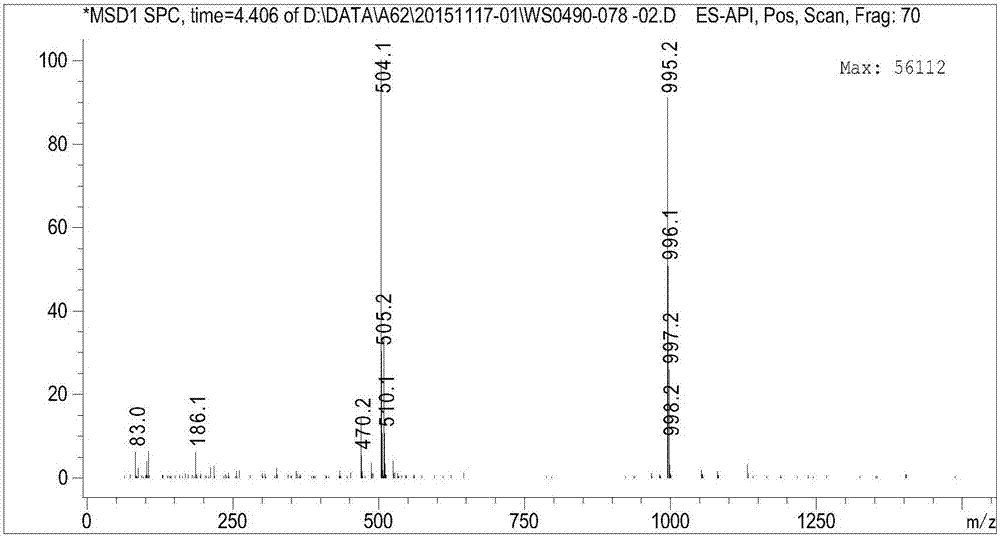
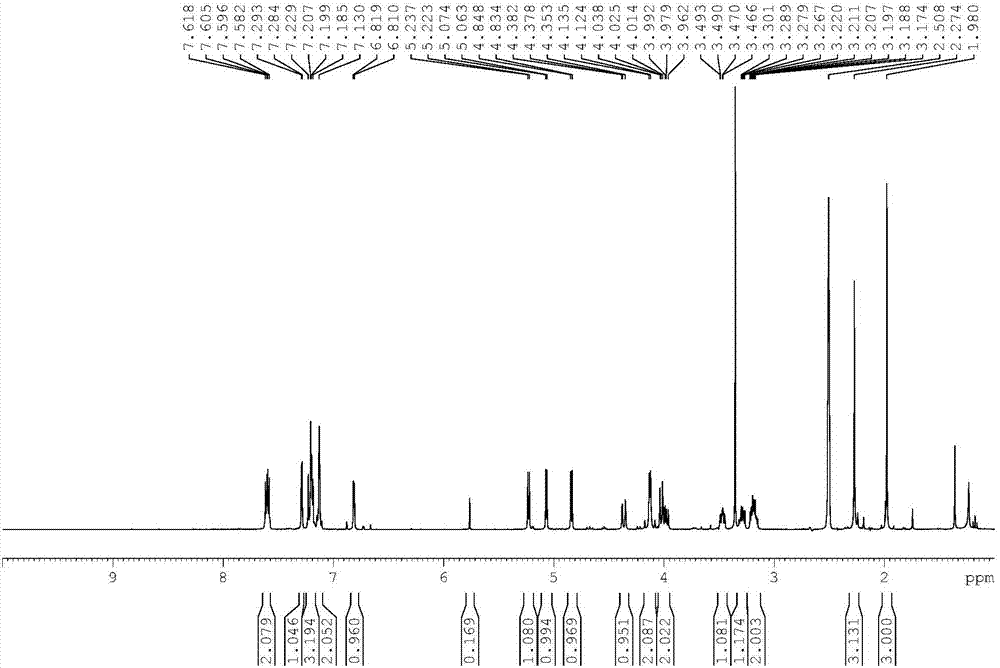
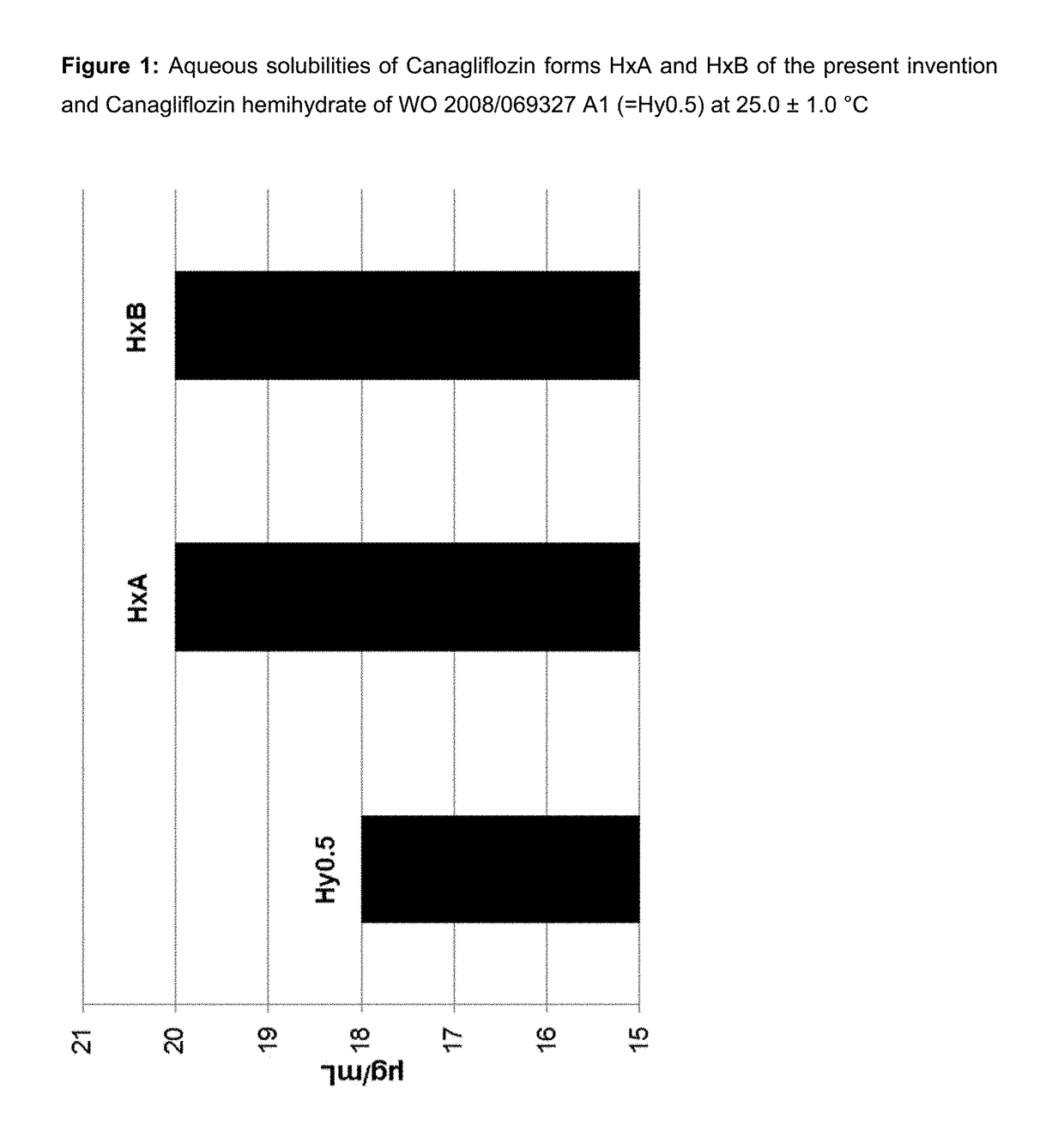
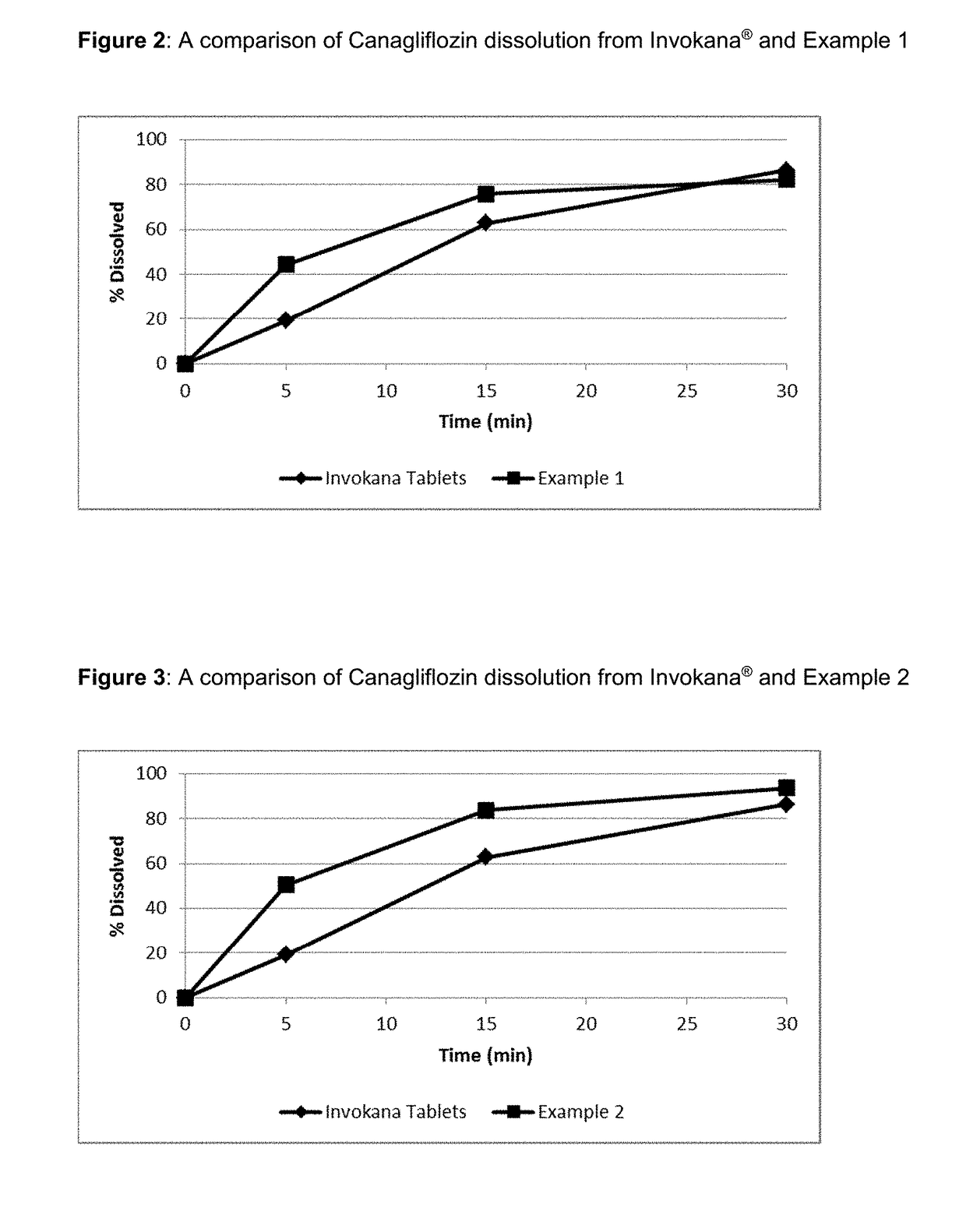
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
133 results about "Canagliflozin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
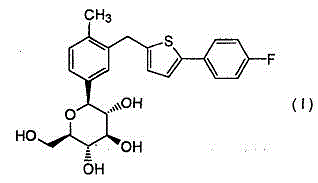
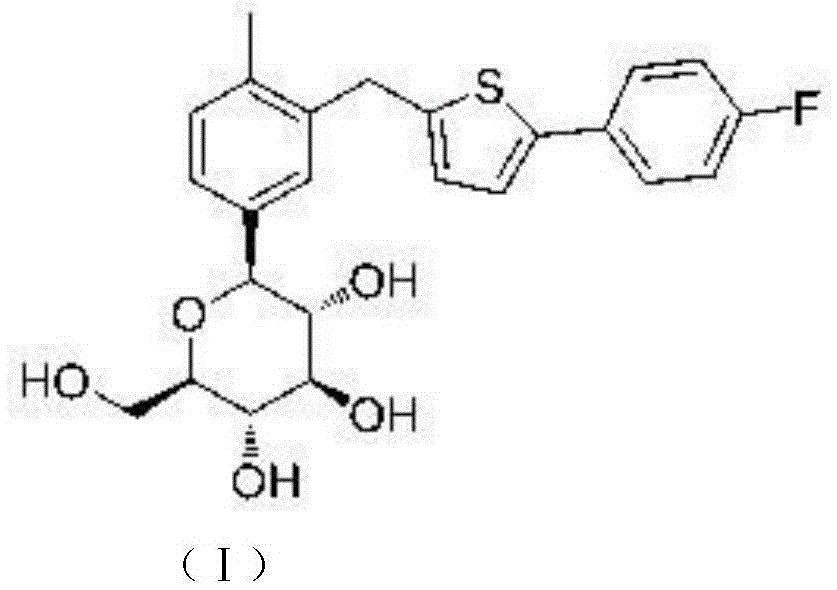
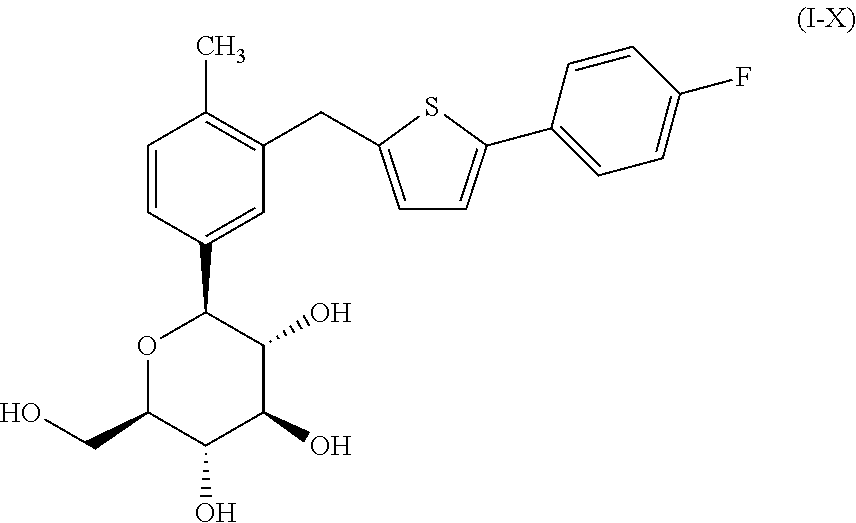
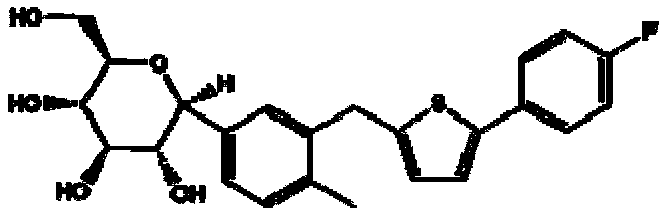
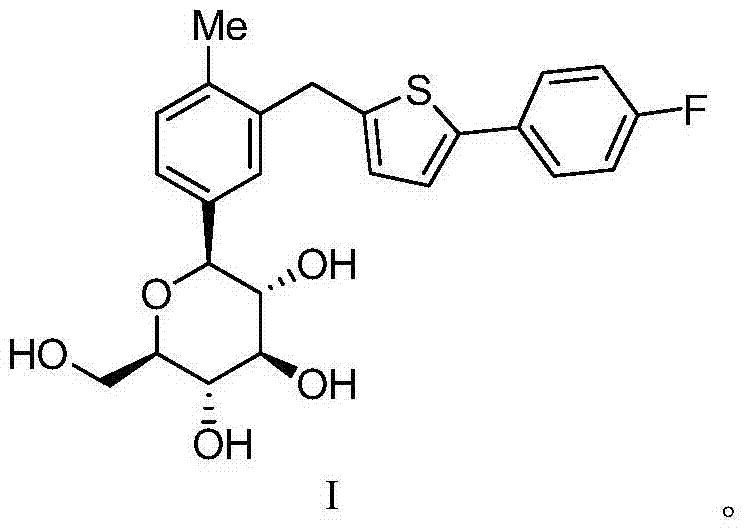
Canagliflozin is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes.
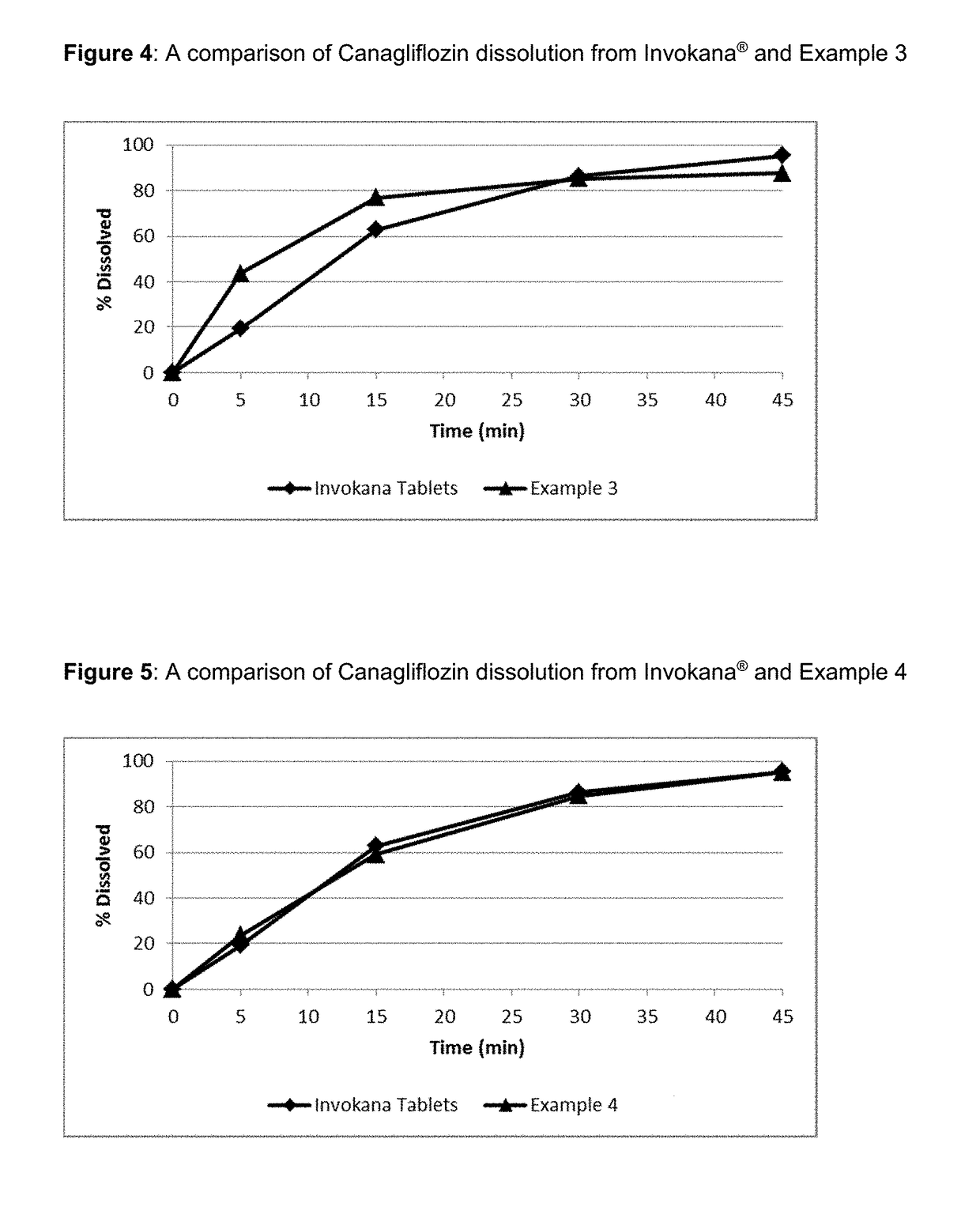
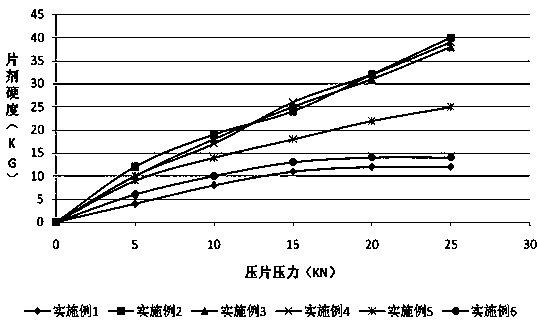
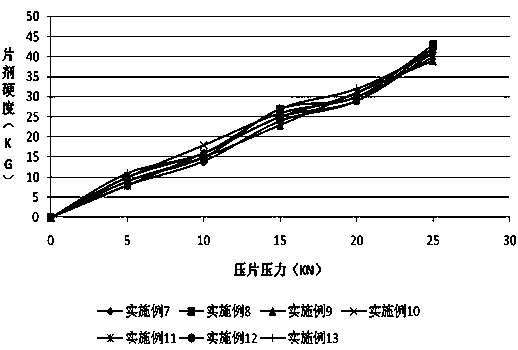
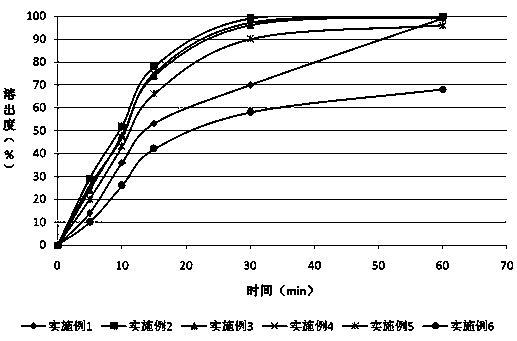
Oral solid preparation of canagliflozin and preparation method thereof
The invention relates to an oral solid medicine composition of canagliflozin and a preparation method thereof. The composition comprises canagliflozin and pharmaceutic adjuvants, wherein the canagliflozin is in an amorphous form, and the average grain size of particles is 2.5-30 microns. The composition can be used for effectively solving the technical problems that the crystal transformation and the compressibility of the canagliflozin in the amorphous form are poor in the preparation process of the solid preparation.
Owner:CHONGQING PHARMA RES INST
High-purity canagliflozin compound and preparation method thereof
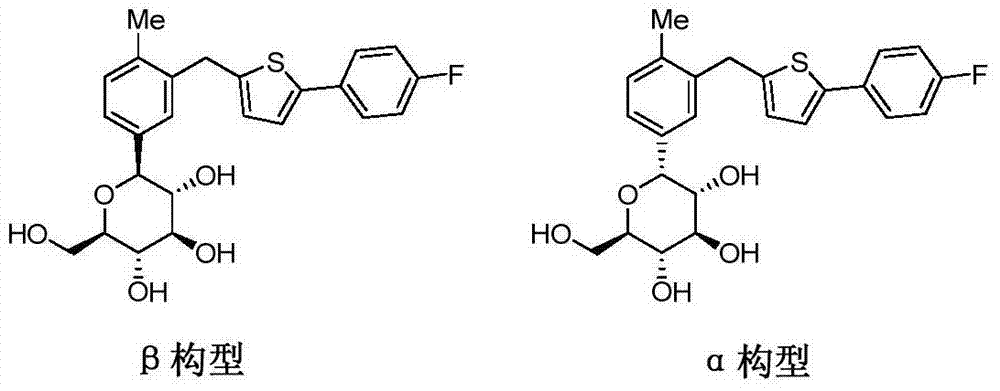
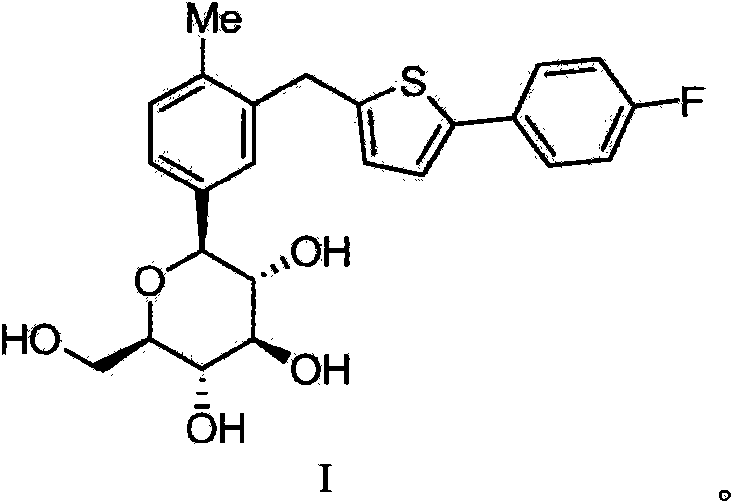
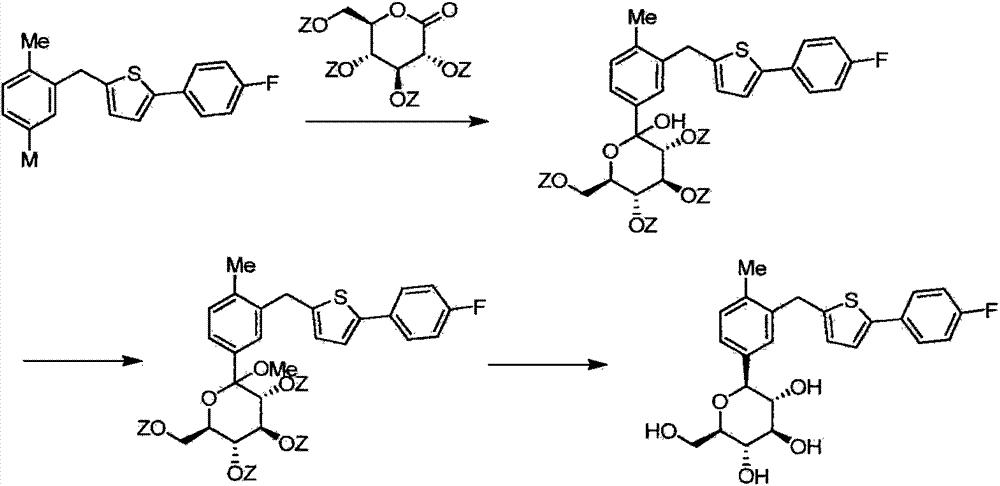
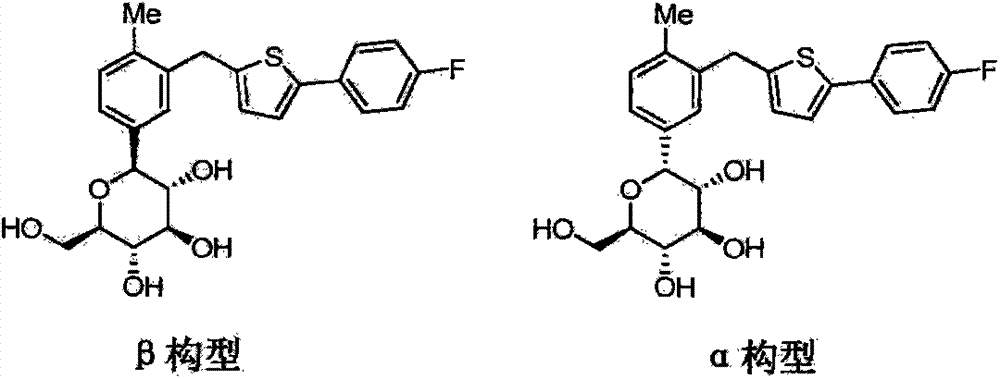
The invention belongs to the field of drug synthesis and relates to a high-purity canagliflozin compound represented by a formula I shown in a drawing and a preparation method thereof. According to the canagliflozin compound provided by the invention, the content of an alpha-configuration impurity represented by a formula II shown in a drawing is lower than 1% and is further lower than 0.5%. The preparation method comprises the steps of preparing a eutectic substance from canagliflozin and amino acid in a solvent, separating the eutectic substance, and then, decomposing the eutectic substance, thereby obtaining the canagliflozin compound.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
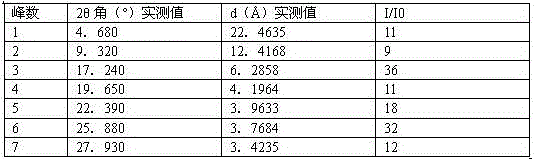
C crystal form of canagliflozin and crystal preparation method of canagliflozin
InactiveCN103936725AImprove stabilityImprove solubilityNervous disorderOrganic chemistrySolubilityX-ray
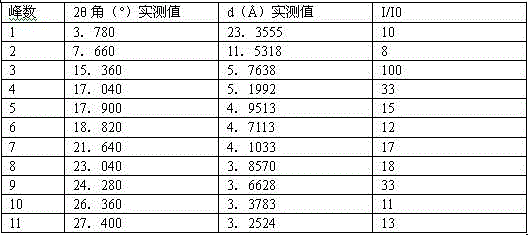
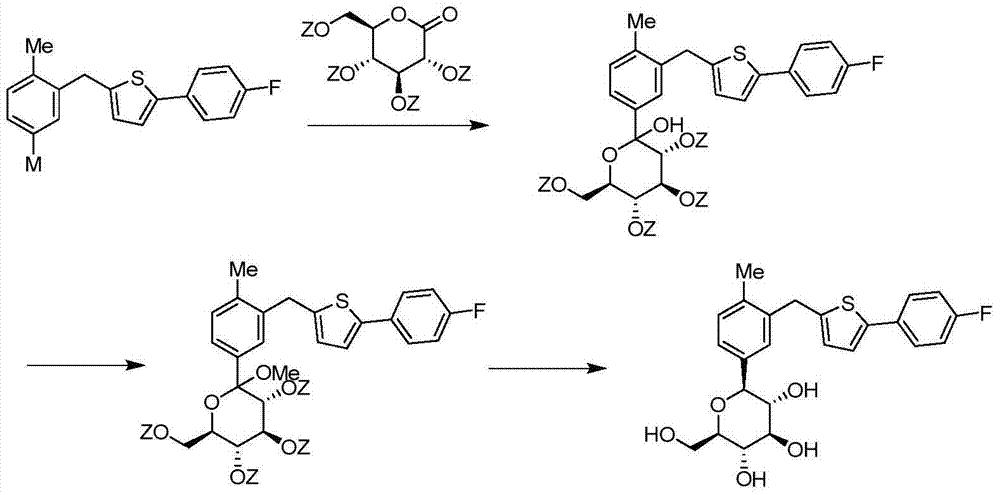
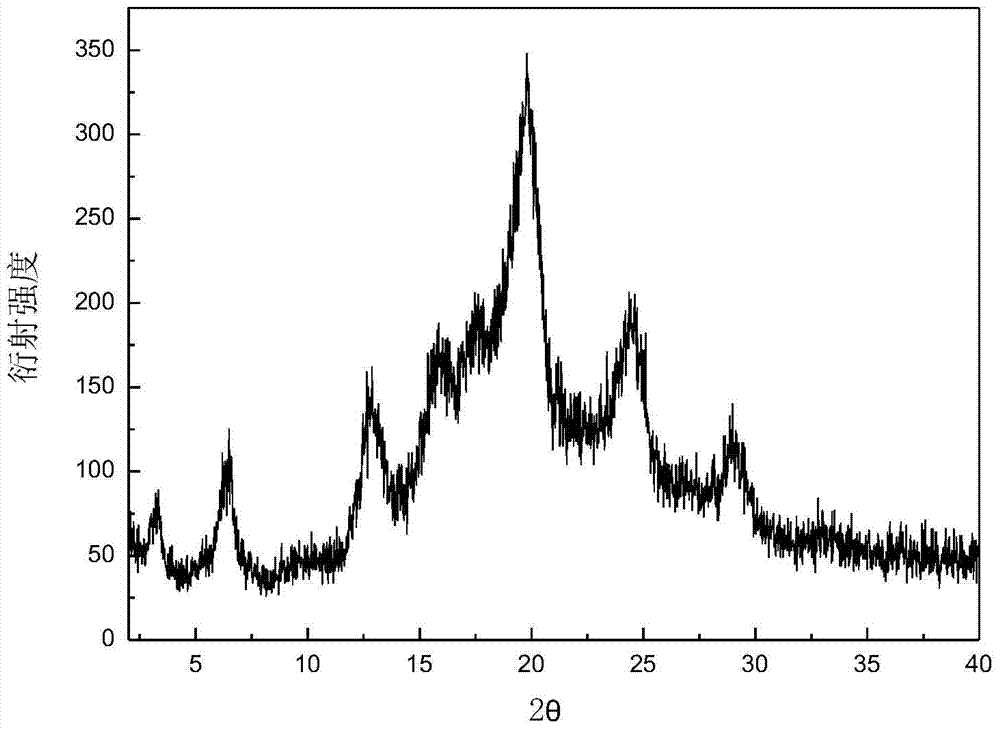
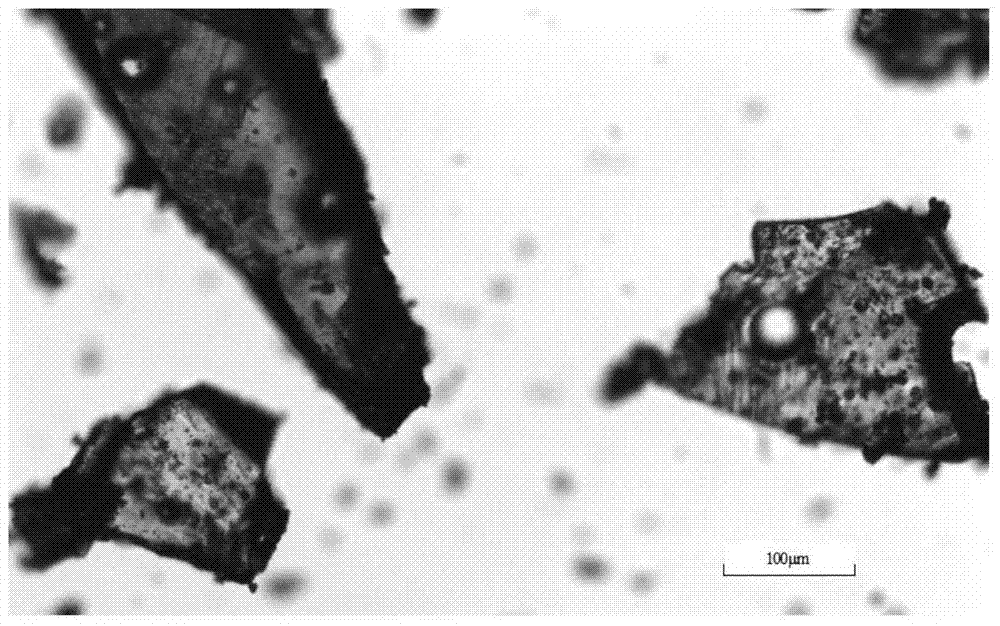
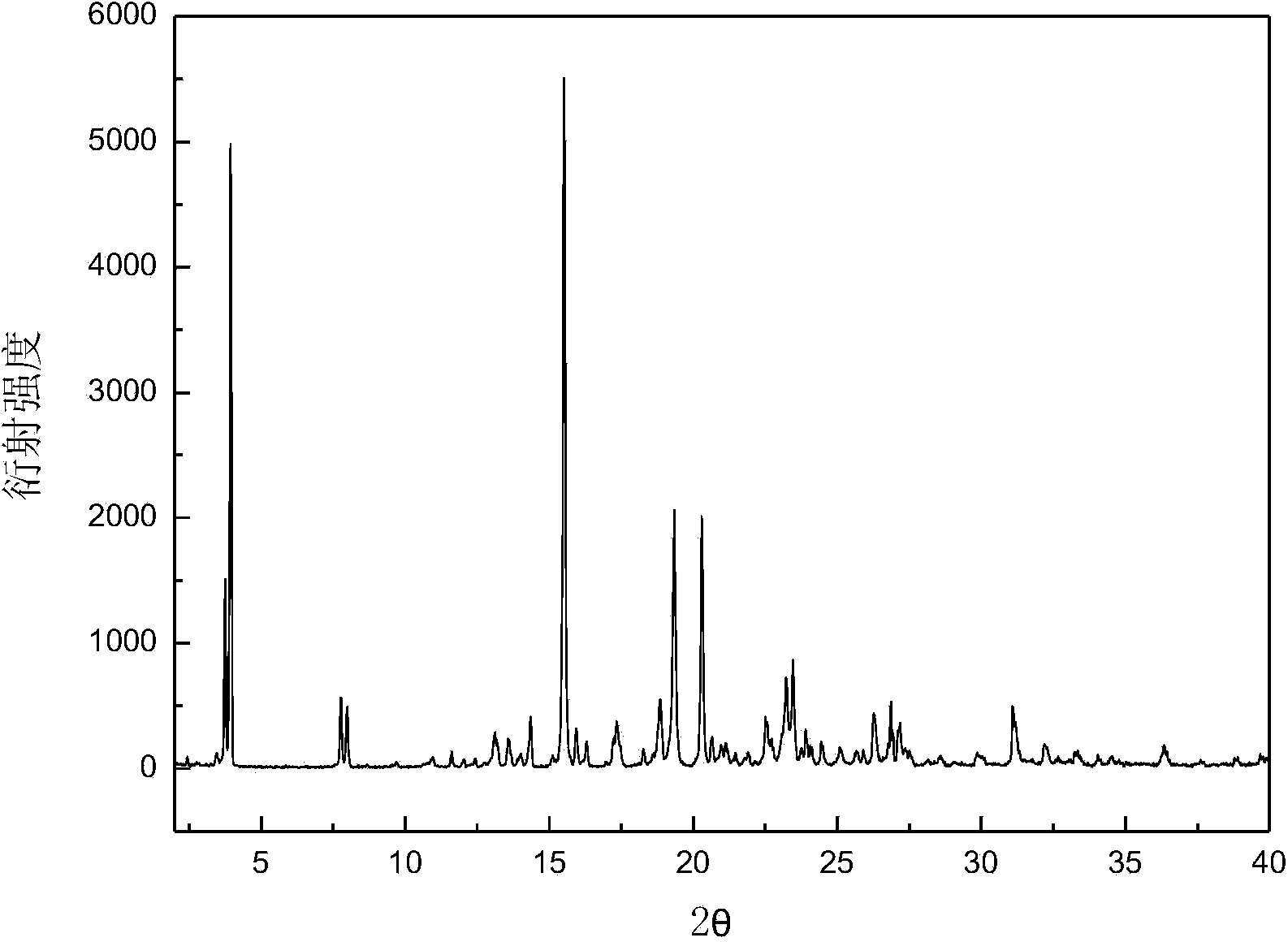
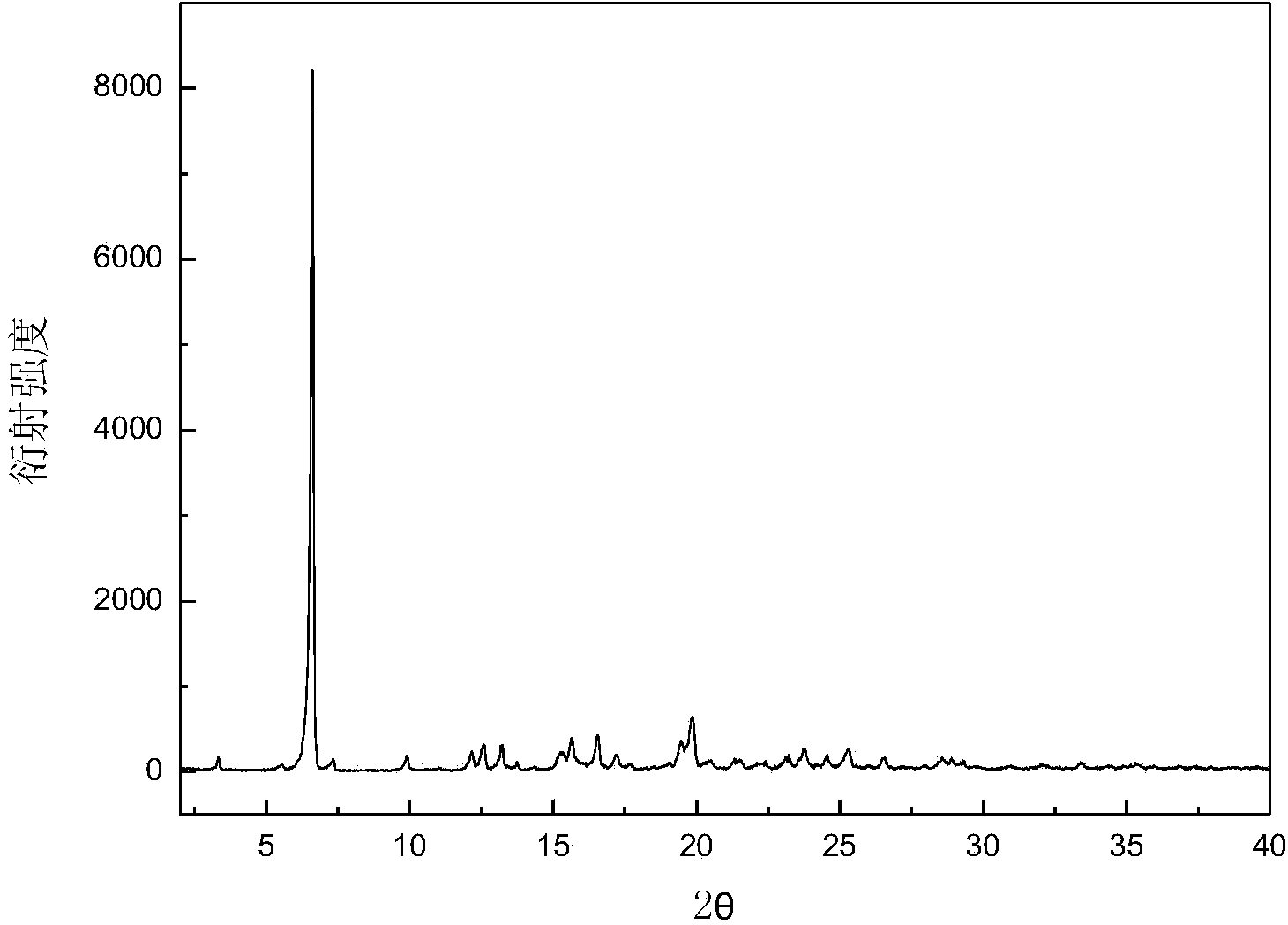
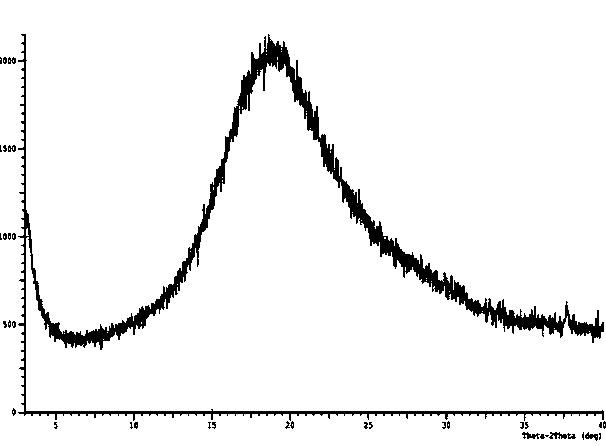
The invention discloses a C crystal form of canagliflozin and a crystal preparation method of the canagliflozin. The X-ray powder of the canagliflozin, which is diffracted at diffraction angles 2 theta which are equal to 3.4 + / - 0.1, 6.5 + / - 0.1, 12.7 + / - 0.1, 15.8 + / - 0.1, 19.8 + / - 0.1, 24.3 + / - 0.1, 24.8 + / - 0.1 and 29.1 + / - 0.1, has characteristic peaks and is called as C crystal form canagliflozin. The method has the beneficial effects of being simple to operate and less in energy consumption. The prepared novel crystal form product is good in stability, large in solubility and rapid in dissolution rate; the purity of the novel crystal product is higher than 99% and the yield of the novel crystal product is 91% or so; the purity, the color and the shape of the novel crystal product are not changed after the novel crystal product is stored for 100 days at a normal temperature under a drying condition. The novel crystal product can be easily smashed and is easily compatible with the dosage forms of medicine compositions as well as is low in cost. Thus, the novel crystal product is easily suitable for large-scale commercial and industrial applications.
Owner:TIANJIN UNIV +1
Canagliflozin of crystal form A, and crystallization preparation method thereof
InactiveCN103980261AEasy to joinEasy to operateSenses disorderNervous disorderCanagliflozinEnergy consumption
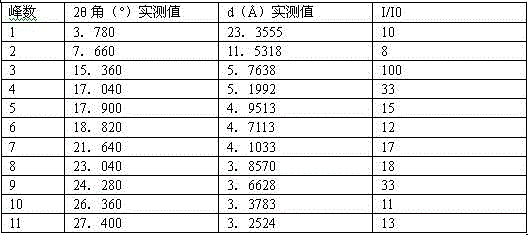
The invention discloses a canagliflozin of crystal form A, and a crystallization preparation method thereof. The X-ray powder diffraction pattern of the canagliflozin of crystal form A has characteristic peaks at diffraction angles 2theta with the values of 3.7+ / -0.1, 3.9+ / -0.1, 7.7+ / -0.1, 7.9+ / -0.1, 11.5+ / -0.1, 13.1+ / -0.1, 13.5+ / -0.1, 14.3+ / -0.1, 15.5+ / -0.1, 17.3+ / -0.1, 18.8+ / -0.1, 19.3+ / -0.1, 20.3+ / -0.1, 22.5+ / -0.1, 22.7+ / -0.1, 23.2+ / -0.1 and 23.4+ / -0.1. The preparation method has the advantages of simplicity and easy operation of operation steps, short time and less energy consumption. The above product prepared in the invention has a good stability, high a purity of above 99%, and has a yield of about 90%, and the purity, the color and the form of the product are unchanged after the product is stored under normal temperature dry conditions for 100d.
Owner:TIANJIN UNIV +1
Canagliflozin of crystal form B, and crystallization preparation method thereof
InactiveCN103980262AImprove solubilityFast dissolution rateSenses disorderNervous disorderSolubilityX-ray
The invention discloses a canagliflozin of crystal form B, and a crystallization preparation method thereof. The X-ray powder diffraction pattern of the canagliflozin of crystal form B has characteristic peaks at diffraction angles 2theta with the values of 3.4+ / -0.1, 6.6+ / -0.1, 12.6+ / -0.1, 13.2+ / -0.1, 15.3+ / -0.1, 15.6+ / -0.1, 16.5+ / -0.1, 19.4+ / -0.1, 19.8+ / -0.1 and 23.7+ / -0.1. The method has the advantages of high efficiency, easy operation and short time. Canagliflozin crystals of crystal form B prepared in the invention have a large solubility and a fast dissolving rate, the above product has a purity of above 99%, and has a yield of about 95%, and the purity, the color and the form of the product are unchanged after the product is stored under normal temperature dry conditions for 100d. The product has the advantages of easy crushing, easy addition of medicinal compositions, low cost, and easy commercial industrial large-scale enforcement.
Owner:TIANJIN UNIV +1
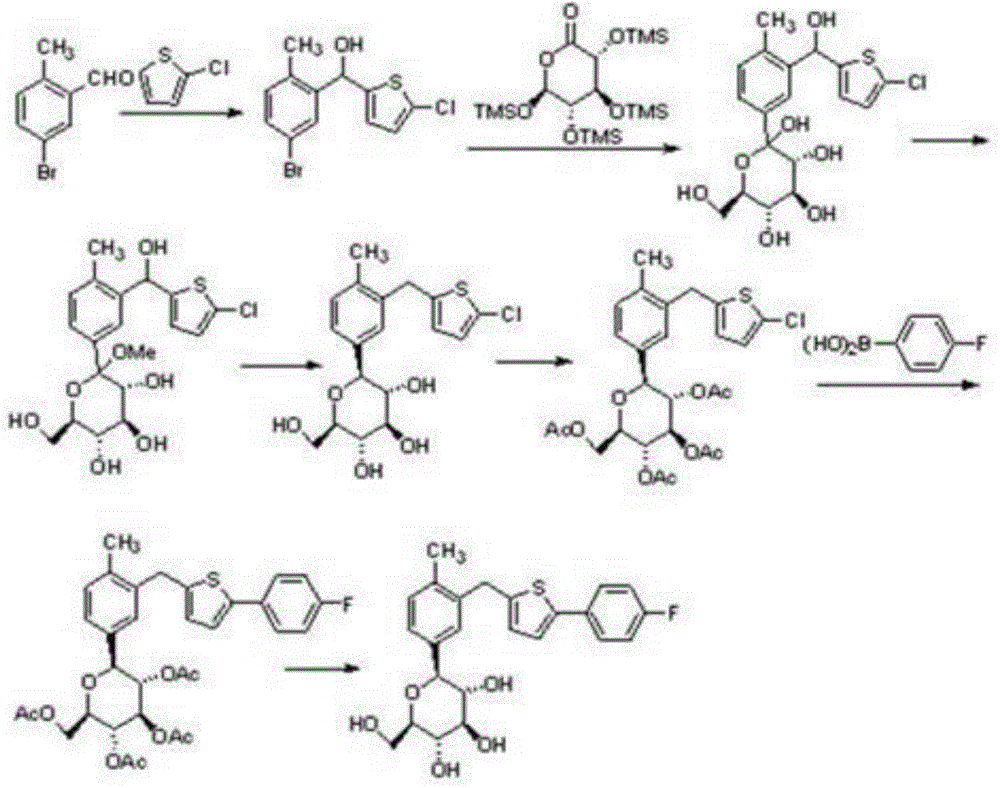
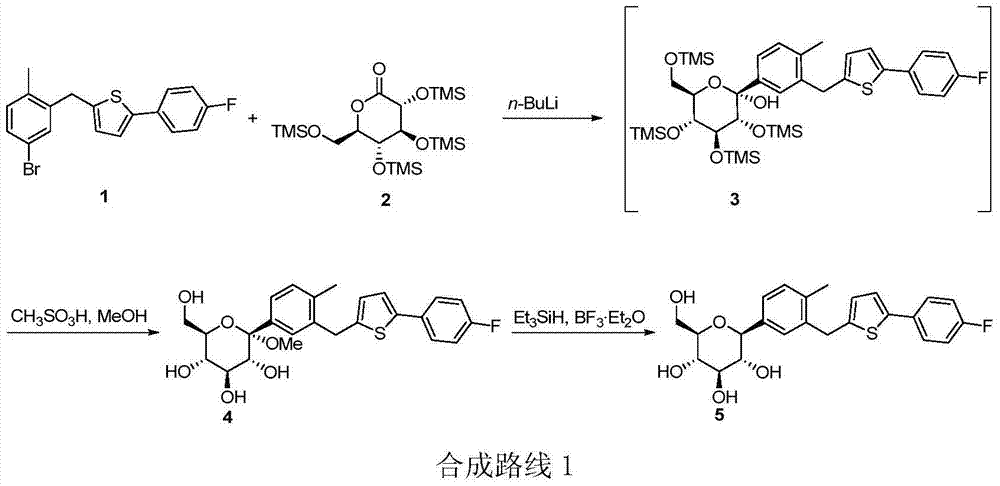
New synthesis process of canagliflozin
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
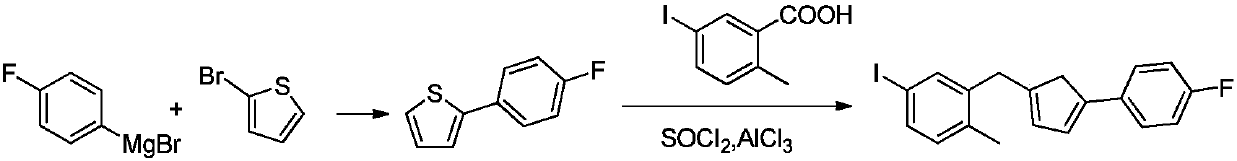
Preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene
InactiveCN104892566AEasy to purifySimple and fast operationOrganic chemistryBromobenzoic AcidsKetone
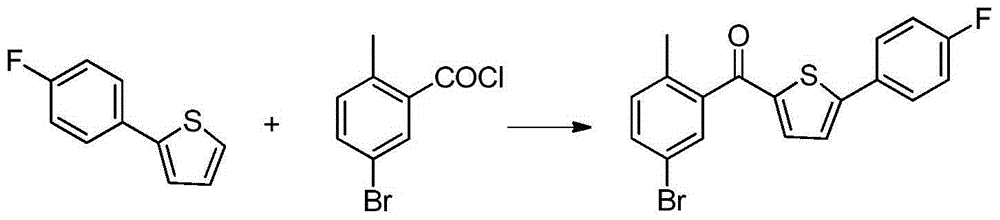
The invention relates to a preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene, which comprises the following steps: carrying out coupling reaction on 2-bromothiophene and p-bromofluorobenzene to obtain 2-(4-fluorophenyl)thiophene; carrying out Friedel-Craft reaction on the 2-(4-fluorophenyl)thiophene and 2-methyl-5-bromobenzoic acid to obtain 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone; and finally, carrying out reduction reaction on the 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone to obtain the 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene. By using the cheap and accessible p-bromofluorobenzene and 2-methyl-5-bromobenzoic acid as the raw materials for synthesizing the canagliflozin intermediate, the product is easy for purification, and the method is simple to operate and has the advantages of low cost and environment friendliness.
Owner:SHANGHAI INST OF TECH
Amorphous substance of canagliflozin and preparation method of amorphous substance
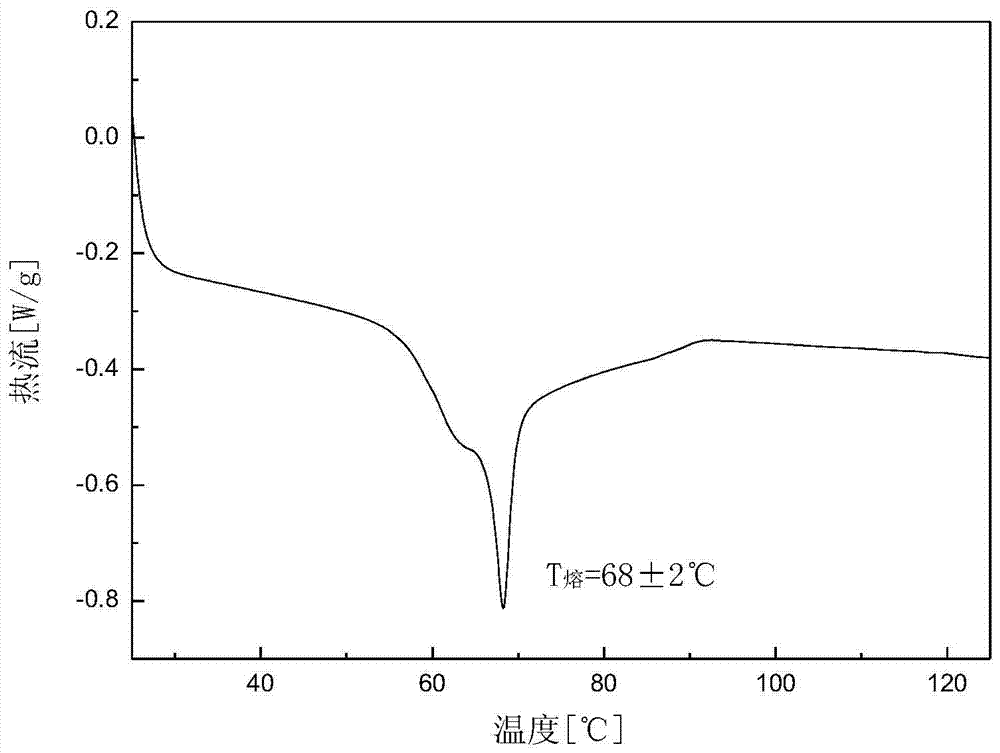
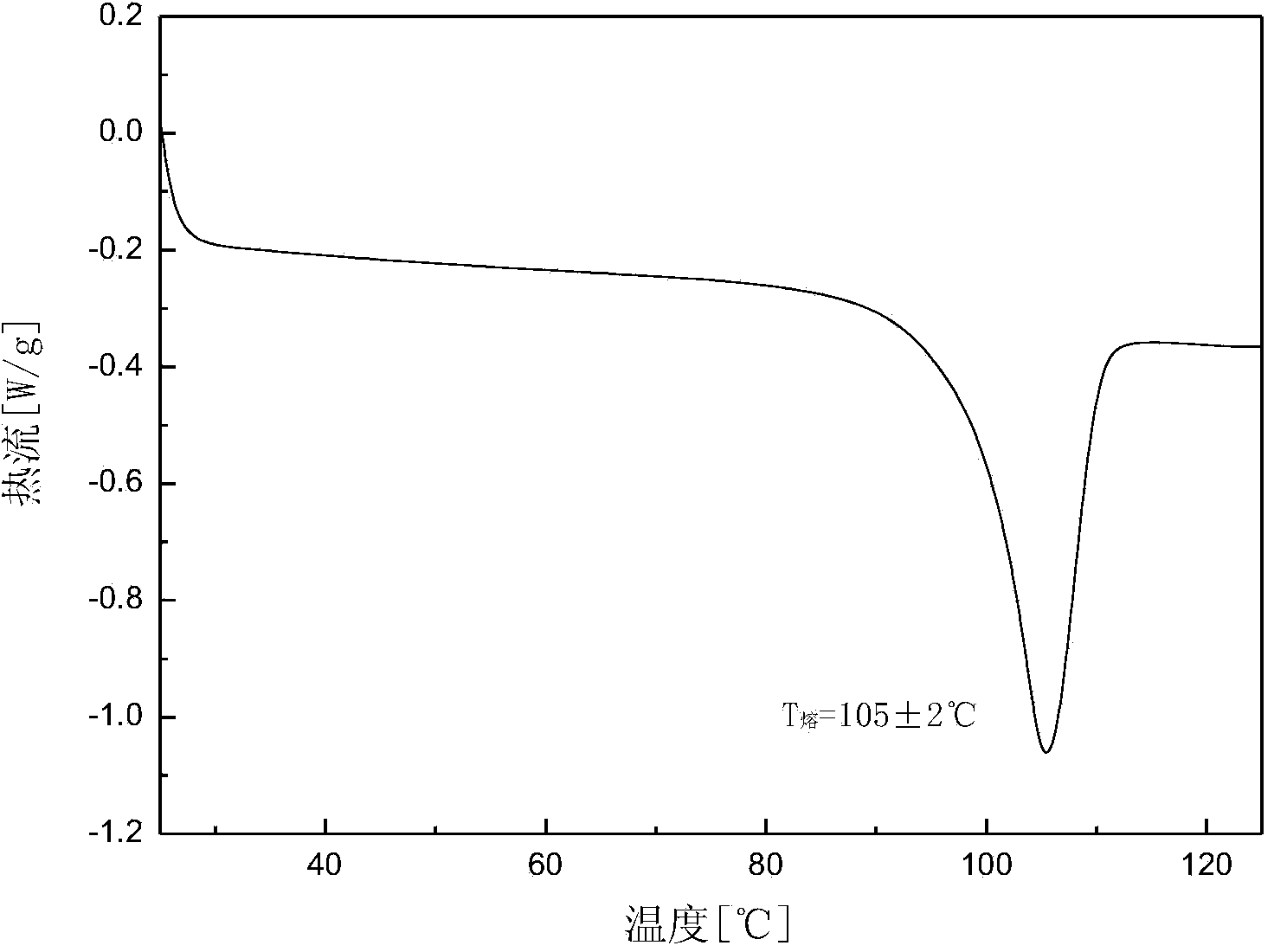
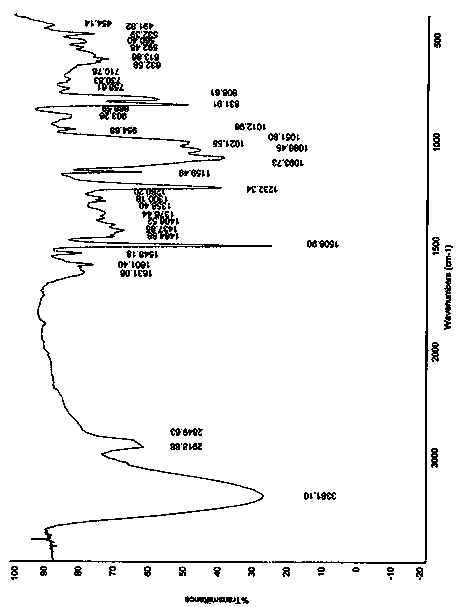
The invention discloses an amorphous substance of canagliflozin and a preparation method of the amorphous substance. Through DSC (Differential Scanning Calorimetry) scanning, the amorphous substance is found to have an endothermic peak within the temperature range of 53-63 DEG C and has characteristic absorption peaks at the wavelengths of about 832cm<-1> and 809cm<-1> in an infrared spectrogram. The invention also discloses an application of the amorphous substance to preparation of drugs for treating type-2 diabetes.
Owner:CHONGQING PHARMA RES INST
High-purity canagliflozin compound and preparation method thereof
The invention belongs to the field of pharmaceutical synthesis and relates to a high-purity canagliflozin compound represented by the formula I and a preparation method thereof. The content of alpha-configuration of impurities in the formula II in the canagliflozin compound provided by the invention is less than 1%, and preferably is less than 0.5%. The preparation method comprises the following steps: enabling the canagliflozin and amino acid to form a eutectic substance in a solvent, separating the eutectic substance, and then decomposing the eutectic substance to obtain the canagliflozin. The formulae I and II are as shown in the specification.
Owner:朱孝云
Canagliflozin new preparation method
InactiveCN104151306AHigh reactivitySimple and fast operationOrganic chemistryBenzeneBiochemical engineering
The invention relates to a new synthetic method of 1-(beta-D-pyran glucosyl)-4-methyl-3-[5-(4-fluorinated phenyl)-2-thienyl methyl] benzene (canagliflozin), and the method is characterized in that: coupling and etherification steps are separately operated, at the same time in the etherification process, usage amount of methanesulfonic acid is greatly reduced compared with the prior art, and the method can reduce the cost, is convenient in operation and improves the quality of products.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Preparation method of canagliflozin
ActiveCN104119324AReduce usageReduce security risksOrganic chemistrySodium methoxideAnhydrous ethanol
The invention discloses a preparation method of canagliflozin. The method comprises the following steps: (1) by using DMF (N,N-dimethylformamide) as a solvent, carrying out protective reaction on the main raw material SM1 by using benzoyl chloride to obtain an intermediate I; (2) by using acetonitrile as a solvent, removing methoxy group from the intermediate I under the action of trimethyl phosphite to obtain an intermediate II; and (3) by using tetrahydrofuran and anhydrous ethanol or methanol as solvents, removing benzoyl protection from the intermediate II under the action of sodium methoxide to obtain the canagliflozin. The purity of the product is at least 99.9%, and the yield is at least 81.6%. The method has the advantages of mild reaction conditions, high yield and high product quality, is economical and environment-friendly, and can easily implement industrial production.
Owner:QILU PHARMA HAINAN +1
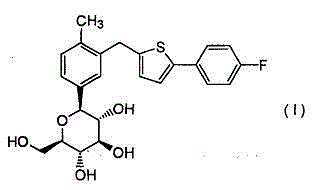
Defluorinated canagliflozin compound, and preparation method and application thereof
The invention relates to a defluorinated canagliflozin compound as shown in a formula (I) which is described in the specification, and a preparation method thereof. Moreover, the invention also provides application of defluorinated canagliflozin as a standard reference substance. According to the invention, the quality of a canagliflozin product is effectively controlled, so safety and validity of a canagliflozin preparation in clinical usage are guaranteed.
Owner:BEIJING PUDEKANGLI PHARMA TECH DEV CO LTD
Synthesis method of canagliflozin intermediate
The invention relates to a synthesis method of a canagliflozin intermediate. Concretely, succinyl oxide and fluorobenzene are used as starting raw materials to be prepared into the canagliflozin crucial intermediate of 2-(4-fluorophenyl)-5-[(5-halogen-2-methyl phenyl)methyl]thiophene through the steps of Friedel-Crafts acylation ring opening, thiophene ring preparation, Friedel-Crafts acylation coupling, reduction and the like. The cheap succinyl oxide is used for preparing the thiophene ring, so that the Suzuki coupling reaction and Grignard reaction are avoided; the use of heavy metal reagents of palladium and the like is avoided; the production process is simplified; the product yield and the quality are improved; the environment pollution is reduced; the production cost is reduced.
Owner:连云港恒运药业有限公司
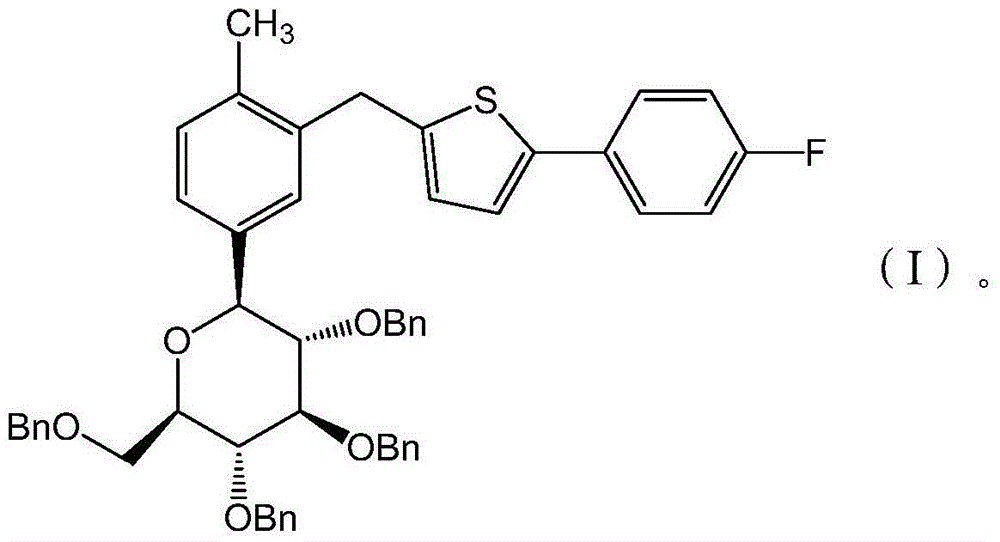
Preparation methods for canagliflozin and intermediate thereof and intermediate
The invention belongs to the field of medicine, and particularly relates to preparation methods for canagliflozin and an intermediate thereof and the intermediate. The preparation method for canagliflozin comprises the following steps: a compound having the structure represented by the formula (I) is subjected to a debenzylation reaction to obtain canagliflozin. The preparation methods provided by the invention have simple reaction route, the intermediate has no need for purification, and the products have high purity and high yield. Experimental results indicate that when the method provided by the invention is used for preparing canagliflozin, the product yield is greater than 88%, and the purity is greater than 99.5%.
Owner:HYBIO PHARMA
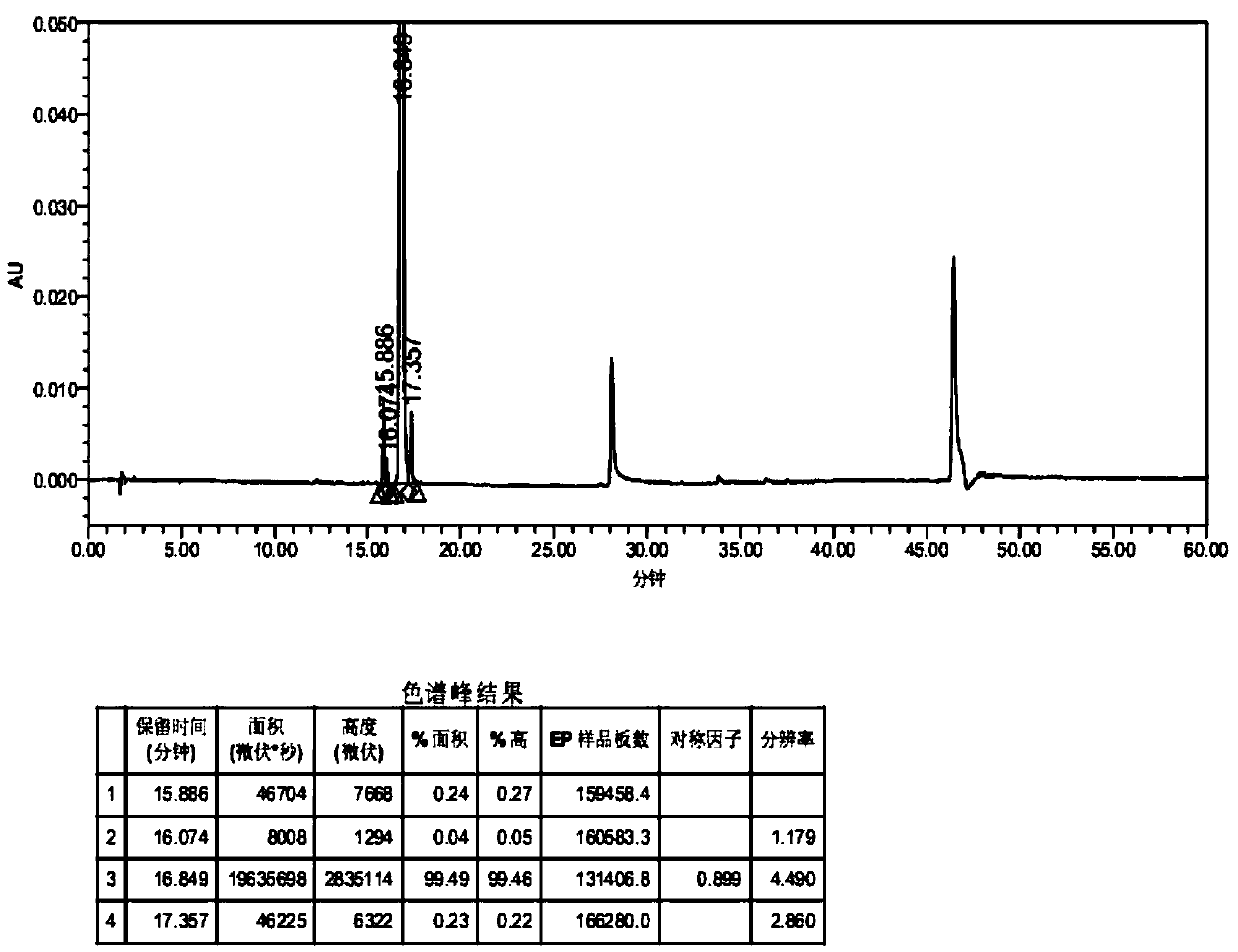
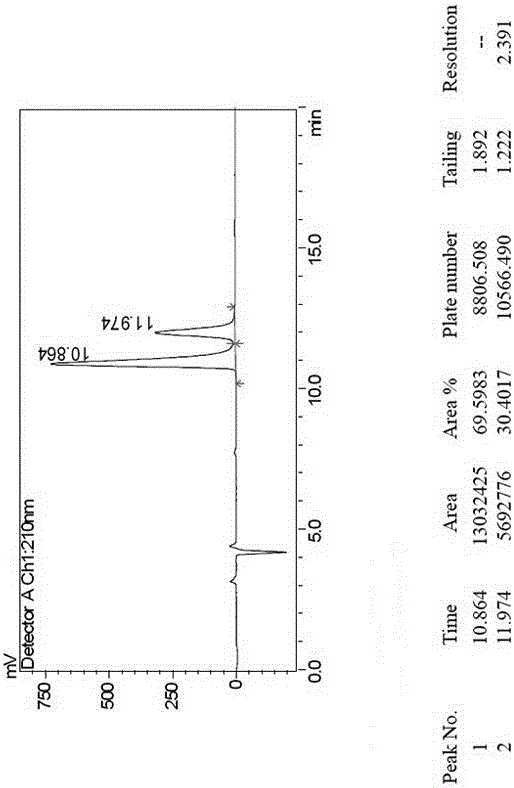
Method for separating canagliflozin five-membered-ring impurity enantiomer through high performance liquid chromatography
ActiveCN106226426AHigh degree of automationImprove production efficiencyComponent separationEnantiomerChirality
The invention discloses a method for separating a canagliflozin five-membered-ring impurity enantiomer through high performance liquid chromatography. The method comprises the steps that a raw material five-membered-ring impurity enantiomer mixture is dissolved in an organic solvent till the concentration is up to 0.1-25 mg / ml; a high performance liquid chromatograph is adopted, and an amylose chiral column serves as a chromatographic column and a mixed liquid prepared from n-hexane and ethanol serves as a mobile phase to perform separating preparation. The canagliflozin five-membered-ring impurity enantiomer separated and prepared by adopting the method is high in yield and can serve as a reference substance for drug research and development, and the purity can be 98% or above. The method effectively achieves separation and preparation of R and S configurations in the five-membered-ring impurity enantiomer, and the separation degree can be 2.6 or above. The automation degree of high performance liquid chromatography separation is high, and the preparation efficiency is high.
Owner:JIANGSU DEYUAN PHARMA
Preparation method of canagliflozin intermediates
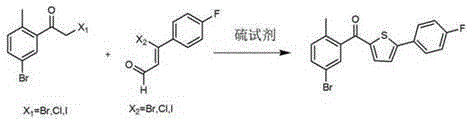
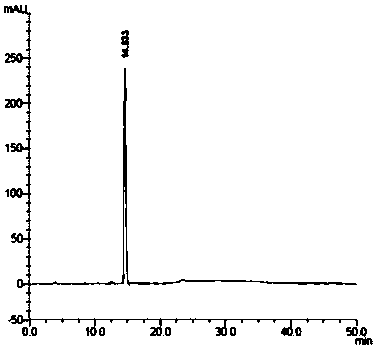
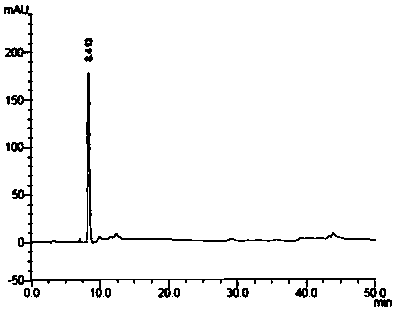
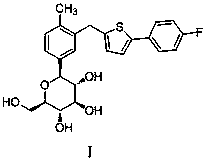
The invention relates to the technical field of preparation of medicine intermediates, and particularly discloses a preparation method of canagliflozin intermediates. High purity 2- ( 4- fluorophenyl)-5[(5- halogenating -2- methyl phenyl carbinol )]thiophene can be prepared, and the preparation method comprises the following steps of: adopting fluorine benzyl halide and 3- halogenating acrolein as raw materials, and under the existence of a sulfur reagent, obtaining 2-p-fluorophenyl thiophene; and enabling the 2-p-fluorophenyl thiophene and 5- halogenating -2- toluyl aldehyde to obtain a product of the 2- ( 4- fluorophenyl)-5[(5- halogenating -2- methyl phenyl carbinol )]thiophene under the condition of n-Butyllithium. Compared with the prior art, according to the method disclosed by the invention, the raw materials are easy to obtain, the yield is high, the reaction condition is easy to control, the product purity is high, the reaction is stable, and the commercialized production is easy.
Owner:CANGZHOU SENARY CHEM SCI TEC
Canagliflozin impurity compound and preparation method thereof
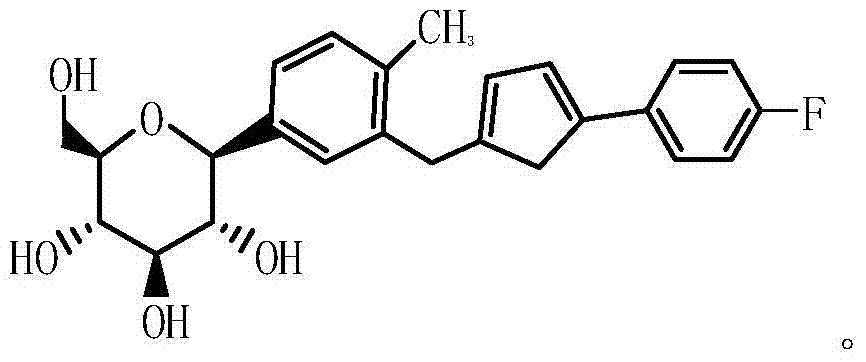
PendingCN108530434AAddressing control deficienciesOrganic chemistryComponent separationMedicineCanagliflozin
The invention relates to a novel type 2 diabetes resistant drug canagliflozin impurity compound [2-methyl-5-(beta-D-glucopyranose)phenyl][5-(4-fluorophenyl)-2-thienyl]methyl hydroperoxide (a compoundof a formula IV) and a preparation method thereof, and application of the impurity compound serving as a canagliflozin quality control reference standard substance.
Owner:CHONGQING PHARMA RES INST
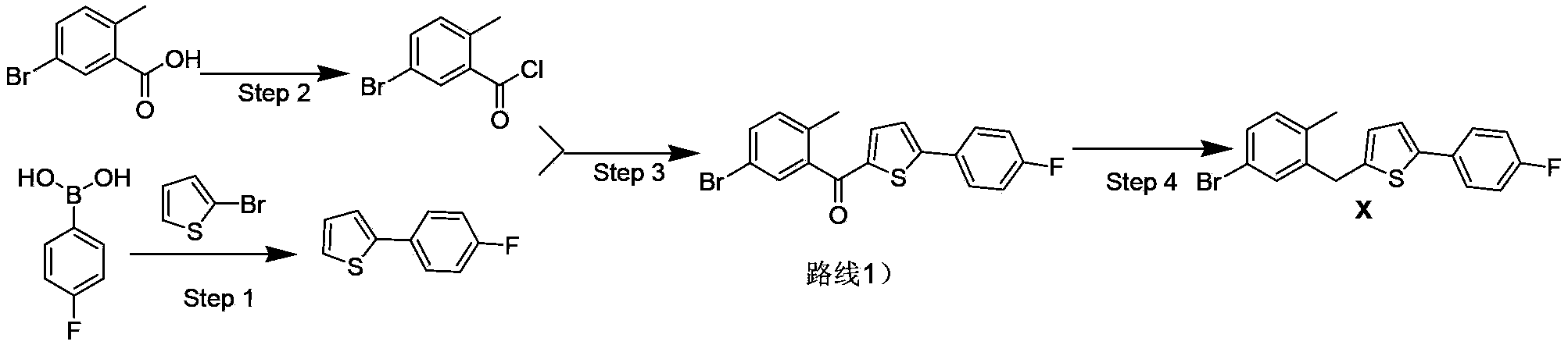
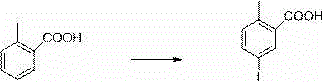
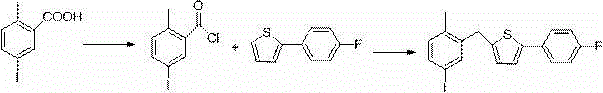
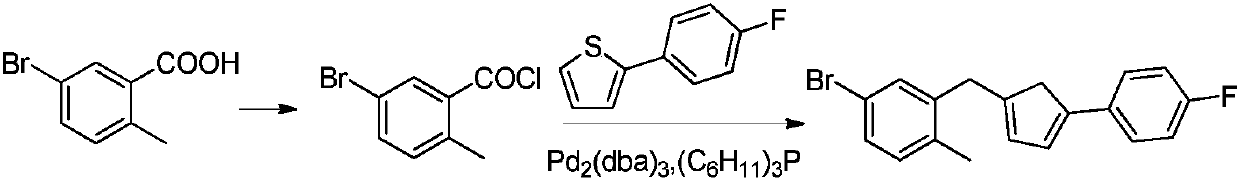
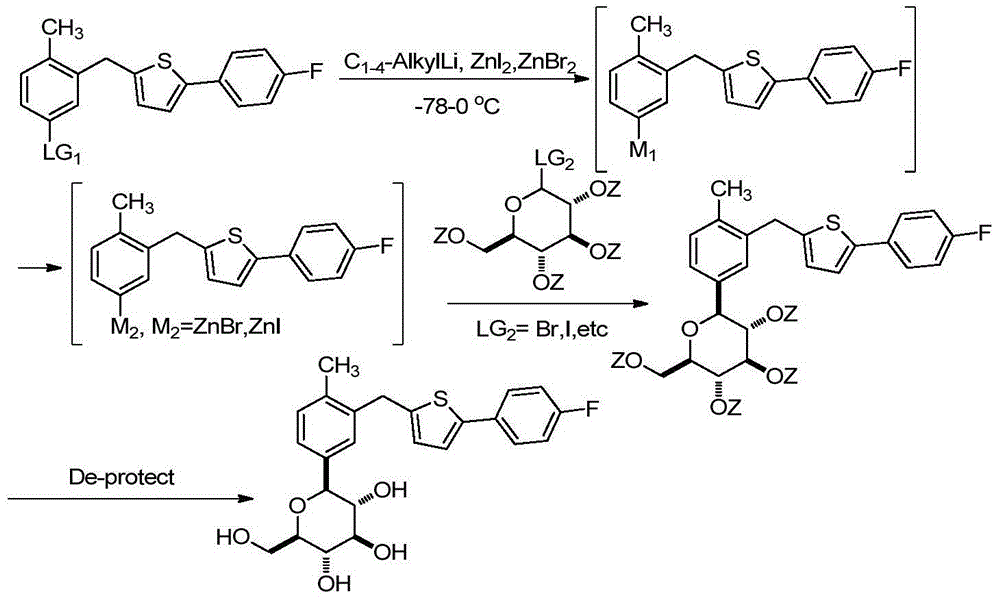
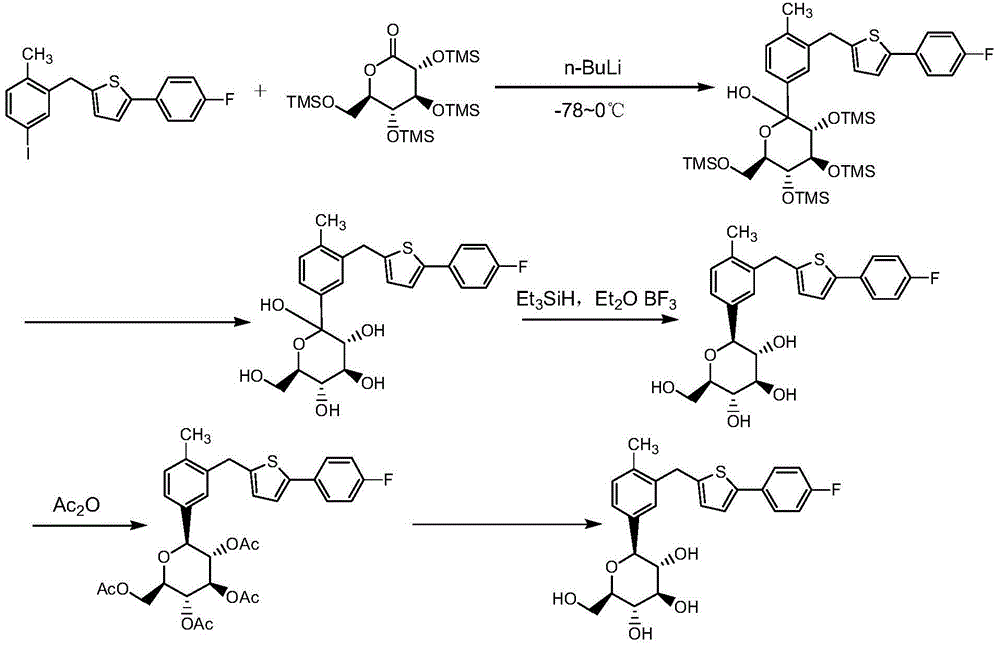
Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene
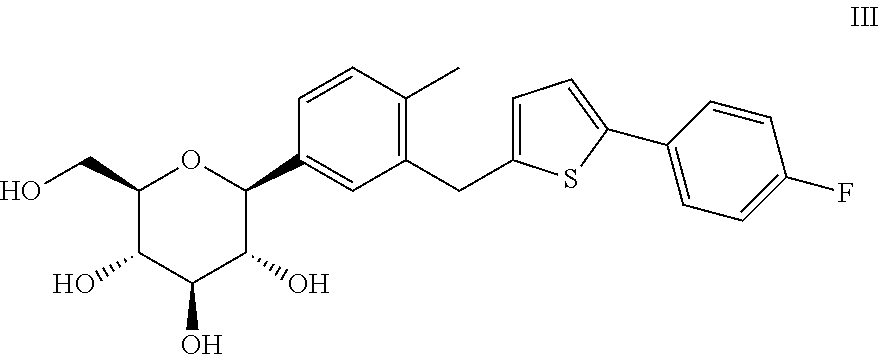
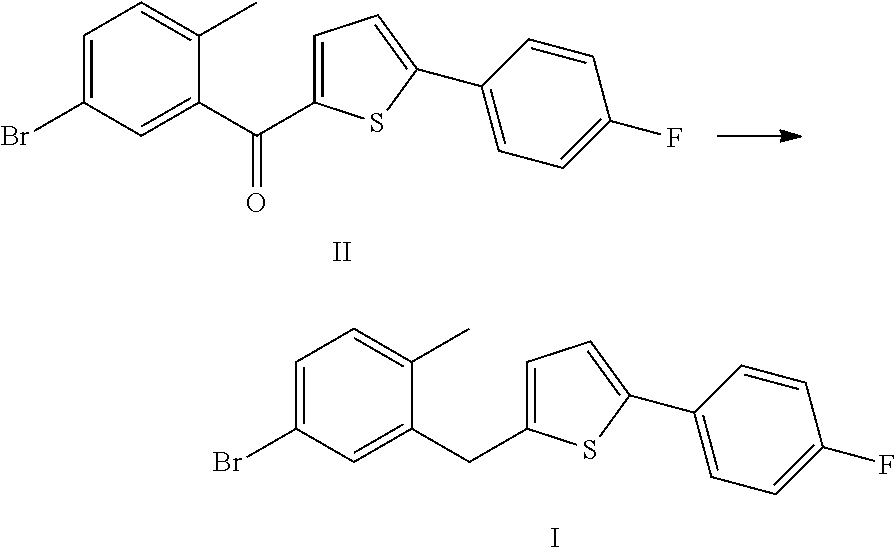
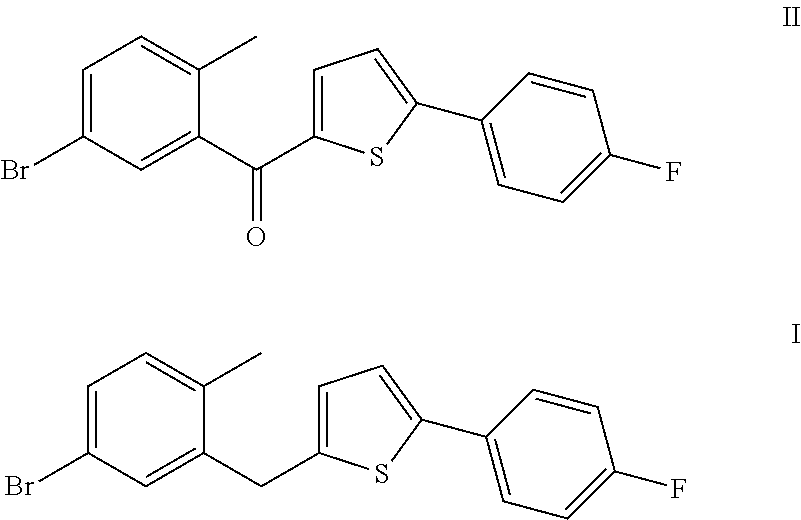
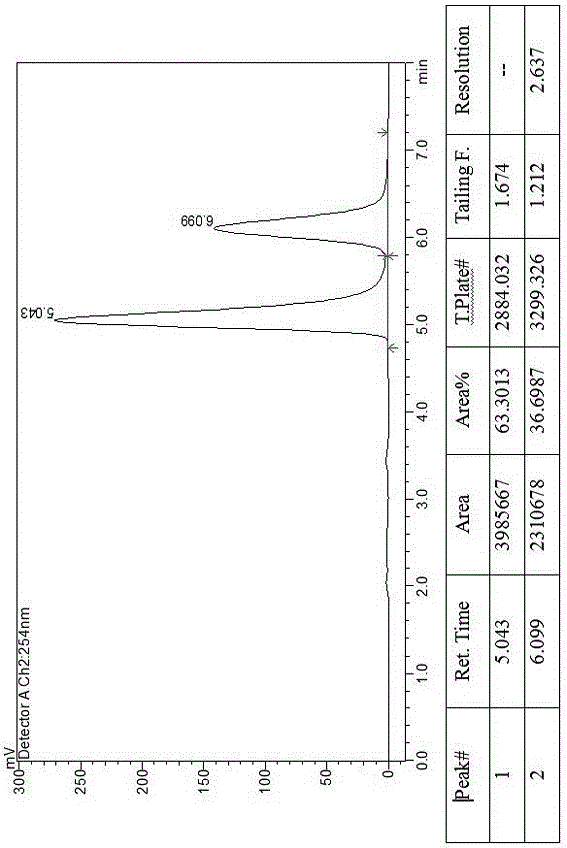
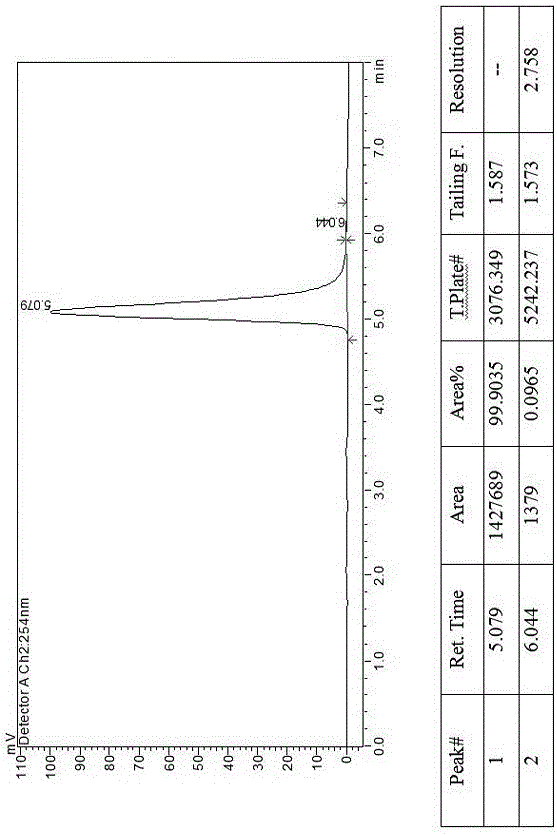
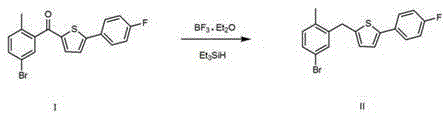
The invention discloses a method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene. A compound, which is represented as formula II, is subjected to a reduction reaction, catalyzed by a protonic acid or a Lewis acid, through a metal borohydride to obtain a compound canagliflozin intermediate I. The intermediate I is a key intermediate for preparing a diabetes drug canagliflozin. According to the method in the invention, triethyl silicane, which is high in dangerousness and has a pungent smell, is replaced by the metal borohydride. The method is safer in technology, is environment-friendly, is low in cost and is suitable for large-scale production.
Owner:SHANGHAI FANGNAN PHARMA
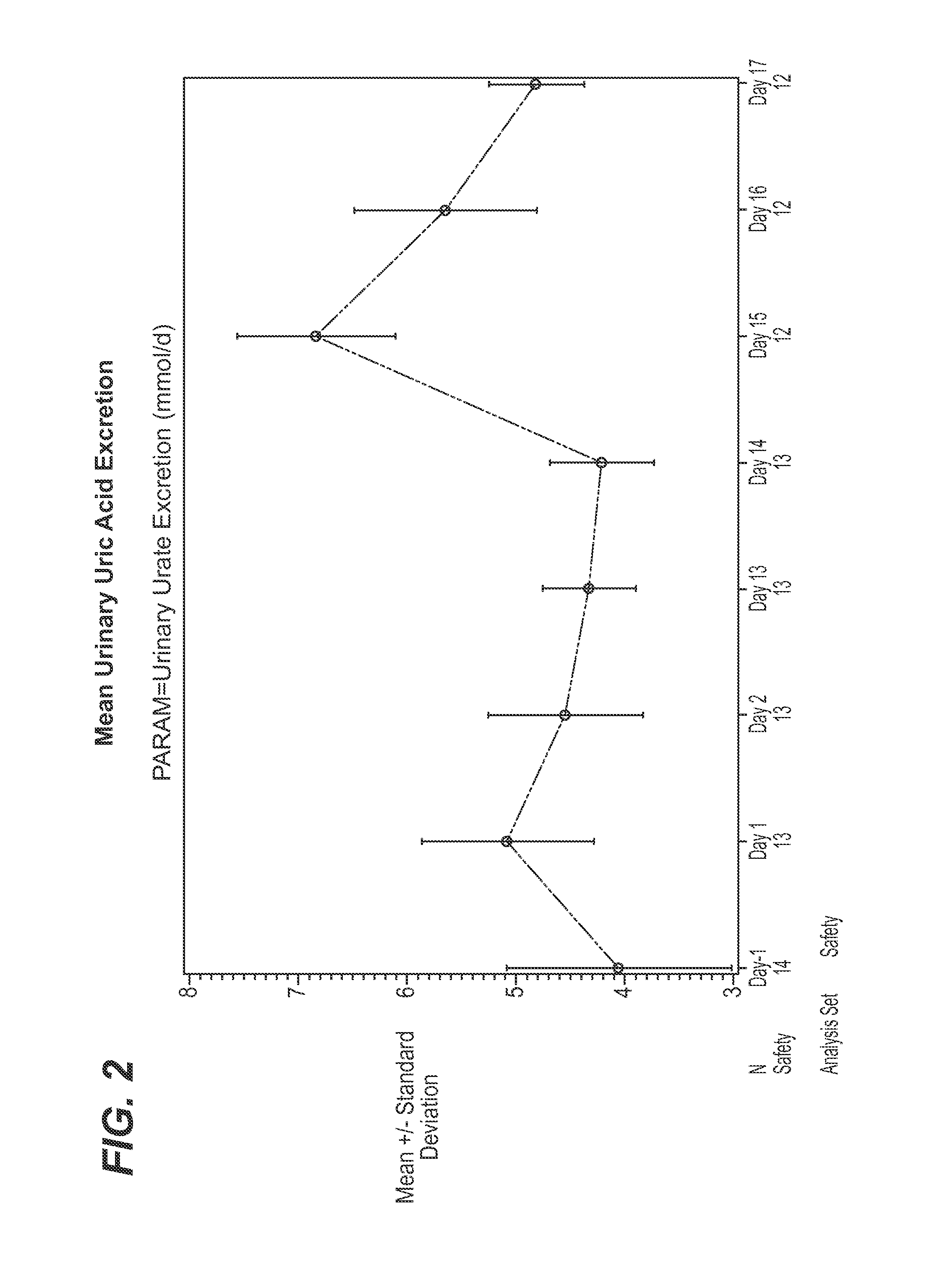
Combination of canagliflozin and probenecid for the treament of hyperuricemia
The present invention is directed to methods for treating hyperuricemia and related disorders, comprising co-therapy with canagliflozin and probenecid.
Owner:JANSSEN PHARMA NV
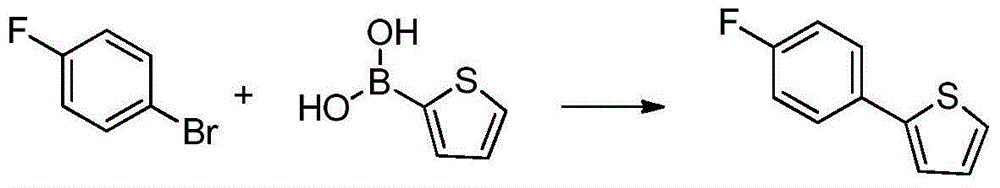
Separation and purification method of 2-(4-fluorophenyl) thiophene
ActiveCN103601715ASimple and fast operationHigh yieldOrganic chemistryPurification methodsOrganic solvent
The invention discloses a separation and purification method of 2-(4-fluorophenyl) thiophene and relates to a separation and purification method of a compound. The method comprises the steps: extracting a mixture obtained in a reaction by using an organic solvent A and drying anhydrous sodium sulfate for later use; carrying out suction filtration on mixed liquor and carrying out fractional distillation at a certain temperature B under reduced pressure; and heating petroleum ether to 60 DEG C, adding the rest residue till the residue is not dissolved, adding little petroleum ether to dissolve most residue, carrying out suction filtration, and cooling to room temperature, thus obtaining a pure substance of the 2-(4-fluorophenyl) thiophene. The 2-(4-fluorophenyl) thiophene is an important intermediate for synthetizing an anti-diabetic medicine Canagliflozin. The method for purifying the 2-(4-fluorophenyl) thiophene through a recrystallization method, which is disclosed by the invention, is simple and convenient in operation and high in yield and is very suitable for industrial production.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
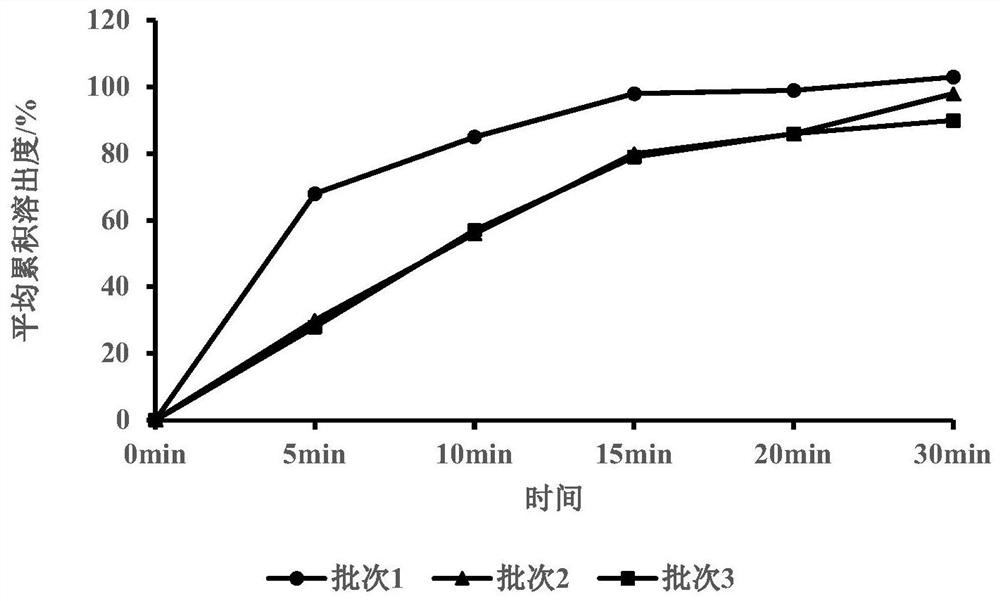
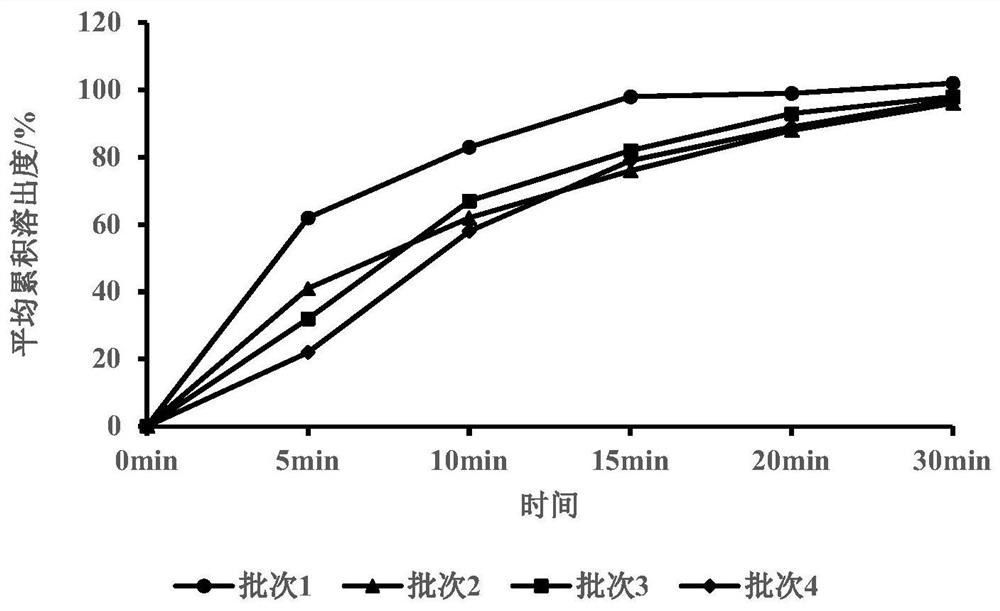
Canagliflozin tablet and preparation method thereof
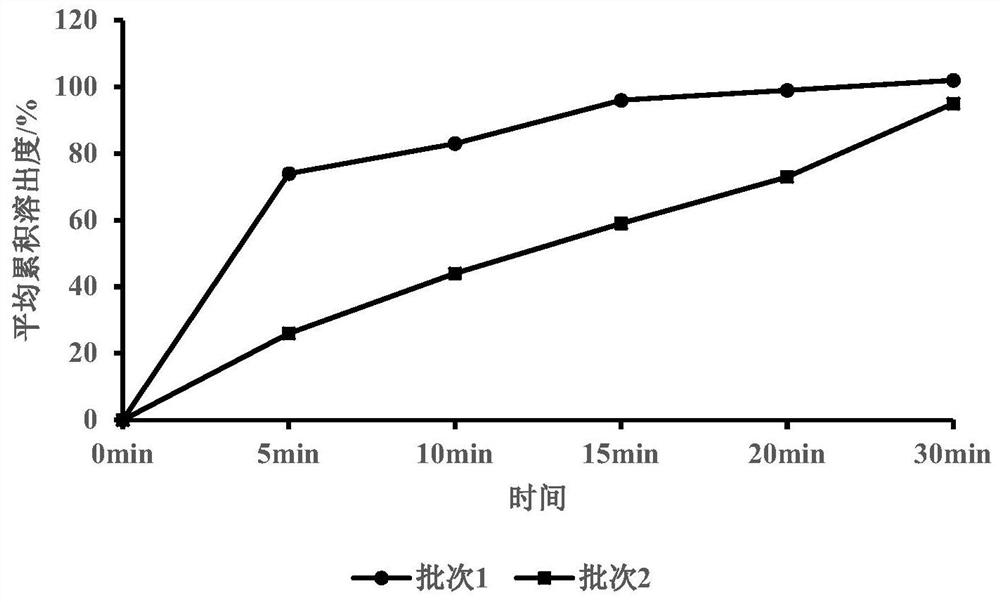
The invention provides a canagliflozin tablet and a preparation method thereof. The canagliflozin tablet is prepared from the following raw and auxiliary materials in percentage by weight: 50%-60% ofcanagliflozin, 32%-40% of a filling agent, 5%-7% of a disintegrating agent, 1%-3% of an adhesive and 0.5%-1% of a lubricating agent. The product adopts a wet granulation process, the preparation process is simple, easy to operate, high in reproducibility and suitable for industrial large-scale production, and the obtained canagliflozin tablet is quick to dissolve out and high in stability of eachbatch.
Owner:CHANGZHOU HANSOH PHARM CO LTD
Canagliflozin anhydrous compound
InactiveCN104447721AGood hypoglycemic effectOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
The invention belongs to the technical field of medicine, and in particular to a Canagliflozin anhydrous crystal form and a preparation method thereof. The Canagliflozin prepared by the invention has the advantages of chemical purity of 99.9%, maximum is less than 1 per thousand, optical purity of up to 99.96% ee and good stability. The invention also relates to application of the crystal form composition to preparation of medicaments for treating type 2 diabetes and related diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
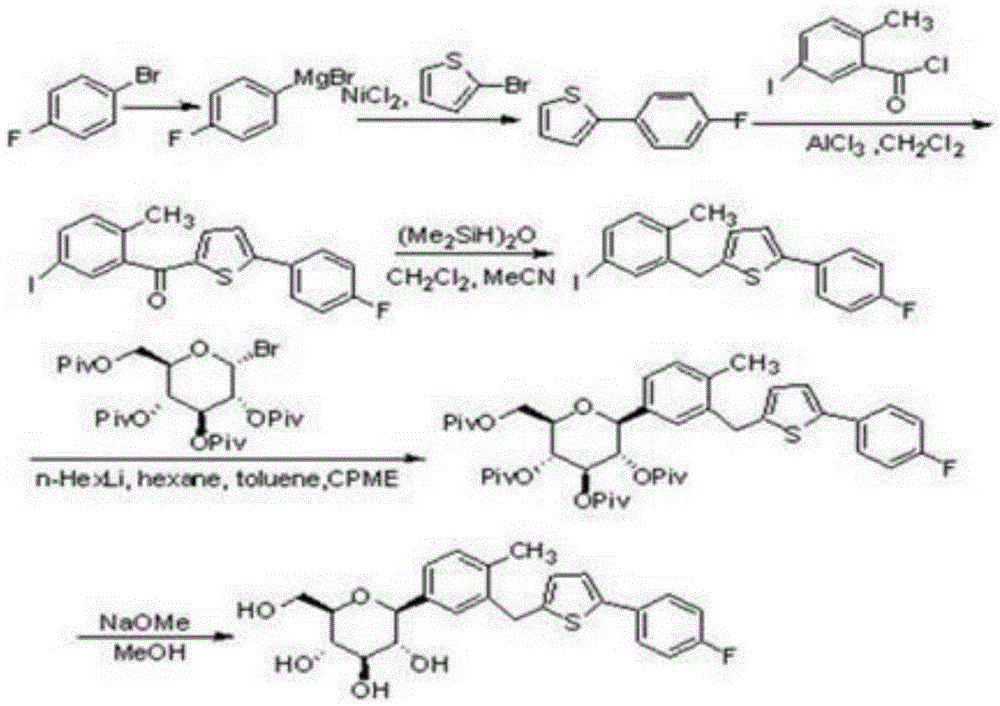
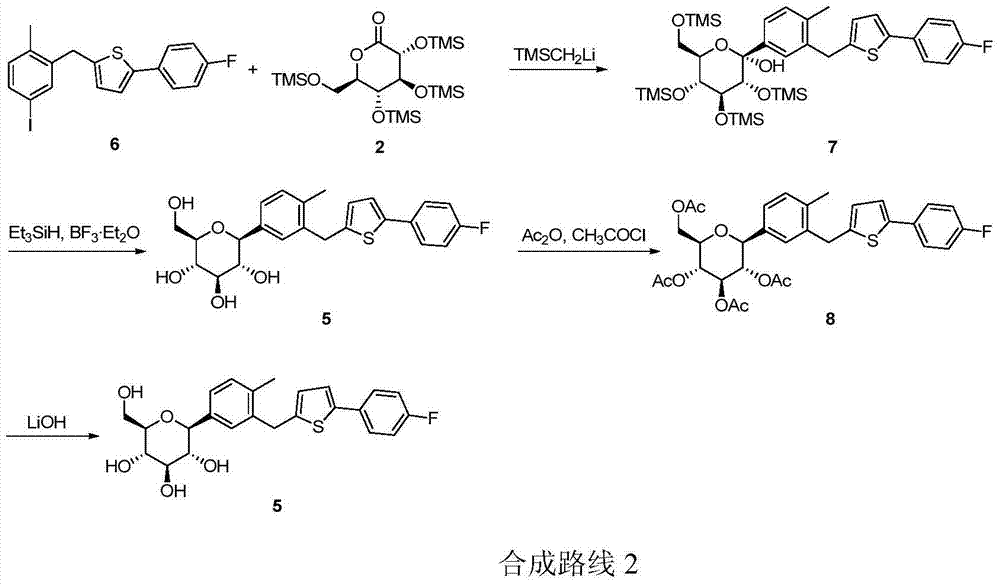
Preparation method for canagliflozin
The invention relates to a novel synthesis method for 1-(Beta-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene (canagliflozin). 2-(4-fluorophenyl)-5-[(5-halogeno-2-methylphenyl)methyl]thiophene and 2,3,4,6-tetrakis-O-(trimethylsilyl)-D-glucono-1,5-lactone are dissolved in organic solvent and carry out condensation reaction under the catalysis of a metallic lithium derivative, and thereby intermediate (II) is produced; the intermediate (II) undergoes catalytic hydrogenation reaction, so that intermediate (III) is further produced, and finally, compound (I), namely the canagliflozin is obtained by acidification hydrolysis. Because the preparation method disclosed by the invention replaces a virulent BF3 / triethyl silicane reduction system with the environment-friendly catalytic hydrogenation technique, the preparation method is technically safer, environment-friendly, low in cost and more suitable for industrial production.
Owner:SHOUGUANG FUKANG PHARMA
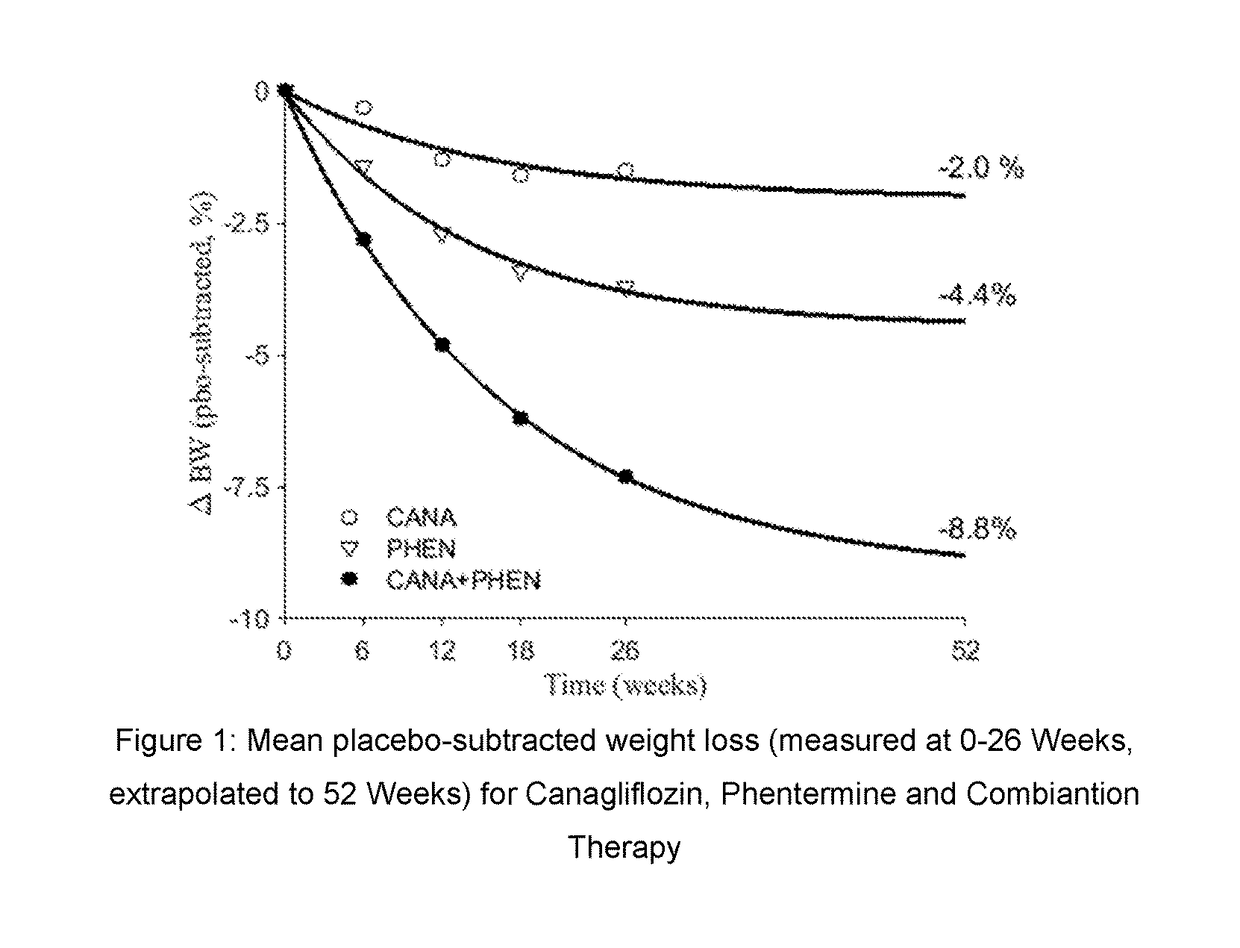
Co-therapy comprising canagliflozin and phentermine for the treatment of obesity and obesity related disorders
InactiveUS20170071970A1Inhibit oxidative stressAltered expressionOrganic active ingredientsMetabolism disorderPhentermineAdjuvant therapy
The present invention is directed to the use of co-therapy comprising administration of canagliflozin and phentermine for the treatment of obesity and obesity related disorders. More particularly, the present invention is directed to co-therapy for treating obesity, for promoting weight loss and / or for suppressing appetite; for treating, delaying, slowing the progression of and / or preventing metabolic disorders (including for example Type 2 diabetes mellitus); for treating, delaying, slowing the progression of and / or preventing renal or fatty liver disorders (including for example NASH, NAFLD, etc.); for treating, delaying, slowing the progression of and / or preventing sleep disorders (including for example sleep apnea); for providing cardiovascular protection; for treating, delaying, slowing the progression of and / or preventing cardiovascular events (including major adverse cardiac events (MACE) such as myocardial infarction, unstable angina, cardiovascular death, revascularization, fatal or non-fatal cerebrovascular accident, peripheral arteriopathy, aortic events, hospitalization due to congestive heart failure, etc.); and / or for extending or prolonging life span.
Owner:JANSSEN PHARMA NV
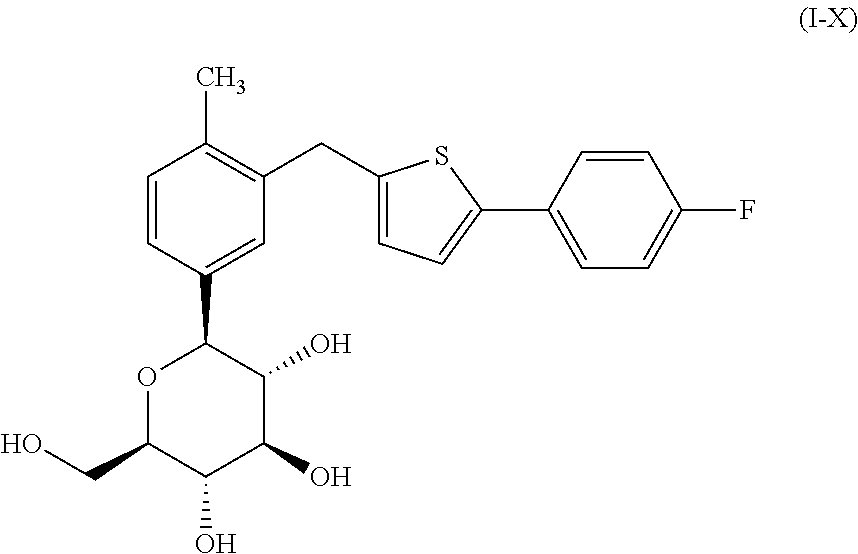
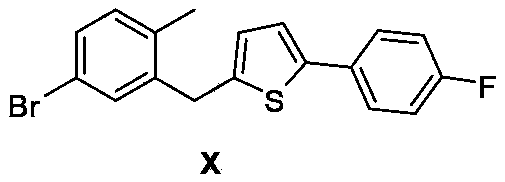
5-(5-bromo-2methylphenyl)-1-(4-fluorophenyl)pentane-1,4-dione, preparation method and applications thereof
ActiveCN104072348ALow priceReduce pollutionOrganic compound preparationCarbonyl compound preparationKetoneCanagliflozin
The invention discloses a compound 5-(5-bromo-2methylphenyl)-1-(4-fluorophenyl)pentane-1,4-dione represented by the formula I, a preparation method and applications thereof. The compound represented by the formula I can be taken as the raw material to carry out reactions in the presence of a vulcanizer so as to obtain an intermediate compound represented by the formula X of a drug Canagliflozin for treating diabetes II. The raw materials and reagents required in the preparation method are relatively cheap, and the preparation method has the advantages of low cost, convenient and safe operation, high yield, little environmental pollution, good economic profits, and suitability for industrial production.
Owner:SHANGHAI FANGNAN PHARMA
Method for preparing canagliflozin intermediate 2-(2-methyl-5-bromobenzyl)-5-(4-fluorophenyl)thiophene
Provide in the present invention is a method for preparing canagliflozin intermediate 2-(2-methyl-5-bromobenzyl)-5-(4-fluorobenzene)thiophene. The method comprises a compound, shown as formula (II), of (5-bromo-2-methylphenyl)[5-(p-fluorophenyl)thiophene-2-yl]ketone being reduced under the action of a directly used borane solution or borane locally produced by reacting alkali metal borohydride with a Lewis acid in a suitable solvent and at a suitable temperature, so as to obtain the compound of formula (I) of 2-(2-methyl-5-bromobenzyl)-5-(4-fluorobenzene)thiophene. The preparation method avoids the use of expensive reductive agents and guarantees the complete conversion of raw materials, wherein the post-treatment is simple, the purity of product obtained is high, the reaction yield is high, in the preparation method is simple and convenient, and can easily be used in industry.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Canagliflozin drug impurity as well as preparation method and application thereof
ActiveCN107286143AStarting materials are cheap and readily availableReduce stepsOrganic chemistry methodsOrganic solventLithium hydroxide
The invention discloses a canagliflozin drug impurity as well as a preparation method and application thereof. The invention provides a compound as well as a preparation method and application thereof. The method comprises the following steps: (1) enabling the compound as shown in formula 2 to be in contact with an alkaline lithium hydroxide aqueous solution to obtain a coarse product containing a compound as shown in formula 3, wherein the coarse product contains a compound as shown in formula 1; (2) crystallizing and filtering the coarse product to obtain mother liquor; (3) concentrating the mother liquor to obtain residues; and (4) crystallizing and filtering the residues in an L-proline-containing organic solvent, thus obtaining the compound as shown in formula 1. The method provided by the invention can realize directed preparation of the compound as shown in formula 1, and a reliable impurity contrast is provided for quality research on industrially produced canagliflozin-series diabetes treatment drug products and quantitative control over impurities.
Owner:WATERSTONE PHARMA WUHAN
Pharmaceutical Compositions Comprising Canagliflozin
InactiveUS20170258761A1Improve solubilityImprove bioavailabilityOrganic active ingredientsMetabolism disorderMedicinePharmaceutical industry
The present invention belongs to the field of pharmaceutical industry and relates to a dry pharmaceutical composition comprising Canagliflozin, as well as to a process for preparing the same. Such dry pharmaceutical composition is useful as a medicament, especially for the normalization of plasma glucose levels.
Owner:SANDOZ AG
Canagliflozin compound
InactiveCN104447722AGood hypoglycemic effectIncrease blood concentrationOrganic active ingredientsOrganic chemistryDiseasePharmaceutical drug
Belonging to the field of medical technologies, the invention in particular relates to a canagliflozin anhydrous compound and a preparation method thereof. The canagliflozin obtained by the invention has the advantage of: chemical purity of 99.9%, maximum impurity of less than 1 per thousand, optical purity up to 99.96 enantiomeric excess percentage, and good stability. The invention also relates to application of the anhydrous crystal composition to preparation of drugs treating type 2 diabetes and related diseases.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Application of canagliflozin in preparation of drugs for adjuvant treatment of idiopathic pulmonary fibrosis
InactiveCN108014106AGood treatment effectImprove the quality of lifeOrganic active ingredientsRespiratory disorderIdiopathic pulmonary fibrosisTrial drug
The invention discloses application of canagliflozin in preparation of drugs for adjuvant treatment of idiopathic pulmonary fibrosis. Clinical trials show that the canagliflozin given as adjuvant therapy for patients with the idiopathic pulmonary fibrosis can significantly treat the idiopathic pulmonary fibrosis, improves breathing difficulties, cough, sputum and other symptoms, and definitely improves the patient's quality of life.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







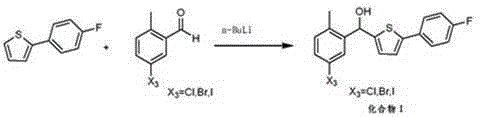
![Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cf1905a7-7c9d-4ce9-934c-e2f28a34d9f1/FDA0000517976460000011.png)
![Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cf1905a7-7c9d-4ce9-934c-e2f28a34d9f1/BDA0000517976470000011.png)
![Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene Method for preparing canagliflozin intermediate 2-(4-fluorophenyl)-5-[(5-halogen-2-methylphenyl)methyl]thiophene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cf1905a7-7c9d-4ce9-934c-e2f28a34d9f1/BDA0000517976470000012.png)