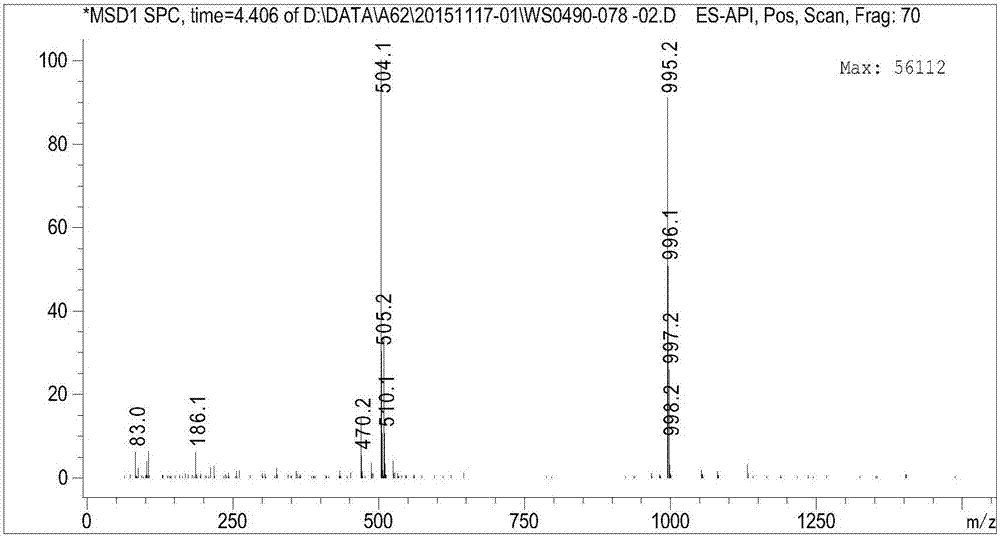
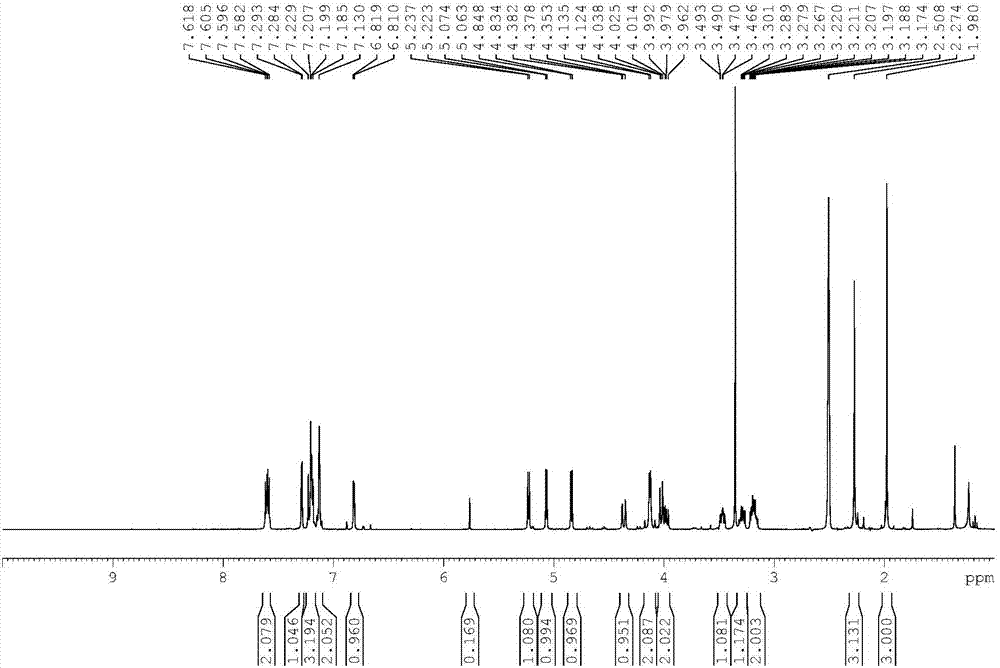
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
158results about How to "Starting materials are cheap and readily available" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
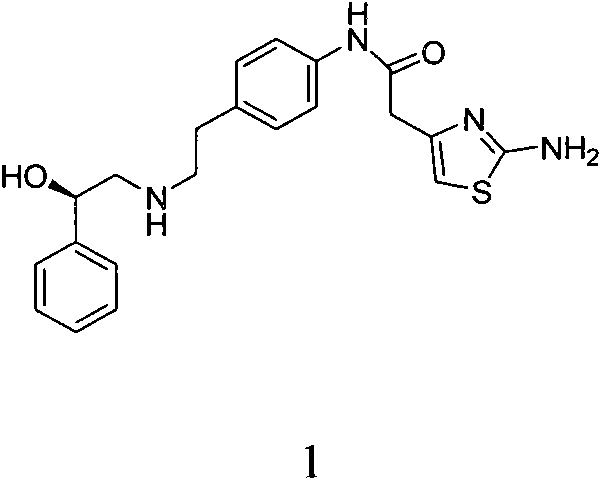
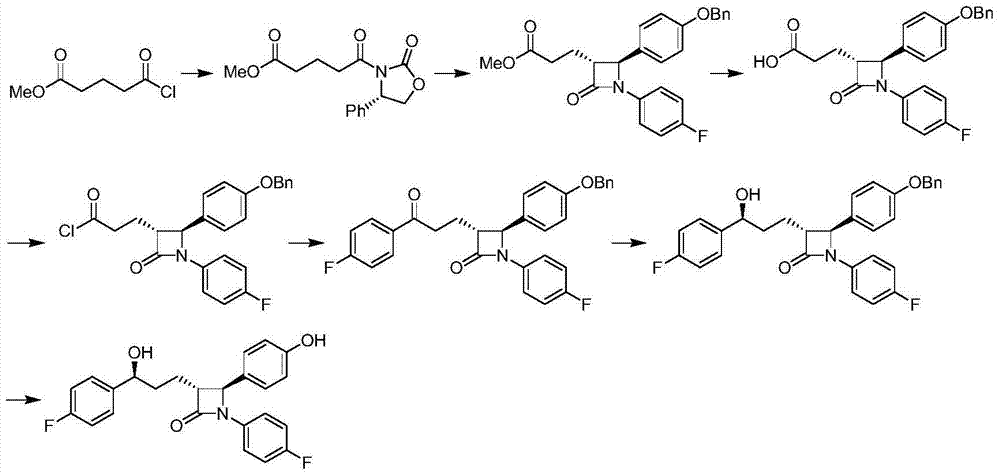
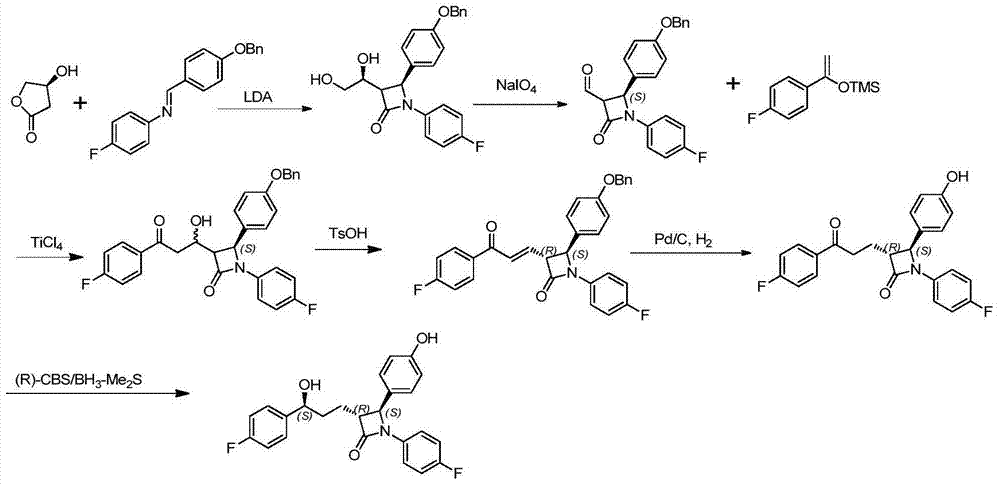
Ezetimibe synthesis method and Ezetimibe intermediate synthesis method
ActiveCN104513187AReduce usageStarting materials are cheap and readily availableGroup 4/14 element organic compoundsTert-butyldimethylsilyl chlorideOrganic solvent
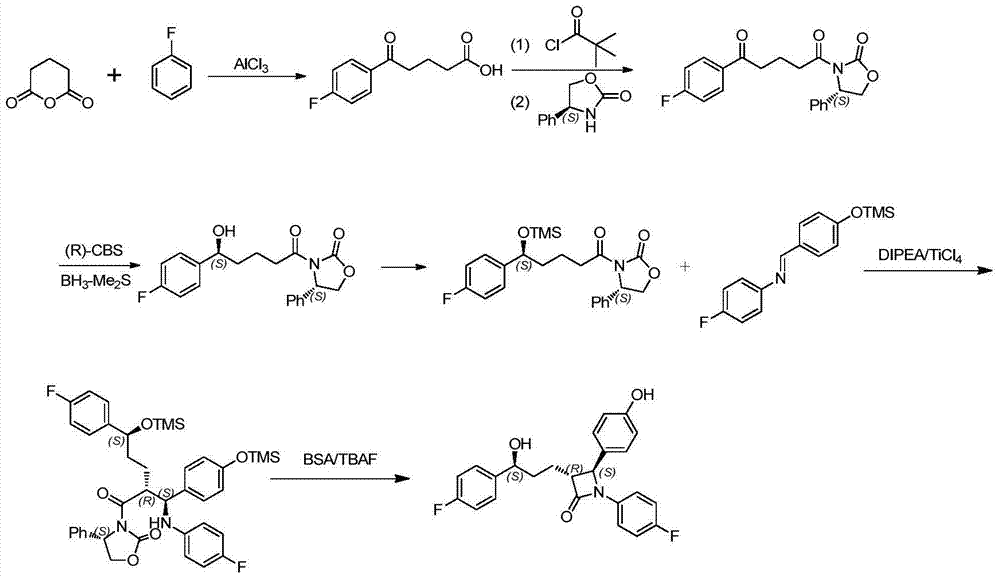
The invention provides an Ezetimibe synthesis method comprising the following steps: (a) a compound (5) is subjected to asymmetric reduction reaction to obtain a compound (6), and the compound (6) and tert-butyldimethylsilyl chloride react in an organic solution under the action of alkali to obtain a compound (7); (b) the compound (7) and diisopropylethylamine are dissolved in the organic solution, titanium tetrachloride is added in the organic solution to react at 20-50 DEG C, and a compound (3) is added in the organic solution at minus 20 to minus 60 DEG C to react to obtain a compound (8); (c) the compound (8) and N,O-bis(trimethylsilyl) acetamide react in the organic solution at 20-80 DEG C, tetrabutylammonium fluoride trihydrate is added into the organic solution to react at 20-80 DEG C to obtain a compound (9); (d) the compound (9) is subjected to off-protection reaction to obtain Ezetimibe, wherein R is equal to TBS, Ac or COOCH2CCl3. The invention further provides an Ezetimibe intermediate and a preparation method thereof.
Owner:ARROMAX PHARMATECH
Method for asymmetrically synthesizing chiral beta-acetenyl ketone from beta-ketonic acid
InactiveCN104513146AHigh reactivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetonic acidsKetone
The invention relates to a method for synthesizing chiral beta-acetenyl ketone by catalytic intermolecular decarboxylation from beta-ketonic acid and a propargyl compound. A chiral copper catalyst adopted in the invention is synthesized in stiu from a copper salt and a chiral P,N,N-tridentate ligand in various polar solvents and nonpolar solvents. According to the invention, various chiral beta-acetenyl ketone compounds with substituent groups can be synthesized conveniently, and can obtain a percent enantiomeric excess as high as 95%. The method in the invention is advantaged by operational simplicity, available raw materials, wide substrate application range, high enantioselectivity, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
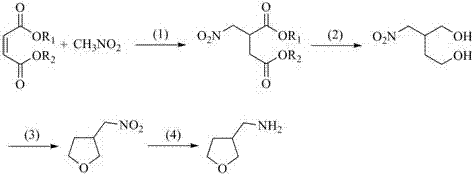
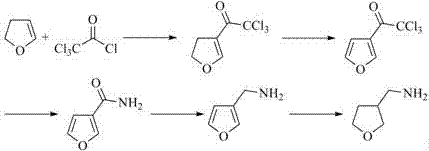
Preparation method for 3-aminomethyltetrahydrofuran
ActiveCN106366056AReduce pollutionStarting materials are cheap and readily availableOrganic chemistryAlcoholAcrylonitrile
The invention discloses a preparation method for 3-aminomethyltetrahydrofuran. The preparation method comprises the following steps: with acrylonitrile as a starting material, carrying out an addition reaction with 2-halogenated ethyl alcohol so as to obtain an intermediate 2-haloethyl-2-nitrile ethyl ether; then subjecting the intermediate 2-haloethyl-2-nitrile ethyl ether to cyclic condensation so as to obtain an intermediate 3-nitrile tetrahydrofuran; and finally subjecting the intermediate 3-nitrile tetrahydrofuran to catalytic hydrogenation so as to obtain 3-aminomethyltetrahydrofuran. The preparation method provided by the invention has the advantages of cheap and easily-available starting material, short synthetic route, simple process operation, low production cost, little pollution to the human body and the environment, good yield and applicability to large-scale industrial production.
Owner:CHANGZHOU SUNLIGHT PHARMA
Method for preparing medetomidine
ActiveCN103664788AEfficient preparationSimple process conditionsOrganic chemistryMedetomidineChemistry
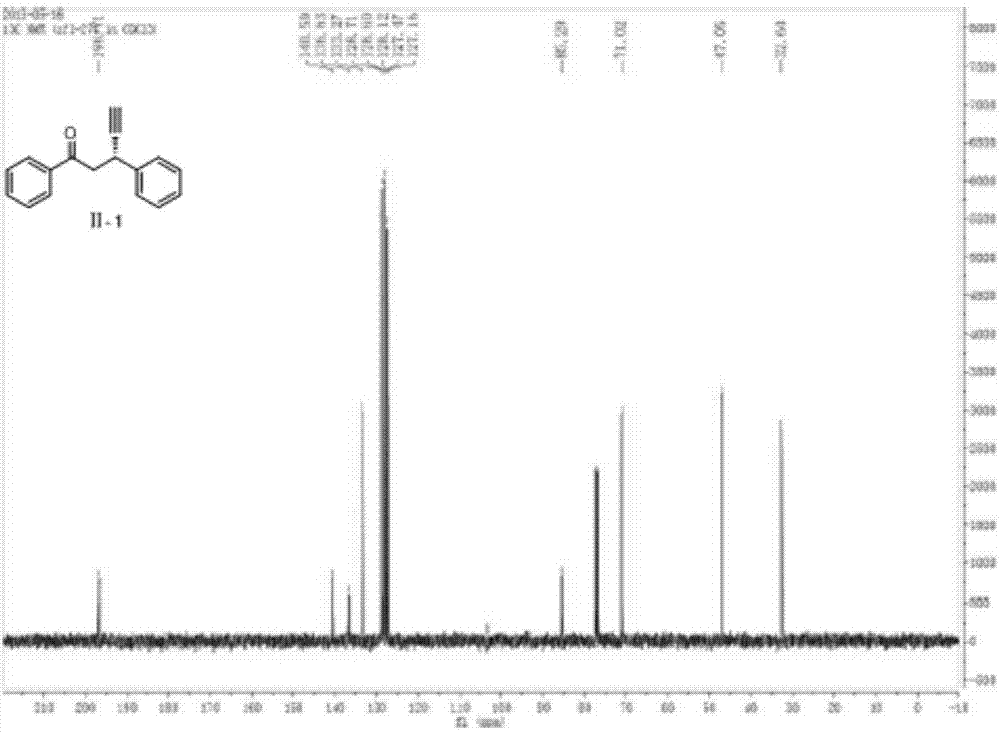
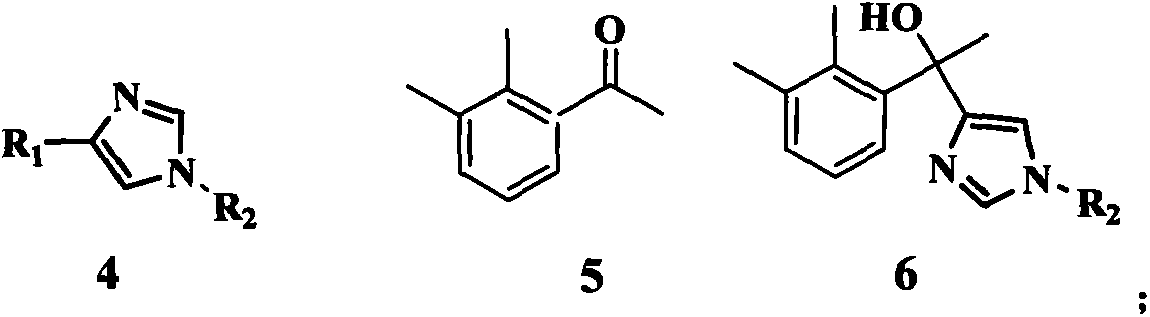
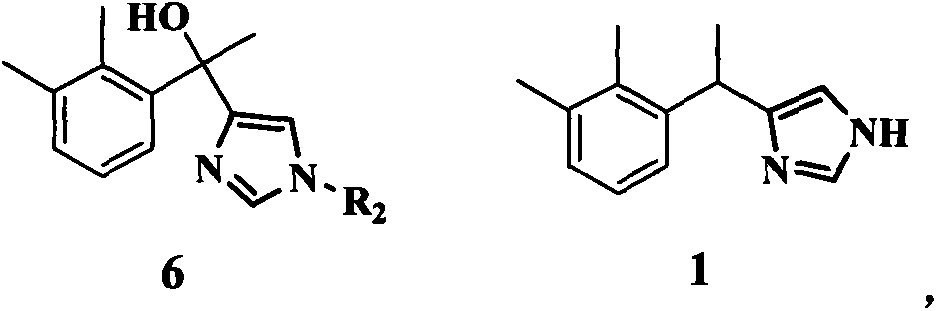
The invention discloses a method for preparing medetomidine. Medetomidine can be effectively prepared according to the method for preparing medetomidine, and by adopting a cheap 4-imidazole derivative and cheap 1-(2,3-dimethylphenyl)ethanone as raw materials, the medetomidine product is prepared only through three-step reactions. The method has simple process conditions, is easy to control, and is conducive to industrialized mass production; and the yield of medetomidine prepared by the method for preparing medetomidine can reach 76%, and the product purity can reach 99.5%.
Owner:YICHANG HUMANWELL PHARMA +1
Preparation method of 2-trifluoromethyl substituted quinazolinone compound
ActiveCN111675662AImprove toleranceSimple and fast operationOrganic chemistryMolecular sievePtru catalyst
The invention discloses a preparation method of a 2-trifluoromethyl substituted quinazolinone compound. The preparation method comprises the following steps: adding ferric trichloride, sodium hydrogen, a molecular sieve, trifluoroethyleneimide chloride and isatin into an organic solvent, reacting at the temperature of 40 DEG C for 10 hours, heating to 120 DEG C, reacting for 20 hours, and after the reaction is completed, carrying out post-treatment to obtain the 2-trifluoromethyl substituted quinazolinone compound. The preparation method is simple and convenient to operate, cheap and easily available in initial raw materials and catalysts, high in reaction efficiency and wide in substrate compatibility range, diversified substituted quinazolinone compounds with trifluoromethyl can be synthesized through substrate design, and the practicability of the method is widened while operation is facilitated.
Owner:ZHEJIANG SCI-TECH UNIV
Method for preparation of Efinaconazole
The invention provides a method for preparation of Efinaconazole. The method comprises the following step: in the presence of iodide or its hydrate, in a reaction solvent and alkali, a compound as shown in the formula 2 is contacted with a compound as shown in the formula 3, so as to obtain a compound as shown in the formula 1. By the method, Efinaconazole can be effectively prepared. In addition, the method has advantages of few steps, simple synthesis process and mild reaction conditions. Meanwhile, excessive use of expensive amine can be avoided; yield and purity of the product are high; there are few by-products; production cost is low; corrosion to the reaction vessel during the production process is little; and industrial "three wastes (waste gas, waste water and industrial residue)" are easy to treat. The method is safe and environment-friendly, and is beneficial to industrial production of Efinaconazole.
Owner:WATERSTONE PHARMA WUHAN
Method for preparing chiral 4,5-dihydropyrazole compounds
ActiveCN105732502AStarting materials are cheap and readily availableEasy to synthesizeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisCycloaddition
The invention relates to the field of organic synthesis, and relates to a method for preparing chiral 4,5-dihydropyrazole compounds. The invention relates to a method for synthesizing the chiral 4,5-dihydropyrazole compounds through a catalyzed asymmetric [2+3] cycloaddition reaction of a hydrazine compound and a propargyl compound. An adopted chiral copper catalyst is prepared through an in-situ reaction of copper salt and a chiral tridentate P,N,N-ligand in various polar and non-polar solvents. With the method, various chiral 4,5-dihydropyrazole compounds with substituent groups can be conveniently synthesized. The percent enantiomer excess is up to 96%. The method has the characteristics of simple operation, easy-to-obtain raw materials, wide substrate application range, high enantioselectively, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of 3-fluorophthalic acid
InactiveCN101381303AHigh purityStarting materials are cheap and readily availablePreparation from nitrilesSolventPhthalic acid
The invention relates to a preparation method for 3-fluorophthalic acid. The preparation method comprises the following steps that: (I) 2,6-difluorobenzonitrile which is used as a starting material is subjected to an ammoximation reaction in an aprotic solvent to obtain 2-amino,6 -fluorobenzonitrile; (II) the 2-amino,6 -fluorobenzonitrile is subjected to a diazotization reaction and then reacts with liquid HBr to form 2- bromo,6 -fluorobenzonitrile; (III) the 2- bromo,6 -fluorobenzonitrile is subjected to a cyanation reaction in an aprotic solvent to form 3-fluorophthalic benzenedicarbonitrile; (IV) the 3-fluorophthalic benzenedicarbonitrile is decomposed in an aqueous solution of inorganic acid to obtain a target substance. The preparation method overcomes the defects of difficult preparation of raw materials, low yield of the target substance, long preparation time or / and low purity and so on in the prior art of preparing the 3-fluorophthalic acid and is capable of meeting commercial preparation requirements.
Owner:EAST CHINA UNIV OF SCI & TECH
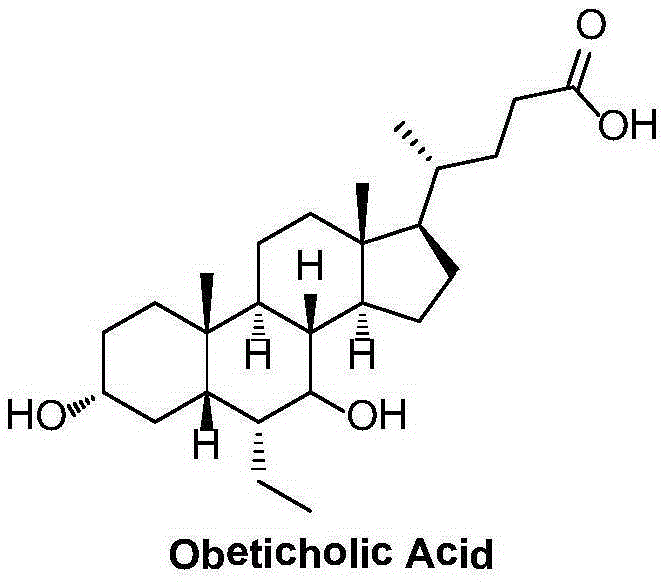
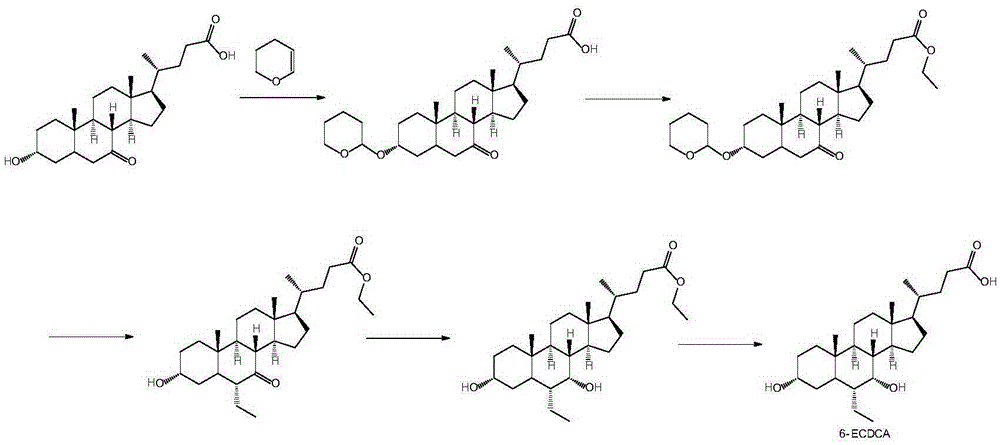
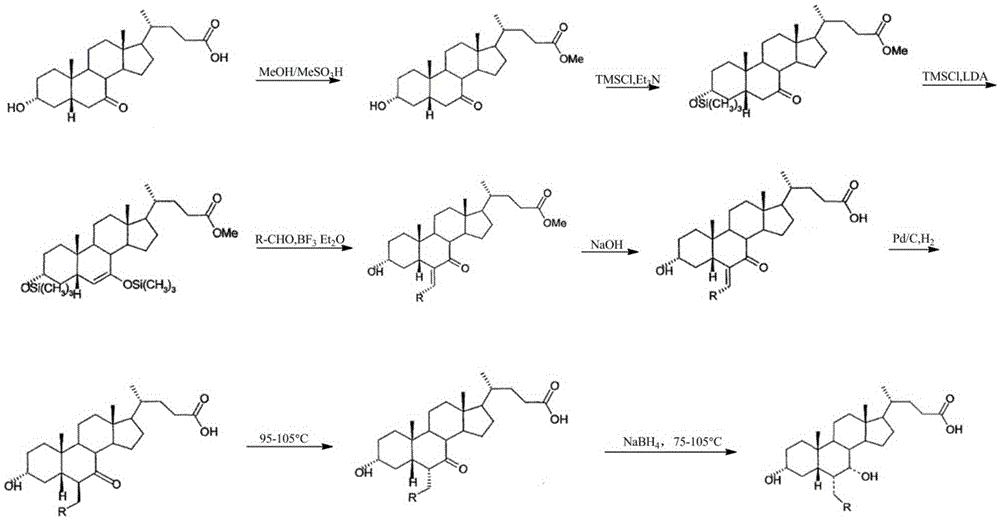
Preparation method of high-purity obeticholic acid
ActiveCN106749466AImprove protectionRaw materials are easy to getSteroidsChenodeoxycholic acidCarbonyl reduction
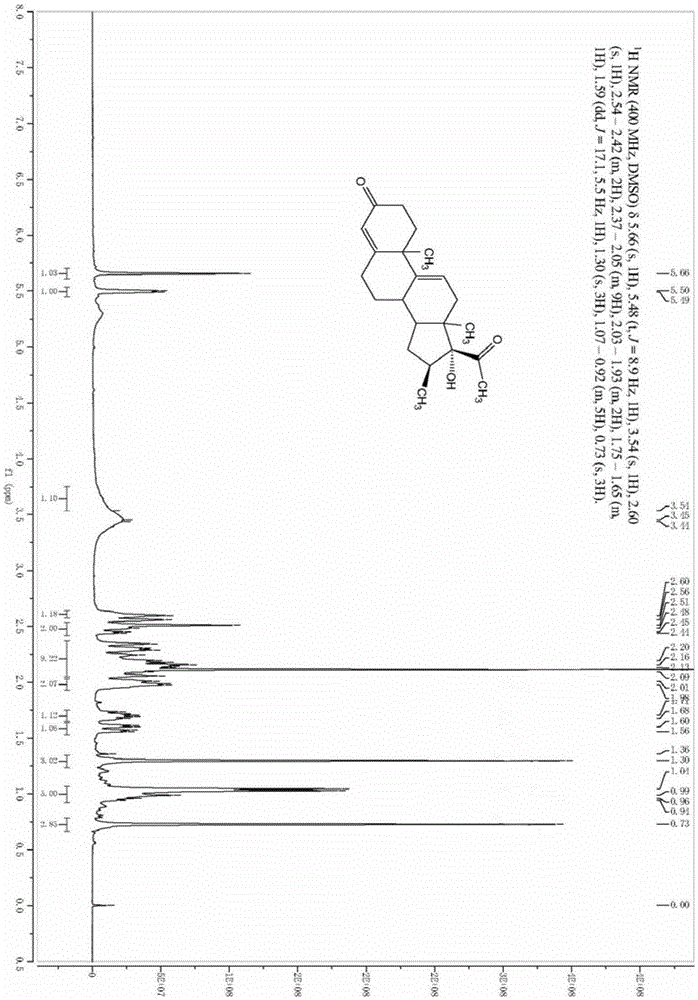
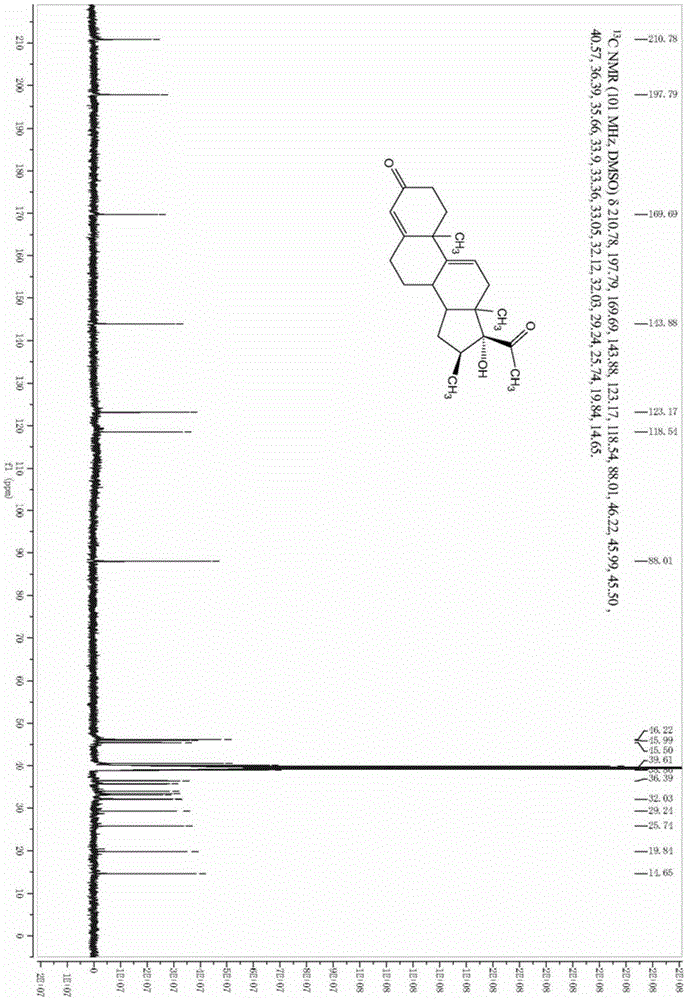
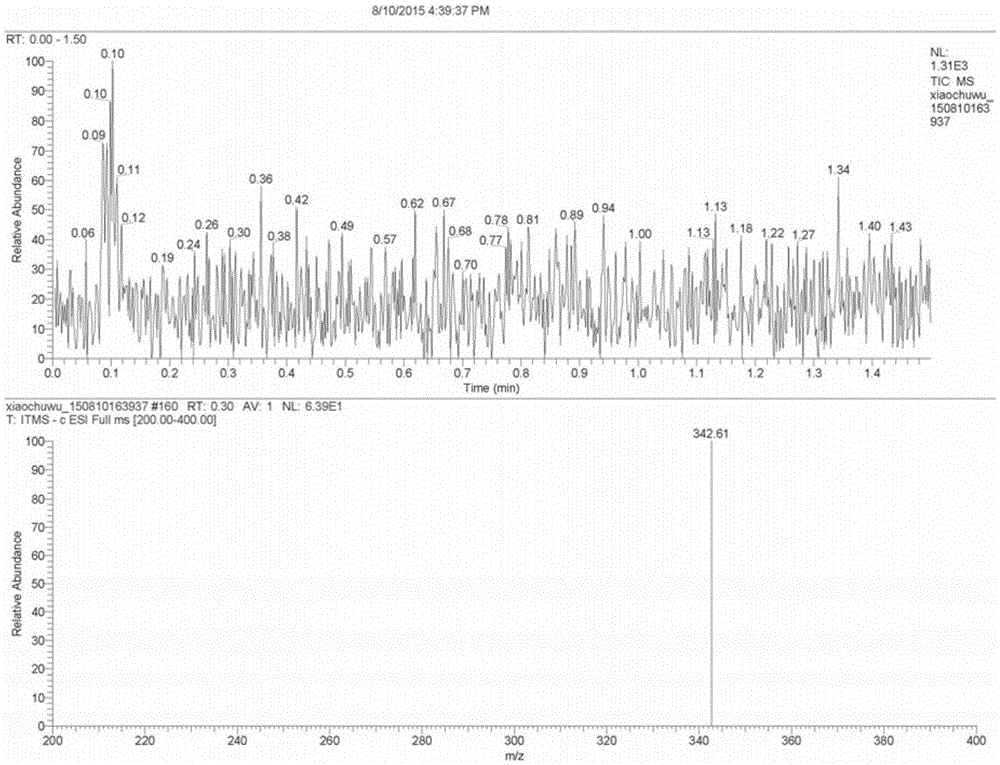
The invention relates to a preparation method of high-purity obeticholic acid. A compound chenodeoxycholic acid (CDCA) shown as a formula II is used as a starting raw material and subjected to oxidation, esterification, hydroxy protection, ethylidene formation, catalytic hydrogenation, carbonyl reduction and esterolysis reaction to obtain the high-purity obeticholic acid. The preparation method of the high-purity obeticholic acid, provided by the invention, has the advantages of low toxicity, low pollution, high purity, good stereoselectivity, low content of impurities, mild reaction conditions, high safety, simplicity and convenience in production operation and the like, and is suitable for industrial production.
Owner:NANJING GRITPHARMA CO LTD +1
Preparation method of betamethasone intermediate
The invention relates to a preparation method of a steroid drug intermediate, and concretely relates to a preparation method of a betamethasone intermediate which is 16beta-methyl-17alpha-hydroxypregna-4,9-diene-3,20-dione. The method comprises the following steps: carrying out a cyanidation reaction on 9alpha-hydroxy-4-ene-pregna-3,17-dione, carrying out a ketal protection reaction, carrying out a double elimination reaction, carrying out an epoxy reaction, and carrying out a Grignard addition hydrolysis reaction to prepare the target product. The method is a brand preparation technology for producing the betamethasone intermediate, and has the characteristics of cheap and easily available initial raw material, simple operations of all above reactions, high yield, suitableness for industrial massive production, and realization of the yield and the quality reaching satisfactory levels.
Owner:JIANGSU YUANDA XIANLE PHARMA
Synthesis method of 3-tetrahydro-furanmethanamine
ActiveCN107417648AReduce pollutionStarting materials are cheap and readily availableOrganic chemistrySynthesis methodsPollution
The invention discloses a synthesis method of 3-tetrahydro-furanmethanamine. According to the method, maleic acid diester is used as a starting material to synthesize 3-tetrahydro-furanmethanamine via four steps of reaction of Michael addition, metal borohydride reduction, dehydration cyclization and catalytic hydrogenation reduction. The synthesis method provided by the invention has the advantages of mild reaction conditions, high product yield, less pollution discharge, simple process operation and suitability for industrialized production.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
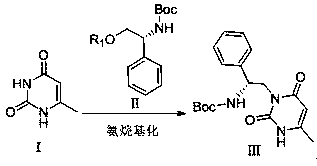
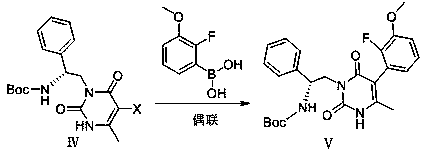
Method for efficiently synthesizing elagolix intermediate
ActiveCN110204498AHigh yieldStarting materials are cheap and readily availableOrganic chemistryLarge applicationsHalogenation
The invention relates to the field of pharmacy, in particular to a method for efficiently synthesizing an elagolix intermediate. The method takes 6-methyluracil as a starting material, and comprises five reactions of aminoalkylation, halogenation, coupling, benzyl halide substitution and deprotection. The synthetic method has the advantages that the starting material is cheap and easy to obtain, areaction reagent is friendly to the environment, the reaction steps are simple, the aftertreatment is convenient, the overall yield is higher, and the method is especially suitable for industrial mass production and has larger application value.
Owner:AURISCO PHARMACEUTICAL CO LTD
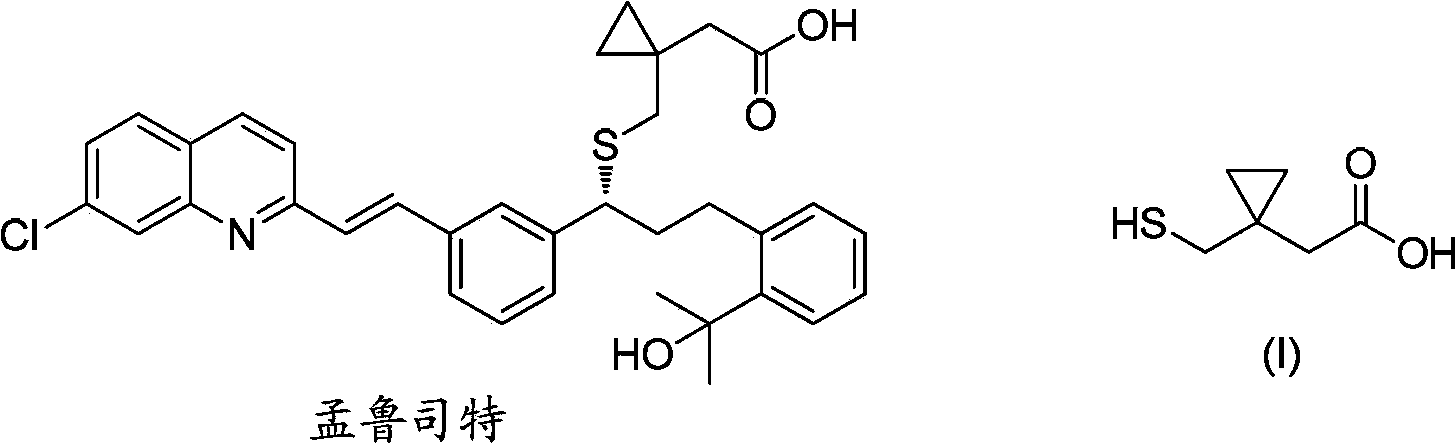
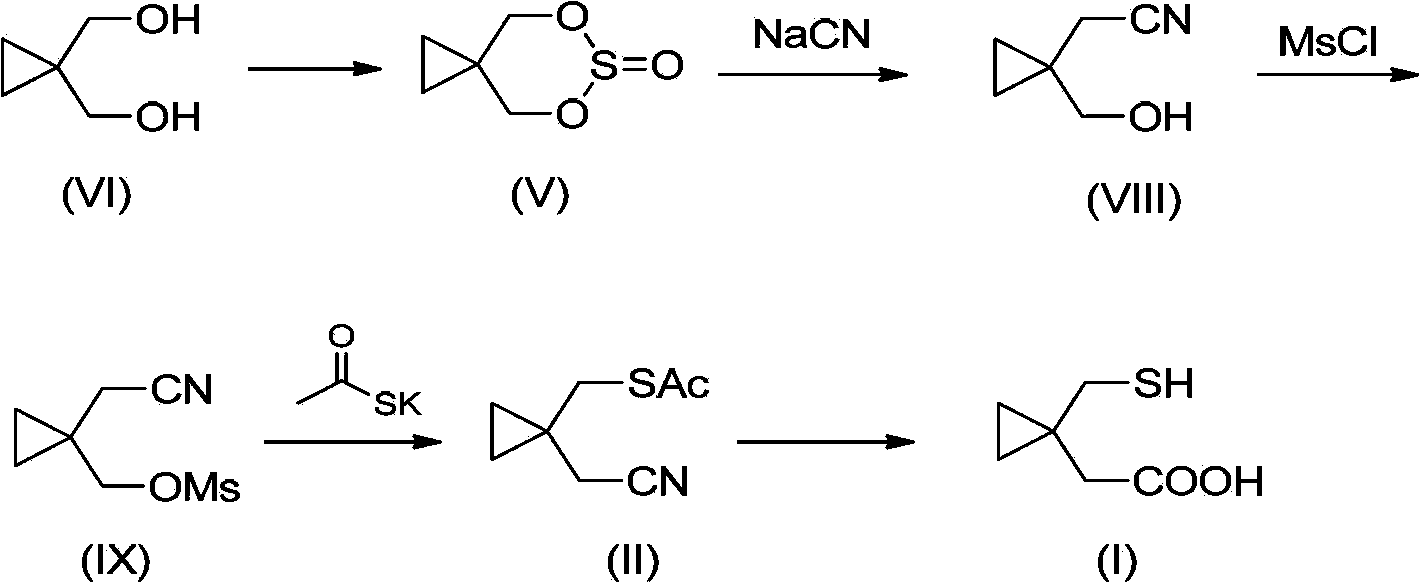
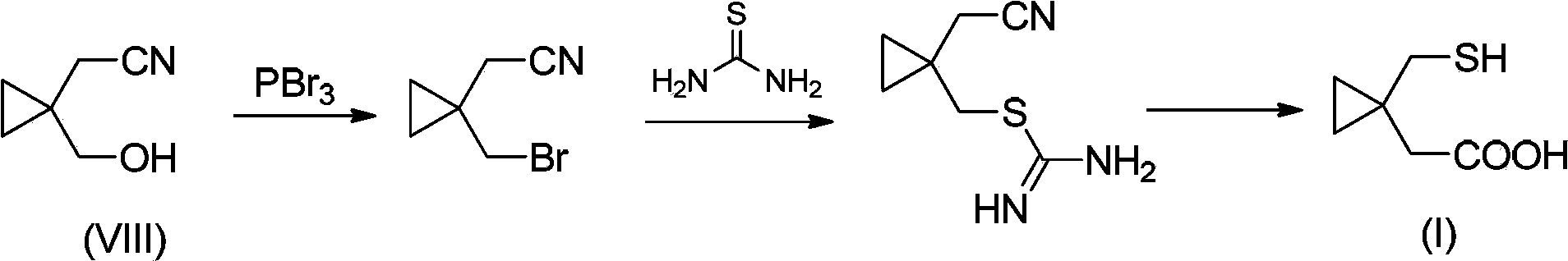
Preparation methods of 1-(mercaptomethyl)cyclopropyl acetic acid and intermediate thereof
InactiveCN103539714AIngenious designStarting materials are cheap and readily availableThiol preparationAcetic acidSulfite
The invention relates to a preparation method of 1-(mercaptomethyl)cyclopropyl acetic acid (I). The preparation method comprises the steps of carrying out ring opening on cyclopropanedimethanol cyclic sulfite (V) with potassium thioacetate, so that a compound (IV) is obtained; carrying out sulfonic acid esterification reaction on the compound (IV) and methanesulfonyl chloride or paratoluensulfonyl chloride to obtain a compound (III); carrying out cyano group substitution on the compound (III) to obtain a compound (II); and hydrolyzing the compound (II) under an alkaline condition so as to obtain the 1-(mercaptomethyl)cyclopropyl acetic acid (I), wherein R is a methyl or a p-methylphenyl. The invention further provides a preparation method of an 1-(mercaptomethyl)cyclopropyl acetic acid intermediate. The preparation methods of the 1-(mercaptomethyl)cyclopropyl acetic acid and the 1-(mercaptomethyl)cyclopropyl acetic acid intermediate are ingenious in design, initial raw materials are low in cost and easily available, and the technological process is simple and practicable, so that the production cost can be greatly reduced; the preparation methods are beneficial to industrial production and suitable for large-scale popularization and application.
Owner:SHANGHAI PUYI CHEM CO LTD
Method for preparing emtricitabine
ActiveCN109438432AStarting materials are cheap and readily availableMild reaction conditionsOrganic chemistry methodsEmtricitabineL menthol
The invention discloses a method for preparing emtricitabine. The method comprises the following steps: refining so as to obtain 5S-(5'-flucytosine-1')-1,3-oxythiacyclopentane-2-carbethoxy-(1'R,2'S,3'R)-menthyl lactate; under a weak alkali and solvent condition, removing a chiral aid L-menthol, thereby obtaining a product of emtricitabine. The initial raw material used in the method is low in price, easy to obtain, mild in reaction condition, high in atom utilization rate and simple and convenient in operation process, the reagent is environmentally friendly, the obtained product is high in chemical purity, meets medicine standards, and is applicable to industrial production of emtricitabine.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
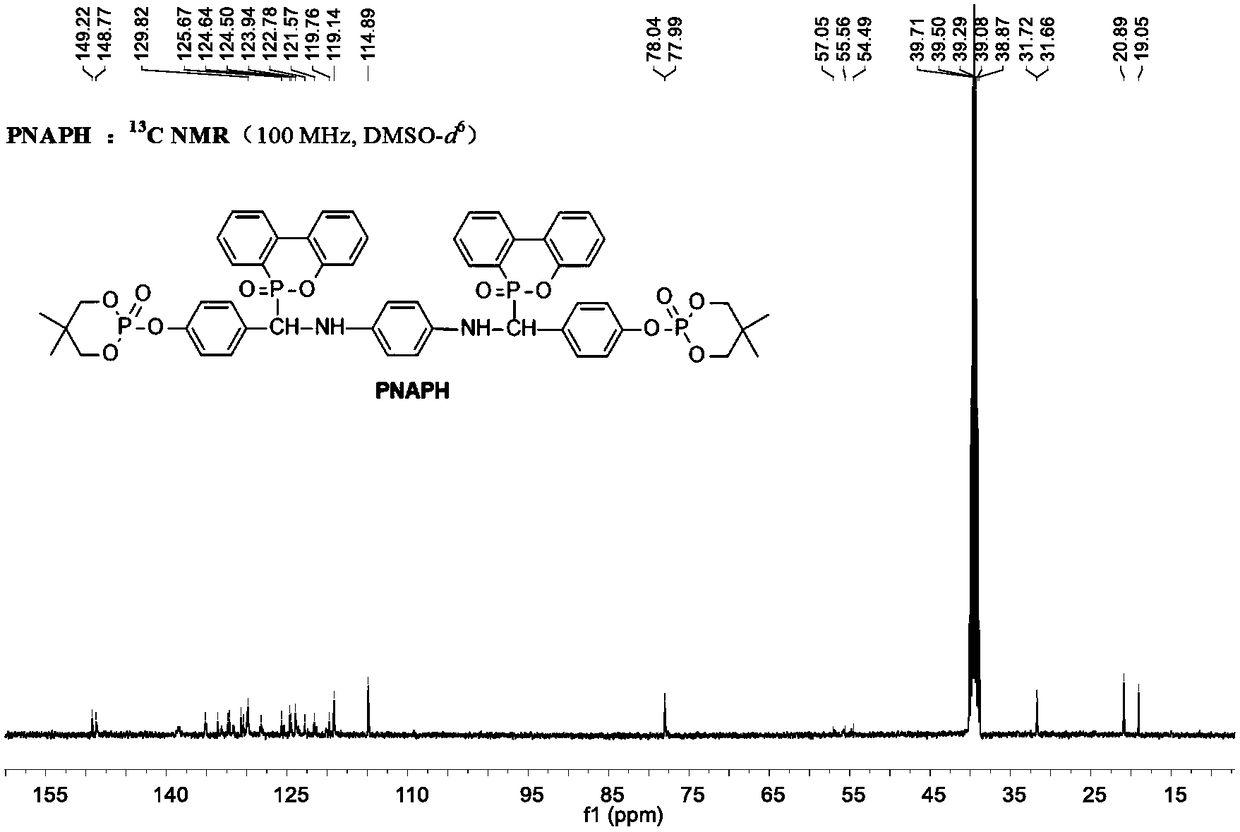
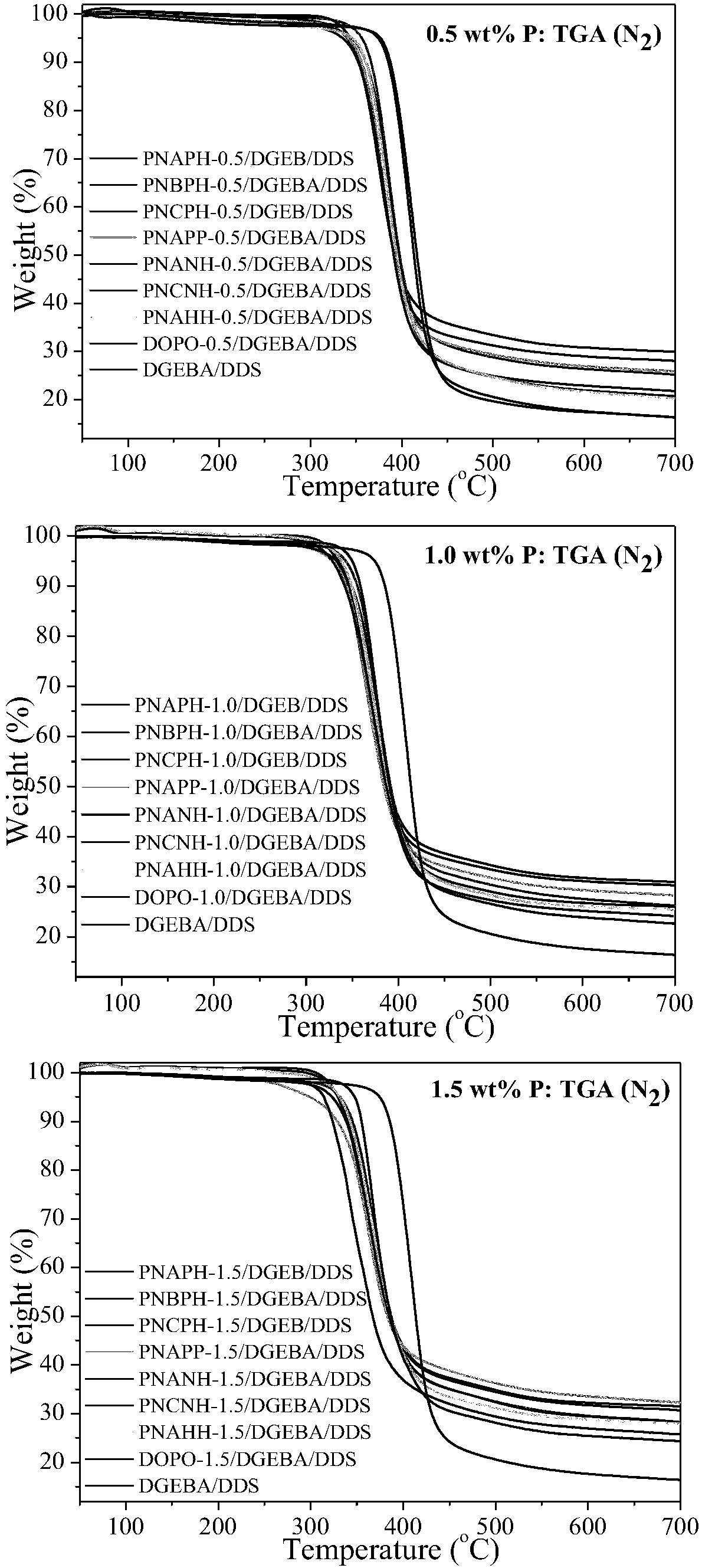
Phosphaphenanthrene compound and preparation method and application thereof
ActiveCN108864193AImprove efficiencyImprove flame retardant performanceGroup 5/15 element organic compoundsEpoxyGeometric isomer
The invention provides a phosphaphenanthrene compound and a preparation method and application thereof. The compound is a compound shown in the formula I or a stereisomer, a geometrical isomer, a tautomer, a hydrate, a solvate, ester and pharmacologically acceptable salt of the compound shown in the formula I, wherein R, X and Y are as defined as in the instruction book. The compound has the goodsynergistic flame retardation effect, can be served as a fire retardant, is suitable for flame retardation in high polymer materials, and is particularly suitable for preparing the high polymer materials such as epoxy resin with the flame retardation property. The formula I is shown in the description.
Owner:HUAZHONG NORMAL UNIV
Synthesis method of lifitegrast intermediate
InactiveCN107857728AImprove responseStarting materials are cheap and readily availableOrganic chemistryBenzyl chloroformateSynthesis methods
The invention discloses a preparation method of a lifitegrast intermediate. The preparation method comprises the following steps: (1) taking a compound (1), treating the compound (1) by using tetramethyldiethylamine and n-butyl lithium, adding benzyl chloroformate, reacting at the temperature of 70 DEG C below zero to obtain a compound (2); and (2) taking the compound (2), reacting the compound (2) with a strong alkaline solution, adjusting pH to be 3-4 after the reaction is ended, and extracting to obtain a compound (3). The prepared lifitegrast intermediate is high in process selectivity, high in controllability, high in stability and high in total yield, and is suitable for commercial large-scale production.
Owner:成都惟邦药业有限公司
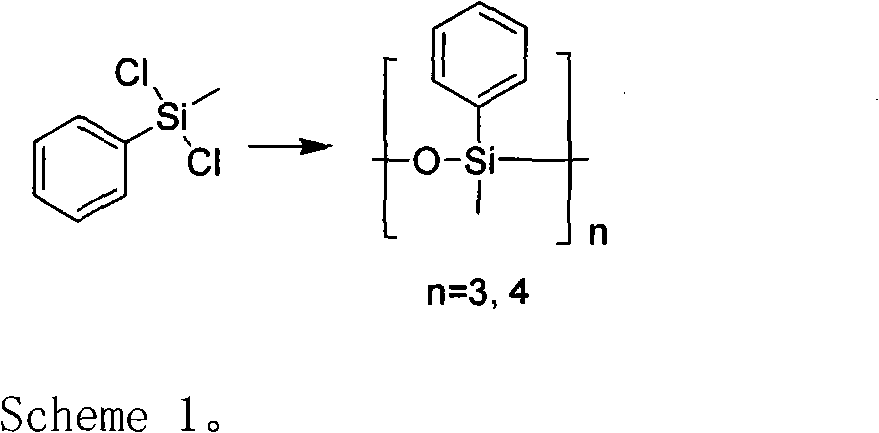
A preparation method for methyl phenyl siloxane unit-containing organosilicone ring bodies
InactiveCN102702248AProcess stabilityShort reaction stepsSilicon organic compoundsGrignard reactionBromobenzene
The invention relates to a preparation method for organosilicone ring bodies. The method comprises that the organosilicone ring bodies are obtained via synthesis and purification processes of synthesis, hydrolysis, pyrolysis and distillation after Grignard reaction of chlorobenzene (bromobenzene). The method has the advantages of readily available and low-price raw materials, stable technology, high security and no environmental pollution, short reaction steps and low cost, and is suitable for industrial production.
Owner:SUZHOU BAILINGWEI HYPERFINE MATERIAL
Compound and preparation method and application thereof
InactiveCN106928186AIncrease productivityStarting materials are cheap and readily availableOrganic chemistryBulk chemical productionReference productCombinatorial chemistry
The invention provides a compound shown in the formula 1 and a preparation method and application thereof. The method comprises the steps that 1, a compound shown in the formula 2 makes contact with trimethylsulfoxonium iodide to obtain a compound shown in the formula 3; 2, the compound shown in the formula 3 is subjected to a ring-opening reaction to obtain a compound shown in the formula 4; 3, an amino group of the compound shown in the formula 4 is subjected to a ring-closure reaction to obtain a compound shown in the formula 5; 4, the compound shown in the formula 5 is subjected to a de-tetrahydropyrane protection group reaction to obtain a compound shown in the formula 6; 5, the compound shown in the formula 6 is subjected to a molecular inner ring oxidation reaction to obtain a compound shown in the formula 7; 6, the compound shown in the formula 7 makes contact with a compound shown in the formula 8 to obtain the compound shown in the formula 1. According to the method, the compound shown in the formula 1 can be effectively prepared and effectively applied to quality control over an Efinaconazole drug by serving as a reference product.
Owner:WATERSTONE PHARMA WUHAN
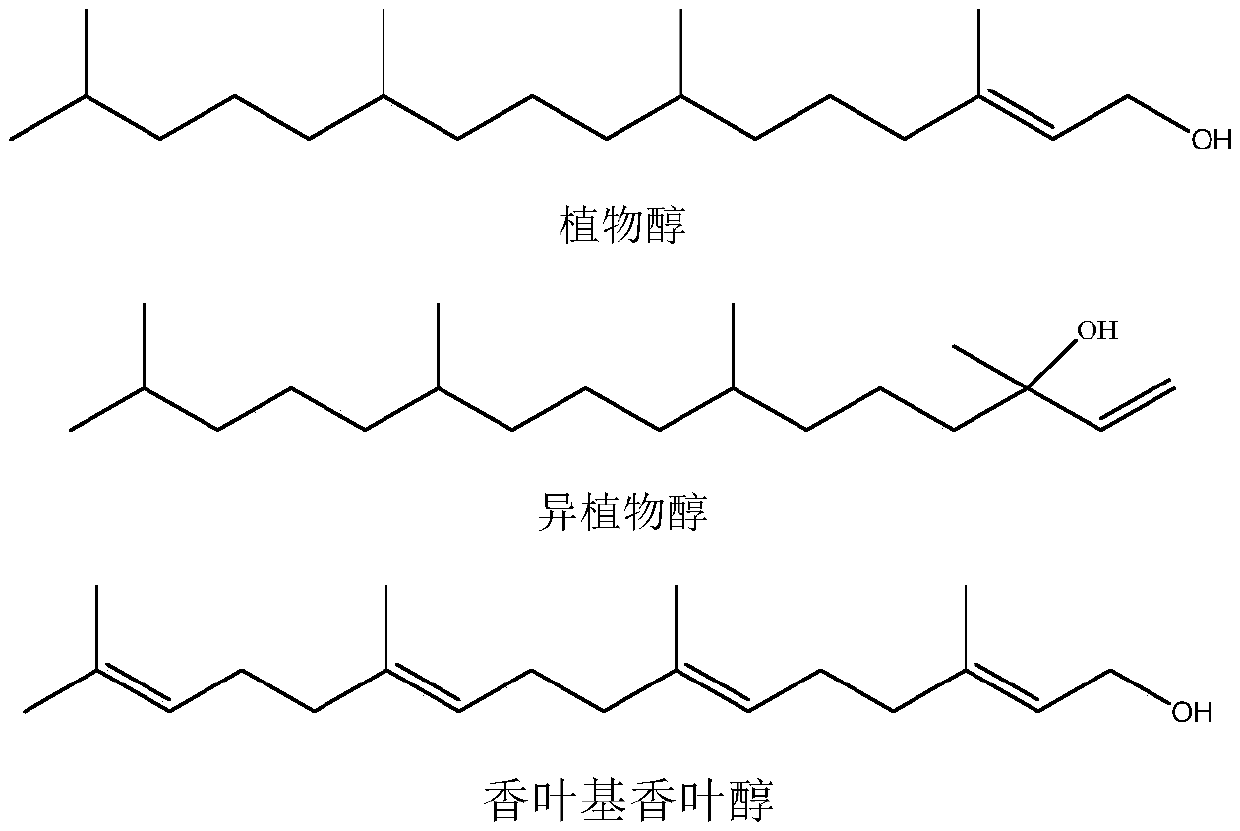
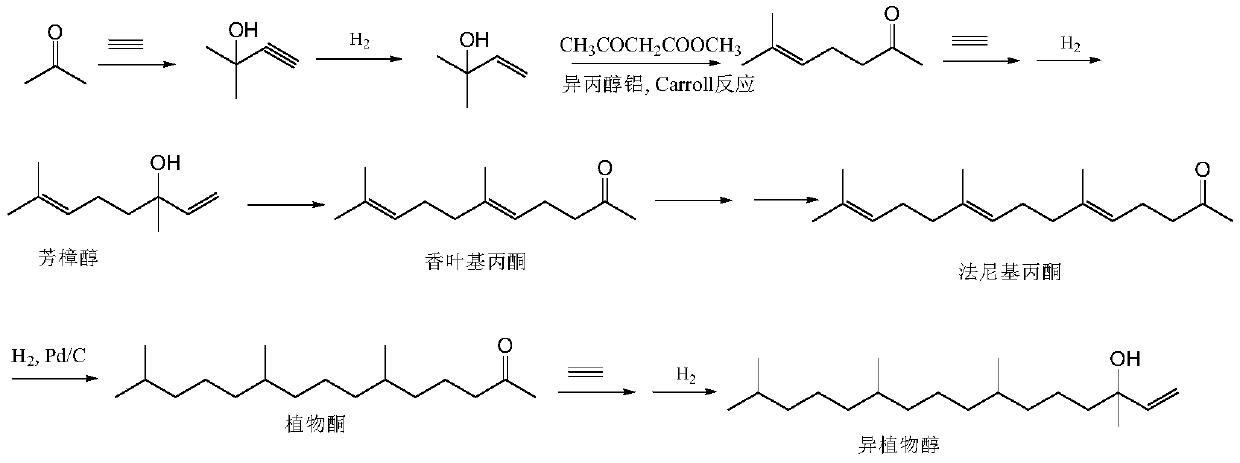
Synthesis method of intermediate farnesyl acetone and method for synthesizing phytol, isophytol and geranyl geraniol by using intermediate farnesyl acetone
ActiveCN111393275AShort synthetic routeReduce dosagePreparation by isomerisationOrganic compound preparationVitamin K2Side chain
The invention relates to a synthesis method of an intermediate farnesyl acetone, and a method for synthesizing vitamin E, vitamin K1, vitamin K2 side chain isophytol, phytol and geranyl geraniol by utilizing farnesyl acetone. Specifically, the method includes: taking acetone and 5-chloro-2-pentanone as the raw materials, carrying out three Grignard reactions to obtain a key intermediate farnesyl acetone, and hydrogenating the farnesyl acetone to obtain phytol ketone; reacting farnesyl acetone with a vinyl chloride Grignard agent to obtain geranyl linalool, and conducting rearrangement under acid catalysis to obtain geranyl geraniol; or reacting the farnesyl acetone directly with a hydroxyl-protected 2-chloroethanol Grignard agent to obtain geranyl geraniol; reacting phytol ketone with a vinyl chloride Grignard agent to obtain isophytol, and rearranging the isophytol under acid catalysis to obtain phytol; or directly reacting the phytol with a hydroxyl-protected 2-chloroethanol Grignardagent to obtain the phytol. The method has the advantages of cheap and easily available initial raw materials, short synthesis process steps, low product cost and the like.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA +1
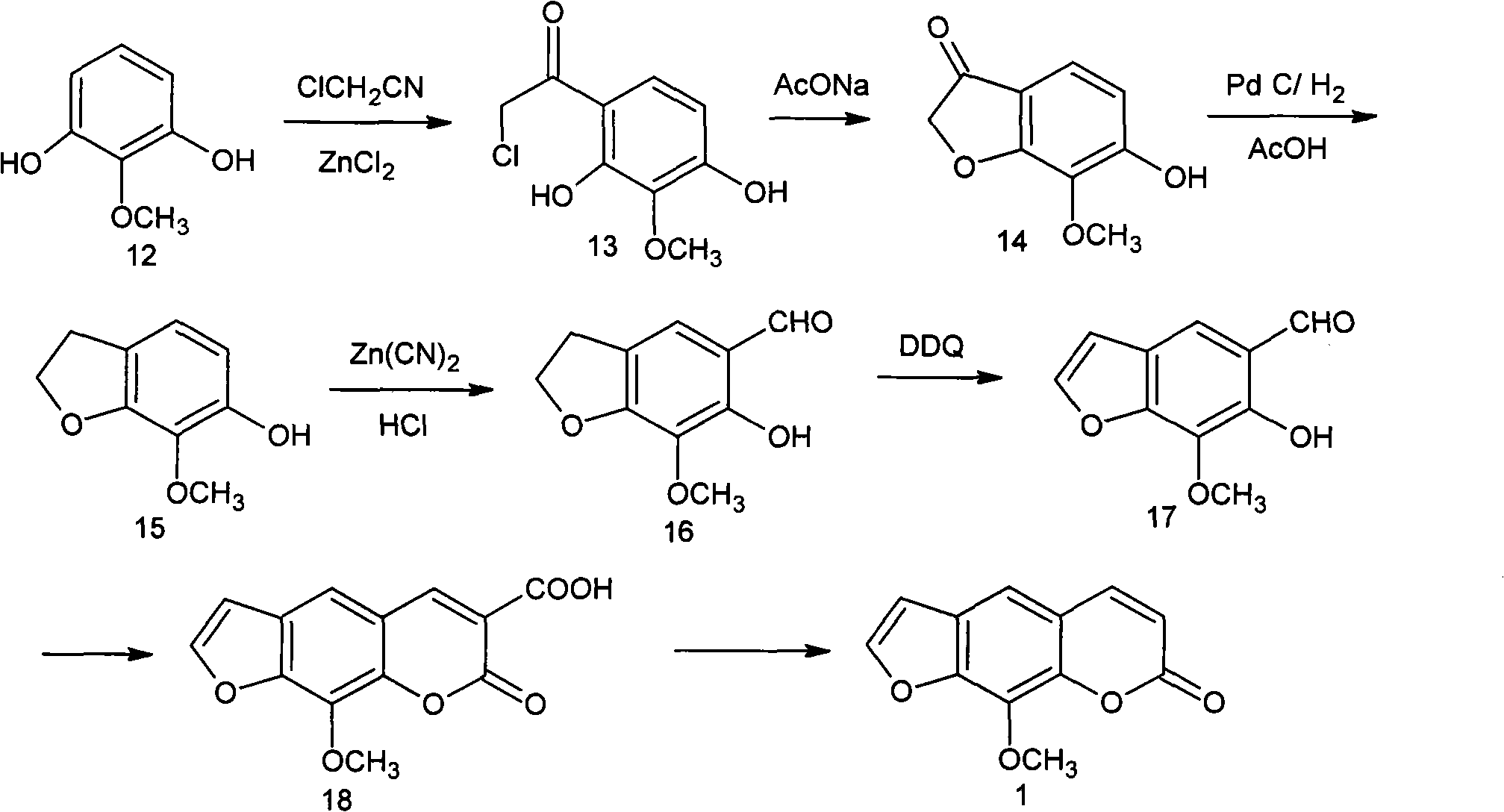
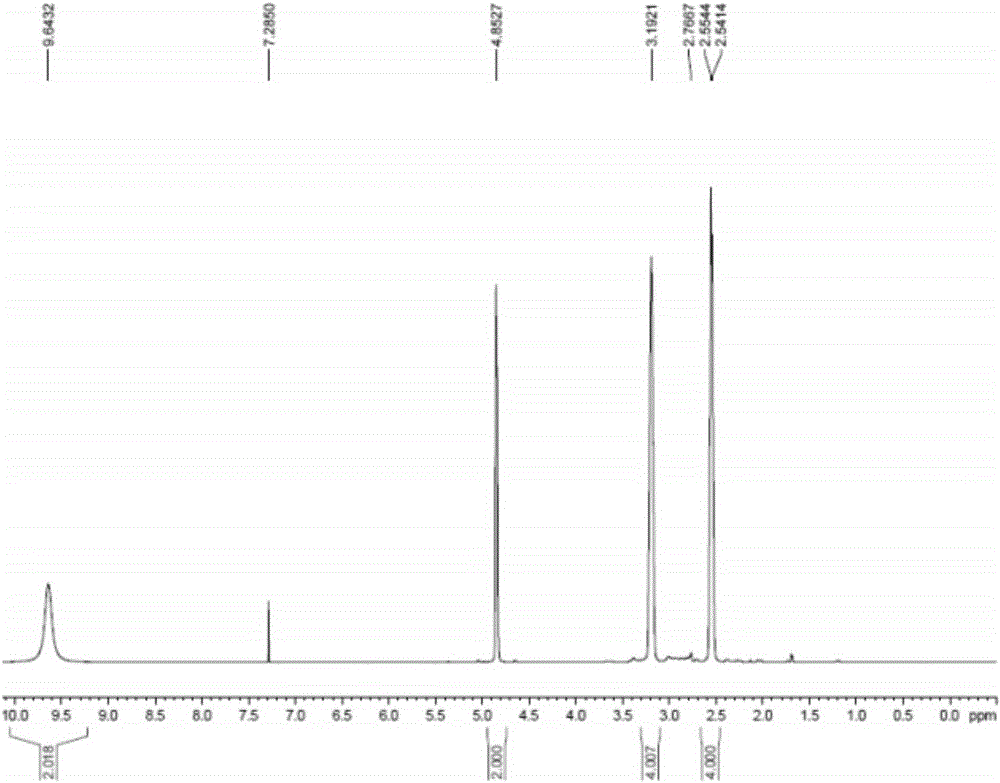
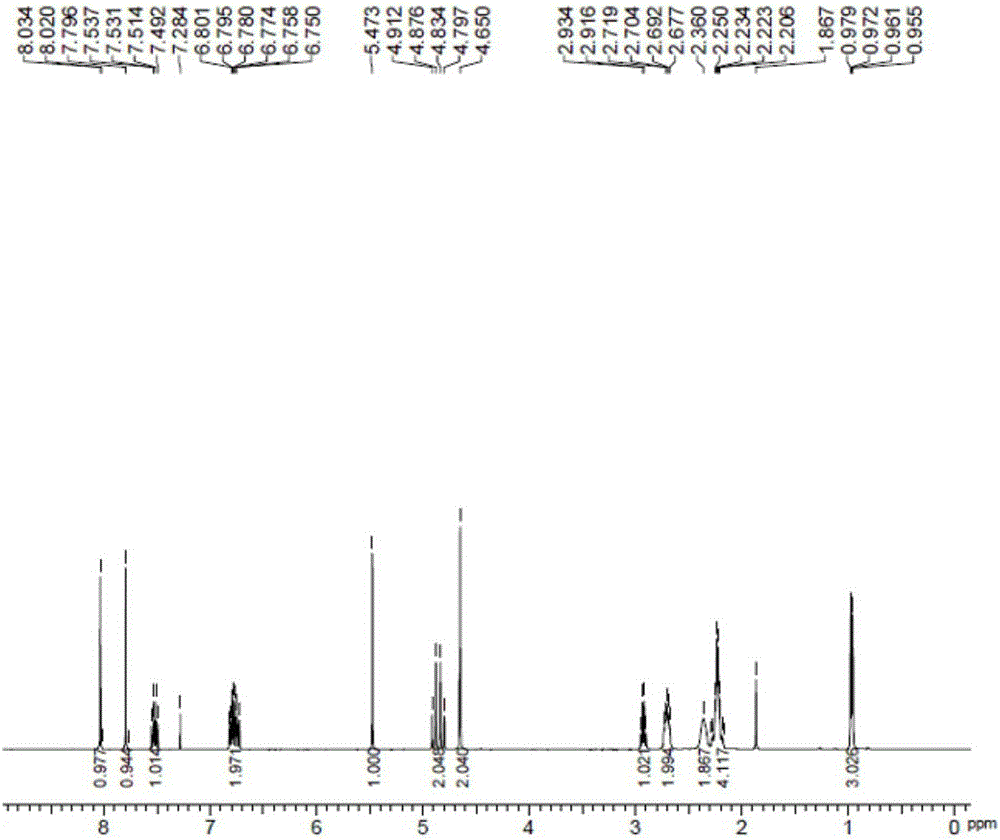
Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate
InactiveCN105111155AStarting materials are cheap and readily availableLow costOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis of N-heterocycle-containing drug intermediates, and particularly relates to a synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate. By using diethyl malonate as a raw material, cyclization reaction, Hofmann reaction, hydrolysis reaction, acylation reaction for recyclization, reduction reaction and the like are performed to conveniently synthesize the target compound product. The method has the advantages of simple synthesis technique, cheap and accessible raw materials, mild reaction conditions, high controllability, low cost and high yield, and is convenient to operate.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
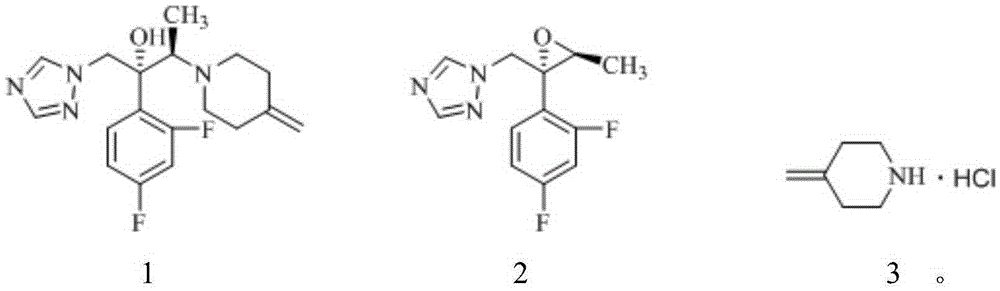
A preparing process of a compound
InactiveCN104876890AEasy to purifyShort synthetic routeOrganic compound preparationAmino-hyroxy compound preparationCompound aAfter treatment
A preparing process of a compound shown as a formula 1 is provided. The process includes (1) bringing a compound shown as a formula 2 into contact with compound shown as a formula 3 to produce a compound shown as a formula 4; (2) subjecting the compound shown as the formula 4 to chiral resolution to produce a compound shown as a formula 5; (3) subjecting the compound shown as the formula 5 to nitro reduction to obtain a compound shown as a formula 6; and (4) bringing the compound shown as the formula 6 into contact with a compound shown as a formula 7 to produce the compound shown as the formula 1. The process is short in route. Initial raw materials are cheap and easily available. All the intermediates are easy to purify and simple in after-treatment. The product is high in purity and high in yield. The process benefits industrial production.
Owner:HUMANWELL HEALTHCARE GRP
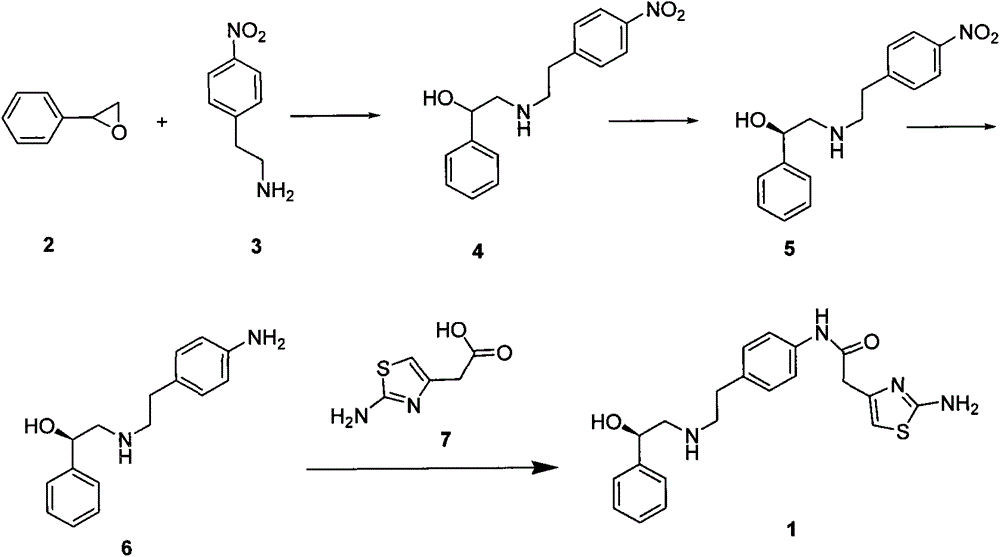
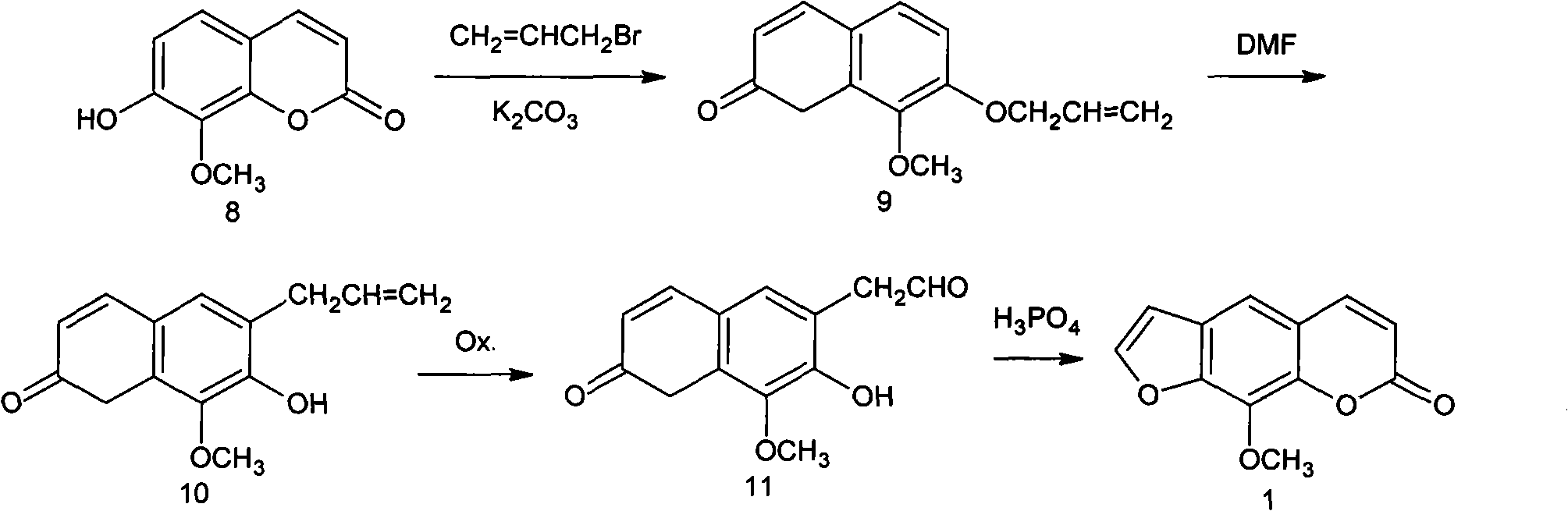
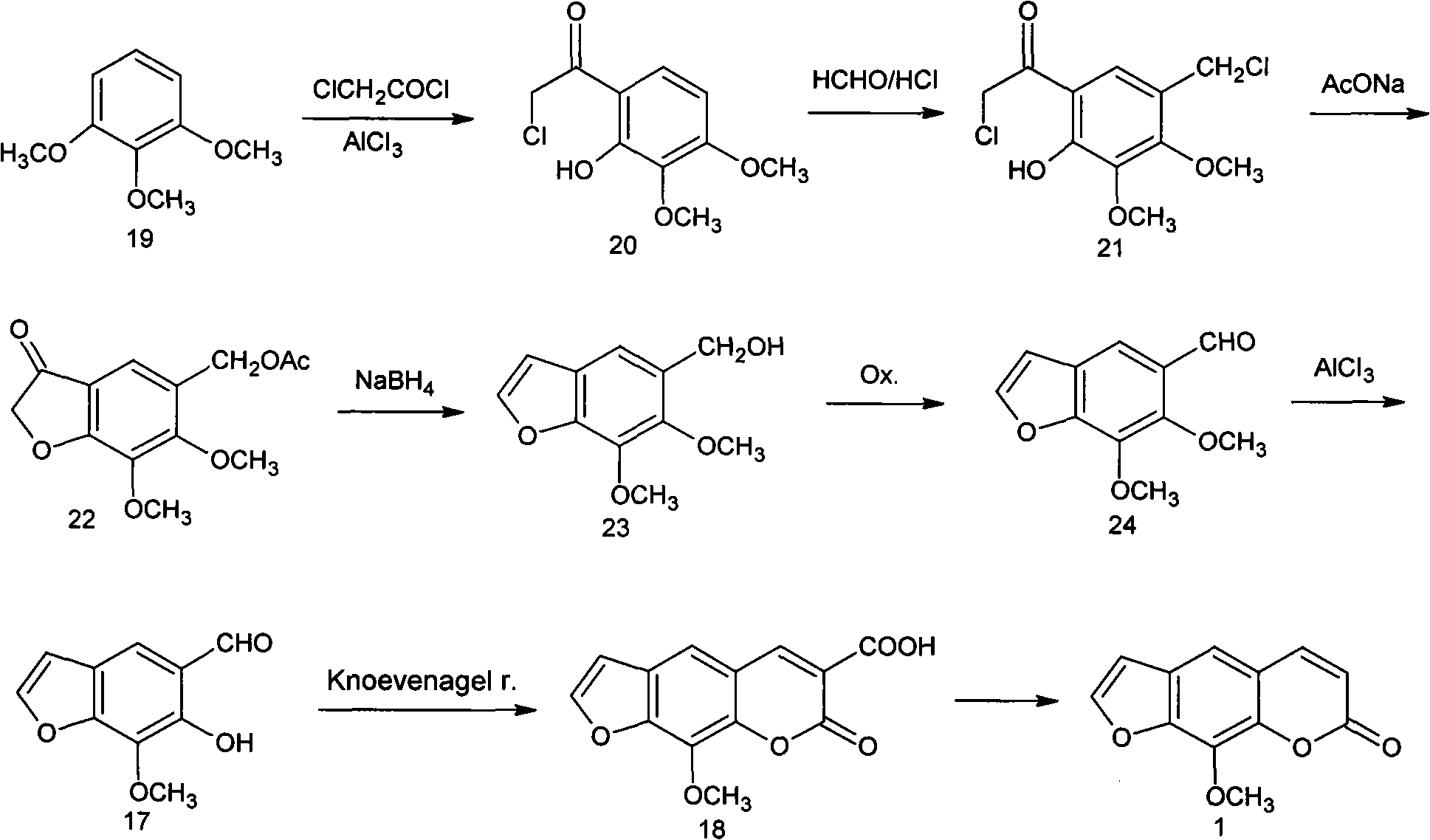
Synthesis process of methoxsalen
The invention relates to a synthesis process of methoxsalen. In the process, pyrogallol is used as an initial raw material, and the methoxsalen is prepared by carrying out six-step reaction on the pyrogallol, wherein the total yield is 29%. The synthesis process has the advantages of short synthesis path, simple method, readily-available raw materials and suitability for industrial production.
Owner:WUHAN WUYAO SCI & TECH
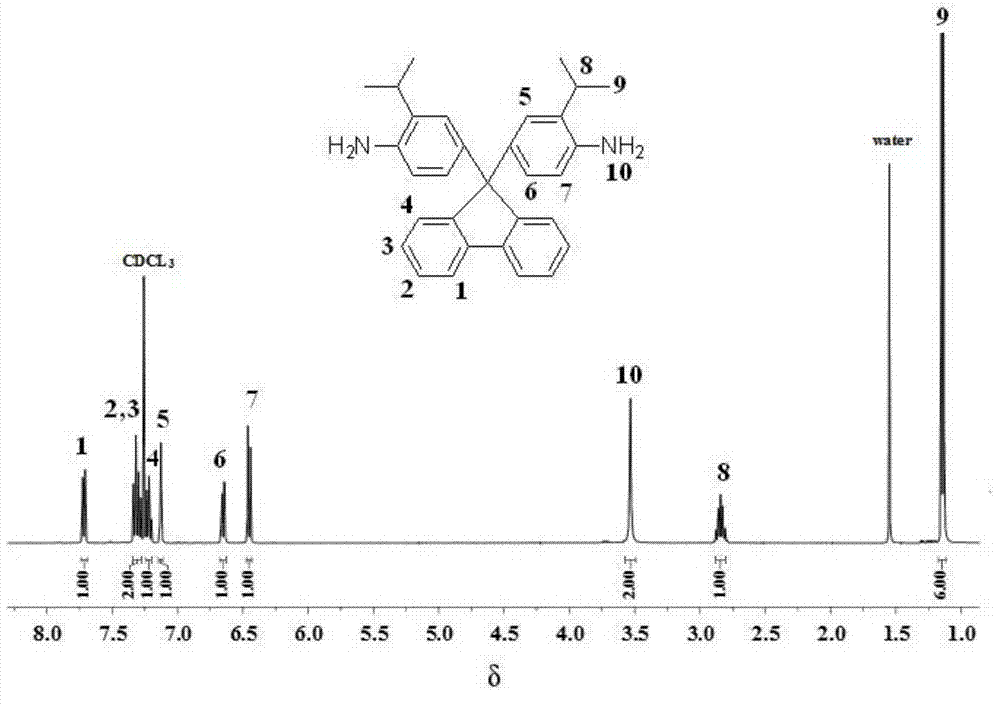
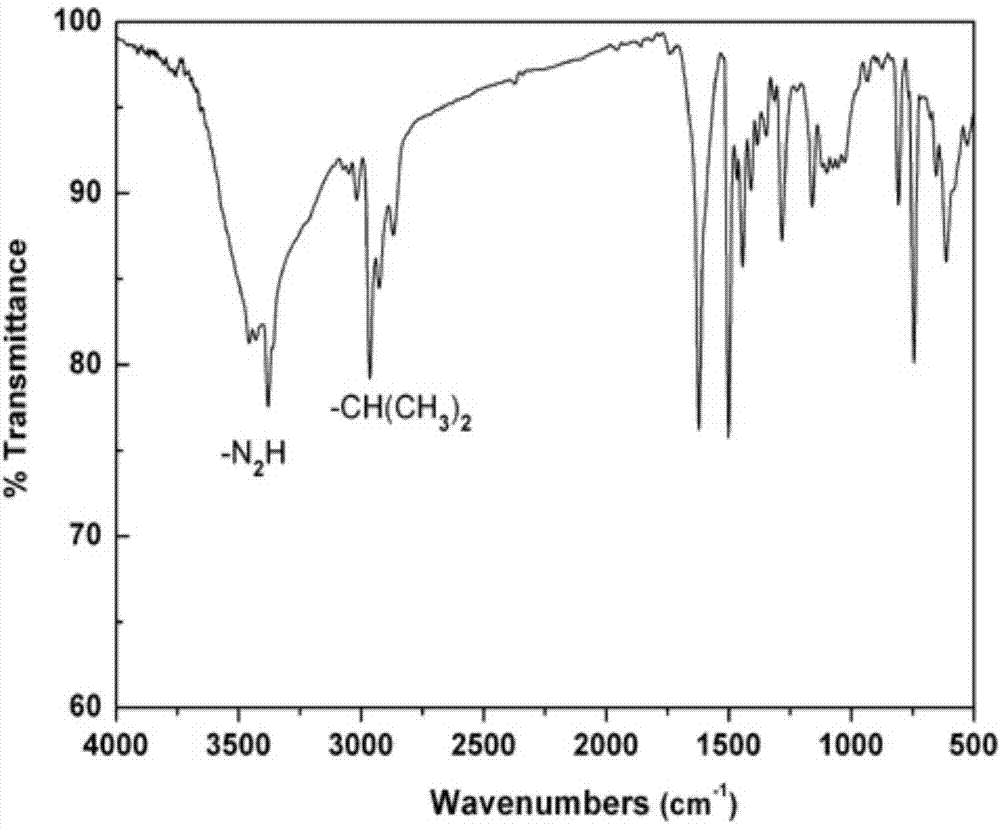
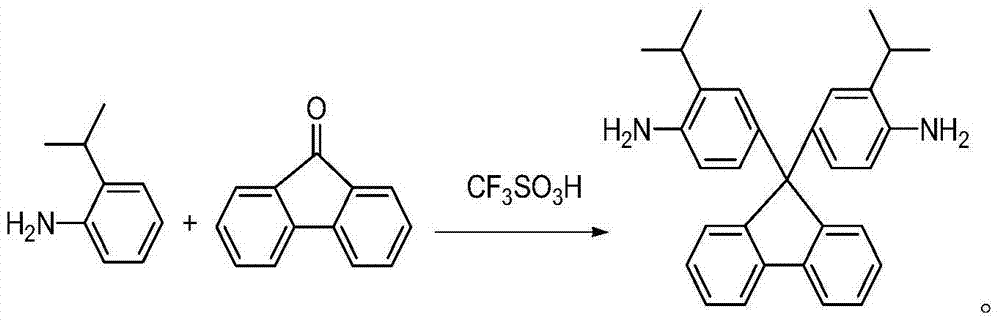
Rigid aromatic diamine monomer as well as preparation method and application thereof
ActiveCN103497110AEasy to separate and purifyStarting materials are cheap and readily availableOrganic compound preparationAmino compound preparationSolubilityPolyamide
The invention relates to the field of technical research of rigid aromatic diamine monomers and in particular relates to a biisopropyl substituted rigid aromatic diamine 9,9-bis(4-amido-3-isopropyl phenyl)fluorene monomer containing a fluorenyl structure. The rigid aromatic diamine monomer is obtained by reacting reactants including 2-isopropylaniline and 9-fluorenone under the condition of an acid catalyst, neutralizing the product with defined amount of aqueous alkali and then transferring the product to a mixed solvent of ethanol and water and carrying out leaching, drying and further recrystallization. The monomer has a simpler synthetic method and high yield, is easy to purify and is stable under room temperature. The obtained biisopropyl substituted rigid aromatic diamine monomer containing the fluorenyl structure can be applied to preparation of soluble aromatic polyamides and the prepared aromatic polyamides have good solubility and excellent film-forming properties.
Owner:大连新阳光材料科技有限公司
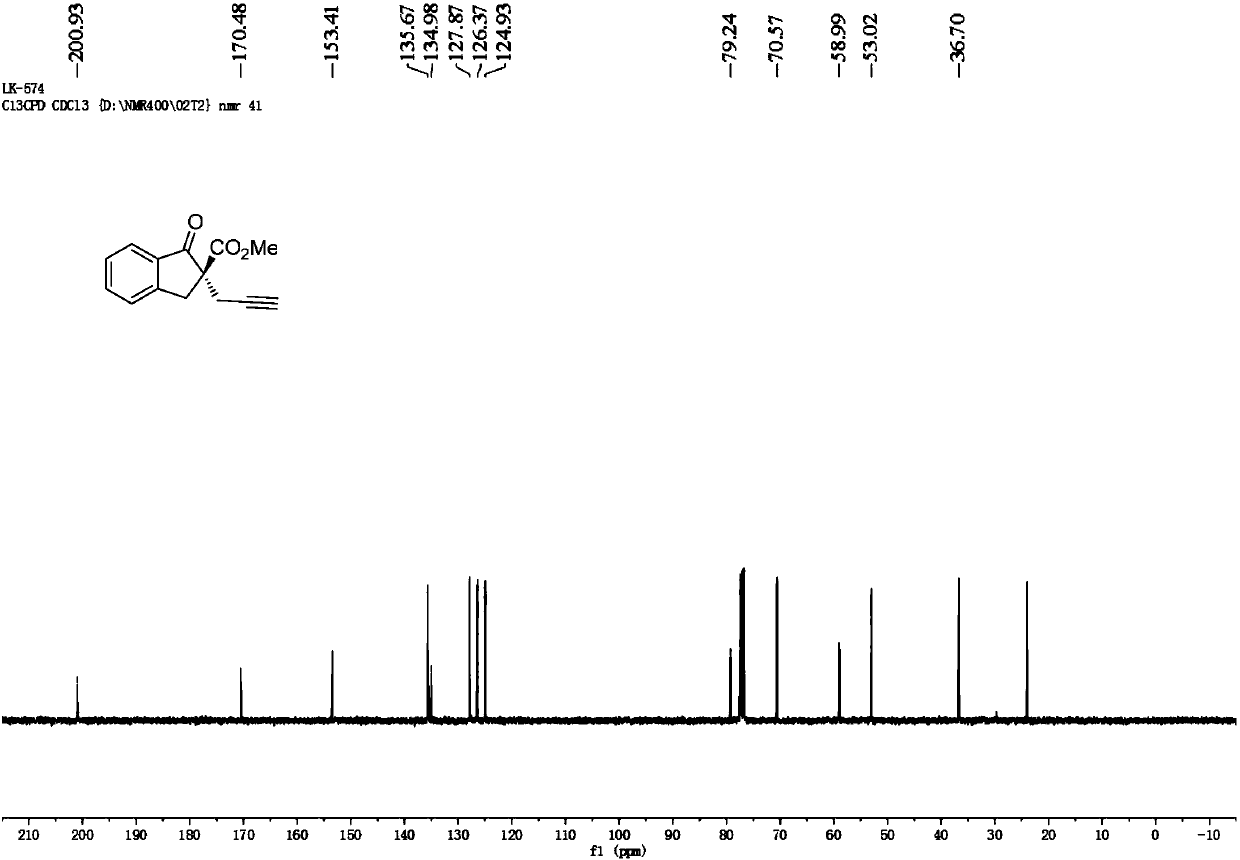
Synthesis method of chiral benzocyclic beta-ketoester compounds
InactiveCN109851504AHigh reactivityHigh stereoselectivityOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsKetone
The invention provides a synthesis method of chiral benzocyclic beta-ketoester compounds, and the compounds are benzocyclic beta-ketoester compounds containing the chiral quaternary carbon center at 2-position. The synthesis method is as follows: in the presence of an alkali additive, a chiral copper catalyst catalyzes an asymmetric propynyl substitution reaction of benzocyclic beta-ketoester compounds and acetylene propyl compounds in a reaction medium to synthesize the benzocyclic beta-ketoester compounds containing the chiral quaternary carbon center at 2-position. The chiral copper catalyst is prepared in situ from copper salts and chiral P, N, N-tridentate ligands in various polar and nonpolar solvents. The method can conveniently synthesize various functionalized benzocyclic beta-ketoester compounds containing the chiral quaternary carbon center at 2-position, and the enantiomer excess percentage is up to 95%. The method has the characteristics of simple operation, easy availability of raw materials, wide application range of substrates, high enantioselectivity and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
One-pot method for preparing crucial intermediate in oseltamivirphosphate synthesizing reaction
InactiveCN102464596AShort reaction pathShort synthesis cycleCarbamic acid derivatives preparationOrganic compound preparationChemistryHydroquinone Compound
The invention discloses a one-pot method for preparing a crucial intermediate in oseltamivirphosphate synthesizing reaction. The method is characterized in that: 1,3-butadiene and ethyl propiolic acid ethyl ester are adopted as raw materials, alcohol and isocyanate / salt are adopted as main reactants, DBU is adopted as a catalyst, and no separation process is required before a finished product is obtained, such that the one-pot method is realized. The method mainly comprises steps that: 1, 1,3-butadiene is cooled to a temperature of -78 DEG C; ethyl propiolic acid ethyl ester and hydroquinone are added to 1,3-butadiene, and the mixture is stirred for 3 days under a temperature of 110 DEG C; the mixture is subject to vacuum distillation, such that 1-ethyl formate-1,4-diene cyclohexane is obtained; 2, I2 is added to a solution of 1-ethyl formate-1,4-diene cyclohexane and AgOCN; the mixture is heated to a temperature of 35-40 DEG C within 4 hours, such that a mixture A is obtained; alcohol and waterless HCl are added to the mixture A, and the mixture is stirred for 6-8 hours under a temperature of 35-40 DEG C, such that a mixture B is obtained; DBU is added to the mixture B, and the mixture is subject to a reaction over night under a temperature of 35-40 DEG C; the mixture is purified, such that the finished product is obtained; or DBU is added to the mixture A, the mixture is subject to a reaction over night under a temperature of 35-40 DEG C, and the mixture is purified, such that the finished product is obtained. Compared to existing technologies, the method is substantially advantaged in that: initial raw materials are cheap and easy to obtain, the reaction route is short, the synthesis period is shortened, the reaction method is improved into a one-pot method, the separation and purification processes are simple, the yield is high, the reaction efficiency is high, and the method has certain potential to be used in large-scaled productions.
Owner:NANKAI UNIV
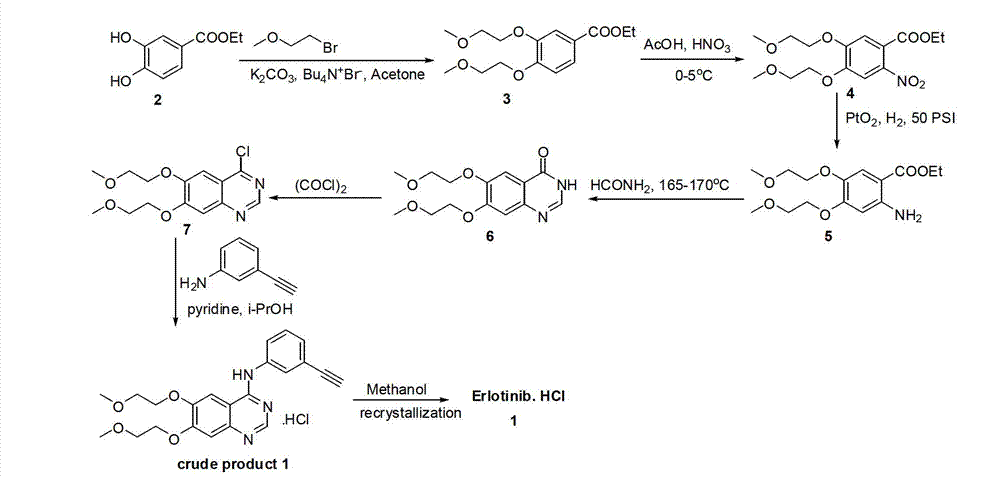
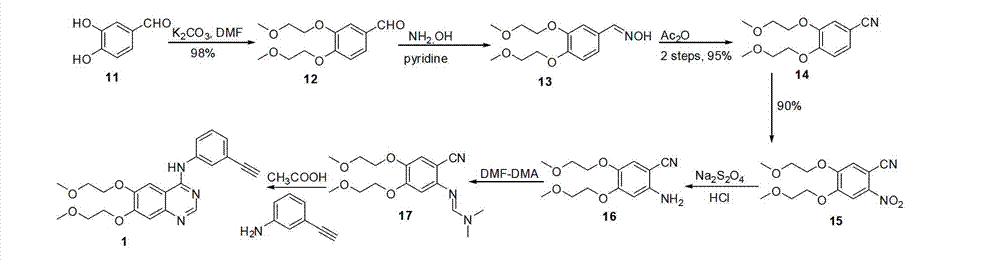
Erlotinib, and preparation method of new intermediate of erlotinib
The invention discloses an erlotinib intermediate compound. The erlotinib intermediate compound is characterized by having a structural general formula (I); in the formula (I), R1 is selected from H, NO2, CN, halogen atom, COOH, CF3 and SO2CF3; the halogen atom is selected from F and Cl. The erlotinib prepared by the intermediate has the advantages of cheap and readily available raw materials, low impurity content, basically no checkout and the like.
Owner:SHANGHAI BOLI BIO TECH
Compound and preparation method and application thereof
InactiveCN106279072AEfficient preparationThe reaction mechanism is clearOrganic chemistryImpurityHplc mass spectrometry
The invention provides a compound shown in the formula I and a preparation method thereof and a method for detecting Gliflozin medicine through high performance liquid chromatography. According to the preparation method, 3,5,6-tribenzyl-D-glucofuranose serves as a raw material, a methylation reaction, a benzyl substitution reaction, demethylating and ketalation are carried out, 3,5,6-tribenzyl-D-glucofuranose and a halide intermediate are subjected to a nucleophilic addition, the material makes contact with triethyl silicane and boron trifluoride diethyl etherate, finally, the material makes contact with ethanethiol and boron trifluoride diethyl etherate, and the target impurity product can be obtained. By means of the method, directional preparation is achieved for synthesis of the target product; the target product provides a reliable impurity reference substance for quality research and impurity quantitative control of industrially-produced Gliflozin series diabetes treatment medicine products. The formula I is shown in the specification.
Owner:WATERSTONE PHARMA WUHAN
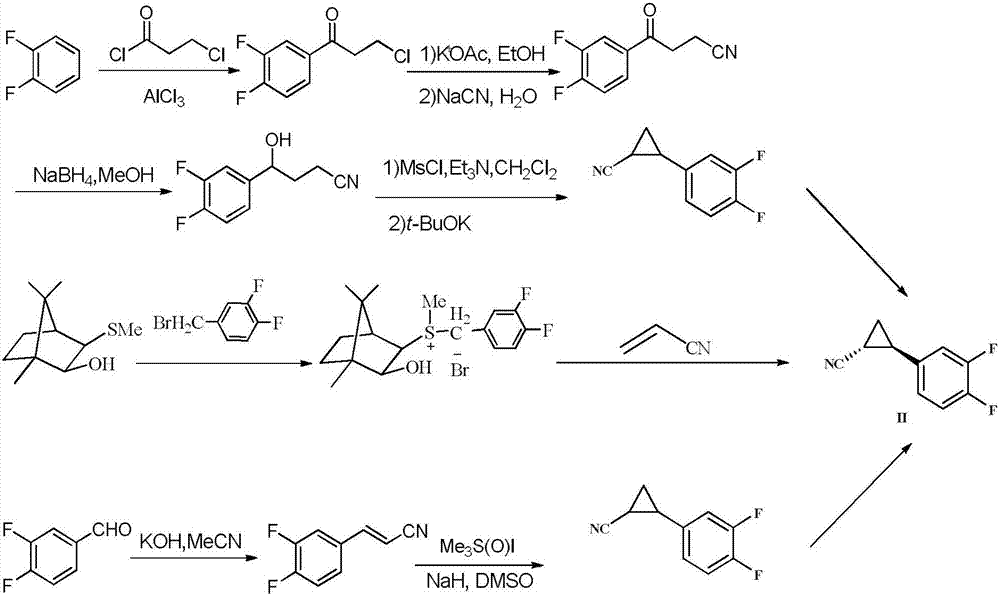
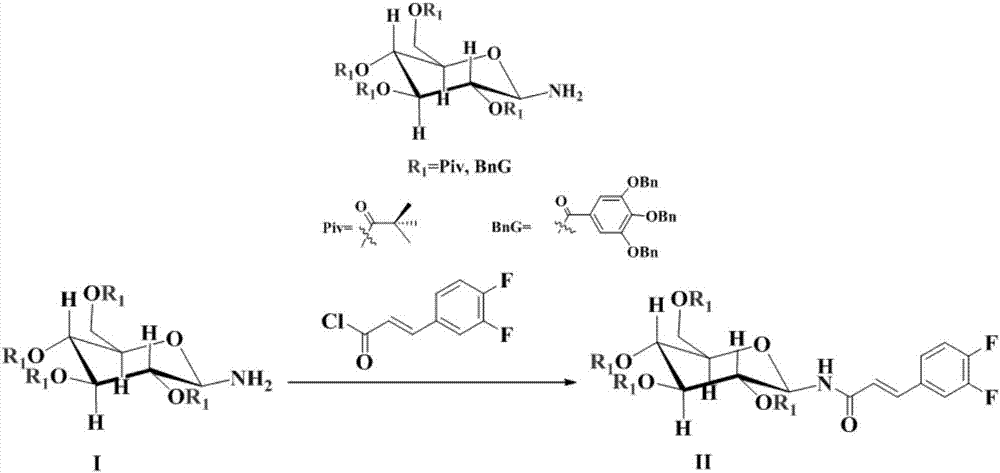
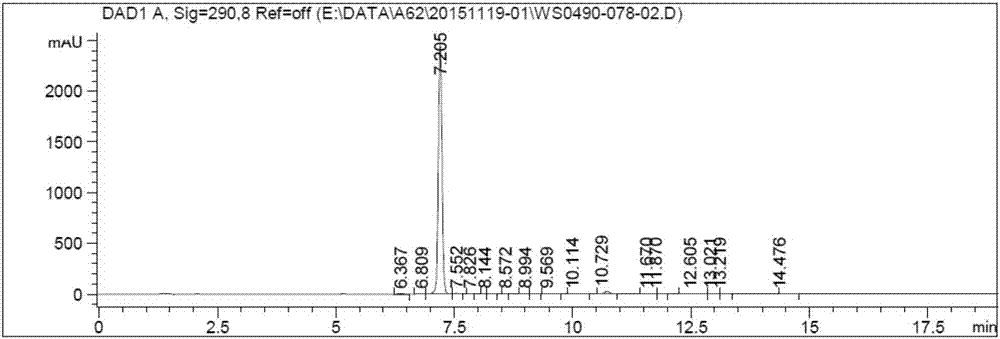
Novel synthesis method of ticagrelor intermediate (1R,2R)-2-(3,4-difluorophenyl) cyclopropane nitrile
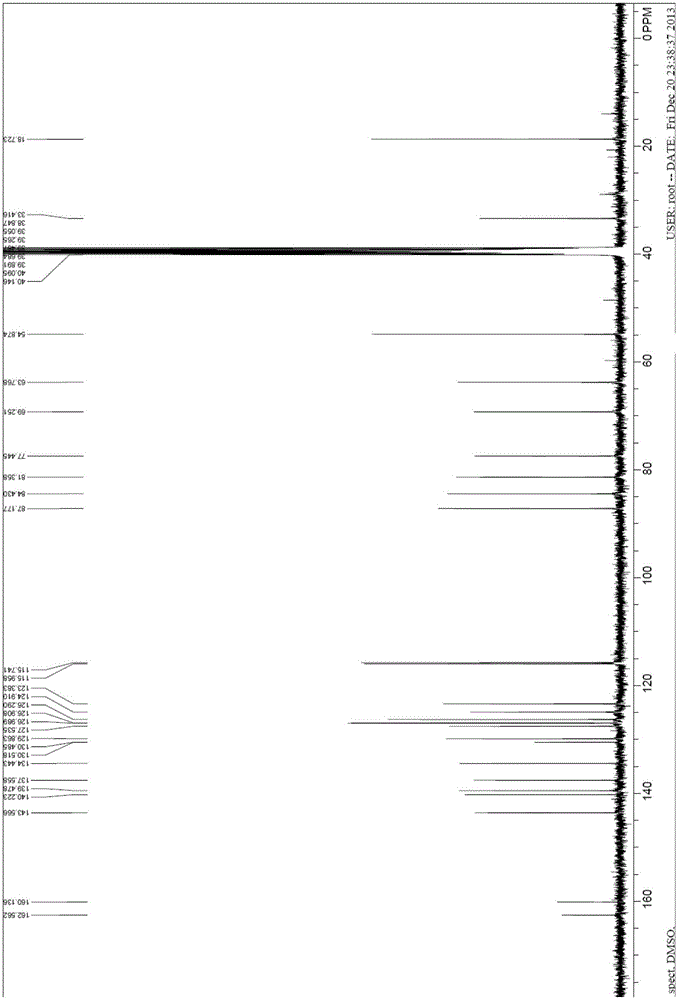
ActiveCN107141236AEasy to separate and purifyMild reaction conditionsEsterified saccharide compoundsSugar derivativesTicagrelorSynthesis methods
The invention discloses a novel synthesis method of a ticagrelor intermediate (1R,2R)-2-(3,4-difluorophenyl) cyclopropane nitrile. The novel synthesis method comprises the following steps: firstly, carrying out amination reaction on a glucose molecule chiral auxiliary reagent and (2E)-3-(3,4-diflurophenyl) acryloyl chloride; secondly, carrying out cyclopropanation reaction on a reactant and a sulfur ylide reagent to obtain a N-glucosyl (1R,2R)-2-(3,4-diflurophenyl) cyclopropanecarboxamide; thirdly, carrying out beta-N-glycosylation reaction on the N-glucosyl(1R,2R)-2-(3,4-diflurophenyl) cyclopropanecarboxamide under the action of PPh3, CBr4, AgOTf and NH3 water. The novel method has the characteristics of shorter process route, low-priced and easily-obtained starting materials, high yield, good stereoselectivity, good enantioselectivity and the like; the production cost is reduced to a great extent and better benefits in economy are obtained.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
Canagliflozin drug impurity as well as preparation method and application thereof
ActiveCN107286143AStarting materials are cheap and readily availableReduce stepsOrganic chemistry methodsOrganic solventLithium hydroxide
The invention discloses a canagliflozin drug impurity as well as a preparation method and application thereof. The invention provides a compound as well as a preparation method and application thereof. The method comprises the following steps: (1) enabling the compound as shown in formula 2 to be in contact with an alkaline lithium hydroxide aqueous solution to obtain a coarse product containing a compound as shown in formula 3, wherein the coarse product contains a compound as shown in formula 1; (2) crystallizing and filtering the coarse product to obtain mother liquor; (3) concentrating the mother liquor to obtain residues; and (4) crystallizing and filtering the residues in an L-proline-containing organic solvent, thus obtaining the compound as shown in formula 1. The method provided by the invention can realize directed preparation of the compound as shown in formula 1, and a reliable impurity contrast is provided for quality research on industrially produced canagliflozin-series diabetes treatment drug products and quantitative control over impurities.
Owner:WATERSTONE PHARMA WUHAN
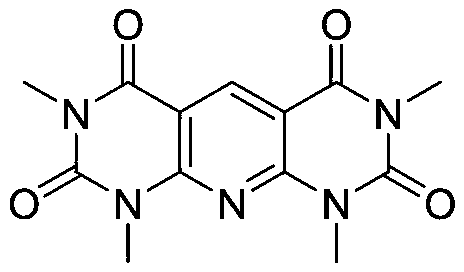
Preparation method of pyridodipyrimidine and pyridodipyrazole derivatives
InactiveCN110790763AReduce consumptionEasy to separateOrganic chemistryMethyl benzeneBiochemical engineering
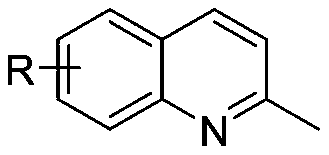
The invention discloses a preparation method of pyridodipyrimidine and pyridodipyrazole derivatives. The method comprises the following steps: carrying out a contact reaction on substituted methylquinoline derivatives, 6-amino-1,3-dimethylpyrimidine-2,4-dione or 3-methyl-1-phenyl-5-aminopyrazole and an iodine elementary substance to synthesize pyridodipyrimidine compounds, pyridodipyrazole compounds and derivatives thereof in one-pot. According to the preparation method, the adopted raw materials are cheap and easy to obtain, the preparation method is simple, steps are relatively short, the yield is up to 97%, and a feasible method is provided for industrially preparing the compounds.
Owner:YANTAI UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

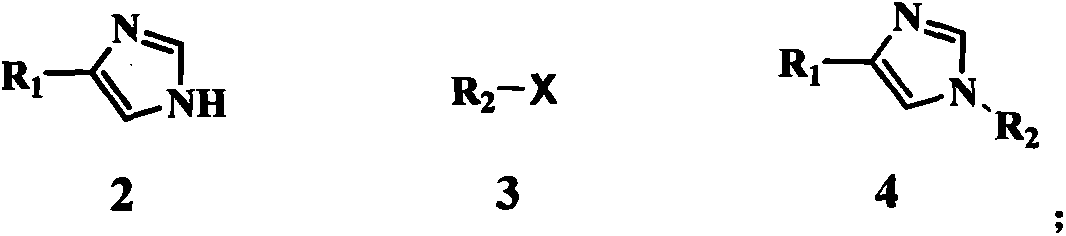


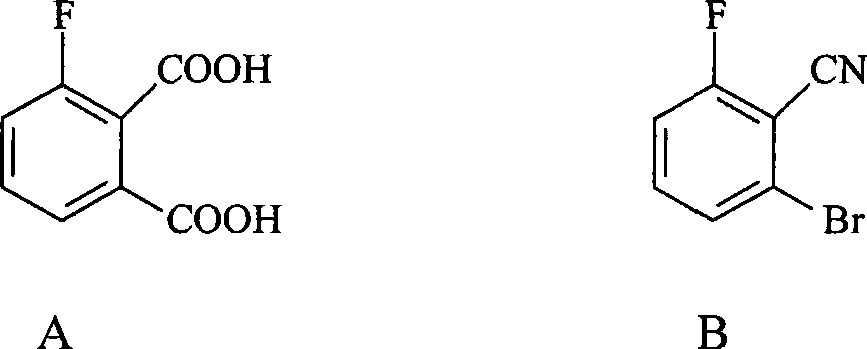
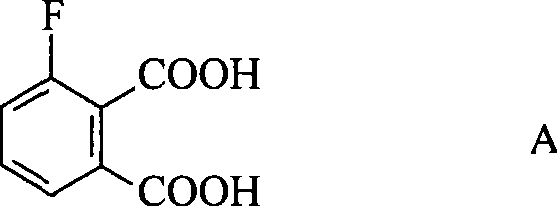






![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000011.PNG)
![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000012.PNG)
![Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate Synthesis method of tert-butyl 4,7-diazaspiro[2.5]octyl-7-formate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/98933b6c-efbc-47d1-a3d7-5eaa2ddeed5c/HDA0000793427800000021.PNG)