Method for preparing emtricitabine
A technology of emtricitabine and reaction time, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of expensive raw materials and reagents, low total yield and high cost, and achieves green environmental protection reagents, mild reaction conditions, and easy operation. The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
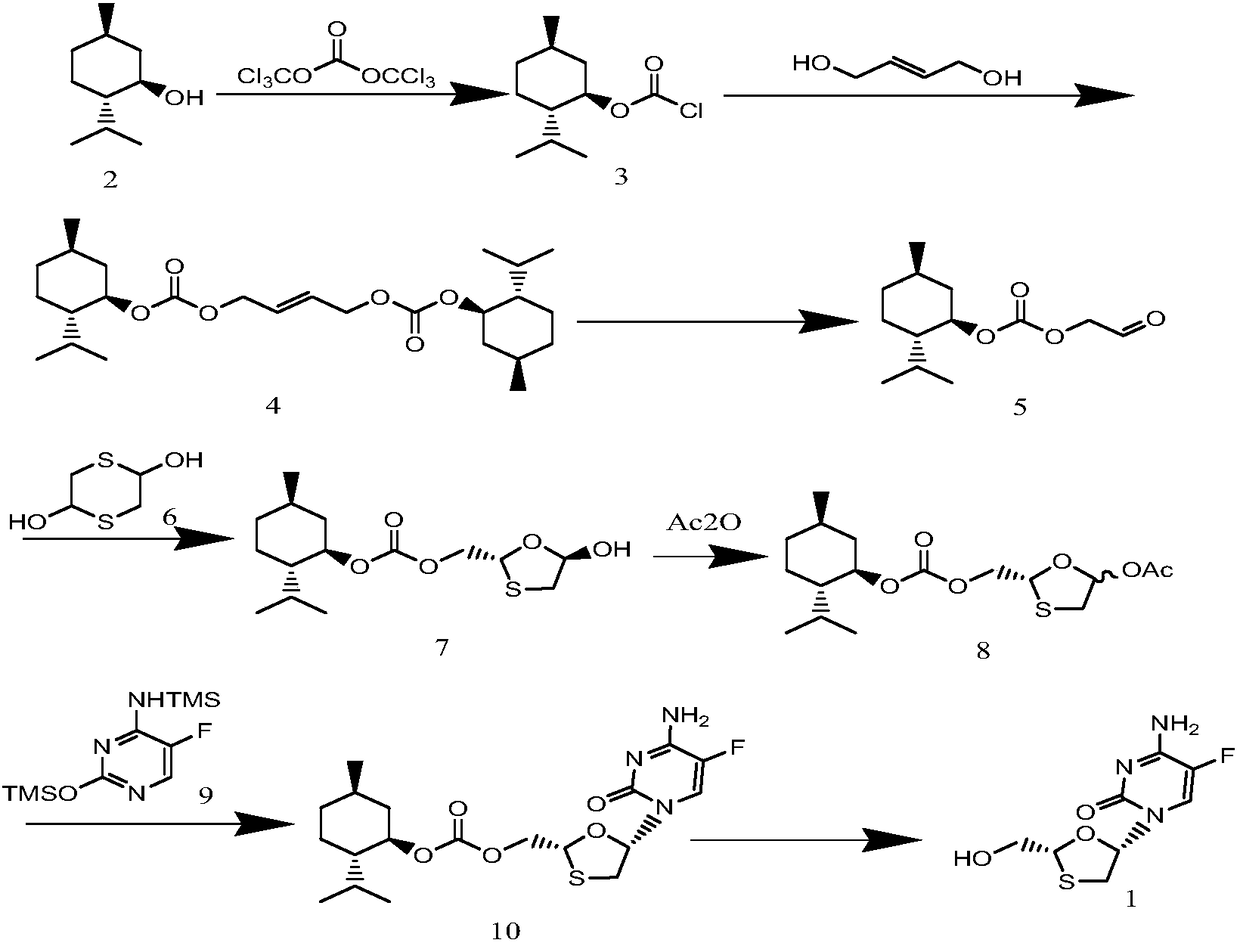
[0047] Preparation of (1R,2S,5R)-2-isopropyl-5-R-methyl-1-cyclohexylchloroformate 2:
[0048] Add 15.60g (0.1mol) L-menthol and 156ml dichloromethane into a round-bottomed three-necked flask equipped with mechanical stirring, thermometer, constant pressure addition funnel and exhaust gas absorption device, stir to make it fully dissolve, and cool to -5°C , add 14.84g (0.05mol) triphosgene and continue to stir to make it fully dissolved, then add dropwise a solution of 37.44g triethylamine and 78ml dichloromethane, and the dropwise addition is completed in about 1.5 hours. Insulate and react for 2 hours, enter the second reaction stage, raise the temperature to 25°C, stir and react for about 6 hours, after the reaction, filter and separate the precipitate, the mother liquor is sequentially passed through distilled water, 5% dilute hydrochloric acid, 5% sodium carbonate, saturated Washed with brine, dried with anhydrous sodium sulfate, finally rectified under reduced pressure, c...
Embodiment 2
[0050] Preparation of but-2-ene-1,4-diylbis((1R,2S,5R)-2-isopropyl-5-methylcyclohexyl)bis(carbonate) 4:
[0051] Add 4.93g (0.052mol) butylene glycol and 30ml dichloromethane into a round-bottomed three-necked flask equipped with mechanical stirring, a thermometer, and a constant-pressure addition funnel, stir well, cool to 0°C in an ice-water bath, add 6.48g triethylene glycol Amine, stirred evenly, dissolved 21.88g (0.10mol) of compound 2 obtained in 45ml of dichloromethane and slowly added dropwise to a three-necked flask, stirred and reacted for 1 hour, filtered to separate the precipitate, and the mother liquor was washed with saturated sodium bicarbonate solution. Then it was dried with anhydrous sodium sulfate, and the solvent was distilled off to obtain 18.98 g of compound 4 with a yield of 84%. 1 H-NMR (CDCl 3 )δ: 5.88(t, 2H), 4.77(d, 4H), 4.51(m, 2H), 1.83(m, 2H), 1.75-1.50(t, 4H), 1.63-1.38(m, 8H), 1.54 (m, 2H), 1.41 (m, 2H), 0.86 (d, 6H), 0.83 (d, 12H). Elementa...
Embodiment 3
[0053] Preparation of (1R, 2S, 5R)-2-isopropyl-5-methylcyclohexyl (2-oxoethyl) carbonate:
[0054] Add 2.52g (0.01mol) tungstic acid, 17.41ml (0.30mol) 50% H 2 0 2 Aqueous solution, 83ml n-butanol solution, stirred for half an hour, then added 22.59g (0.05mol) of the obtained compound (4), stirred evenly, raised the temperature to 80°C, reacted for 5 hours, removed the catalyst by centrifugation, extracted, and used anhydrous After drying over magnesium sulfate, the solvent was distilled off under reduced pressure to obtain 18.83 g of compound 5, with a yield of 79%. 1 H-NMR (CDCl 3 ) δ: 9.65(s, 2H), 4.67(s, 2H), 4.51(m, 1H), 2.10(m, 1H), 2.00(m, 1H), 1,70(m, 2H), 1.51(m , 2H), 1.28 (m, 1H), 1.12 (m, 2H), 0.9 (m, 6H), 0.81 (d, 3H). Elemental Analysis C 13 h 22 o 4 Measured value (%): C65.48, H9.15, 026.40; theoretical value (%) C64.44, H9.15, O26.41.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com