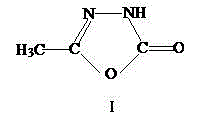
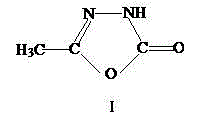
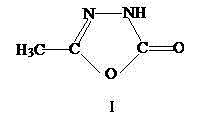
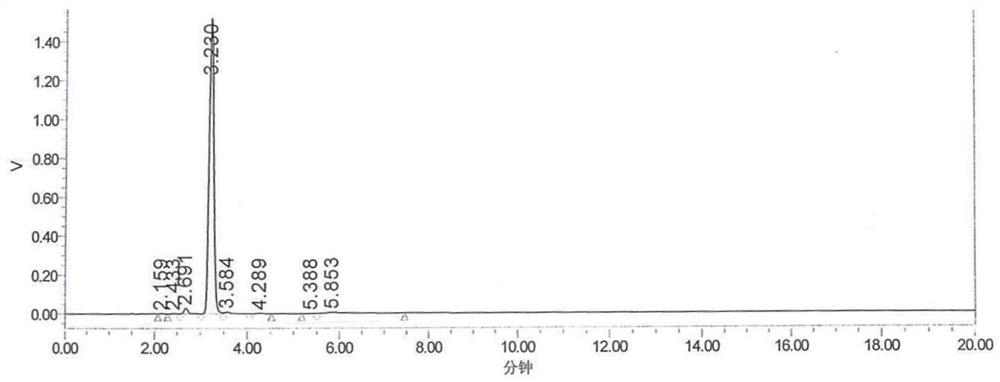
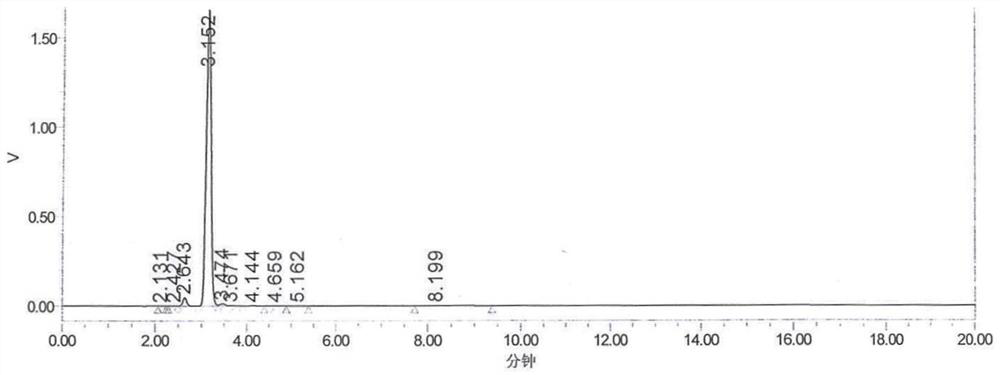
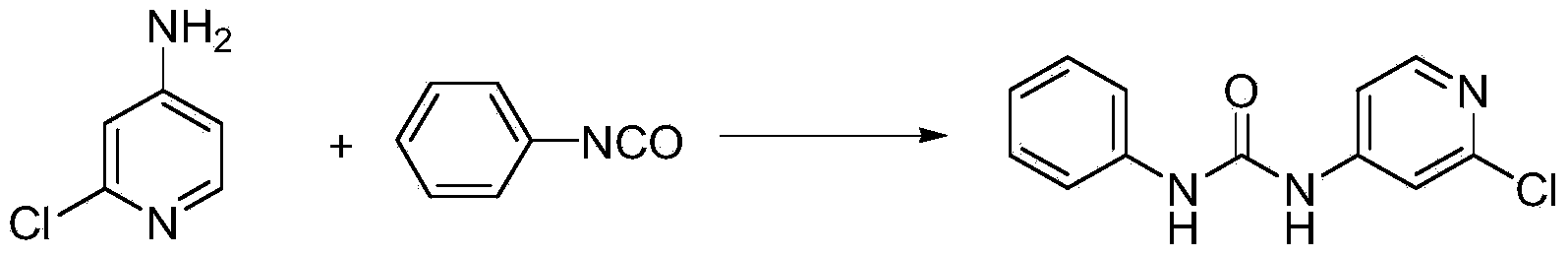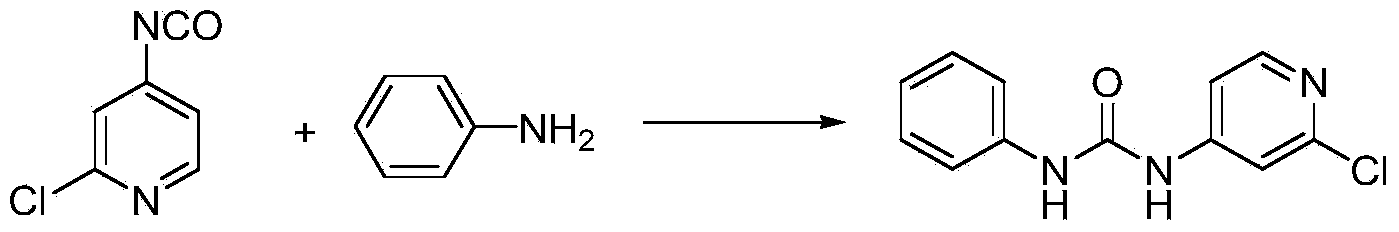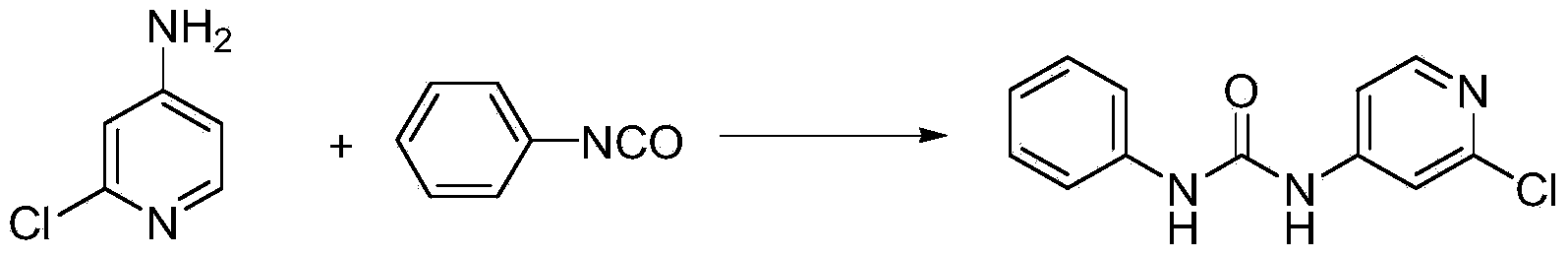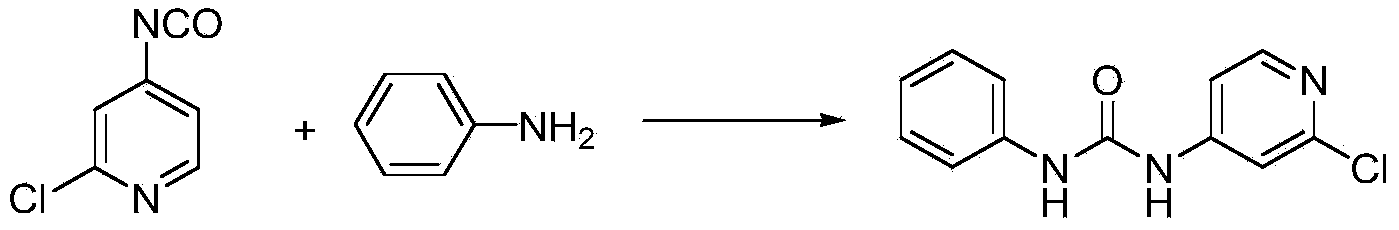Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Phenyl chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenyl chloroformate has been used in the synthesis of poly(2-(phenoxycarbonylox y)ethylmethacrylate) and phenyl-(4-vinylphenyl)car bonate. General description Kinetics of spontaneous hydrolysis of phenyl chloroformate has been studied in water-ethylene glycol, cationic, zwitterionic, nonionic and anionic micellar solutions.
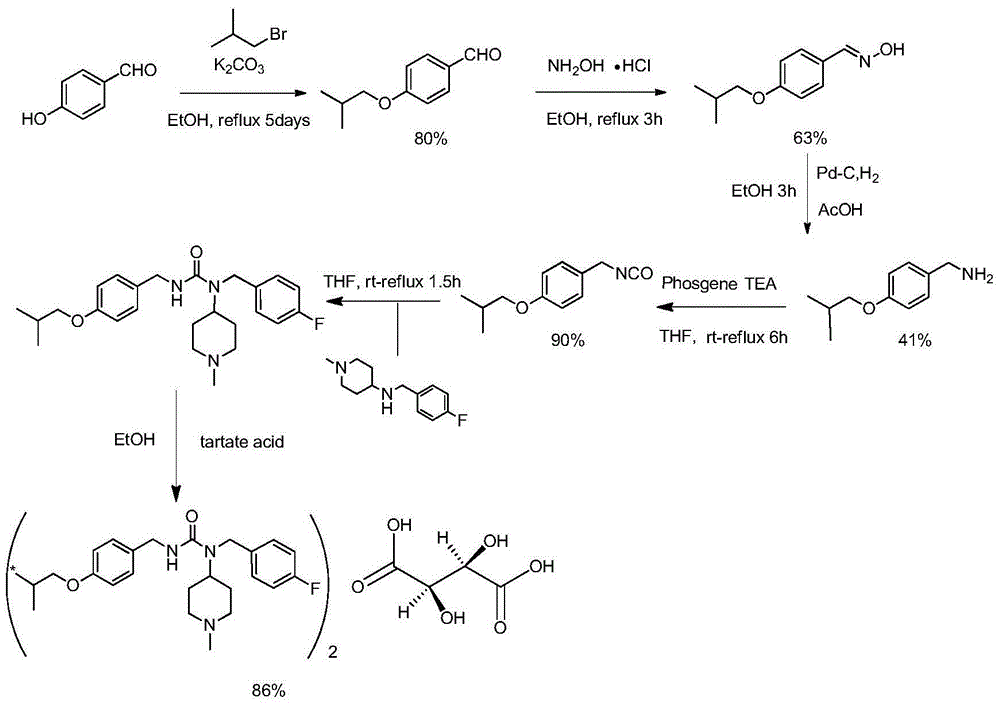
Synthetic method of tartrate of N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-isobutoxybenzyl)urea
ActiveCN104961672ARaw materials are easy to getSimple and fast operationCarbamic acid derivatives preparationOrganic compound preparationCarbamate4-hydroxybenzylamine
The invention belongs to the technical field of medicine, and relates to a synthetic method of a tartrate of N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-isobutoxybenzyl)urea. According to the synthetic method, 4-hydroxybenzylamine is taken as a raw material, and N-protecting group-4-isobutoxybenzylamine is obtained via amino protection and hydrocarbylation reaction; key intermediate 4-isobutoxybenzylamine is obtained via deprotection reaction; N-(4-isobutoxybenzyl)phenyl carbamate is obtained via reaction with phenyl chloroformate; N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-isobutoxybenzyl)urea is prepared via ammonolysis reaction with another intermediate N-(4-fluorobenzyl)-1-methyl-4-piperidylamine; and the tartrate of N-(4-fluorobenzyl)-N-(1-methylpiperidine-4-yl)-N'-(4-isobutoxybenzyl)urea is obtained via salifying with tartaric acid. The raw materials are easily available; operation is simple and mild; production period is short; yield is high; safety is high; cost is low; and the synthetic method is suitable for industrialized production.
Owner:SHENYANG PHARMA UNIVERSITY +1
Method for synthesizing Nexavar
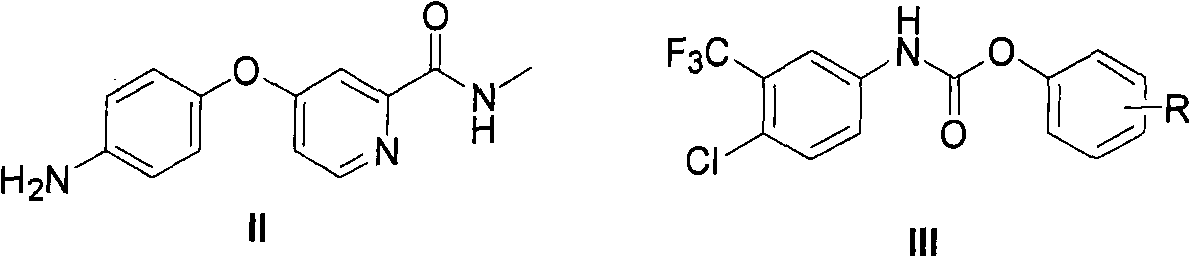
InactiveCN101671299AReduce usageHigh purityOrganic chemistryAntineoplastic agentsOrganic solventHydrogen
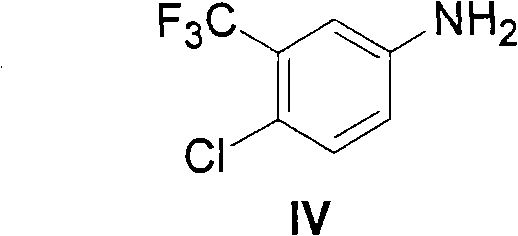
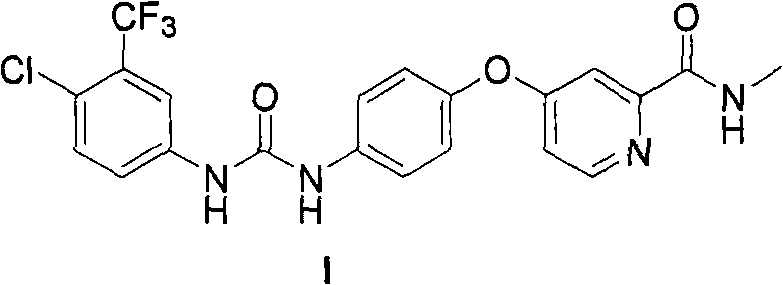
The invention provides a method for synthesizing Nexavar. The method comprises the following steps: a, dissolving a compound II and a compound III in an organic solvent inert to the compound III and adding appropriate base; b, performing reaction at a reaction temperature between 40 and 150 DEG C so as to generate a crude Nexavar product; and c, performing conventional post-treatment on the crudeproduct, wherein R is hydrogen, methyl, nitro or chlorine; the nitro is on site 4; the methyl or the chlorine is on site 2, 3 or 4; and the compound III is prepared through the reaction of a compoundIV and phenyl chloroformate or the phenyl chloroformate containing substituents on benzene rings in an organic reaction solvent inert to chloroformate at a temperature between 10 DEG C below zero and50 DEG C. The method has the advantages of applying to the preparation of Nexavar on an industrial scale, meeting standards in pharmaceutical industrial production, improving the purity and environmental compatibility of products and improving operability, safety and yield.
Owner:SHANGHAI PUYI CHEM CO LTD
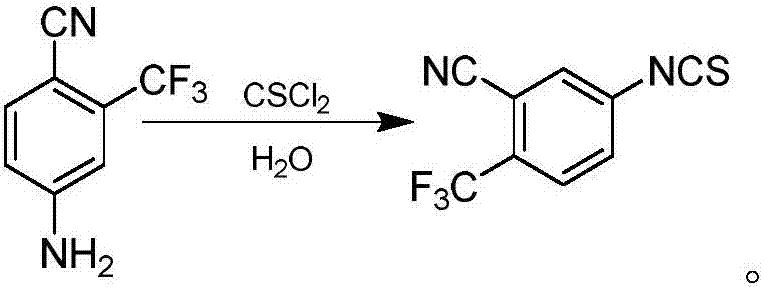
Synthetic method of 4-isothiocyanato-2-(trifluoromethyl) benzonitrile
InactiveCN107400073ALow priceSuitable for industrial productionOrganic chemistryOrganic solventToluene
The invention discloses a method for synthesizing 4-isothiocyano-2-(trifluoromethyl)benzonitrile. The method is divided into the following two steps: step 1, using 3-trifluoromethyl-4-cyano Aniline (I) is the starting material, reacts with phenyl thiochloroformate in organic solvents such as dichloromethane to obtain intermediate (II); step 2, intermediate (II) reflux reaction in toluene to remove a molecule Phenol obtains formula (III) product 4-isothiocyanato-2-(trifluoromethyl)benzonitrile, and the reaction formula is as follows: the inventive method has low cost, safety and environmental protection, simple and easy operation, and is suitable for industrialized production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
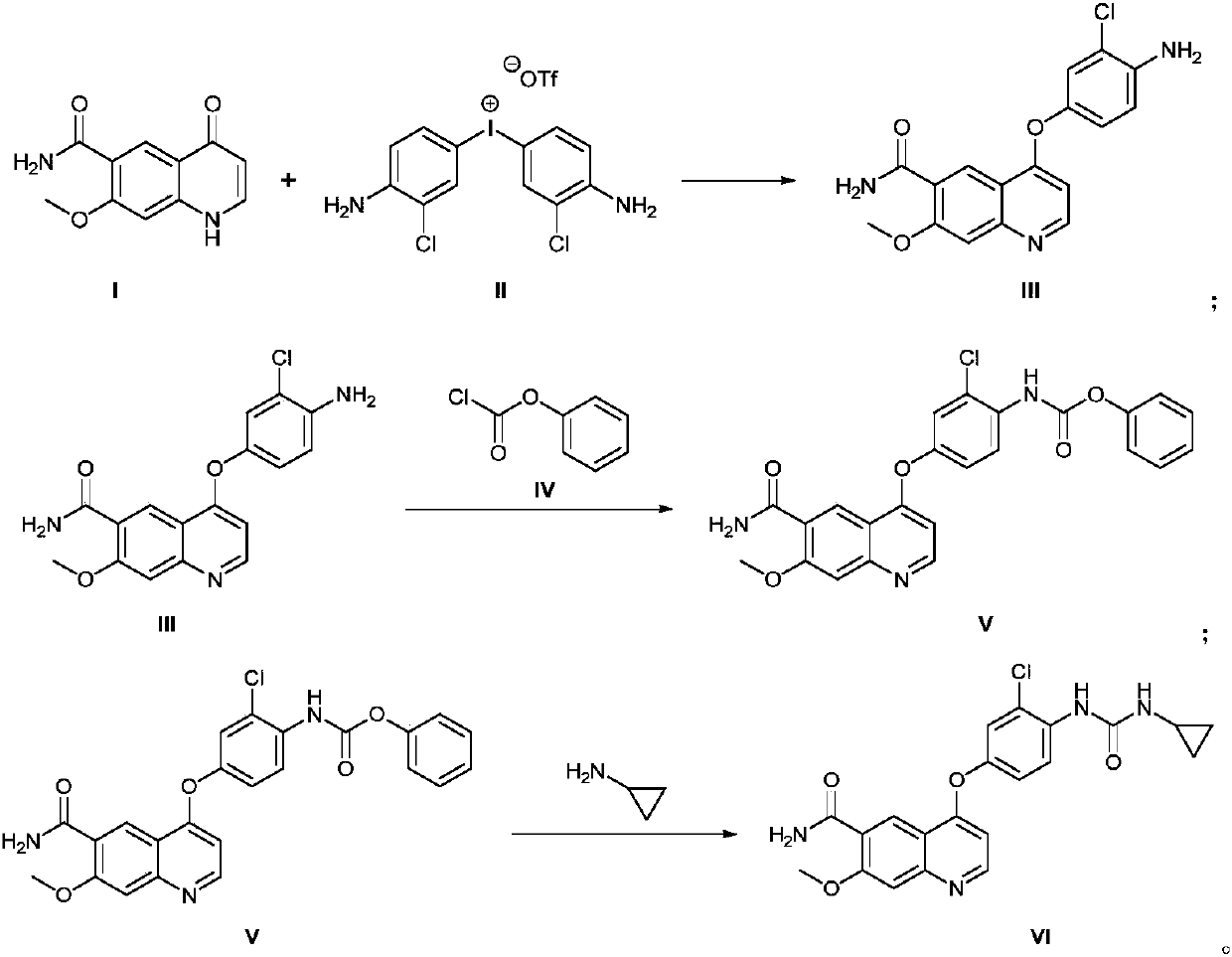
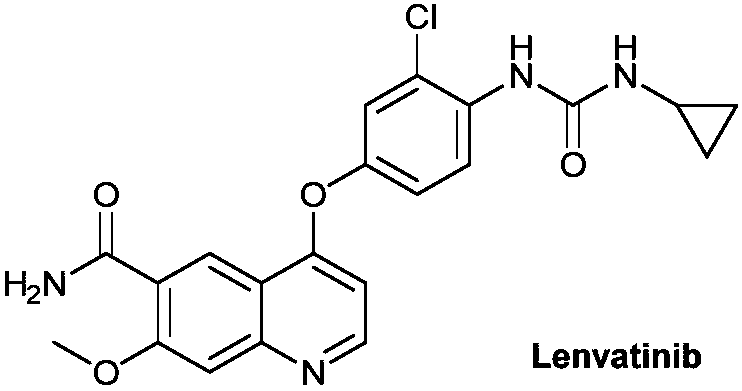
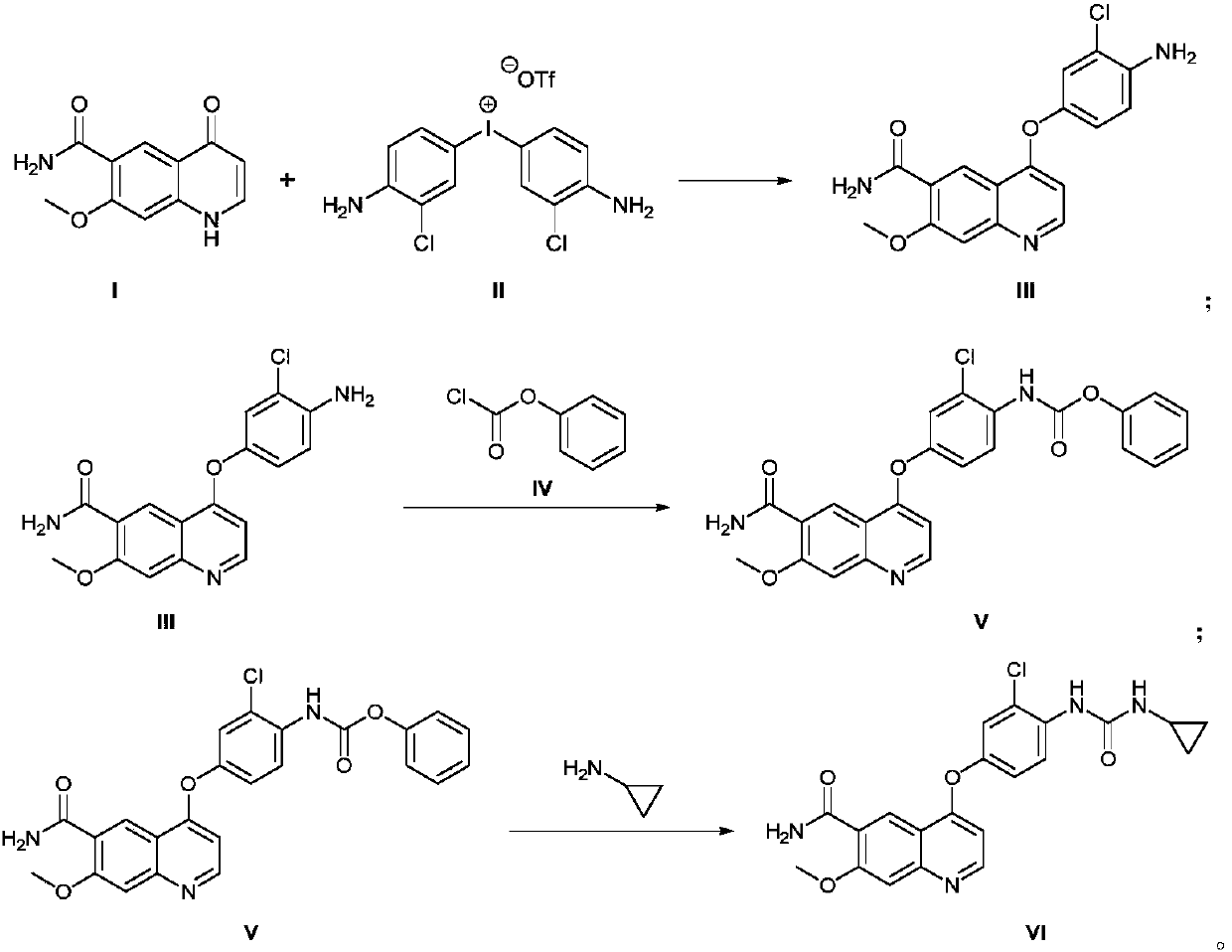
Method for synthesizing lenvatinib
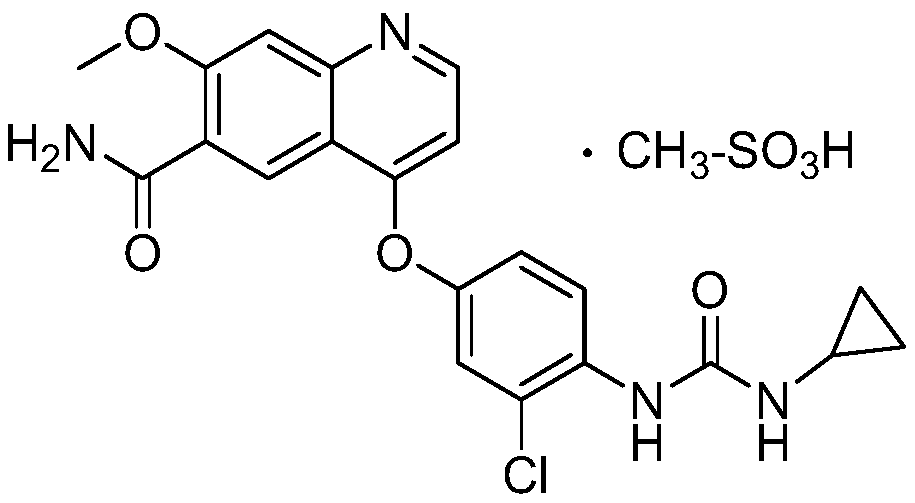
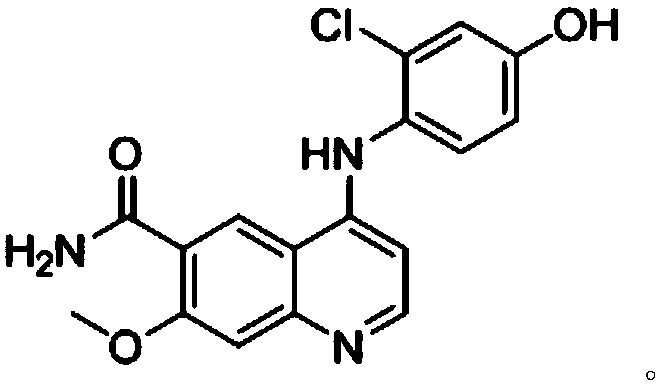
The invention discloses a method for synthesizing lenvatinib. The method comprises the following steps: with 7-methoxy-4-oxo-1,4-dihydro-quinoline-6-formamide as an initial raw material, carrying outan oxo-arylation reaction on the initial raw material and bis(3-chloro-4-amino)phenyl iodine trifluoro-methanesulfonate, carrying out an amidation reaction between 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-formamide, a product of oxo-arylation reaction, and phenyl chloroformate, and carrying out a urea reaction on a product of amidation reaction and cyromazine, thereby obtaining lenvatinib. The synthetic method has few process route steps and is simple in operation, 7-methoxy-4-oxo-1,4-dihydro-quinoline-6-formamide innovatively replaces 4-chloro-7-methoxyquinoline-6-formamide to serve as the initial raw material, and the method is environmental-friendly and is applicable to industrialized production.
Owner:NANJING CHICO PHARMA CO LTD
Method for synthesizing lenvatinib
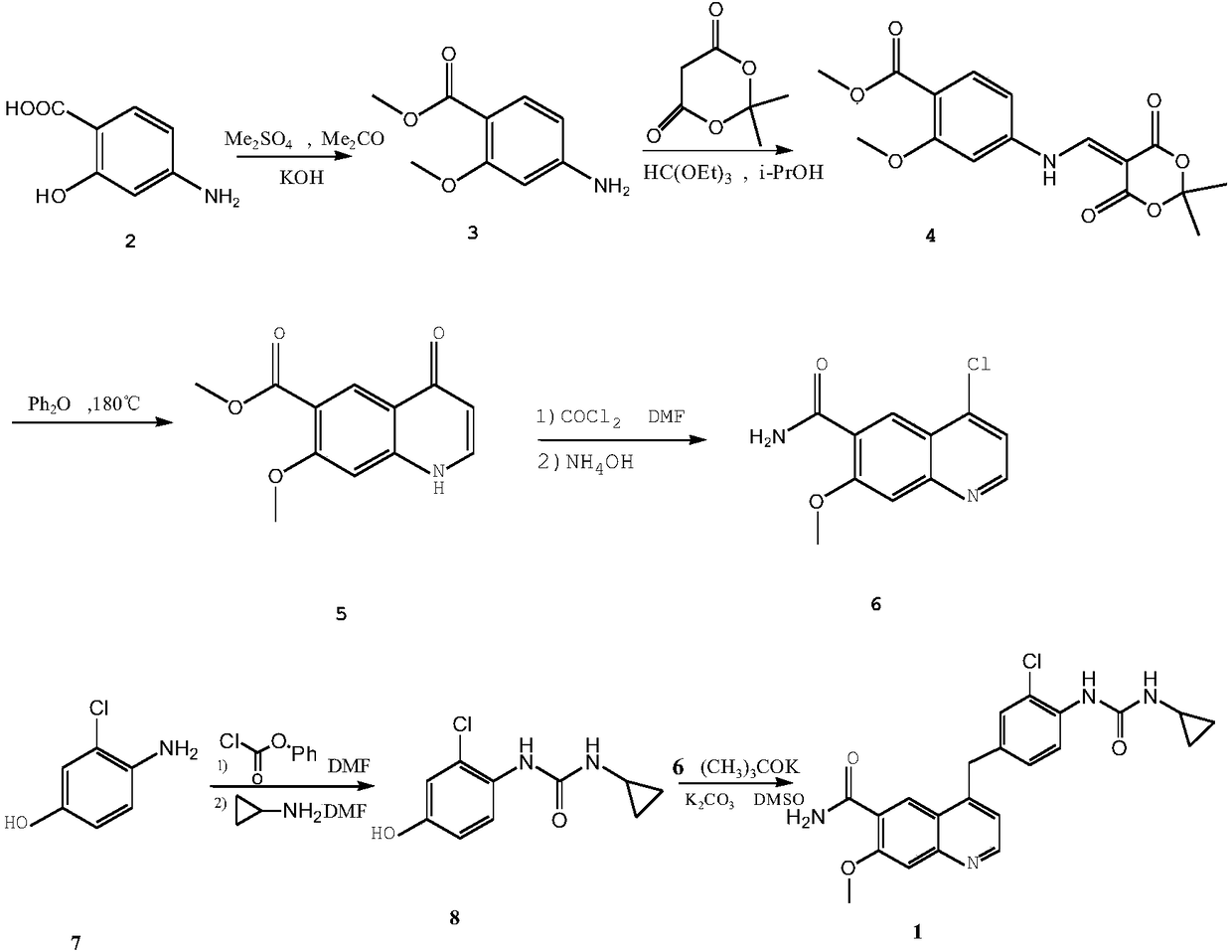
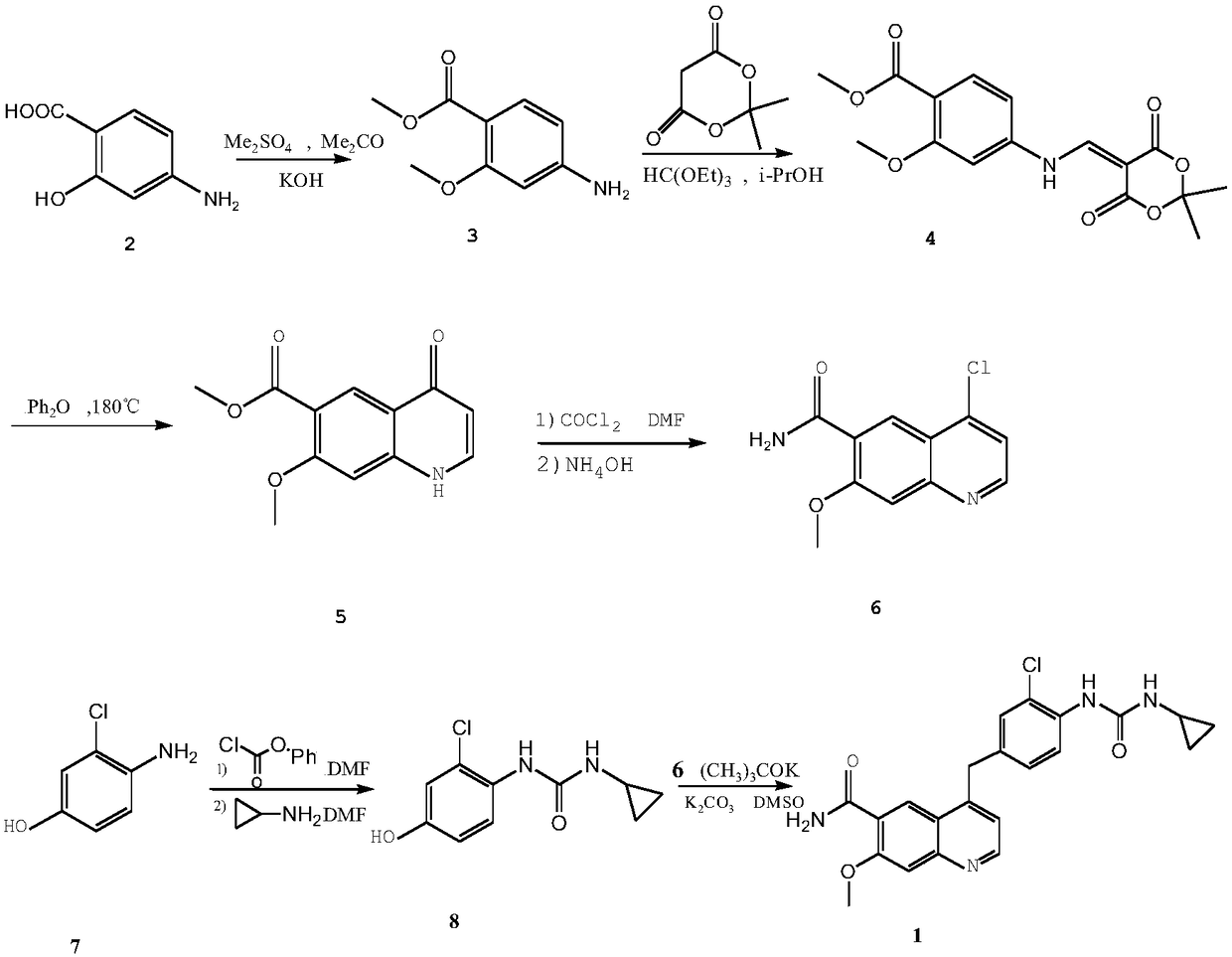
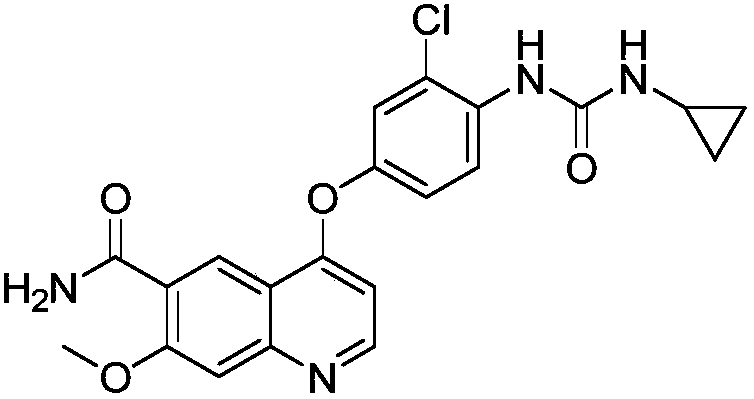
The invention belongs to the field of chemical pharmacy, and specifically relates to a method for synthesizing lenvatinib. The method comprises the following steps: step 1, taking 4-aminosalicylic acid as a raw material, and preparing 4-chloro-7-methoxyquinoline-6-formamide through methylation, condensation with meldrum's acid, high-temperature cyclization, chlorination and ammoniation; step 2, taking 3-chloro-4-aminophenol as a raw material, and reacting with phenyl chloroformate and cyclopropylamine to obtain 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea; and step 3, enabling the 4-chloro-7-methoxyquinoline-6-formamide prepared in step 1 to react with the 1-(2-chloro-4-hydroxy phenyl)-3-cyclopropyl urea prepared in step 2 under action of potassium tert-butoxide to obtain the lenvatinib. The invention provides a brand-new route for synthesising the lenvatinib. The used reagent is cheap and is easily available, is simple in operation, has a yield higher than that of other methods, and is easy for industrial production.
Owner:南京天越星生物技术有限公司
Environment-friendly novel technology for synthesizing high content nicosulfuron
InactiveCN103483318ADoes not affect product qualityThe reaction device is simpleOrganic chemistryEthyl chloroformatePtru catalyst
The invention provides an environment-friendly novel technology for synthesizing high content nicosulfuron. According to the invention, phenyl chloroformate or diphenyl carbonate is adopted to substitute ethyl chloroformate or methyl clhlorofonmate, which is a toxic substance; organic catalyst or inorganic catalyst is adopted; dichloromethane, dichloroethane, or acetonitrile is used as a solvent; N,N-dimethyl-2-amidosulfonyl-picolinamide, 2-amidogen-4,6,-dimethoxy-pyrimidine, catalyst I, and the solvent are added into a reaction kettle as whole; adding phenyl chloroformate or diphenyl carbonate and catalyst II sequentially at room temperature; performing heating reflux to obtain the product. The yield is above 90%, and the content is above 98%.
Owner:ANHUI FENGLE AGROCHEM
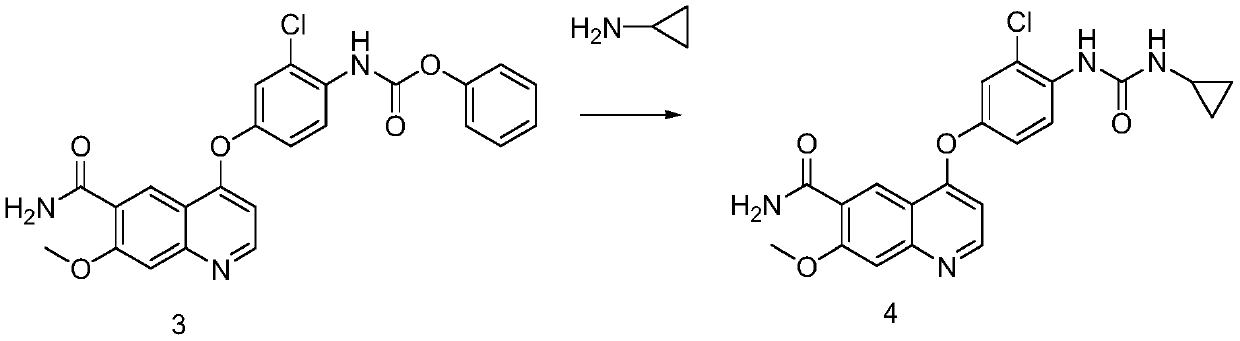
Preparation method of lenvatinib and its salts
The invention discloses a preparation method of lenvatinib and its salts, wherein N-methylpyrrolidone is used as a reaction solvent in the step of condensation with phenyl chloroformate. The preparation method has the advantages that the danger is successfully avoided that N,N-dimethylformamide and phenyl chloroformate react to produce massive gas during large-scale production; the preparation method is stable, the reaction time is short so that better safety is ensured, posttreatment is facilitated, and quality control is facilitated for the production of active pharmaceutical ingredients.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Complex reagent capable of removing tin in sewage
InactiveCN103086491AGood processing effectNo secondary pollutionWater/sewage treatmentMultistage water/sewage treatmentTriethyl orthoacetateCompound organic
The invention discloses a complex reagent capable of removing tin in sewage, belonging to the field of sewage treatment in environment protection. The complex reagent is formed by compounding organic ester and inorganic salt, wherein the organic ester refers to two or more of ethyl cyclopropanecarboxylate, trithyl orthoacetate, ethyl cyclopropanecarboxylate, phenyl chloroformate, and diisopropyl phosphate; and the inorganic salt refers to one or two of sodium bisulfide, ammonium sulfide, sodium hydrogen phosphate, sodium dihydrogen phosphate, sodium carbonate and magnesium carbonate. The complex reagent has the characteristics of being wide in available raw materials, low in cost, lower in reagent feeding amount, low in running cost, free from secondary pollution in a treatment process, high in tin removal efficiency, capable of achieving the removal rate by more than 99.9%, and the like.
Owner:CHANGZHOU UNIV
Preparation and separation purification methods of drug intermediate
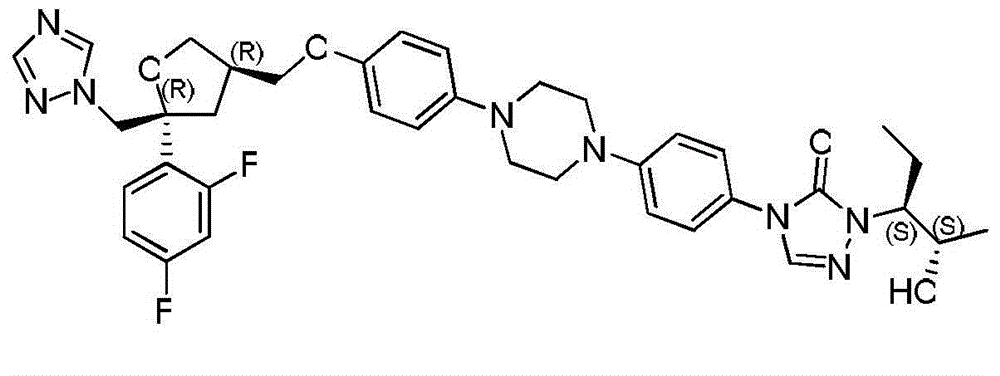
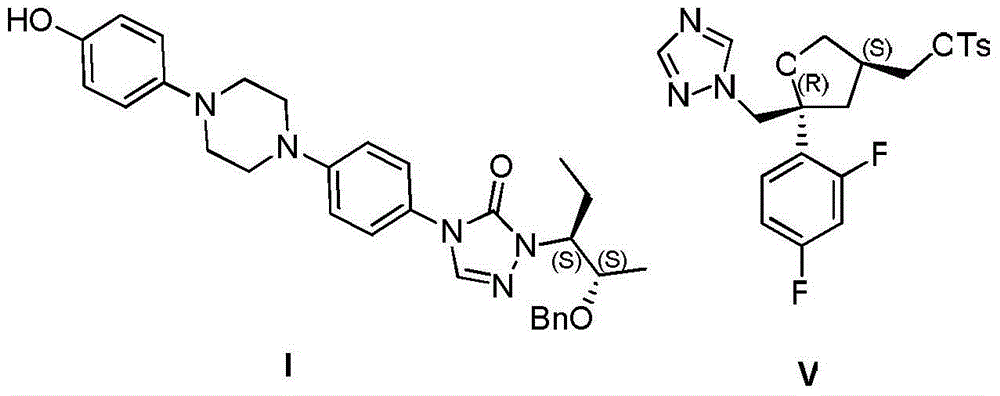
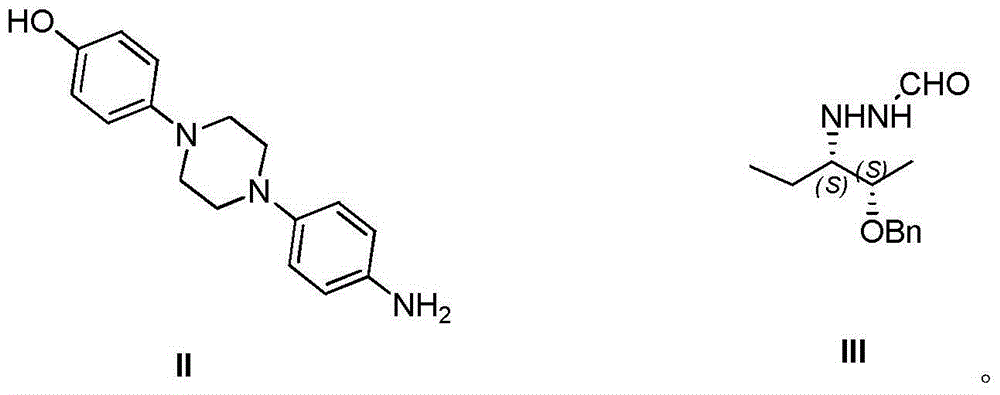
The invention provides a preparation method of 1-((2S,3S)-2-(benzyloxy) amyl-3-)-4-(4-(4-(4-hydroxyphenyl) piperazine-1-yl)-1H-1,2,4-triazole-5-(4H) ketone. The preparation method comprises: adding substituted or unsubstituted phenyl chloroformate into a reaction solution of a compound II for reaction for 12 hours at a temperature of -10 to 30 DEG C; and then adding a compound III and an alkali into the reaction system, and heating to 80 to 120 DEG C to continuously carry out reflux reaction for 5-24 hours. In comparison with the prior art, according to the preparation method provided by the invention, the process time is greatly saved, the total yield is improved by 10-15%, the discharge of three wastes is reduced, the energy source and cost are saved, and certain contributions can be generated for reduction of the synthetic cost of an important drug intermediate.
Owner:上海欣生源药业有限公司
Preparation method of tert-butyl carbazate
ActiveCN102911084AReduce pollutionSimple processOrganic chemistryTert-butyl carbazateMethyl carbazate
The invention discloses a preparation method of tert-butyl carbazate. The method includes: using phenyl chloroformate and tert-butanol as raw materials, performing esterification in ionic liquid at the temperature of 30-40 DEG C under the action of solid base catalysts, adding hydrazine hydrate into esterification liquid after esterification is completed, performing substitution reaction at the temperature of 60-75 DEG C, and subjecting the reaction liquid to separation and purification after the substitution reaction is finished so that the tert-butyl carbazate is obtained. The preparation method is simple in process, high in yield, easy to operate, free of phosphine ligands and less in environmental pollution.
Owner:苏州卫优知识产权运营有限公司
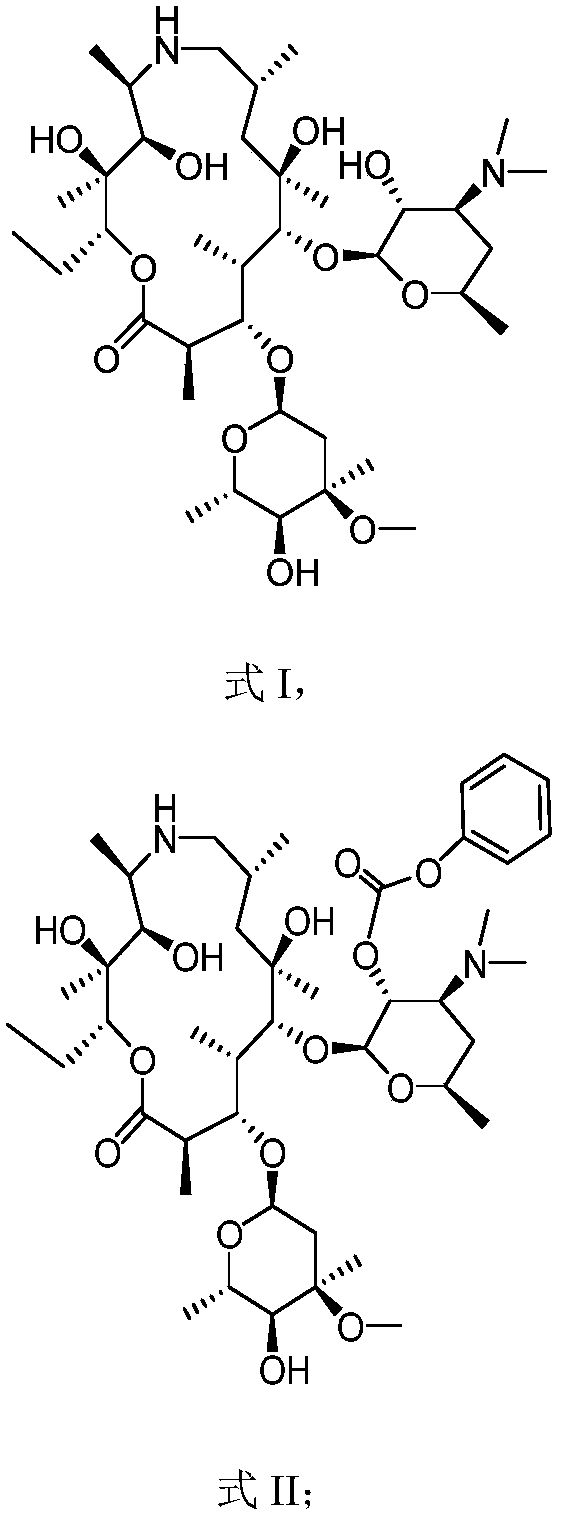
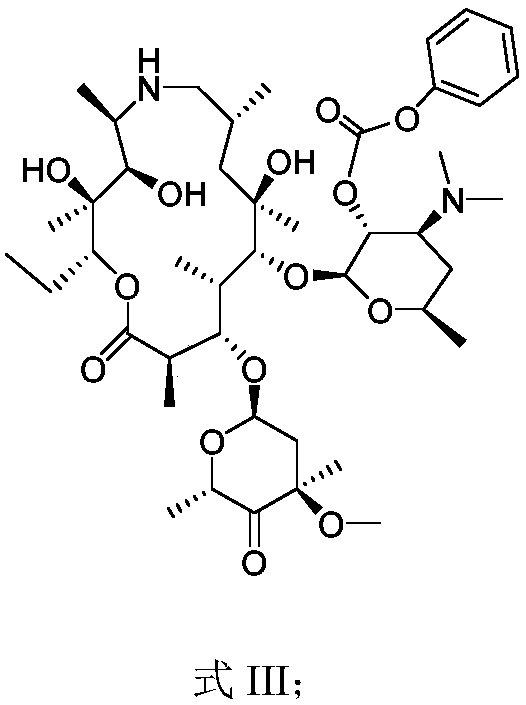
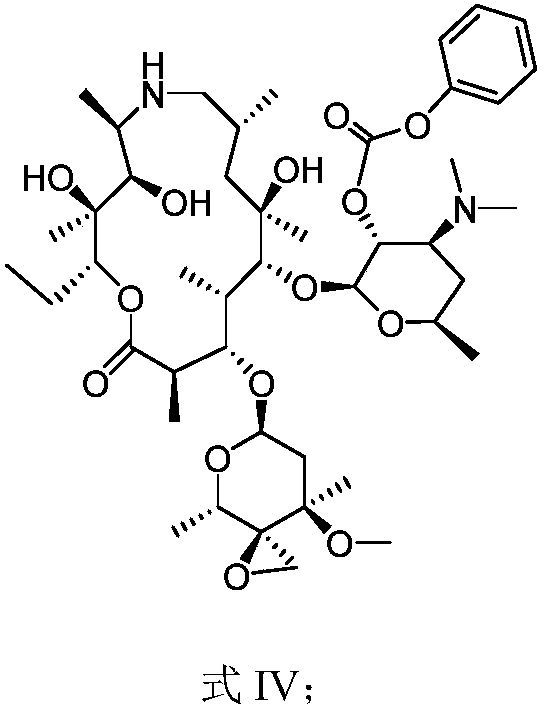
Preparation method of tulathromycin
InactiveCN110903335AHigh purityHigh yieldSugar derivativesSugar derivatives preparationAzithromycinMedicine
The invention provides a preparation method of tulathromycin, and belongs to the field of pharmaceutical chemicals. The method comprises the following steps of: reacting azithromycin serving as a rawmaterial with phenyl chloroformate to protect hydroxyl to obtain protected nitrogen azithromycin, oxidizing the hydroxyl into a ketone group by oxidation, epoxidizing, deprotecting, and reacting withn-propylamine to obtain tulathromycin. The product produced by the method has the characteristics of high purity, high yield, low cost, simple operation and stable process.
Owner:SUNSHINE LAKE PHARM CO LTD
Synthesis method of lenvatinib and new intermediate
InactiveCN111349045AStarting materials are cheap and readily availableOrganic chemistryChlorobenzenePhenyl chloroformate
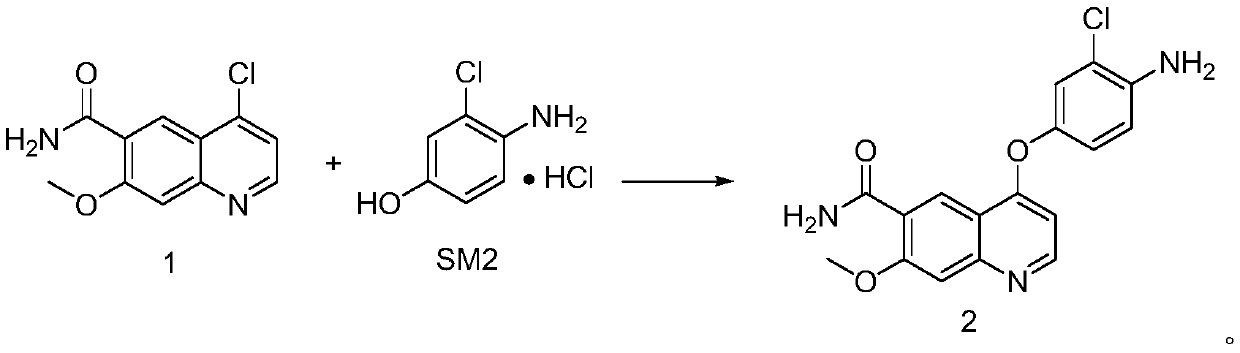
The invention discloses a synthesis method of lenvatinib and a new intermediate. The method comprises the following steps: step 1, taking 4-amino-3-chlorophenol hydrochloride and 4-chloro-7-methoxy-6-amido quinoline as initial raw materials, and carrying out a condensation reaction, so as to obtain a target product 4-(4-amino-3-chloro-phenoxy)-7-methoxy-6-carboxamide quinoline; carrying out amidation reaction on the obtained product and phenyl chloroformate to obtain 4-(4-(phenoxycarbonyl) amino-3-chloro-phenoxy)-7-methoxy-6-formamide quinoline, carrying out amidation reaction on the 4-(4-(phenoxycarbonyl) amino-3-chloro-phenoxy)-7-methoxy-6-formamide quinoline and cyclopropylamine to form urea to obtain lenvatinib, and carrying out salifying reaction with methanesulfonic acid to obtain lenvatinib mesylate. The synthesis method has few steps, is simple and convenient to operate, and omits Boc protection and deprotection steps by selecting a solvent. In the research process, it is foundthat for the reaction in the first step, a new compound 4-(4-hydroxy-2-chloro-anilino)-7-methoxy-6-carboxamide quinoline is obtained in a high-yield mode by replacing a solvent.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Preparation of duloxetine hydrochloride
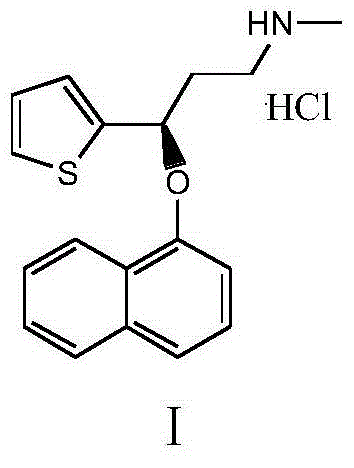
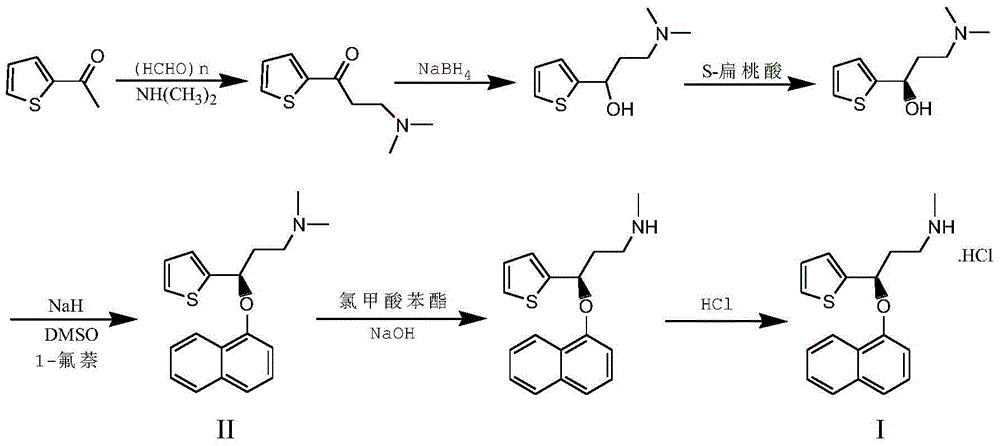
ActiveCN104829587AReduce salt forming stepsShorten the production cycleOrganic chemistryChloroformatePhenyl chloroformate
The invention belongs to the fields of organic chemistry and pharmaceutical chemistry, and particularly relates to a synthesis technique of duloxetine hydrochloride. By converting the R configuration compound into S configuration, compared with the duloxetine hydrochloride prepared from the single S-configuration compound, the total yield is enhanced by nearly 47%, and the production cost is lowered. 1-chloroethylchloroformate is used instead of phenyl chloro-formate to directly generate the duloxetine hydrochloride during demethylation, thereby reducing the salification step, shortening the production cycle and saving the cost.
Owner:SHANGHAI WANXIANG PHARMA
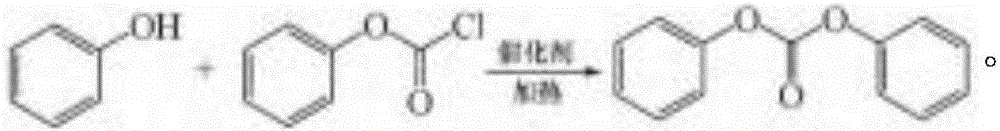
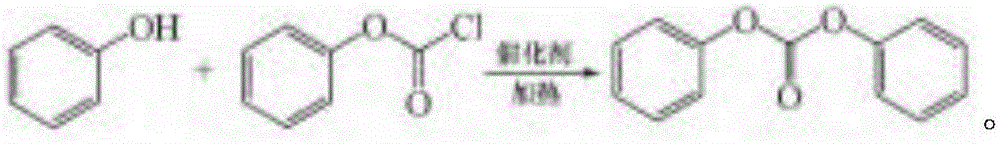
Synthetic method of phenyl chloroformate by taking phenol as raw material
ActiveCN106083584AAvoid it happening againHigh purityPreparation from phosgene or haloformatesMolten stateDistillation
The invention provides a synthetic method of phenyl chloroformate by taking phenol as a raw material. The method comprises the following steps: processing 94 Kg of phenol to a fusion state for standby; transferring the above phenol to a phenol addition pot, transferring a catalyst to a catalyst spraying pot; reducing the temperature at the bottom of a reaction pot to 0 DEG C, then adding triphosgene to the bottom of the reaction pot; finally employing nitrogen for expelling gas for the above reaction pot, and performing underpressure distillation to obtain phenyl chloroformate. The method can avoid the generation of a by-product diphenyl carbonate, avoids waste water generation, and further increases the purity of the product phenyl chloroformate.
Owner:ANHUI GUANGXIN AGROCHEM
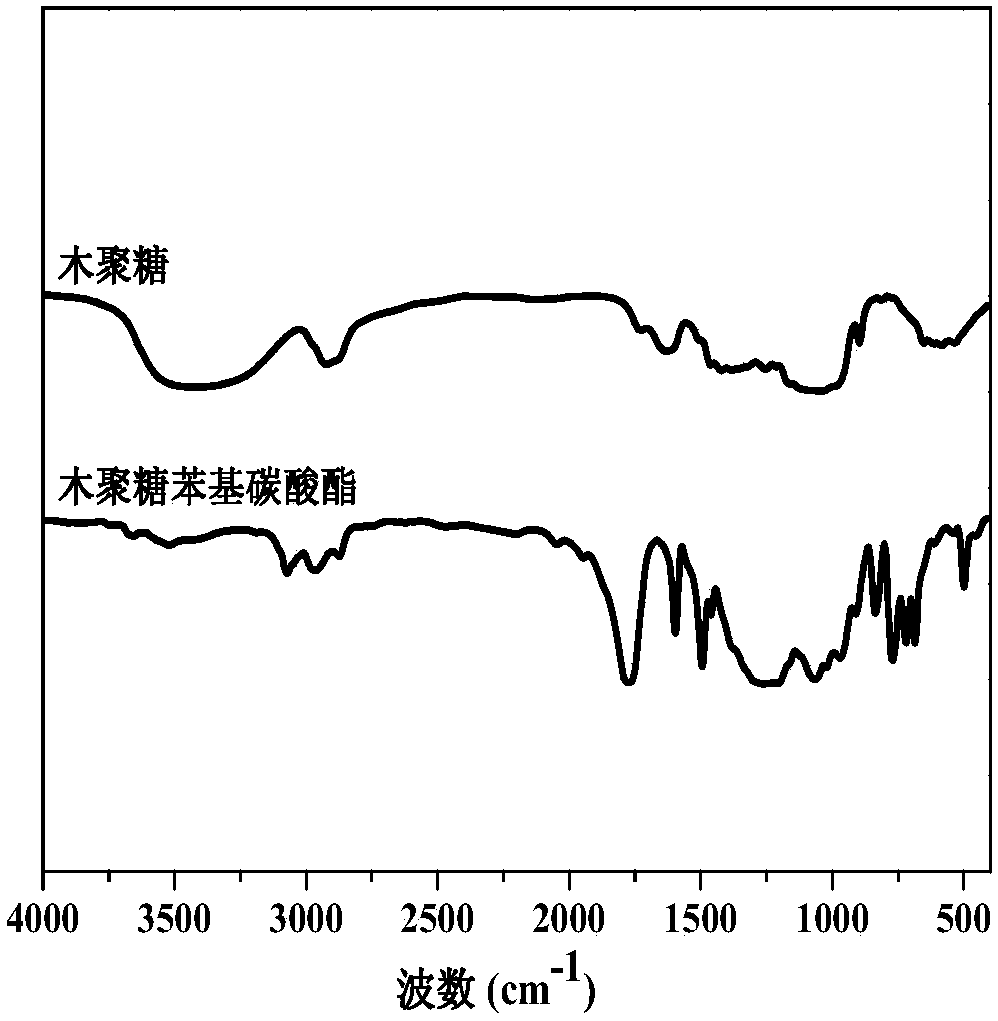
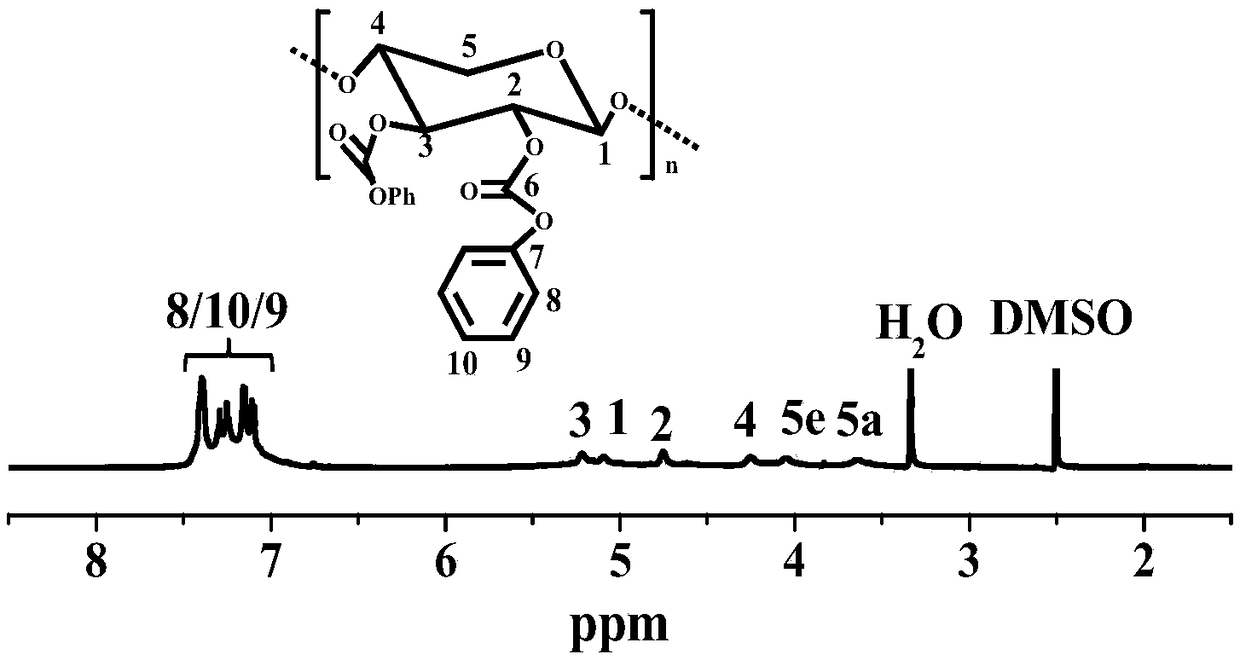
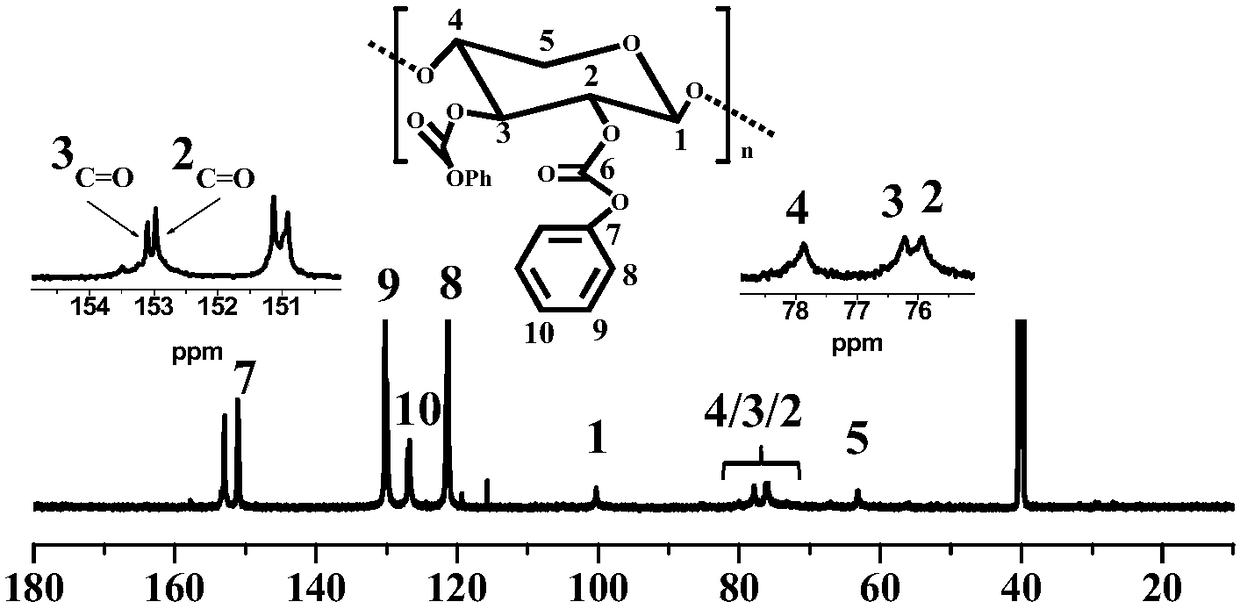
Preparation method of hemicellulose phenyl carbonate
The invention discloses a preparation method of hemicellulose phenyl carbonate, and belongs to the field of biomass material modification. The method comprises the following steps: firstly, dissolvinghemicellulose in ionic liquid, and adding pyridine; then, reacting with phenyl chloroformate to obtain a hemicellulose phenyl carbonate crude product; precipitating, washing and centrifuging the crude product with an organic solvent to obtain a final product. The preparation method of the hemicellulose phenyl carbonate disclosed by the invention has the advantages of high economical efficiency, high efficiency, simple preparation process, no side reactions, high yield of product and controllable degree of substitution, and can meet the needs of different substitution degrees of hemicellulosephenyl carbonate in different application scenes. Compared with hemicellulose, the hemicellulose phenyl carbonate has significantly higher solubility, thermal stability and the like; moreover, as a structurally-controllable active intermediate, the hemicellulose phenyl carbonate can further realize various modifications of hemicellulose through nucleophilic substitution, and has a certain application value in the field of biomass high-value conversion.
Owner:SOUTH CHINA UNIV OF TECH
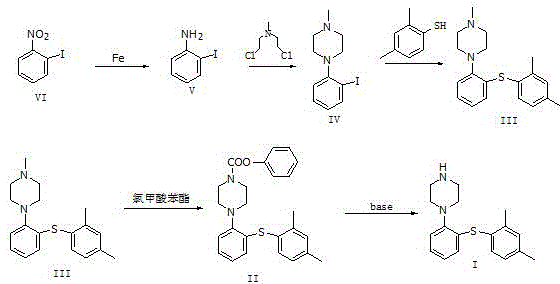
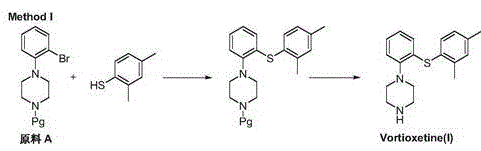
Novel method for preparing vortioxetine
InactiveCN105541759ASuitable for industrial productionEco-friendly economyOrganic chemistryPhenylpiperazineCarboxylic acid
The invention relates to a novel method for preparing vortioxetine. Reduction, nucleophilic substitution and coupling are conducted on a 2-nitroiodobenzene compound, and a compound of 1-[2-(2,4-dimethyl phenyl sulfanyl)-phenyl]methyl piperazine; then the obtained compound reacts with phenyl chloroformate to obtain 1-[2-(2,4-dimethyl phenyl sulfanyl)-phenyl]piperazine-1-carboxylic acid phenyl ester which generates vortioxetine after being hydrolyzed under the alkaline condition.
Owner:万全万特制药(厦门)有限公司
Preparation of 4,6-dimethoxy-2-((phenoxy carbonyl) amino) pyrimidine
The invention relates to a Process for preparing 4,6-dimethoxy-2-((phenoxycarbonyl)amino)pyrimidine comprising reacting 2-amino-4,6-dimethoxy pyrimidine and phenyl chloroformate in an inert solvent in the presence of an acid receptor.
Owner:EI DU PONT DE NEMOURS & CO
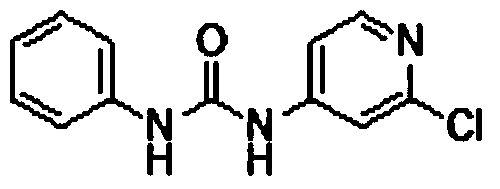
Preparation method of 1-(2-chloro-4-pyridyl)-3-phenylurea
The invention discloses a preparation method of a phenylurea-type cytokinin high-efficiency plant growth regulator pyridyl phenylurea of which the chemical name is 1-(2-chloro-4-pyridyl)-3-phenylurea (also called CPPU), which comprises the following steps: reacting 2-chloro-4-aminopyridine and phenyl chloroformate to obtain phenyl 2-chloropyridyl-4-carbamate, and reacting with aniline to obtain the CPPU. The method has the advantages of accessible raw materials, fewer byproducts, high reaction yield and high product content, is simple to operate, greatly lowers the production cost, and is suitable for industrial production.
Owner:ZHEJIANG TIDE CROP TECH
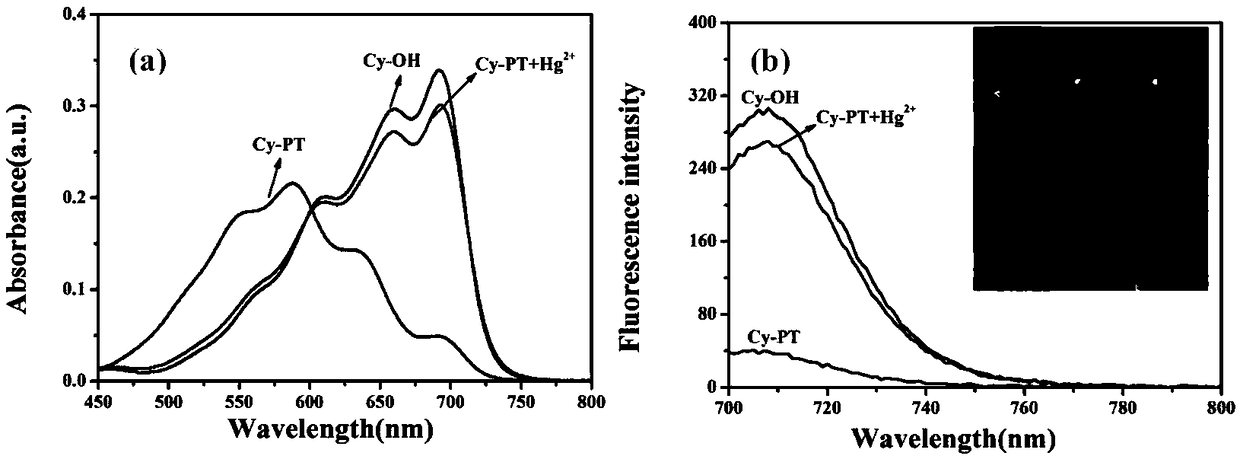
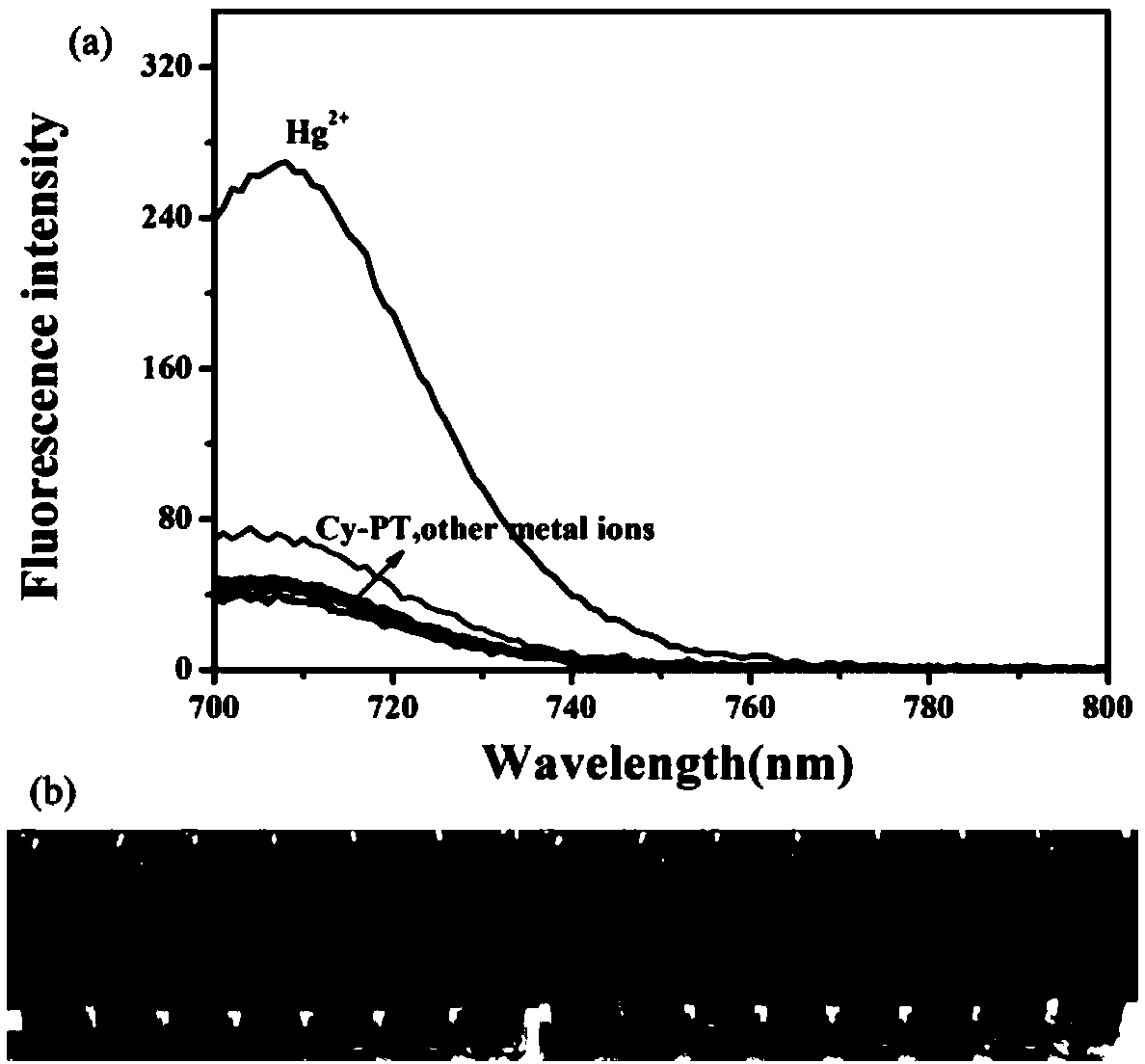
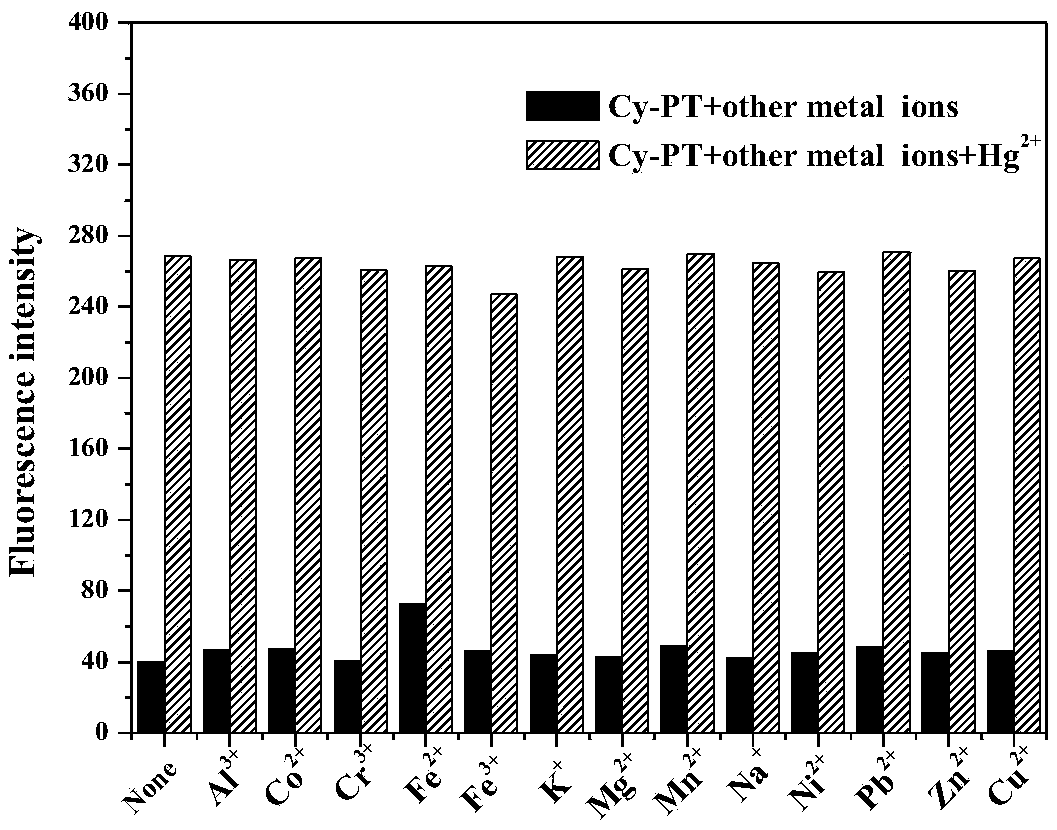
Hemicyanine-based reactive Hg<2+> fluorescent probe Cy-PT and preparation method and application thereof
InactiveCN109438426ALow biological toxicityHigh selectivityOrganic chemistryFluorescence/phosphorescenceFluorescenceLiving body
The invention discloses a reactive Hg<2+> fluorescent probe Cy-PT and a preparation method and application thereof. In the reactive Hg<2+> fluorescent probe Cy-PT, phenyl thiochloroformate serving asan identification unit is coupled to a stable hemicyanine framework via an ester bond to form the fluorescent probe Cy-PT; and generated thioester is hydrolyzed under the catalysis of Hg<2+>, and theester bond is broken to expose a hydroxyl group. The probe fluorescence is enhanced due to an intramolecular ICT mechanism. The probe can specifically recognize Hg<2+> in a physiological pH environment, and can be used for colorimetric detection of Hg<2+> as the solution turns from indigo to lake blue. In addition, the cytotoxicity test of the reactive Hg<2+> fluorescent probe Cy-PT proves that the probe has low biotoxicity, and a cell imaging experiment shows that the probe can detect mercury ions in a living complex biological system, which indicate that the reactive Hg<2+> fluorescent probeCy-PT has a development potential in living body detection.
Owner:HUBEI UNIV
Method for synthesizing pymetrozine intermediate (oxadiazole ketone) by utilizing halogenated formate ester
The invention discloses a method for synthesizing a pymetrozine intermediate (oxadiazole ketone) by utilizing halogenated formate ester. According to the method, oxadiazole ketone is generated through a ring-closure reaction between acethydrazide and halogenated formate ester under certain conditions. Phosgene, diphosgene or triphosgene which can easily trigger group safety accidents is prevented from being utilized as a process route for the ring-closure reaction; such halogenated formate ester as phenyl chloroformate or phenyl chloroformate is utilized for replacing previously common virulent phosgene, diphosgene or triphosgene as a carbonylation ring-closure reagent; under the action of catalysts, a ring-closure cyclization reaction is performed for preparation of oxadiazole ketone; generation of virulent phosgene during a production process is avoided, so that major hidden dangers are reduced; the method has the advantages that technology is safe and reliable, reaction conditions are mild and easy to control, after-treatment is convenient, and an environmental-friendly effect is achieved.
Owner:兰州鑫隆泰生物科技有限公司
Preparation method of 1-(2-chloro-4-pyridyl)-3-phenylurea
The invention discloses a preparation method of a phenylurea-type cytokinin high-efficiency plant growth regulator pyridyl phenylurea of which the chemical name is 1-(2-chloro-4-pyridyl)-3-phenylurea (also called CPPU), which comprises the following steps: reacting aniline and phenyl chloroformate, and reacting with 2-chloro-4-aminopyridine to obtain the target compound. The method has the advantages of accessible raw materials, fewer byproducts, high reaction yield and high product content, is simple to operate, greatly lowers the production cost, and is suitable for industrial production.
Owner:ZHEJIANG TIDE CROP TECH
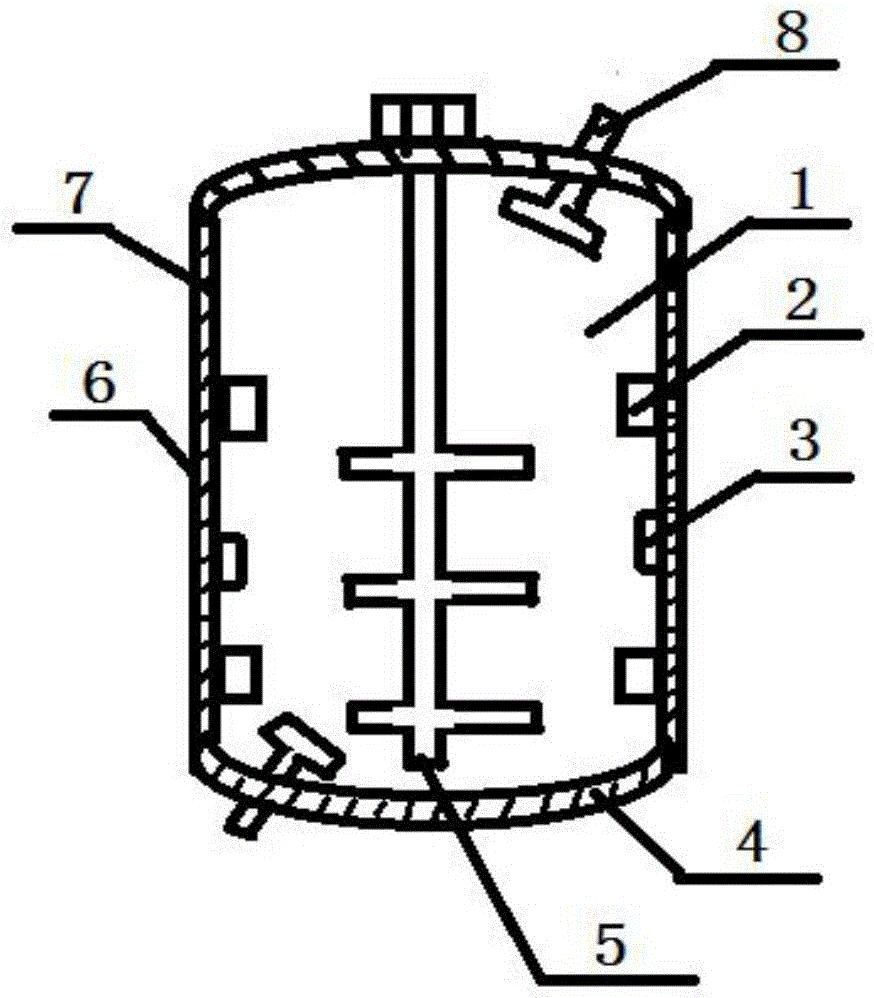
Reaction kettle for phenyl chloroformate
InactiveCN106076231AExtended service lifeAvoid problemsOrganic compound preparationChemical/physical/physico-chemical stationary reactorsChemistryPhenyl chloroformate
A reaction kettle for phenyl chloroformate is characterized by comprising a reaction kettle body, four heating devices, a temperature measuring device, a stirrer and fans; the four heating devices are located on the left wall and the right wall of the reaction kettle respectively and are symmetrical; the outer wall of the reaction kettle is coated with a heat preservation and heat insulation coating, and the inner wall of the reaction kettle is coated with a high-temperature-resisting anticorrosive coating; the space between the outer wall and the inner wall of the reaction kettle is full of jacket steam; the fans are arranged on the upper right portion and the lower left portion of the reaction kettle. The stirrer is provided with a plurality of stirring paddles, materials can be mixed more evenly, and therefore the reaction process is accelerated. Due to the fact that the fans are installed at the different positions in the reaction kettle, the temperatures at all positions in the reaction kettle can be kept consistent as much as possible, and the situation that local temperature is too high, consequently side reactions are caused is avoided.
Owner:ANHUI GUANGXIN AGROCHEM
Phenyl chloroformate production technique
ActiveCN105949060AAvoid it happening againHigh purityPreparation from phosgene or haloformatesDistillationNitrogen
The invention provides a phenyl chloroformate production technique. The technique comprises the steps of firstly, slowly increasing the temperature of a stirring tank, then introducing phosgene constantly into the stirring tank from the middle of the side wall of the stirring tank, opening a phenol dropwise adding tank, and opening the stirring tank and a catalyst spray tank at the same time; secondly, continuing to introduce phosgene, and taking a central control sample; thirdly, continuously extracting the central control sample every other 30 min; fourthly, conducting air expelling with nitrogen on the stirring tank after final sampling is stopped; finally, conducting reduced pressure distillation on materials left after air expelling to obtain phenyl chloroformate. Compared with the prior art, the production technique has the advantages that reaction time is shortened, and purity and reaction efficiency are improved.
Owner:ANHUI GUANGXIN AGROCHEM
Synthetic method for preparing pure phenyl chloroformate with two-step method
InactiveCN105949058AAvoid using effectsAvoid recyclingPreparation from phosgene or haloformatesMolten stateState of art
The invention provides a synthetic method for preparing pure phenyl chloroformate with a two-step method. The method comprises main steps as follows: (1) phenol in a molten state and a small quantity of a catalyst DMF (dimethyl formamide) are added to a flask, stirring is started, phosgene is introduced at a certain temperature, activated carbon is added after complete reaction, nitrogen is introduced to a reaction solution, and crude phenyl chloroformate is obtained after the reaction ends; (2) obtained crude phenyl chloroformate is subjected to reduced pressure distillation, and pure phenyl chloroformate is obtained. Compared with the prior art, the method has the advantages as follows: aftertreatment is simple, use of large quantities of acid-binding agents and solvents is effectively avoided, no industrial waste water is produced, the cost is relatively low, the method is suitable for industrial production, and meanwhile, possible harm to the phosgene can be reduced through improvement of equipment.
Owner:ANHUI GUANGXIN AGROCHEM
Synthetic method of phenyl chloroformate as pesticide intermediate
InactiveCN106083594ASimple post-processingLow costPreparation from phosgene or haloformatesDistillationNitrogen
Owner:ANHUI GUANGXIN AGROCHEM
A kind of synthetic method of 4,6-dimethoxy-2-((phenoxycarbonyl)amino)-pyrimidine
ActiveCN103848791BEasy to recycleEmission reductionOrganic chemistryDimethylaniline N-oxideSynthesis methods
The invention provides a synthesis method for 4,6-dimethoxy-2-((phenoxy carbonyl) amino)-pyrimidine. According to the method, 2-amino-4,6-dimethoxy pyrimidine and phenyl chloroformate are used as raw materials, 1,4-dioxane, dimethyl carbonate or methyl tetrahydrofuran is used as a solvent, and in the presence of N,N-dimethylaniline, 4-(N,N- dimethylamino) pyridine (DMAP) is as a catalyst. The method disclosed by the invention is mild in reaction conditions; the average purity and yield of obtained products are high; and the recovery of the solvent is simple, so that the treatment pressure of the three wastes is small, and the production cost is further reduced.
Owner:INSIGHT FINECHEM +1
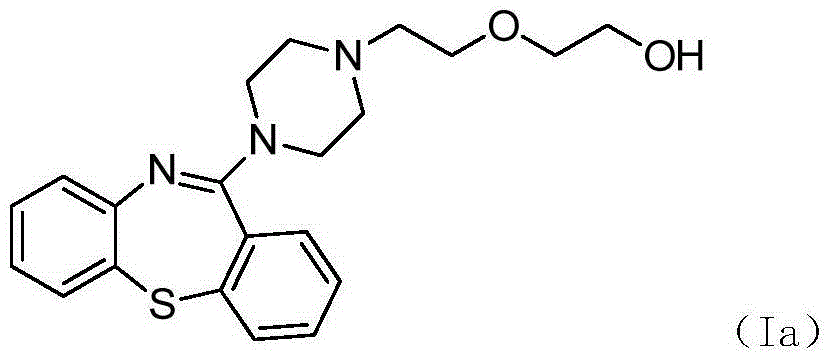
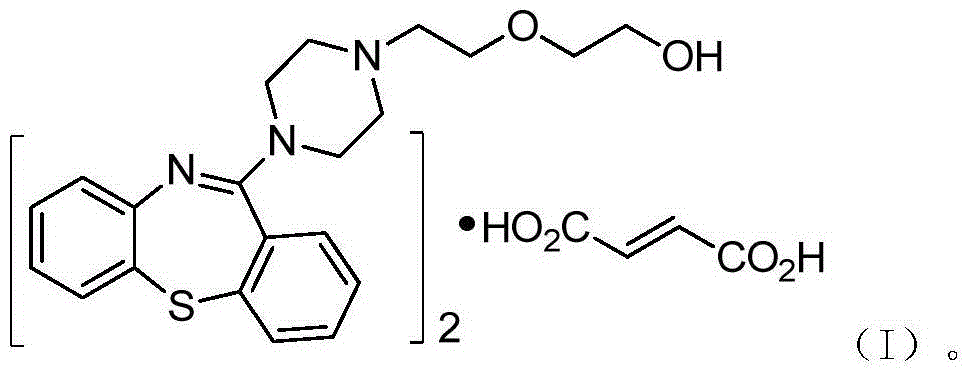
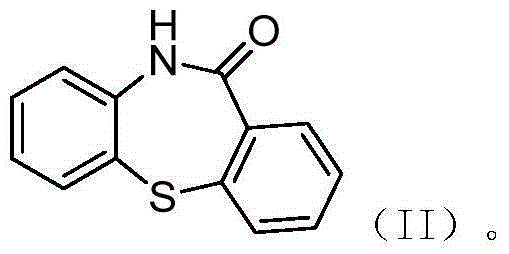
Preparation method of quetiapine intermediate
The invention relates to a method for preparing a compound as shown in a formula (II), namely 10H-dibenzo[b,f][1,4]thiazepine-11one, through 'one-pot process'. The formula is as shown in the specification and the 10H-dibenzo[b,f][1,4]thiazepine-11one comprises a compound as shown in a formula (III) as shown in the specification. The method comprises the following steps: carrying out an acylation reaction on 2-amino diphenyl sulfide and phenyl chloroformate; and after the reaction, without post-treatment or separation of a target intermediate product in the period, adding polyphosphoric acid (PPA) for a cyclization reaction, wherein the formula III is as shown in the specification. In a process of preparing the 10H-dibenzo[b,f][1,4]thiazepine-11one, the 'one-pot process' disclosed by the invention can be used for preventing a phenyl(2-thiophenyl)phenyl carbamate solid from being moved out of a reaction kettle, simplifying the operation course of the process and reducing the consumption of the reaction intermediate product on one aspect, and on the other aspect, the 'one-pot process' can be used for achieving the yield above 90%, preferably above 95%, and achieving the high performance liquid chromatography (HPLC) purity above 99.0%.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for preparing high purity 4,6-dimethoxy-2-((phenoxy carbonyl)amido)-pyrimidine
InactiveCN101423499BLow boiling pointResidue reductionOrganic chemistryDimethylaniline N-oxideBenzyl chloroformate
The invention relates to a method for preparing 4, 6-dimethoxy-2(phenoxyl carboxide)amido)-pyrimidine) (DPAP for short). The method is characterized by comprising the following steps: adopting tetrahydrofuran, ethyl acetate or glycol dimethyl ether as a reaction solvent; with the ratio of grams of the reaction to reaction initiator 2-amido-4, 6-dimethoxy pyrimidine of between 2 to 1 and 10 to 1, in the presence of N, N-dimethylaniline with the molar weight of between 1.1 and 1.5, preparing the DPAP by a method of leading the 2-amido-4, 6-dimethoxy pyrimidine with 1 molar weight to react with benzyl chloroformate with molar weight of between 1.1 and 1.5 at normal pressure and at a temperature of between 20 and 40 DEG C; distilling solvent after the reaction; then adding water to the solvent to separate out a product; or dissolving the reaction mixture of the distilled solvent by methanol, and adding water with 1 to 5 times amount of the methanol to separate out the product; and washingthe crude product by 1 time amount of the methanol to obtain the product. The method has the advantages that the average purity of the product is higher than the average yield; and the used solvent can be reclaimed, thereby not only saving production cost, but also reducing discharge of waste water.
Owner:JIANGSU POLYTECHNIC UNIVERSITY +1
Synthesis process of OAB-14
ActiveCN113754566AUrea derivatives preparationCarbamic acid derivatives preparationEthylene diaminePotassium carbonate
The invention provides a synthesis process of OAB-14. The synthesis process comprises the steps of (1) adding anhydrous potassium carbonate and dichloromethane into an intermediate 7 of OAB-14, uniformly stirring, dropwise adding phenyl chloroformate, heating to reflux, and keeping the temperature and refluxing until the reaction is finished; (2) stopping heating, adding purified water, cooling to room temperature in an ice bath, centrifuging, washing the solid with dichloromethane, pulping with dichloromethane, centrifuging, and drying to obtain a phenyl ester intermediate; and (3) mixing triethylamine, ethidene diamine and tetrahydrofuran, adding the phenyl ester intermediate prepared in the step (2) in batches at room temperature, heating to reflux, determining a reaction endpoint by TLC, cooling, cooling to the temperature of 5-10 DEG C in the ice bath, cooling and crystallizing for 1 hour, centrifuging, washing with dichloromethane, and drying to obtain the OAB-14.
Owner:SHANDONG XINHUA PHARMA CO LTD
Simple and efficient production method for phenyl chloroformate
InactiveCN105949059AReduce generationHigh purityPreparation from phosgene or haloformatesMolten stateTemperature control
The invention provides a simple and efficient production method for phenyl chloroformate. The production method comprises the following steps that firstly, phenol is treated into a molten state for use; secondly, phenol is transferred into a phenol spray tank, and a catalyst is transferred into a catalyst spray tank; thirdly, a circulating refrigerating machine is started, then a temperature control pipeline is controlled, a reaction tank is heated, phosgene is introduced into the reaction tank continuously, then the phenol spray tank is started, stirring is started, the catalyst spray tank is started, and central control samples are taken; finally, gas dispelling is carried out on the reaction tank with nitrogen, and phenyl chloroformate is obtained through reduced pressure distillation. Compared with an existing production technology, by means of the simple and efficient production method for phenyl chloroformate, the reaction time is shortened, and the purity and the reaction efficiency are improved.
Owner:ANHUI GUANGXIN AGROCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com