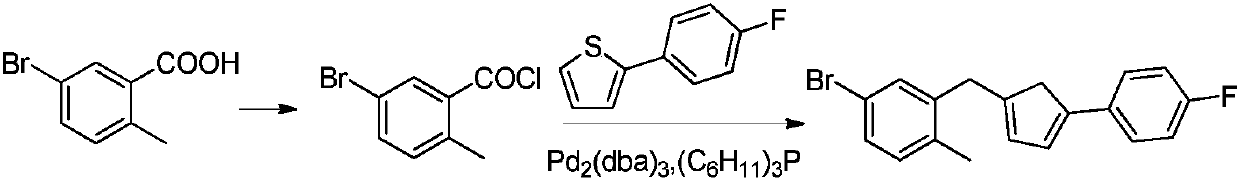
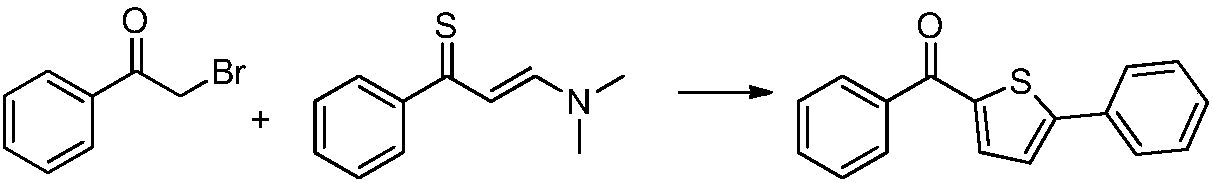
Synthesis method of canagliflozin intermediate
A synthesis method and compound technology, which are applied in the field of synthesis of canagliflozin intermediates, can solve the problems of reducing production cost, complicated process and high price, and achieve the effects of reducing production cost, avoiding lattice reaction and simplifying production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
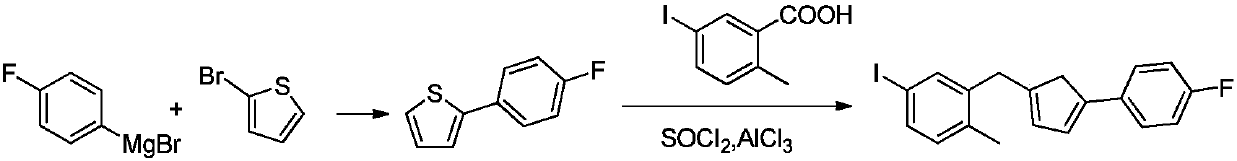
[0037]
[0038]Add 50ml of dichloromethane into a 250ml reaction bottle, add 10.6g (0.11mol) of fluorobenzene, 14.7g (0.11mol) of anhydrous aluminum trichloride, stir the reaction solution evenly in an ice-water bath, and slowly add 10.1g of succinic anhydride g (0.1mol) of dichloromethane solution in 100ml, after the dropwise addition was completed, the temperature was slowly raised to reflux and reacted for 2 hours. After the reaction is complete, add 150ml of water, cool to 0°C, add 100ml of concentrated hydrochloric acid, stir at room temperature for 1 hour, let stand to separate layers, wash the organic layer with 150ml of saturated aqueous sodium bicarbonate solution and saturated brine successively, concentrate to dryness under reduced pressure, and perform HPLC The detection purity was 98.0%, and 16.3 g of 4-(4-fluorophenyl)-4-oxobutanoic acid was obtained by recrystallization and purification with toluene, with a yield of 83%. The purity by HPLC was 99.8%, and the ...
Embodiment 2
[0040] Add 50ml of chloroform into a 250ml reaction bottle, add 10.6g (0.11mol) of fluorobenzene, 14.7g (0.11mol) of anhydrous aluminum trichloride, stir the reaction solution evenly in an ice-water bath, slowly add 10.1g of succinic anhydride g (0.1mol) of a chloroform solution in 100ml, after the dropwise addition was completed, the temperature was slowly raised to reflux, and the reaction was carried out for 1.5 hours. After the reaction is complete, add 150ml of water, cool to 0°C, add 100ml of concentrated hydrochloric acid, stir at room temperature for 1 hour, let stand to separate layers, wash the organic layer with 150ml of saturated aqueous sodium bicarbonate solution and saturated brine successively, concentrate to dryness under reduced pressure, and perform HPLC The detection purity was 97.9%, and 15.9 g of 4-(4-fluorophenyl)-4-oxobutanoic acid was obtained by recrystallization and purification with toluene, with a yield of 81%. The purity by HPLC was 99.7%, and the...
Embodiment 3
[0042]
[0043] Add 4-(4-fluorophenyl)-4-oxobutanoic acid 19.6g (0.1mol), phosphorus pentasulfide 17.8 (0.04mol), toluene 200ml prepared by Example 1 in the microwave reactor, adjust the microwave radiation power to 160 Watts, react for 30 minutes. After the reaction was completed, toluene was recovered by distillation under reduced pressure, the residue was 200ml of ethyl acetate, 200ml of 5% aqueous sodium hydroxide solution, and after stirring, it was allowed to stand for stratification, and the organic layer was washed successively with 100ml of 5% aqueous sodium hydroxide solution, 100ml of water, and 100ml of saturated brine. After that, it was concentrated to dryness under reduced pressure to obtain 15.1 g of 2-(4-fluorophenyl)thiophene with a yield of 85% and a purity of 98.4% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com