Coumarin-acridone fluorescent probe, and preparation method and application thereof
A fluorescent probe and acridone technology, applied in the field of coumarin-acridone fluorescent probe and its preparation, can solve the problems of unsuitable real-time and on-site detection, expensive equipment, complicated operation, etc., and achieve short reaction time , low detection limit and high anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of coumarin-acridone fluorescent probe:
[0039] Put o-bromobenzoic acid (1mmoL) and 1-amino-4-methylcoumarin (2moL) into a 100mL three-neck flask, add DMFmL, after the dissolution is complete, add copper powder and potassium carbonate, and the temperature rises to 180°C Stir and reflux the reaction for 8 hours. After the reaction is completed, suction filter while it is hot. Add 400 mL of pure water to the filtrate, then add an appropriate amount of hydrochloric acid to adjust the pH to 2, and let it stand overnight. After suction filtration, a yellow solid is obtained, and chloroform is recrystallized to obtain intermediate 1. .
[0040] Dissolve Intermediate 1 (1moL) in Eaton’s reagent (1.14moL), heat up to 90°C and stir under reflux for 4h. After the reaction is complete, pour the reaction solution into saturated NaHCO 3 The solution was extracted with chloroform, the upper layer liquid was suction-filtered, recrystallized with acetic acid, and the coum...
Embodiment 2
[0043] Preparation of coumarin-acridone fluorescent probe:
[0044] Put o-bromobenzoic acid (1mmoL) and 1-amino-4-methylcoumarin (1.5moL) into a 100mL three-necked flask, add DMFmL, after the dissolution is complete, add copper powder and potassium carbonate, and the temperature rises to 180 Stir and reflux at ℃ for 8 hours. After the reaction is over, filter while hot. Add 400mL of pure water to the filtrate, then add an appropriate amount of hydrochloric acid to adjust the pH to 5, and let it stand overnight. After suction filtration, a yellow solid is obtained, and chloroform is recrystallized to obtain an intermediate 1.
[0045] Dissolve Intermediate 1 (1moL) in Eaton’s reagent (2moL), raise the temperature to 90°C and stir under reflux for 4h. After the reaction is complete, pour the reaction solution into saturated NaHCO 3 The solution was extracted with chloroform, the upper layer liquid was suction-filtered, recrystallized with acetic acid, and the coumarin-acridone ...
Embodiment 3
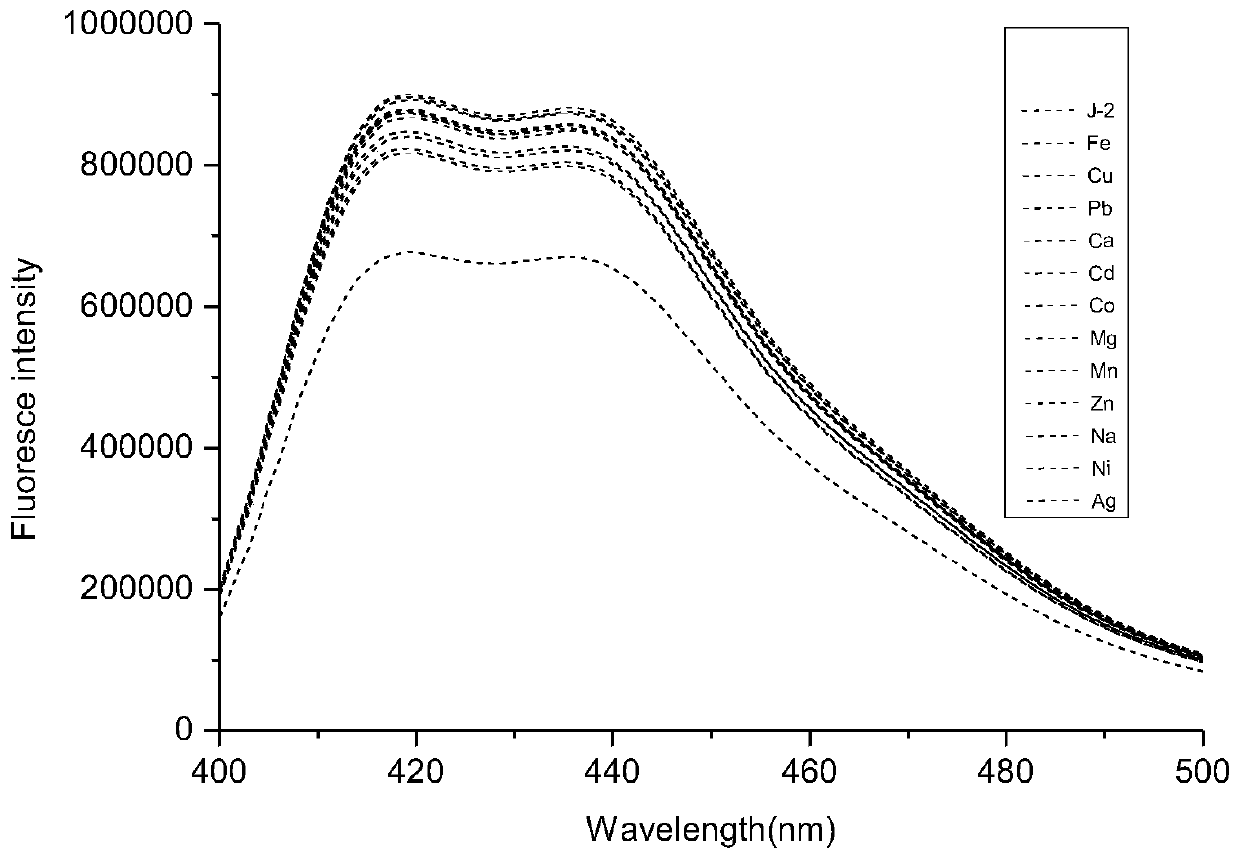
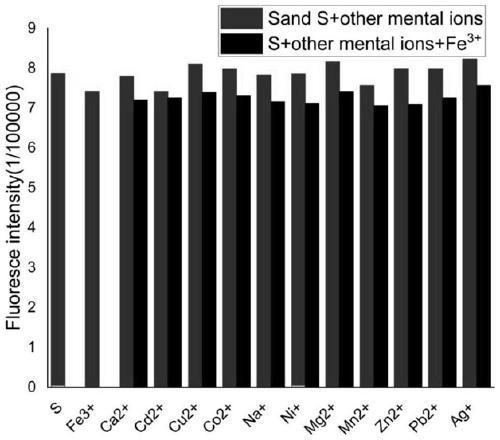
[0047] Selective detection of iron ions by coumarin-acridone fluorescent probe S
[0048] Use acetonitrile: buffer solution=8:2 (v / v) solution to configure molar concentration 2×10 -5 moL / L of coumarin-acridone fluorescent probe, adding Mn 2+ , Mg 2+ 、Ag + 、Na + 、Ni 2+ , Ca 2+ 、Cd 2+ 、Co 2+ 、 Cu 2+ , Fe 3+ , Zn 2+ , Pb 2+ Mix 10 ul of the acetonitrile solution of ion perchlorate, let it react for 5 minutes, and measure its fluorescence emission spectrum with 355 nm as the excitation wavelength.
[0049] Such as figure 1 As shown, the fluorescent probe has an emission peak at 420-450nm, when adding Fe 3+ After that, the emission peak of the fluorescent probe solution at 420-450nm was significantly weakened, and when other cations were added, such as Mn 2+ , Mg 2+ , Ag + 、Na + 、Ni 2+ , Ca 2+ 、Cd 2+ 、Co 2+ 、Cu 2+ , Fe 3+ , Zn 2+ , Pb 2+ After ionization, the fluorescent probe solution has no obvious change at 420-450nm. Therefore, the experimental result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com