Preparation method of regorafenib hydrate
A regorafenib hydrate and regorafenib technology are applied in the field of preparation of regorafenib hydrate, which can solve the problems of low overall yield, poor stability, poor economy and the like, and achieve low impurity content and high stability. The effect of lowering and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
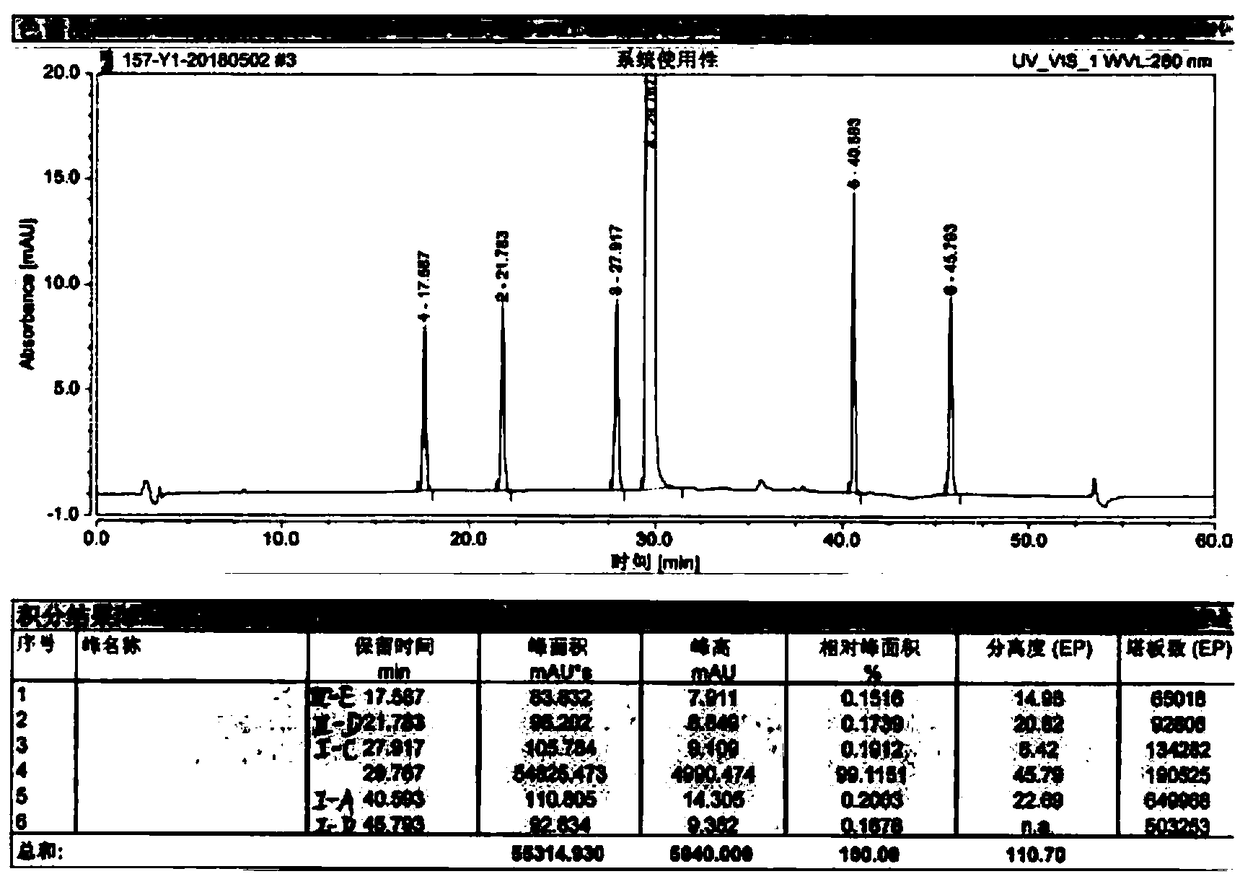
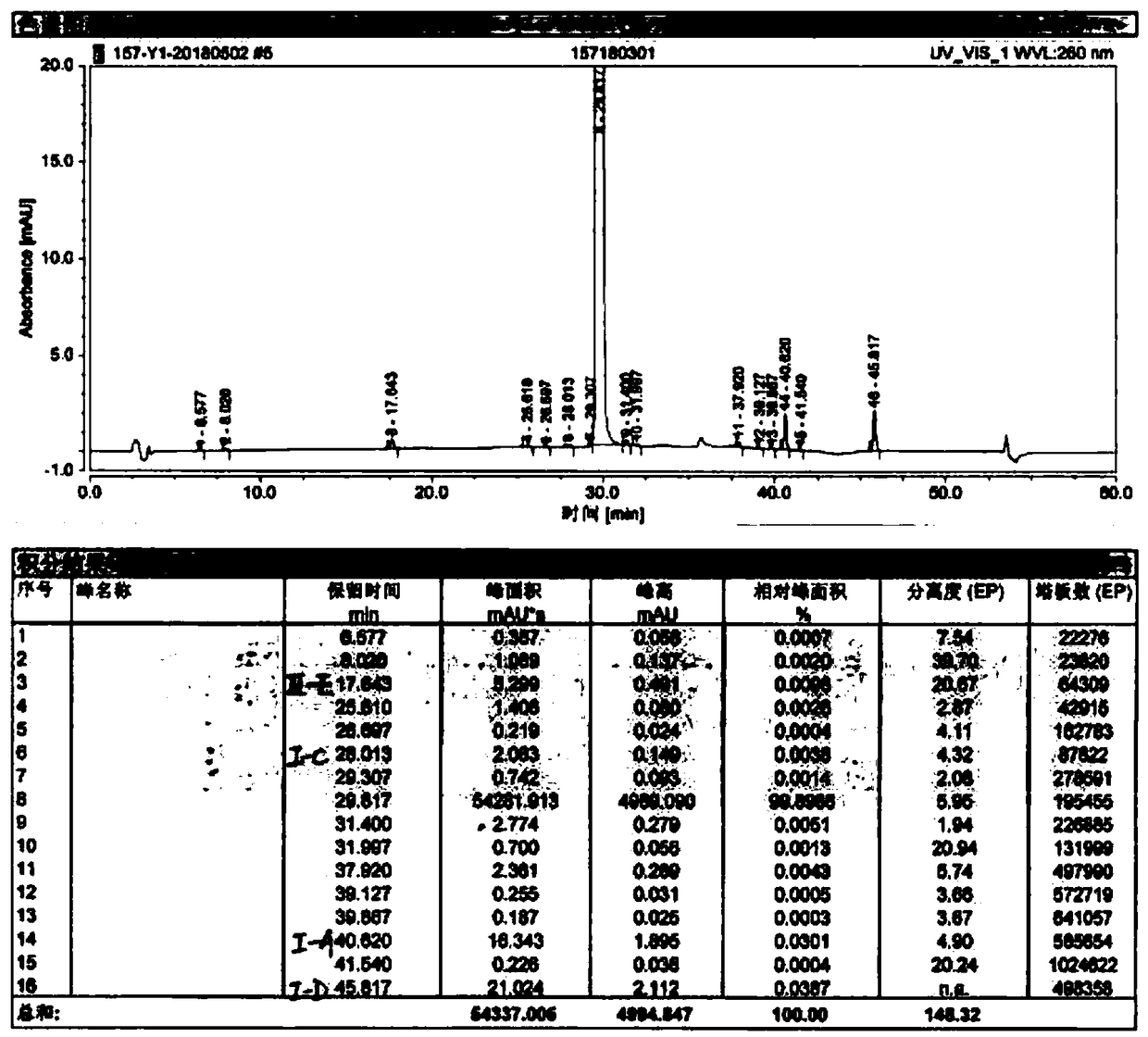
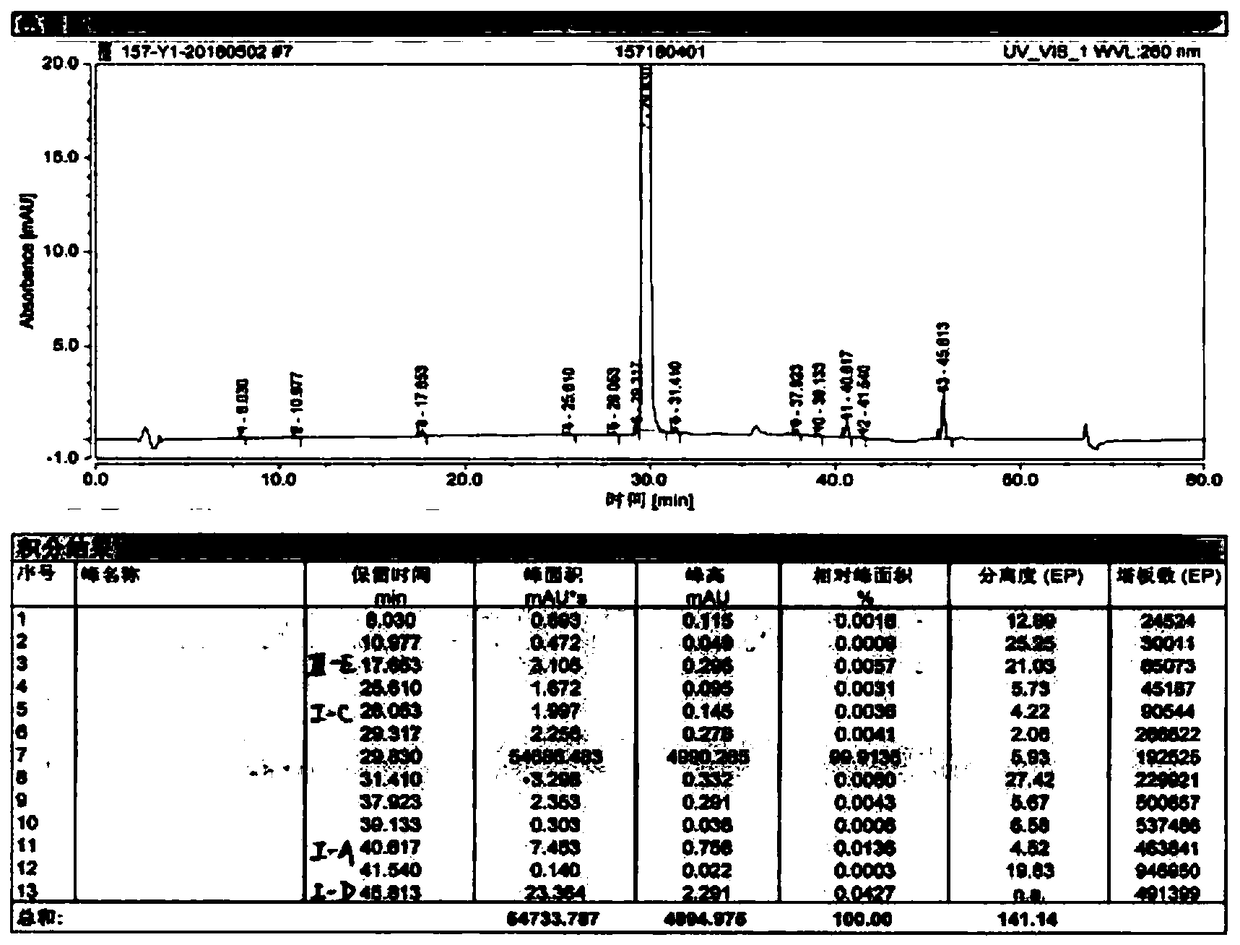
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 regorafenib hydrate
[0047] 1. Preparation of Regorafenib Intermediate 1
[0048] The chemical reaction equation is:
[0049]
[0050] Preparation:
[0051] While stirring, add 26.140kg of toluene, 10.320kg of 4-methyl-2-pentanone, and 4.300kg of 4-amino-3-fluorophenol into the reaction kettle, raise the temperature to 112°C, reflux and separate water, and monitor the reaction by TLC. Cool the reaction solution to 85°C, place it in a rotary evaporator, control the water temperature to 70°C, and vacuum to -0.10MPa, concentrate under reduced pressure until no solvent drops out; fill the rotary evaporator with nitrogen, add N-formazol 12.900 kg of pyrrolidone, stir to dissolve, transfer to the reactor, start stirring, nitrogen protection, lower the temperature to 15 ° C, take N-methyl-4-chloro-2-pyridine carboxamide 5.830 kg into the reactor, control the internal temperature 15 ℃, add 3.980 kg of potassium tert-butoxide in five time...
Embodiment 2~3
[0063] The preparation of embodiment 2~3 regorafenib hydrate
[0064] The difference from Example 1 is that the raw material dosages of the Examples 2 to 3 are different, see Table 1 for details.
[0065] Table 1 Example 2-3 Regorafenib Hydrate Raw Materials and Auxiliary Materials Feeding Amount
[0066]
[0067]
[0068] The preparation method refers to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com