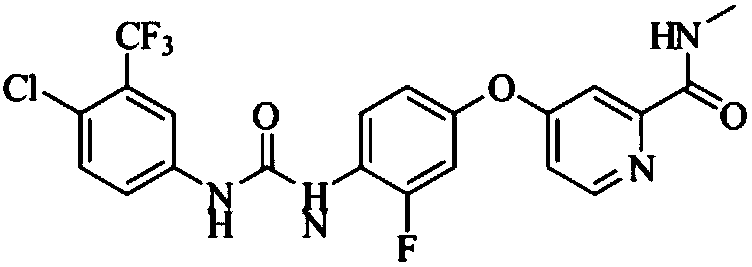
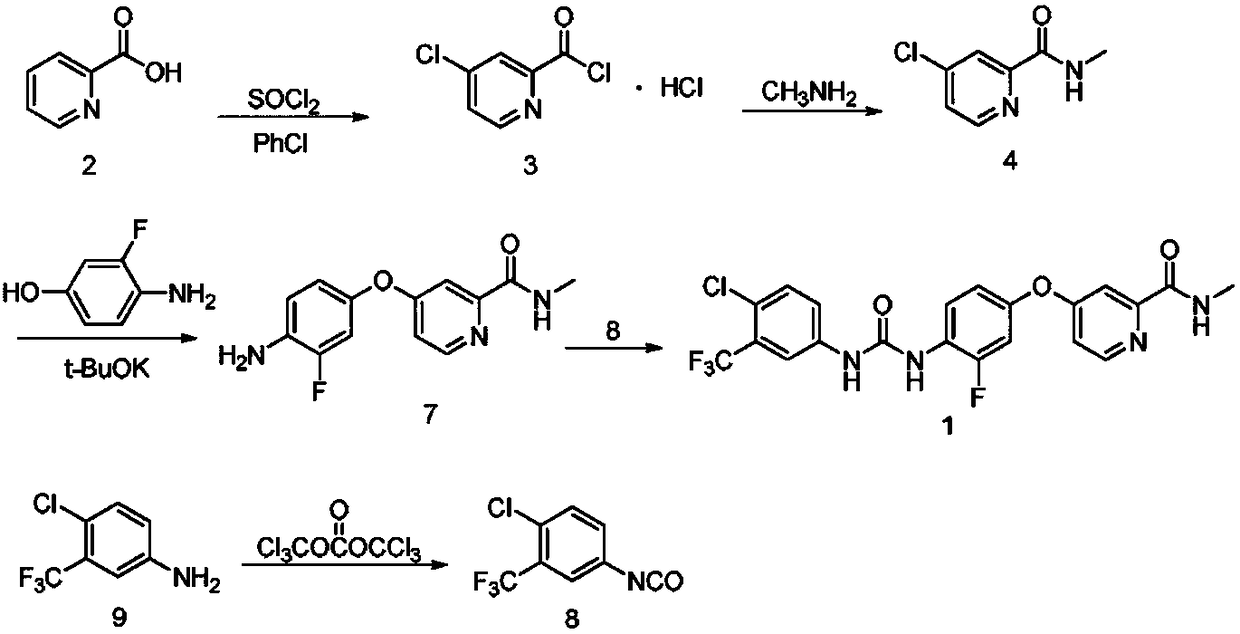
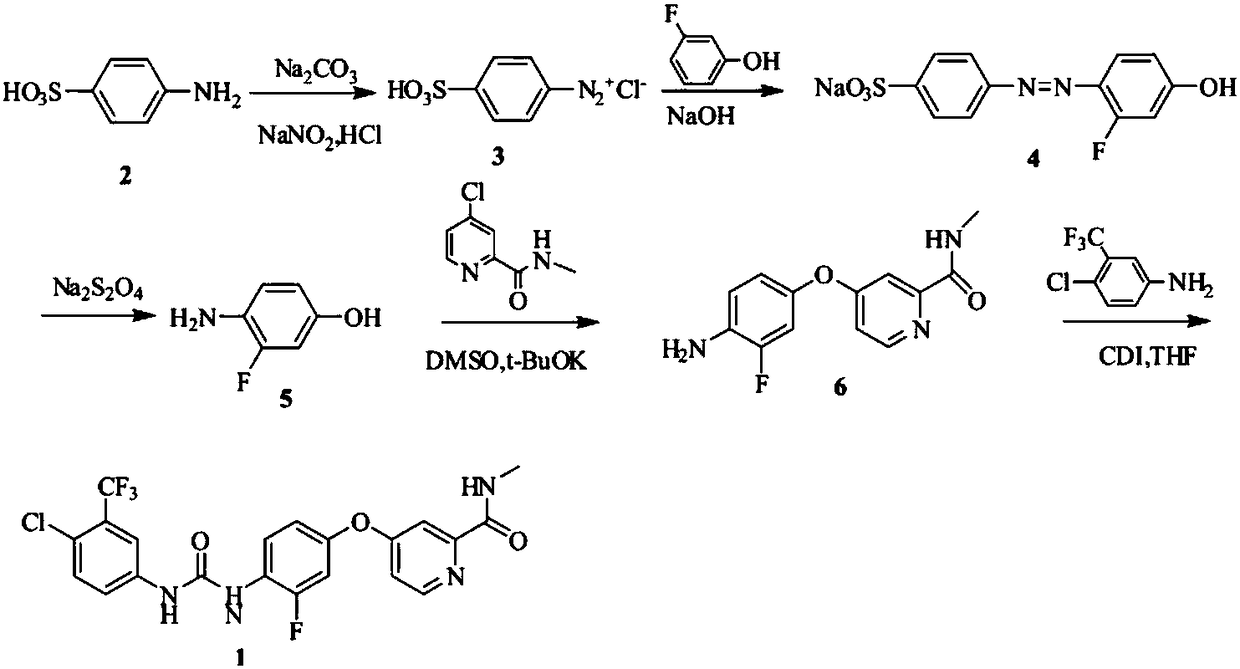
Preparation method of regorafenib
A technology of regorafenib and quantitative ratio, which is applied in the field of preparation of regorafenib, can solve the problems of unsuitability for industrial production, expensive catalyst, difficult operation, etc., and achieve reduced production costs, mild reaction conditions, and high conversion rate of the method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0024] The synthesis of embodiment 1-1 intermediate I
[0025] In a 250mL three-neck flask with a magnetic stirring device, a thermometer and a reflux condenser, add 0.09mol of 3-fluoro-4-nitrophenol, 0.10mol of anhydrous potassium carbonate, and 0.10mol of 4-chloro-N-methyl Pyridine-2-carboxamide, 0.003 mol of PEG-400 and 100 mL of acetonitrile were stirred with electromagnetic force, and heated to reflux in a water bath for 4 hours. Cool, filter, and evaporate acetonitrile under reduced pressure with a water pump. Mix the obtained residue, 1 g of activated carbon for sugar, 0.1 mmol of ferric chloride, and 50 mL of methanol in a 250 mL four-necked flask, and add 0.33 mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. , Reflux 2h. After stopping the reaction, filter, wash the activated carbon with 40mL ether, and distill the filtrate to remove methanol at the same time. After evaporation, extract the still liquid with 240mL ether, combine the ether laye...
Embodiment 1-2
[0026] The synthesis of embodiment 1-2 intermediate I
[0027]In a 250mL three-neck flask with a magnetic stirring device, a thermometer and a reflux condenser, add 0.09mol of 3-fluoro-4-nitrophenol, 0.10mol of anhydrous potassium carbonate, and 0.10mol of 4-chloro-N-methyl Pyridine-2-carboxamide, 0.003 mol of PEG-400 and 100 mL of acetonitrile were stirred with electromagnetic force, and heated to reflux in a water bath for 4 hours. Cool, filter, and evaporate acetonitrile under reduced pressure with a water pump. Mix the obtained residue, 1 g of activated carbon for sugar, 0.12 mmol of ferric chloride, and 50 mL of methanol in a 250 mL four-necked flask, and add 0.30 mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. , Reflux 2h. After stopping the reaction, filter, wash the activated carbon with 40mL ether, and distill the filtrate to remove methanol at the same time. After evaporation, extract the still liquid with 240mL ether, combine the ether laye...
Embodiment 1-3
[0028] Synthesis of Example 1-3 Intermediate I
[0029] In a 250mL three-necked flask with a magnetic stirring device, a thermometer and a reflux condenser, add 0.08mol of 3-fluoro-4-nitrophenol, 0.10mol of anhydrous potassium carbonate, and 0.09mol of 4-chloro-N-methyl Pyridine-2-carboxamide, 0.002 mol of PEG-400 and 100 mL of acetonitrile were stirred with electromagnetic force, and heated to reflux in a water bath for 4 hours. Cool, filter, and evaporate acetonitrile under reduced pressure with a water pump. Mix the obtained residue, 1 g of activated carbon for sugar, 0.12 mmol of ferric chloride, and 50 mL of methanol in a 250 mL four-necked flask, and add 0.30 mol of 85% hydrazine hydrate dropwise at reflux temperature for 1 hour. , Reflux 2h. After stopping the reaction, filter, wash the activated carbon with 40mL ether, and distill the filtrate to remove methanol at the same time. After evaporation, extract the still liquid with 240mL ether, combine the ether layers, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com