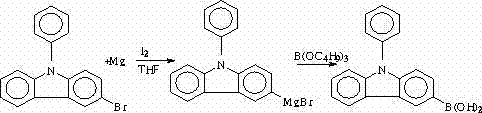
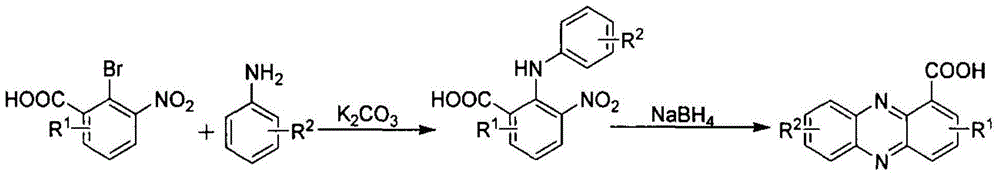
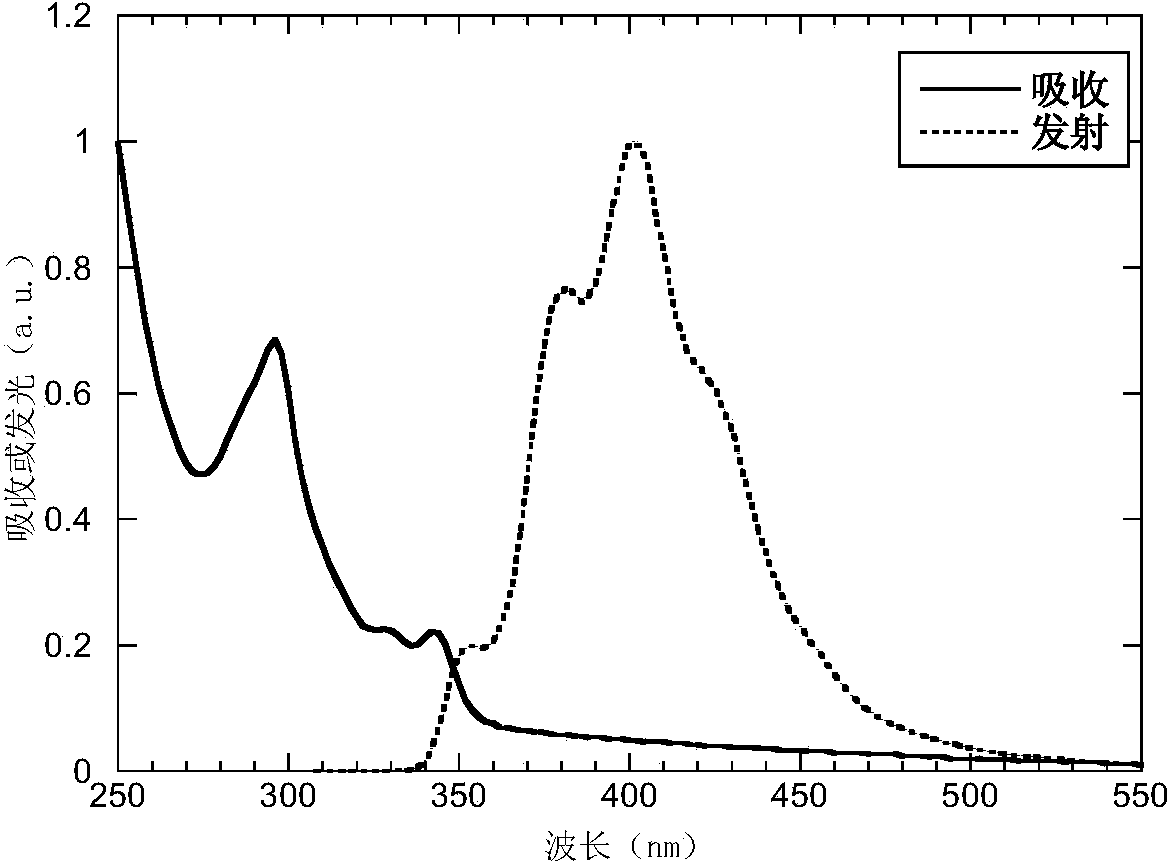
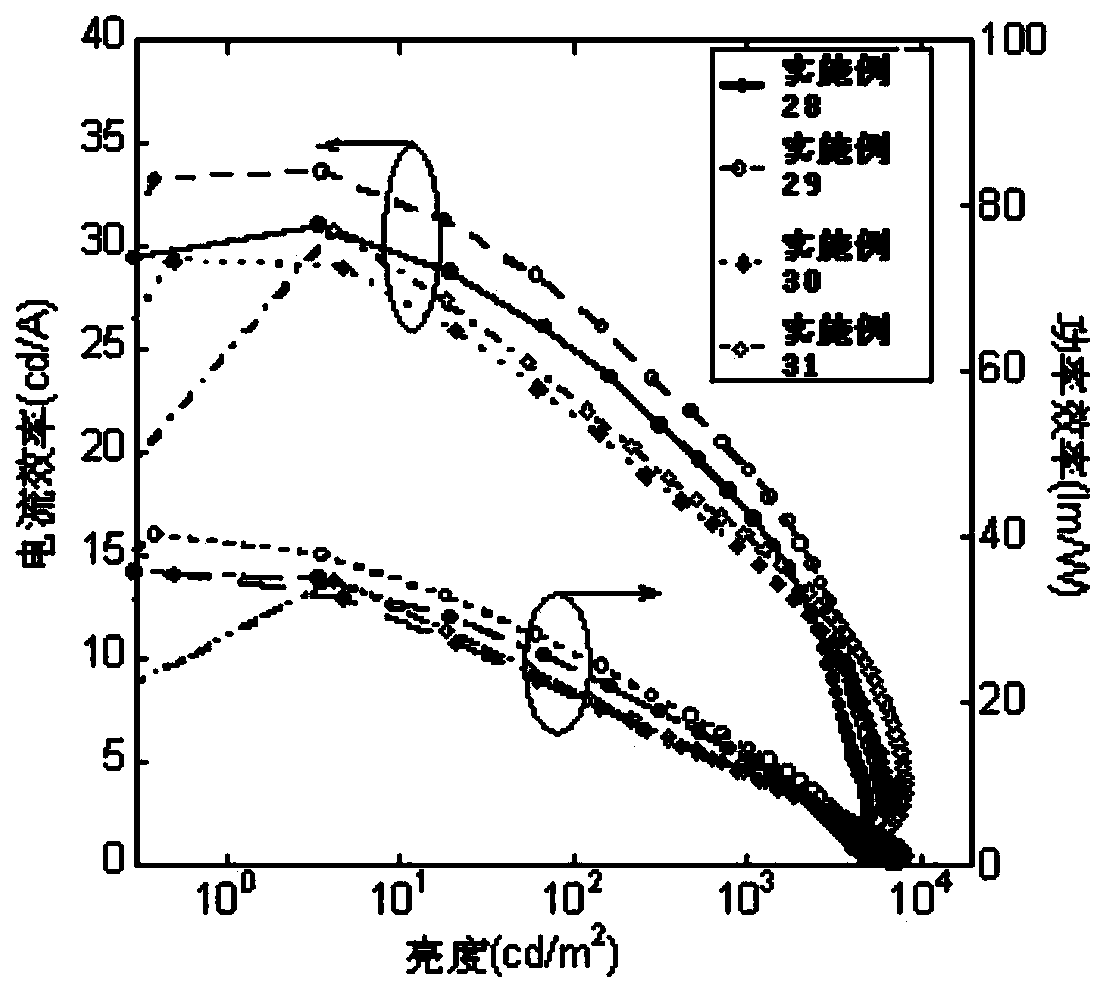
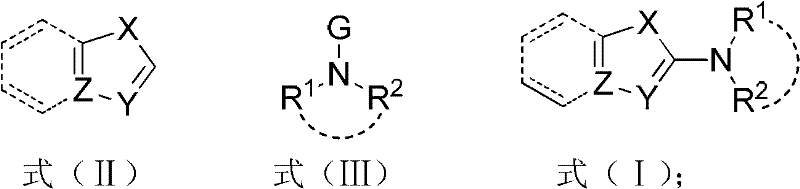Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Ullmann reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides and copper. The reaction is named after Fritz Ullmann.
Polyelectrolyte membranes made of poly(perfluorocyclobutanes) with pendant perfluorosulfonic acid groups and blends with poly(vinylidene fluoride)
A polymer useful as an ion conductor in fuel cells includes a perfluorocyclobutyl moiety and pendant PFSA side groups. The polymer is made by a variation of the Ullmann reaction. Ion conducting membranes incorporating the polymer are provided.
Owner:GM GLOBAL TECH OPERATIONS LLC
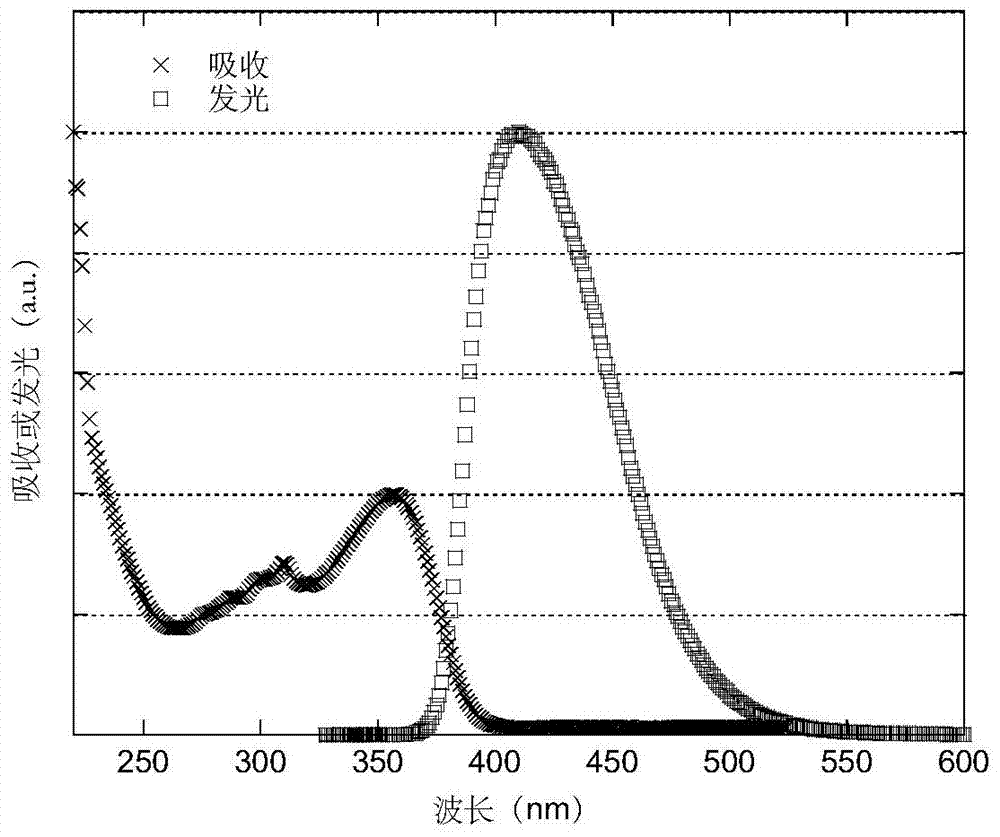
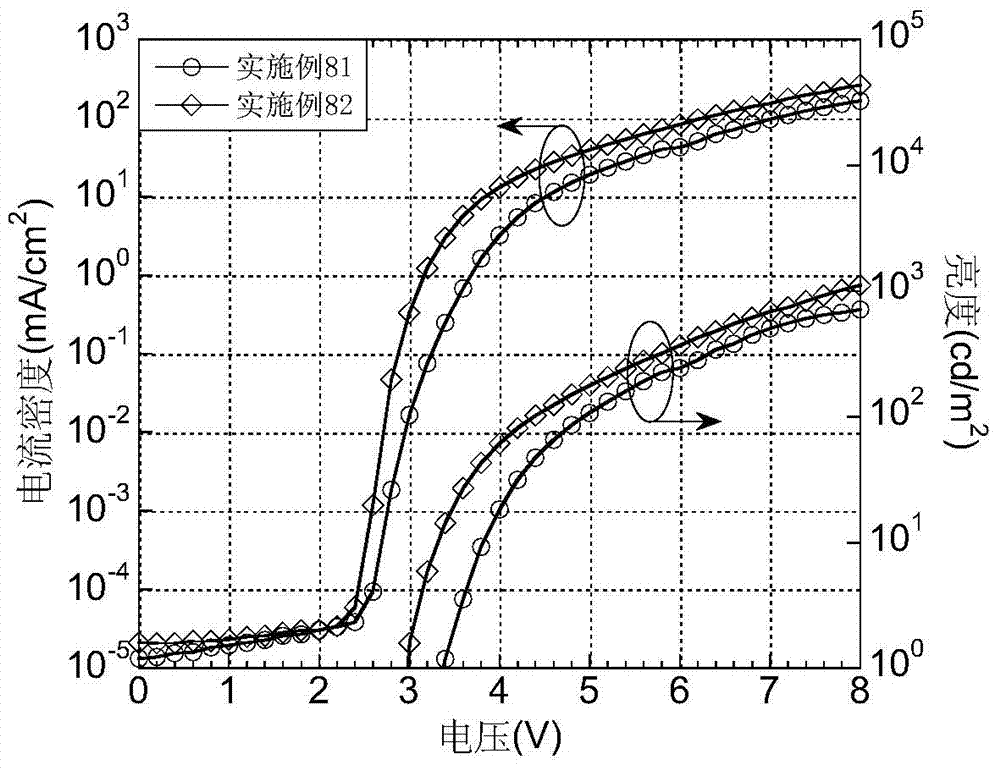
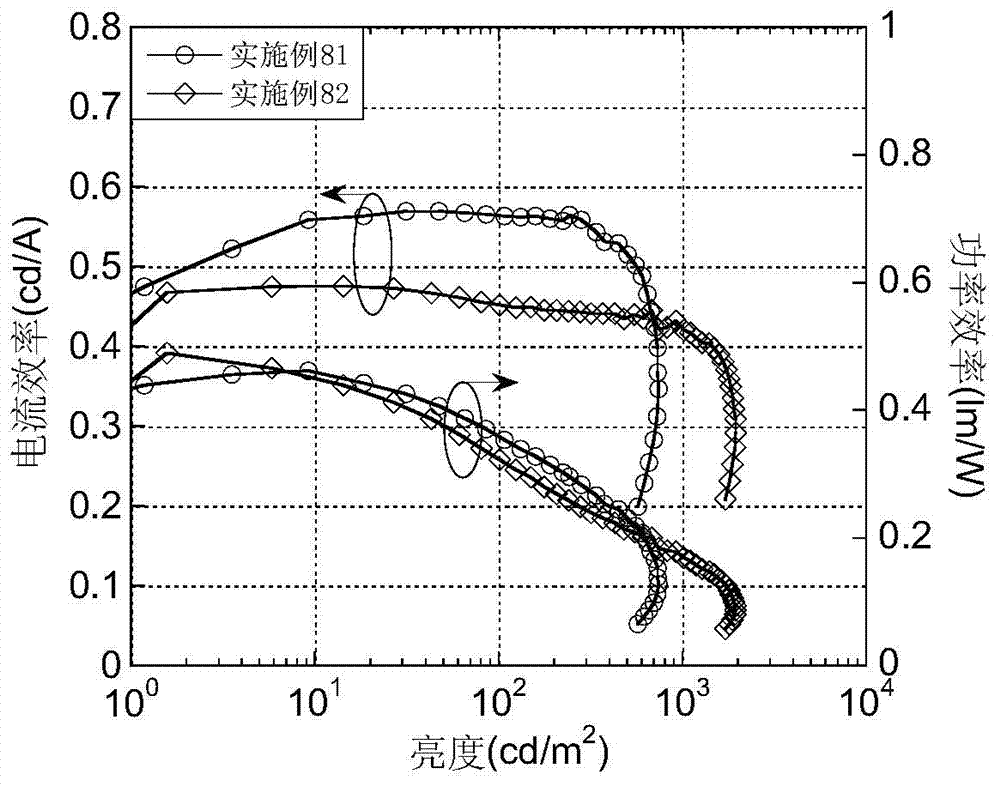
Luminescent materials based on thioxanthene-fluorene spiral structures and organic optoelectronic devices adopting the materials as luminescent layers
ActiveCN104263351ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSuzuki reactionLight-emitting diode
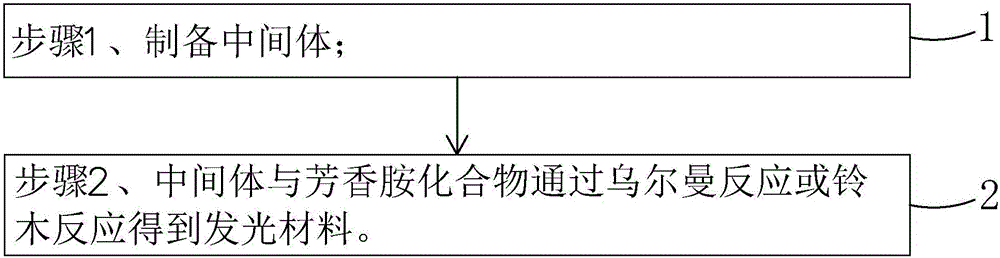
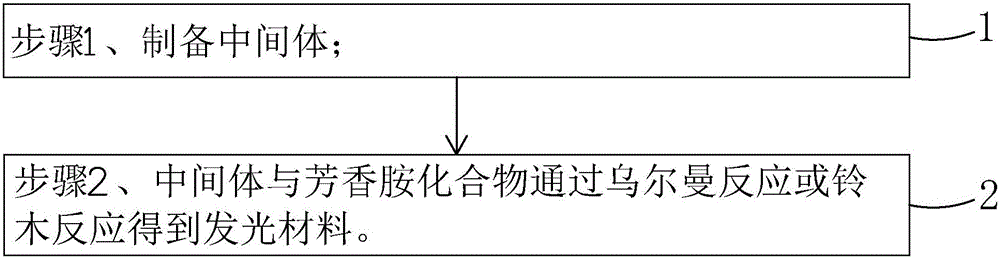
The invention belongs to the technical field of photoelectric materials, and particularly relates to luminescent materials based on thioxanthene-fluorene spiral structures and organic photoelectric devices adopting the materials as luminescent layers. The luminescent materials adopts the thioxanthene-fluorene spiral structures as skeleton units. Intermediates are prepared through Ullmann reactions, and then the target compounds are obtained through Ullmann reactions or Suzuki reactions. The materials are single in structure and definite in molecular weight. The spiral structures of the materials can adjust an intermolecular accumulation manner, thus effectively inhibiting exciplex luminescence. The materials have deep meaning for development of high-effect devices. The organic photoelectric devices adopting the materials as the luminescent layers have good luminescent properties and can be used for small organic molecule light emitting diodes.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing chlorinated diphenyl ether
The invention discloses a method for preparing chloro-substituted diphenyl ether or polychloro-substituted diphenyl ether. A target compound is generated by dichlorobenzene and phenol or fortified phenol which are added or not added with an organic solvent in the presence of a chlorine hydride absorbent for an Ullmann reaction under the catalysis of a catalyst; the chlorine hydride absorbent is one or more of sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate; the solvent is a polar or non-polar organic solvent, and the using amount of the solvent accounts for 0 to 50 percent of total reaction materials; and the catalyst is iodide or a copper compound or a mixture of the iodide and the copper compound. The method has the advantages that the method adopts the optimized mixture ratio of raw materials, a mixed solvent system and a copper catalyst with a nano-grain diameter, and ensures that the reaction rate is accelerated and a product has high yield.
Owner:ANHUI LIXING CHEM
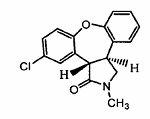
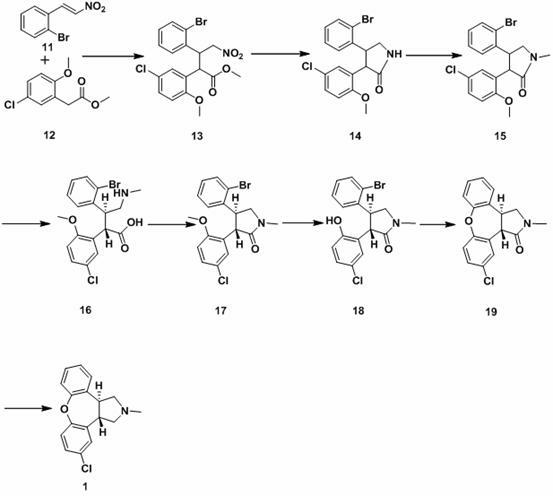
New process for synthesis of asenapine
ActiveCN102229613AReaction transposition effect is goodHigh yieldOrganic chemistryLoop closingMethyl group
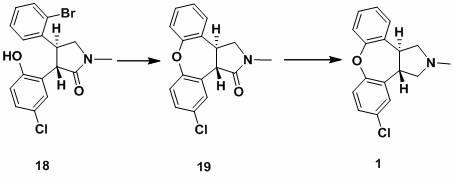
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
Benzene-substituted phenothiazine unit-based luminescent material, intermediate thereof and organic photoelectric device made by luminescent material
ActiveCN104293349ASingle structureGood reproducibility for multiple synthesisOrganic chemistrySolid-state devicesBenzeneSuzuki reaction
The invention belongs to the technical field of photoelectric material, and concretely relates to a benzene-substituted phenothiazine unit-based luminescent material, an intermediate thereof and an organic photoelectric device made by the luminescent material. The luminescent material takes phenothiazine as an initial reaction raw material, the intermediate is prepared through an Ullmann reaction, and a target compound is obtained through the Ullmann reaction or a Suzuki reaction. The material has the advantages of single structure and definite molecular weight; has high decomposition temperature and low sublimation temperature, and the luminescent material with high purity cab can be easily obtained through sublimation. The material has good luminescence performance as a luminescent layer of the organic photoelectric device and can be used for an organic micromolecule light emitting diode.
Owner:SOUTH CHINA UNIV OF TECH
Polyelectrolyte Membranes Made Of Poly(Perfluorocyclobutanes) With Pendant Perfluorosulfonic Acid Groups and Blends With Poly(Vinylidene Fluoride)
A polymer useful as an ion conductor in fuel cells includes a perfluorocyclobutyl moiety and pendant PFSA side groups. The polymer is made by a variation of the Ullmann reaction. Ion conducting membranes incorporating the polymer are provided.
Owner:GM GLOBAL TECH OPERATIONS LLC
Donor-acceptor compound light-emitting material based on dicyanopyrazine and dicyanobenzopyrazine derivative, and electroluminescent device for preparation
The invention relates to a donor-acceptor compound light-emitting material based on a dicyanopyrazine and dicyanobenzopyrazine derivative, and an electroluminescent device for preparation, and belongsto the technical field of organic electroluminescence. According to the present invention, the dicyanopyrazine and dicyanobenzopyrazine derivative as the good electron acceptor nucleus can be linkedto different donor groups through a Suzuki reaction or Ullmann reaction to synthesize a series of electron donor-acceptor type organic small molecule light-emitting materials, wherein the materials have characteristics of large rigid planar skeleton, good thermal stability and high TADF fluorescence quantum yield, can achieve deep red light emission and near infrared light emission, are used for preparing deep red light and near infrared electroluminescent devices, and further have characteristics of simple synthesis, high yield, easy purification and the like. According to the present invention, the electroluminescent device has one or a plurality of light-emitting layers, and at least one layer of the light-emitting layer of the electroluminescent device contains one or a plurality of the compounds of the present invention, wherein further 0.1-100.0% by mass of the compound of the present invention is doped in the main body material so as to prepare the light emitting layer.
Owner:JILIN UNIV
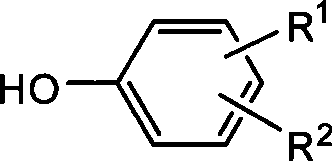
Method for synthetizing 2-fluoro phenol compound
ActiveCN104844399AWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationHydroxy group formation/introductionOrganic solventOrtho position
The present invention provides a method for synthetizing a 2-fluoro phenol compound shown in a formula IV. The phenol compound shown in the formula I is prepared into a 2-pyridine oxygroup arene compound shown in a formula II through an Ullmann reaction, the 2-pyridine oxygroup arene compound shown in the formula II is mixed with a palladium catalyst, a fluorinating reagent, an additive and an organic solvent, the mixture is stirred under the temperature of 30-160 DEG C to perform a fluorination reaction to obtain an ortho-position fluoridated 2-pyridine oxygroup arene compound shown in a formula III, and the ortho-position fluoridated 2-pyridine oxygroup arene compound shown in the formula III is prepared into the 2-fluoro phenol compound shown in the formula IV through the action of alkali. The method provided by the present invention has the advantages of mild reaction conditions, simplicity in operations, good substrate adaptability, high fluorination selectivity and the like. The 2-fluoro phenol compound is shown in the figure below.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing apalutamide and intermediate thereof
ActiveCN108383749AHigh crystallinityImprove stabilityOrganic compound preparationCarboxylic acid amides preparationMethyl groupHydrochloride
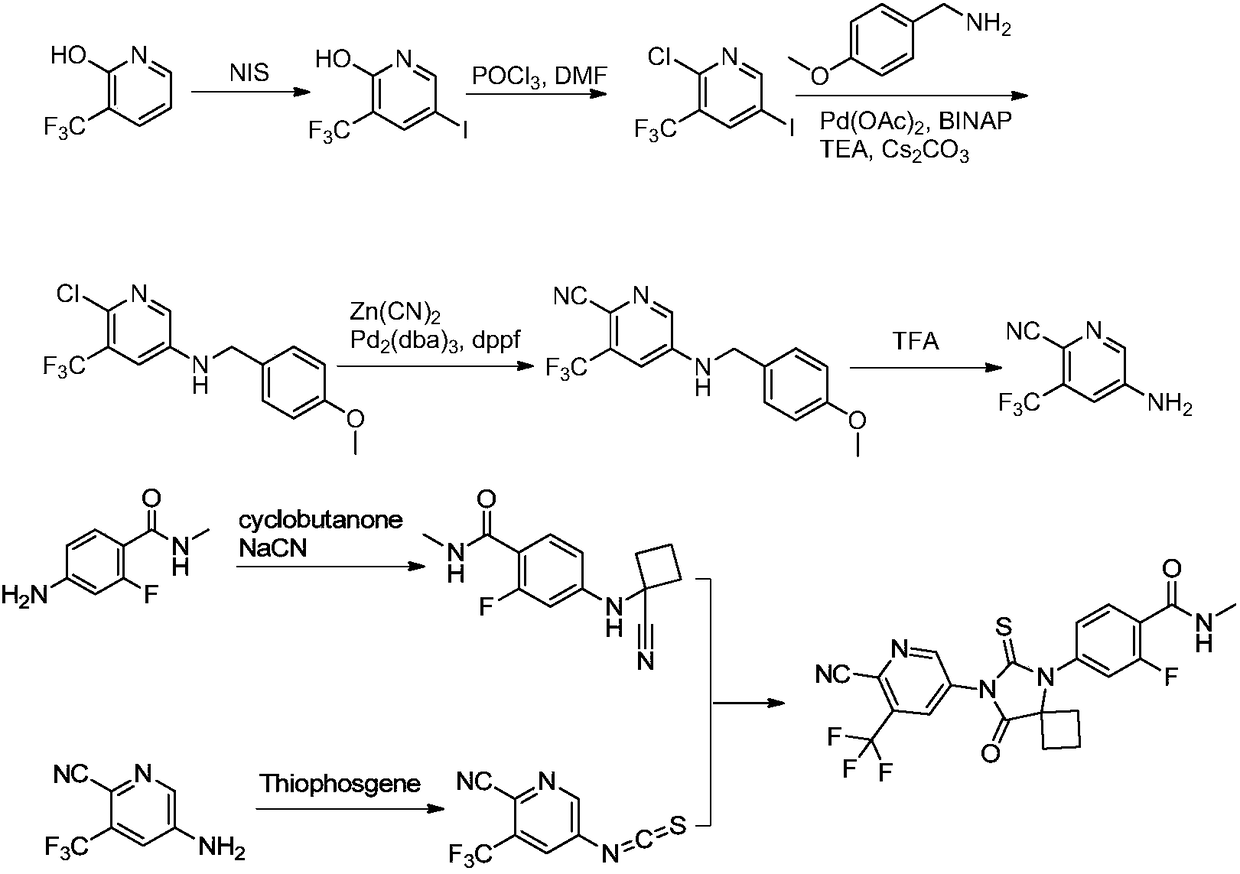
The invention discloses a method for synthesizing apalutamide. The method includes carrying out condensation on N-methyl-2-fluorine-4-halogenated-benzamide compounds 1 and amino-1-cyclobutanecarboxylic acid hydrochloride 2 by means of Ullmann reaction to obtain to obtain intermediate compounds 3 and esterifying the intermediate compounds 3 to obtain intermediate compounds 4; carrying out cyclization by means of reaction on the intermediate compounds 4 and thiocyanide to obtain compounds 5; carrying out condensation by means of coupling the compounds 5 to obtain the apalutamide. The N-methyl-2-fluorine-4-halogenated-benzamide compounds 1 and the amino-1-cyclobutanecarboxylic acid hydrochloride 2 are used as starting materials. A path is shown, an R in the path represents alkyl, includes butis not limited to methyl or ethyl. The method has the advantages that route steps can be shortened to a great extent, the route efficiency can be improved, precious metal catalysts are omitted, and accordingly the process cost can be lowered; byproduct generation can be reduced, and accordingly the method is favorable for improving the purity of ultimate products.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Loaded aurum palladium catalyst and preparation method and application thereof
ActiveCN102806105AEvenly dispersedHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsPalladium catalystIon exchange
The invention relates to a loaded aurum palladium catalyst for coupling benzene chloride in an aqueous solution. According to the loaded aurum palladium catalyst, active components comprise Au and Pd, and a carrier is ionic exchange resin, wherein the content of the active components is 0.1 to 5 weight percent, aurum and palladium form an alloy, the grain diameter is 1 to 6 nanometers, and the relative molar ratio of aurum to palladium is 1 / 6-6 / 1. A preparation method comprises the following processes of: performing ionic exchange on resin which is subjected to ball-milling and sieving and a metal precursor; and reducing loaded metal precursor. The catalyst is used for Ullmann Reaction and Suzuki Cross-coupling in the aqueous solution, and has high catalytic performance. The catalyst is easy to prepare, and has high catalytic activity and stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis method of N-phenyl-3(4-bromophenyl) carbazole
The invention discloses a synthesis method of N-phenyl-3(4-bromophenyl) carbazole. The synthesis method is characterized in that carbazole is selected as a raw material and is subjected to Ullmann reaction with iodobenzene to prepare N-phenyl carbazole; N-phenyl-3-bromine carbazole is synthesized by NBS (N-bromosuccinimide) bromination; N-phenyl-3-boric acid base carbazole is prepared through Grignard coupling; and N-phenyl-3-boric acid base carbazole and p-bromoiodobenzene are subjected to cross coupling to obtain N-phenyl-3(4-bromophenyl) carbazole. The method provided by the invention has the characteristics of high yield, low production cost, less three-waste emission and high target compound selectivity and yield, a high-efficiency loaded catalyst is used, and isomer is reduced.
Owner:山东盛华电子新材料有限公司
Preparation method for phenazine-1-carboxylic acid
ActiveCN104829544AReaction raw materials are readily availableHigh yieldOrganic chemistrySandmeyer reactionHydroxylamine
The invention relates to a preparation method for phenazine-1-carboxylic acid. The preparation method comprises the following steps: reacting aniline with chloral hydrate and hydroxylamine to produce alpha-oximidoacetanilide, treaing alpha-oximidoacetanilide with concentrated sulfuric acid to obtain isatin, reacting isatin with hydrogen peroxide so as to obtain 2-amino-3-nitrobenzoic acid and then carrying out Sandmeyer reaction to prepare 2-bromo-3-nitrobenzoic acid; and subjecting prepared 2-bromo-3-nitrobenzoic acid and aniline to Jourdan-Ullmann reaction so as to obtain substituted diphenylamine and carrying out ring closure to prepare phenazine-1-carboxylic acid. Compared with the prior art, the preparation method provided by the invention has the advantages of easy availability of raw materials, easy control of reaction, mild reaction conditions, easy post-treatment, high overall yield, as high as 32 to 47%, and suitability for industrial production; and compared with conventional method for production of shenqinmycin from ferment powder, the method provided by the invention enables cost to be greatly reduced.
Owner:SHANGHAI TAIHE INT TRADE CO LTD +1
Organic small molecular material based on spiro thioxanthene and organic photoelectric device using material as light emitting layer
ActiveCN104193736AImprove solubilityRegulated conjugation lengthGroup 5/15 element organic compoundsSolid-state devicesSolubilityChemical compound
The invention belongs to the technical field of photoelectric materials and particularly relates to an organic small molecular material based on spiro thioxanthene and an organic photoelectric device using the material as a light emitting layer. By using spiro thioxanthene as a framework unit, the organic small molecular material can be used for obtaining a target compound by virtue of friedel craft reaction and Ullmann reaction. The material is single in structure and certain in molecular weight and has good solubility and film forming property in common solvents. The spiro structure of the material can adjust the accumulation mode of molecules, so that an exciplex is effectively prevented from emitting light, therefore, the material is of profound significance in development of high-effect devices. The photoelectric device using the material as the light emitting layer has a good light emitting performance and can be applied to organic small molecular light emitting diodes.
Owner:SOUTH CHINA UNIV OF TECH
Luminescent material and preparation method thereof and organic light emitting diode using same
InactiveCN105968104ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSolubilitySuzuki reaction
The invention provides a luminescent material and a preparation method thereof and an organic light emitting diode using the same. The luminescent material provided by the invention has a single structure, determined molecular weight, relatively good solubility and film forming property while the film form is stable; and with very high decomposition temperature and relatively low sublimation temperature, the luminescent material can be easily sublimated into a high-purity luminescent material and can be applied to a small-molecule organic light emitting diode. In the preparation method of the luminescent material provided by the invention, p-bromophenol and 2-fluoro-4-bromobenzonitrile are used as start raw materials, an intermediate of the luminescent material is obtained through a series of simple reactions, and finally, the luminescent material is obtained through Ullman reaction or Suzuki reaction; and the steps are simple, and the yield is high. In the organic light emitting diode provided by the invention, a luminescent layer contains the luminescent material and has relatively high luminescent efficiency and stability.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Acridine-1, 2, 3-triazole type compound and preparation method and application thereof
ActiveCN104277029ASignificant in vitro antitumor activityGood potential medicinal valueOrganic chemistryAntineoplastic agentsVitamin CFiltration
The invention discloses an acridine-1, 2, 3-triazole type compound and a preparation method and application thereof. The preparation method of the acridine-1, 2, 3-triazole type compound comprises the following steps: 1) performing ullmann reaction by taking o-bromobenzoic acid and p-methoxyaniline as raw materials, taking potassium carbonate and copper powder as catalysts and taking isoamyl alcohol or n-amyl alcohol as a solvent to obtain a compound 1; 2) cyclizing the compound 1 with phosphorus oxychloride to prepare a compound 2; 3) dissolving the compound 2 in DMF (dimethylformamide) and performing nucleophilic substitution reaction with sodium azide to prepare a compound 3; and 4) taking p-methoxyphenylacetylene, dissolving in a tert-butyl alcohol / water solution, adding vitamin C sodium, copper sulfate pentahydrate and the compound 3 to react, performing suction filtration, and recrystallizing for preparation. In-vitro anti-tumor test results show that the acridine-1, 2, 3-triazole type compound has significant in-vitro anti-tumor activity against three subjects, namely MGC80-3, BEL-7404 and T24, has relatively good potential medicinal values, and is expected to be used for preparing various anti-tumor medicines.
Owner:广西新桂环保科技集团有限公司
Synthetic method of Fuan mycin skeleton
InactiveCN107235952AEasy to operateResponse requirements are lowOrganic chemistryBulk chemical productionNatural productKetone
The invention discloses a synthetic method of a Fuan mycin skeleton. The method adopts a compound of a formula 1 as a starting material, and a compound of a formula 3 is obtained by removing a silicon protection group in a two-step conversion manner by virtue of Sonogashira coupling reaction. The compound of formula 4 is also used as a starting raw material to obtain the compound of formula 6 by virtue of pinic oxidization and methyl esterification. The compound of formula 3 and the compound of formula 6 have the Sonogashira coupling reaction, a phenolic hydroxyl group is protected by a silicon group, methoxyl is selectively removed, an acetylenic bond is reduced, 6pai electrocyclization is carried out, the phenolic hydroxyl group is activated to have the secondary Sonogashira coupling reaction, the silicon-group protective group is removed, a Ullmann reaction is carried out, methyl protection, pinic oxidization and Fourier-krafz acylation are carried out, esters are hydrolyzed and condensated with amino acid, the acetylenic bond is hydrolyzed into ketone under the gold catalysis, thus obtaining Fuan mycin skeleton protected by pentamethoxyl, and synthesizing the Fuan mycin skeleton. The synthetic route is reasonable in design, raw materials are cheap and easy to get, the operation is simple and easy, a key reaction midbody is easy to modify, and the synthetic research of various polycyclic xanthenone natural products can be realized by utilizing the method.
Owner:EAST CHINA NORMAL UNIV
Uses of 2-pyridine-beta ketone compounds
ActiveCN101402537ALow pricePromotes N-arylation reactionOrganic-compounds/hydrides/coordination-complexes catalystsAmino group formation/introductionRoom temperatureKetone
The invention relates to application of a 2-pyridyl-beta ketone compound taking a structure of formula I as an additive in the process of promoting N-arylation reaction. In the invention, because the 2-pyridyl-beta ketone compound is used as the additive, the N-arylation reaction can be promoted well, and the catalyst used in the N-arylation reaction is just CuI which is cheap and available; and the additive 2-pyridyl-beta ketone compound is novel and is stable in air; compared with the same type of reaction reported in a literature, the ullmann reaction of iodo compounds can be performed at room temperature, and the temperature of a bromo compound reaction can be reduced by about 20 DEG C on average, thus the reaction condition is very mild and the application prospect is good.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
A luminescent material, a preparing method thereof and an organic light-emitting diode using the luminescent material
InactiveCN106167487ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSolubilityDecomposition
A luminescent material, a preparing method thereof and an organic light-emitting diode using the luminescent material are provided. The luminescent material has a single structure, a definite molecular weight, and good solubility and film forming ability and is stable in film shape. The luminescent material has a high decomposition temperature and a low sublimation temperature, can easily sublimate into a high-purity luminescent material, and can be used for the small-molecule organic light-emitting diode. The method adopts bromothiophenol and 4-bromo-2-fluorobenzonitrile as initial raw materials, prepares an intermediate of the luminescent material through a series of simple reactions, prepares the luminescent material finally through a Ullmann reaction or a Suzuki reaction, and is simple in steps and high in yield. A luminescent layer of the organic light-emitting diode contains the luminescent material. The organic light-emitting diode has a high luminescent efficiency and stability.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Method for synthesizing 2,3,4,5,6-pentafluorophenol
ActiveCN103787839AReduce usageLow costOrganic compound preparationEther preparation by ester reactionsPalladium on carbonPtru catalyst
The invention provides a method for synthesizing 2,3,4,5,6-pentafluorophenol. The method comprises the steps of (a) carrying out Ullmann reaction on bromopentafluorobenzene as shown in a formula (I) and benzyl alcohol in an inert gas atmosphere under the action of a catalyst cuprous iodide, a ligand and an inorganic base at 100-120 DEG C, and then generating a compound (II), and (b) reacting the compound (II) in an alcoholic solvent in the presence of hydrogen under the action of a palladium carbon catalyst at 20-50 DEG C, and removing the benzyl group, thereby obtaining a compound (III), namely, the 2,3,4,5,6-pentafluorophenol. The method is simple, low in cost and high in yield, and applicable to industrial production. The synthetic route of the method is as shown in specification.
Owner:SUZHOU HIGHFINE BIOTECH
Luminescent material and preparation method thereof and organic light-emitting diode using luminescent material
InactiveCN106279130ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSuzuki reactionDecomposition
The invention provides a luminescent material and a preparation method thereof and an organic light-emitting diode using the luminescent material. The luminescent material is single in structure and certain in luminescent material, is good in dissolvability and film-forming ability, and is stable in film shape. The luminescent material is high in decomposition temperature and low in sublimation temperature, can easily sublimate to a luminescent material with high purity, and can be applied to a micromolecule organic light-emitting diode. The preparation method comprises: taking 3-bromophenol and 2-fluoro-4-bromobenzonitrile as initial raw materials, carrying out a series simple reactions to obtain an intermediate of the luminescent material, and carrying out an Ullmann reaction or a Suzuki reaction to obtain the luminescent material. The method is simple in steps and high in yield. A luminescent layer of the organic light-emitting diode contains the luminescent material, and the organic light-emitting diode is high in luminous efficiency and stability.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Method for preparing diphenyl ether
The invention discloses a method for preparing diphenyl ether, which comprises the following steps of: generating saline solution of phenol through phenol and alkali; removing water in a system by back flow of benzene chloride; and under the condition of the existence of a catalyst, performing Ullmann reaction by dripping benzene chloride to generate a target compound. In the method, phenol is used as the raw material and is also used as a solvent; and the catalyst is a wet material of blue vitriol or cuprous chloride or a mixture of the wet materials of blue vitriol or cuprous chloride. The method has low production cost. No solid waste is generated. The yield is high. The product has high quality.
Owner:SHANDONG TIANYI CHEM
Luminescent material and the preparation method thereof and organic luminous diode using the luminescent material
InactiveCN106317008ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSolubilityDecomposition
The invention provides a luminescent material and the preparation method thereof and organic luminous diode using the luminescent material. The luminescent material has a single structure, definitive molecular weight, as well as favorable solubility and film forming property; the film formed has stable conformation; besides, the luminescent material also has a very high decomposition temperature and a comparatively low sublimation temperature, and can be easily sublimated to high-purity luminescent material, so the luminescent material can be used in small molecule organic luminous diodes. The luminescent material is prepared with m-bromophenol and 2-Fluoro-4-bromobenzonitrile as the starting material; intermediate of the luminescent material can be obtained through a series of simple reactions, and finally the luminescent material can be obtained through Ullmann reaction or Suzuki reaction. The preparation method is simple in step, but high in productivity. Luminescent layer of the organic luminous diode provided in the invention contains the luminescent material, which ensures a higher luminous efficiency and stability.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Method for processing sludge from papermaking and pulping by using earthworms, method for separating sludge and earthworms
ActiveCN102229461APromote circulationImprove fertilityClimate change adaptationSewage/sludge fertilisersLoop closingSludge
The invention discloses a new process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to a condensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:GUIZHOU CHITIANHUA
Synthesis method of axially chiral dinaphthalene ligand precursor (s)-2,2'-dinaphthyl-1,1'-dicarboxylic acid
InactiveCN105541605AEasy to operateHigh yieldOrganic compound preparationOrganic chemistry methodsOrganic synthesisSynthesis methods
The invention relates to the technical field of bromination, hydrolysis, oxidation and coupling reaction in organic synthesis, particularly a synthesis method of an axially chiral dinaphthalene ligand precursor (s)-2,2'-dinaphthyl-1,1'-dicarboxylic acid. The method comprises the following steps: synthesizing a compound 1-bromo-2-methylnaphthalene from an initial raw material 2-bromomethylnaphthalene, carrying out bromination, hydrolysis and oxidation on the 1-bromo-2-methylnaphthalene to obtain 1-bromo-2-naphthoic acid, generating an ester under the actions of methanol and thionyl chloride, protecting the carboxyl group, carrying out Ullmann reaction under the action of copper powder to perform coupling, carrying out hydrolysis to obtain 2,2'-dinaphthyl-1,1'-dicarboxylic acid, and finally, resolving to obtain the (s)-2,2'-dinaphthyl-1,1'-dicarboxylic acid. The method is simple to operate, and has the advantages of higher yield and favorable economical efficiency.
Owner:YANGZHOU UNIV
Method for synthesizing 2-amino five-membered heterocyclic derivative
InactiveCN102503753AGentle preparationEfficient preparationAmino group formation/introductionReaction temperaturePalladium catalyst
The invention discloses a method for synthesizing a 2-amino five-membered heterocyclic derivative. In the method, a route which is opposite to the conventional amination reaction is adopted, nitrogen polar reversed amine is taken as an amination reagent, a cheap cupric salt is taken as a catalyst, triphenylphosphine is taken as a ligand, lithium tert-butoxide is taken as an alkali, toluene is taken as a solvent, and the amination reagent undergoes a C-N coupling reaction with five-membered heterocyclic molecules, so that a five-membered heterocyclic two-position amination reaction is realized. The method has the characteristics of mild conditions, quick reaction, low cost, large-scale preparation, and the like. Due to the adoption of the method, the defects of harsh reaction conditions (high reaction temperature and long term), large quantity of side reactions, low yield, and the like existing in the conventional Ullmann reaction are avoided to a large extent, and the limitation on the use of a high-cost noble metal palladium catalyst is avoided.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method and application of TPD hole transport material with crystallization resistance
InactiveCN101723838AAddress bugs that degrade performance or even lose functionalityGood hole transport propertiesOrganic compound preparationElectrography/magnetographyStructural formulaOrganic photoconductor
The invention relates to a preparation method and application of a TPD hole transport material with crystallization resistance. TPD compounds of the invention have the following structural formula, wherein N-TPD-1:R1=Me, and R2=4-Me; N-TPD-2:R1=H, and R2=H; and N-TPD-3:R1=H, and R2=3-Me. TPD molecules formed by using 2,2'-dimethylbenzidine segments with larger stereoscopic effect as an intermediate connecting structure can generate obvious molecular twist effect, and 2,2'-dimethyl-4,4'-diiodobiphenyl is selected to serve as a key material and reacts with a diarylamine material under the ullmann reaction condition to obtain the target product of the invention. The TPD hole transport material is used for an organic photoconductor (OPC) of a laser printer. The TPD hole transport material has excellent crystallization resistance when keeping excellent hole transporting capacity, and can well overcome the defect that when the TPD material is used in an organic photoconductor device, crystallization of the TPD material makes the performance of the device worsened even makes the functions of the device lost.
Owner:中船汉光科技股份有限公司
Method for synthesizing 3-iodine-phenylcarbazole
The invention discloses a method for synthesizing 3-iodine-phenylcarbazole, which comprises the following steps of: synthesizing 9-phenylcarbazole by performing Ullmann reaction on carbazole serving as an initial raw material and phenyl iodide under the action of cuprous oxide serving as a catalyst; and synthesizing a target compound 3-iodine-phenylcarbazole by performing iodo-reaction on the 9-phenylcarbazole and iodine under the catalysis of iodic acid and concentrated sulfuric acid. The synthesis method has the characteristics of high yield, low production cost and low pollution, and is simple.
Owner:山东盛华新材料科技股份有限公司
Process for synthesizing chiral methoxybenzylamine
InactiveCN101462970ANo need to worry about optical purityEasy to crystallize and purifyOrganic compound preparationAmino-hyroxy compound preparationBenzylaminePara position
The invention discloses a method for synthesizing chiral p-methoxy benzylamine. The method, with chiral benzylamine as original starting material, replaces halogen atom with methoxy by amidocyanogen protection, para position halogenating and Ullmann reaction; and finally, the protection is removed to obtain chiral p-methoxy benzylamine with pure optical enantiomorphism. The method is low in production cost, easy in operation and applicable to industrial production.
Owner:BAILING PHARMA SHANGHAI
Luminescent material and the preparation method thereof and organic luminous diode using the luminescent material
InactiveCN106317041ASingle structureMolecular weight determinationOrganic chemistrySolid-state devicesSolubilityDecomposition
The invention provides a luminescent material and the preparation method thereof and organic luminous diode using the luminescent material. The luminescent material has a single structure, definitive molecular weight, as well as favorable solubility and film forming property; the film formed has stable conformation; besides, the luminescent material also has a very high decomposition temperature and a comparatively low sublimation temperature, and can be easily sublimated to high-purity luminescent material, so the luminescent material can be used in small molecule organic luminous diodes. The luminescent material is prepared with m-Bromothiophenol and 2-Fluoro-4-bromobenzonitrile as the starting material; intermediate of the luminescent material can be obtained through a series of simple reactions, and finally the luminescent material can be obtained through Ullmann reaction or Suzuki reaction. The preparation method is simple in step, but high in productivity. Luminescent layer of the organic luminous diode provided in the invention contains the luminescent material, which ensures a higher luminous efficiency and stability.
Owner:TCL CHINA STAR OPTOELECTRONICS TECH CO LTD
Synthesis method of relugolix or salt thereof
The invention provides a synthesis method of relugolix or a salt thereof, wherein thesynthesis method comprises the steps: taking 1-(dimethylamino)-3-(4-nitrophenyl)-2-acetone and cyanoacetate as initial raw materials, and carrying out condensation cyclization, alkylation reaction, Ullmann reaction and coupling reaction to obtain a relugolix 4 free alkali form or salt form. The synthetic route optimized the process, the route steps are shortened, the route efficiency is improved, the use of noble metal catalysts can be reduced, and the process cost is greatly reduced. The route is simple to operate, the total yield is high, the purity of the obtained product is high, and the method is suitable for large-scale production. The forms of the three salts of relugolix are also found, the crystallinity is good, the purification is easy, and the product purity is favorably improved.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com