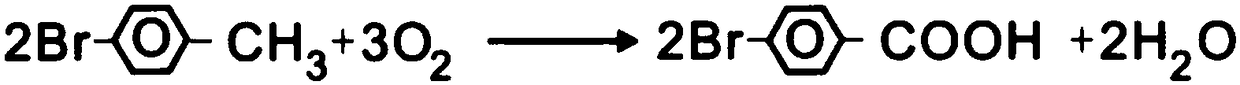Method for preparing 4-bromobenzoic acid
A technology of bromobenzoic acid and p-bromotoluene, which is applied in the field of preparation of 4-bromobenzoic acid, can solve the problems of many reaction steps, only yield, and high cost, and achieve the effects of low cost, few steps, and reduced raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation method of 4-bromobenzoic acid of the present embodiment specifically comprises the steps:
[0030] (1) Put 40g p-bromotoluene, 600g glacial acetic acid, 4.8g cobalt acetate, 4.8g manganese acetate, 2.4 g potassium bromide, warming up to 80 ° C, open oxygen 0.6L / min, until reflux, keep the reflux state and react with oxygen for 7 hours, remove the sample and detect that p-bromotoluene is less than 0.5% as the reaction end point, cool and filter to get the crude product and mother liquor, The crude product is to be refined, and the mother liquor is applied mechanically after being adsorbed by activated carbon;
[0031] (2) Add 10 g of activated carbon to the mother liquor in the upper batch, heat up to 80-90° C. for insulation and adsorption for 1 hour, cool to 60° C., filter, weigh and use as the next batch. Add 40g of p-bromotoluene to the 1000mL reaction bottle, measure the water content of the treated mother liquor and replenish acetic anhydride...
Embodiment 2
[0035] A kind of preparation method of 4-bromobenzoic acid of the present embodiment specifically comprises the steps:
[0036] (1) Put 30g of p-bromotoluene, 500g of glacial acetic acid, 3g of cobalt acetate, 3g of manganese acetate, and 1g of bromide into a 1000mL reaction flask equipped with mechanical stirring, oxygen-passing glass tube, constant pressure dropping funnel, thermometer, and reflux condenser. Potassium, heat up to 75°C, turn on oxygen 0.5L / min, until reflux, keep reflux and react with oxygen for 9 hours, remove the sample and detect that p-bromotoluene is less than 0.5% as the reaction end point, cool and filter to obtain the crude product and mother liquor, the crude product is to be refined , the mother liquor is applied mechanically after being adsorbed by activated carbon;
[0037] (2) Add 10 g of activated carbon to the mother liquor in the upper batch, heat up to 80-90° C. for adsorption for 0.5 hours, cool to 50° C. to filter, weigh and use for the nex...
Embodiment 3
[0041] A kind of preparation method of 4-bromobenzoic acid of the present embodiment specifically comprises the steps:
[0042](1) Put 50g of p-bromotoluene, 700g of glacial acetic acid, 7g of cobalt acetate, 7g of manganese acetate, 4g of Sodium, heat up to 80°C, turn on oxygen 0.7L / min, until reflux, maintain the reflux state and react with oxygen for 6.5 hours, remove the sample and detect that p-bromotoluene is less than 0.5% as the reaction end point, cool and filter to obtain the crude product and mother liquor, the crude product is to be refined , the mother liquor is applied mechanically after being adsorbed by activated carbon;
[0043] (2) Add 10 g of activated carbon to the mother liquor of the previous batch, heat up to 80-90° C. for adsorption for 2 hours, cool to 60° C. to filter, weigh and apply mechanically for the next batch. Add 50g of p-bromotoluene to the 1000mL reaction bottle, measure the water content of the treated mother liquor and supplement the acet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com