Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Bromobenzoic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
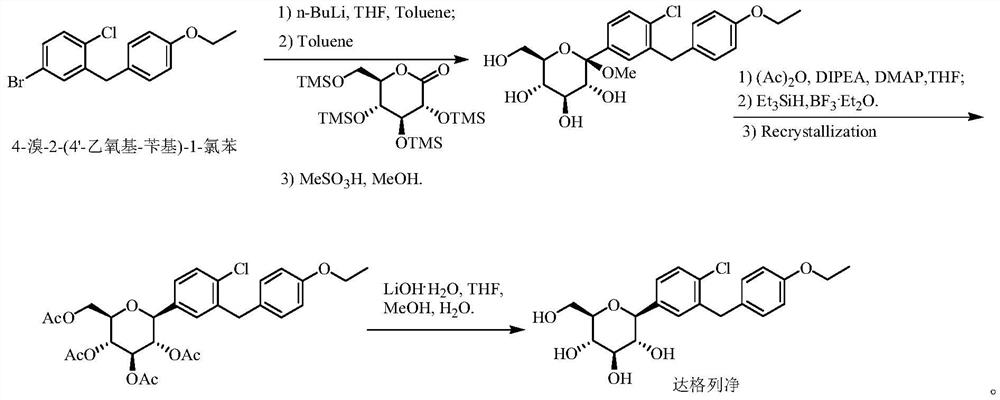
Synthesis method of 5-bromo-2-chloro benzoic acid
ActiveCN105622382AEasy to operateHigh purityOrganic compound preparationCarboxylic compound preparationBenzoic acidChlorobenzilate
The invention provides a synthesis method of 5-bromo-2-chloro benzoic acid.The method includes the following steps of A, making 2-chlorine benzotrichloride and bromide reagents react under the effect of a catalyst to obtain 2-chloro-5-bromine benzotrichloride, wherein bromide reagents include one or more of bromine, N-bromosuccinimide, dibromohydantoin and hydrobromic acid; B, conducting hydrolysis reaction on 2-chloro-5-bromine benzotrichloride in the step A under the acid condition to obtain 5-bromo-2-chloro benzoic acid.According to the method, 2-chlorine benzotrichloride which is low in price and easy to obtain is adopted as the raw material, operation is easy, intermediates do not need to be purified, 5-bromo-2-chloro benzoic acid is synthesized through a one-pot method, purity is high, yield is high, three-waste emission is little, and production cost is low.It is shown through experiment results that 2-chloro-5-benzoic acid obtained according to the synthesis method has yield larger than 95% and purity of 80-92%.
Owner:苏州正济药业有限公司
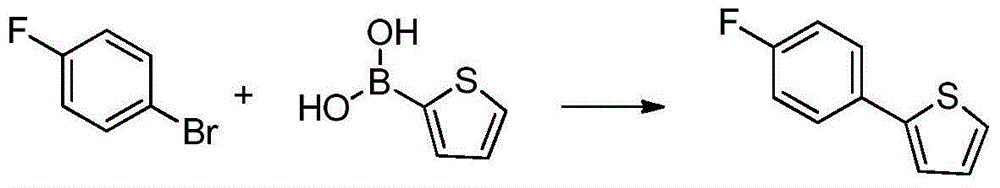
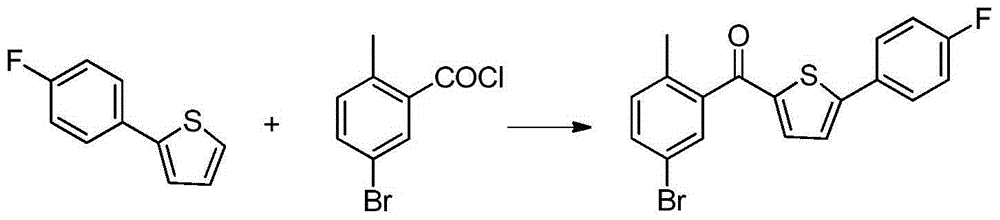
Preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene
InactiveCN104892566AEasy to purifySimple and fast operationOrganic chemistryBromobenzoic AcidsKetone
The invention relates to a preparation method of 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene, which comprises the following steps: carrying out coupling reaction on 2-bromothiophene and p-bromofluorobenzene to obtain 2-(4-fluorophenyl)thiophene; carrying out Friedel-Craft reaction on the 2-(4-fluorophenyl)thiophene and 2-methyl-5-bromobenzoic acid to obtain 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone; and finally, carrying out reduction reaction on the 5-bromo-2-methylphenyl-2-(4-fluorophenyl)thienyl ketone to obtain the 2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene. By using the cheap and accessible p-bromofluorobenzene and 2-methyl-5-bromobenzoic acid as the raw materials for synthesizing the canagliflozin intermediate, the product is easy for purification, and the method is simple to operate and has the advantages of low cost and environment friendliness.
Owner:SHANGHAI INST OF TECH
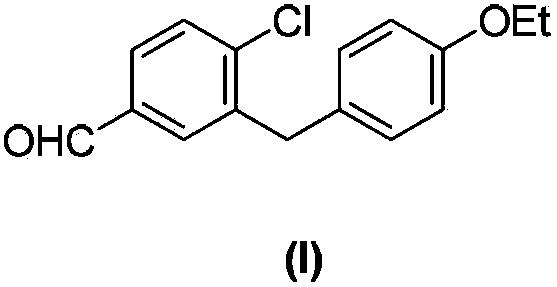
Preparation method of 4-chloro-3-(4-ethoxybenzyl)benzaldehyde
InactiveCN103896752ASimple and fast operationMild reaction conditionsOrganic compound preparationCarbonyl compound preparation by condensationBenzaldehydeKetone
The invention discloses a preparation method of 4-chloro-3-(4-ethoxybenzyl)benzaldehyde. The preparation method comprises the following steps that 2-chloro-5-bromobenzoic acid is dissolved in an organic solvent, an acylating chlorination reagent is added into the solution, the mixed solution undergoes a reaction to produce 5-bromo-2-chlorobenzoyl chloride, 5-bromo-2-chlorobenzoyl chloride is dissolved in an organic solvent, the solution is cooled, the cooled solution is added with phenetole and acid catalysts, the mixed solution undergoes a reaction to produce (5-bromo-2-chlorphenyl)(4-ethoxyphenyl)ketone, (5-bromo-2-chlorphenyl)(4-ethoxyphenyl)ketone is dissolved in an organic solvent, the solution is added with a reduction reagent system, the solution undergoes a reaction to produce 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene, 4-bromo-1-chloro-2-(4-ethoxybenzyl)benzene is dissolved in an organic solvent, a formylation reagent is added into the solution and the mixed solution undergoes a reaction to produce 4-chloro-3-(4-ethoxybenzyl)benzaldehyde. The preparation method utilizes cheap and easily available raw materials and reagents and has a simple synthesis route, simple processes and a high yield.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD
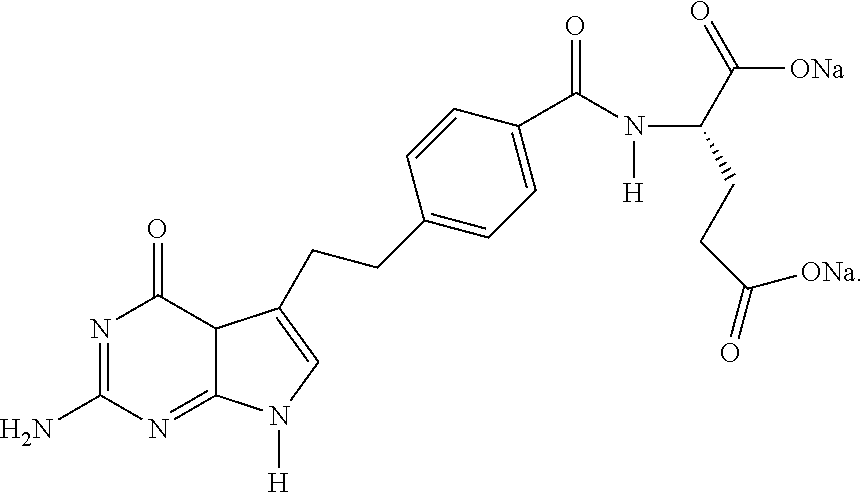
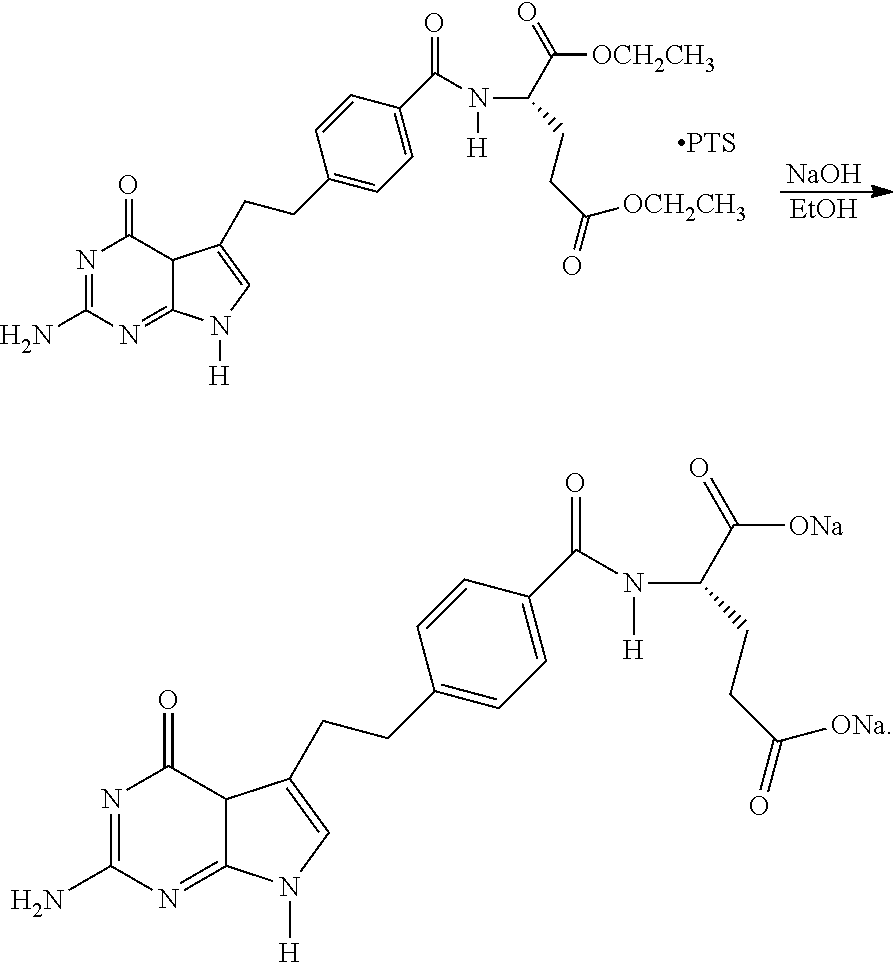
Intermediate of pemetrexed disodium, preparation method thereof and method for preparing pemetrexed disodium thereby
ActiveCN101560206AHigh yieldReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidGlutaric acid
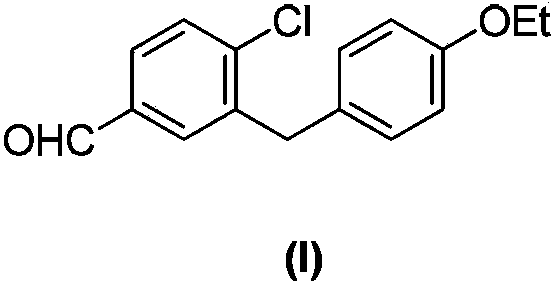
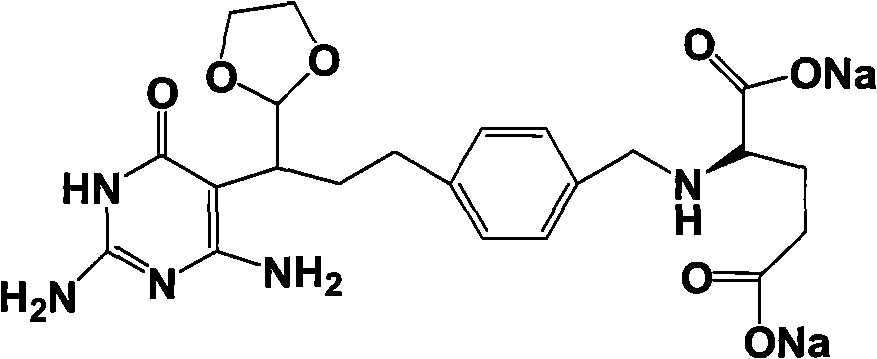
The invention relates to an intermediate of pemetrexed disodium, a preparation method thereof and a method for preparing pemetrexed disodium thereby; and the intermediate is (2-(4-(3-(2,4-diamino-6-oxy-1,6-dihydro-pyridine-5-group)-3-(1,3)dioxolane-2-group-propyl) benzylamine)sodium glutaric acid. The synthesis of the intermediate comprises the following steps: firstly, condensation reaction is conducted on 4-bromobenzoic acid or 4-iodobenzoic acid and L-glutamate diethylester, then Hack reaction is conducted, 4-bromo is replaced and 4-butyraldehyde is formed, then selective bromo replacement is conducted and the 4-butyladehyde is converted into 2-bromobutyraldehyde, and then condensation reaction of aldehyde and ethylene glycol is utilized for protecting the aldehyde, and pyrimidine ring is further synthesized, and finally the intermediate is obtained. Acid hydrolysis ring-closing reaction and sodium hydroxide salification are respectively conducted for once on the intermediate so as to obtain the pemetrexed disodium. The method for preparing pemetrexed disodium in the invention has high yield, low cost and easy operation and is applicable to industrialized production.
Owner:山东立新制药有限公司
One-pot synthesis method for 5-bromo-2-chloro-4'-ethoxy diphenylmethane
InactiveCN103570510AReduce consumptionImprove recycling effectOrganic compound preparationCarbonyl compound preparation by condensationDiphenylmethaneSolvent
The invention discloses a one-pot synthesis method for 5-bromo-2-chloro-4'-ethoxy diphenylmethane. The method comprises the following continuous steps of: taking 2-chloro-5-bromobenzoic acid as a raw material, reacting it with thionyl chloride to generate 2-chloro-5-bromobenzoyl chloride, and adding an organic solvent, Lewis acid, phenetole and borohydride to undergo Friedel-Crafts acylation reaction and hydroboration reduction reaction so as to obtain the 5-bromo-2-chloro-4'-ethoxy diphenylmethane. The process involved in the invention has the advantages that the same Lewis acid is used as a catalyst in the Friedel-Crafts acylation reaction and the hydroboration reduction reaction, so that the catalyst consumption is greatly reduced, the operation is simple, and the intermediate needs no separation and purification. By effectively controlling the reaction conditions, and the charging sequence and proportion, the 5-bromo-2-chloro-4'-ethoxy diphenylmethane can be synthesized by one step, and fewer three wastes are discharged, the solvent recovery effect is good, and the production cost is low.
Owner:苏州中科天马肽工程中心有限公司
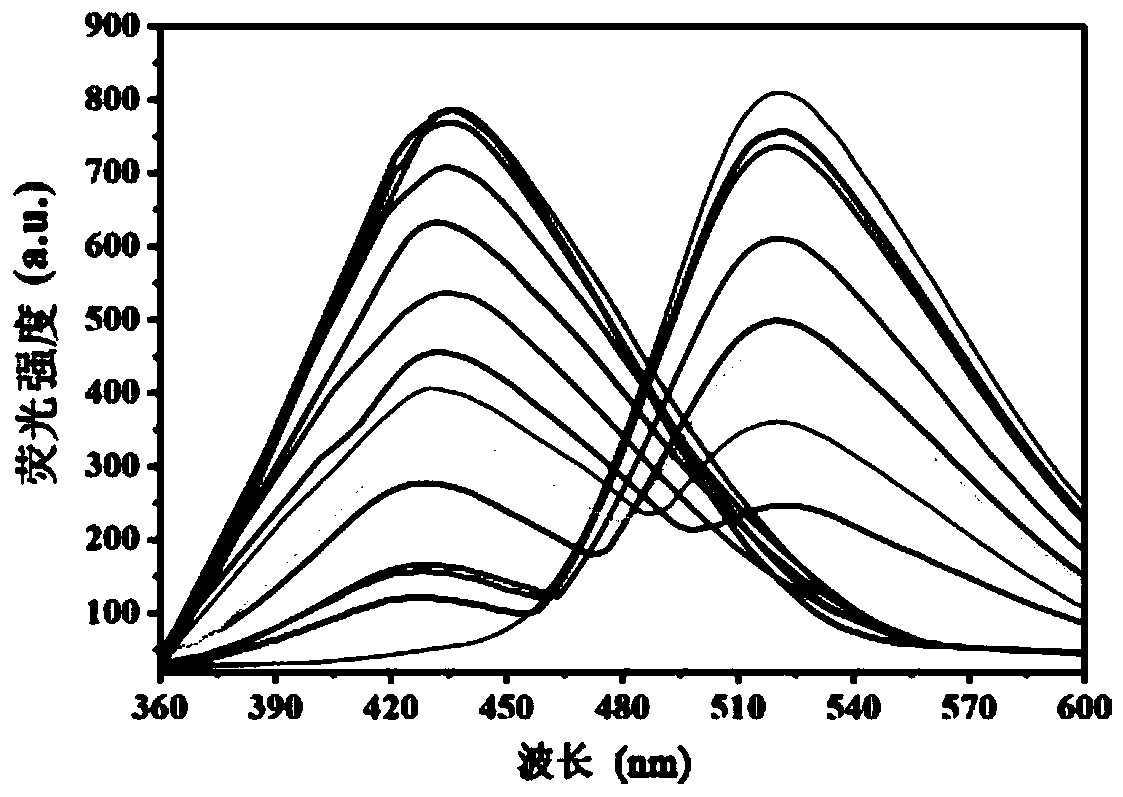
Fluorescent probe capable of quickly detecting hydrogen sulfite ions and preparation method and application thereof
InactiveCN109734738ASpecific complexationHigh selectivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescenceClaisen condensation
The invention discloses a fluorescent probe capable of quickly detecting hydrogen sulfite ions and a preparation method and application thereof. The preparation method includes: taking beta-pinene oxidation derivative nopinone which is one of main ingredients of natural renewable natural resource turpentine as a raw material, and allowing Claisen condensation reaction with 4-methyl bromobenzoate to obtain 3-(4'-bromobenzoyl) nopinone; allowing 3-(4'-bromobenzoyl) nopinone to be in coupling reaction with 4-formylbenzeneboronic acid to obtain 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone;allowing 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone to react with boron trifluoride etherate to obtain a 3-(4''-formyl-1', 1''-biphenyl-4'-carbonyl) nopinone based fluoroboron complex. Thecompound serving as a high-selectivity high-sensitivity color ratio type fluorescent probe has good application in the aspect of detecting the hydrogen sulfite ions.
Owner:NANJING FORESTRY UNIV
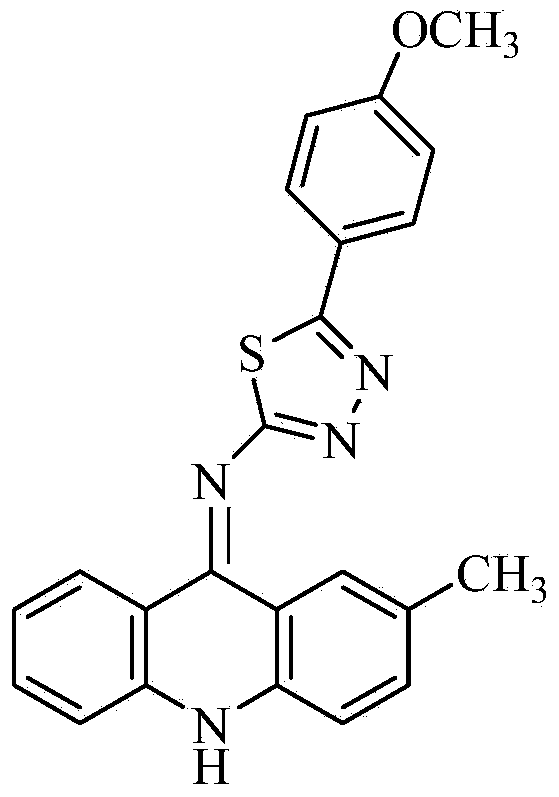
Acridine-1, 2, 3-triazole type compound and preparation method and application thereof
ActiveCN104277029ASignificant in vitro antitumor activityGood potential medicinal valueOrganic chemistryAntineoplastic agentsVitamin CFiltration
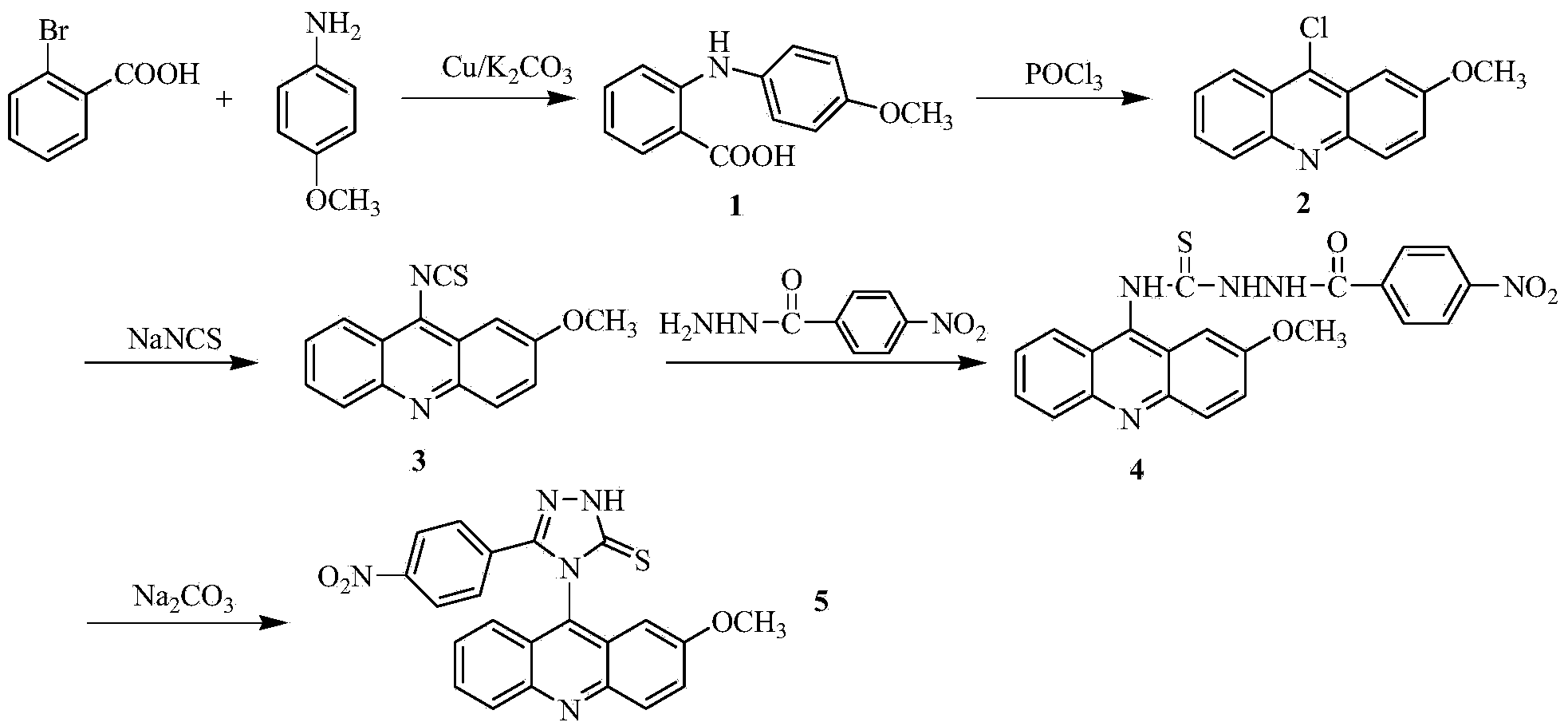
The invention discloses an acridine-1, 2, 3-triazole type compound and a preparation method and application thereof. The preparation method of the acridine-1, 2, 3-triazole type compound comprises the following steps: 1) performing ullmann reaction by taking o-bromobenzoic acid and p-methoxyaniline as raw materials, taking potassium carbonate and copper powder as catalysts and taking isoamyl alcohol or n-amyl alcohol as a solvent to obtain a compound 1; 2) cyclizing the compound 1 with phosphorus oxychloride to prepare a compound 2; 3) dissolving the compound 2 in DMF (dimethylformamide) and performing nucleophilic substitution reaction with sodium azide to prepare a compound 3; and 4) taking p-methoxyphenylacetylene, dissolving in a tert-butyl alcohol / water solution, adding vitamin C sodium, copper sulfate pentahydrate and the compound 3 to react, performing suction filtration, and recrystallizing for preparation. In-vitro anti-tumor test results show that the acridine-1, 2, 3-triazole type compound has significant in-vitro anti-tumor activity against three subjects, namely MGC80-3, BEL-7404 and T24, has relatively good potential medicinal values, and is expected to be used for preparing various anti-tumor medicines.
Owner:广西新桂环保科技集团有限公司
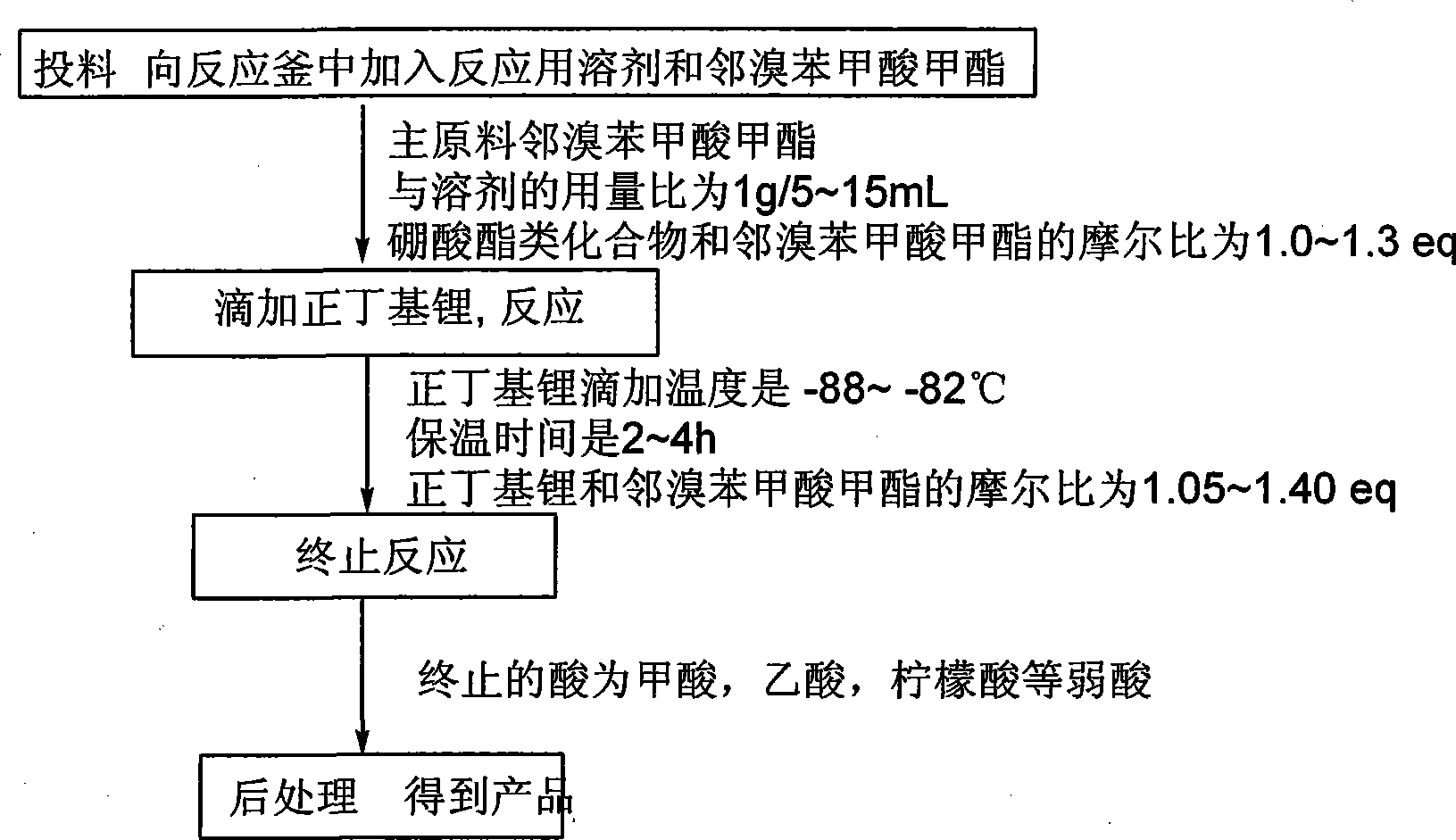
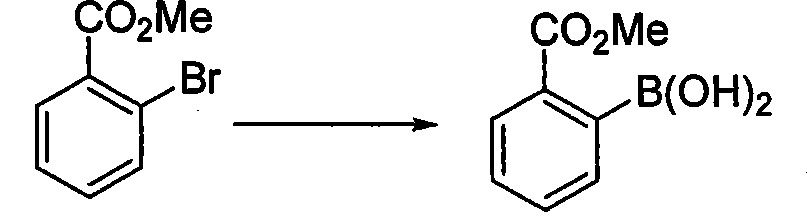
Method for preparing phenylboronic acid-2-methyl formate
ActiveCN101519411AInhibit side effectsMeet the needs of large-scale productionGroup 3/13 element organic compoundsPhenylboronic acidN-Butyllithium
The invention relates to a method for preparing phenylboronic acid-2-methyl formate. A starting material is a commercial raw material on the market or easy-to-prepare bromobenzoic acid methyl ester; and under a condition of low temperature, n-butyl lithium is dripped into a mixture of the main raw material of bromobenzoic acid methyl ester and a boric acid ester compound to form the phenylboronic acid-2-methyl formate through hydrolysis. The method has the advantages of readily available raw materials, high reaction purity and yield, stable process conditions, simple operation and suitability for large-scale production and provides a new thought and means for preparing the phenylboronic acid-2-methyl formate.
Owner:JILIN ASYMCHEM LAB CO LTD
Selective enrichment media and uses thereof
ActiveUS9029118B1Preventing undesirable false positive responseBacteriaMicrobiological testing/measurementSulfur drugAminocoumarins
Selective enrichment media and methods for selectively growing and detecting Salmonella spp. The media comprise a carbon and nitrogen source, an inorganic salt, a fermentable sugar, one or more selective agents, and an efflux pump inhibitor. Various selective agents include sulfa drugs, surfactants, aminocoumarins, cycloheximide, supravital stains, ascorbic acid, bromobenzoic acid, myricetin, rifamycins, polyketides, and oxazolidinones. Various efflux pump inhibitors include arylpiperazines, such as 1-(1-naphthylmethyl)piperazine, and quinoline derivatives, such as 4-chloroquinoline. The selective agents and efflux pump inhibitors are provided in the media in combinations and amounts that inhibit growth of non-Salmonella microorganisms without substantially affecting growth and metabolism of Salmonella species. Methods of selectively growing and detecting Salmonella species are provided.
Owner:PARADIGM DIAGNOSTICS
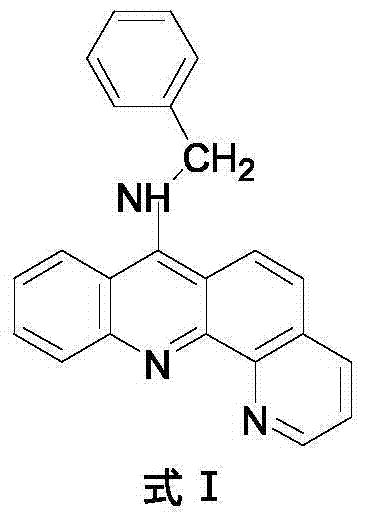
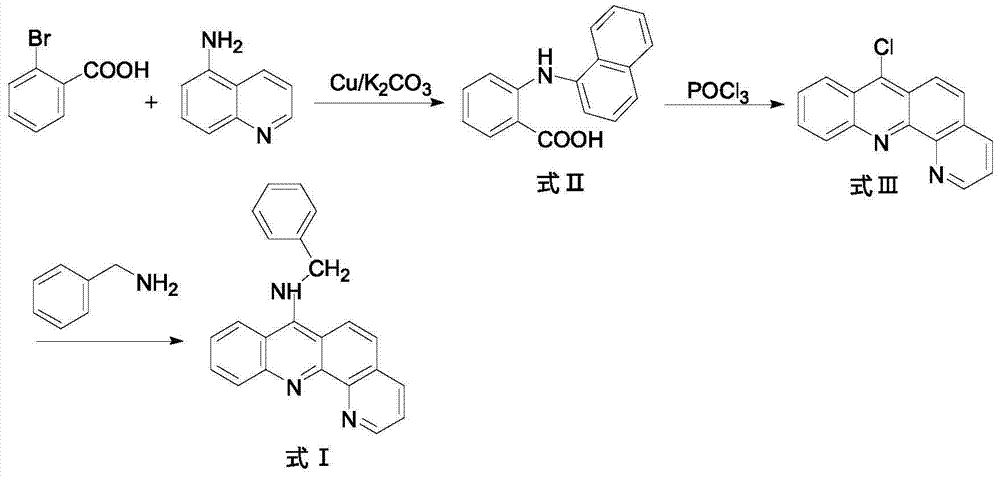
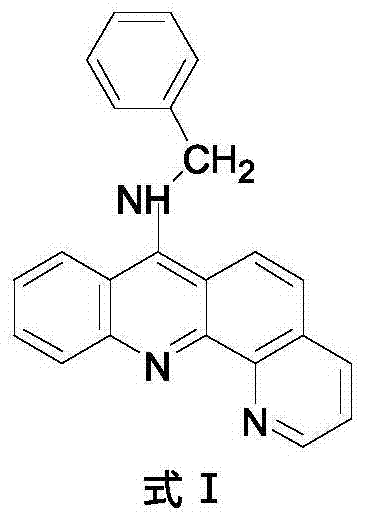
9-amino substituted pyrido acridine derivative, preparation method and uses thereof
ActiveCN104327075AStrong acetylcholinesterase inhibitory activityEasy to prepareSenses disorderNervous disorderQuinolineNucleophilic substitution
The present invention relates to a 9-amino substituted pyrido acridine derivative, a preparation method and uses thereof, wherein the structure formula of the derivative is represented in the instruction. The preparation method comprises that 2-bromobenzoic acid and 8-amino quinoline are adopted to prepare N-(quinolyl)anthranilic acid, the product is subjected to cyclization with phosphorus oxychloride to obtain 9-chloro pyrido acridine, the 9-chloro pyrido acridine is dissolved in ethanol, and benzylamine is added to carry out a nucleophilic substitution reaction so as to prepare the target product. The derivative can be used as an acetylcholinesterase inhibitor so as to be used for the treatment of Alzheimer's disease, cerebrovascular dementia and other diseases.
Owner:SHANGHAI RONA THERAPEUTICS CO LTD
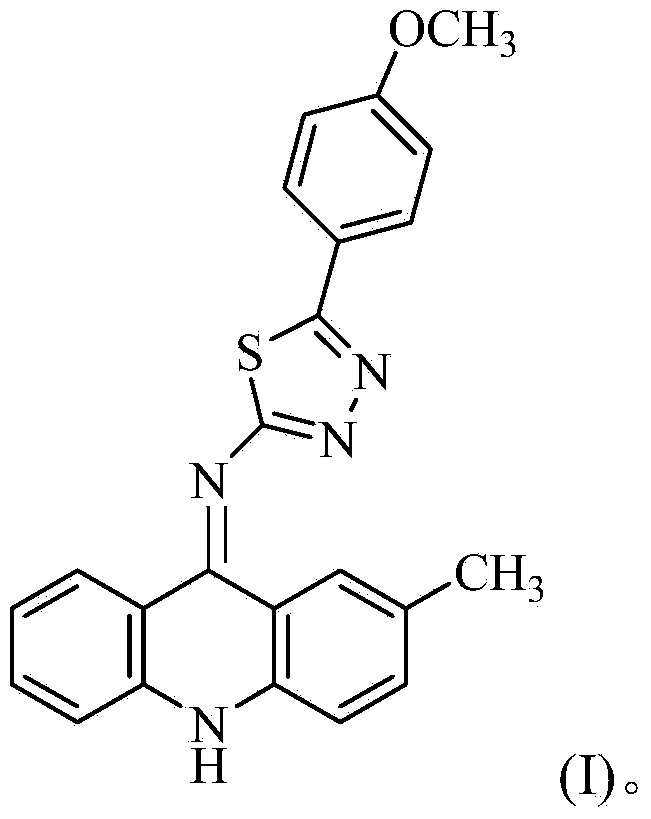
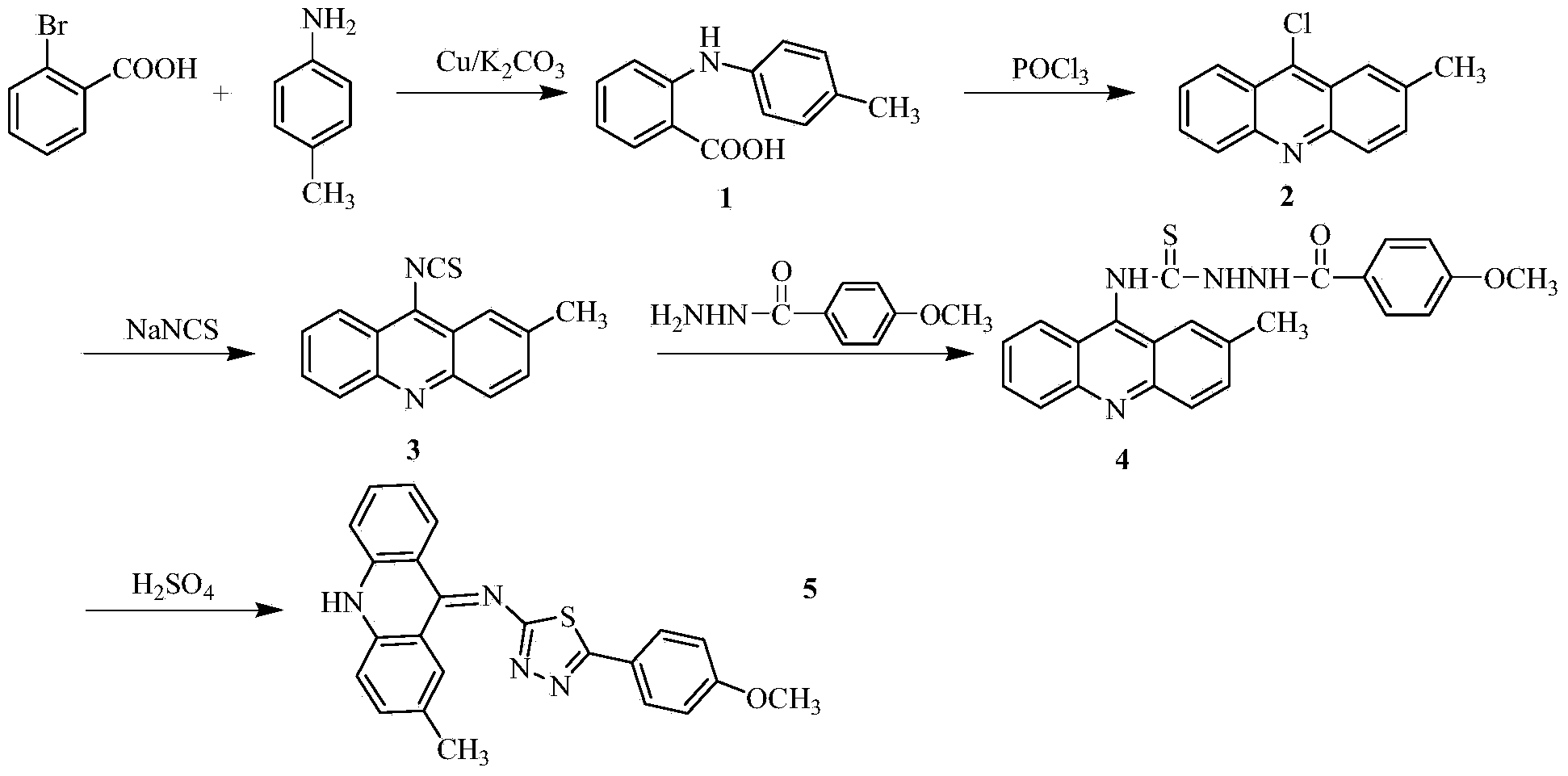
Acridine-1, 3, 4-thiadiazole type compound and preparation method and application thereof
ActiveCN104277035AIncrease the conjugate areaEasy to embedOrganic chemistryAntineoplastic agentsBenzoic acidMethylaniline
The invention discloses an acridine-1, 3, 4-thiadiazole type compound and a preparation method and application thereof. The preparation method of the acridine-1, 3, 4-thiadiazole type compound comprises the following steps: 1) reacting by taking o-bromobenzoic acid and p-methyl aniline as raw materials, taking potassium carbonate and copper powder as catalysts and taking isoamyl alcohol or n-amyl alcohol as a solvent to obtain a compound 1; 2) cyclizing the compound 1 with phosphorus oxychloride to prepare a compound 2; 3) dissolving the compound 2 in an organic solvent, and then reacting with sodium thiocyanate in the presence of tetrabutyl ammonium bromide to prepare a compound 3; 4) taking the compound 3, dissolving in the organic solvent, and reacting with p-methoxybenzhydrazide to prepare a compound 4; and 5) taking the compound 4, reacting with concentrated sulfuric acid or acetic acid, adding water into a reactant to precipitate solids, and performing suction filtration for preparation. In-vitro anti-tumor test results show that the acridine-1, 3, 4-thiadiazole type compound has significant in-vitro anti-tumor activity against four subjects, namely MGC80-3, BEL-7404, NCI-H460 and T24.
Owner:广西新桂环保科技集团有限公司
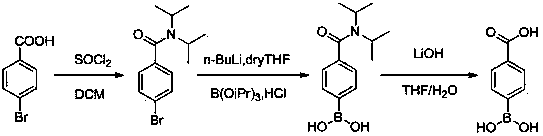
Preparation method of p-carboxyphenylboronic acid
ActiveCN103724366AHigh yieldSimple and fast operationGroup 3/13 element organic compoundsBenzoic acidHydration reaction
The invention discloses a preparation method of p-carboxyphenylboronic acid. The preparation method comprises the steps of carrying out an amidation reaction on bromobenzoic acid and diisopropylamine to obtain a reaction intermediate 4-bromo-N,N-diisopropylbenzamide; carrying out the substitution reaction on the 4-bromo-N,N-diisopropylbenzamide at a reaction temperature ranging from -75 to -80 DEG C in a solvent which is dry tetrahydrofuran, thereby obtaining (4-(diisopropylcarbamyl)phenyl) boric acid; hydrolyzing (4-(diisopropylcarbamyl)phenyl) boric acid in the presence of lithium hydroxide monohydrate to obtain p-carboxyphenylboronic acid. The preparation method provided by the invention is high in product yield, simple and convenient to operate, low in reaction cost and applicable to industrial production.
Owner:贵州威顿晶磷电子材料股份有限公司
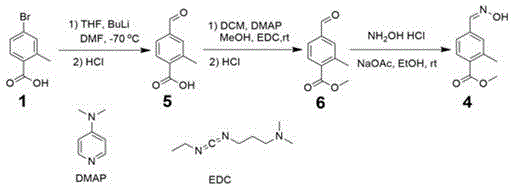
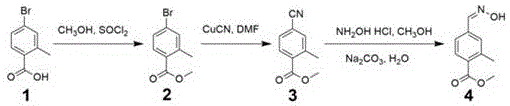
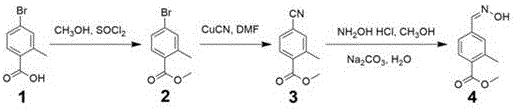
Method for preparing 2-methyl-4-formaldoxime methyl benzoate
ActiveCN106431969AReduce manufacturing costMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationBromobenzoic AcidsHydroxylamine Hydrochloride
The invention discloses a method for preparing 2-methyl-4-formaldoxime methyl benzoate. The method comprises the following steps: performing acylating chlorination on 2-methyl-4-bromobenzoic acid, carrying out methanol esterification and cyano substitution, and then performing nucleophilic addition elimination with hydroxylamine hydrochloride under alkaline conditions to obtain a target product. The method has the advantages that the process route is simple; reaction conditions are mild; the product yield is high; the total yield reaches 61 percent; the product quality is high; the appearance is white solid; the purity reaches 99.2 percent.
Owner:荆门医药工业技术研究院 +1
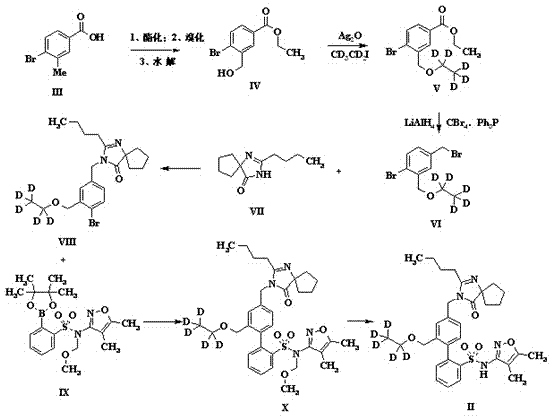
Preparation method of sparsenatan for stabilizing isotope labeling
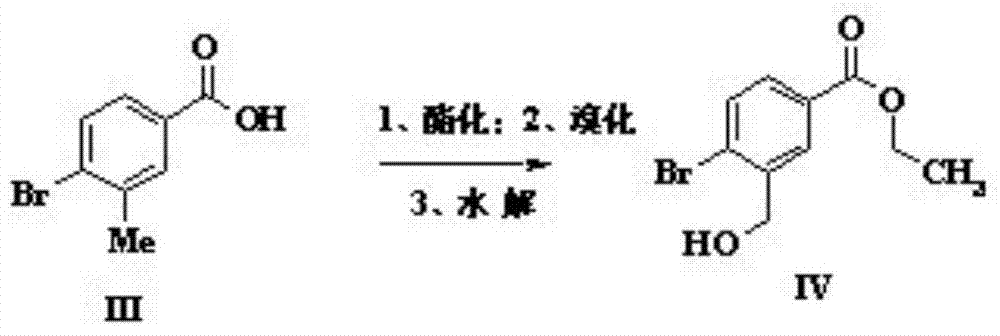
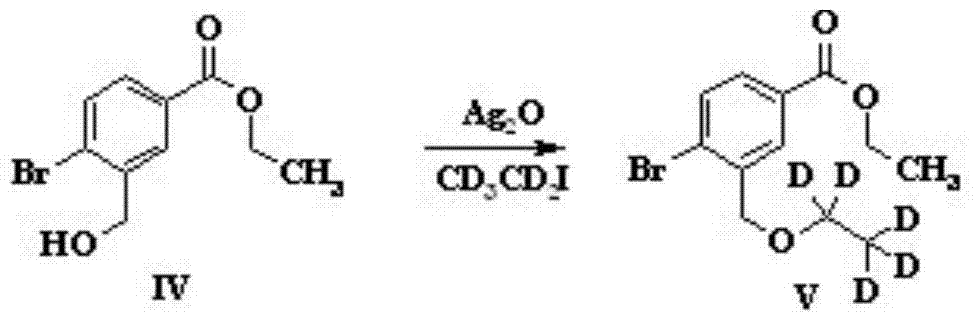
The invention discloses a preparation method of sparsenatan for stabilizing isotope labeling. According to the method, 3-methyl-4-bromobenzoic acid is used as a raw material, iodoethane for deuterium labeling serves as a deuterium labeling initiator, and the sparsenatan is obtained through six-step reaction. Optimal preparation steps and reaction conditions are screened out through a large number of experiments, and the whole preparation method is reasonable in design and good in operability. The purity of the sparsenatan prepared by means of the method and used for stabilizing the isotope labeling can be higher than 99%, and the isotope abundance is higher than 99%. The sparsenatan prepared by means of the method and used for stabilizing the isotope labeling is a standard product for research on the metabolic mechanism of the sparsenatan, can be used for tracing the metabolic process of the sparsenatan in a living body and has great application and research value on clinic pharmacokinetic study.
Owner:TLC NANJING PHARMA RANDD CO LTD
Acridine-1,2,4-triazole-5-thioketone compound and preparation method and applications of acridine-1,2,4-triazole-5-thioketone compound
ActiveCN104277028AIncrease the conjugate areaEasy to embedOrganic active ingredientsOrganic chemistrySodium sulfocyanateThioketone
The invention discloses an acridine-1,2,4-triazole-5-thioketone compound and a preparation method and applications of the acridine-1,2,4-triazole-5-thioketone compound. The preparation method of the compound comprises the following steps: 1) by taking an o-bromobenzoic acid and p-methoxyaniline as raw materials, taking potassium carbonate and copper powder as catalysts, and taking isopentyl alcohol or n-amyl alcohol as a solvent, reacting so as to obtain a compound 1; 2) carrying out cyclization on the compound 1 by using phosphorus oxychloride so as to obtain a compound 2; 3) after the compound 2 is dissolved by using an organic solvent, in the presence of tetrabutylammonium bromide, reacting the dissolved compound 2 with sodium sulfocyanate so as to obtain a compound 3; 4) after the compound 3 is dissolved by using an organic solvent, reacting the dissolved compound 3 with m-nitrobenzoylhydrazine so as to obtain a compound 4; and 5) reacting the compound 4 with sodium carbonate, carrying out suction filtration on a reactant, collecting filter liquor, adjusting the pH value of the filter liquor to be less than 4, separating out precipitates, and carrying out suction filtration on the precipitates. In-vitro antitumor test results show that the compound has a significant in-vitro antitumor activity to tested MGC80-3, NCI-H460 and T24.
Owner:广西新桂环保科技集团有限公司
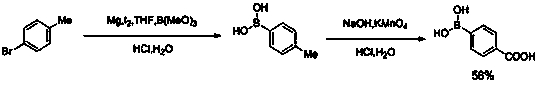
Method for preparing 4-bromobenzoic acid
InactiveCN108558636AHigh yieldHigh purityOrganic compound preparationCarboxylic compound separation/purificationAcetic acidBromobenzoic Acids
The invention relates to a method for preparing 4-bromobenzoic acid and belongs to the technical field of compound production and preparation. The method comprises the following steps: by taking bromotoluene as an initial raw material, glacial acetic acid as a solvent and oxygen as an oxidant, carrying out catalytic oxidation on parabromotoluene by using a liquid-phase oxidation method under the action of a catalyst, controlling a reaction temperature to 75-85 DEG C, terminating the reaction when the content of the parabromotoluene in the reaction system accounts for 0.5wt% of an initial content, cooling, filtering so as to obtain a crude product and filtrate of 4-bromobenzoic acid, and further purifying the crude product of the 4-bromobenzoic acid at one step, thereby obtaining a finishedproduct. The melting point of the finished product of the 4-bromobenzoic acid is 252-254 DEG C. In addition, the 4-bromobenzoic acid prepared by using the method is high in yield, that is, the yieldis up to 98% or greater, meanwhile, the finished product is high in purity, that is, the purity is up to 99% or greater, the defects that a product prepared by using a conventional preparation methodis low in purity and low in yield can be overcome, and in addition, the method provided by the invention is simple and easy in raw material obtaining, low in cost and wide in application prospect.
Owner:黄石市利福达医药化工有限公司
A kind of synthetic method of 5-bromo-2-chlorobenzoic acid
ActiveCN105622382BEasy to operateHigh purityOrganic compound preparationCarboxylic compound preparationBenzoic acidSynthesis methods
The invention provides a synthesis method of 5-bromo-2-chloro benzoic acid.The method includes the following steps of A, making 2-chlorine benzotrichloride and bromide reagents react under the effect of a catalyst to obtain 2-chloro-5-bromine benzotrichloride, wherein bromide reagents include one or more of bromine, N-bromosuccinimide, dibromohydantoin and hydrobromic acid; B, conducting hydrolysis reaction on 2-chloro-5-bromine benzotrichloride in the step A under the acid condition to obtain 5-bromo-2-chloro benzoic acid.According to the method, 2-chlorine benzotrichloride which is low in price and easy to obtain is adopted as the raw material, operation is easy, intermediates do not need to be purified, 5-bromo-2-chloro benzoic acid is synthesized through a one-pot method, purity is high, yield is high, three-waste emission is little, and production cost is low.It is shown through experiment results that 2-chloro-5-benzoic acid obtained according to the synthesis method has yield larger than 95% and purity of 80-92%.
Owner:苏州正济药业有限公司
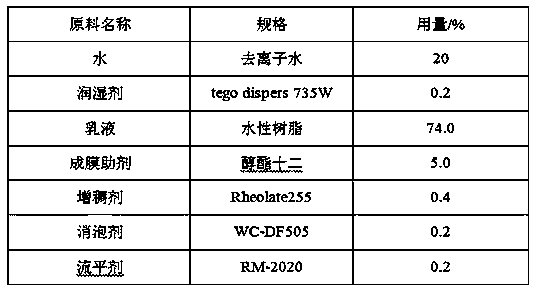
Self-flame retardant antibacterial aqueous polyester resin and preparation method thereof
InactiveCN110563936ANot easy to dissociateDurable Flame RetardancyFireproof paintsAntifouling/underwater paintsCarboxylic acidFire retardant
The invention relates to a self-flame retardant antibacterial aqueous polyester resin and a preparation method thereof. The resin comprises the following components in parts by weight: 6.0-12.0 partsof organic anhydride, 3.0-5.0 parts of carboxylic acid Schiff base, 3.0-8.0 parts of polybasic acid, 6.0-20.0 parts of polyhydric alcohols, 1.2-3.5 parts of 2-chloro-5-bromobenzoic acid, 1.5-3.5 partsof dimethylolpropionic acid, 2.0-5.0 parts of xylene, 2.0-5.0 parts of a neutralizing agent and 45.0-65.0 parts of deionized water. The self-flame retardant antibacterial aqueous polyester resin provided by the invention is good in coloring property, low in viscosity, high in hardness, good in fullness, good in brightness, and the like, is free of external flame retardant or antibacterial agent when an aqueous coating is prepared, is long in flame retardant and antibacterial effect lasting and high in efficiency, and can be applied to inner and outer wall coatings, aqueous industrial coatings, aqueous wood product coatings, and the like.
Owner:UNION FOSHAN CHEM +1
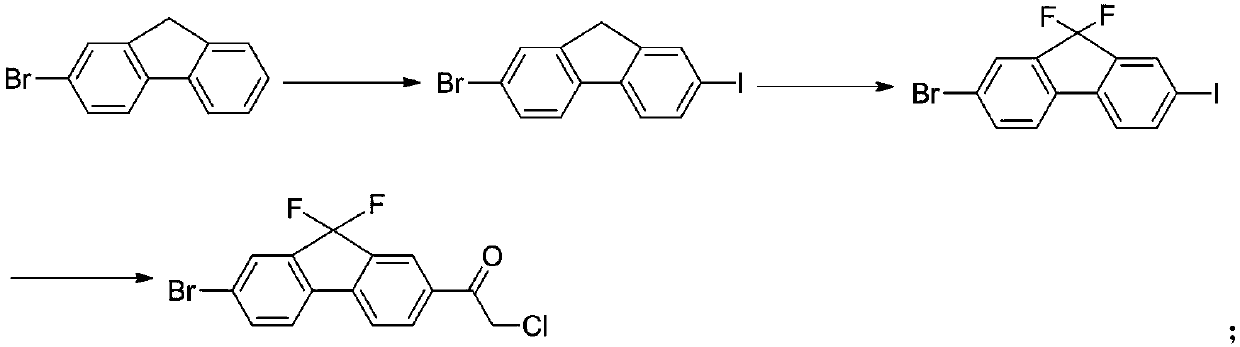
Preparation method of key intermediate of anti-hepatitis C drug ledipasvir
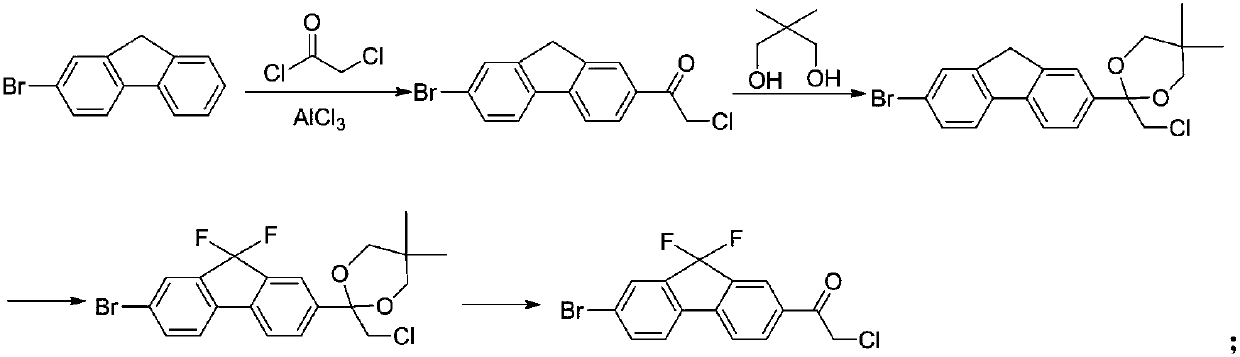
InactiveCN109678686ARaw materials are easy to getLow priceOrganic compound preparationCarboxylic acid esters preparationN-methylacetamideIobenzamic acid
The invention provides a preparation method of a key intermediate 1-(7-bromo-9,9-difluoro-9H-fluoren-2-yl)-2-chloroethanone. The method comprises the steps as follows: 2-amino-5-bromobenzoic acid is taken as a raw material, and subjected to diazotization, iodination,synthesis of 5-bromo-2-iodobenzoic acid, methylation, coupling reaction with phenylboronic acid, ester hydrolysis, acyl chlorination,intramolecular Friedel-Crafts alkylation, carbonyl reduction, iodization, fluorination and final reaction with 2-chloro-N-methoxy-N-methylacetamide to prepare the target product. The process adopts easily available starting raw materials, is low in price and free of hazardous process and has mild reaction conditions..
Owner:IANGSU COLLEGE OF ENG & TECH
Artificial synthesis method of neomarchantin A
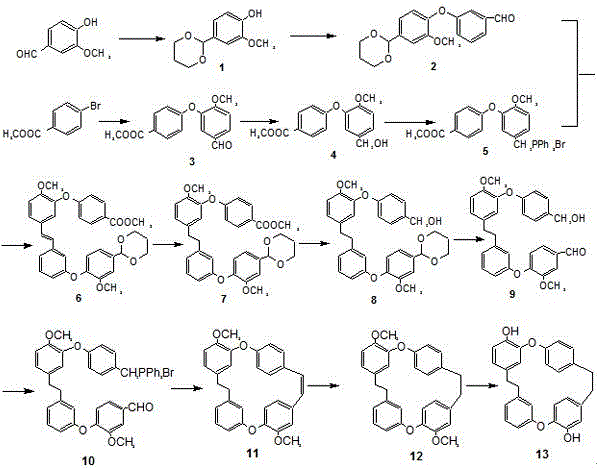
The invention relates to an artificial synthesis method of neomarchantin A, which comprises the following step: by using 4-hydroxy-3-methoxybenzaldehyde and methyl parabromobenzoate as initial raw materials, carrying out chemical reaction to finally obtain the neomarchantin A. The method provided by the invention utilizes the artificial synthesis process to obtain the neomarchantin A for the first time and can continuously and abundantly synthesis neomarchantin A, and the overall yield is 18.9%, thereby providing favorable technical supports for subsequent large-scale production of neomarchantin A.
Owner:SUZHOU HEALTH COLLEGE
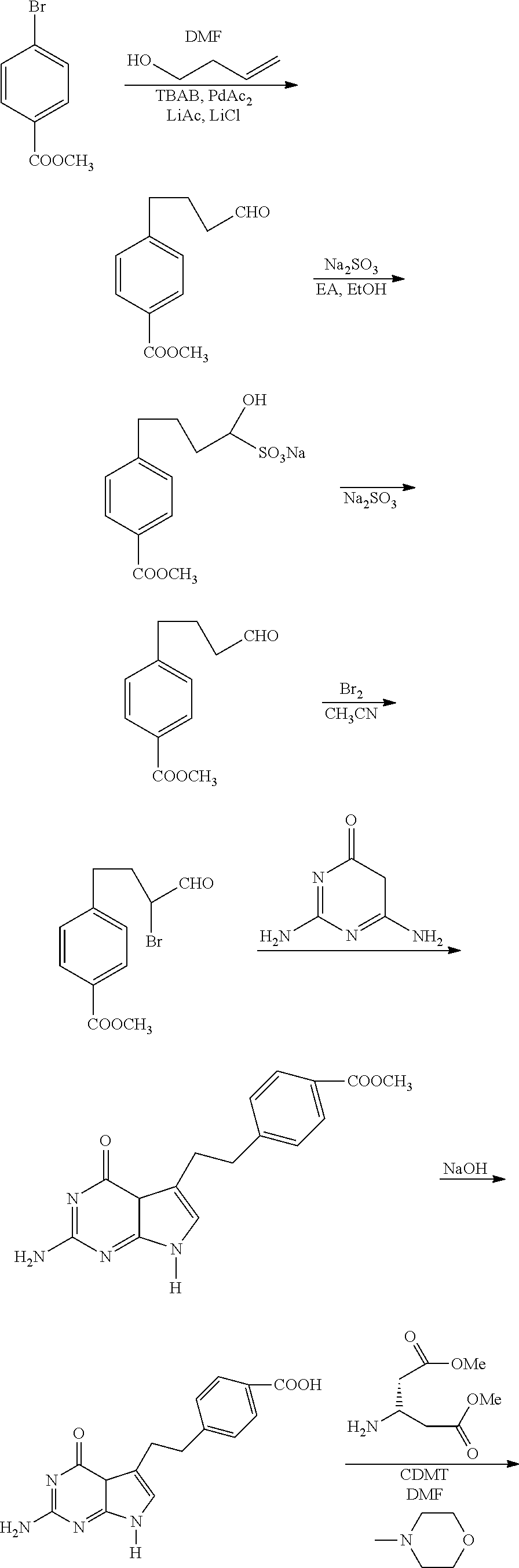
Process for Preparing Pemetrexed Disodium and Its Intermediate, 4-(4-Carbomethoxyphenyl) Butanal
InactiveUS20110124861A1Speed up the processOrganic compound preparationCarboxylic acid esters preparationBenzoic acidOrganic solvent
The present invention provides a process for preparing pemetrexed disodium and its intermediate, 4-(4-carbomethoxyphenyl)butanal. The process for preparing the intermediate comprises the following steps: condensing methyl 4-bromobenzoate with 3-buten-1-ol; extracting with an organic solvent during the work-up; adding silica gel to decolorize; and evaporating the organic solvent to give 4-(4-carbomethoxyphenyl)butanal. The product obtained by the present process, with a yield of higher than 80%, and a purity measured by GC of higher than 95%, may be directly used in the next bromination reaction for synthesizing pemetrexed disodium without purification. The present process is suitable for industrial production, as the operation is simple and the reagents used are cheap and readily available.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
3-chloro-7(5)-bromo-benzo-isoxazole compounding method
The invention discloses a 3-chloro-7(5)-bromo-benzo-isoxazole compounding method which includes utilizing bromofluorobenzene as a raw material, acidizing the raw material to obtain 2-fluoro-3-bromobenzoic-acid, esterifying the 2-fluoro-3-bromobenzoic-acid to obtain 2-fluoro-3-methyl-bromobenzoate, enabling the 2-fluoro-3-methyl-bromobenzoate to react with N-acetyl-hydroxylamine to obtain 7-bromo-3-benzo-isoxazolone, and finally chloridizing the 7-bromo-3-benzo-isoxazolone to obtain 3-chloro-7-bromo-benzo-isoxazole; utilizing 2-fluoro-5-bromobenzoic-acid as a raw material, esterifying the 2-fluoro-5-bromobenzoic-acid to obtain 2-fluoro-5-methyl-bromobenzoate, enabling the 2-fluoro-5-methyl-bromobenzoate to react with the N-acetyl-hydroxylamine to obtain 5-bromo-3-benzo-isoxazolone, and finally chloridizing the 5-bromo-3-benzo-isoxazolone to obtain the 3-chloro-5-bromo-benzo-isoxazole. The method is low in raw material price and cost, fewer in reaction steps, simple in post treatment, high in total recovery and capable of reducing environment pollution.
Owner:SYNTHESIS MED SHANGHAI
Modified cotton straw adsorbent for removing pyrazinamide through adsorption
InactiveCN106975459AStrong selective adsorptionImprove adsorption capacityOther chemical processesWater contaminantsLithium chlorideSorbent
The invention discloses a modified cotton straw adsorbent for removing pyrazinamide through adsorption. The modified cotton straw adsorbent is prepared through the following steps of selecting cotton straws of which the particle size is 4-6mm, washing the cotton straws with deionized water, absolute ethyl alcohol and an NaOH solution and drying to obtain a substance Q; modifying the substance Q with a mixed liquid prepared from urea sulfate, guanidine nitrate, rhodium nitrate, cobalt sodium nitrate and iridous chloride, thus obtaining a substance R; modifying the substance R with a mixed liquid prepared from ceric ammonium nitrate, pyridine sulfur trioxide, duroquinol, pyridine sulfur trioxide, duroquinol, 1,5-naphthalene disulfonic acid, 2-amino-3-bromobenzoic acid and N-ethyl acetamide, thus obtaining a substance S; and modifying the substance S with a mixed liquid prepared from cupric nitrate, cadmium nitrate, lithium chloride, magnesium chloride and stannous chloride, thus obtaining a substance, namely the modified cotton straw adsorbent for removing the pyrazinamide through adsorption.
Owner:北京益净环保设备科技有限公司
Synthetic method of fluralana
PendingCN114315748AReduce dosageReduce intermediate lossesOrganic chemistryBenzoic acidBromobenzoic Acids
According to the method, 2-methyl-5-bromobenzoic acid is adopted as a raw material, and Suzuki coupling reaction, condensation reaction, dehydration cyclization reaction and amide condensation reaction are performed to finally obtain the fluralan. According to the synthesis method, the reaction cost is reduced, the yield is improved, and the reaction period is shortened.
Owner:JIANGSU TIANHE PHARMA CO LTD
Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method
ActiveCN107759461AServing market needsHigh yieldOrganic compound preparationCarboxylic compound preparationBromobenzoic AcidsPalladium catalyst
The invention belongs to the field of compound preparation and discloses a preparation method for synthesizing an anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through a one-step method. The method comprises the following steps: under a condition of an alkali solution, by taking N,N-dimethyl formamide as a solvent, catalyzing with a palladium catalyst, adding 4-bromobenzoic acid, 1,4-phenylenediboronic acid pinacol ester and 4-(n-pentyloxy) bromobenzene as raw materials, and performing an SUZUKI coupling reaction, thereby obtaining a target product, namely the anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-). The raw materials used in the method are all industrial products available in the market, the raw materials are easily available, in addition, the steps are simple, the reaction conditions can be controlled, wastes are greatly reduced in the production process of the product, and chemical environmental-friendly requirements of the current society are met.
Owner:TIANJIN DERCHEMIST SCI TECH
Coumarin-acridone fluorescent probe, and preparation method and application thereof
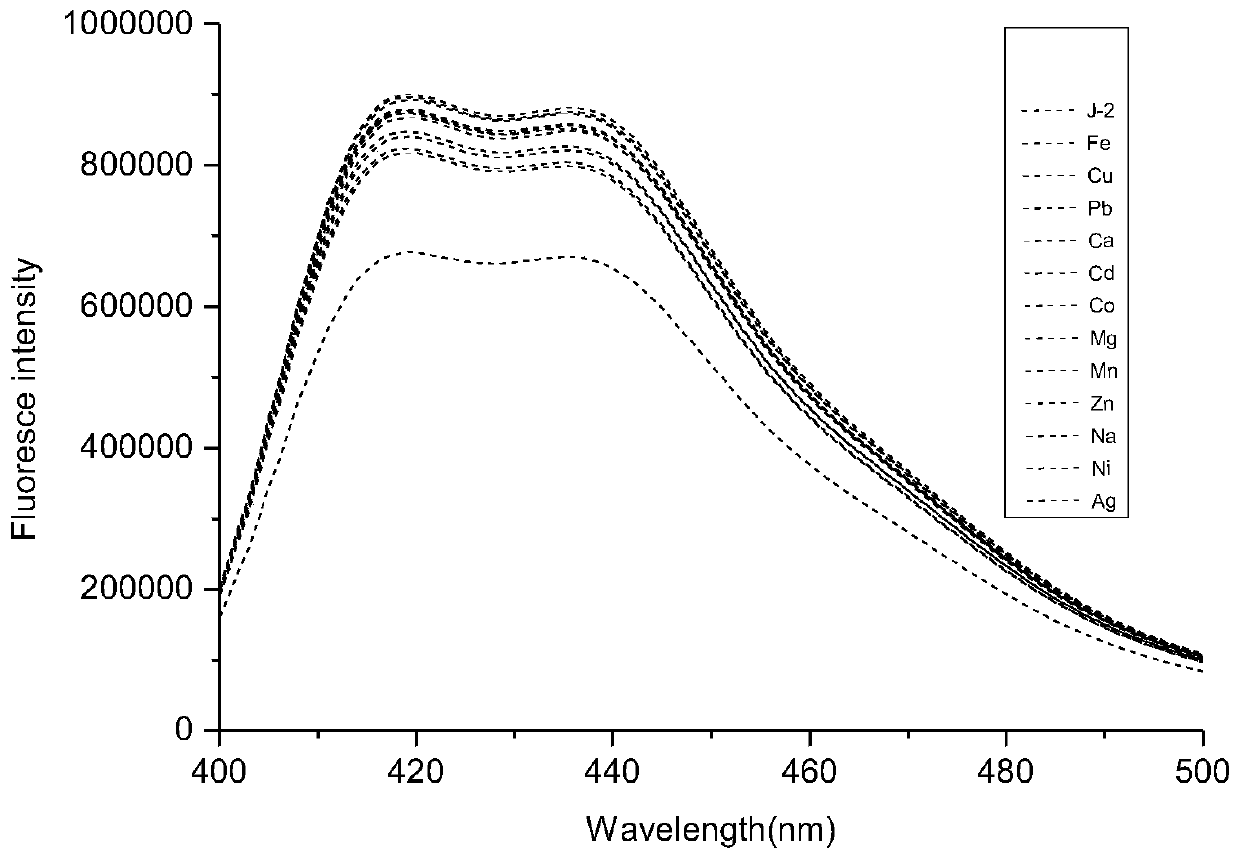
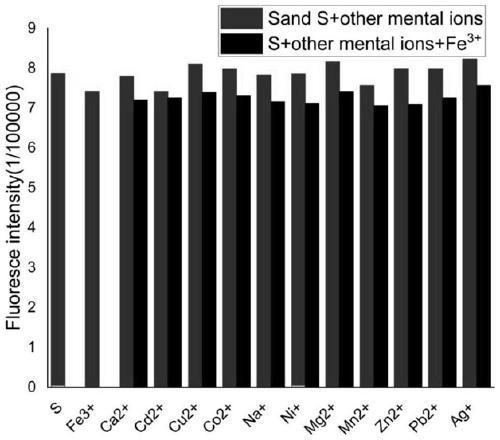
ActiveCN111217820AGood choiceImprove anti-interference abilityOrganic chemistry methodsFluorescence/phosphorescenceFluoProbesBenzoic acid
The invention discloses a coumarin-acridone fluorescent probe, and a preparation method and an application thereof. The chemical name of the coumarin-acridone fluorescent probe is 4-methyl-2H-pyrano[2,3a]acridin-2,12(7H)-dione acetate. The preparation method is characterized in that o-bromobenzoic acid and 1-amino-4-methylcoumarin which are taken as initial raw materials undergo a Cu-catalyzed Ullmann reaction and a ring closing reaction to synthesize the coumarin-acridone fluorescent probe. The fluorescent probe has specific selectivity to iron ions, basically has no change with other commonmetal ion fluorescence signals, and has high sensitivity and low detection limit. The coumarin-acridone fluorescent probe can be applied to in-vitro fluorescence detection of iron ions.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
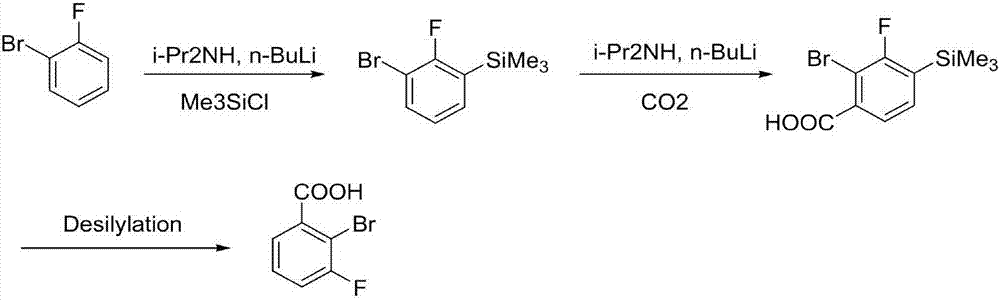
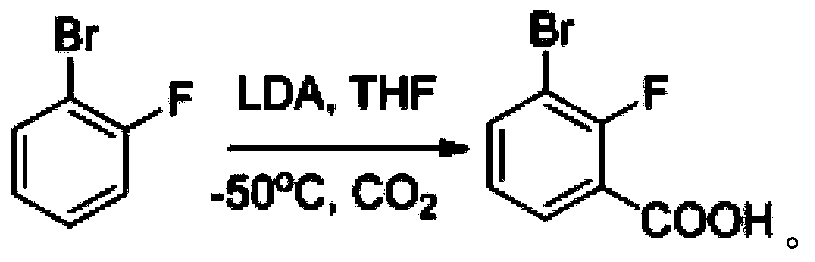
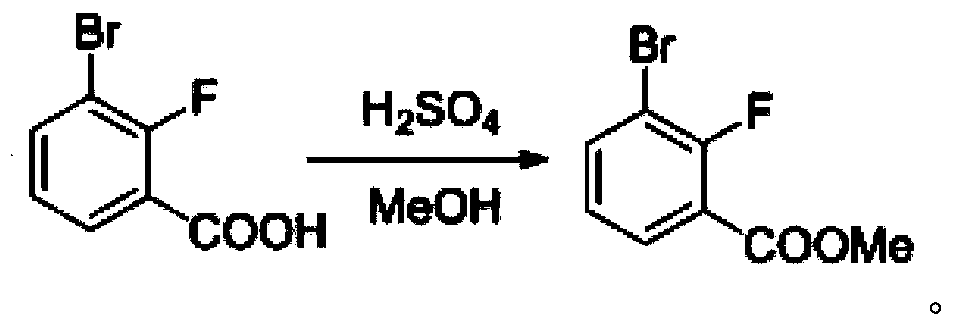
Synthesis method of 2-bromo-3-fluobenzoic acid
ActiveCN108003016AReasonable designNo need for separation and purificationSilicon organic compoundsOrganic compound preparationSynthesis methodsLithium diisopropylamide
The invention discloses a synthesis method of 2-bromo-3-fluobenzoic acid. The method comprises the following steps of (1) using o-bromo fluorobenzene as starting raw materials; performing proton abstraction through lithium diisopropylamide; performing reaction with trimethylchlorosilane to generate 3-bromo-2-fluorophenyl trimethylsilane; (2) forming an intermediate of 4-trimethylsilyl-3-bromo-2-bromobenzene lithium saltsby 3-bromo-2-fluorophenyl trimethylsilane under the effect of lithium diisopropylamide; then, performing reaction with carbon dioxide to generate 4-trimethylsilyl-3-bromo-2-bromobenzoic acid; (3) performing reaction on 4-trimethylsilyl-3-bromo-2-bromobenzoic acid and fluorine-containing reagents for desilylation to obtain the 2-bromo-3-fluobenzoic acid. The intermediate obtained by the method does not have isomer; the separation is easy; the obtained product has high purity; a simple and compact path is provided for the synthesis of compounds.
Owner:杭州逸翔化工科技有限公司
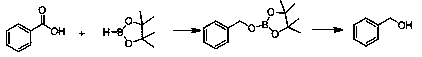
Method for preparing alcohol compound through uncatalyzed reaction of aromatic carboxylic acid
InactiveCN109574808AImprove conversion ratePost-processing is simpleOrganic compound preparationGroup 3/13 element organic compoundsBenzoic acidCarboxylic acid
The invention discloses a method for preparing alcohol compound through uncatalyzed reaction of aromatic carboxylic acid. The method includes: in an inert gas atmosphere, well stirring and mixing pinacolborane and carboxylic acid in a reaction bottle after dehydration deoxygenation, allowing reaction for 6-12h to obtain borate, and further hydrolyzing into alcohol, wherein the carboxylic acid is benzoic acid, 4-bromobenzoic acid, 4-fluorobenzoic acid, 1-naphthoic acid and 2-methoxybenzoic acid. The carboxylic acid is utilized to be in hydroboration reaction with borane efficiently in a catalyst-free condition for the first time, and a carbonyl compound is enabled to be in hydroboration reaction with borane to prepare borate which is further hydrolyzed into alcohol, in this way, a new scheme is provided.
Owner:SUZHOU UNIV
Method for preparing conjugated microporous polymer TiO2 composite nano antibacterial agent by in-situ method
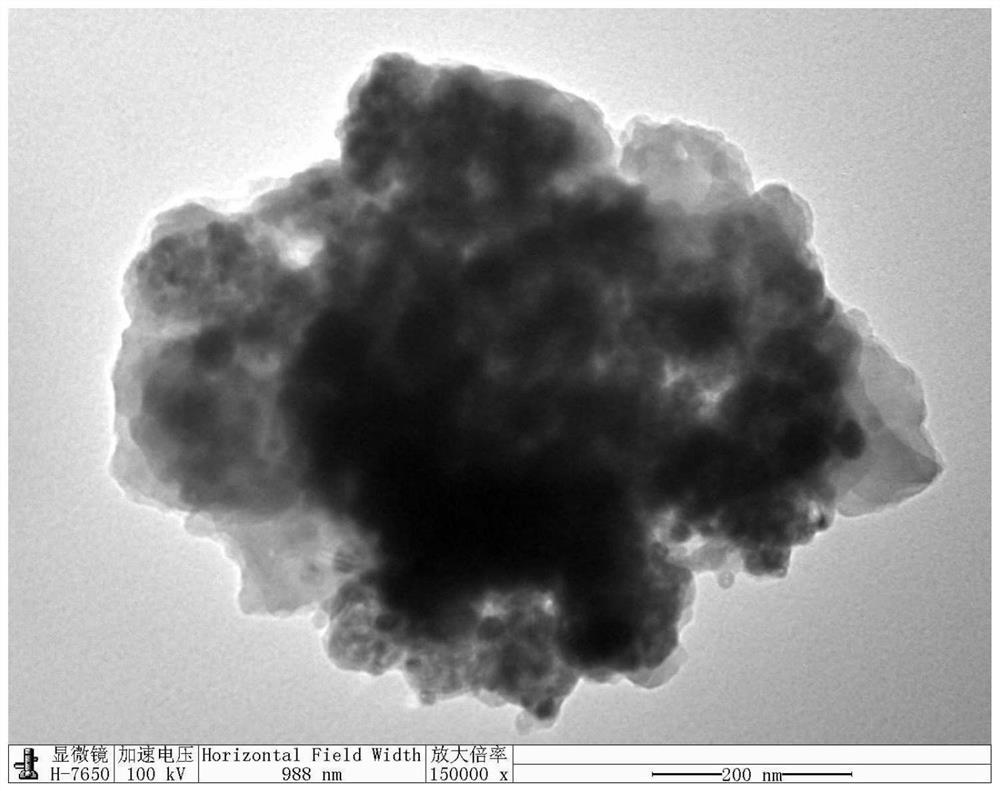
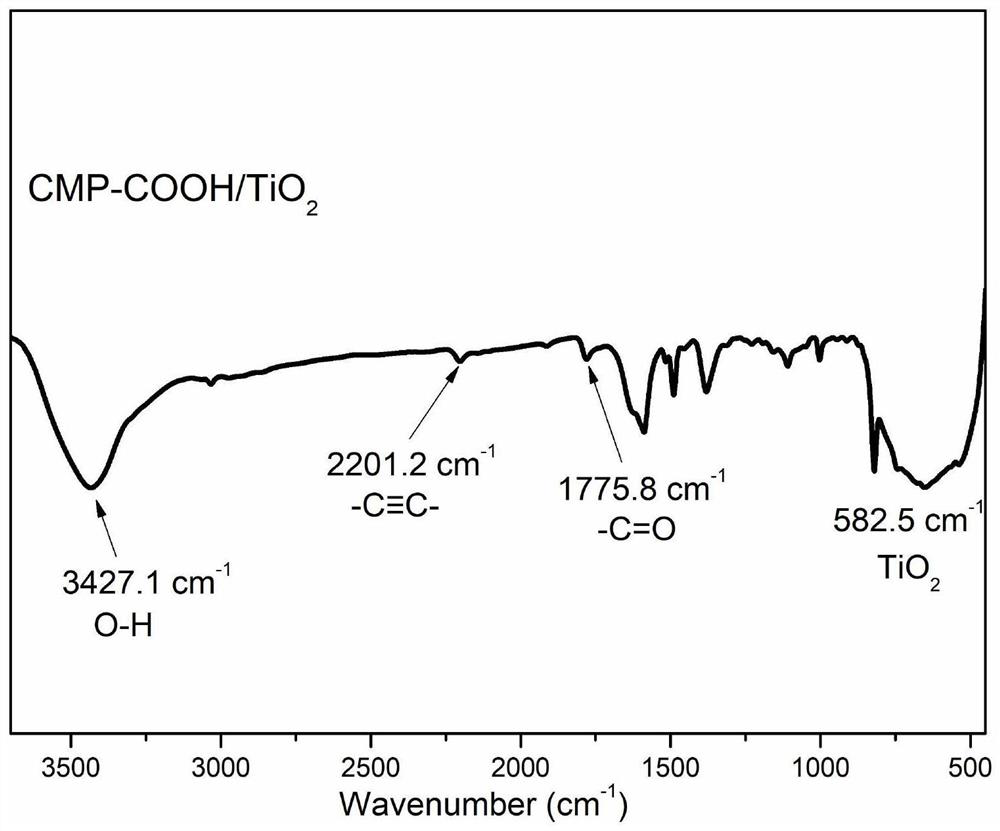
The invention belongs to the technical field of TiO2 composite materials, and discloses a method for preparing a conjugated microporous polymer TiO2 composite nano antibacterial agent by an in-situ method. The method comprises the following steps: ultrasonically dispersing TiO2 particles: adding a certain amount of TiO2 into a beaker containing toluene, sealing the beaker by using a preservative film, and putting the sealed beaker into an ultrasonic cleaner for oscillation to obtain a mixture A; adding 4, 4 '-diethynyl biphenyl, 2, 4, 6-tribromobenzoic acid, CuI, Pd (PPh3) 2Cl2 and PPh3 into a three-necked bottle filled with triethylamine and methylbenzene; according to the method for preparing the conjugated microporous polymer TiO2 composite nano antibacterial agent, the TiO2 particles grow on the surface and inside of a conjugated microporous polymer in an in-situ polymerization mode, the conjugated microporous polymer has the characteristics of high stability, high hydrophobicity, high photon activity and the like, the band gap of TiO2 can be effectively widened, and the antibacterial property of the conjugated microporous polymer is improved. Therefore, the photocatalytic antibacterial activity of the composite material is improved, and bacterial inactivation is accelerated.
Owner:QIQIHAR UNIVERSITY
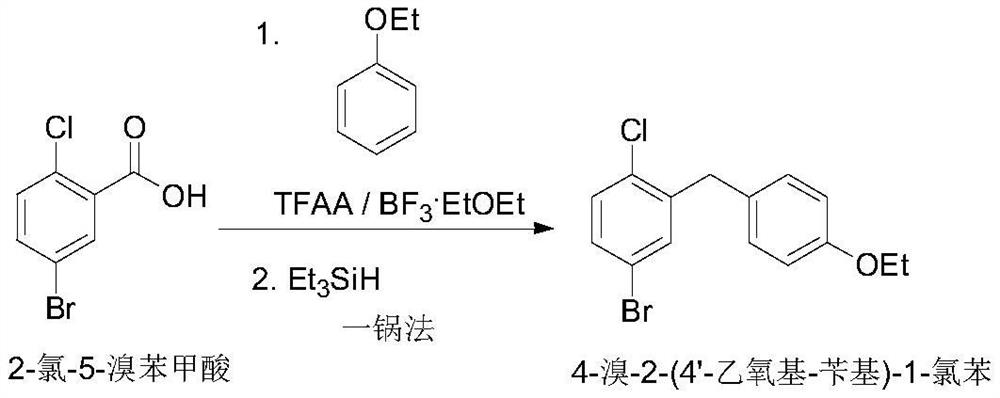
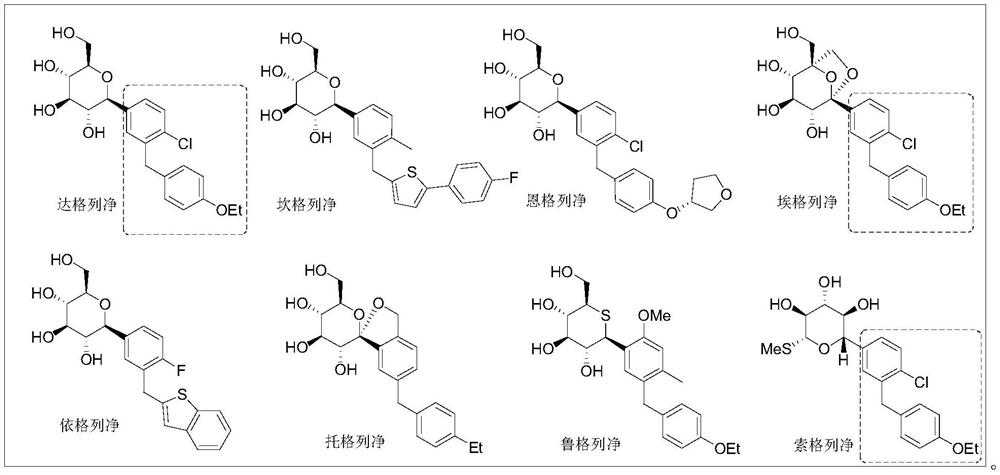
Preparation of 4-bromo-2-(4 '-ethoxy-benzyl)-1-chlorobenzene
InactiveCN112500267AShort stepsEfficient synthesisOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidChlorobenzene
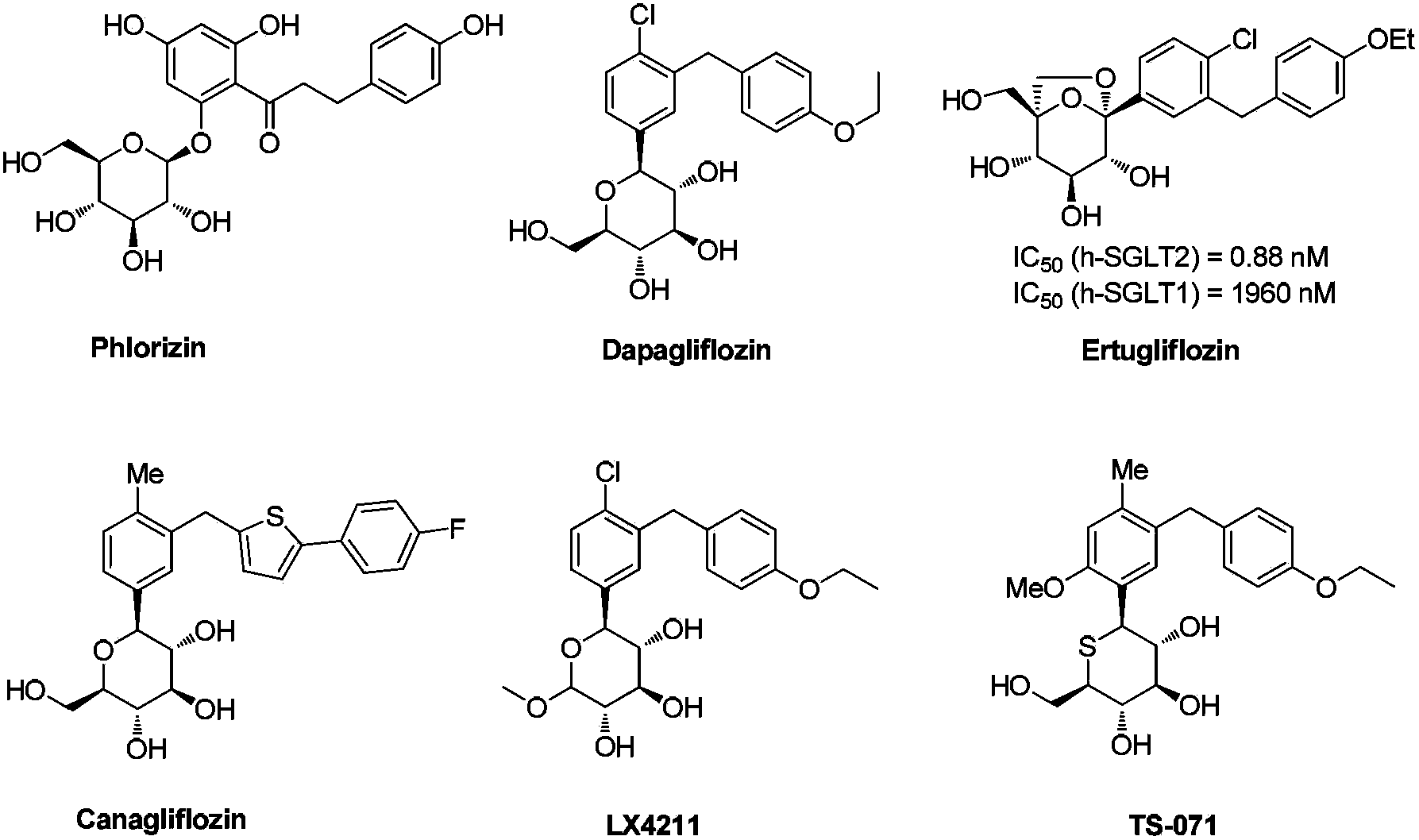
The invention aims to provide a method for synthesizing 4-bromo-2-(4 '-ethoxy-benzyl)-1-chlorobenzene, which has shorter steps and is greener, and provides a more effective synthesis strategy for production of key intermediates of SGLT-2 inhibitor drugs such as dapagliflozin, sotagliflozin, ertugliflozin and the like. The method comprises the following steps: selecting 2-chlorine-5-bromobenzoic acid and phenethyl ether, completing a direct acylation reaction in the presence of trifluoroacetic anhydride by taking boron trifluoride diethyl ether as a catalyst, adding triethylsilane without treatment, and carrying out a one-pot reaction to obtain the target compound 4-bromo-2-(4 '-ethoxy-benzyl)-1-chlorobenzene.
Owner:WISDOM PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







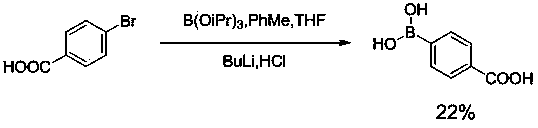

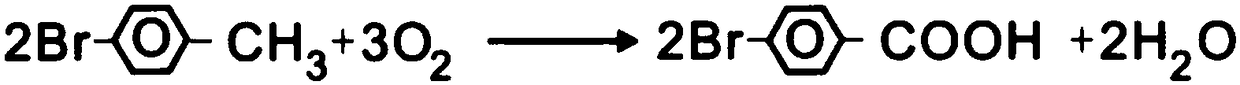


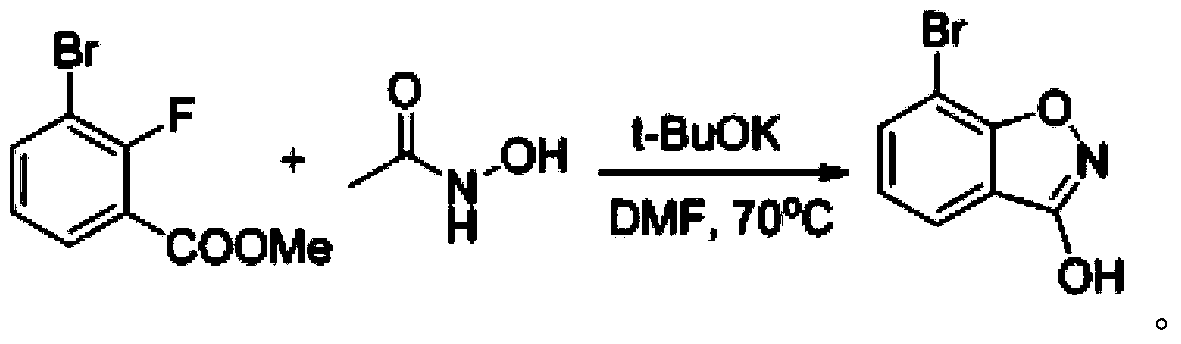
![Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31ac0688-2a46-4734-a53b-736fdbd65695/FDA0001461731410000011.png)
![Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31ac0688-2a46-4734-a53b-736fdbd65695/BDA0001461731420000011.png)
![Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method Preparation method for synthesizing anidulafungin intermittent ([1,1':4',1''-Terphenyl]-4-carboxylic acid, 4''-(pentyloxy)-) through one-step method](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/31ac0688-2a46-4734-a53b-736fdbd65695/BDA0001461731420000031.png)