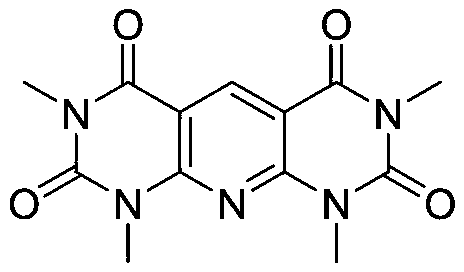
Preparation method of pyridodipyrimidine and pyridodipyrazole derivatives
A technology for pyridodipyrazole and derivatives, which is applied in the field of synthesis of pyridodipyrimidine derivatives and pyridodipyrazole derivatives, can solve problems such as unfavorable industrial production, high reaction temperature, complicated procedures, etc. Low cost, high reaction efficiency, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis:
[0058] The reaction formula is:
[0059]
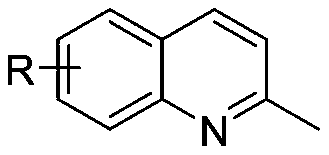
[0060] The specific steps are: add 0.3mmol 6-bromo-2-methylquinoline, 0.66mmol 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, 0.45 mmol I 2 , 2mLDMSO, and the reaction mixture was stirred at 110° C. for 6 hours in an air environment, and the reaction solution was extracted with ethyl acetate, and the organic layer was washed with 10% sodium thiosulfate solution (w / w), dried over anhydrous sodium sulfate, Concentrate under reduced pressure and dry in vacuo to get the crude product, use ethyl acetate / petroleum ether=2:1 (V / V) as the eluent for column separation and purification of the crude product to get the desired product, the product is a white solid, the yield was 86%.
[0061] The NMR result of gained product is: 1 H NMR (400MHz, CDCl 3 )δ8.16(dd, J=8.9,0.7Hz,1H),8.06(d,J=2.5Hz,1H),7.88-7.82(dt,J=8.9,0.7Hz,1H),7.75(dd,J =9.0, 2.3Hz, 1H), 7.41(d, J=8.6Hz, 1H), 3.78(s, 6H), 3.24(s, 6H). 13 C NMR (10...
Embodiment 2
[0063] Synthesis:
[0064] The reaction formula is:
[0065]
[0066] The specific steps are: add 0.3mmol 7-chloro-2-methylquinoline, 0.66mmol 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, 0.45 mmol I 2 , 2mL DMSO, the reaction mixture was stirred at 110°C for 6 hours in air, and the reaction solution was extracted with ethyl acetate, the organic layer was washed with 10% sodium thiosulfate solution (w / w), dried over anhydrous sodium sulfate , concentrated under reduced pressure and vacuum dried to obtain the crude product, the crude product was separated and purified by column separation and purification with ethyl acetate / petroleum ether=2:1 (V / V) as the eluent to obtain the desired product, the product was a white solid, and The rate is 78%.
[0067] The NMR result of gained product is: 1 H NMR (400MHz, CDCl 3 )δ8.23(dd, J=8.7,1.0Hz,1H),7.99(d,J=2.4,1H),7.84(d,J=8.8Hz,1H),7.51(dd,J=8.8,2.2Hz , 1H), 7.40(d, J=8.5Hz, 1H), 3.78(s, 6H), 3.25(s, 6H). 13 C NMR (100...
Embodiment 3
[0069] Synthesis:
[0070] The reaction formula is:
[0071]
[0072] The specific steps are: add 0.3mmol 6-chloro-2-methylquinoline, 0.66mmol 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione, 0.45 mmol I 2 , 2mLDMSO, and the reaction mixture was stirred at 110° C. for 10 hours in an air environment, and the reaction solution was extracted with ethyl acetate. The organic layer was washed with 10% sodium thiosulfate solution (w / w), and dried over anhydrous sodium sulfate. Concentrate under reduced pressure and dry in vacuo to get the crude product, use ethyl acetate / petroleum ether=1:2 (V / V) as the eluent for column separation and purification to get the desired product, the product is a white solid, the yield was 52%.
[0073] The NMR result of gained product is: 1 H NMR (400MHz, CDCl 3 )δ8.17(m,1H),7.92(d,J=9.0Hz,1H),7.88(d,J=2.6Hz,1H),7.62(dd,J=9.0,2.5Hz,1H),7.42( d,J=8.6Hz,1H),3.78(s,6H),3.24(s,6H). 13 C NMR (100MHz, CDCl 3 )δ159.14, 157.20, 156.60, 153.67, 150....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com