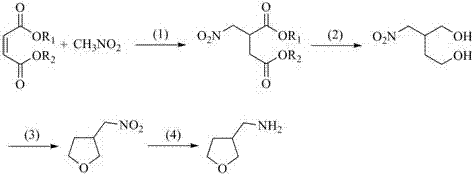
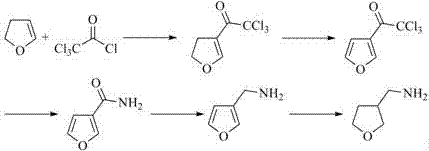
Synthesis method of 3-tetrahydro-furanmethanamine
A technology of aminomethyltetrahydrofuran and nitromethyltetrahydrofuran, which is applied in the field of synthesizing 3-aminomethyltetrahydrofuran, can solve the problems of unsuitability for industrial production, long synthesis route, improper operation, etc., and achieves low cost of raw materials and short synthesis route. , The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of 2-nitromethyl-1,4-diethyl succinate:
[0038] Add potassium carbonate (10.4 g, 0.075 mol) to nitromethane (100 mL), then add diethyl maleate (43.0 g, 0.25 mol), after the addition is complete, stir the reaction at room temperature for 12 h, filter off potassium carbonate , concentrated to recover nitromethane, and the residue was distilled under reduced pressure to obtain 54.0 g of diethyl 2-nitromethyl-1,4-butanedioate with a yield of 92.6%. 1 H NMR (400MHz, CDCl 3 ) δ 4.82-4.77 (dd, J = 8.0, 4.0 Hz, 1H), 4.71-4.66 (dd, J = 8.0,4.0 Hz, 1H), 4.21-4.17 (q, J = 4.0 Hz, 2H), 4.16-4.12 (q, J = 4.0 Hz, 2H),3.55-3.49 (m, 1H), 2.84-2.79 (dd, J = 4.0, 4.0 Hz, 1H), 2.71-2.65 (dd, J =8.0, 8.0 Hz, 1H), 1.26-1.22 (t, J = 8.0 Hz, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ170.49, 170.23, 74.41, 61.84, 61.16, 39.05, 32.87, 14.02, 13.88.
[0039] Synthesis of 2-nitromethyl-1,4-butanediol:
[0040]A solution of 2-nitromethyl-1,4-butanedioic acid diethyl ester (46....
Embodiment 2
[0046] Synthesis of 2-nitromethyl-1,4-diethyl succinate:
[0047] Add potassium carbonate (6.9 g, 0.05 mol) to nitromethane (75 mL), then add diethyl maleate (43.0 g, 0.25 mol), after the addition is complete, stir the reaction at room temperature for 14 h, filter off potassium carbonate , concentrated to recover nitromethane, and the residue was distilled under reduced pressure to obtain 52.9 g of diethyl 2-nitromethyl-1,4-butanedioate with a yield of 90.7%.
[0048] Synthesis of 2-nitromethyl-1,4-butanediol:
[0049] A solution of 2-nitromethyl-1,4-butanedioic acid diethyl ester (46.6 g, 0.20 mol) in MeOH (42 mL) was slowly added dropwise to sodium borohydride (13.6 g, 0.36 mol) in tetrahydrofuran (180 mL) suspension, keep the reaction temperature at 20~30°C during the dropwise addition o Between C. The dropwise addition was complete, and the reaction was maintained at this temperature with stirring for 4 h, and then the temperature was raised to 60 o C was refluxed and ...
Embodiment 3
[0055] Synthesis of 2-nitromethyl-1,4-dibutyl succinate:
[0056] Potassium fluoride (3.5 g, 0.06 mol) was added to nitromethane (100 mL), and then dibutyl maleate (45.7 g, 0.2 mol) was added. After the addition was complete, the reaction was stirred at room temperature for 13 h, concentrated to recover nitrate Dichloromethane, the residue was dissolved in dichloromethane (100 mL), then washed with water, the organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and distilled under reduced pressure to obtain 2-nitromethyl-1,4-butane 49.5 g of dibutyl diacid, the yield is 85.5%.
[0057] Synthesis of 2-nitromethyl-1,4-butanediol:
[0058] A solution of 2-nitromethyl-1,4-butanedioic acid dibutyl (57.9 g, 0.2 mol) in MeOH (42 mL) was slowly added dropwise to potassium borohydride (21.6 g, 0.4 mol) in tetrahydrofuran (180 mL) suspension, keep the reaction temperature at 15~30°C during the dropwise addition o Between C. The dropwise addition was complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com