Preparation method of betamethasone intermediate
A technology of betamethasone and intermediates, which is applied in the field of preparation of steroid drug intermediates, can solve the problems of long steps and high costs, and achieve the effect of simple reaction operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
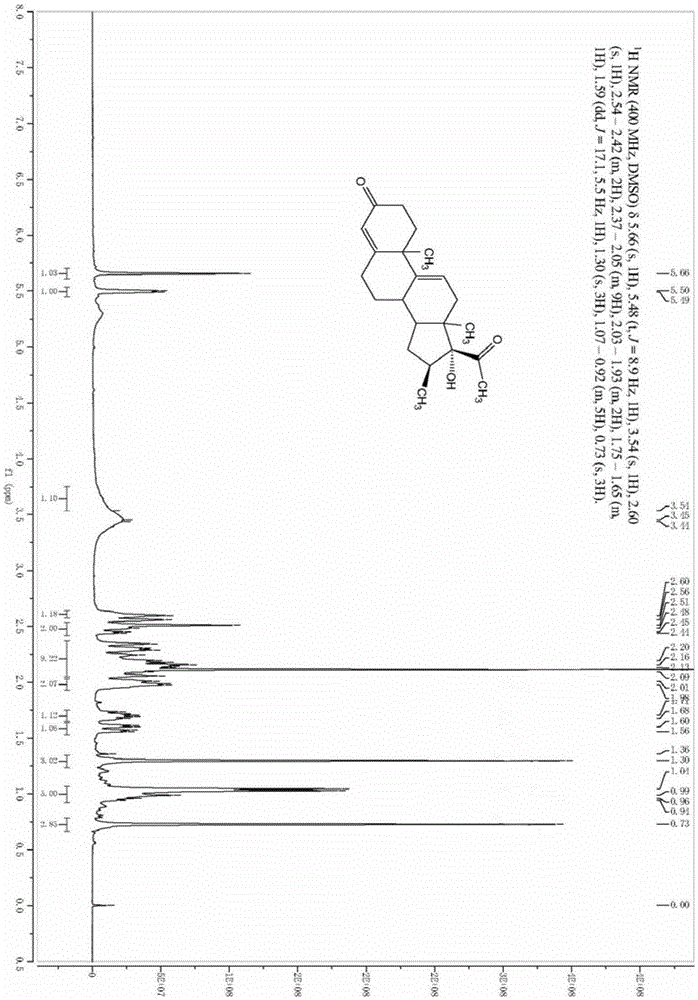
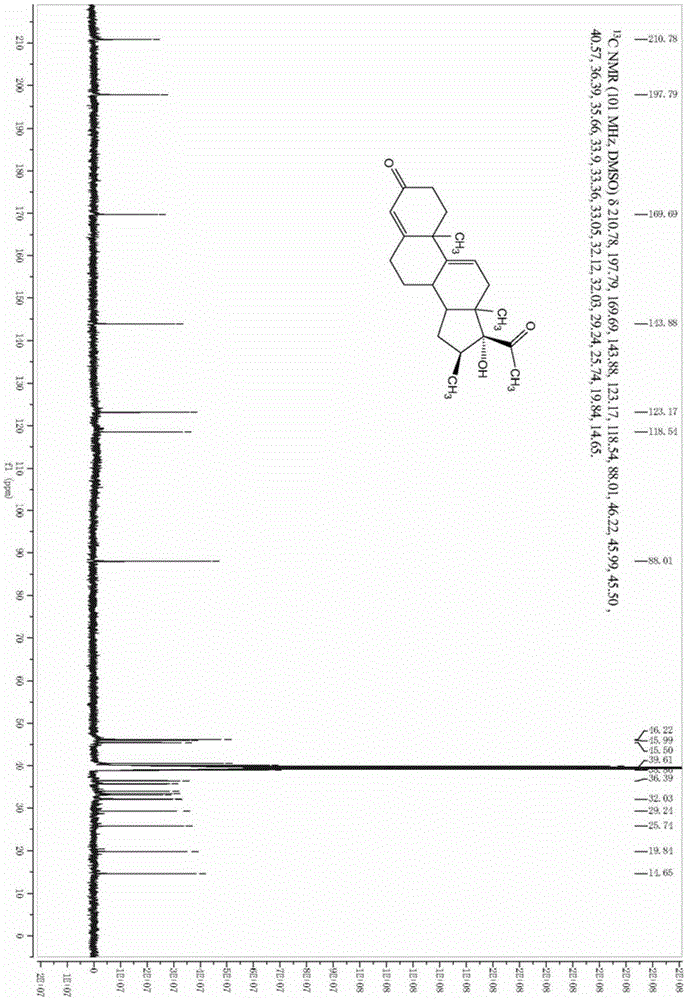
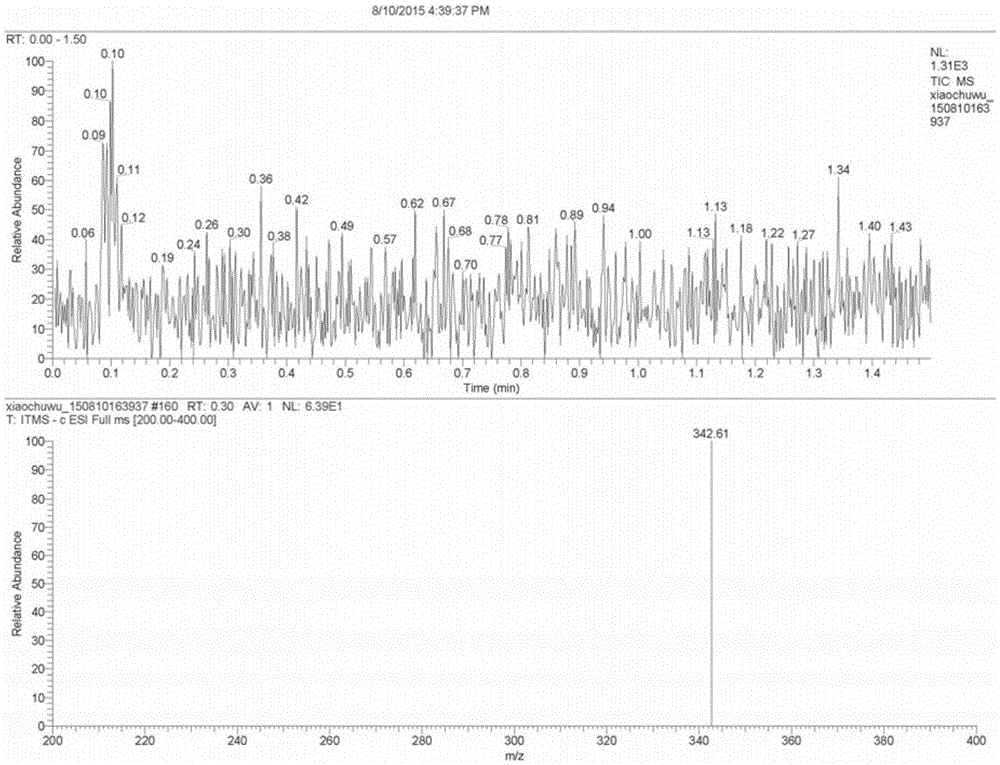
[0034] A preparation method of a betamethasone intermediate, the betamethasone intermediate is 16β-methyl-17α-hydroxypregna-4,9-diene-3,20-dione, the structural formula is as shown in formula VI Shown, described preparation method comprises:
[0035] (1) Cyanide reaction
[0036] Under nitrogen protection, put 100g of the compound of formula I into a clean four-necked reaction flask, add 200ml of methanol, stir, then add 50ml of acetone cyanohydrin, stir to raise the temperature to 40-45°C, and dropwise add 100ml of carbonic acid with a mass concentration of 5%. Potassium aqueous solution, the dropwise addition time is about 30 minutes, after the dropwise addition is completed, keep warm and stir for 24 hours, take samples for TLC tracking, until the raw materials are completely reacted. Dilute the reaction solution into 1000ml of water, stir for 1 hour, let stand for 2 hours, filter with suction, wash the filter cake with a large amount of water until it is neutral, and dry ...
Embodiment 2
[0046] A preparation method of a betamethasone intermediate, the betamethasone intermediate is 16β-methyl-17α-hydroxypregna-4,9-diene-3,20-dione, the structural formula is as shown in formula VI Shown, described preparation method comprises:
[0047] (1) Cyanide reaction
[0048] Under nitrogen protection, put 100g of the compound of formula I into a clean four-necked reaction flask, add 100ml of methanol, stir, then add 150ml of acetone cyanohydrin, stir to raise the temperature to 45-50°C, and dropwise add 200ml of carbonic acid with a mass concentration of 5%. Potassium aqueous solution, the dropwise addition time is about 30 minutes, after the dropwise addition is completed, keep warm and stir for 24 hours, take samples for TLC tracking, until the raw materials are completely reacted. Dilute the reaction solution into 1000ml of water, stir for 1 hour, let stand for 2 hours, filter with suction, wash the filter cake with a large amount of water until neutral, and dry the f...
Embodiment 3
[0058] A preparation method of a betamethasone intermediate, the betamethasone intermediate is 16β-methyl-17α-hydroxypregna-4,9-diene-3,20-dione, the structural formula is as shown in formula VI Shown, described preparation method comprises:
[0059] (1) Cyanide reaction
[0060] Under the protection of nitrogen, put 100g of the compound of formula I into a clean four-necked reaction flask, add 300ml of methanol, stir, then add 250ml of acetone cyanohydrin, stir to raise the temperature to 40-45°C, and dropwise add 250ml of carbonic acid with a mass concentration of 5%. Potassium aqueous solution, the dropwise addition time is about 30 minutes, after the dropwise addition is completed, keep warm and stir for 24 hours, take samples for TLC tracking, until the raw materials are completely reacted. Dilute the reaction solution into 1000ml of water, stir for 1 hour, let stand for 2 hours, filter with suction, wash the filter cake with a large amount of water until neutral, and dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com