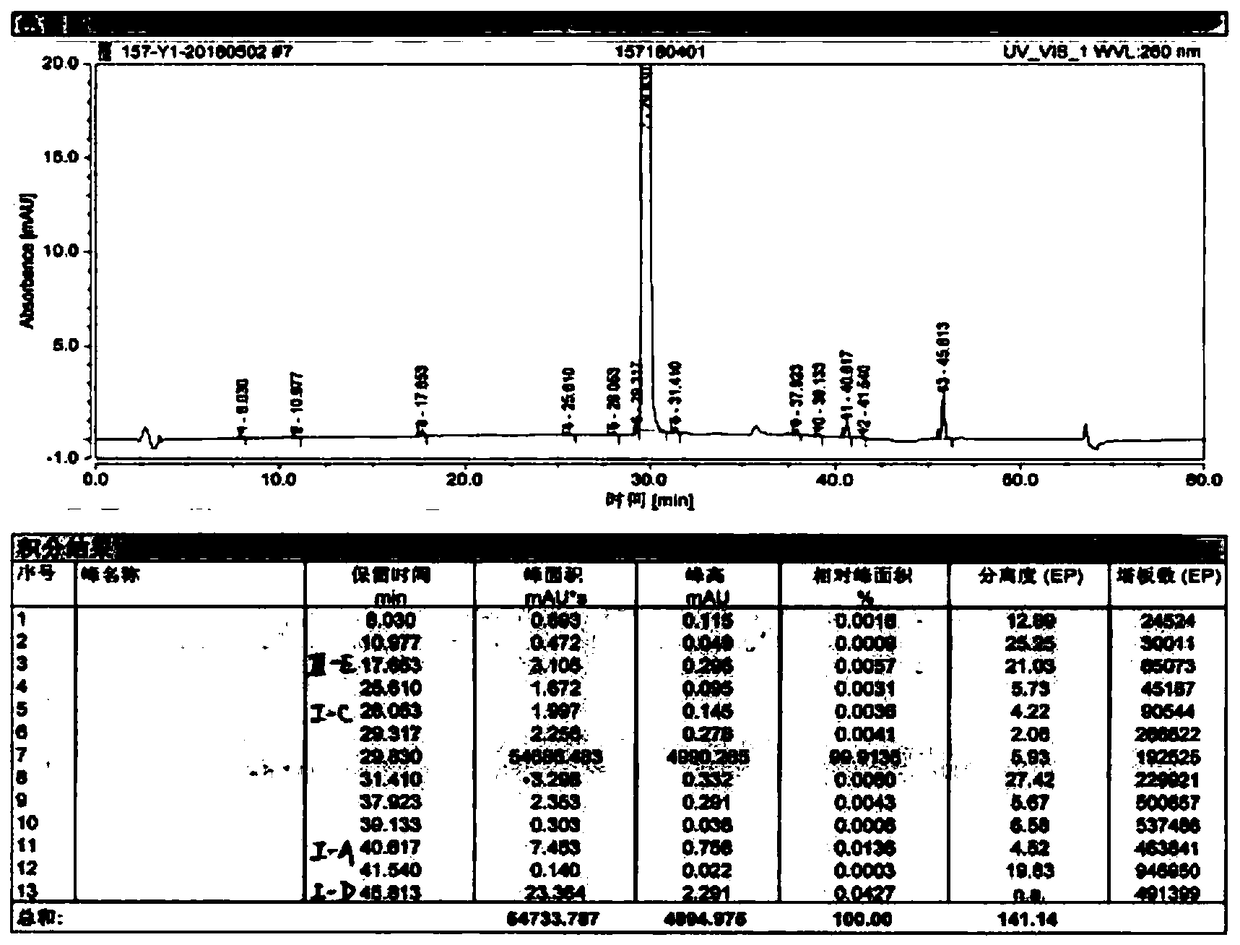
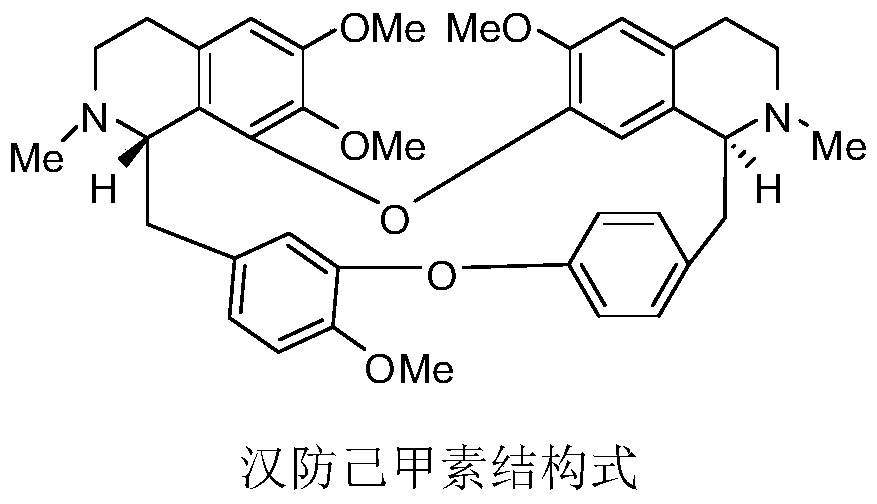
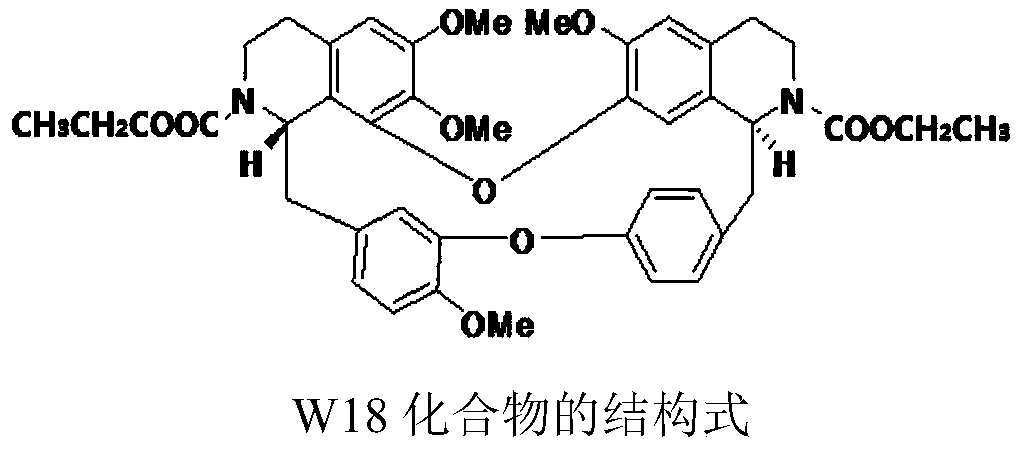
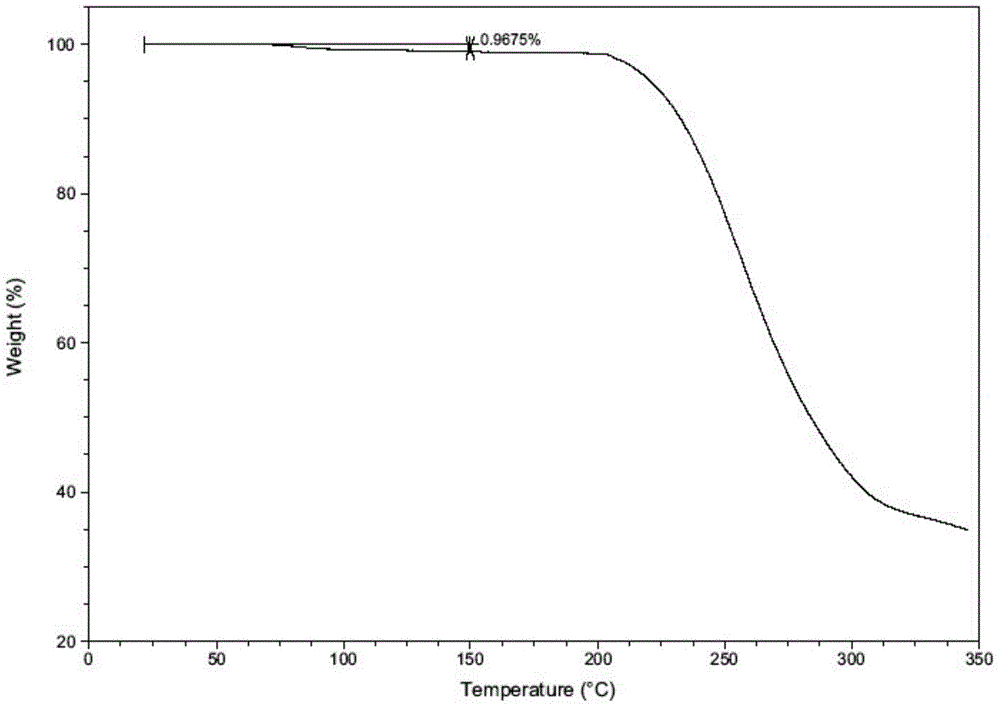
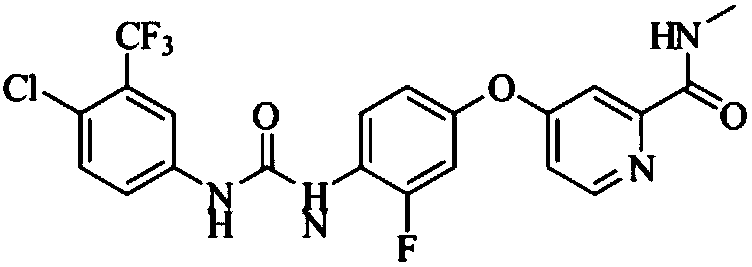
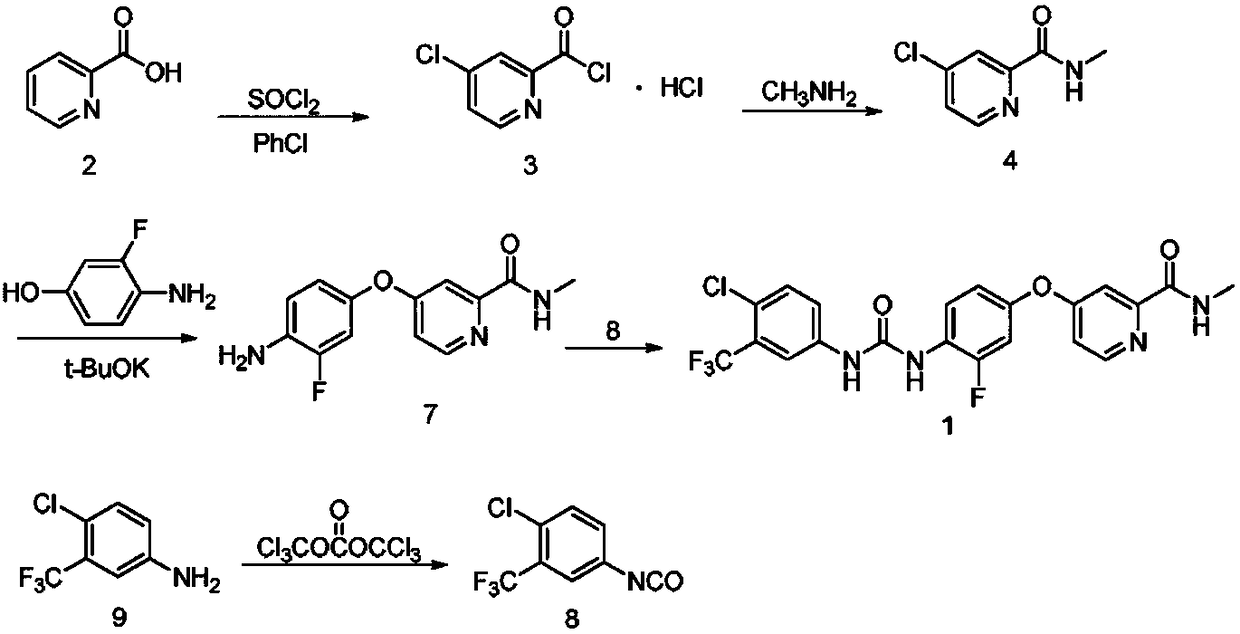
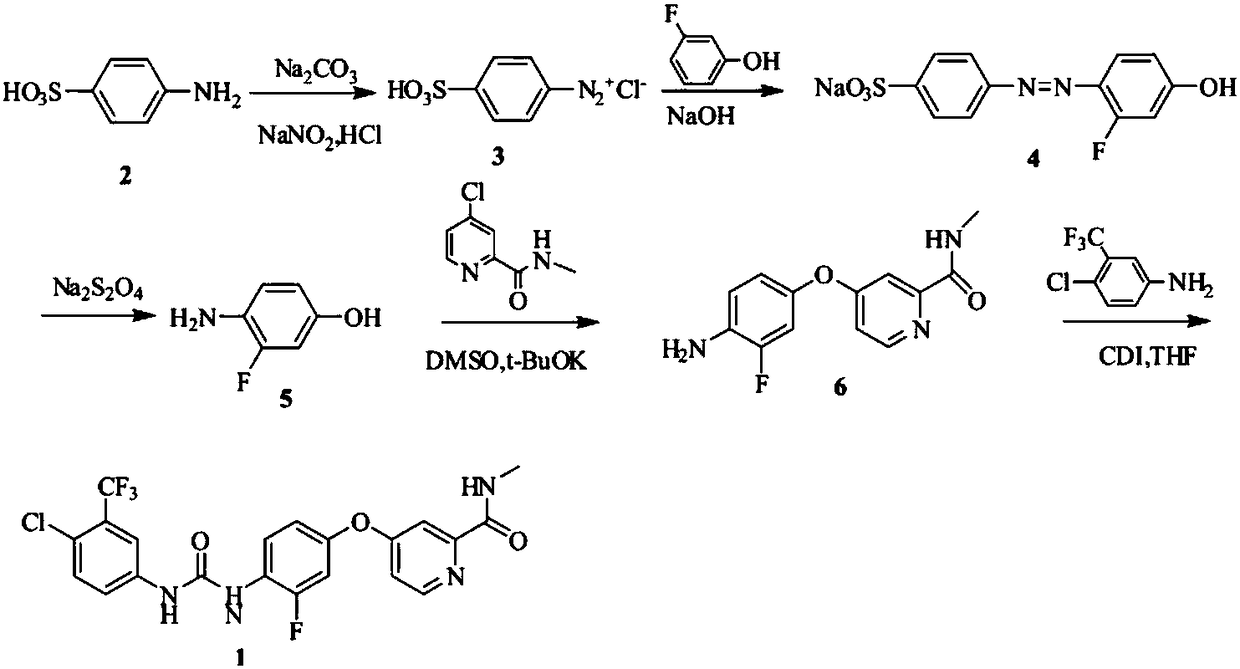
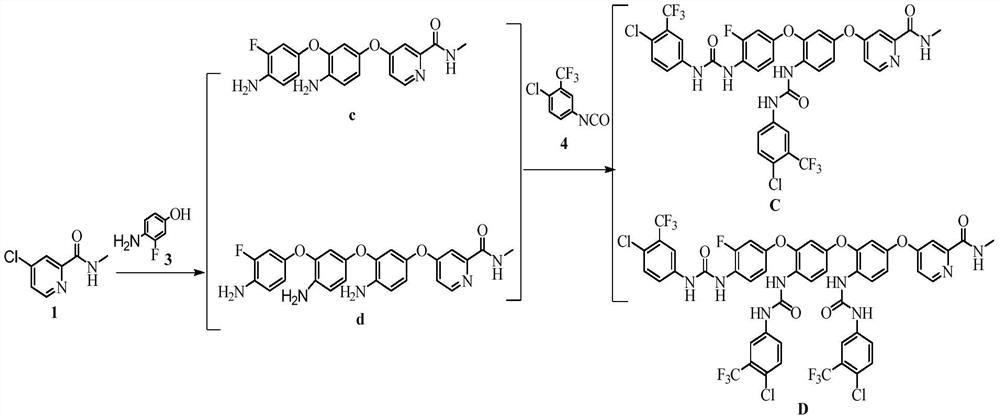
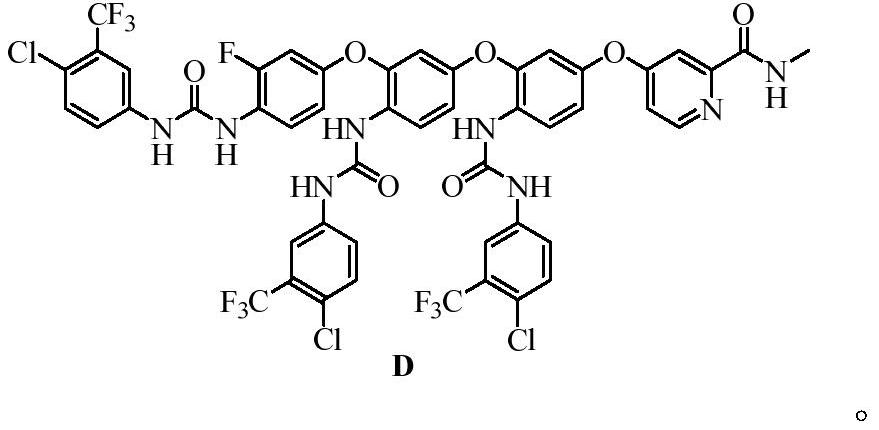
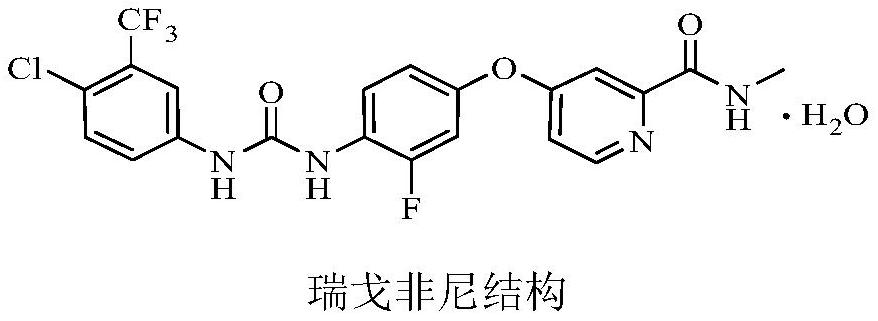
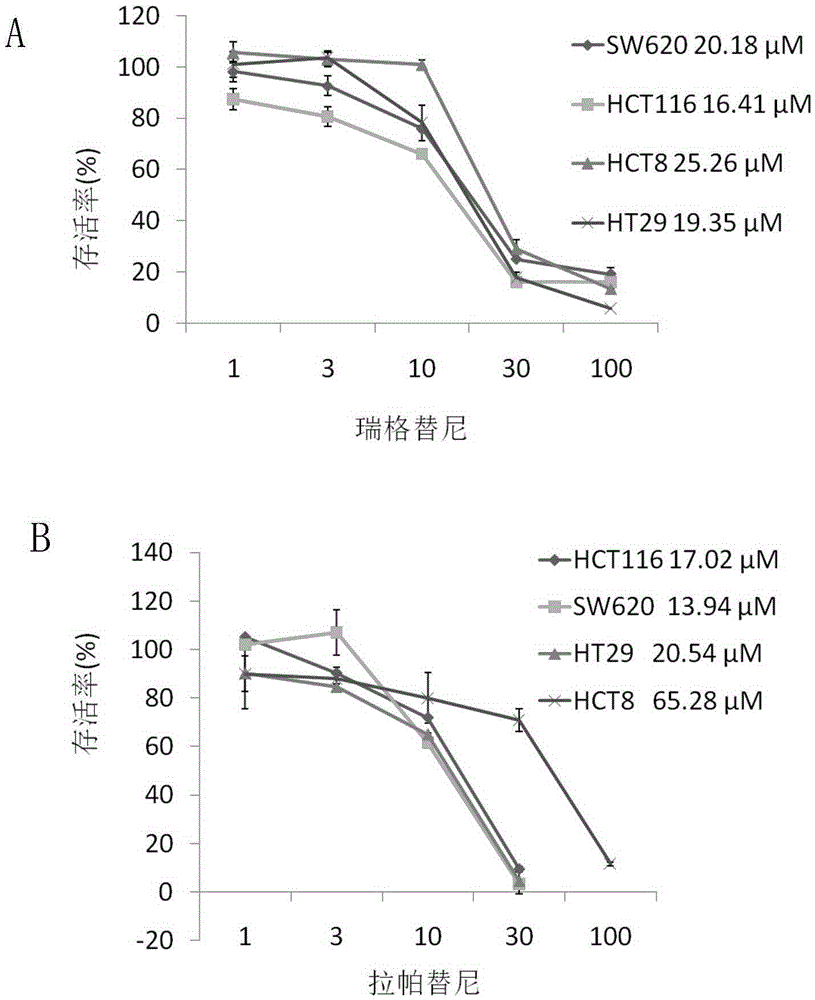
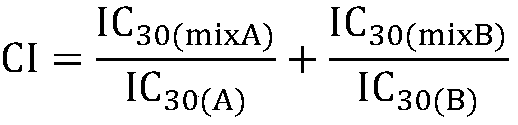Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Regorafenib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
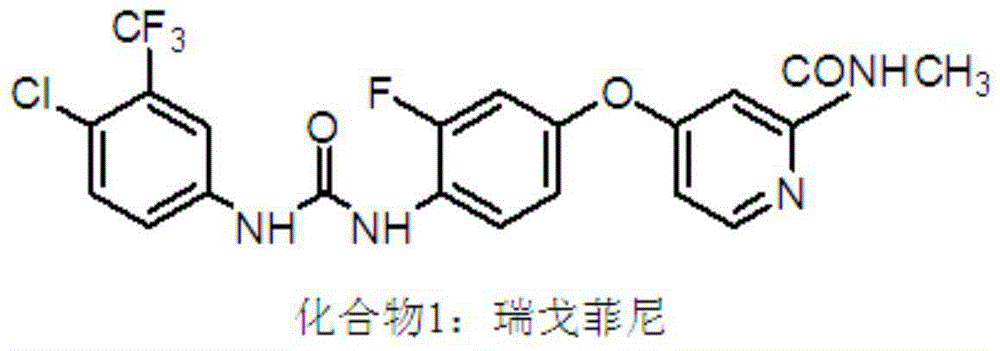
Regorafenib (BAY 73-4506, commercial name Stivarga) is an oral multi-kinase inhibitor developed by Bayer which targets angiogenic, stromal and oncogenic receptor tyrosine kinase (RTK). Regorafenib shows anti-angiogenic activity due to its dual targeted VEGFR2-TIE2 tyrosine kinase inhibition. Since 2009 it was studied as a potential treatment option in multiple tumor types. By 2015 it had 2 US approvals for advanced cancers.
Liver targeted medicine
InactiveCN107929273AImproved Liver TargetingGood curative effectDigestive systemPharmaceutical non-active ingredientsDiseaseCitrulline
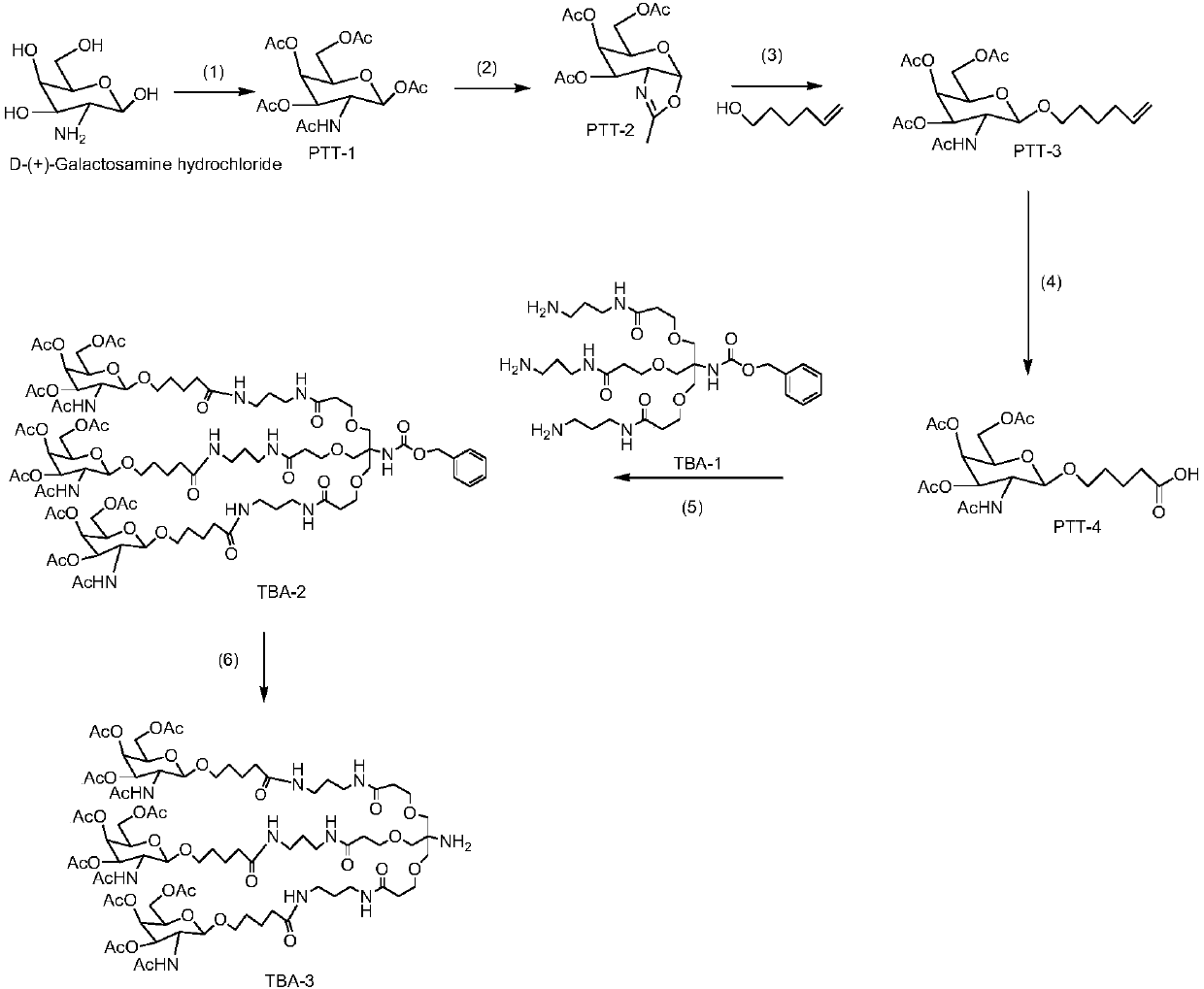
The invention relates to the field of biological medicine, and in particular relates to liver targeted medicine. The medicine is chemical micromolecular medicine connecting galactosamine. The chemicalmicromolecular medicine is medicine for treating liver diseases or liver-related diseases. The chemical micromolecular medicine is prepared from but not limited to thyroxine T3, sorafenib, taxol, regorafenib, lamivudine, entecavir, telbivudine, statins, fibrates, niacin, bile acid sequestrants, other hepatitis virus DNA (RNA) polymerase inhibition compounds and the like. The galactosamine is tervalent acetylgalactosamine. The connection is the direct connection of the galactosamine and the chemical micromolecular medicine or the connection through linking fragments; the linking fragments comprise but not limited to carbon chains, disulfide bonds, pyrophosphate diester, cysteic acid, polypeptide and thioether or valine-citrulline. The medicine provided by the invention has the advantages that the liver targeted performance is improved; the medicine curative effect is enhanced; the toxic and side reactions on other non-targeted tissues are few.
Owner:崔坤元
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Targeting mesoporous polydopamine multi-purpose nanometer diagnosis and treatment preparation as well as preparation method and applications thereof
ActiveCN109276721ARealize the loadImprove tumor treatment effectPharmaceutical non-active ingredientsEmulsion deliveryTreatment effectMass ratio
The invention discloses a targeting mesoporous polydopamine multi-purpose nanometer diagnosis and treatment preparation as well as a preparation method and applications of the targeting mesoporous polydopamine multi-purpose nanometer diagnosis and treatment preparation. The targeting mesoporous polydopamine multi-purse nanometer diagnosis and treatment preparation is composed of a water-soluble folic-acid-targeting mesoporous polydopamine medicine carrier, hydrophobic regorafenib and manganese sulfate, wherein the mass ratio of regorafenib to the folic-acid-targeting mesoporous polydopamine medicine carrier is (0.5-4): 1, and the mass ratio of manganese sulfate to the folic-acid-targeting mesoporous polydopamine medicine carrier is (1-6):1. The targeting mesoporous polydopamine diagnosis and treatment preparation can recognize positive tumor cells of a folic acid receptor, so that a targeting effect is achieved during treatment. For the diagnosis and treatment preparation, the chemotherapy under the guidance of MRI imaging is realized, the tumor treatment effect is expected to be improved, meanwhile, the biocompatibility is good, and therefore, the targeting mesoporous polydopaminemulti-purpose nanometer diagnosis and treatment preparation has the clinical application potential.
Owner:SUN YAT SEN UNIV
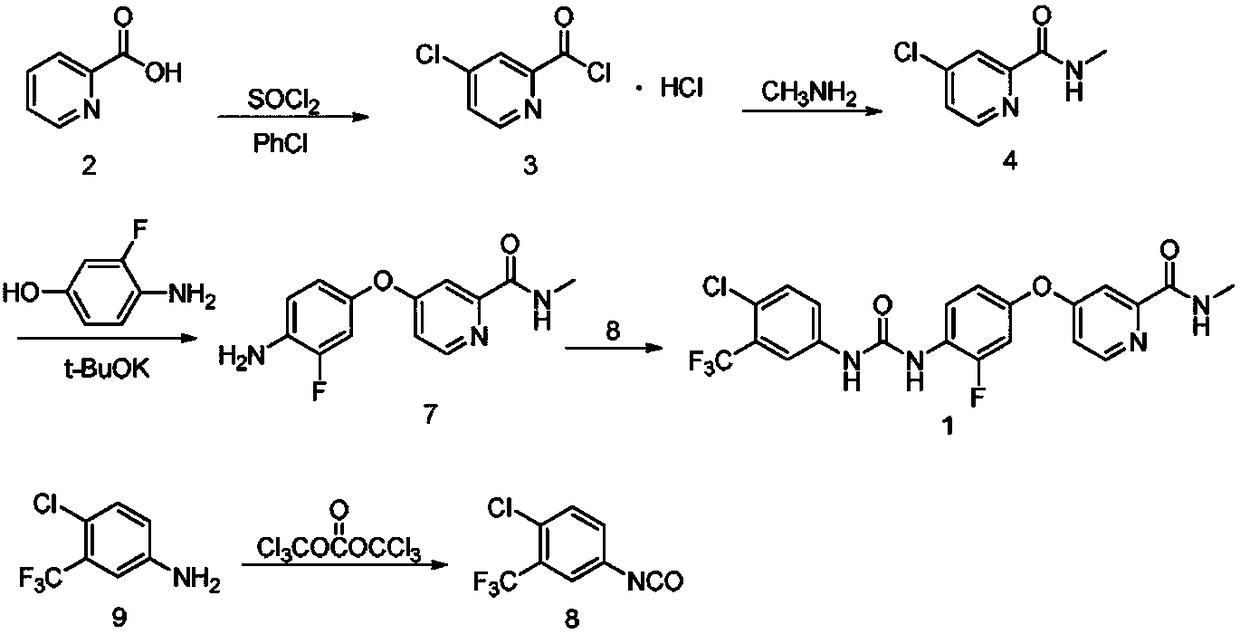
Regorafenib synthesis method by one kettle way
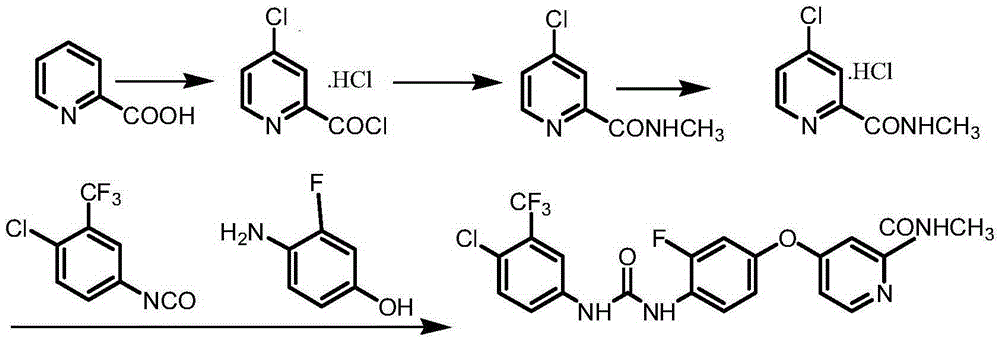
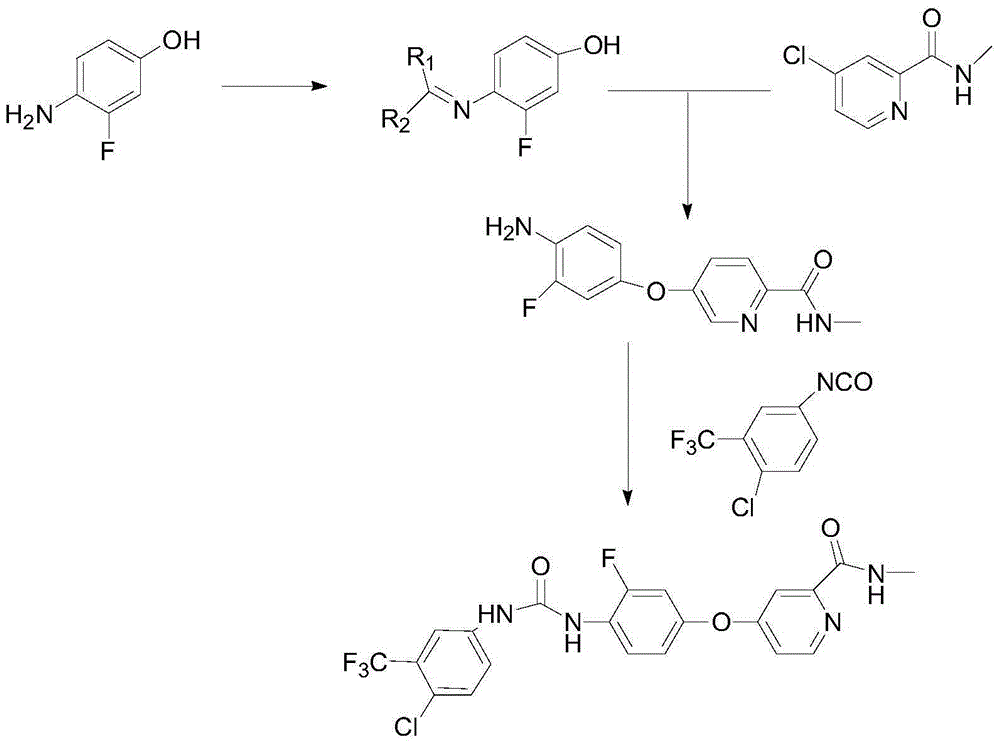
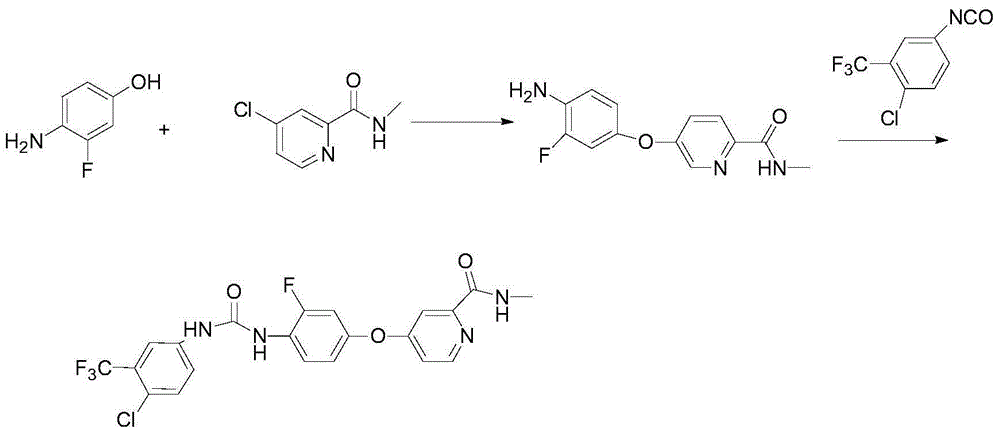
The invention discloses a regorafenib synthesis method by one kettle way, which is characterized in that 4-chlorine-trifluoro- tolyl isocyanate and 3-fluorine-4-aminophenol are added in a solvent, the temperature is controlled, a condensation reaction is carried out to generate a regorafenib intermediate 1-(4-chlorine-3-(trifluoromethyl)phenyl)-3-(2-fluorine-4-hydroxyphenyl)carbamide, then alkali is added in a same reactor for stirring, 4-chlorine-N-methyl-2-pyridine methanamide hydrochloride or 4-chlorine-N-methyl-2-pyridine methanamide are subjected to a replacement reaction, and finally purifying processing is carried out to obtain regorafenib. The method has the advantages of short process flow, simple operation, high utilization rate of raw material, and low generation cost, and has better production and utility value.
Owner:NANJING UNIV OF TECH
Preparation method for high-purity regorafenib
InactiveCN105218440ALower reaction conditionsAvoid demandingOrganic chemistryRegorafenibPhase-transfer catalyst
The invention discloses an improved preparation method for high-purity regorafenib (a compound I), particularly a preparation method for a compound 3 (a regorafenib intermediate) with the structure shown in the description, wherein the compound 3 is further used for industrial preparation of regorafenib with extremely excellent purity. By adopting a phase transfer catalyst, the method is simple and convenient to operate and environmental-friendly, can effectively inhibit generation of impurities, is low in requirement on equipment and is suitable for industrial production of high-purity regorafenib on a large scale.
Owner:HENAN UNIV OF CHINESE MEDICINE
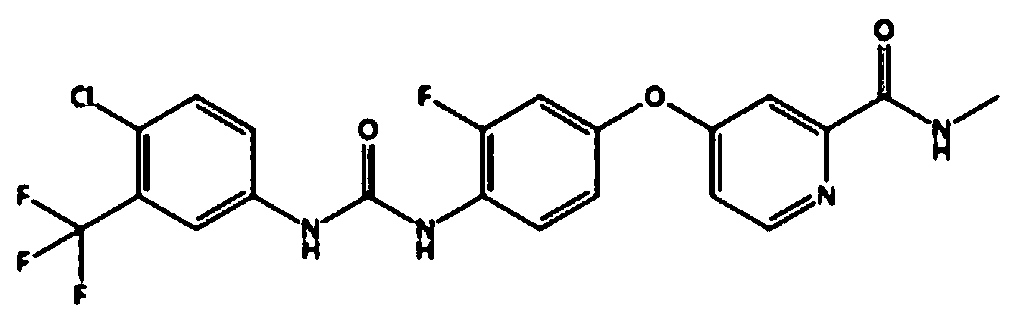
Regorafenib tablet pharmaceutical composition and preparation method thereof
ActiveCN104546776AReduced range of distribution in the bodyTumor size reductionPill deliveryAntineoplastic agentsKinase signalingAdhesive
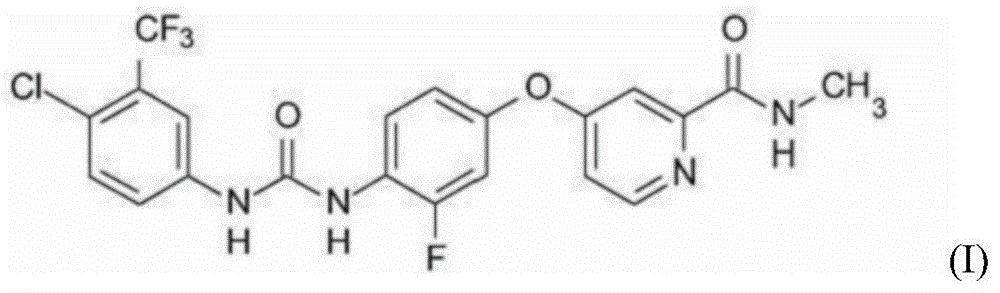
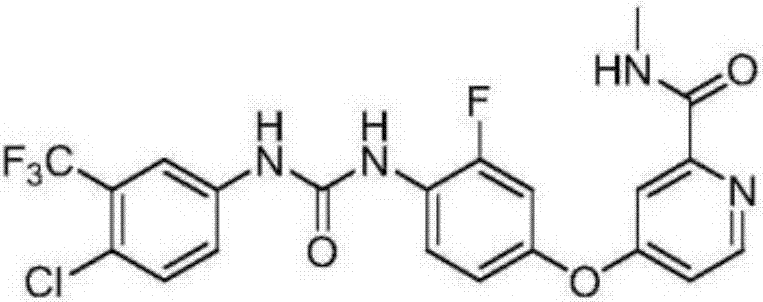
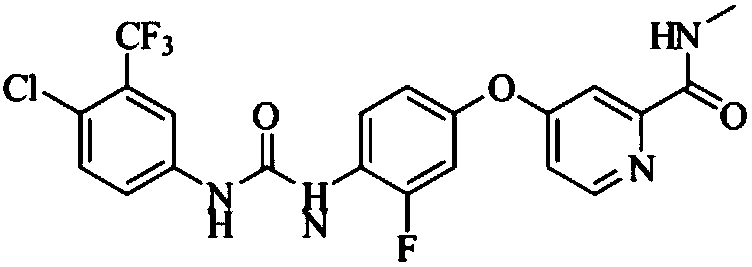
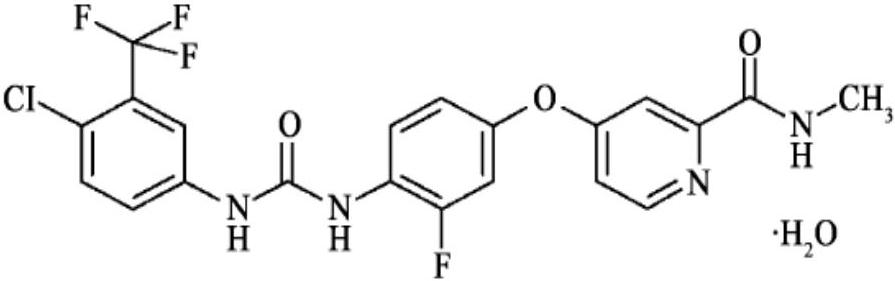
The invention relates to a regorafenib tablet pharmaceutical composition and a preparation method thereof and in particular relates to a pharmaceutical composition. The pharmaceutical composition comprises an active pharmaceutical compound, a diluent, a disintegrating agent, an adhesive and a lubricating agent, wherein the active pharmaceutical compound refers to 4-[4-({4-chloro-3-(trifluoromethyl)phenyl]carbamyl}amino)-3-chlorophenoxy]-N-methylpyridine-2-formamide or pharmaceutically acceptable salts, solvates and polymorphism. The pharmaceutical composition has a contact angle of 40-100 degrees, particularly a contact angle of 40-90 degrees, preferably a contact angle of 40-80 degrees, and in particular a contact angle of 40-70 degrees. The pharmaceutical composition disclosed by the invention can be used for treating diseases and disease symptoms mediated by abnormal VEGFR, PDGFR, raf, p38 and / or flt-3 kinase signals. The invention also relates to a method for preparing an anti-tumor pharmaceutical composition. The pharmaceutical composition disclosed by the invention has excellent preparation performance.
Owner:HANGZHOU ZHUYANGXIN PHARMA
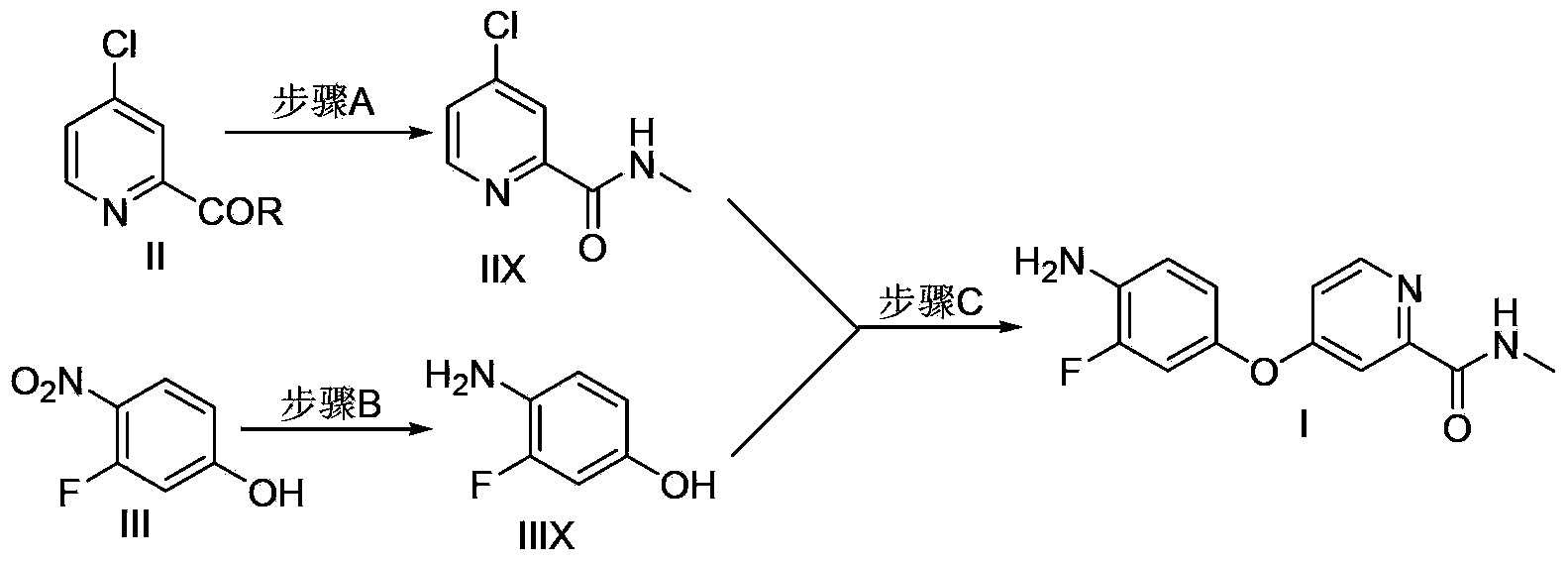
Method for preparing regorafenib intermediate
ActiveCN104250226AImprove recycling efficiencyReduced post-processingOrganic chemistryThree stageRegorafenib
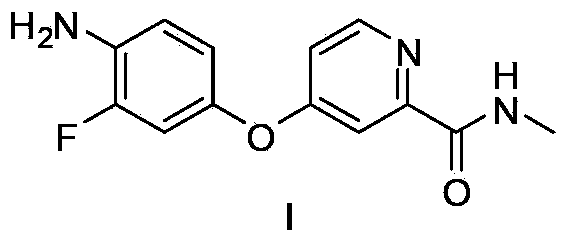
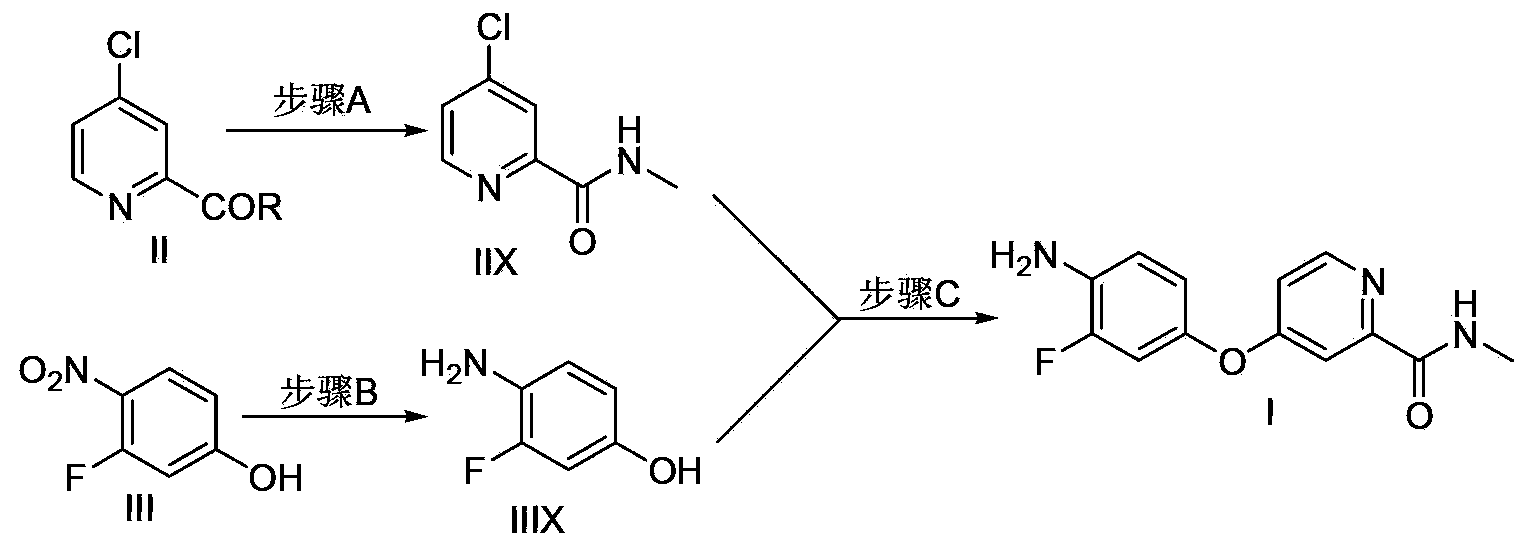
The invention discloses a method for preparing a regorafenib intermediate; a compound represented by the formula II and a compound represented by the formula III are subjected to three stages of a step A, a step B and a step C, intermediates of all the stages are not separated, and thus the regorafenib intermediate I is prepared. According to the method, the intermediates of all the steps are not separated, post-treatment procedures are reduced, the process operational flow is saved, the solvent recovery and utilization efficiency is improved, pollution emissions and energy consumption are reduced, the requirements on green chemical process are met, and the method is suitable for industrialized production.
Owner:SHANGHAI FANGNAN PHARMA
Preparation method for Regorafenib hydrate
The invention relates to a preparation method for Regorafenib hydrate. The method includes the steps that creatively, 4-chloro-1-trifluoromethyl phenylamine (compound 2) and allyl chloroformate (compound 3) react to generate (4-chloro-3-trifluoromethyl phenylamine)-formic acid allyl ester (compound 4); (4-chloro-3-trifluoromethyl phenylamine)-formic acid allyl ester (compound 4) and 4-(4-amino-3-trifluoromethyl)-N-methylpyridine-2-formamide (compound 9) are subjected to a substitution reaction under the catalysis of N-methyl pyrrolidine or trialkylaluminium to obtain Regorafenib hydrate. According to the method, cost is low, operation is easy, few reaction steps are needed, the period is short, energy consumption is low, the yield is high, purity is high, the process is safe, no high-toxicity reagent is used, and the obtained product has no potential safety problem and is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Composition for promoting hair cell regeneration and hearing recovery and application thereof
ActiveCN111643671APromote regenerationPromote maturitySenses disorderNervous system cellsHair cell differentiationRegorafenib
The invention belongs to the field of biological medicines, and discloses a composition for promoting hair cell regeneration and hearing recovery and an application thereof. The composition comprisesa Wnt agonist and one or more of the following reagents: (a) a VEGFR inhibitor; (b) a Tgfbr inhibitor; and (c) an ERG inhibitor. The VEGFR inhibitor comprises regorafenib, apatinib mesylate, cabozantinib, pazopanib hydrochloride and medicinal salts or derivatives of the regorafenib, the apatinib mesylate, the cabozantinib and the pazopanib hydrochloride. An organoid platform verifies that after any inhibitor in a signal axis of EGFR-TGFB1-ERG is combined with the Wnt agonist to prepare the composition, efficient hair cell differentiation, maturation and survival can be realized, and the composition has important value for hearing recovery.
Owner:NANJING UNIV
Composition containing protein kinase inhibitor and resveratrol
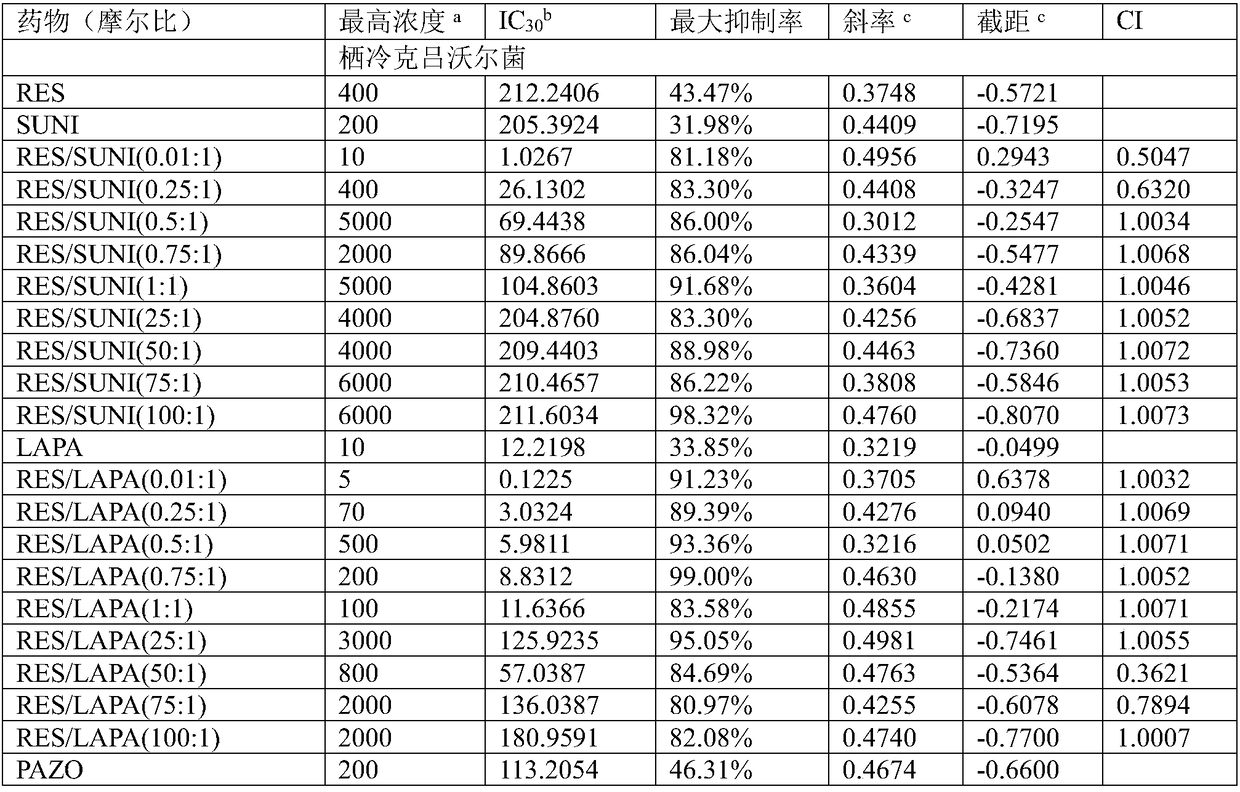
The invention firstly provides a composition containing a protein kinase inhibitor and resveratrol. The composition is characterized in that the protein kinase inhibitor is one, or its pharmaceutically-acceptable salt or solvate or pharmaceutically-acceptable salt solvate, of sunitinib, lapatinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib, and regorafenib; the molar ratio of resveratrolto the protein kinase inhibitor is (0.01-100):1. It is discovered through in-vitro antibacterial tests that the composition of the protein kinase inhibitor and resveratrol, in the molar ratio of (0.01-100):1, and can provide synergic antibacterial action against Kluyvera cryocrescens and other bacteria (interaction index CI<1 upon 30% of inhibition).
Owner:黄泳华
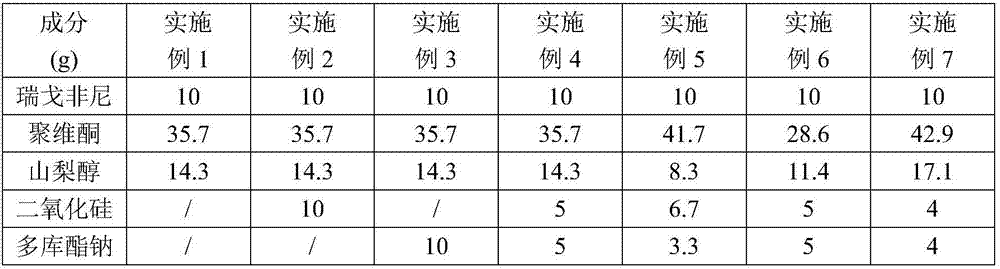
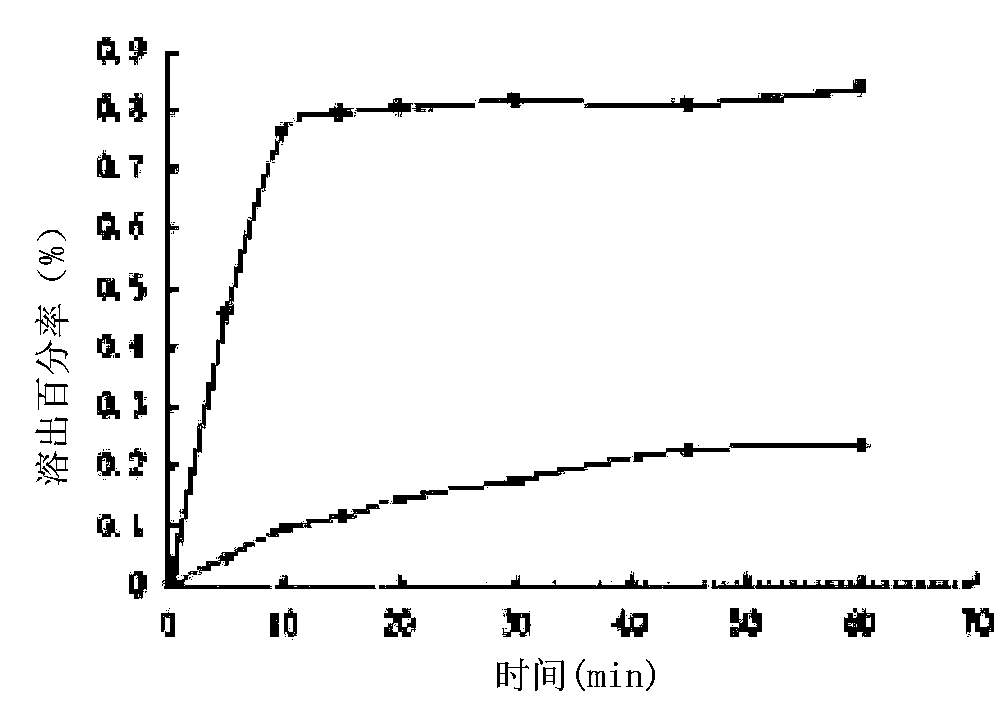
Regorafenib solid dispersion and preparation thereof
ActiveCN107213127AGood water solubilityImprove solubilityPill deliveryPharmaceutical non-active ingredientsSolubilityDocusate Sodium
The invention discloses a regorafenib solid dispersion, which comprises regorafenib, a carrier material and a synergistic agent in the mass ratio of 1: (2-20): (0.2-5), the carrier material comprises povidone and sorbitol in the mass ratio of 1: (0.1-0.5), and the synergistic agent comprises silica and Docusate sodium in the mass ratio of 1: (0.5-1). Through a certain process, the regorafenib is prepared into the sorbitol-regorafenib-povidone sandwich structure solid dispersion, dissolution of the regorafenib from inside to outside is promoted, the solubility of the regorafenib is greatly improved, safe, non-toxic and volatile ethanol is used as a solvent in the preparation process, and no security risk is caused by residual solvent. At the same time, the aging phenomenon in the long time storage process of the regorafenib solid dispersion can be avoided, and stability is improved.
Owner:广东安诺药业股份有限公司
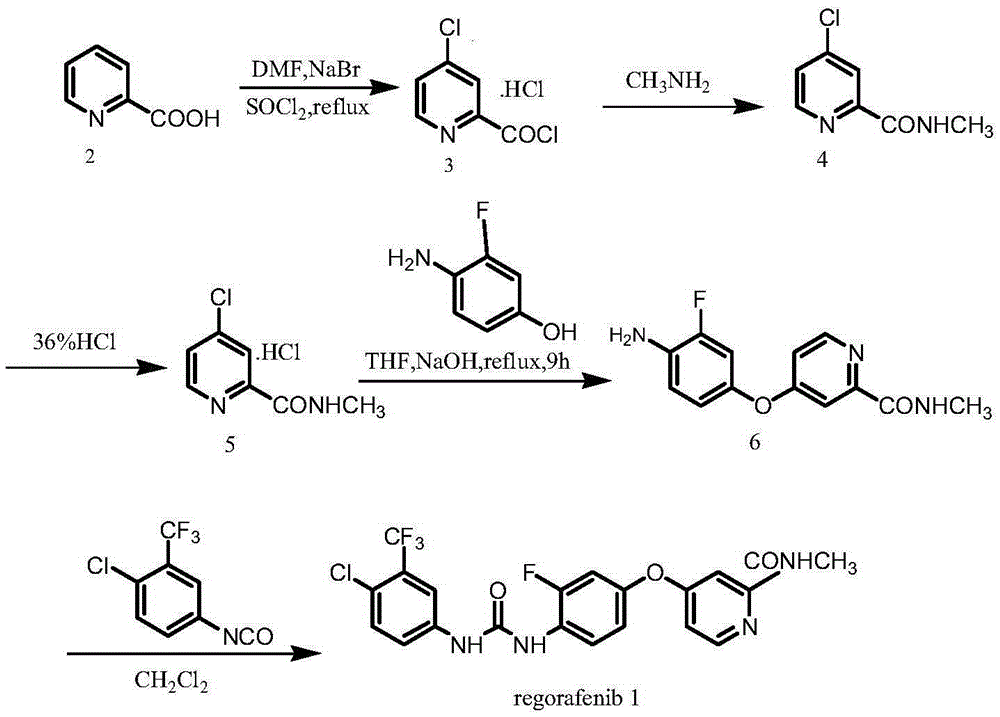
Preparation method of regorafenib
InactiveCN108558747AAvoid breakingReduce consumption costsOrganic chemistryHydrazine compoundFormamide
The invention relates to a preparation method of regorafenib. The method comprises the following steps: heating and melting 3-fluoro-4-nitrophenol and reacting with 4-chloro-N-methylpyridine-2-formamide under the action of KOH; reducing through hydrazine hydrate to obtain an intermediate I; finally enabling the intermediate I and 3-trifluoromethyl-4-chloroaniline, and ethylene carbonate to be subjected to one-pot reaction under the catalysis of a catalyst; carrying out post-treatment to obtain a regorafenib pure product. The method provided by the invention has the advantages of high conversion rate, safety and no harms, no pollution, moderate reaction conditions, high yield and high product purity, and is applicable to industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
New application of sorafenib, regorafenib and analogues or derivatives thereof
ActiveCN111870600AExempt from transplantSmall burdenAntineoplastic agentsHeterocyclic compound active ingredientsChemical synthesisRed blood cell
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Regorafenib preparation method
InactiveCN105130887ASimple methodSimple processOrganic chemistryBulk chemical productionRegorafenibProtecting group
The invention discloses a regorafenib preparation method, and belongs to pharmacy field. According to the method, on the basis of a conventional routine, the routine defects are avoided, a raw material 4-amino-3-fluorophenol, which is low in price and easy to obtain or prepare, is employed for replacing 4-chloro-3-(trifluoromethyl)phenyl isocyanate, a protection group for protection is ingeniously employed during reaction and unnecessary impurities are prevented from generating, the whole routine is simple in operation, and the product is high in yield, stable and easy to purify.
Owner:JIANGSU ZHONGBANG PHARMA
Regorafenib-coated nano-porous hydroxyapatite sustained-release microsphere as well as preparation method and application thereof
ActiveCN112999169AIncrease profitImprove membrane penetrationPharmaceutical non-active ingredientsGranular deliveryDrug release rateMicrosphere
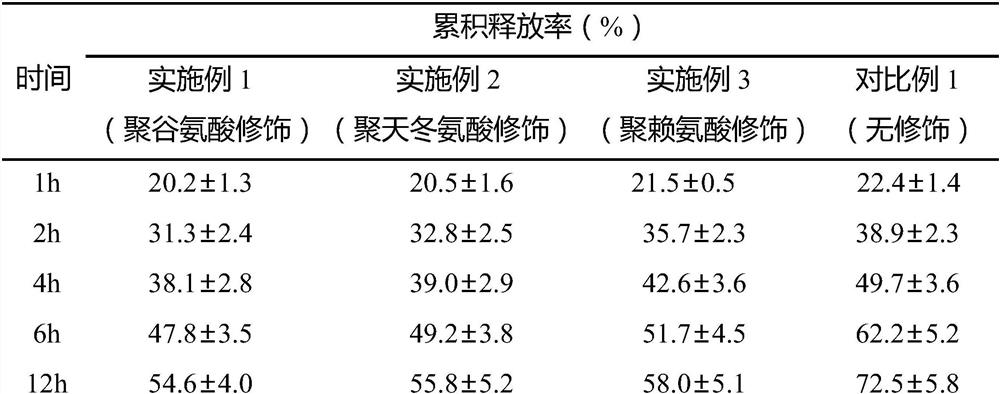
The invention relates to the field of drug sustained-release preparations, and in particular, relates to a regorafenib-coated nano-porous hydroxyapatite sustained-release microsphere as well as a preparation method and application thereof. The sustained-release microsphere disclosed by the invention contains 5 to 10 parts of regorafenib and 10 to 20 parts of nano-porous hydroxyapatite; the nano porous hydroxyapatite is modified by polyglutamic acid, polyaspartic acid or polylysine. The nano-porous hydroxyapatite is subjected to polyamino acid modification, so that the release rate of the active ingredient regorafenib can be effectively controlled, the drug release rate and the treatment effect are improved, the side effects of drugs on patients can be remarkably reduced, and the nano-porous hydroxyapatite has wide application prospects in the field of drug sustained-release carriers.
Owner:GUANGDONG MEDICAL UNIV
Regorafenib oral solid pharmaceutical composition preparation method
InactiveCN105267167ASolve the problem of low solubility and slow dissolution rateImprove stabilityPill deliveryPharmaceutical non-active ingredientsSolubilityMedicine
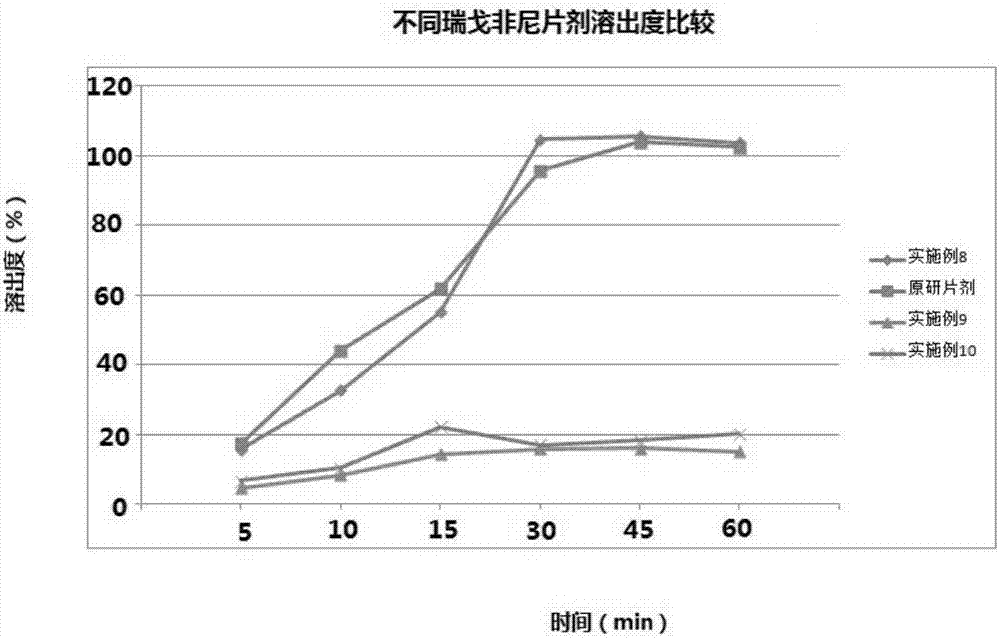
The present invention discloses a regorafenib oral solid pharmaceutical composition preparation method, a binder solution is first prepared by solvent method, then regorafenib is added into the binder solution, and sufficiently stirred to obtain a clear granulating liquid; the clear granulating liquid is then sprayed into other auxiliary material to prepare into a suitable solid dispersion; further the suitable solid dispersion is compressed into the smallest pharmaceutical dosage unit, the tablet may further be coated; the composition contains a binder, a disintegrating agent, a lubricant and a wetting agent. Advantages of the method are that: the method mainly solves the problems of small regorafenib solubility andslow dissolution rate to further improve the bioavailability and enhance the stability of the drug.
Owner:JIANGSU SINOBIOPHARMA
Application of OPAGANIB in preparation of medicine for treating regorafenib-tolerant liver cancer
ActiveCN111557932AReverse drug resistanceGrowth inhibitionOrganic active ingredientsAntineoplastic agentsChemical compoundPharmaceutical drug
The invention relates to the field of treatment of drug-resistant tumors, in particular to an application of a small molecular compound in preparation of a drug for treating regorafenib-tolerant livercancer. The invention discloses a SphK2 inhibitor OPAGANIB capable of reversing the drug resistance of liver cancer to regorafenib. On the basis of a regorafenib liver cancer drug-resistant cell strain and an in-vivo liver cancer drug-resistant model, it is found that the sensitivity of the OPAGANIB treated drug-resistant cell strain to regorafenib is remarkably improved; and after overexpressionof SphK2, the sensitivity of the drug-resistant cell strain to regorafenib is significantly reduced, the results show that the sensitivity of liver cancer cells to regorafenib can be effectively improved by inhibiting the activity of SphK2, so that the problem of drug resistance of liver cancer to regorafenib can be solved. According to the invention, the activity of SphK2 is inhibited by using OPAGANIB, so that the drug resistance of liver cancer to regorafenib is reversed.
Owner:NANJING UNIV
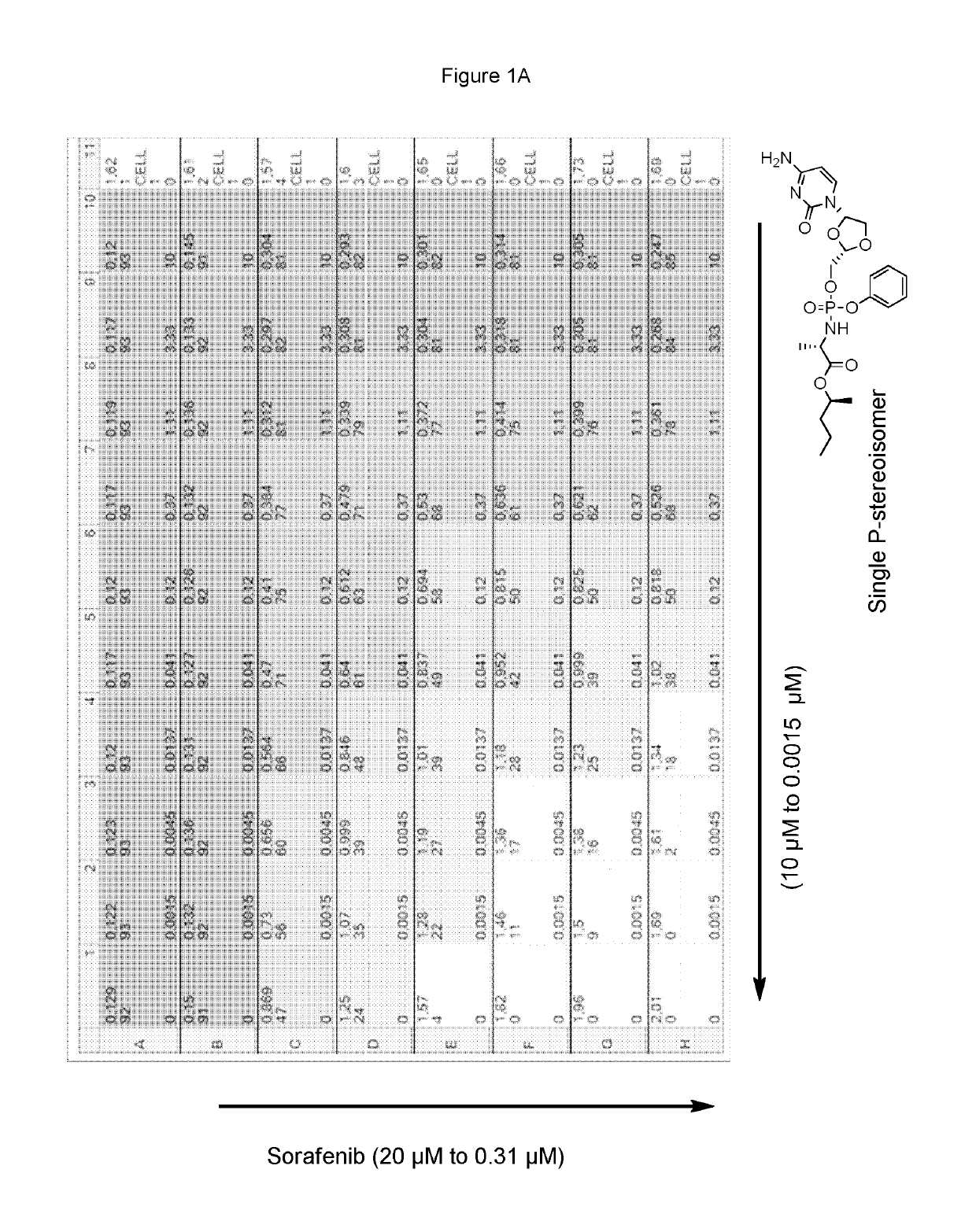
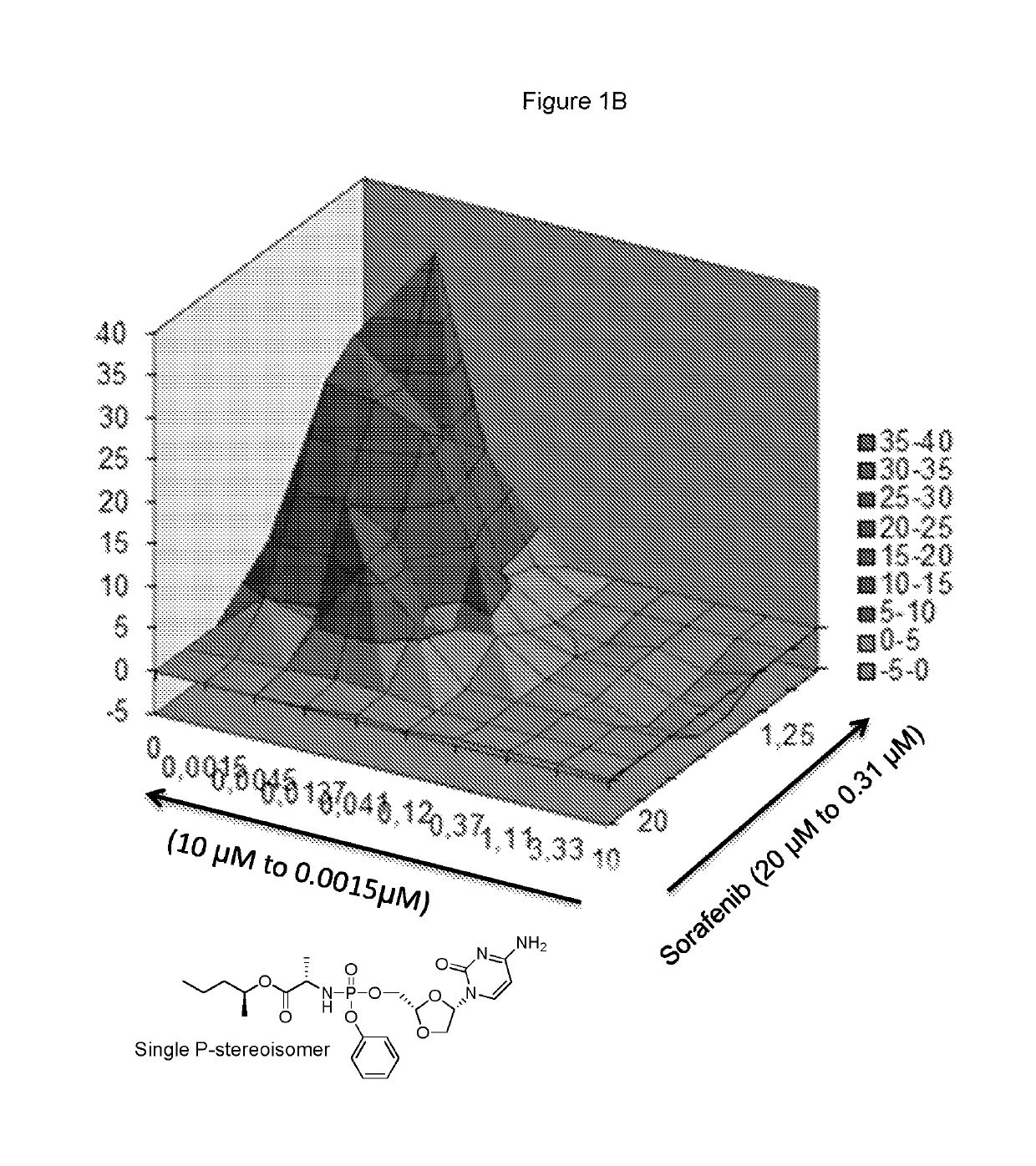
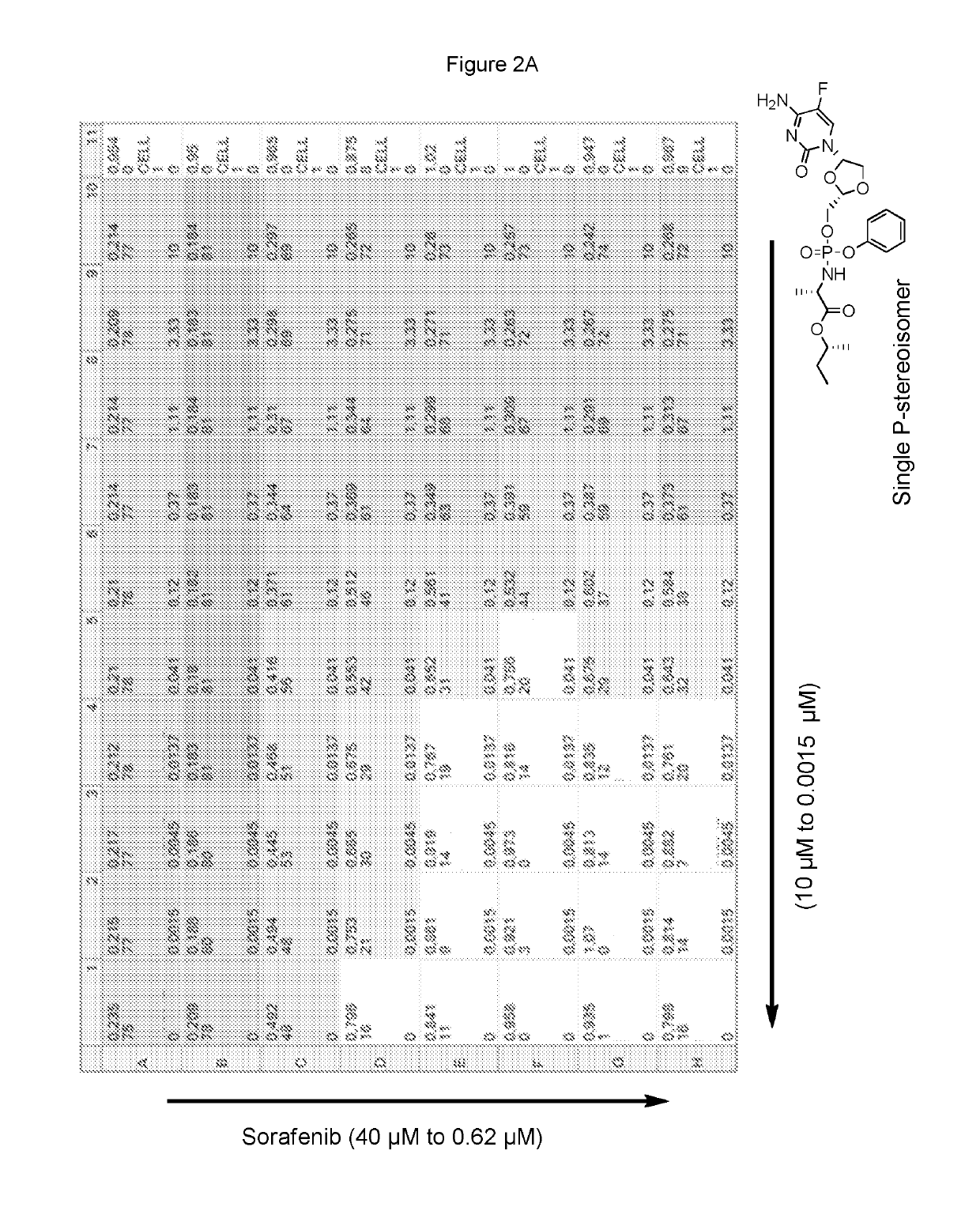
Combination therapy with sorafenib or regorafenib and a phosphoramidate prodrug of troxacitabine
ActiveUS10456413B2Profound anti-oncogenic activityIncreased activationPharmaceutical delivery mechanismAntineoplastic agentsTroxacitabineLymphatic Spread
Combination therapy with sorafenib or regorafenib and a phosphoramidate prodrug of troxacitabine with the formula: where Y is C1-C8 straight or branched chain alkyl, X is H, halo, C3-C4cycloalkyl or C1-C4alkyl and Z is H or fluoro, or a pharmaceutically acceptable salt thereof, shows surprising utility in the treatment of liver cancer or liver metastasis.
Owner:MEDIVIR AB
Novel regorafenib anti-tumor platinum (II) complex and preparation method and application thereof
ActiveCN108997436AStrong antitumor activity in vitroSignificant in vitro antitumor activityOrganic active ingredientsPlatinum organic compoundsChemical structurePlatinum
The invention discloses a novel regorafenib anti-tumor platinum (II) complex. A chemical structural formula of the platinum (II) complex is as shown in the specification. The platinum (II) complex expresses excellent anti-tumor activity in vitro, has a potential medicinal value and is hopefully used for preparing various anti-tumor drugs. The invention further discloses a preparation method and the application of the platinum (II) complex.
Owner:YULIN NORMAL UNIVERSITY
Preparation method of regorafenib hydrate
ActiveCN109438336AHigh yieldLow impurity contentOrganic chemistryAntineoplastic agentsRegorafenibSolvent
The invention belongs to the field of drug synthesis and particularly relates to a preparation method of regorafenib hydrate. 4-methyl-2-pentanone, 4-amino-3-fluorophenol and N-methyl-4-chloro-2-pyridine carboxamide are condensed to obtain a regorafenib intermediate 1, 4-chloro-3-(trifluoromethyl)isocyanate is added for condensation and salt-forming reactions to produce a regorafenib intermediate2, and finally, free hydration is performed in an alkaline acetone aqueous solution to obtain the regorafenib hydrate. The preparation method of the regorafenib hydrate is simple to operate and easy to monitor, has yield up to 90% or above and high efficiency and is applicable to mass industrial production. The prepared regorafenib hydrate has low impurity content, has no solvent residues basically, significantly shortens purification time, has stable properties, keeps stable in accelerated stability tests and has high safety.
Owner:广东安诺药业股份有限公司
Medical composition of didemethyl tetrandrine dual ethyl formate and tyrosine kinase inhibitor
ActiveCN110025617AImprove anti-tumor activityImprove anti-tumor effectAntineoplastic agentsHeterocyclic compound active ingredientsTreatment effectTyrosine-kinase inhibitor
The invention relates to the technical field of medicines, specifically discloses a medical composition of didemethyl tetrandrine dual ethyl formate (W18) and a tyrosine kinase inhibitor and the use of the medical composition for treating medicine-resisting liver cancer. Particularly, a medical composition of didemethyl tetrandrine dual ethyl formate (W18) and sorafenib or regorafenib has the effects of synergistically increasing sensitivity and reversing medicine resistance in the respect of treating drug tolerance liver cancer, and can significantly increase the treatment effects of treatingthe drug tolerance liver cancer.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
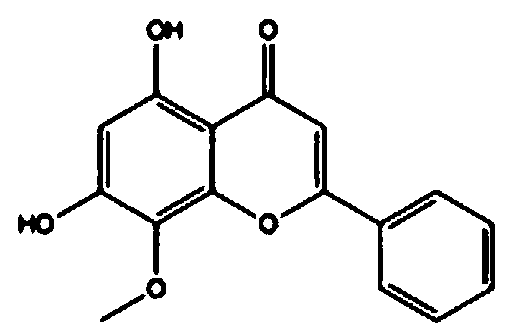
Regorafenib and wogonin eutectic and application thereof
PendingCN111116462AImprove solubilityImprove bioavailabilityOrganic chemistryAntineoplastic agentsAnticarcinogenic EffectPharmaceutical drug
The invention relates to a pharmaceutical composition for preventing and / or treating cancer and application thereof. The pharmaceutical composition takes regorafenib and wogonin as active ingredients,and the regorafenib and the wogonin exist in the form of eutectic. The compound disclosed by the invention has a good anti-cancer effect, particularly improves the solubility and bioavailability of an insoluble drug regorafenib for cocrystals of colon cancer, regorafenib and wogonin, and has a more remarkable anti-cancer effect compared with a physical mixture of the colon cancer, the regorafeniband wogonin.
Owner:QINGDAO CENT HOSPITAL
Refining method of regorafenib
InactiveCN111410632AHigh purityReduce adverse reactionsOrganic chemistryActivated carbonPhysical chemistry
The invention relates to a refining method of regorafenib. The preparation method comprises the following specific steps: (1) heating and dissolving regorafenib by using a solvent at the heating temperature of 20-60 DEG C, wherein the volume-mass ratio of the solvent to the regorafenib is less than or equal to 40: 1; wherein the unit of the solvent is g, and the unit of the regorafenib is g; (2) adding activated carbon, wherein the adding amount of the activated carbon is 2-6% of the mass of the regorafenib; (3) heating for decarburization, and then performing hot filtration; and (4) performing standing on a filtrate to room temperature, crystallizing in an environment of -20 to 30 DEG C, filtering, and collecting a filter cake to obtain the product. The method has the beneficial effects that the purity of the regorafenib is greatly improved, and adverse reactions caused by the existence of impurities are reduced.
Owner:西安百宁医药科技有限公司
Application of Regorafenib hydrate in preparing drug for treating pulmonary fibrosis
InactiveCN109820851AGood effectNo adverse reactionRespiratory disorderHeterocyclic compound active ingredientsDiseaseRegorafenib
The invention provides application of a Regorafenib hydrate in preparing a drug for treating pulmonary fibrosis and further provides a drug composition. The drug composition comprises the Regorafenibhydrate, a pharmaceutically acceptable salt or a pharmaceutically acceptable ester or a pharmaceutically acceptable hydrate of the Regorafenib hydrate or a composition of the pharmaceutically acceptable salt, ester and hydrate and accessories. The Regorafenib hydrate has a good effect on the pulmonary fibrosis, causes no adverse reactions, is capable of reducing the pulmonary fibrosis induced by bleomycin in mice, and provides a good application prospect for treating, easing or improving the pulmonary fibrosis.
Owner:NANKAI UNIV
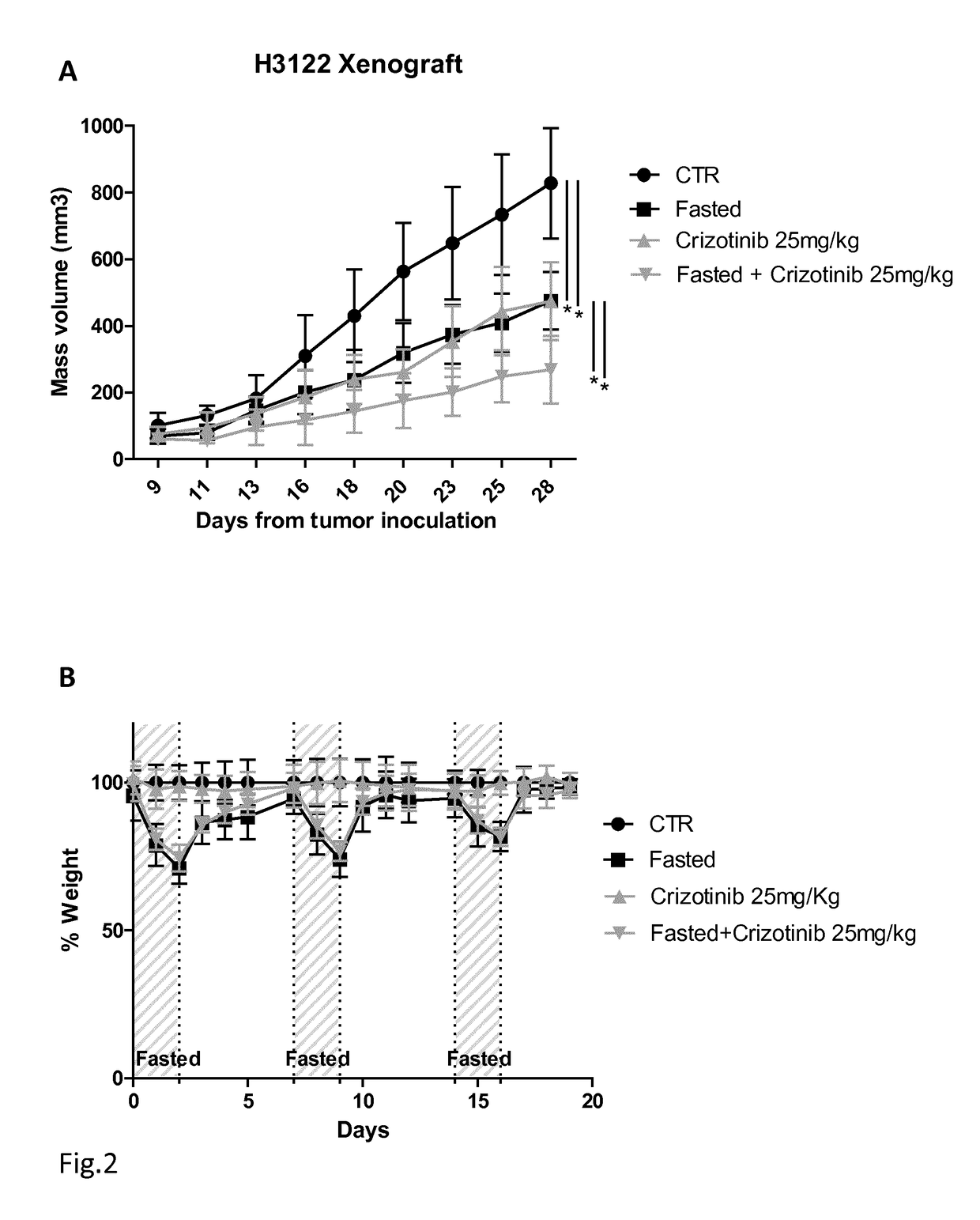
Tyrosine kinase inhibitors for use in a method of treating cancer in association with a reduced caloric intake
ActiveUS10117872B2Reduced caloric intakeReduce contentAntineoplastic agentsHeterocyclic compound active ingredientsMedicineTyrosine-kinase inhibitor
A tyrosine kinase inhibitor (TKI) for use in a method for the treatment of cancer in a patient, wherein the method comprises subjecting the patient to reduced caloric intake, i.e a daily caloric intake reduced by 10-100%, including starvation, for a period of 24-190 hours and administering the tyrosine kinase inhibitorto the patient during such period; the tyrosine kinase inhibitor is preferably selected among Lapatinib, Crizotinib, Gefitinib, Erlotinib, Afatinib and Regorafenib.
Owner:UNIV DEGLI STUDI DI GENOVA +1
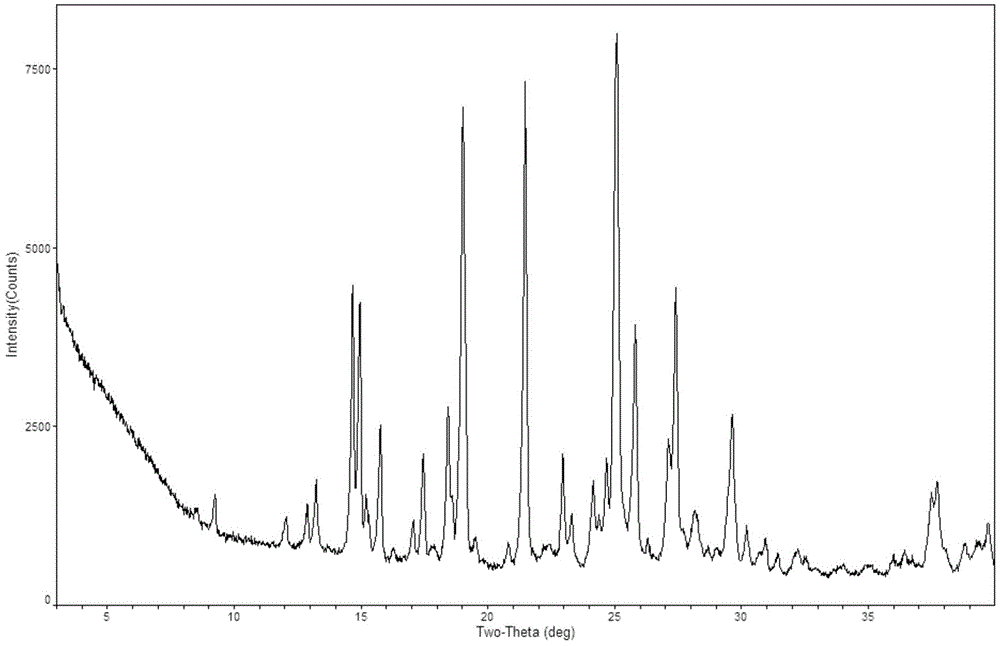
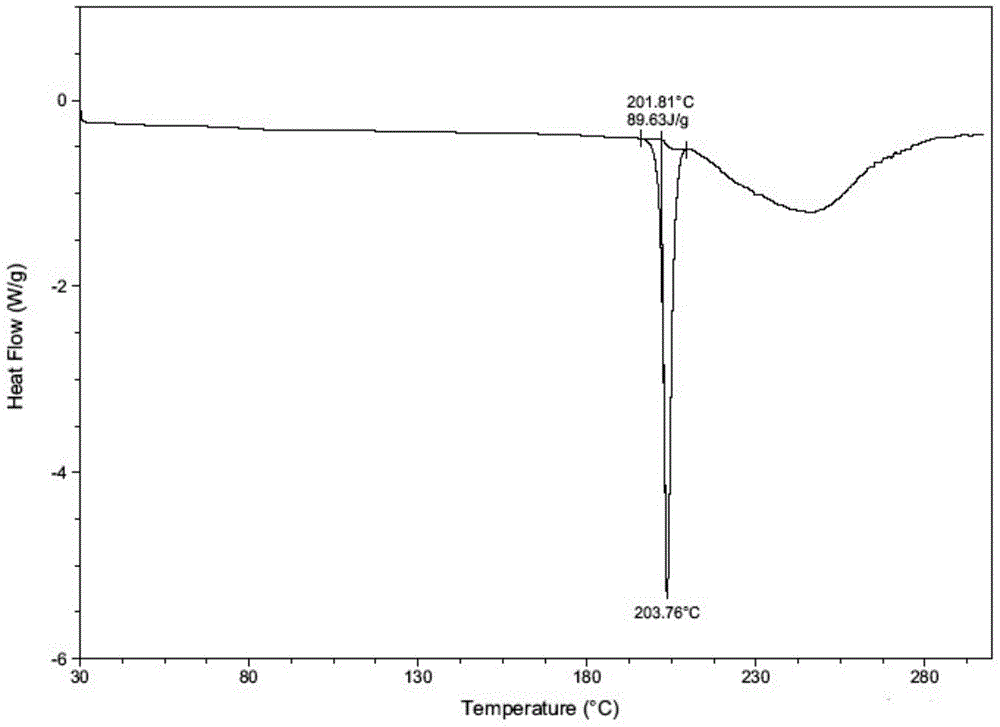
Novel crystal form of regorafenib
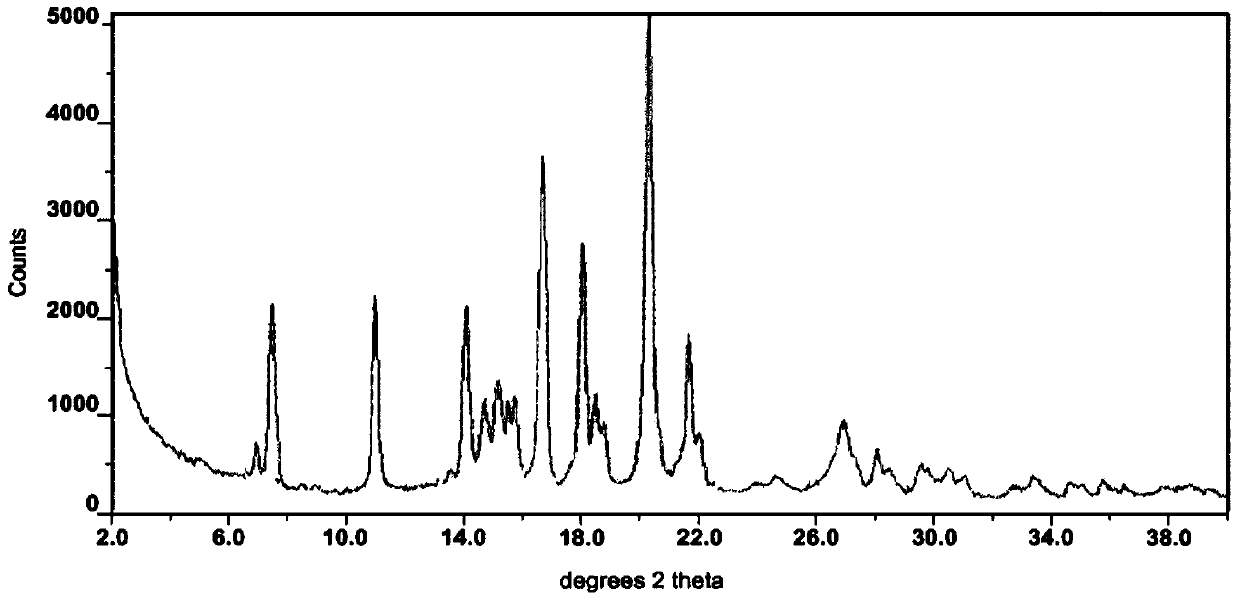
InactiveCN105985287ASimple preparation processGood crystal stabilityOrganic chemistryCrystallographyRegorafenib
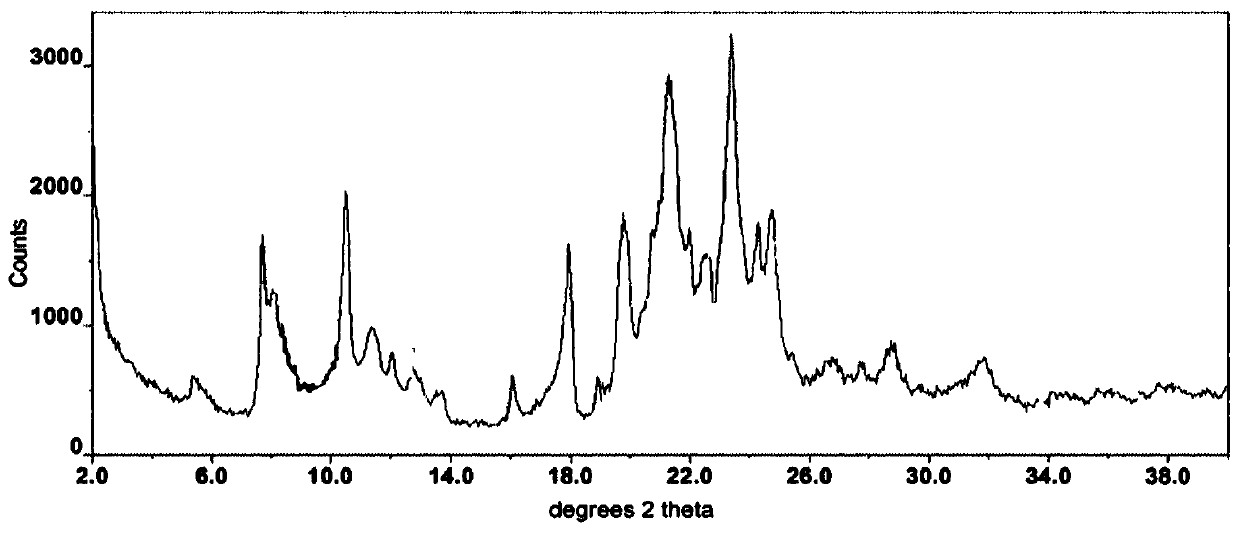
The invention provides a novel crystal form of regorafenib. Characteristic peaks occur in the X-ray powder diffraction pattern of the crystal form when 2theta is about 9.25, 12.06, 12.88, 13.23, 14.68, 14.96, 15.76, 17.46, 18.44, 19.02, 21.47, 22.97, 24.69, 25.11, 25.83, 27.12, 27.41 and 29.66. The crystal form of regorafenib has good stability.
Owner:SHANGHAI JINGXIN BIOLOGICAL MEDICAL +1
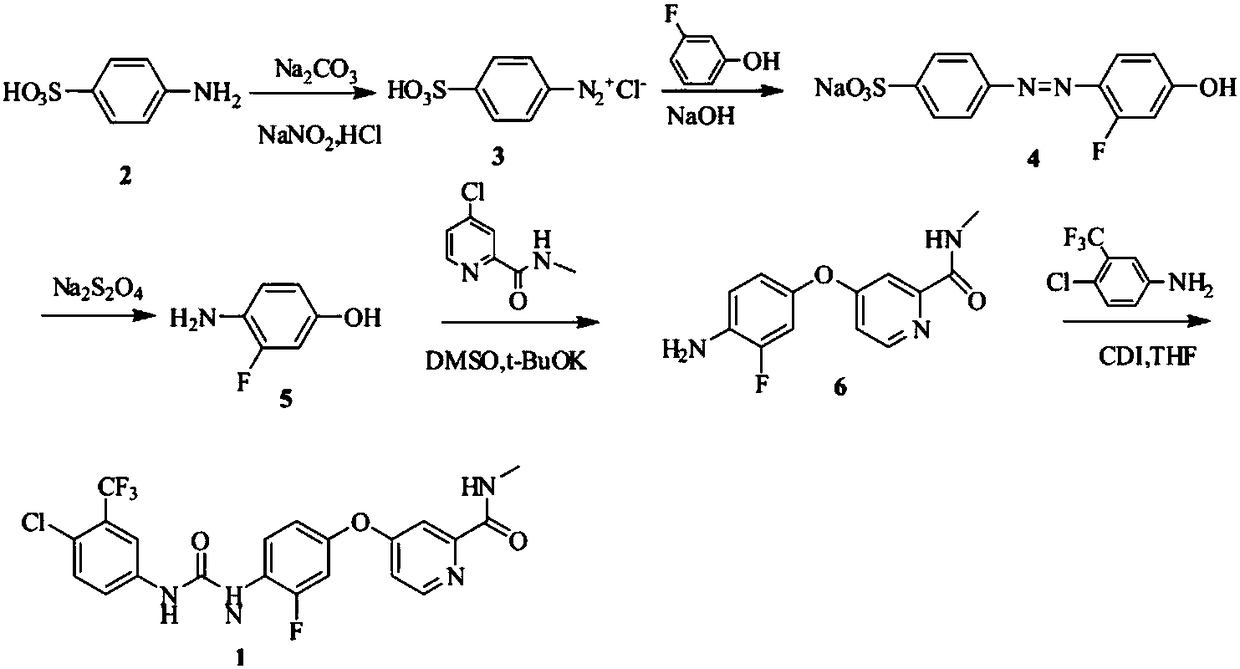
Preparation method of regorafenib
The invention relates to a preparation method of regorafenib. The method comprises the following steps: performing reaction on 3-fluoro-4-nitrophenol and 4-chloro-N-methyl pyridine-2-methylformamide under the effects of anhydrous potassium carbonate and PEG-400, and performing hydrazine reduction to generate an intermediate I; then performing one-pot reaction on the intermediate I, 3-trifluoromethyl-4-chlorophenylamine and dimethyl dithiocarboxylate under the effect of a catalyst, then performing post-treatment to obtain a regorafenib crude product, and further purifying the regorafenib crudeproduct to obtain a regorafenib purified product. The method disclosed by the invention has the advantages of high conversion ratio, security, no harm, no pollution, mild reaction conditions, high yield and high purity of the product; moreover, the method is suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Regorafenib related substances as well as preparation method and application thereof
PendingCN111892533AShorten the timeEase of industrial productionOrganic chemistryComponent separationPotassium tert-butoxideEngineering
The invention relates to a synthesis method of regorafenib related substances B and C. According to the method, 4-chloro-N-methylpyridine-2-formamide, 4-amino-3-fluorophenol and potassium tert-butoxide are used as starting materials, and the two related substances are obtained through one synthesis route, so time and cost are saved, and industrial production is easier.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
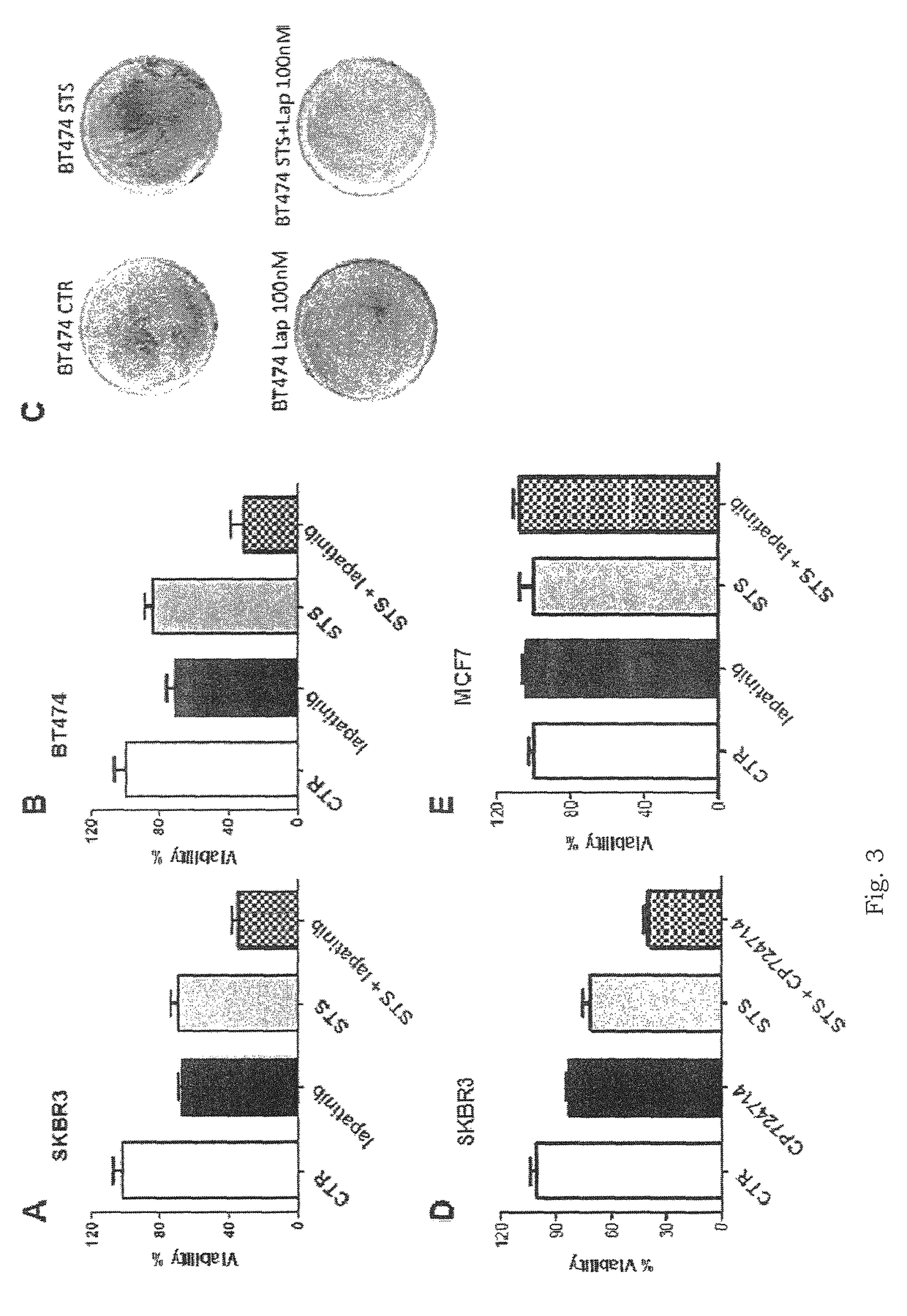
Application of regorafenib and lapatinib in preparation of antitumor combination drug
InactiveCN105343095AInhibit progressIncrease lethalityAntineoplastic agentsHeterocyclic compound active ingredientsSide effectCombined use
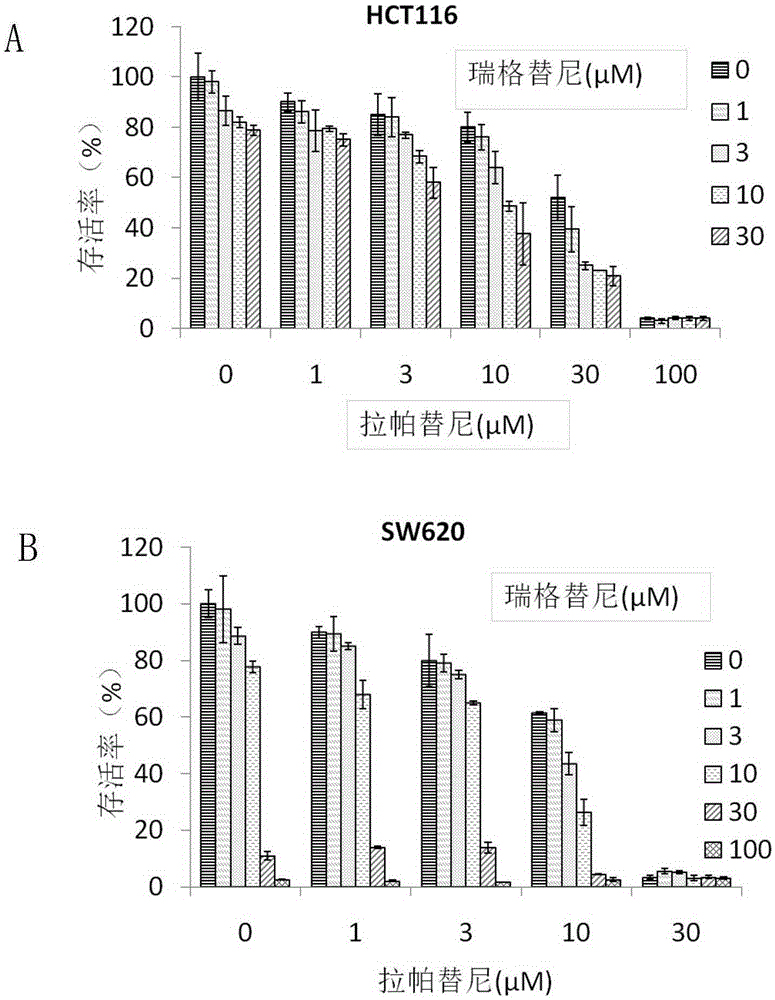
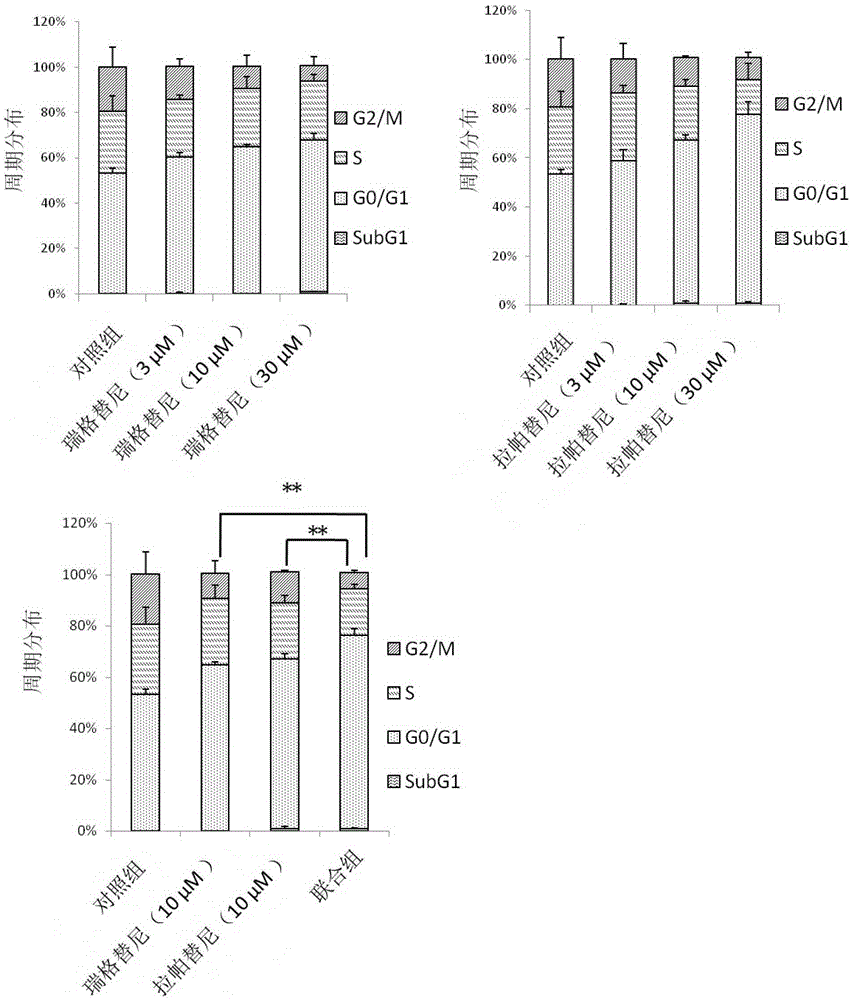
The invention belongs to the technical field of preparation of antitumor drugs and particularly relates to application of regorafenib and lapatinib in the preparation of an antitumor combination drug. The combination drug of regorafenib and lapatinib is significant in causing tumor cell cycle arresting, thus arresting tumor cells in the stage G0 / G1 and inhibiting the development of colon cancer; the ability to kill tumor cells can be effectively improved, and tumor cell apoptosis can be greatly promoted. The regorafenib and lapatinib are significant in synergistic action, effectively improving the curative effect, and has more significant effect than the single effect of a single component or double components, thus improving the ability to kill tumor cells; usage of the regorafenib and lapatinib is effectively reduced, thus reducing toxic or side effect. The combined use of the regorafenib and lapatinib can also save the cost and relieve the economic burden of a patient, a new way to control tumors is provided, and this drug has a promising application prospect in the field of medicine.
Owner:JINAN UNIVERSITY
Pharmaceutical composition for treating liver cancer and application thereof
InactiveCN111001004AIncreased sensitivityReduce doseDigestive systemAntineoplastic agentsUse medicationPharmaceutical drug
The invention discloses an application of combined use of regorafenib and a PD-1 / PD-L1 inhibitor in preparation of a medicine for treating liver cancer. The pharmaceutical composition (regorafenib andthe PD-1 / PD-L1 inhibitor) provided by the invention can generate a synergistic anti-liver cancer effect; according to the scheme of combined administration of the two, the sensitivity of liver cancercells to the regorafenib can be improved, the inhibition effect on the liver cancer cells is improved, the dosage of the regorafenib can be reduced, the treatment cost of each liver cancer patient isdirectly reduced, and more liver cancer patients are benefited; the pharmaceutical composition is low in price, high in safety and affirmative in curative effect; the pharmaceutical composition disclosed by the invention is convenient to produce, convenient for large-scale production, good in curative effect and low in cost, and is also beneficial to social public interests.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com