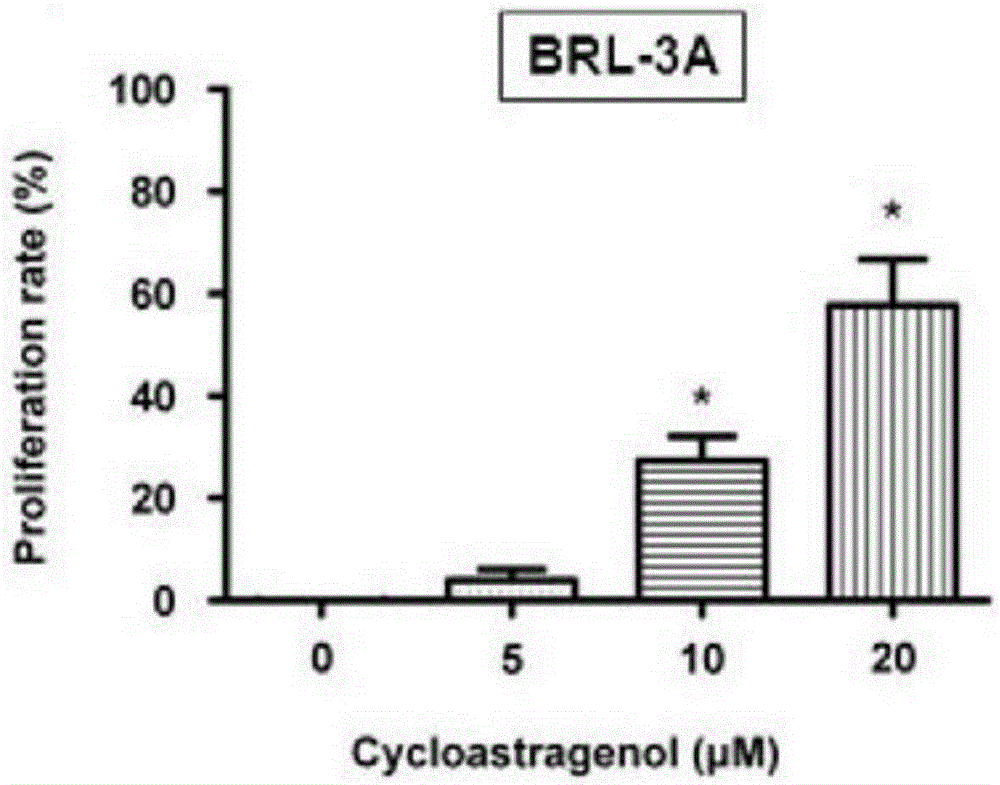
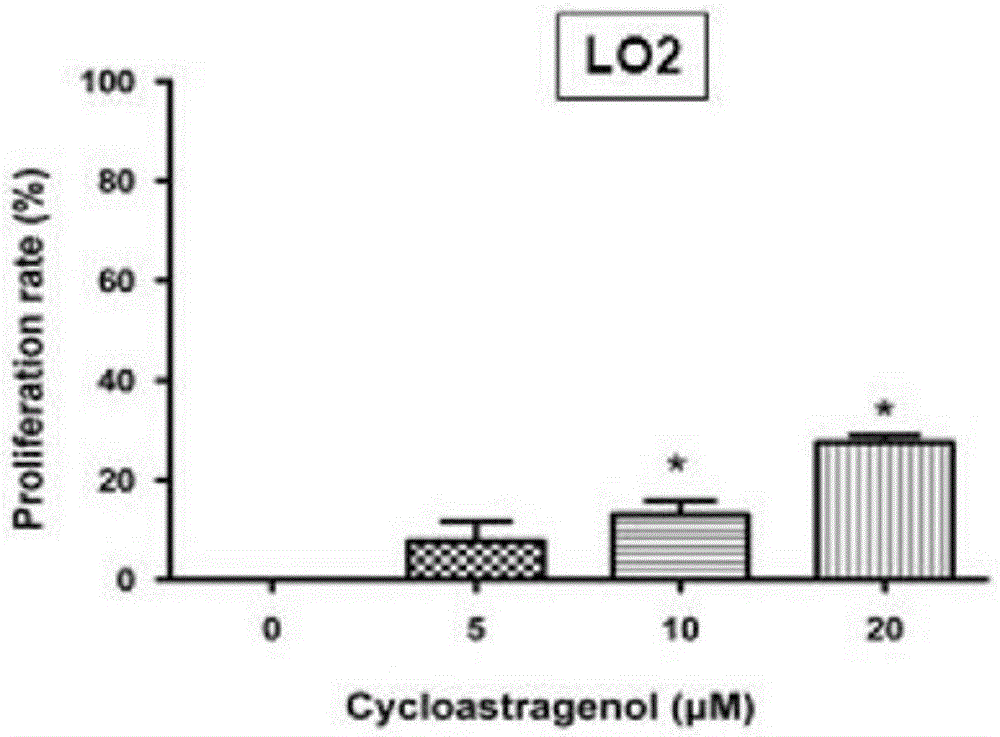
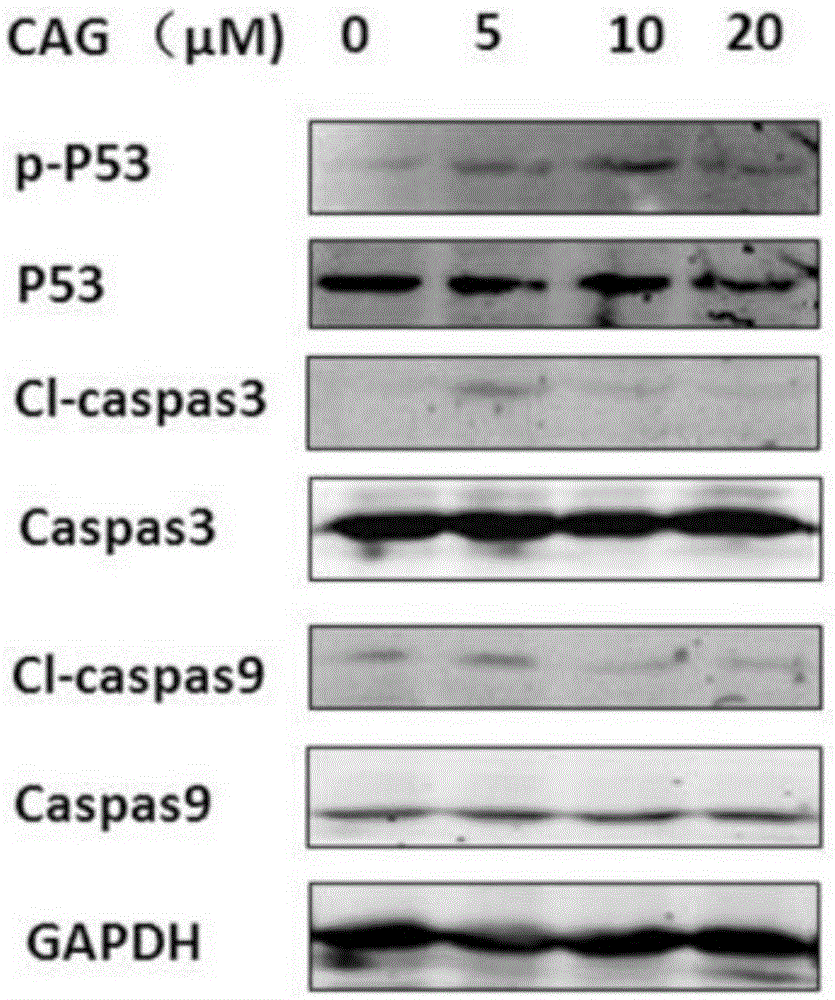
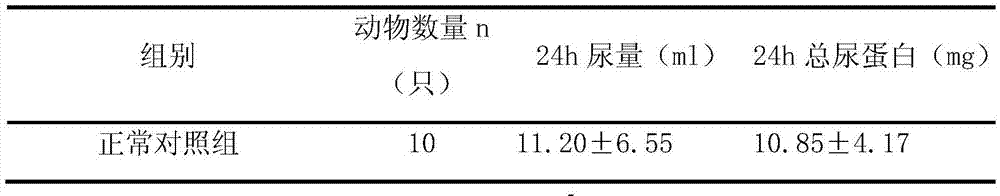
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Cycloastragenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
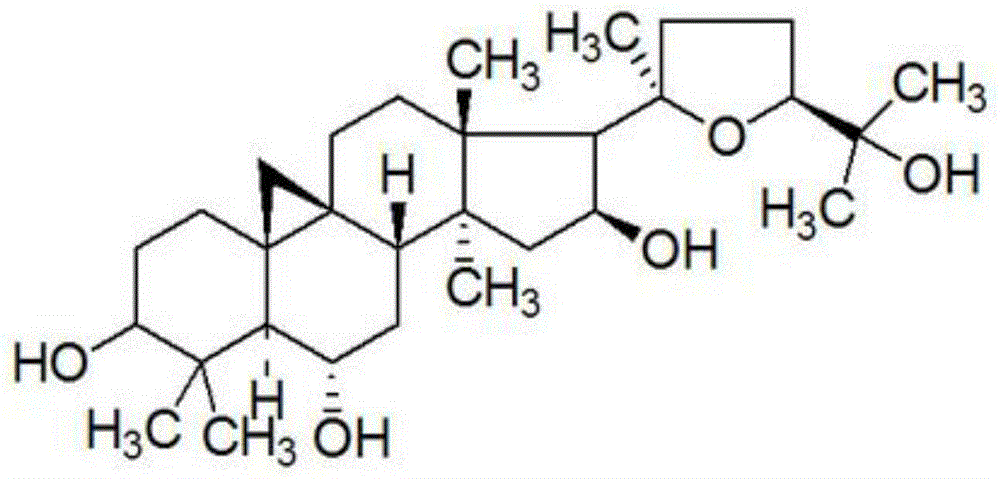
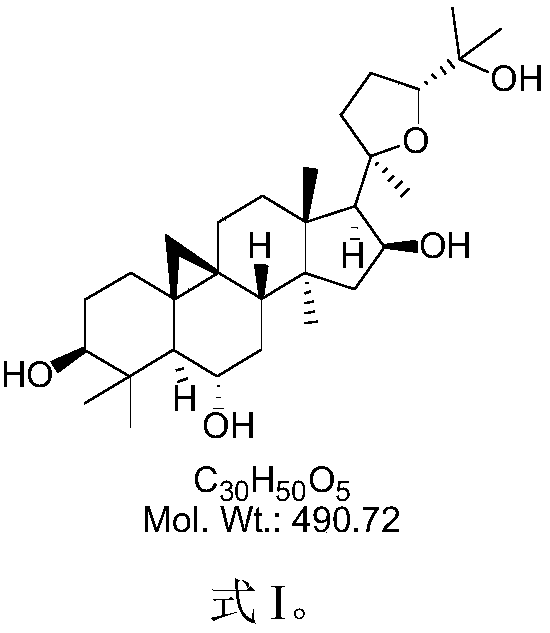
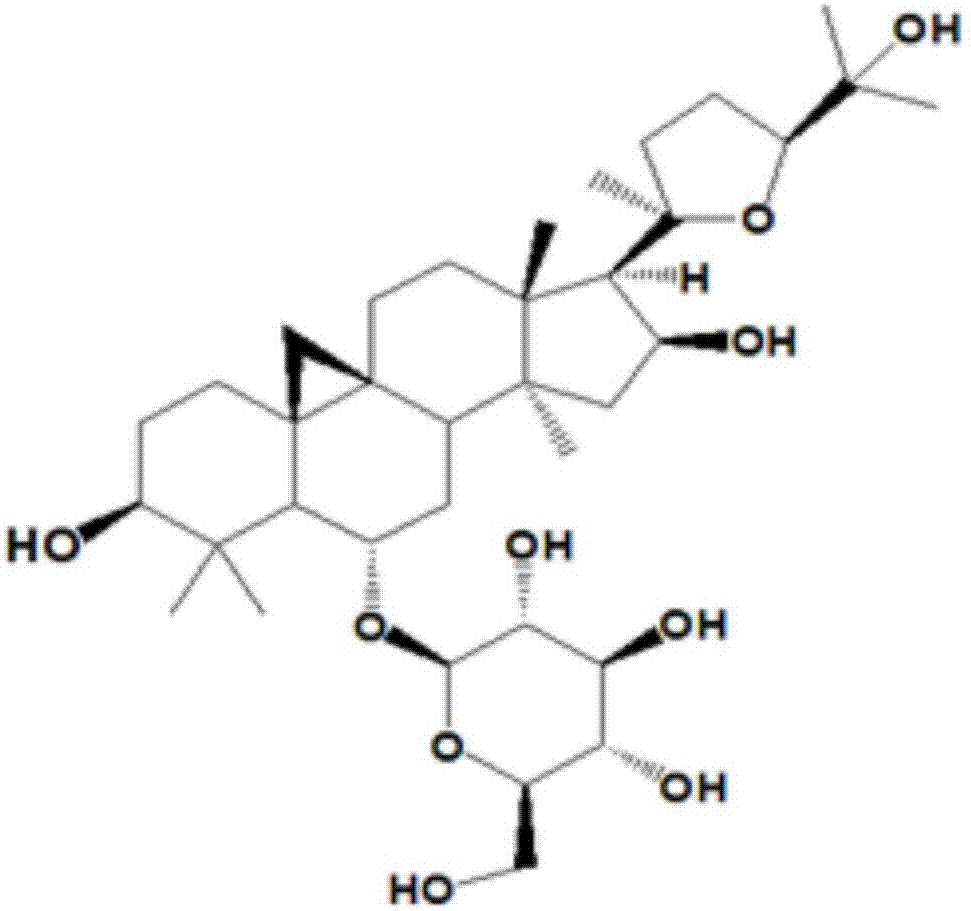
Cycloastragenol is a molecule isolated from various species in the genus Astragalus that is purported to have telomerase activation activity. The U.S. Federal Trade Commission issued an order preventing TA Sciences from advertising products containing cycloastragenol as "anti-aging". A single in vitro study on human CD4 and CD8 T cells led to claims that cycloastragenol may activate telomerase, leading to controversial claims for its role in reducing the effects of aging.
Preparation method and application of cycloastragenol
ActiveCN103880910AImprove qualityEfficient removalSteroidsAntineoplastic agentsAstragalosideAdjuvant
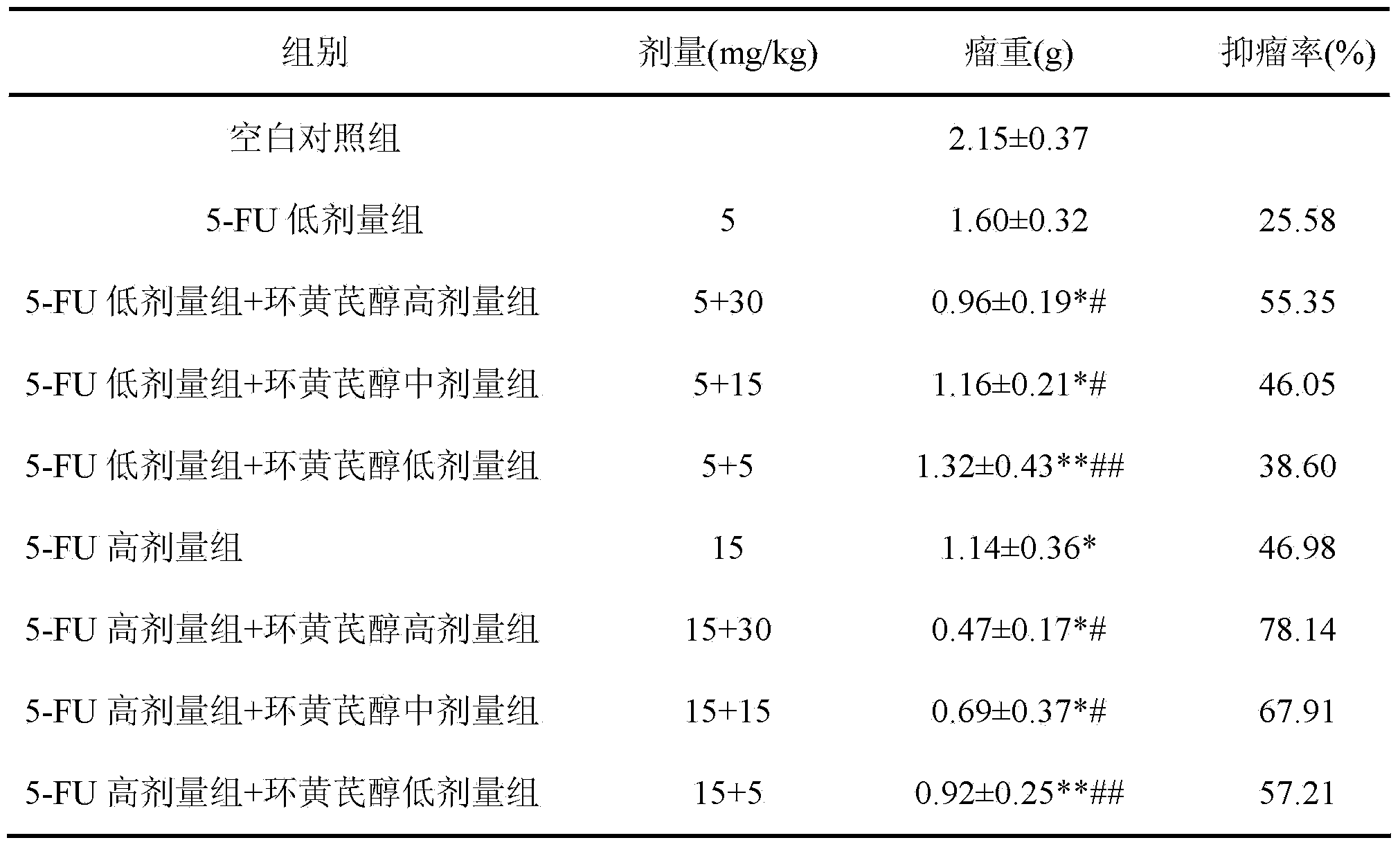
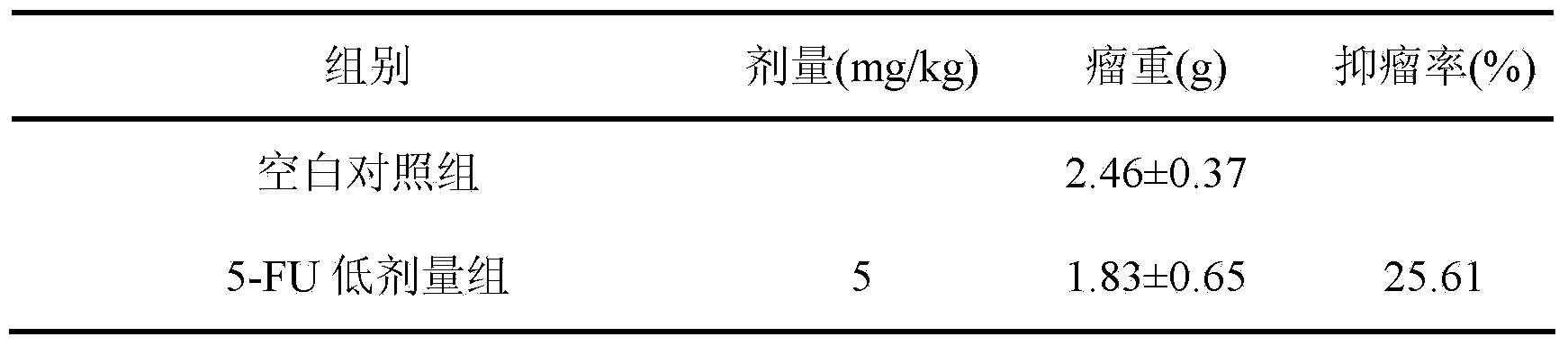
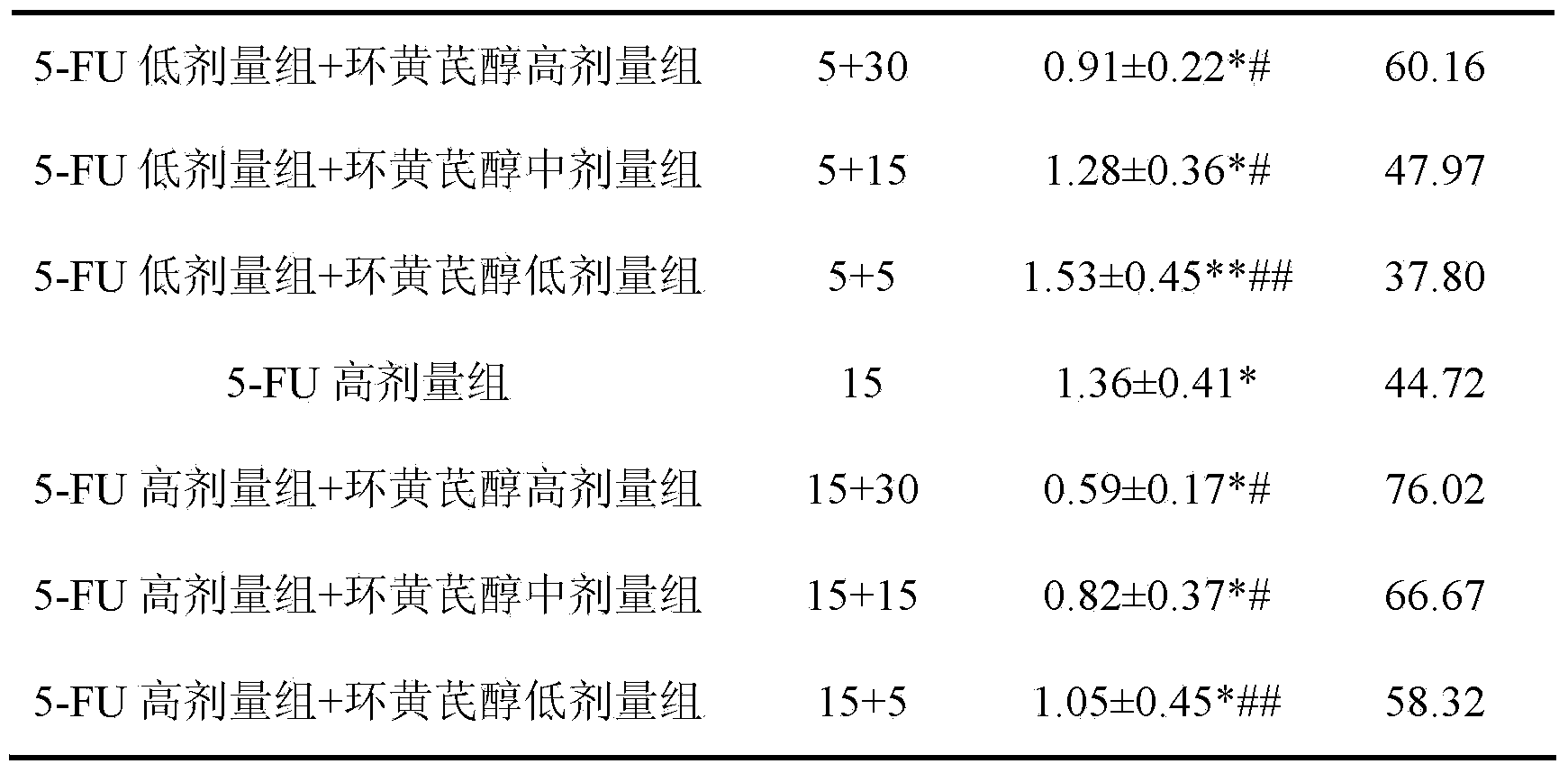
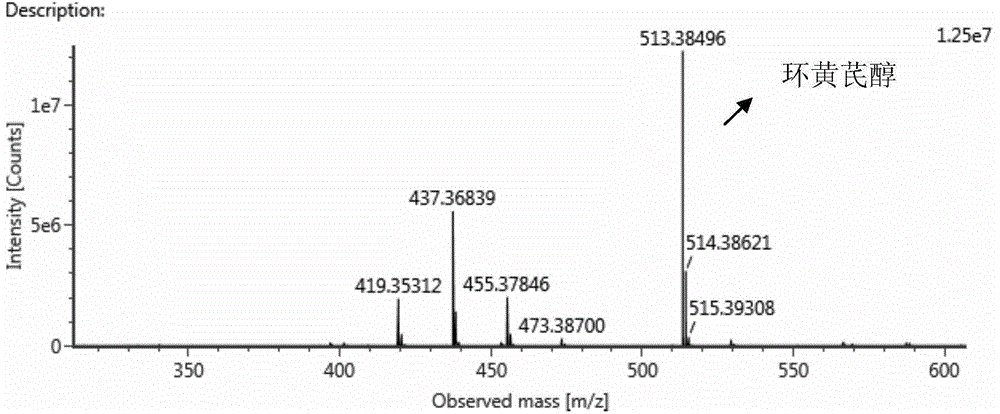
The invention discloses a preparation method and application of cycloastragenol. The preparation method of the cycloastragenol comprises the following steps: by using astragaloside or astrasieversianin as a raw material, oxidizing, reducing, hydrolyzing, extracting and purifying to obtain high-purity cycloastragenol. The method is simple to operate, gentle in condition, good in product quality and high in yield. The cycloastragenol can be applied to preparation of anticancer adjuvant therapeutic medicine. An anticancer adjuvane therapeutic medicine prepared by using the cycloastragenol as a pharmaceutical active ingredient has the anticancer adjuvant therapeutic effect of enhancing the anticancer therapeutic effect, reducing the toxicity of the anticancer medicine, preventing and treating the neutropenia caused by the anticancer medicine therapy.
Owner:SOUTHWEST JIAOTONG UNIV
Method for preparation of Cycloastragenol by sulfuric acid hydrolysis
The invention relates to a method for preparation of Cycloastragenol by sulfuric acid hydrolysis. The method provided by the invention utilizes sulfuric acid to hydrolyze the Chinese medicine astragalus crude extract astragaloside, and realizes conversion of astragaloside to Cycloastragenol under certain hydrolysis condition, then performs impurity separation on the Cycloastragenol crude extract obtained by acid hydrolysis by chloroform and n-butanol extraction, and further makes use of silica gel column chromatography purification to finally obtain high purity Cycloastragenol. The invention aims to provide the method that can overcome the disadvantages of complex production process, low yield, high cost and the like of Cycloastragenol production and is suitable for large-scale production of different purity Cycloastragenol.
Owner:BEIJING UNIV OF CHEM TECH
Producing and refining method for high-purity cycloastragenol
InactiveCN105734109AFew reaction stepsImprove conversion rateSteroidsFermentationAstragalosideHydrolysis
The invention relates to a method for producing and refining cycloastragenol with a purity greater than or equal to 95%. Using astragaloside IV as a raw material, through two-phase enzymatic hydrolysis, extraction, and recrystallization, cycloastragenol products with a purity greater than or equal to 95% are obtained. Compared with the existing cycloastragenol production method, the present invention has simple production process, mild reaction conditions, high reaction conversion rate, is more suitable for large-scale production, and solves the problem of astragaloside IV being hydrolyzed under acid-base conditions. The easy ring opening leads to the difficulty of generating astragalol.
Owner:CHENGDU KING TIGER PHARM CHEM TECH CO LTD
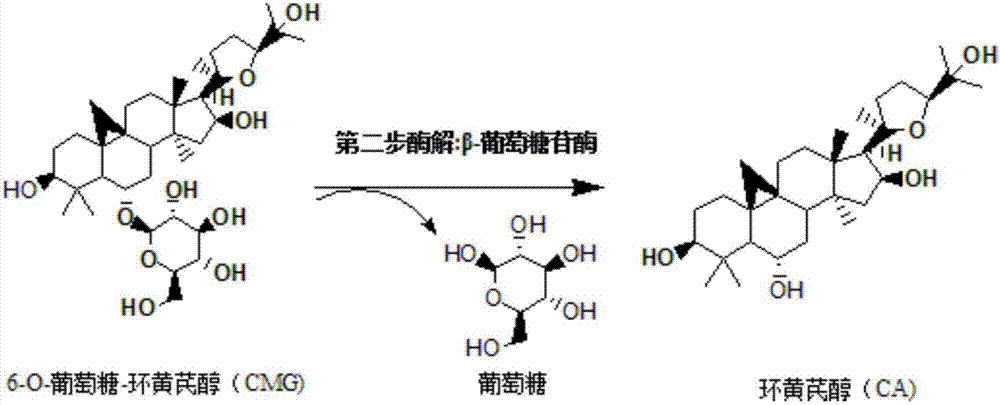
Method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis
The invention discloses a method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis; the method comprises the specific steps of using astragalosides as a substrate, using two different hydrolases to hydrolyze and break beta-xyloside bond at site C3 of the astragalosides and beta-glucoside bond at the site C6 respectively to obtain aglycone cycloastragenol of the astragalosides, extracting, performing silica-gel column chromatography, recrystallizing with ethanol, and purifying to obtain the finished cycloastragenol up to 95% and higher in purity. The problem that damage of three-membered ring structure of astragalosides during the preparation of cycloastragenol by a chemical process causes massive byproducts is solved, the defects of traditional cycloastragenol preparation methods, such as low substrate conversion rate, step complexity and environmental pollution, are overcome. The method has the advantages that substrate specificity is high, the substrate astragalosides is completely converted, the steps are simple, the conditions are mild, the cost is low, and the method is a mild biological preparation method, has no environmental pollution and is suitable for industrial production.
Owner:BEIJING UNIV OF CHEM TECH
Method for efficiently preparing cycloastragenol
InactiveCN105566434AMaintain biological activityHigh puritySteroidsFermentationRadix Astragali seu HedysariFermentation
The invention provides a method for efficiently preparing cycloastragenol. The method sequentially comprises the following steps of A, fermenting: filling total saponins of radix astragali seu hedysari into a fermentation tank, injecting a buffer solution containing compound enzyme into the fermentation tank until a sample is completely immersed, stirring and then performing catalytic fermentation; B, regulating alkali: after catalysis is ended, regulating fermentation liquid obtained in the step A into an alkali solution, and standing for one hour; C, performing counter-current extraction: pumping the fermentation liquid obtained in the step B and an extraction agent into a counter-current extraction tower for extracting; D, performing vacuum concentration on a mixed solution, which is obtained by extracting, of the cycloastragenol and the extraction agent. The method provided by the invention has the beneficial effects that firstly, a compound enzyme fermentation technology and a continuous counter-current extraction technology are combined for extracting and purifying to obtain high-purity cycloastragenol, and high conversion rate and higher purity of products are realized; secondly, the finishing time of the whole process does not exceed eight hours; the temperature is low in the operation process, so that biological activity of the cycloastragenol is guaranteed; the production efficiency is improved, energy consumption is reduced, and the production cost is reduced by 50 percent or above 50 percent.
Owner:安徽本森堂生物科技有限公司
Cycloastragenol extract and preparation method and application thereof
InactiveCN106083979ASuitable extraction methodGood reproducibilityAntinoxious agentsSteroidsAstragalosideAlcohol
The invention provides a cycloastragenol extract. A preparation method of the cycloastragenol extract comprises the steps that coarse astragalus membranaceus powder is extracted by using an alkaline alcohol water solution with a pH value of 11-13 to obtain a coarse astragaloside extract; the obtained coarse astragaloside extract is enriched and purified through macroporous resin to obtain an astragaloside extract; the obtained astragaloside extract is subjected to Smith degradation to obtain the cycloastragenol extract. For cycloastragenol extract preparation, an optimum extraction method is obtained through multiple times of tests conducted on the pH value, sodium periodate dosage and methanol concentration in the extraction conditions. The method is good in reproducibility, simple and easy to operate. The prepared cycloastragenol extract can obviously retard senility hurt of H2O2 to PC12 cells, and improve cell vitality, and the cycloastragenol extract obtained by adopting the preparation method has certain anti-aging activity and can be applied to preparation of anti-aging medicines and health-care products.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing cycloastragenol by means of biological conversion and degradation of astragaloside
ActiveCN106011213AHigh purityHigh transformation specificityMicroorganism based processesFermentationMicroorganismAstragaloside
The invention discloses a method for preparing cycloastragenol by means of biological conversion and degradation of astragaloside. The method is characterized in that astragaloside serves as a substrate, and cycloastragenol is prepared by means of fermentation of the strain of Absidia sp.CGMCC 3.2834. According to the method for preparing cycloastragenol by means of biological conversion and degradation of astragaloside, a microbiologic fermentation technology is adopted for degrading astragaloside to obtain high-purity cycloastragenol, the technology is simple and easy to carry out, the cost is low, the conversion rate of cycloastragenol reaches 60% or above, the purity reaches up to 95% or above, and the conversion specificity is high; the whole technological process is conducted at normal temperature and atmospheric pressure, the required equipment conditions are low, and the method is suitable for mass production.
Owner:安徽中鸣科技有限公司
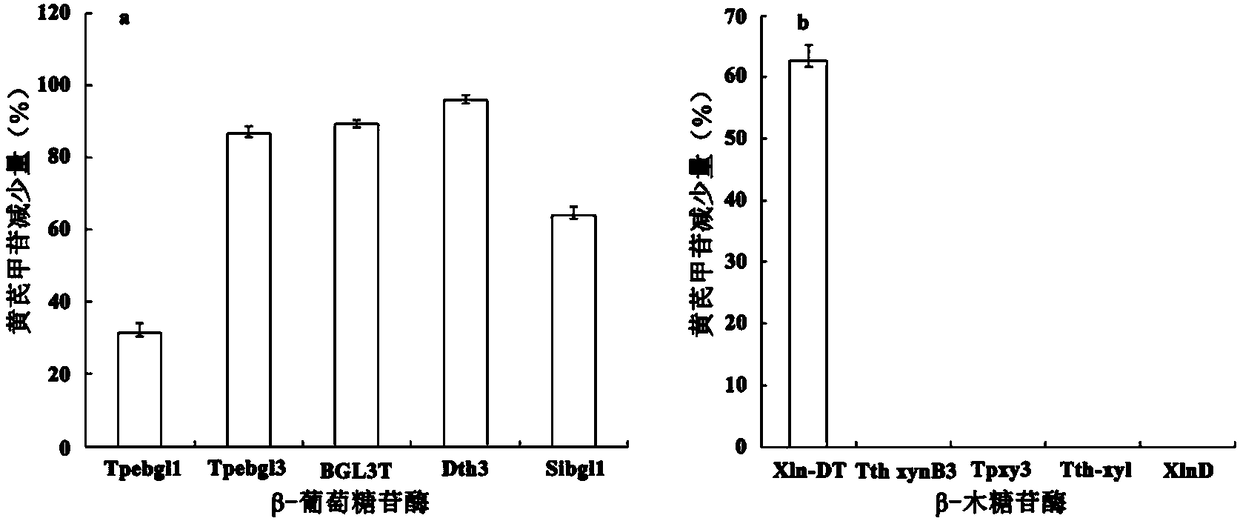
High-temperature-resistant compound enzyme and applications thereof
ActiveCN108384769AImprove heat resistanceImprove temperature stabilityFermentationGlycosylasesAstragalosideAlglucerase
The invention provides a high-temperature-resistant compound enzyme and applications of the high-temperature-resistant compound enzyme. The high-temperature-resistant compound enzyme is composed of beta-glucosidase and beta-xylosidase. The compound enzyme provided by the invention is strong in specificity, and can completely convert astragaloside, so that the conversion recovery rate of cycloastragenol is improved. The whole process is completed within 3h, the preparation process of cycloastragenol is greatly shortened, and the production efficiency is improved; in the reaction process, the reagents including strong reducing agents, oxidizing agents, strong acid and the like do not need to be added, so that the environmental pollution is reduced; self-making of the two enzymes can be realized, thus the production cost is greatly reduced, and the process is suitable for industrial production.
Owner:NANJING FORESTRY UNIV
Application of cycloastragenol in preparation of drugs for protecting liver and promoting liver injury restoration
ActiveCN105343109AImprove protectionGood treatment effectOrganic active ingredientsDigestive systemPharmaceutical drugPharmacology
The invention discloses an application of cycloastragenol in preparation of liver-protecting drugs or liver-caring drugs or liver injury treating drugs. Animal and cell experiments indicate that cycloastragenol has the effects of protecting liver, caring liver, and promoting liver injury restoration, thereby having strong application prospects.
Owner:TAIZHOU DANDING BIOTECH CO LTD
Uses of cycloastragenol in preparation of chronic renal failure treatment drugs
PendingCN106943410ADefinite curative effectFully functionalOrganic active ingredientsUrinary disorderPharmaceutical drugAglycone
The present invention belongs to the field of medicine, and particularly relates to uses of cycloastragenol in preparation of chronic renal failure treatment drugs. According to the present invention, cycloastragenol is the aglycone of astragaloside A, and has advantages in biological membrane permeation and gastrointestinal tract absorption compared to astragaloside A due to the relatively small molecular weight and the strong lipophilicity, and the test results show that cycloastragenol can change various physiologic indexes of chronic renal failure rates so as to provide great application prospects in the treatment of chronic renal failure.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing cycloastragenol by double-enzyme compounding conversion of astragaloside
ActiveCN111893158AIncreased substrate toleranceHigh substrate conversion rateMicroorganism based processesFermentationAstragalosideAlglucerase
The invention relates to the technical field of biotransformation, in particular to a method for preparing cycloastragenol through double-enzyme compounding transformation of astragaloside. Accordingto the method, the astragaloside is used as a substrate, xylosidase and glucosidase are subjected to double-enzyme compounding, then xyloside bonds at the C3 position of the substrate and glucoside bonds at the C6 position of the substrate are broken through one-step hydrolysis, and the cycloastragenol is obtained. The purity of the obtained cycloastragenol can reach 98% or above, and the method is easy to operate, free of pollution, milder in reaction temperature, clear in enzyme conversion mechanism, and wider in enzyme substrate adaptability and is suitable for industrial production.
Owner:WEIHAI BAIHE BIOTECH +1
Application of cycloastragenol derivatives to preparation of medicament with anti-hepatic-fibrosis effect
The invention discloses a category of cycloastragenol derivatives or pharmaceutically acceptable salt thereof and application thereof to preparation of an anti-hepatic-fibrosis medicament. According to the invention, a microbial conversion technology is utilized to successfully carry out structural modification on cycloastragenol, a plurality of novel compounds are obtained, and in-vitro anti-hepatic-fibrosis cell tests prove that the compounds have good anti-hepatic-fibrosis activities, can be used as active ingredients of the anti-hepatic-fibrosis medicament, and have wide application.
Owner:义乌国信白蚁防治有限公司
Derivative of cyclo membranousol kind and application thereof
InactiveCN100384830CGood water solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityAnti virus
The invention supplies eye-astragalus alcohols compounding ramification that could improve the water solubility of parent substance compounding. The invention could improve the biology availability of the drug, and make it possible to develop the constituent into oral dosing agent. It could be used in curing cardiovascular disease and cerebrovascular disease, myocarditis, nephritis, diabetes, rheumatism and hepatitis. It could be also used to anti virus.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
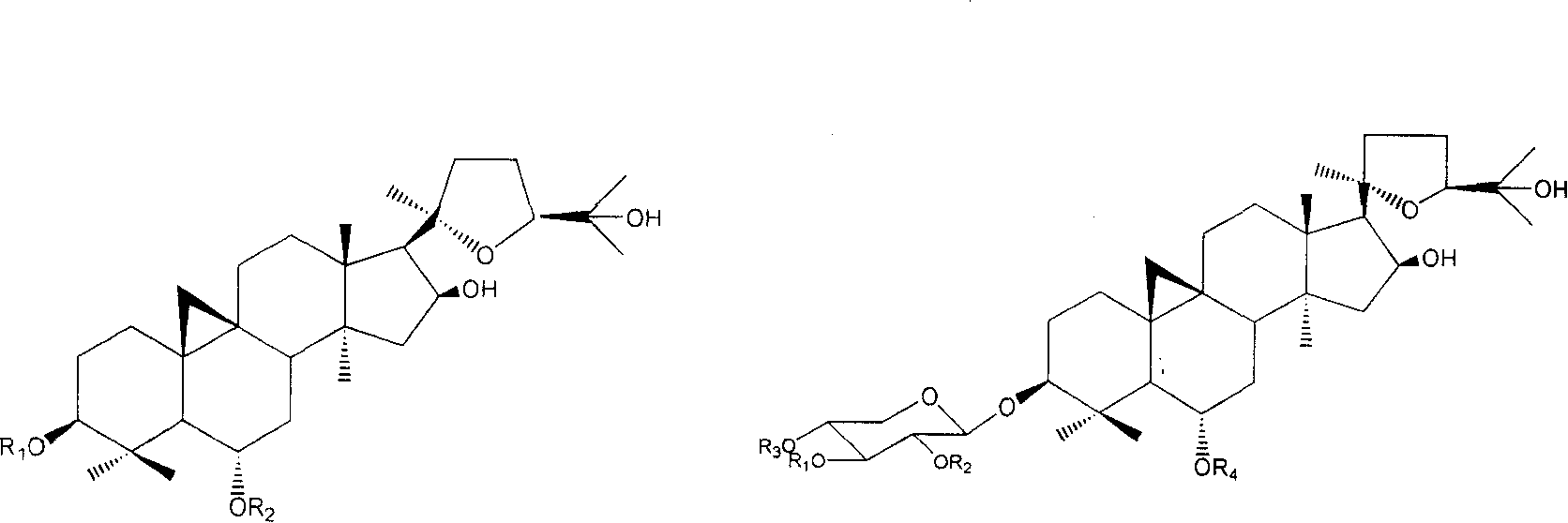
Cycloastragenol-6-O-beta-D glucoside monohydrate and crystal thereof
The invention provides cycloastragenol-6-O-beta-D glucoside monohydrate and a crystal thereof and preparation methods of the cycloastragenol-6-O-beta-D glucoside monohydrate and the crystal thereof and a drug composition. Studies show that the prepared cycloastragenol-6-O-beta-D-glucoside hydrate containing crystal water is white crystalline powder, can exist stably and is convenient to store and transport and to prepare drug preparations. The cycloastragenol-6-O-beta-D-glucoside containing crystal water is easier to dissolve in water and alcohols solvents, has good room temperature storage stability and lower moisture absorption and is convenient for preparing the directly dissolved and absorbed preparations.
Owner:天津天诚新药评价有限公司
Synthesis method of astragaloside
ActiveCN106831927APromoting active mechanism researchAdvance the development processOrganic chemistry methodsSteroidsAstragalosideSynthesis methods
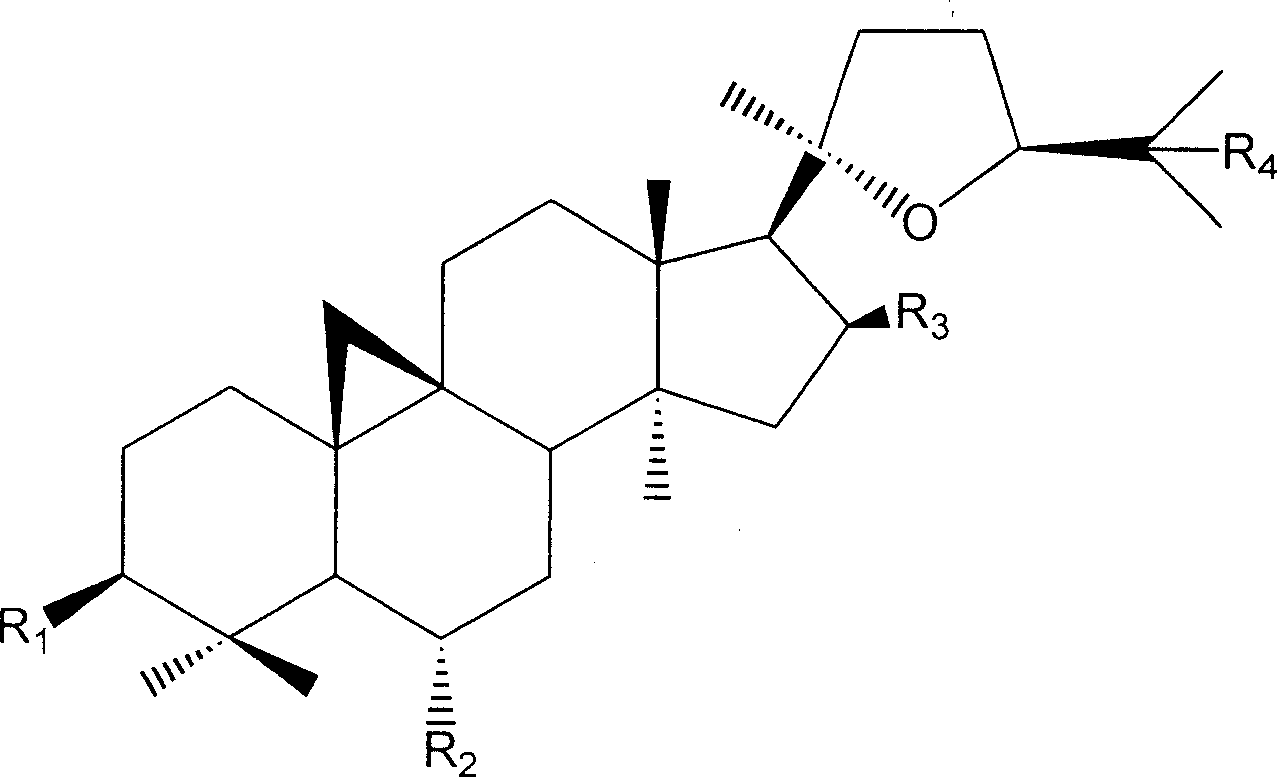
The invention discloses a synthesis method of astragaloside. The synthesis method of the astragaloside comprises the following steps of using protecting groups R1 for protecting 3-site hydroxy and 6-site hydroxy of cycloastragenol; after using protecting groups R2 for protecting 16- site hydroxy and 25- site hydroxy, removing the protecting groups R1 for the 3- site hydroxy and the 6- site hydroxy, and obtaining a compound 4; using a protecting group R3 for protecting 3- site hydroxy of the compound 4, and obtaining a compound 5; carrying out glycosylation on the compound 5, then removing the protecting group R3 for the 3- site hydroxy, continuously carrying out glycosylation, and obtaining a compound 8; removing all protecting groups to obtain the astragaloside. According to the synthesis method of the astragaloside provided by the invention, the astragaloside is high-efficiently and high-stereoselectively prepared, a gap in the prior art is filled, and the progresses of the activity mechanism research of astragalus membranaceus saponins and the medicinal development thereof are greatly improved.
Owner:JIANGXI NORMAL UNIVERSITY
Method for preparing cycloastragenol through microorganism mixed fermentation, transformation and degradation of astragaloside iv
InactiveCN109609581AHigh purityImprove conversion rateMicroorganism based processesFermentationMicroorganismHigh volume manufacturing
The invention relates to a method for preparing cycloastragenol through microorganism mixed fermentation, transformation and degradation of astragaloside iv. Two strains of fusarium proliferatum 0821(CGMCC NO.16482 ) and Fusarium verticillioides 0213 (CGMCC NO.16481 ) which are screened out are used, the astragaloside iv is used as a substrate, and through mixed fermentation of the two strains, the cycloastragenol is prepared. A technique for mixed fermentation with two microorganisms is adopted to degrade the astragaloside iv so as to obtain the cycloastragenol high in purity. The technological flow is simple, the cost is low, and the entire technological process is performed at normal temperature and normal pressure. The required equipment condition is low, the method is suitable for mass production, the conversion rate of the final cycloastragenol is as high as 80% or above, and the purity is as high as 95% or above.
Owner:BEIJING UNIV OF CHEM TECH
A kind of preparation method and application of cycloastragenol
ActiveCN103880910BImprove qualityEfficient removalSteroidsAntineoplastic agentsAstragalosideAdjuvant
The invention discloses a preparation method and application of cycloastragenol. The preparation method of the cycloastragenol comprises the following steps: by using astragaloside or astrasieversianin as a raw material, oxidizing, reducing, hydrolyzing, extracting and purifying to obtain high-purity cycloastragenol. The method is simple to operate, gentle in condition, good in product quality and high in yield. The cycloastragenol can be applied to preparation of anticancer adjuvant therapeutic medicine. An anticancer adjuvane therapeutic medicine prepared by using the cycloastragenol as a pharmaceutical active ingredient has the anticancer adjuvant therapeutic effect of enhancing the anticancer therapeutic effect, reducing the toxicity of the anticancer medicine, preventing and treating the neutropenia caused by the anticancer medicine therapy.
Owner:SOUTHWEST JIAOTONG UNIV
Monoglucoside injection of cycloastragenol and preparation method and application of injection
InactiveCN102652731ASatisfy pharmacology and toxicologyFulfil requirementsOrganic active ingredientsPharmaceutical delivery mechanismSolventGlucoside
The invention provides monoglucoside injection of cycloastragenol and a preparation method and application of the injection. The injection consists of monoglucoside of the cycloastragenol, non-aqueous solvent, solubilizer and water for injection. According to the injection, the solubilizer and the non-aqueous solvent are utilized to perform combined solubilization on the single glucoside of the cycloastragenol; and compared with the single solubilization of the non-aqueous solvent, the combined solubilization can be used for reducing the dosage of the non-aqueous solvent, relieving the stimulus of the non-aqueous solvent on a patient, and effectively avoiding the medicine precipitation in a medicine diluting process.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for promoting in-vitro amplification of umbilical cord blood T cell and maintaining high-proportion TSCM subpopulation
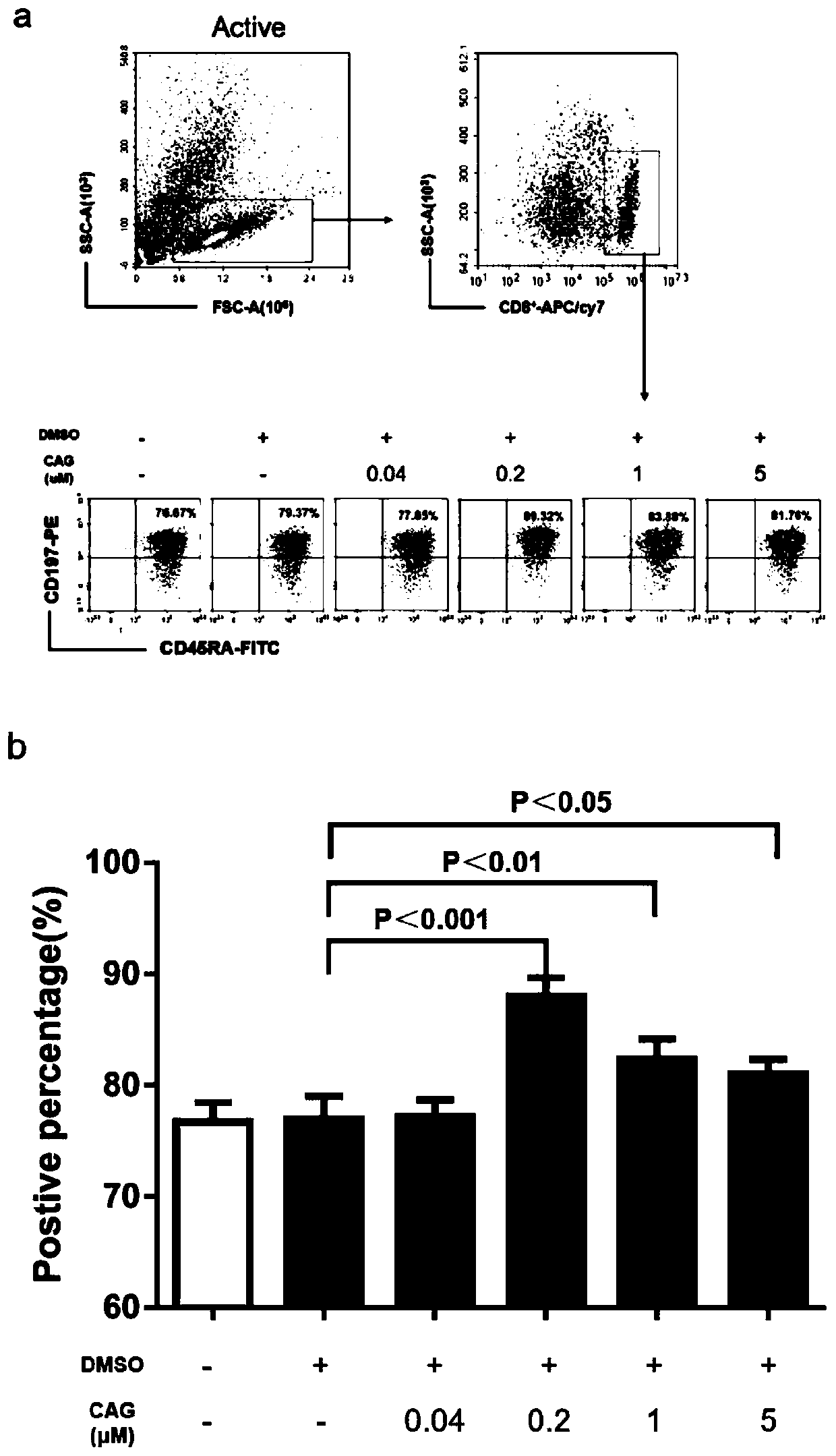
InactiveCN109943527APromote amplificationRaise the ratioBlood/immune system cellsCAR T-cell therapyCulture fluid
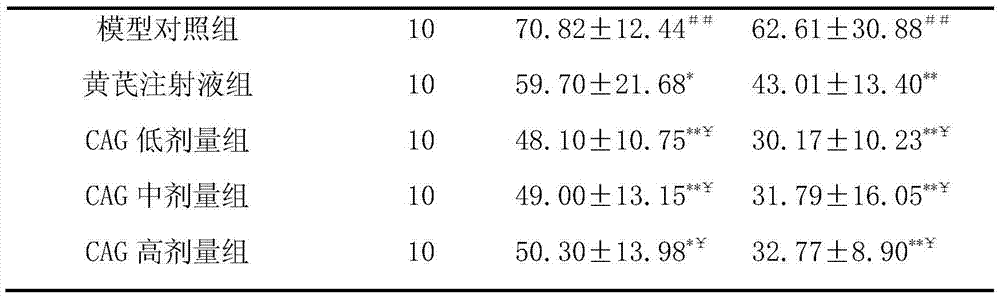
The invention discloses a method for promoting in-vitro amplification of an umbilical cord blood T cell and maintaining a high-proportion TSCM subpopulation. The method comprises the steps that cycloastragenol, a cell factor IL-7 and a cell factor IL-15 are combined and added into an in-vitro culture solution of the T cell to promote the amplification of the T cell and maintain the high-proportionTSCM subpopulation. The method for promoting the in-vitro amplification of the umbilical cord blood T cell and maintaining the high-proportion TSCM subpopulation has the advantages that the high-proportion TSCM cell subpopulation can be maintained while the low-concentration CAG combined with the IL-7 and the IL-15 to promote the amplification of the umbilical cord blood derived T cell is successively explored; the novel in-vitro culture method lays an experimental foundation for the in-vitro long-term culture of the T cell and an immunization therapy containing a CAR-T cell therapy.
Owner:四川药智联恒科技有限公司
Complex microbial inoculant for regulating gynecological micro-ecological balance
ActiveCN111281896AIngredient safetyEasy to useAntibacterial agentsMilk preparationBiotechnologyLactobacillus salivarius
The invention discloses a complex microbial inoculant for regulating gynecological micro-ecological balance. The complex microbial inoculant is prepared from lactobacillus crispatus, lactobacillus salivarius SIL1 (Lactobacillus salivarius SIL1) and other acceptable drug excipients. The invention further discloses a preparation method of the complex microbial inoculant. The preservation number of the Lactobacillus crispatus strain is CGMCC No. 6364, and the preservation number of the lactobacillus salivarius SIL1 is CCTCC M2010281. The complex microbial inoculant can be prepared into oral liquid or fermented food, preferably capsules, bacterial powder, milk tablets, fermented food and the like. Preferably, the complex microbial inoculant also comprises cycloastragenol.
Owner:KUIMING JIAJIANING BIOLOGICAL PROD CO LTD
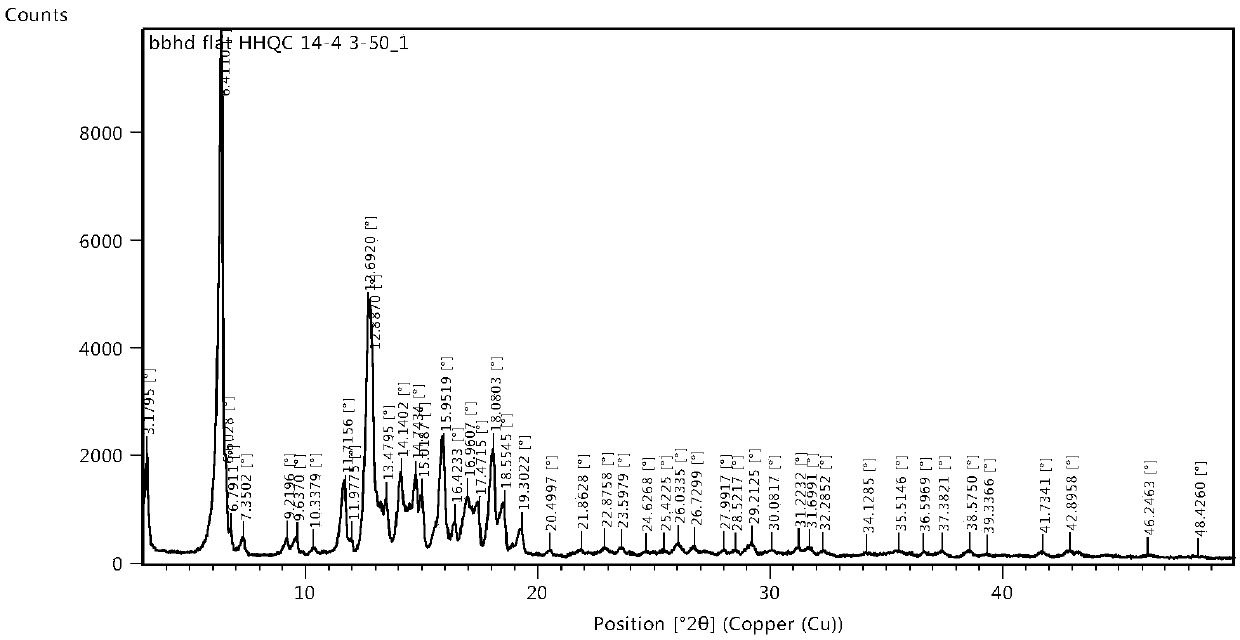
Cycloastragenol crystal form B and preparation method thereof
PendingCN111377999AHigh purityGood chemical stabilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistryPharmaceutical formulation
The invention provides a cycloastragenol crystal form B as well as a preparation method and application thereof. The cycloastragenol crystal form B provided by the invention has higher purity and better chemical stability, and a better dissolution and release effect can be achieved when the crystal form B is prepared into a pharmaceutical preparation, and meanwhile, the preparation method of cycloastragenol is simple, convenient and easy to industrialize.
Owner:LUNAN PHARMA GROUP CORPORATION
Cycloastragenol crystal form E and preparation method thereof
PendingCN111378001AHigh purityGood chemical stabilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistryDrugs preparations
The invention belongs to the technical field of organic chemical drug preparation, and provides a cycloastragenol crystal form E as well as a preparation method and application thereof. The cycloastragenol crystal form E provided by the invention has higher purity and better chemical stability, the crystal form E has better dissolution and release behaviors when being prepared into a pharmaceutical preparation, and meanwhile, the preparation method of the cycloastragenol is simple, convenient, easy to industrialize and high in production adaptability.
Owner:LUNAN PHARMA GROUP CORPORATION
Cycloastragenol new crystal form A and preparation method thereof
PendingCN111378002AExcellent physical and chemical propertiesImprove stabilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistryDrugs preparations
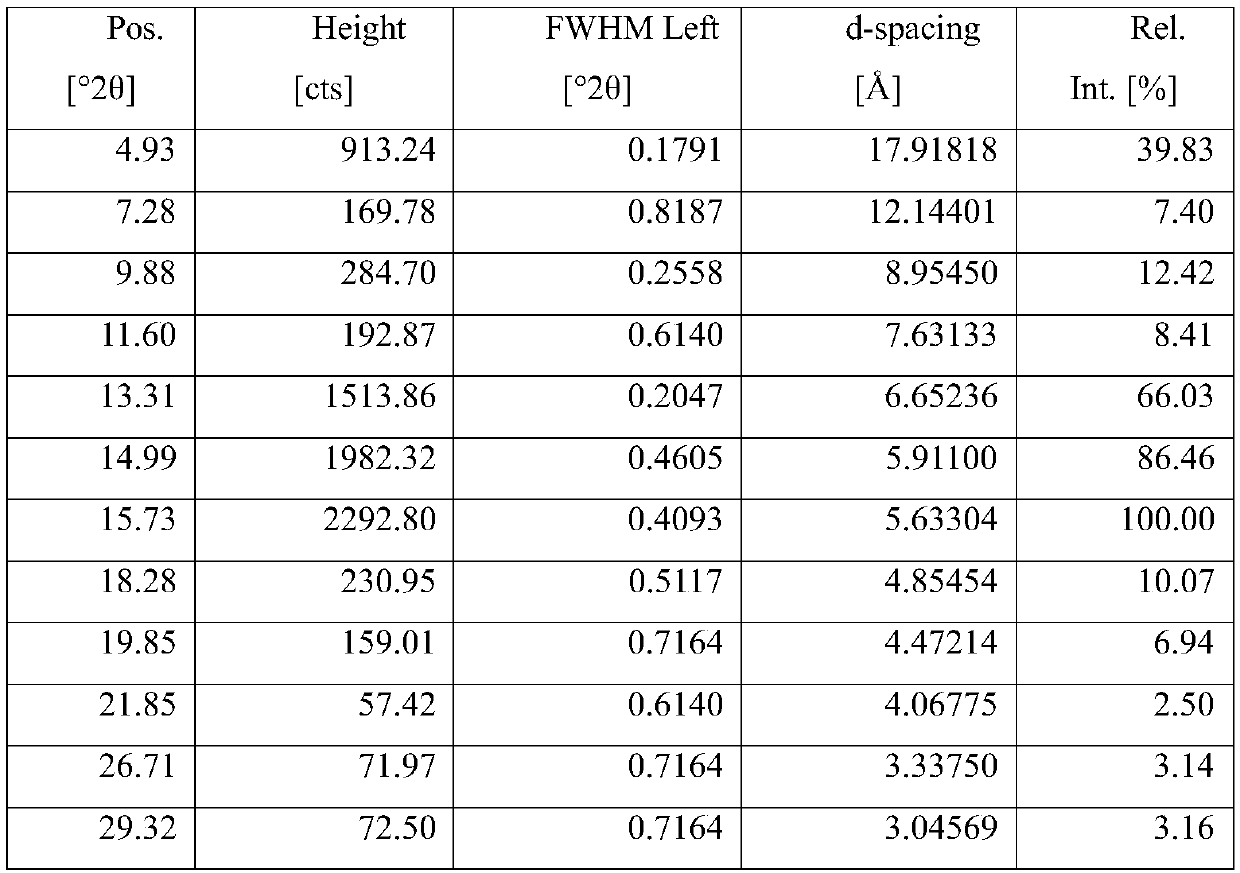
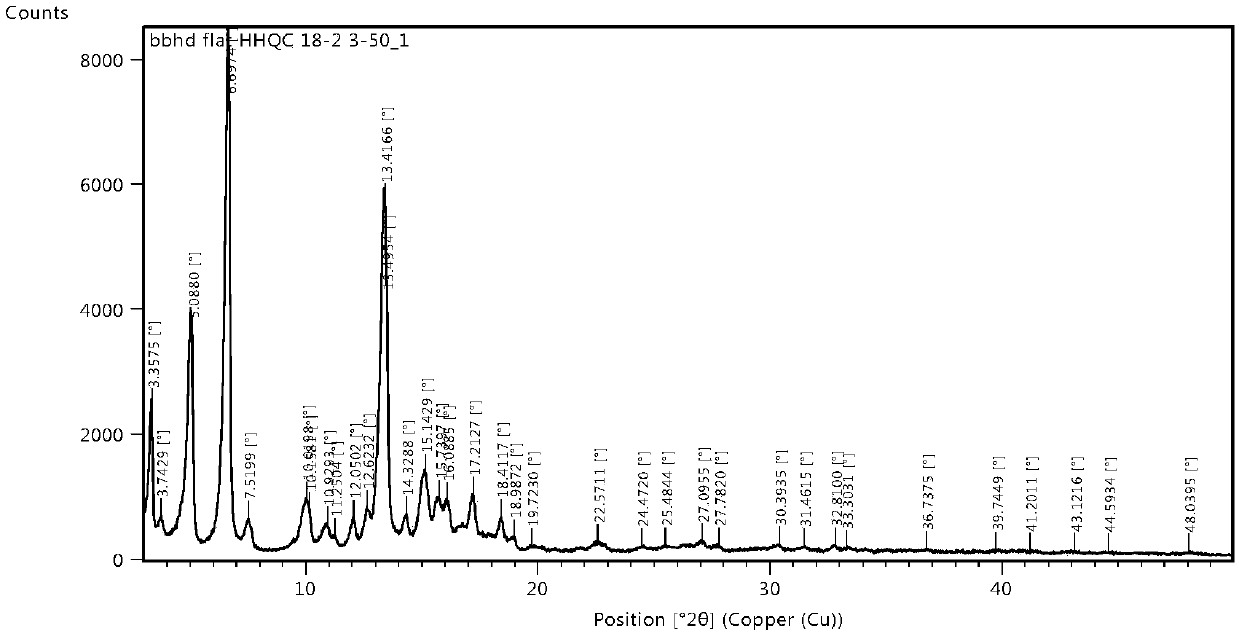
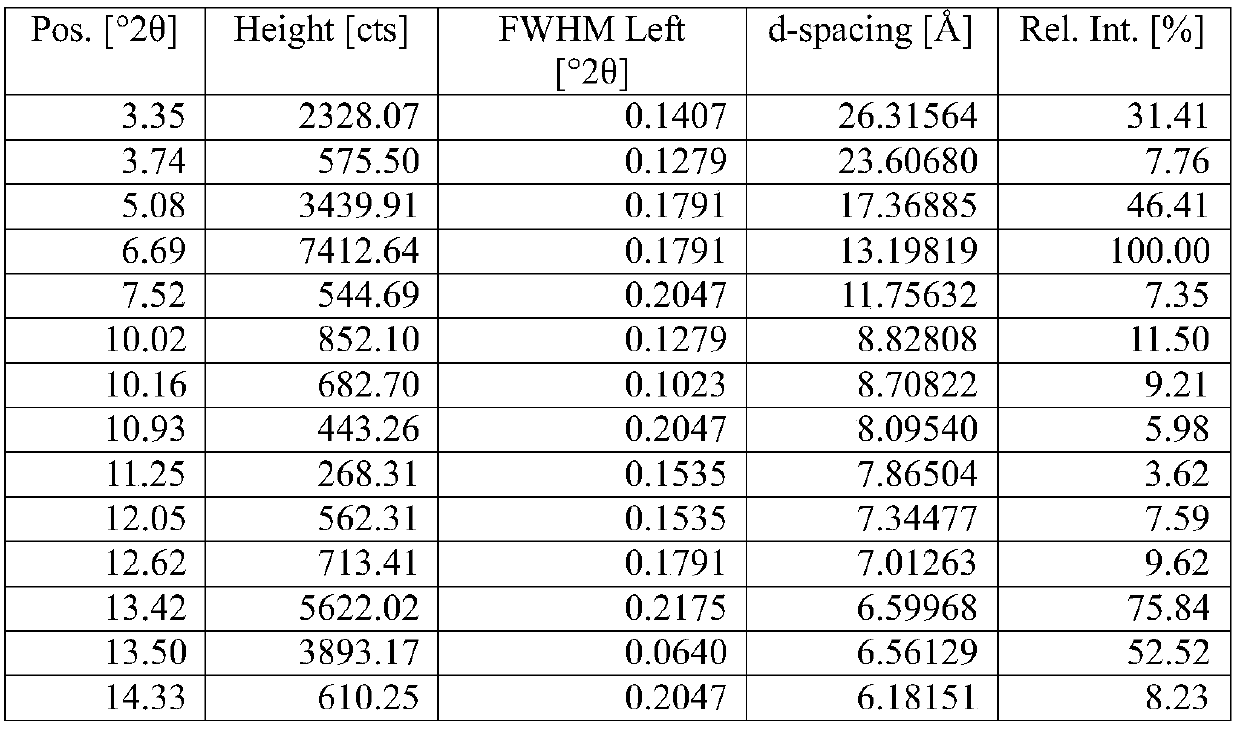
The invention belongs to the technical field of organic chemical medicine preparation, and specifically relates to a cycloastragalol crystal form A and a preparation method thereof. The crystal form uses Cu-Kalpha radiation, and X-ray powder diffraction (X-RPD) represented by a 2theta angle has characteristic diffraction peaks when 2theta is equal to 4.9 + / -0.2 degrees, 9.8 + / -0.2 degrees, 13.2 + / -0.2 degrees, 15.7 + / -0.2 degrees and the like. The crystal form has good chemical stability and crystal form purity, large-scale preparation is easy, and the preparation method is simple to operate,can be better suitable for preparation of pharmaceutical preparations and large-scale production, and has broad application prospects.
Owner:LUNAN PHARMA GROUP CORPORATION
Cycloastragenol crystal form C and preparation method thereof
PendingCN111377998AHigh purityGood chemical stabilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistryDrugs preparations
The invention belongs to the technical field of organic chemical drug preparation, and provides a cycloastragenol crystal form C as well as a preparation method and application thereof. The cycloastragenol crystal form C provided by the invention has higher purity and better chemical stability, the crystal form C has better dissolution and release effects when being prepared into a pharmaceuticalpreparation, and meanwhile, the preparation method of cycloastragenol is simple, convenient, easy to industrialize and high in production adaptability.
Owner:LUNAN PHARMA GROUP CORPORATION
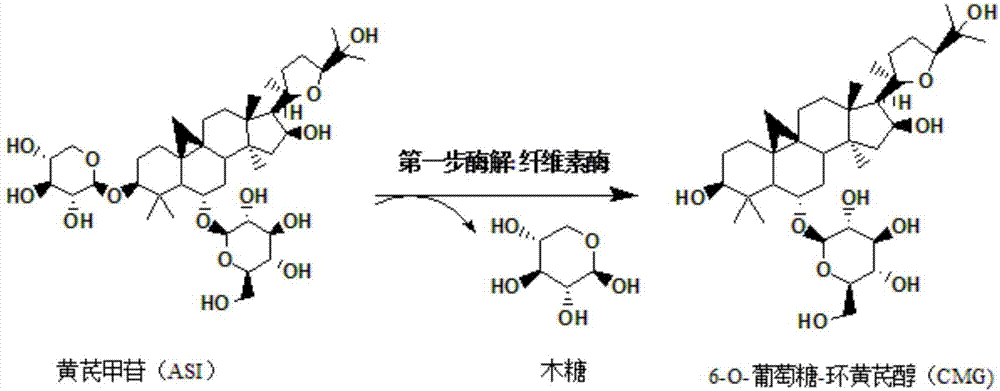
Method for preparing 6-O-glucose-cycloastragenol by transforming astragaloside through aspergillus carbonarius
ActiveCN107034261AWon't happenImprove conversion efficiencyMicroorganism based processesFermentationAstragalosideMicrobial transformation
The invention discloses a method for preparing 6-O-glucose-cycloastragenol by transforming astragaloside through aspergillus carbonarius, and particularly relates to a method for preparing the 6-O-glucose-cycloastragenol in a transformed manner by using the astragaloside as a substrate to ferment through aspergillus carbonarius. CICC 41254 on the premise of using a proper amount of astragalus root powder as an inducer. Fermentation liquor obtained by fermentation is extracted through water saturated n-butyl alcohol, is subjected to column chromatography on silica gel and is recrystallized with ethanol, and finally, the 6-O-glucose-cycloastragenol product with the purity being 95% or above is obtained finally. The 6-O-glucose-cycloastragenol is prepared by a microbe transformation method. The method has the characteristics that the astragaloside as a substrate is almost transformed totally, the transformation rate is high, the method is simple and practicable, the cost is low, and the method is pollution-free to environment, and is suitable for industrialized production.
Owner:BEIJING UNIV OF CHEM TECH
Method for preparing cycloastragenol by catalyzing astragaloside through co-immobilized double enzymes
ActiveCN111849959AReduce manufacturing costSimple processFermentationOn/in inorganic carrierAstragalosideAlglucerase
The invention belongs to the technical field of catalysis, and provides a method for preparing cycloastragenol by catalyzing astragaloside through co-immobilized double enzymes. The method comprises the following steps of: carrying out a reaction among beta-glucosidase, a phosphate buffer solution, a Fe3O4 solution, a copper chloride solution and xylosidase to obtain the co-immobilized double enzymes; and carrying out a catalytic reaction by using co-immobilized double enzymes and astragaloside to obtain cycloastragenol. The method provided by the invention overcomes the problem of extractionand separation of intermediates in a process for preparing cycloastragenol by adopting a two-step reaction in the prior art, and meanwhile, the co-immobilized double enzymes can be recycled, so that the production cost is reduced, the process is simple, economic and economical, and the method is very suitable for large-scale industrial production. The conversion rate of the co-immobilized double enzymes to astragaloside basically reaches 100%, and the substrate astragaloside is completely converted into cycloastragenol. The purity of the cycloastragenol product obtained by the method can reach78.3% or above.
Owner:WEIHAI BAIHE BIOTECH +1
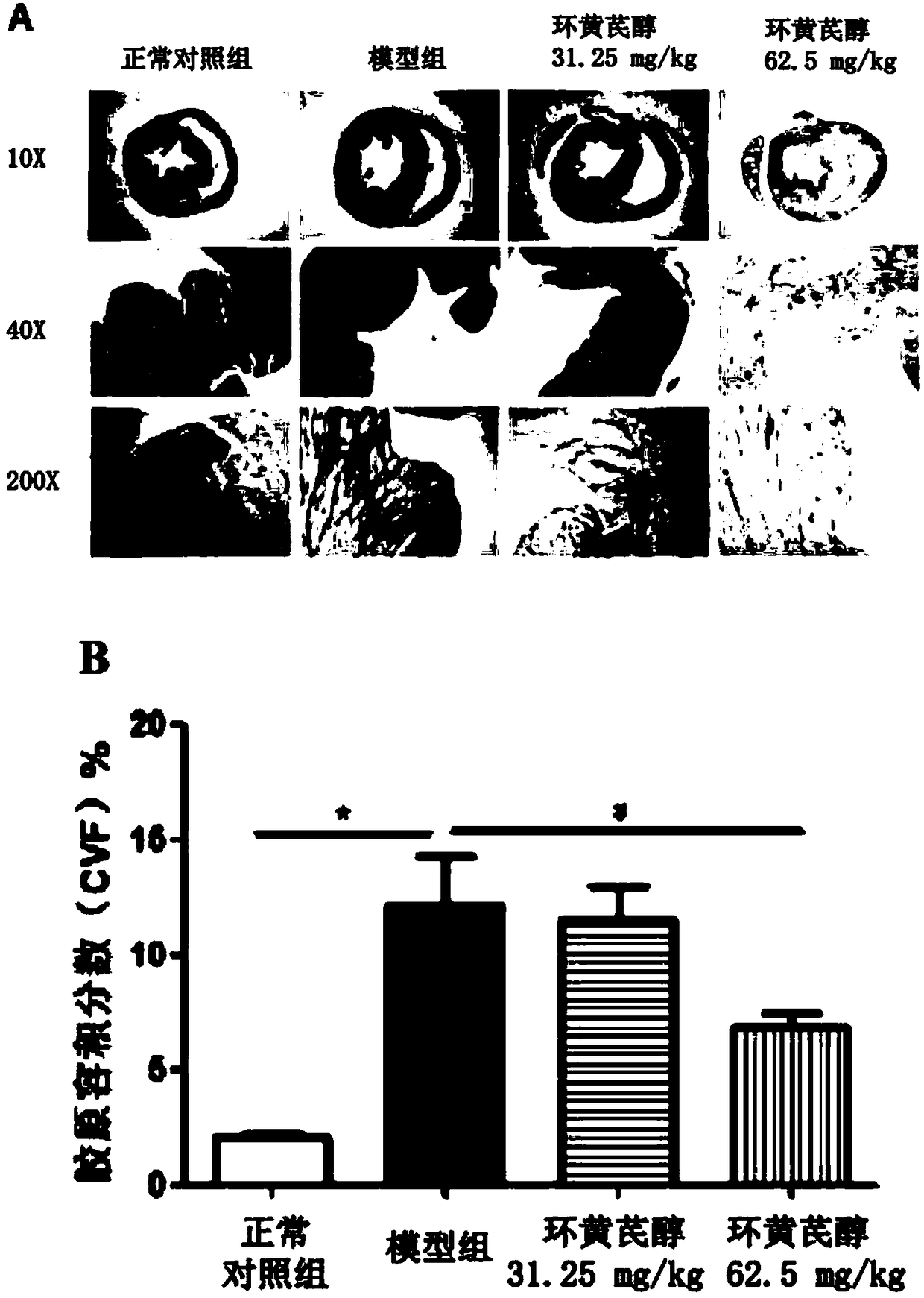
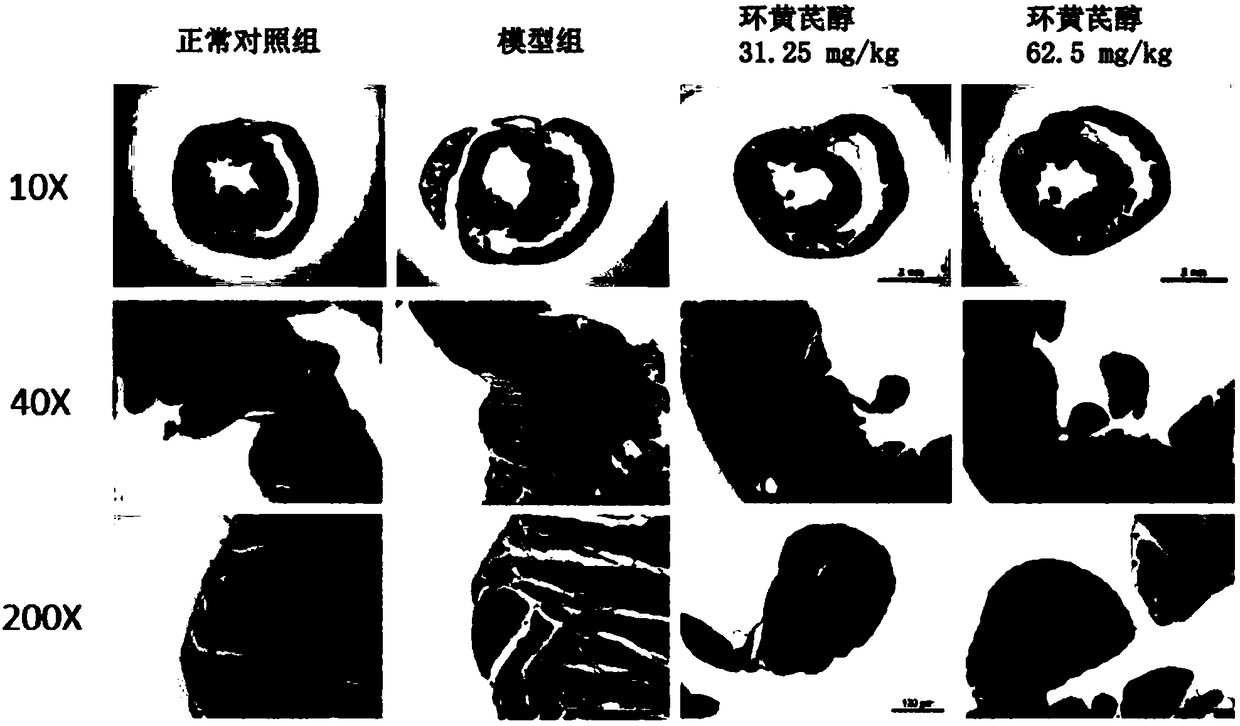
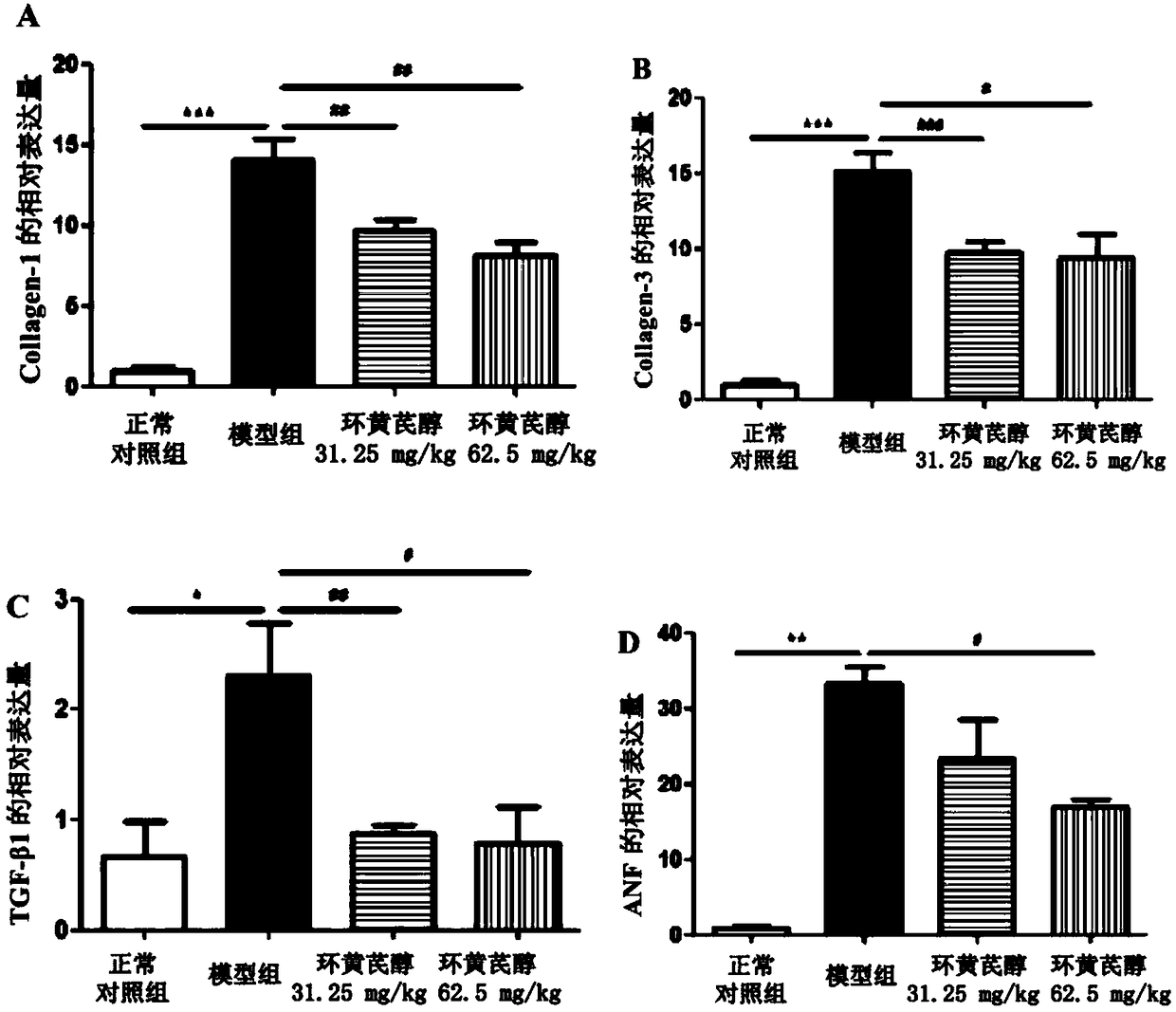
Application of cycloastragenol in prevention and treatment of myocardial fibrosis
InactiveCN108992452AElevated volume fractionNo symptoms of irritating dry coughOrganic active ingredientsCardiovascular disorderIrritationFactor ii
The invention discloses an application of cycloastragenol in prevention and treatment of myocardial fibrosis. The cycloastragenol is applied to one of the following steps (a1) and (a2): (a1) preparinga product for preventing and / or treating myocardial fibrosis; (a2) preventing and / or treating myocardial fibrosis. The cycloastragenol can effectively inhibit a myocardial collagen volume fraction increase, an expression level increase of myocardial fibrosis marker genes in myocardial tissues, an mRNA expression level increase of ANF genes in myocardial tissues, an expression level increase of related inflammatory cytokine genes and the degree of infiltration of inflammatory cells in the heart of the body due to the myocardial fibrosis, and the symptom of irritating dry cough does not occur in experiments of the cycloastragenol. In summary, the cycloastragenol plays an important role in preventing and treating the myocardial fibrosis, and has a great application prospect in the preventionand treatment of the myocardial fibrosis.
Owner:PEKING UNIV
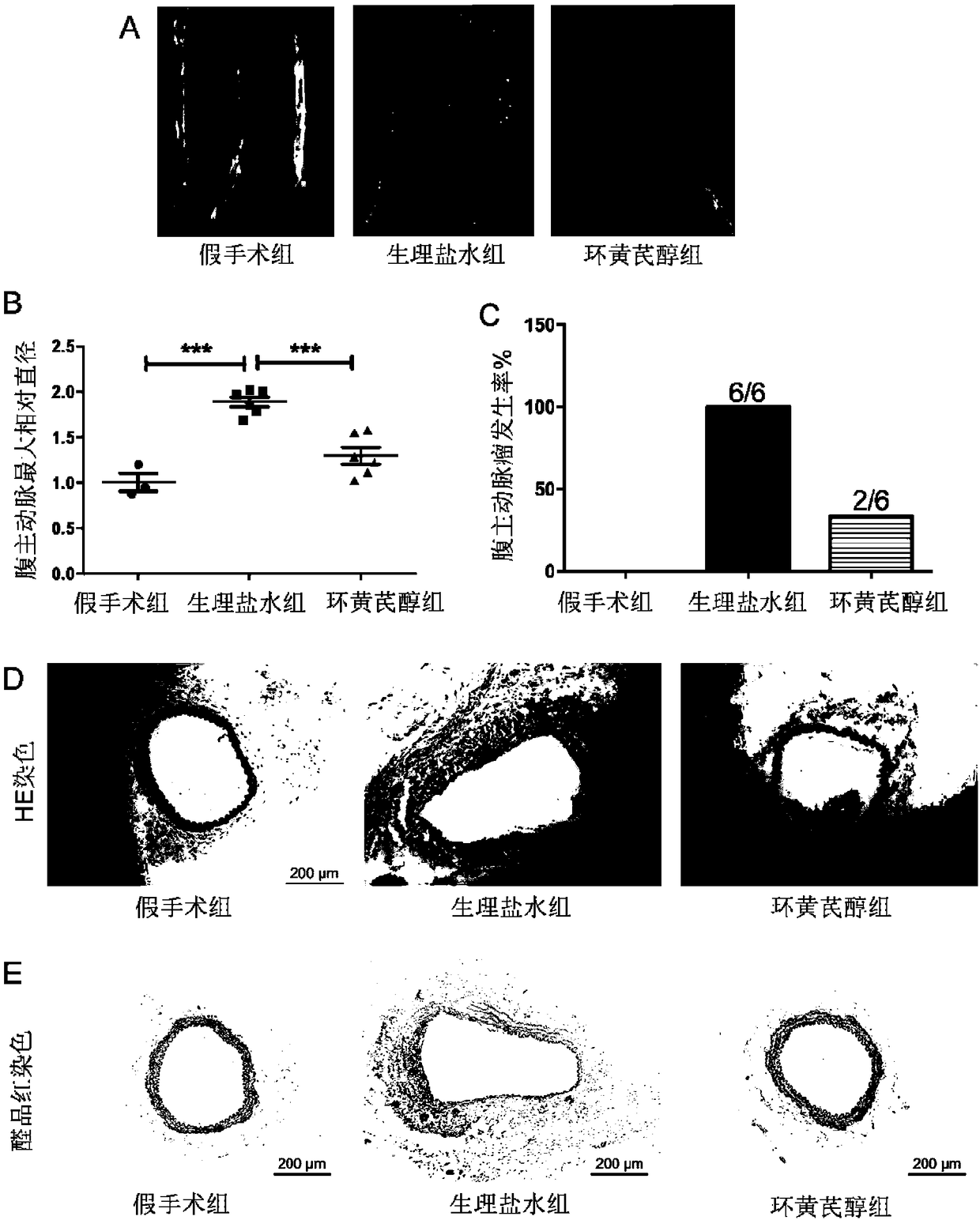
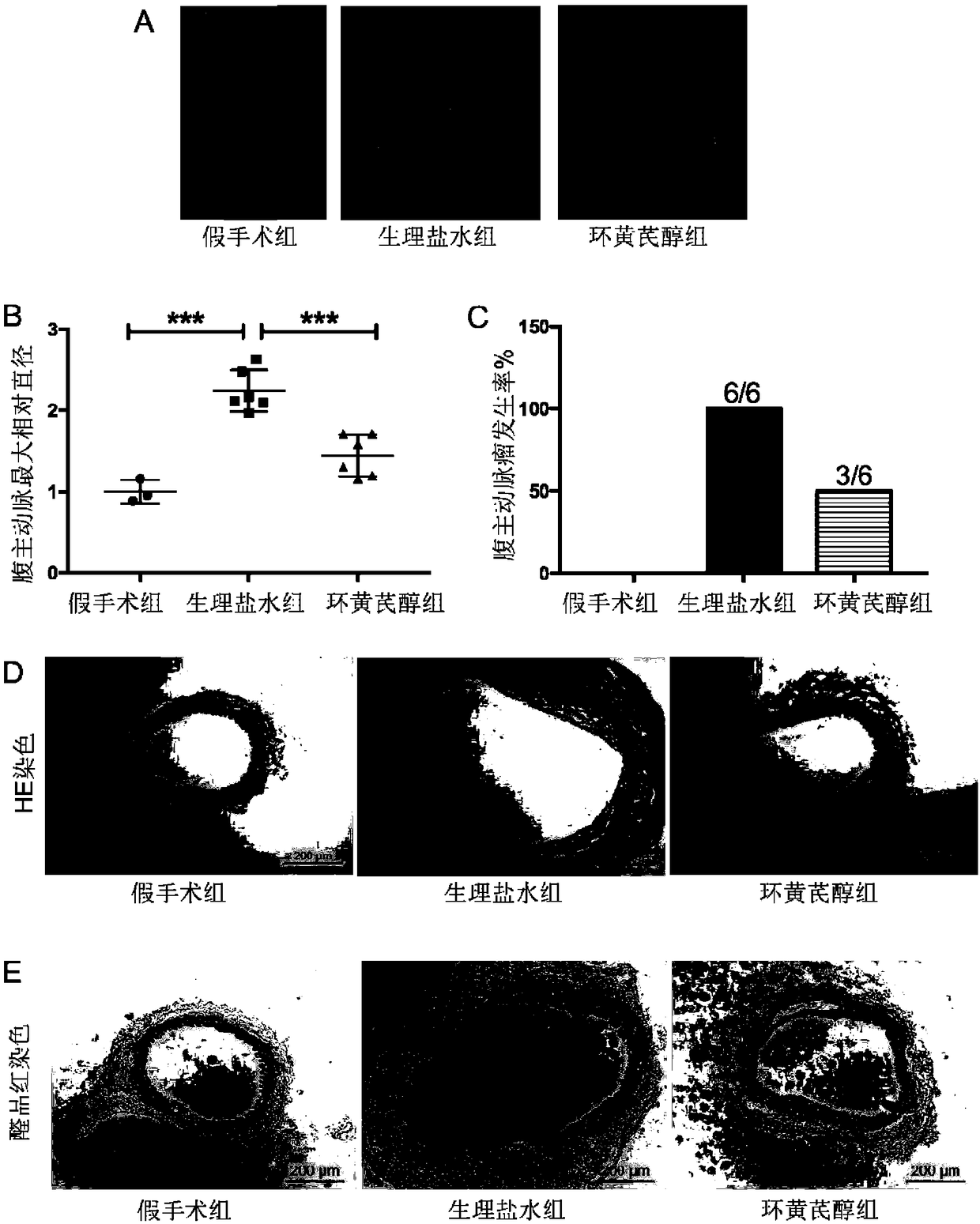
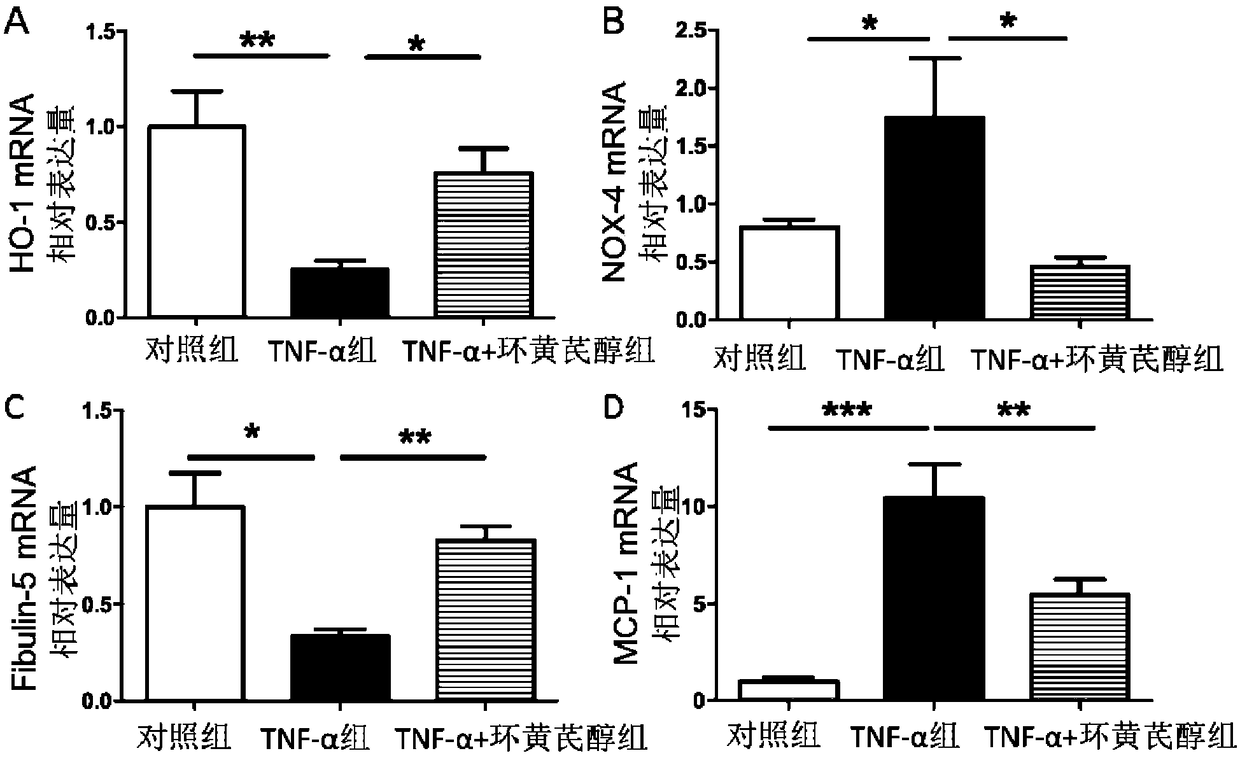
Application of cycloastragenol to preparation of drug for inhibiting abdominal aortic aneurysm
ActiveCN108498521AEnhance pharmacological effectsWide variety of sourcesOrganic active ingredientsAntinoxious agentsDiseaseAstragaloside
The invention discloses application of cycloastragenol to preparation of a drug for inhibiting abdominal aortic aneurysm. The invention provides application of cycloastragenol to preparation of products for preventing and / or treating abdominal aortic aneurysm, and / or to prevention and / or treatment abdominal aortic aneurysm. The invention also provides a drug for preventing and / or treating abdominal aortic aneurysm. The active component of the drug is cycloastragenol. The drug for inhibiting abdominal aortic aneurysm is safe and has low toxicity and strong pharmacological action; the raw material of the drug is widely available and low in price, and can be prepared through hydrolysis of the astragalus extract astragaloside; a preparation method for the drug is low in cost, simple in processand high in yield; and the curative effect of the drug is definite. According to the invention, a novel drug source is provided for preventing, diagnosing, detecting, protecting, treating and researching abdominal aortic aneurysm diseases, and is easy to promote and apply and capable of generating enormous social and economic benefits in a short period of time.
Owner:PEKING UNIV
Cycloastragenol crystal form D and preparation method thereof
PendingCN111378004AHigh purityGood chemical stabilityOrganic active ingredientsOrganic chemistry methodsDrugs preparationsCombinatorial chemistry
The invention belongs to the technical field of organic chemical drug preparation, and provides a cycloastragenol crystal form D and a preparation method and application thereof. The cycloastragenol crystal form D provided by the invention has higher purity and better chemical stability, the crystal form D has better dissolution and release behaviors when being made into a pharmaceutical preparation, and meanwhile, the preparation method of the cycloastragenol is simple, convenient, easy to industrialize and high in production adaptability.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing cycloastragenol by fermenting and converting astragaloside IV by utilizing fusarium oxysporum
PendingCN114438158AResolve by-productsSolve the pollution of the environmentMicroorganism based processesSteroidsBiotechnologyAstragaloside
The invention relates to a method for preparing cycloastragenol by fermenting and converting astragaloside IV by using fusarium palustris 0821 (CGMCC (China General Microbiological Culture Collection Center) No.16482). The invention also relates to a method for preparing the cycloastragenol by using the fusarium palustris 0821). According to the method disclosed by the invention, the cycloastragenol is prepared by utilizing the screened Fusarium proliferum and taking astragaloside as a substrate through fermentation. According to the method, astragaloside is converted to obtain high-purity cycloastragenol by utilizing a Fusarium profermentum fermentation technology, the whole technological process is carried out at normal temperature and normal pressure, required equipment conditions are low, the method is suitable for mass production, the conversion rate of the cycloastragenol reaches 80% or above finally, and the purity of the cycloastragenol reaches 95% or above finally.
Owner:厦门北化生物产业研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com