Uses of cycloastragenol in preparation of chronic renal failure treatment drugs
A technology of cycloastragalus and chronic renal failure, applied in the field of medicine, can solve the problems such as the clinical application of the biological activity of cycloastragalus against renal failure, which can improve the treatment effect and the quality of life, reduce the renal coefficient and the effect of full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
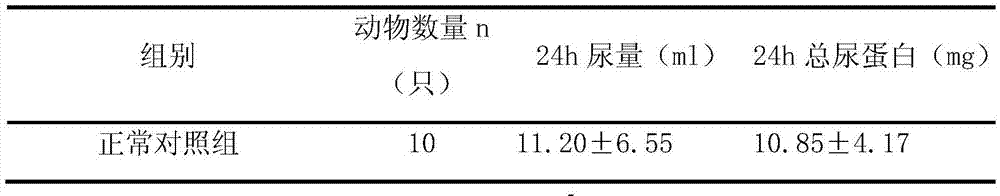
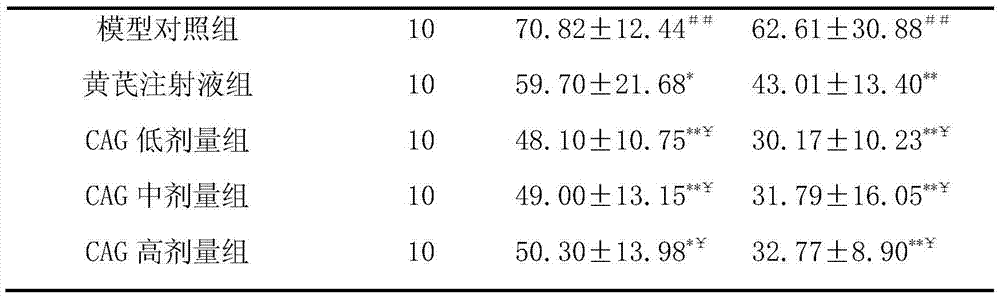
[0024] Example 1 In vivo pharmacodynamics test of cycloastragenol in treating renal failure
[0025] 1. Purpose of the test
[0026] To investigate the effectiveness of cycloastragenol in the treatment of renal failure, and to provide a basis for the research, development and clinical application of cycloastragenol.
[0027] 2. Test site
[0028] The barrier system of the Institute of New Drug Pharmacodynamics, Pharmacology Center, Shandong New Times Pharmaceutical Co., Ltd.
[0029] 3. Test materials
[0030] 3.1 Test drugs
[0031] Cycloastragenol 98% HHQC-20150119 Xi'an Haoxuan Biotechnology.
[0032] 3.2 Positive control drug
[0033] Astragalus injection 141126A3 Shineway Pharmaceutical Group.
[0034] 3.3 Other drugs and reagents
[0035] Adenine: purity ≥ 98%, Shanghai Bojing Chemical, batch number: 20120510. Urine protein quantitative kit: Nanjing Jiancheng Biotechnology Co., Ltd., batch number: 20141220.
[0036] 3.4 Test animals
[0037] SPF grade SD rats, ...
Embodiment 2
[0106] The preparation of embodiment 2 injection
[0107]
[0108] Preparation process: mix the prescribed amount of propylene glycol and ethanol evenly, add cycloastragenol, stir to dissolve, add the prescribed amount of 0.9% sodium chloride solution, stir evenly, add 0.5% activated carbon for needles, stir, and decarbonize to obtain the product.
Embodiment 3
[0109] The preparation of embodiment 3 injection
[0110]
[0111] Preparation process: add cycloastragenol to the mixed solution of PEG-400 and ethanol in the prescribed amount, stir to dissolve, add 0.9% sodium chloride solution to 10L, stir evenly, add 0.5% activated carbon for needles, stir, decarbonize, and obtain .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com