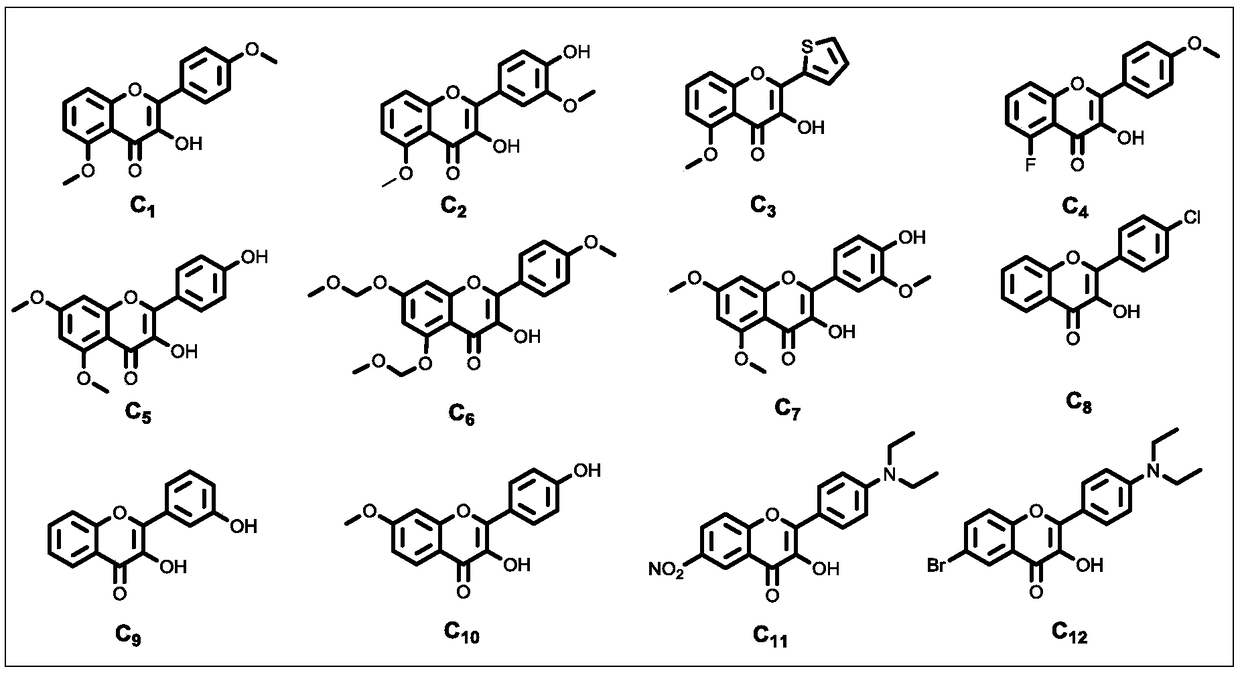
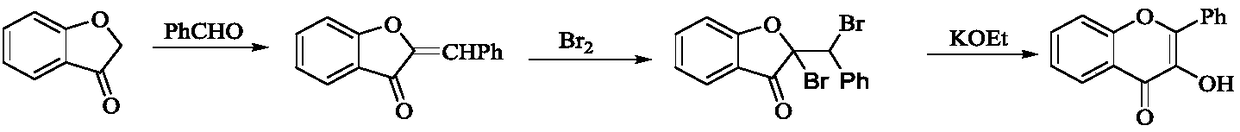
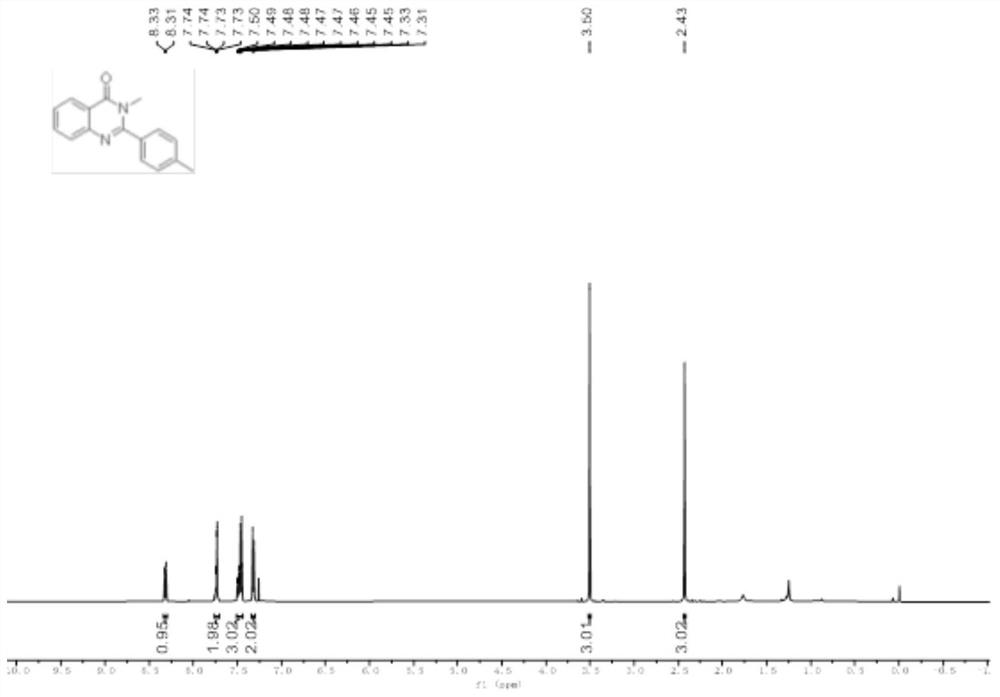
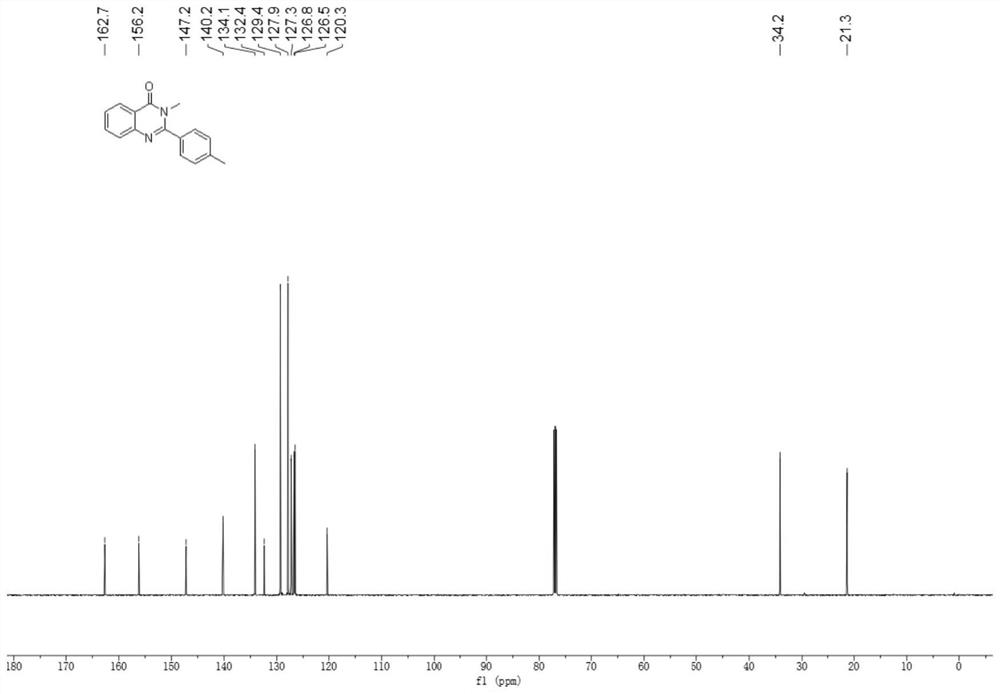
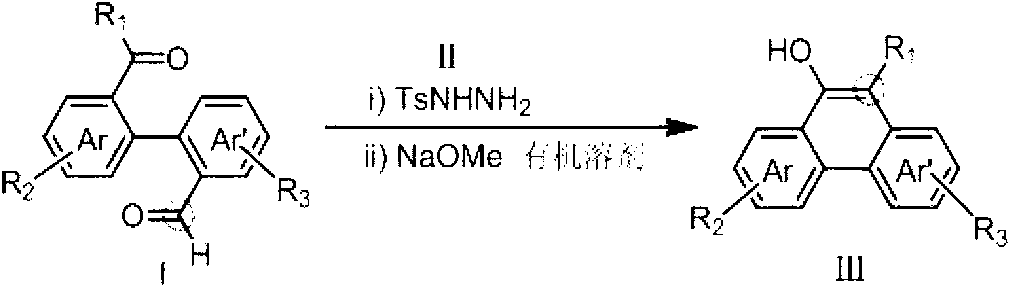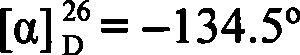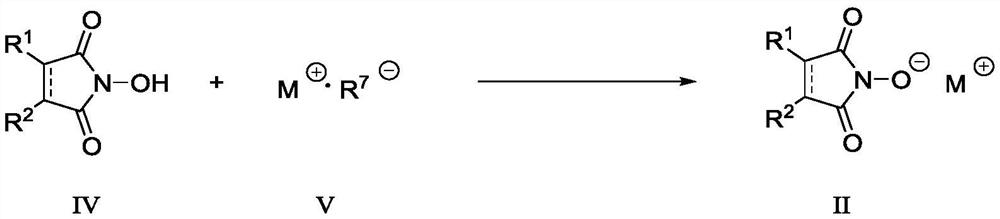Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75results about How to "Wide substrate adaptability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
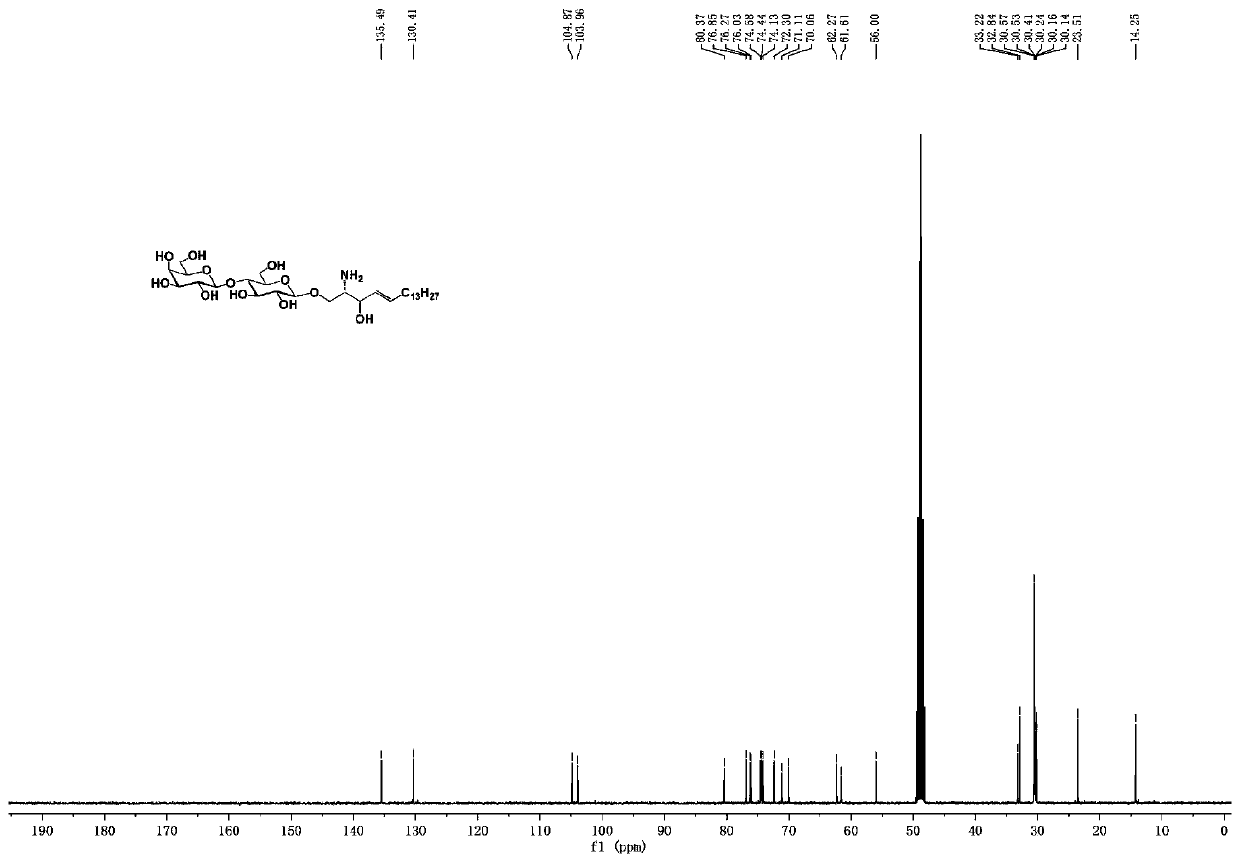
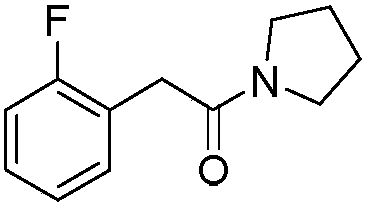
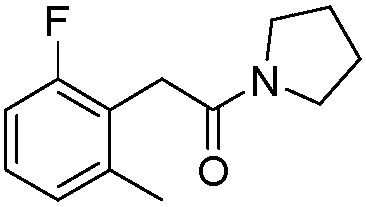
Lipase, its gene, yalulipolytic geast for producing said enzyme and its application
ActiveCN1948470AWide substrate adaptabilityImprove stabilityImmobilised enzymesFungiConservative mutationAmino acid
This invention relates to a preparation of a kind of lipase and the method of using the lipase to compound ester. Exactly is that amino acid sequence is SEQ ID NO:1 or its lipase of conservative mutation sequence, it also relates to the gene coding this lipase, Yarrowia lipolytica generating this lipase.
Owner:BEIJING UNIV OF CHEM TECH
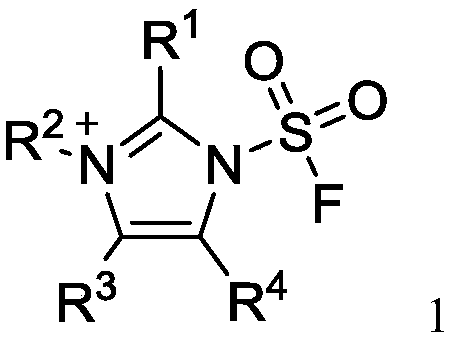
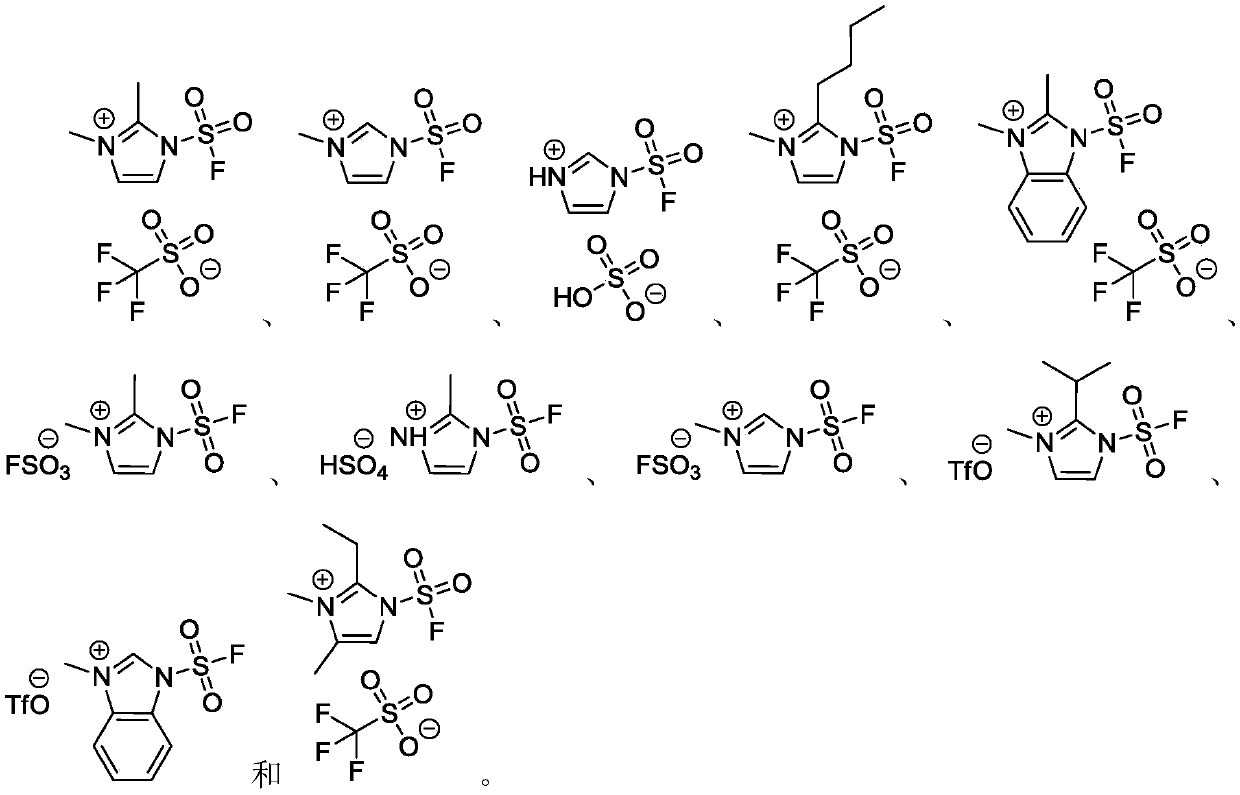
Fluorine-containing sulfonyl compound as well as intermediate, preparation method and application thereof
ActiveCN107857730AWide substrate adaptabilityLow toxicityOrganic chemistry methodsSulfonic acids salts preparationStable stateFluorine containing
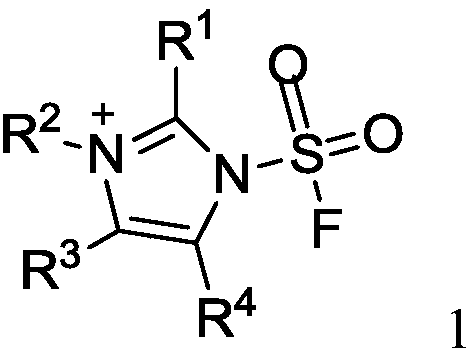
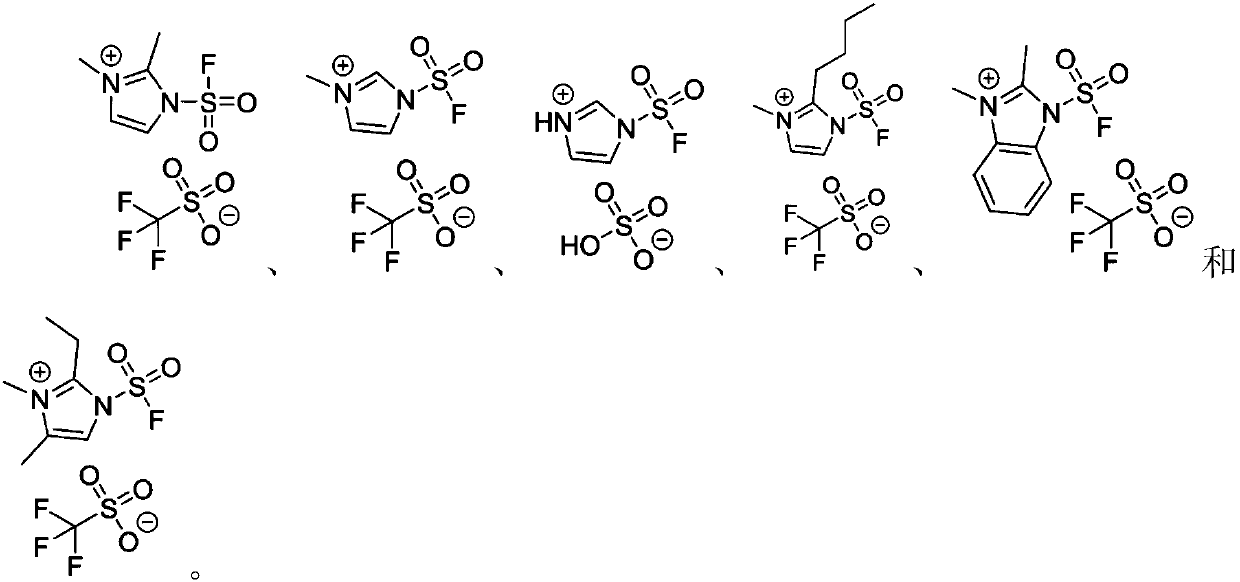
The invention discloses a fluorine-containing sulfonyl compound as well as an intermediate, a preparation method and application thereof. The fluorine-containing sulfonyl compound disclosed by the invention comprises positive ions and negative ions, wherein the positive ions are shown as the formula I. The fluorine-containing sulfonyl compound can react with substrates to effectively synthetize the fluorine-containing sulfonyl compound; the toxicity is low; the preparation is simple; the use is convenient; the fluorine-containing sulfonyl compound is in a solid stable state at normal temperature. In addition, the substrate applicability of the compound is extremely wide, and a phenol compound and an amine compound can be included; the fluorine-containing sulfonyl compound is a unique solidformation reagent capable of realizing the chemical conversion at present, so that important academic and application values are realized. The formula I is shown in the description.
Owner:中宏鑫投资控股(深圳)有限公司
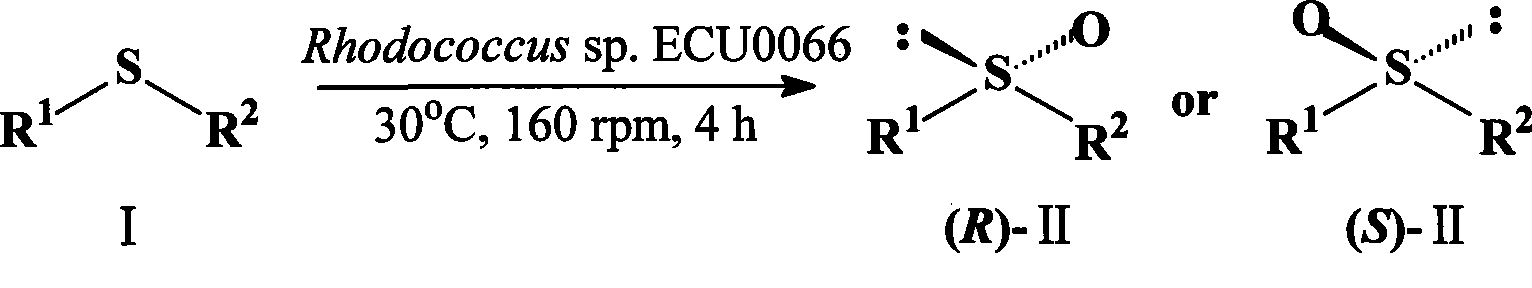
A strain of Rhodococcus and use thereof for preparing optical pure chiral sulphoxide
InactiveCN101372676AEasy to manufactureMild reaction conditionsBacteriaMicroorganism based processesBiological oxidationCatalytic oxidation
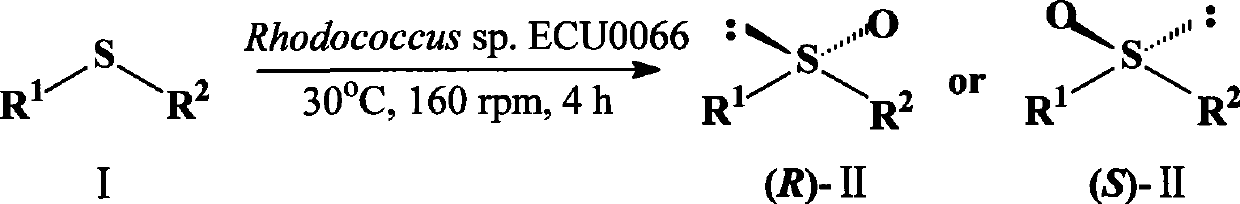
The invention discloses a Rhodococcus (Rhodococcus sp.ECU0066) and the use thereof, and the culture collection number of the strain is CGMCC No.2547. The resting cells of the strain is taken as a biological catalyst, and prochiral phenyl alkyl sulfide and a derivative thereof are carried out catalytic oxidation asymmetrically to obtain benzoylate sulfoxide and a derivative thereof with optical activity. The strain and the stereoselective biological oxidation process have the advantages of better catalysis effect, simple and safe operation and low cost, the product is easily purified and is friendly to environment.
Owner:EAST CHINA UNIV OF SCI & TECH
Mutant short-chain dehydrogenase, recombinant expression vector, genetic engineering bacterium and application
InactiveCN106636020AHigh catalytic activityEasy to manufactureOxidoreductasesFermentationAlcoholKetone
The invention provides a mutant of short-chain dehydrogenase, a recombinant expression vector, a genetic engineering bacterium, a preparation method of the mutant and application of the mutant or the genetic engineering bacterium generating the mutant in asymmetrically reducing a series of prochiral ketones to prepare optical homochiral alcohol. The chiral alcohol prepared by asymmetric reduction of the mutant of the short-chain dehydrogenase or the genetic engineering bacterium containing the mutant has high catalytic activity, and high-optical-purity chiral alcohol (ee>99%) can be synthesized.
Owner:ZHEJIANG UNIV
Fluorosulfuryl-containing compound, and intermediate, preparation method and application thereof
InactiveCN110590609AWide substrate adaptabilityLow toxicityOrganic compound preparationSulfuric acid amide preparationChemical transformationReagent
The invention discloses a fluorosulfuryl-containing compound, and an intermediate, preparation method and application thereof. The fluorosulfuryl-containing compound disclosed by the prevention includes cations and anions, and the cations are shown as a formula 1 (please see the specifications for the formula). The fluorosulfuryl-containing compound can react with a substrate to efficiently synthesize a fluorosulfuryl product, is low in toxicity, easy to prepare and convenient to use, and is in a stable solid state at the normal temperature; and in addition, the substrate of the compound has extremely high adaptability and can include a phenolic compound and an amine compound, and the fluorosulfuryl-containing compound is a unique solid form reagent capable of realizing chemical transformation at present and thus has important academic and application value.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
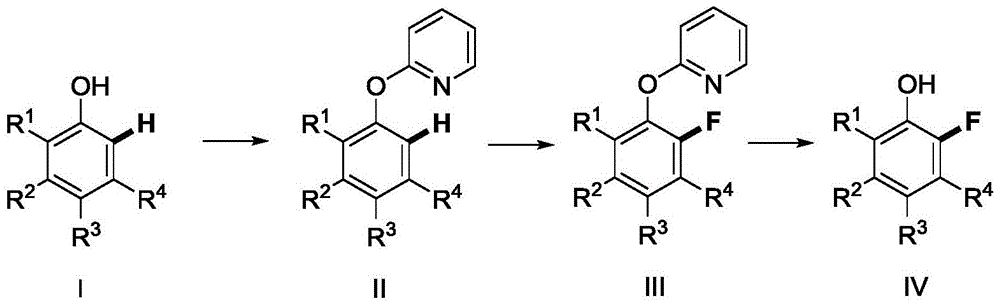
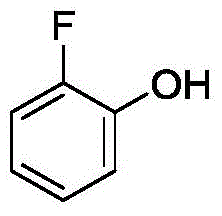
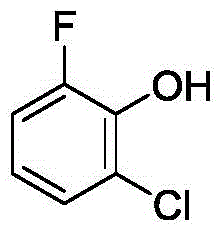
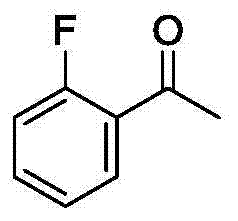
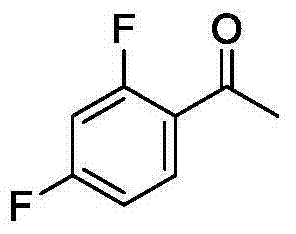
Method for synthetizing 2-fluoro phenol compound
ActiveCN104844399AWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationHydroxy group formation/introductionOrganic solventOrtho position
The present invention provides a method for synthetizing a 2-fluoro phenol compound shown in a formula IV. The phenol compound shown in the formula I is prepared into a 2-pyridine oxygroup arene compound shown in a formula II through an Ullmann reaction, the 2-pyridine oxygroup arene compound shown in the formula II is mixed with a palladium catalyst, a fluorinating reagent, an additive and an organic solvent, the mixture is stirred under the temperature of 30-160 DEG C to perform a fluorination reaction to obtain an ortho-position fluoridated 2-pyridine oxygroup arene compound shown in a formula III, and the ortho-position fluoridated 2-pyridine oxygroup arene compound shown in the formula III is prepared into the 2-fluoro phenol compound shown in the formula IV through the action of alkali. The method provided by the present invention has the advantages of mild reaction conditions, simplicity in operations, good substrate adaptability, high fluorination selectivity and the like. The 2-fluoro phenol compound is shown in the figure below.
Owner:ZHEJIANG UNIV OF TECH
Short-chain dehydrogenase, gene of short-chain dehydrogenase, recombinant expression vector, genetically engineered bacterium and application
InactiveCN105238768AAsymmetric reductionHigh optical purityBacteriaOxidoreductasesPtru catalystKetone
The invention provides short-chain dehydrogenase obtained from Empedobacter brevis, a gene of the short-chain dehydrogenase, recombinant short-chain dehydrogenase, a recombinant expression vector with the gene, a genetically engineered bacterium, a preparation method of the recombinant short-chain dehydrogenase and application of the short-chain dehydrogenase or the genetically engineered bacterium containing the short-chain dehydrogenase to asymmetric reduction of prochiral ketones for preparation of optical pure chiral alcohols. The short-chain dehydrogenase for preparation of the chiral alcohols by asymmetric reduction has remarkable advantages and can be used for synthesis of high-optical-purity chiral alcohols (ee>99%). Moreover, easiness in preparation of catalysts, mild reaction conditions, high substrate adaptability and environment friendliness are realized, asymmetric reduction of the prochiral ketones can be efficiently catalyzed by recombinant cells in an isopropanol-containing reaction system without any coenzymes, and industrial application and development prospect is promising.
Owner:ZHEJIANG UNIV
Method for synthesizing mephenesin carbamate by using carbon dioxide, amine and aryl diazoacetate
ActiveCN107674044AImprove economyImprove adaptabilityCarbamic acid derivatives preparationOrganic compound preparationCarbamateDistillation
The invention discloses a method for synthesizing mephenesin carbamate by using carbon dioxide, amine and aryl diazoacetate. The method comprises the following steps: in a high-pressure reactor, dissolving the aryl diazoacetate and the amine into a solvent, then adding a silver catalyst, introducing the carbon dioxide, heating and reacting under the stirring condition; after the reaction is ended,stopping heating and stirring, cooling, and slowly releasing unreacted carbon dioxide; diluting the reaction solution with ethyl acetate, filtering and removing the solvent by reduced pressure distillation to obtain a crude product; then carrying out column chromatography purification to obtain the mephenesin carbamate. The synthetic method disclosed by the invention has the advantages of simpleoperation, low-price and easily-obtained raw materials such as the carbon dioxide and the amine, high economy of reaction atoms, good adaptability of functional groups, wide applicability of a substrate, relative environment friendliness, facilitation for industrial production and good application prospect in organic synthesis.
Owner:SOUTH CHINA UNIV OF TECH
Synthesis method of 4-allyl-3,5-disubstituted isooxazole
ActiveCN108863969ARaw materials are easy to getInnovativeOrganic chemistryOrganic synthesisSynthesis methods
The invention discloses a synthesis method of 4-allyl-3,5-disubstituted isooxazole, and belongs to the technical field of organic synthesis. The synthesis method is characterized in that in a reactor,acetyenic ketone oxime ether substrates, 3-bromopropylene, palladium catalysts, additives and solvents are added; stirring reaction is performed at 70 to 80 DEG C; a reaction product is separated andpurified to obtain the 4-allyl-3,5-disubstituted isooxazole. The method has the advantages that a product obtained by performing Sonogashira coupling on simple and easy-to-obtain acyl chloride and alkyne, and methoxylamine hydrochloride react to obtain a series of norethisteroneoxime ether; the reaction conditions are mild; no environment pollution exists; a potential functional 4-allyl-3,5-disubstituted isooxazole compound is built. The method has innovativeness and atom economy; the conditions are mild; the operation is safe; the scale can be magnified to 5g level scale without influencingthe yield, so that potential practical values are realized.
Owner:SOUTH CHINA UNIV OF TECH
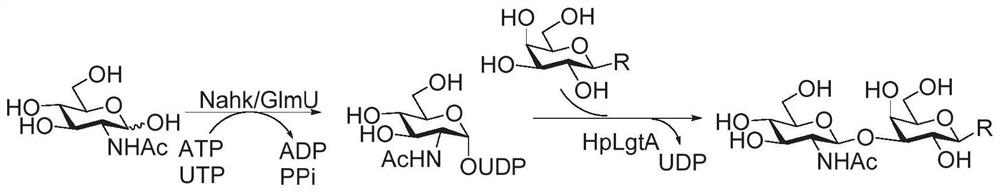
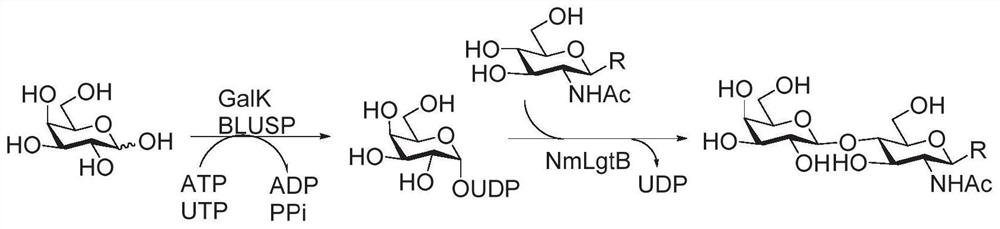
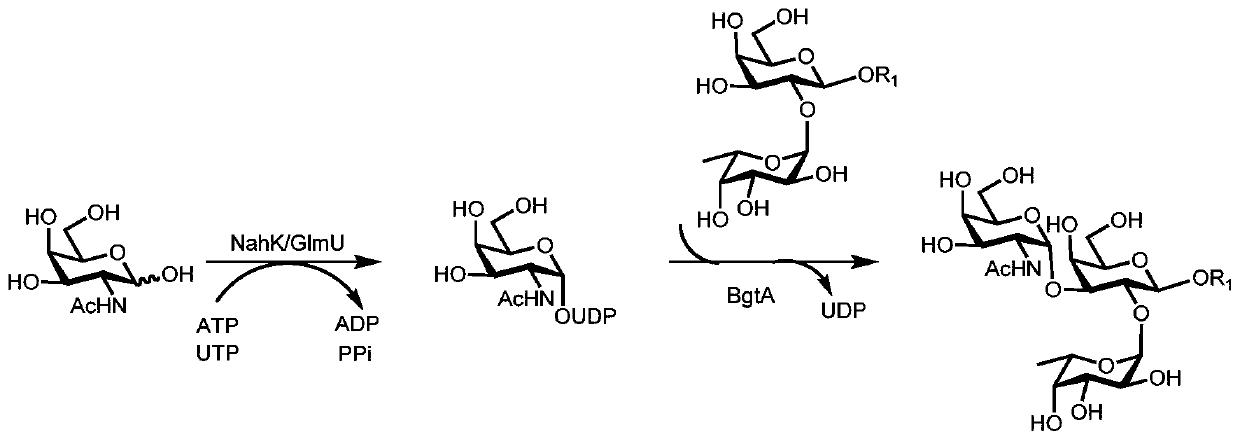
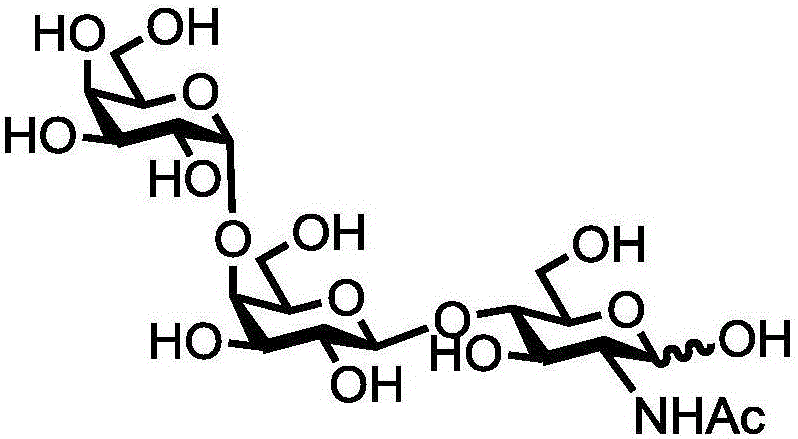
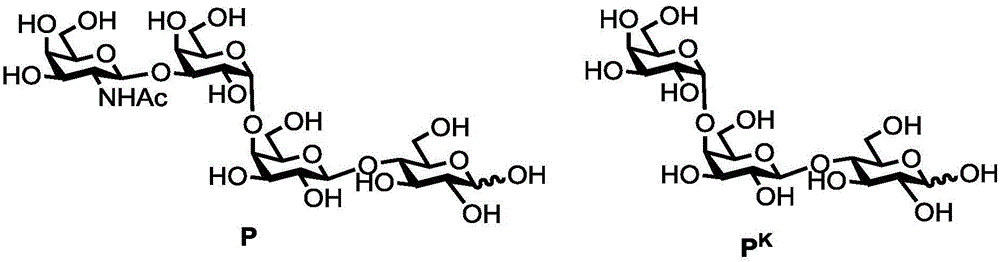
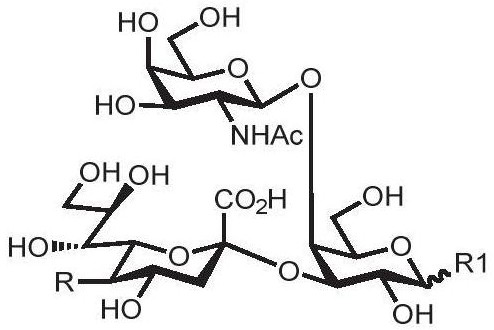
Human blood group antigen P1 pentasaccharide synthesis method
The invention discloses a human blood group antigen P1 pentasaccharide synthesis method. The method includes steps: adopting a one-pot multienzyme system for coupling galactose to trisaccharide as shown in a formula (III) through a beta1-4 glucosidic bond to synthesize tetrasaccharide as shown in a formula (IV); adopting the one-pot multienzyme system for coupling galactose to the tetrasaccharide as shown in the formula (IV) through an alpha1-4 glucosidic bond to synthesize pentasaccharide as shown in a formula (I), wherein in the formula (I), the formula (III) and the formula (IV), R1 refers to hydroxyl, azide substituted alkyl, alkynyl substituted alkyl, sulfydryl substituted alkyl, alpha- or beta- configuration substituted alkyl, alpha- or beta- configuration serine residue and alpha- or beta- configuration threonine residue. By integration of high regioselectivity and high efficiency of enzymatic synthesis, P1 antigen pentasaccharide is synthesized for the first time. Glycosyltransferase, glucose nucleoside generating enzymes and glucokinse adopted in the synthesis method are all derived from prokaryotes, and high protein expression quantity, high substrate adaptability and high catalytic efficiency are realized.
Owner:SHANDONG UNIV
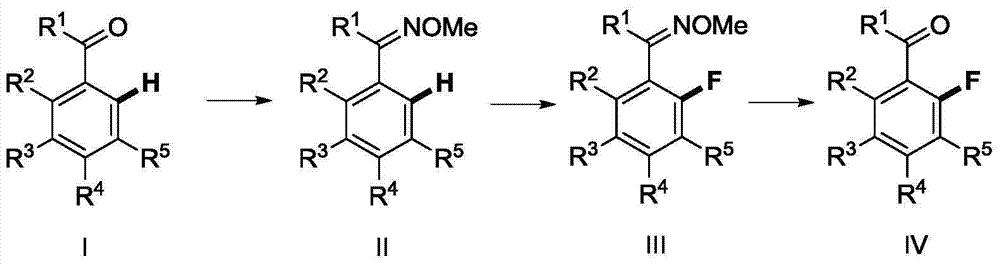
Method for synthesizing 2-fluoroarylcarbonyl compounds
ActiveCN103922904AWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationCarbonyl compound preparation by hydrolysisArylOrtho position
The invention provides a method for synthesizing 2-fluoroarylcarbonyl compounds, which comprises the following steps: converting arylcarbonyl compounds into corresponding carbonyl oxime ether compounds, mildly implementing aryl hydrocarbon chain direct fluoridation of high-selectivity oximido substituent group ortho-position in the presence of a palladium catalyst, a fluoridation reagent and additives, and finally, rehydrolyzing oxime ethers under the action of acid to obtain the 2-fluoroarylcarbonyl compounds. The fluoridation method has the advantages of mild reaction conditions, high substrate adaptability, high fluoridation selectivity and the like, is simple to operate, and has higher application research value.
Owner:ZHEJIANG UNIV OF TECH
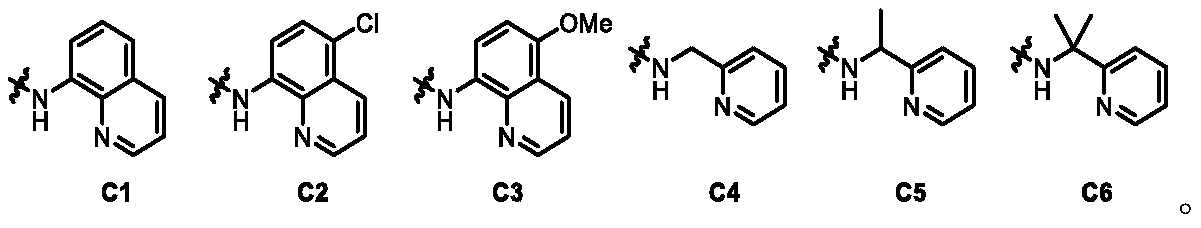
Amino acid chiral ligand containing bidentate coordination group, chiral catalyst, and corresponding preparation methods and applications thereof
ActiveCN109970713AIncrease diversityEasy to manufactureIndium organic compoundsOrganic chemistry methodsStereochemistryAMINO ACID PREPARATION
The present invention relates to an amino acid chiral ligand containing a bidentate coordination group, a chiral catalyst, and corresponding preparation methods and applications thereof. The chiral ligand is prepared from a cheap and easily available amino acid, and the development of the chiral ligand can improve the diversity of the chiral ligand. The chiral Ir (III) catalyst is simply and efficiently prepared from the chiral ligand only through a one-step reaction. The chiral Ir (III) catalyst is characterized in that a bidentate guiding group is introduced to an amino acid framework to change the original coordination mode of the amino acid and Ir in order to enhance the chiral control ability of the amino acid to the Ir(III) catalyst. The chiral Ir(III) catalyst is designed and synthesized for the first time, and the selectivity reaches up to 99% ee when the catalyst is successfully applied to the high-efficiency asymmetric synthesis of chiral gamma-cyclolactam, so the catalyst has superior stereo control ability.
Owner:NANKAI UNIV
Method for preparing cycloastragenol by double-enzyme compounding conversion of astragaloside
ActiveCN111893158AIncreased substrate toleranceHigh substrate conversion rateMicroorganism based processesFermentationAstragalosideAlglucerase
The invention relates to the technical field of biotransformation, in particular to a method for preparing cycloastragenol through double-enzyme compounding transformation of astragaloside. Accordingto the method, the astragaloside is used as a substrate, xylosidase and glucosidase are subjected to double-enzyme compounding, then xyloside bonds at the C3 position of the substrate and glucoside bonds at the C6 position of the substrate are broken through one-step hydrolysis, and the cycloastragenol is obtained. The purity of the obtained cycloastragenol can reach 98% or above, and the method is easy to operate, free of pollution, milder in reaction temperature, clear in enzyme conversion mechanism, and wider in enzyme substrate adaptability and is suitable for industrial production.
Owner:WEIHAI BAIHE BIOTECH +1
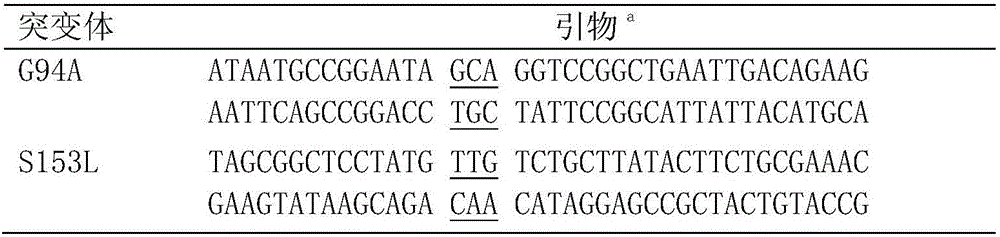
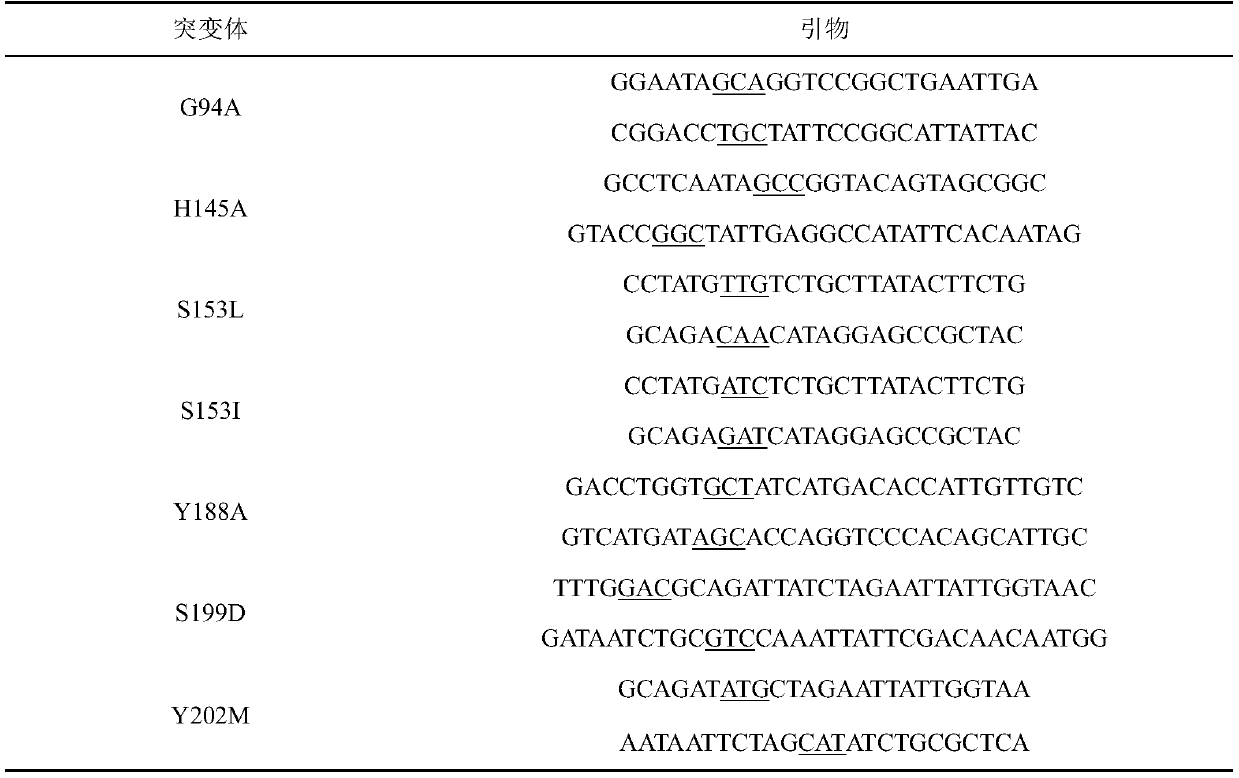
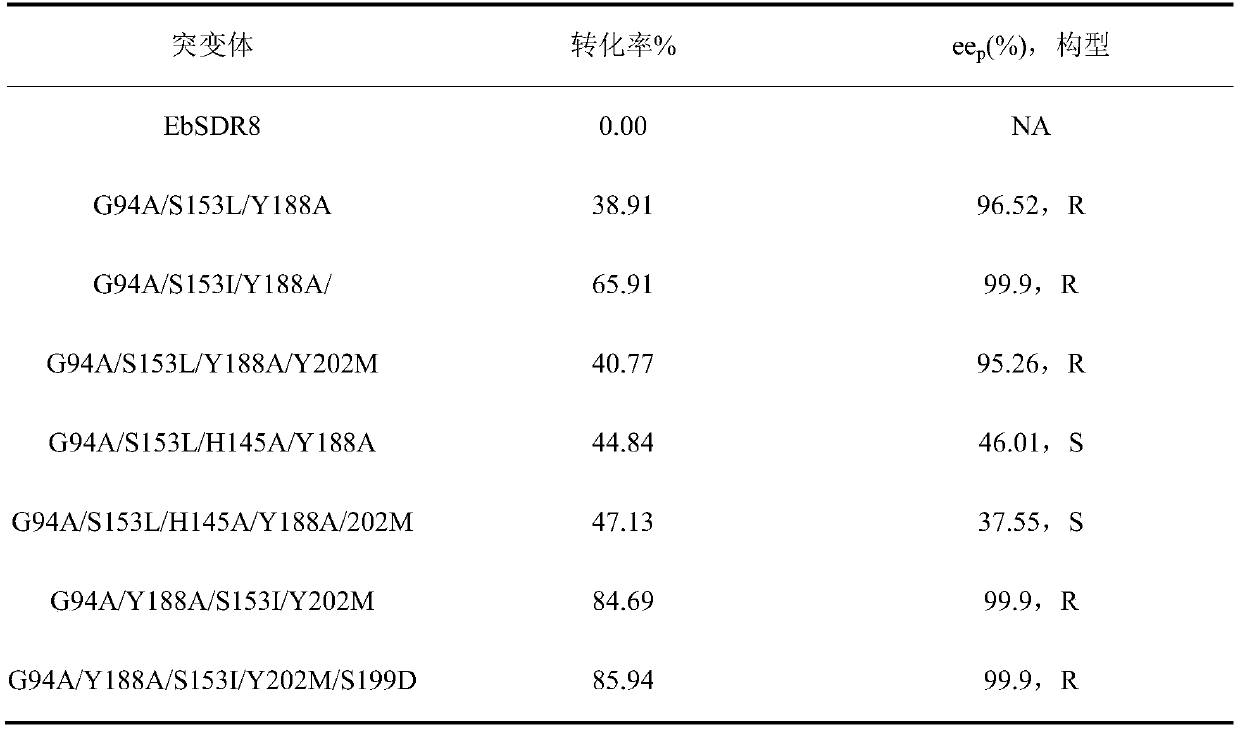
Carbonyl reductase mutant, recombinant expression carrier and application of carbonyl reductase mutant and recombinant expression carrier to production of chiral alcohol
ActiveCN110592035AEasy to manufactureMild reaction conditionsOxidoreductasesFermentationHigh concentrationMulti site
The invention discloses a carbonyl reductase mutant, a recombinant expression carrier and an application of the carbonyl reductase mutant and the recombinant expression carrier to production of chiralalcohol. The mutant is a single-site mutant or multi-site combined mutant containing the following sites based on the amino acid sequence of carbonyl reductase EbSDR8: 94-site glycine, 145-site histidine, 153-site serine, 188-site tyrosine, 199-site serine and 202-site tyrosine. The carbonyl reductase mutant or the recombinant expression carrier containing the mutant can efficiently catalyze asymmetric reduction of high-concentration latent chiral ketone in a reaction system without addition of any coenzymes to generate chiral alcohol high in optical purity (e.e.>99%), and has good industrialization application prospects.
Owner:ZHEJIANG UNIV +1
Enzymatic modules and Sda carbohydrate antigen synthesis method
ActiveCN111909910AWide variety of sourcesBuild accuratelyAcyltransferasesFermentationEscherichia coliAntigen epitope
The invention relates to enzymatic modules and an Sda carbohydrate antigen synthesis method, and relates to six enzymatic modules. One of the six enzymatic modules comprises bifidobacterium longum N-acetylglucosamine kinase, escherichia coli glyconucleoside generating enzyme and campylobacter jejuni [beta]1-4-N-acetylglucosamine transferase. According to the Sda carbohydrate antigen synthesis method, the campylobacter jejuni [beta]1-4-N-acetylglucosamine transferase is utilized to replace human-derived [beta]1-4-N-acetylglucosamine transferase to introduce GalNAc, and Sda carbohydrate antigenepitope is constructed; and Sda carbohydrate antigen and ABH antigen correlated hybridSda carbohydrate antigen is rapidly and efficiently synthesized by helicobacter pylori [alpha]1-2 fucosyltransferase,helicobacter pylori [alpha]1-3-N-acetylglucosamine transferase and human-derived [alpha]1-3-galactotransferase.
Owner:SHANDONG UNIV
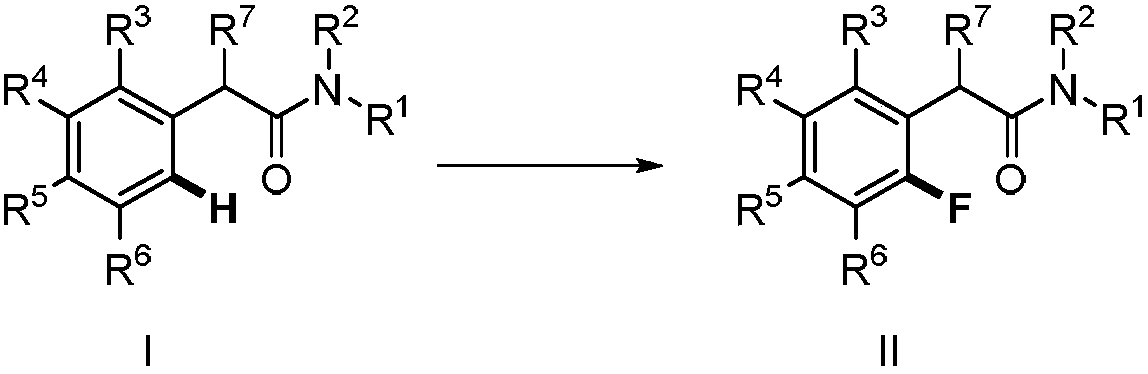
Method for synthesizing 2-fluoro-N-substituted aryl acetamide compound
The invention provides a method for synthesizing a 2-fluoro-N-substituted aryl acetamide compound. The method comprises the following steps that a N-substituted aryl acetamide compound reacts at 20 to150 DEG C under the condition of existence of palladium catalysts, fluorinated reagents and additives; TLC tracking detection is performed until the reaction is thoroughly performed; through aftertreatment, the compound shown as a formula II is obtained; the amide substituent ortho-position high-selectivity aryl hydrocarbon bond direct fluorination can be mildly realized. The method has the advantages that the reaction conditions are mild; the operation is simple; the substrate applicability is high; the fluoridation selectivity is high, and the like. High application study values are realized.
Owner:ZHEJIANG UNIV OF TECH
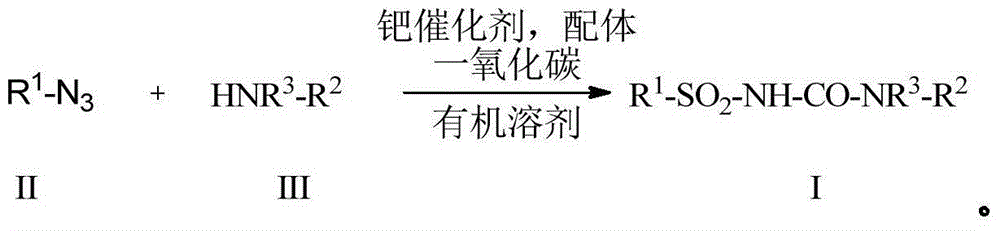
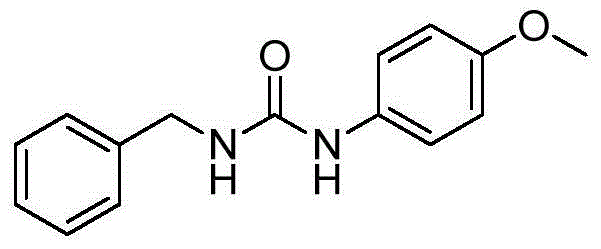
Preparation method for alkyl/benzyl/aryl urea compounds through heterogeneous-phase catalysis
ActiveCN105481723AWide substrate adaptabilityReduce manufacturing costGroup 4/14 element organic compoundsUrea derivatives preparationArylPalladium catalyst
The invention discloses a preparation method for formula (I) compounds, and the compounds are obtained by reacting a formula (II) compound with a formula (III) compound in a solvent in carbon monoxide atmosphere under catalysis of a heterogeneous-phase palladium catalyst. The reaction related in the method does not need strict waterless oxygen-free condition and does not need high pressure, is convenient and simple to operate, and possesses extremely good tolerance and universality on functional groups. Also the catalyst is extremely small in usage amount and is recoverable, reaction cost is low, and the preparation method is widely applicable to prepare alkyl / benzyl / aryl urea compounds. The formula (I) is R<1>-NH-CO-NR<3>-R<2>, the formula (II) is R<1>-N3, and the formula (III) is HNR<3>-R<2>, wherein R<1> and R<2> are same or different, and are mutually independently selected from aryl, heteroaryl, cycloalkyl, heterocyclic groups, alkyl, alkenyl, alkynyl, arylalkyl, heteroaryl alkyl, cycloalkyl alkyl, heterocyclic-group alkyl, aryl alkenyl, heteroaryl alkenyl, cycloalkyl alkenyl and heterocyclic-group alkenyl; and R<3> is selected from H or R<2>, or R<3> and R<2> are in connection for forming a ring.
Owner:CHINA AGRI UNIV
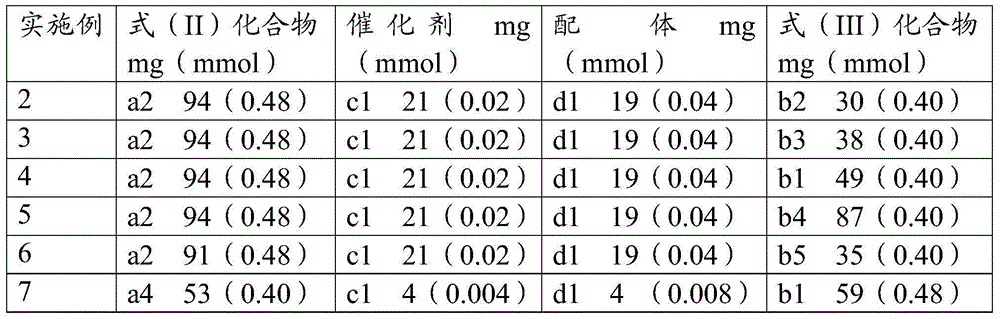
Thieno[3,4-b] indole derivative and synthesis method thereof
ActiveCN111285881AGood varietyRaw materials are easy to getOrganic chemistryArylCombinatorial chemistry
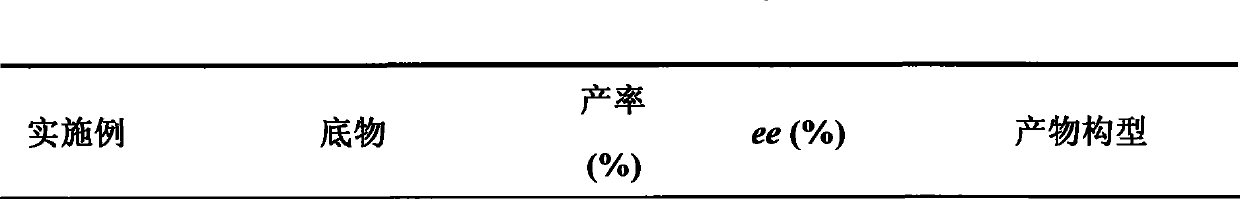
The invention discloses a thieno[3,4-b]indole derivative and a synthesis method thereof. The preparation method comprises the following steps: by taking 4-alkylthio-4-((2-(phenyl ethynyl)aryl)amino)butyl-3-ene-2-one as a raw material, catalyzing a self-cyclization reaction of 4-alkylthio-4-((2-(phenyl ethynyl)aryl)amino)butyl-3-ene-2-one with an oxidizing agent by catalyzing with a cheap metal toconstruct the thieno[3,4-b] indole derivative in one step, wherein the obtained thieno[3,4-b] indole derivative is widely applied to the fields of photoelectric materials, medicines and the like. Themethod of the invention has characteristics of cheap metal catalysis, elemental sulfur oxidation, easily available raw materials, simple operation, high efficiency, wide substrate adaptability and diversified functional groups.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
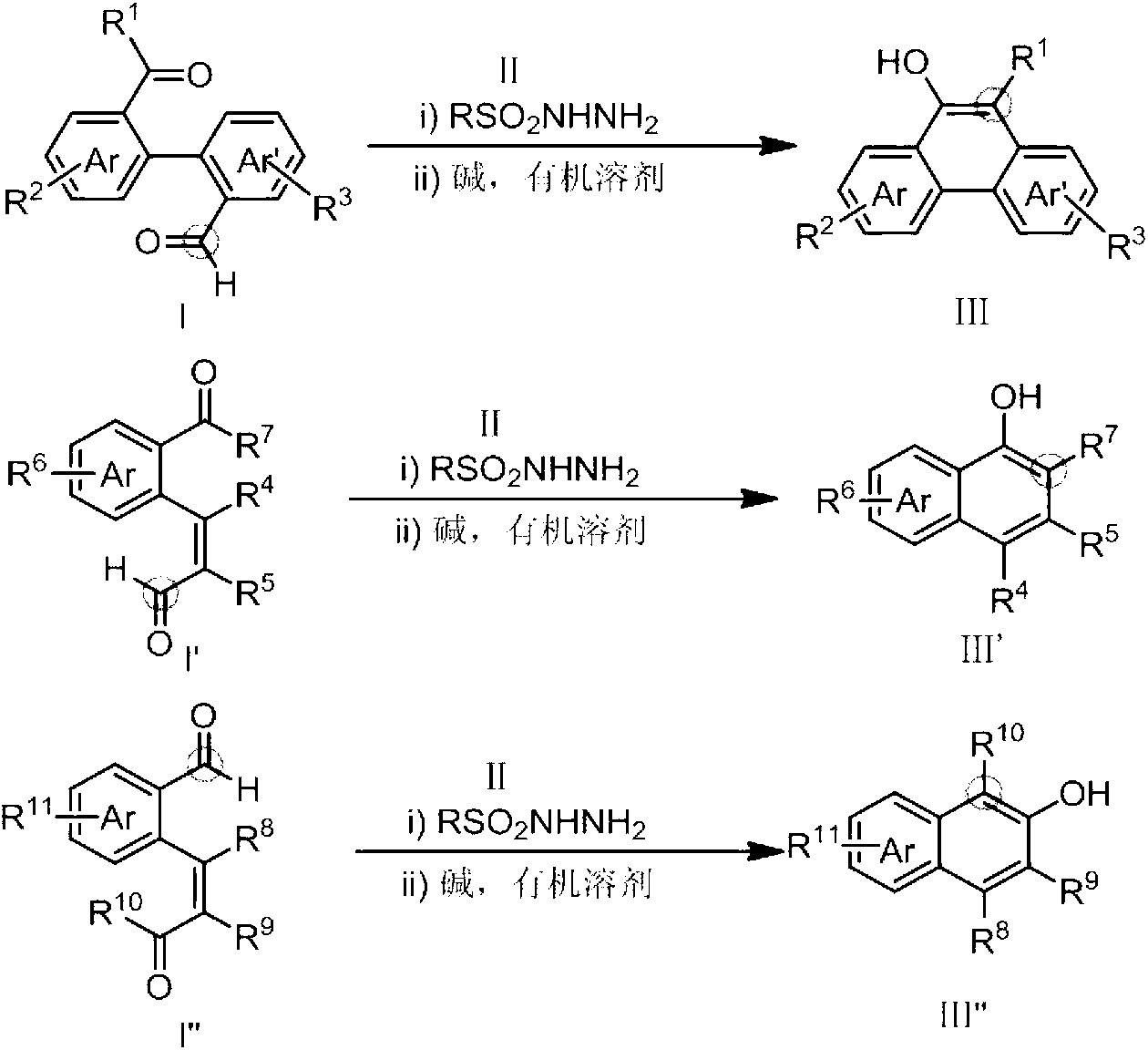
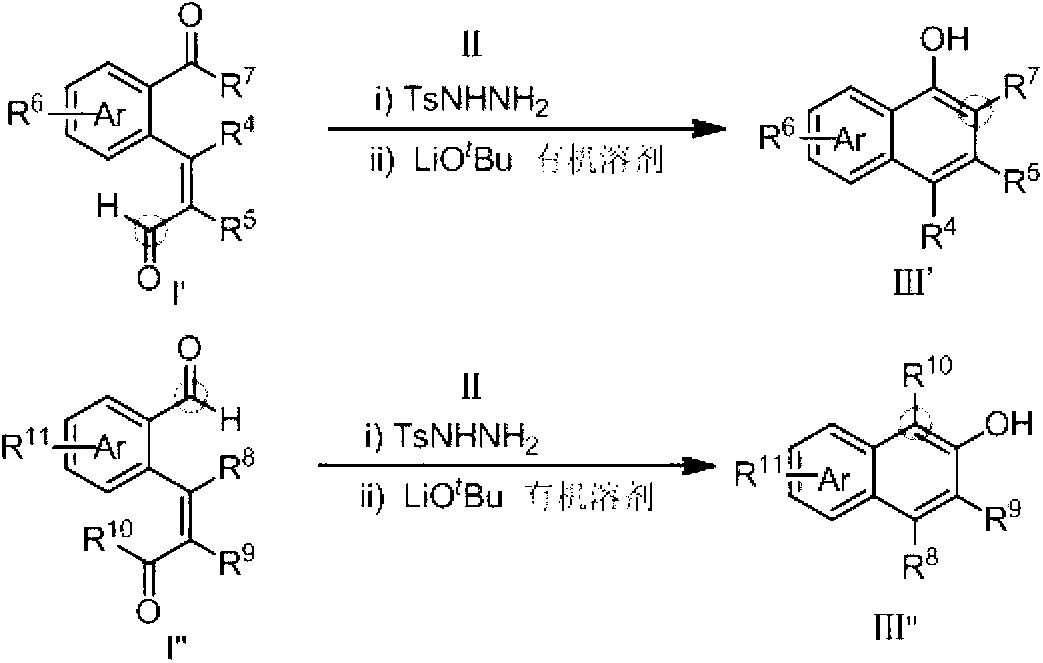
Method for preparing hydroxy-substituted polycyclic aromatic compound
ActiveCN103073393AWide substrate adaptabilityOrganic compound preparationCarbonyl compound preparation by condensationPolycyclic compoundHydrazone
The invention discloses a method for preparing hydroxyl-substituted polycyclic aromatic compound. Dicarbonyl compounds such as intramolecular aldehyde ketone react with sulfohydrazide to obtain single N-sulfonyl-hydrazone, and the single N-sulfonyl-hydrazone reacts in an organic solvent under the alkaline condition, as a result, the hydroxy-substituted polycyclic aromatic compound is obtained. The method utilizes the activity difference between aldehyde and ketone, comprises a procedure of intramolecular carbon-carbon bond insertion-aromatization of the intermediate of the single N-sulfonyl-hydrazone, is short in reaction time and high in reaction yield, can well forbear and universally adapt to functional groups, and can be widely used for preparing various hydroxyl-substituted polycyclic armomatic compounds.
Owner:PEKING UNIV
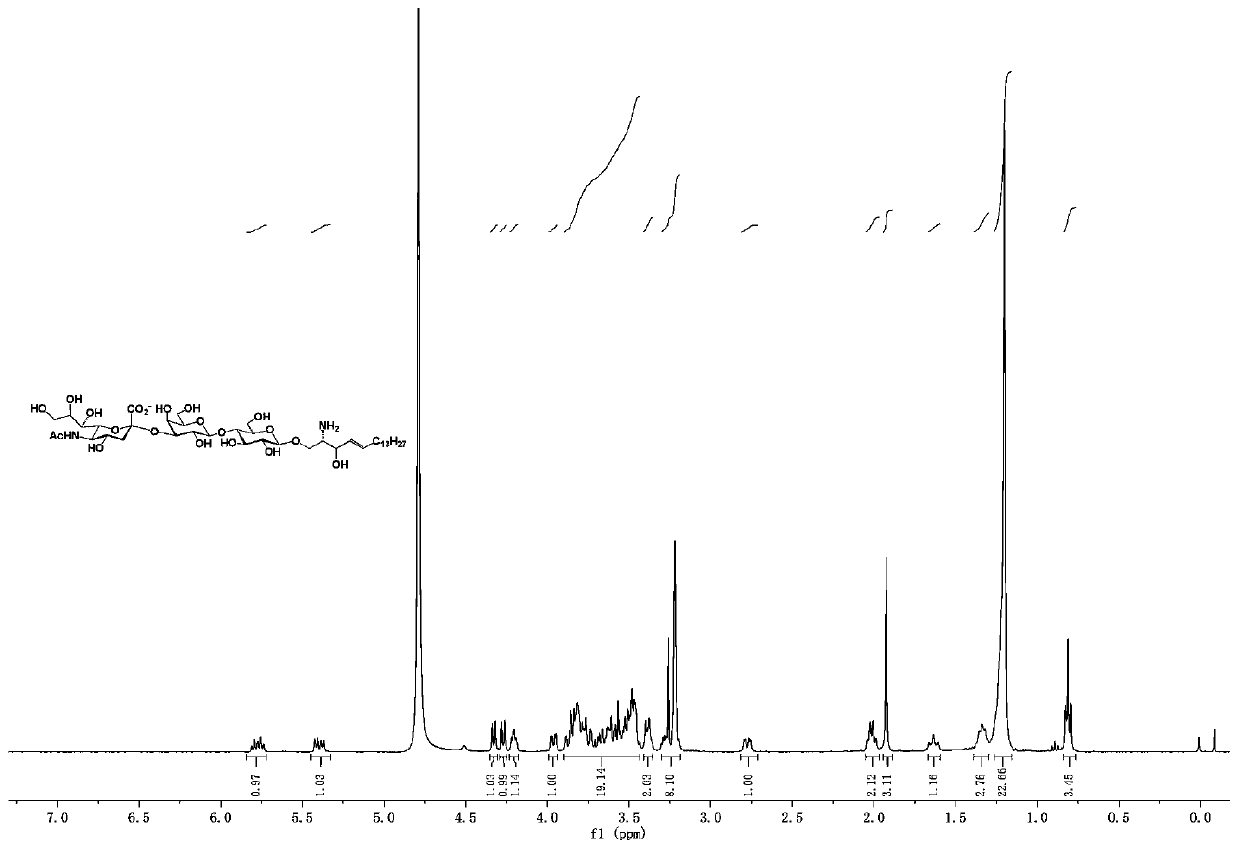
Ganglioside GM3 and/or analogue thereof, synthesis method and application of ganglioside GM3 and/or analogue thereof
InactiveCN111233949AEfficient synthesisSynthesis fastSugar derivativesSugar derivatives preparationFatty acidPharmaceutical Substances
The invention relates to a method for synthesizing ganglioside GM3 and / or an analogue thereof. The method includes the following steps: (1) selecting a lactose donor represented by a general formula Iand / or an analogue thereof and selecting a sphingosine derivative represented by a general formula II; (2) synthesizing lactosphingosine and / or a derivative thereof from the compounds shown in the general formula I and the general formula II by using a glycosidation reaction, and then removing a protecting group; (3) synthesizing sialylated lactosphingosine and / or derivative thereof by using a one-pot three-enzyme method; and (4) carrying out condensation reaction on the general formula IV and a fatty acid to synthesize the ganglioside GM3 and / or analogue thereof. The method can be used for efficiently, rapidly and simply synthesizing ganglioside GM3 and analogues thereof. The method adopts a chemical enzymatic method, can be applied in preparing ganglioside GM3 and analogues thereof, andcan be used for drug development.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
A kind of method of synthesizing 2-fluorophenol compound
ActiveCN104844399BWide substrate adaptabilityGood substrate adaptabilityOrganic compound preparationHydroxy group formation/introductionOrganic solventOrtho position
The present invention provides a method for synthetizing a 2-fluoro phenol compound shown in a formula IV. The phenol compound shown in the formula I is prepared into a 2-pyridine oxygroup arene compound shown in a formula II through an Ullmann reaction, the 2-pyridine oxygroup arene compound shown in the formula II is mixed with a palladium catalyst, a fluorinating reagent, an additive and an organic solvent, the mixture is stirred under the temperature of 30-160 DEG C to perform a fluorination reaction to obtain an ortho-position fluoridated 2-pyridine oxygroup arene compound shown in a formula III, and the ortho-position fluoridated 2-pyridine oxygroup arene compound shown in the formula III is prepared into the 2-fluoro phenol compound shown in the formula IV through the action of alkali. The method provided by the present invention has the advantages of mild reaction conditions, simplicity in operations, good substrate adaptability, high fluorination selectivity and the like. The 2-fluoro phenol compound is shown in the figure below.
Owner:ZHEJIANG UNIV OF TECH
Fluorine label and preparation method thereof, and method for synthesizing oligosaccharide chain by adopting assisted enzymic method
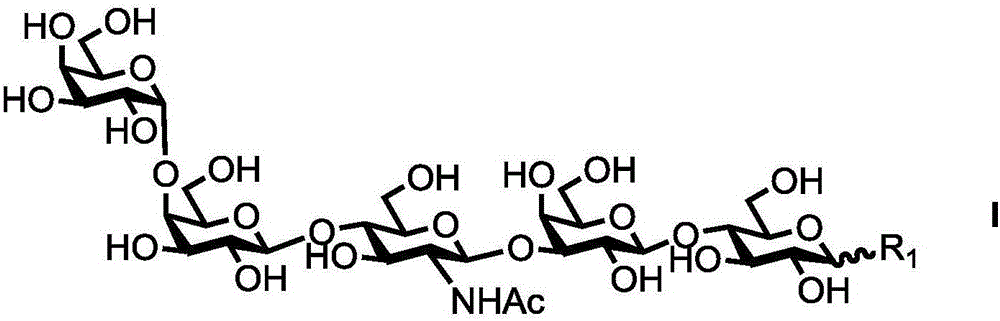
ActiveCN112940058AHigh modular assembly efficiencyEfficient preparationSugar derivativesOligosaccharidesReceptorCombinatorial chemistry
The invention relates to a fluorine label and a preparation method thereof, and a method for synthesizing an oligosaccharide chain by adopting an assisted enzyme method. The structural formula of the fluorine label is G-R(I) as shown in the formula I. G represents monosaccharide or oligosaccharide; and R is one of the following formulas II and III, wherein R1 is one of C6H13 and C8H17. When G represents lactose and R represents a C8F17 difluoro chain, the formula II is shown in the specification. The fluorine label has the advantages of being easy to remove and recycle. The glycosyl receptor is used for synthesizing various different oligosaccharide chains, so that the oligosaccharide chain with a determined structure is quickly and efficiently synthesized.
Owner:SHANDONG UNIV
Water-phase one-pot synthesis method of 3-flavonol and 3-flavonol derivative
InactiveCN109320488AStrong substrate adaptabilityGood substrate adaptabilityOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the field of chemical synthesis, and particularly relates to a water-phase one-pot synthesis method of 3-flavonol and a 3-flavonol derivative. According to the method, 2-hydroxyacetophenone, 2-hydroxyacetophenone derivatives, benzaldehyde and benzaldehyde derivatives are used as reaction substrates, or 2-hydroxy-chalcone and hydroxy-chalcone derivatives are used as reaction substrates; water or ethanol water solution is used as a solvent; under the aerobic condition at 20 to 100 DEG C, reaction is performed to obtain the 3-flavonol and the 3-flavonol derivative. The invention provides a fire-new reaction mechanism, and develops a novel 3-flavonol synthesis method with the advantages of high efficiency, convenience, high speed and wide substrate adaptability. The invention also provides a fire-new 3-flavonol derivative synthesized by the novel method; important application values are realized in the field of medical care sanitation.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
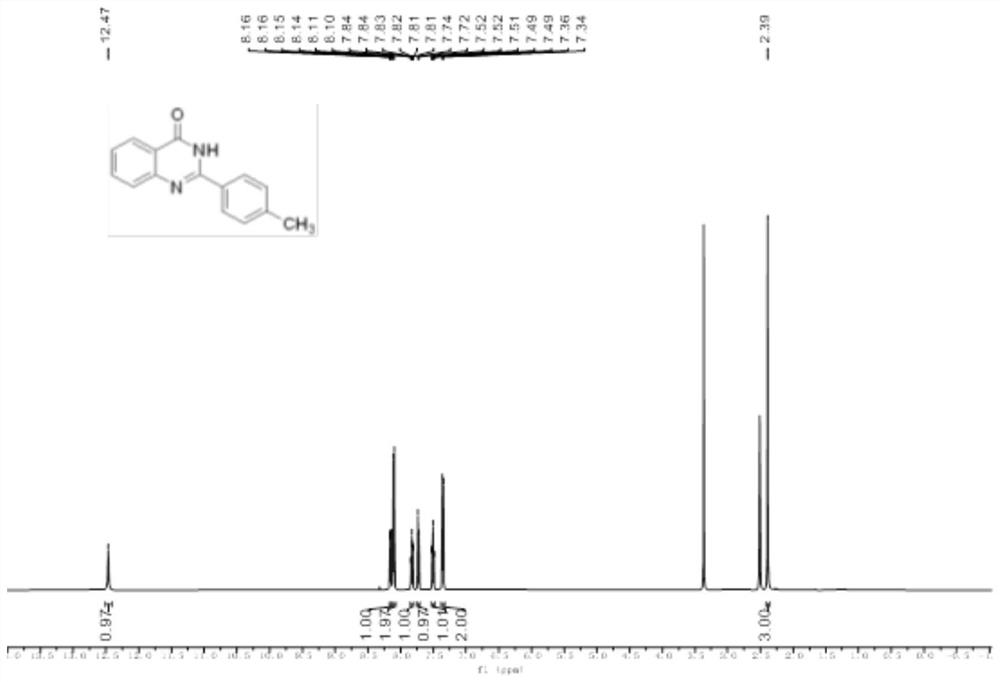
Method for synthesizing quinazolinone compound through visible light induction
The invention provides a method for synthesizing a quinazolinone compound through visible light induction. By taking an anthranilamide compound (I) and an aldehyde compound (II) as raw materials, and taking glacial acetic acid as a solvent, reaction is performed under the irradiation of purple light to obtain the quinazolinone compound (III). The method for synthesizing the quinazolinone compound through visible light induction is mild in reaction condition, simple and convenient in post-treatment operation, free of additional additives, efficient in reaction, wide in substrate adaptability, high in product purity, green and environmentally friendly.
Owner:WUHAN UNIV OF TECH
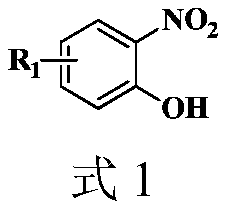
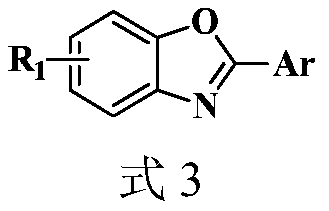
Synthesis method of 2-aryl benzoxazole derivative
The invention discloses a synthesis method of a 2-aryl benzoxazole derivative, wherein the method comprises the steps: carrying out ultrasonic reaction on an o-nitrophenol derivative and an aryl formaldehyde derivative in the presence of a pyridine accelerator and an elemental sulfur reducing agent to obtain the 2-aryl benzoxazole derivative. The method has the advantages of one-pot reaction synthesis, simple steps, easily available accelerant, low cost, high yield and environmental friendliness, and is beneficial to industrial production and application.
Owner:HUNAN BIOLOGICAL & ELECTROMECHANICAL POLYTECHNIC
A strain of Rhodococcus and use thereof for preparing optical pure chiral sulphoxide
InactiveCN101372676BEasy to manufactureMild reaction conditionsBacteriaMicroorganism based processesBiological oxidationCatalytic oxidation
The invention discloses a Rhodococcus (Rhodococcus sp.ECU0066) and the use thereof, and the culture collection number of the strain is CGMCC No.2547. The resting cells of the strain is taken as a biological catalyst, and prochiral phenyl alkyl sulfide and a derivative thereof are carried out catalytic oxidation asymmetrically to obtain benzoylate sulfoxide and a derivative thereof with optical activity. The strain and the stereoselective biological oxidation process have the advantages of better catalysis effect, simple and safe operation and low cost, the product is easily purified and is friendly to environment.
Owner:EAST CHINA UNIV OF SCI & TECH
N-substituted carbonyl fluorine sulfonamide compound as well as preparation method and application thereof
ActiveCN113121401AWide substrate adaptabilityLow toxicitySulfuric acid amide preparationCombinatorial chemistrySynthetic amine
The invention discloses an N-substituted carbonyl fluorine sulfonamide compound as well as a preparation method and application thereof. The N-substituted carbonyl fluorine sulfonamide compound comprises positive ions and negative ions, and the negative ions are shown in the formula I. The N-substituted carbonyl fluorine sulfonamide compound is low in toxicity, simple to prepare and convenient to use, is in a solid stable state at normal temperature, and reacts with a substrate to efficiently synthesize an N-amino carbonyl fluorine sulfonamide product; and besides, the compound is wide in substrate adaptability and can comprise primary amine and secondary amine compounds, so that the compound has important academic and application values.
Owner:中宏鑫投资控股(深圳)有限公司
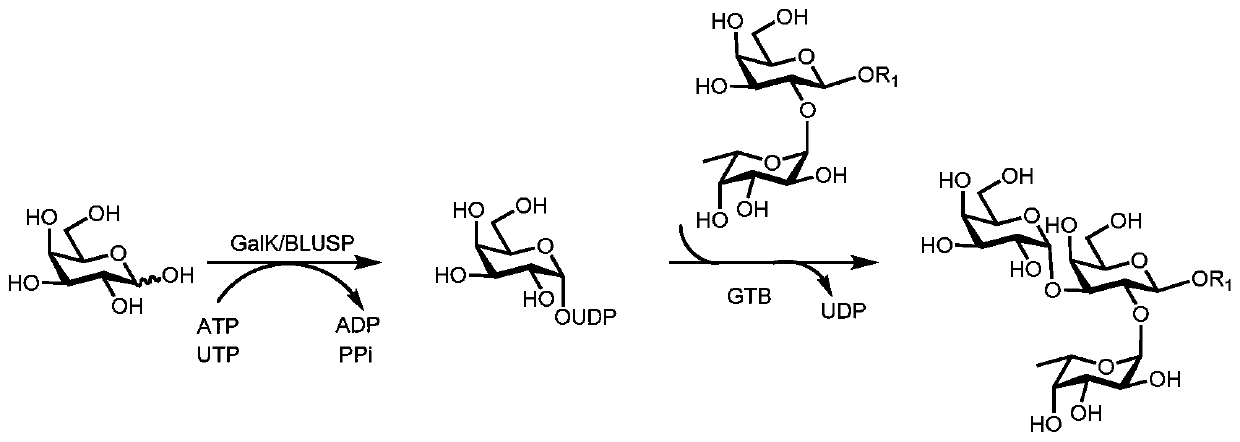
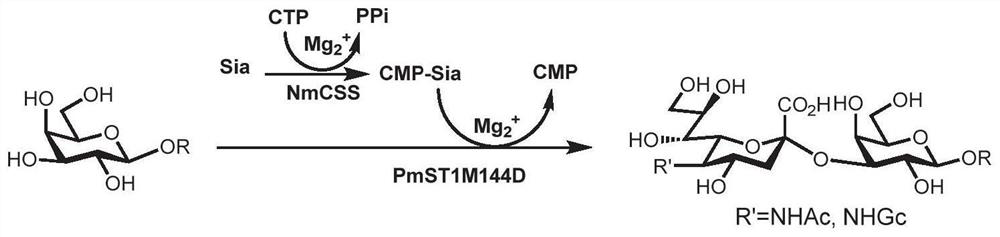
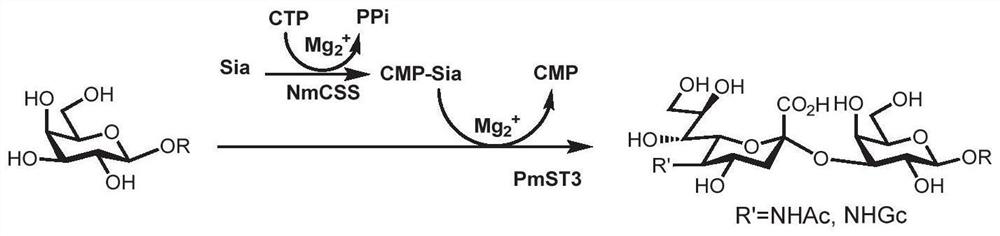
Synthesis method of GalNAc<alpha>1, 3Gal or Gal<alpha>1, 3Gal glycosidic bond oligosaccharide
ActiveCN111235128AHigh protein expressionWide substrate adaptabilityFermentationGlycosyltransferasesEnzymatic synthesisFucosylation
The invention discloses a synthesis method of GalNAc<alpha>1, 3Gal or Gal<alpha>1, 3Gal glycosidic bond oligosaccharide. The method comprises the following steps: a non-reductive tail end Gal of target oligosaccharide is subjected to <alpha>1, 2-fucosylation modification, so that a substrate can be recognized by <alpha>1, 3-N-acetylaminogalatose glycosyltransferase or <alpha>1, 3-galactose glycosyltransferase to synthesize GalNAc (Fuc<alpha>1, 2)<alpha>1, 3GAL or Gal (Fuc<alpha>1, 2)<alpha>1, 3Gal glycosidic, fucose is removed to synthesize target oligosaccharide containing a GalNAc<alpha>1, 3Gal or Gal<alpha>1, 3Gal glycosidic bond. The method combines high region selectivity and high efficiency of enzymatic synthesis together and realizes synthesis of a target molecule with a higher yield. The glycosyltransferase, glycoribosidase and glucokinse used in the method are all derived from prokaryotes, and the method has the advantages of high protein expression quantity, wide substrate adaptability, high catalysis efficiency and the like and can be applied to mass preparation.
Owner:SHANDONG UNIV
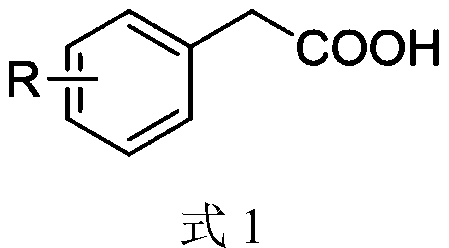
Synthesis method of aryl aldehyde compound
ActiveCN110963900ALow costMild reaction conditionsOrganic compound preparationCarbonyl compound preparationArylPtru catalyst
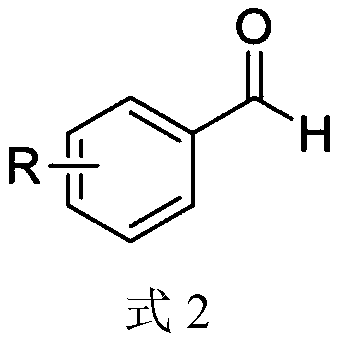
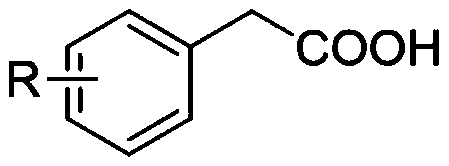
The invention discloses a synthesis method of an aryl aldehyde compound. The method comprises the following step: carrying out an oxidative decarboxylation reaction on an aryl acetic acid compound andan organic alkali under the catalytic action of a visible light catalyst to obtain the aryl aldehyde compound. According to the method, the aryl aldehyde compound is generated through one-step oxidative decarboxylation of aryl acetic acid at room temperature under visible light in an open system, so that the method is simple to operate, mild in reaction condition, cheap and easily available in raw materials and catalysts and high in reaction yield, and is an environment-friendly synthesis method.
Owner:HUAIHUA UNIV
Phosphate compound, and preparation method and application thereof
PendingCN111560037AMild reaction conditionsWide substrate adaptabilityGroup 5/15 element organic compoundsSteroidsPhosphoric Acid EstersPhosphate
The invention discloses a phosphate compound, and a preparation method and application thereof. The phosphate compound has a molecular structure represented by formula (I); and in the formula (I), R1is one of a phenyl group, a biphenyl group, a fluorophenyl group, a dimethoxy substituted phenyl group and a naphthyl group, R2 is one of a phenyl group, a biphenyl group, a fluorophenyl group, a dimethoxy substituted phenyl group and a naphthyl group, R3 is one of (3S,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptane-2-yl)hexahydro-1H-cyclopenta[a]phenanthrene-3-yl group, a dimethoxy substituted phenyl group, a 2,3-dihydroxypropyl group and a methyl group, and when R3 is the methyl group, R1 and R2 are not the phenyl group. The phosphate compound is prepared from a phosphine oxide compound and a hydroxyl-substituted organic matter in a certain ratio under mild conditions, is high in yield and can be applied to the field of drug synthesis.
Owner:GUANGDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Thieno[3,4-b] indole derivative and synthesis method thereof Thieno[3,4-b] indole derivative and synthesis method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c03ff2c0-3d10-41ec-812a-509d0a7dbc11/FDA0001896969650000011.png)
![Thieno[3,4-b] indole derivative and synthesis method thereof Thieno[3,4-b] indole derivative and synthesis method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c03ff2c0-3d10-41ec-812a-509d0a7dbc11/FDA0001896969650000012.png)
![Thieno[3,4-b] indole derivative and synthesis method thereof Thieno[3,4-b] indole derivative and synthesis method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c03ff2c0-3d10-41ec-812a-509d0a7dbc11/FDA0001896969650000013.png)