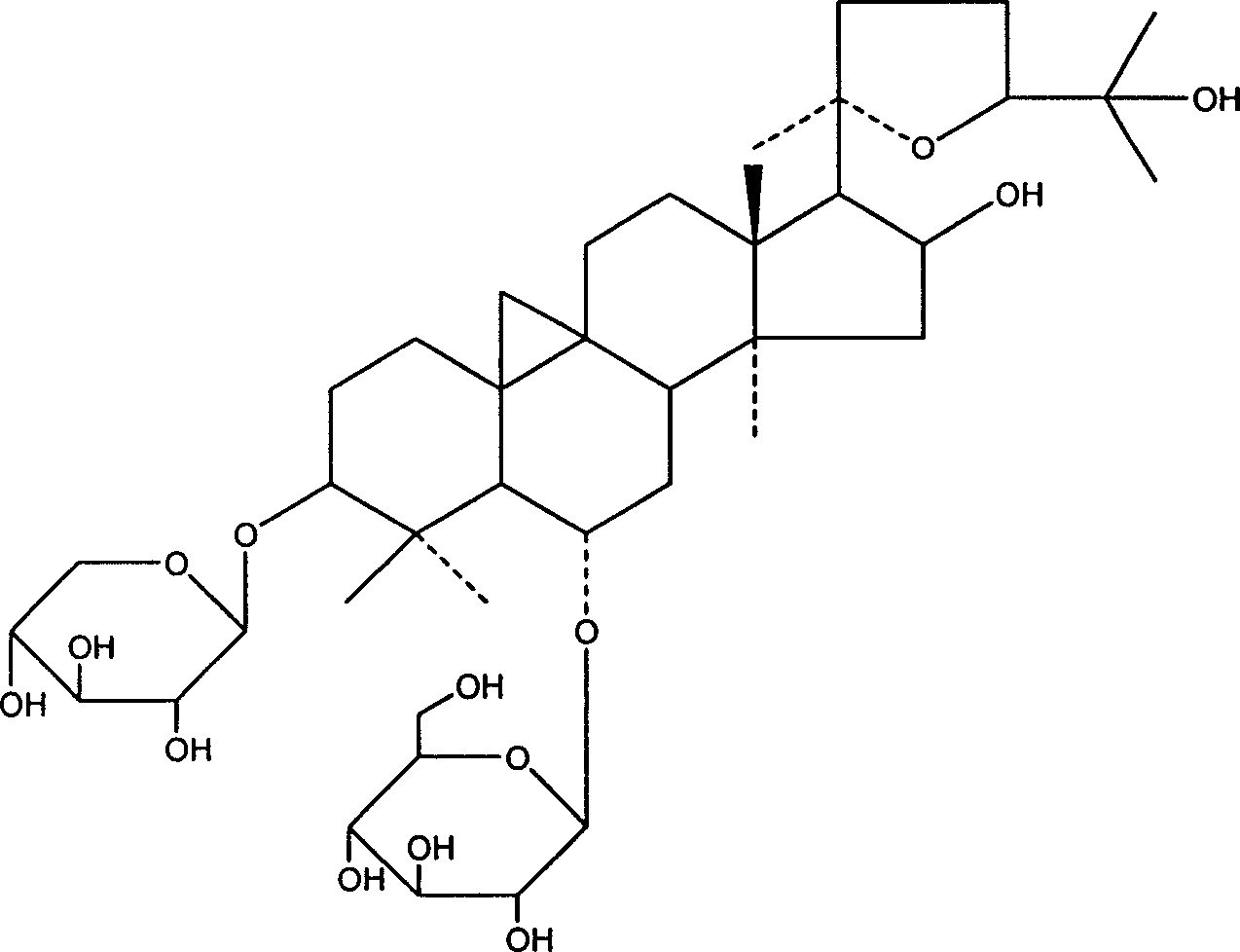
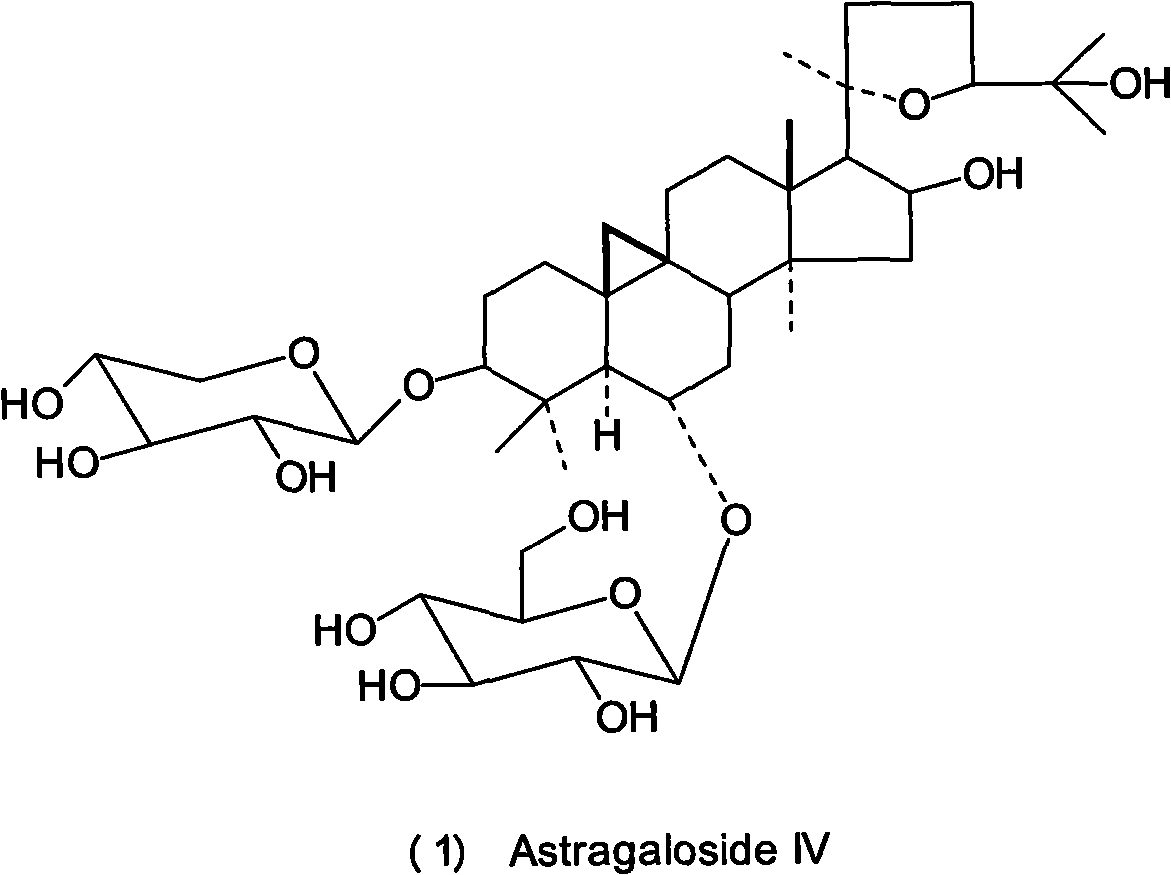
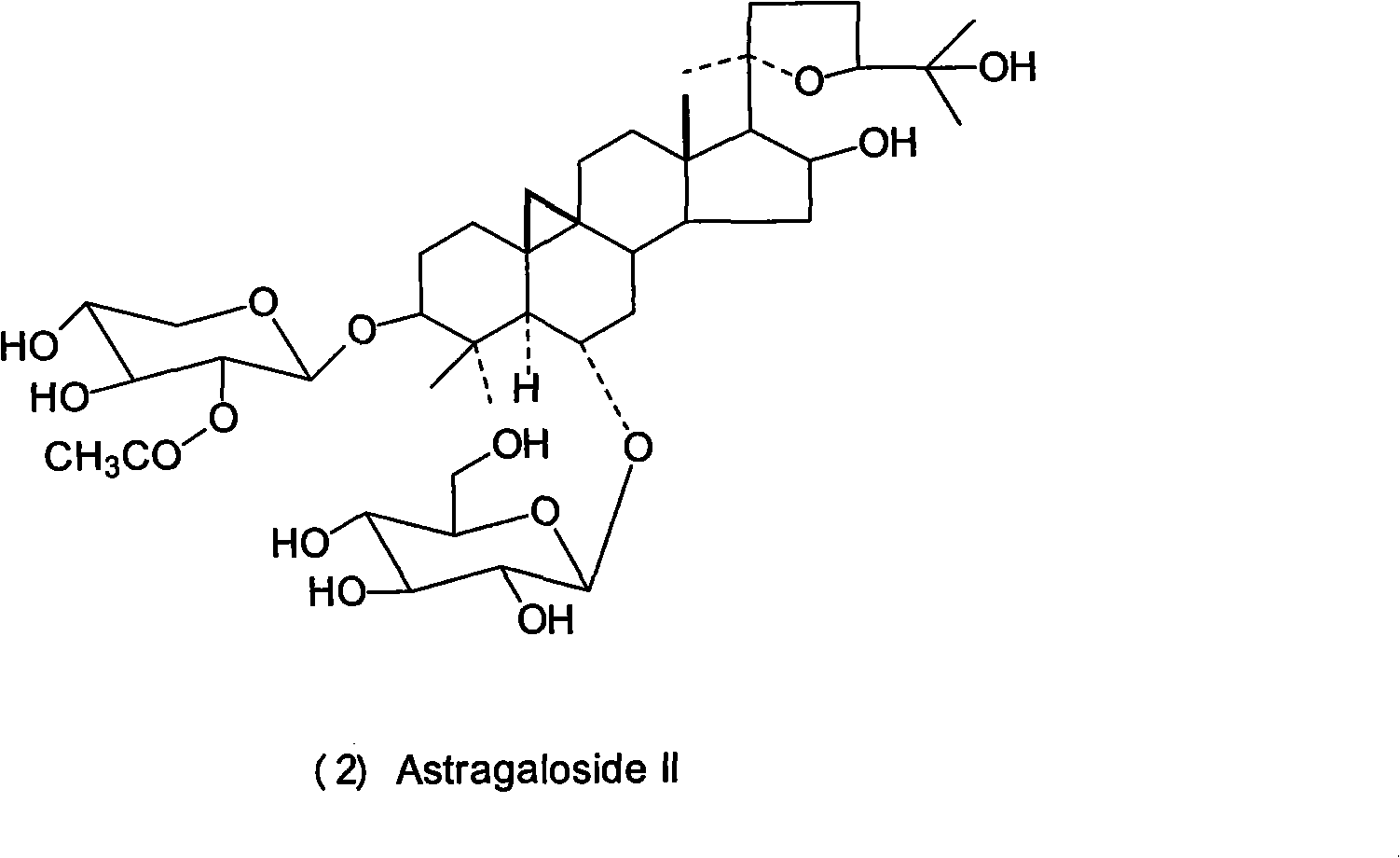
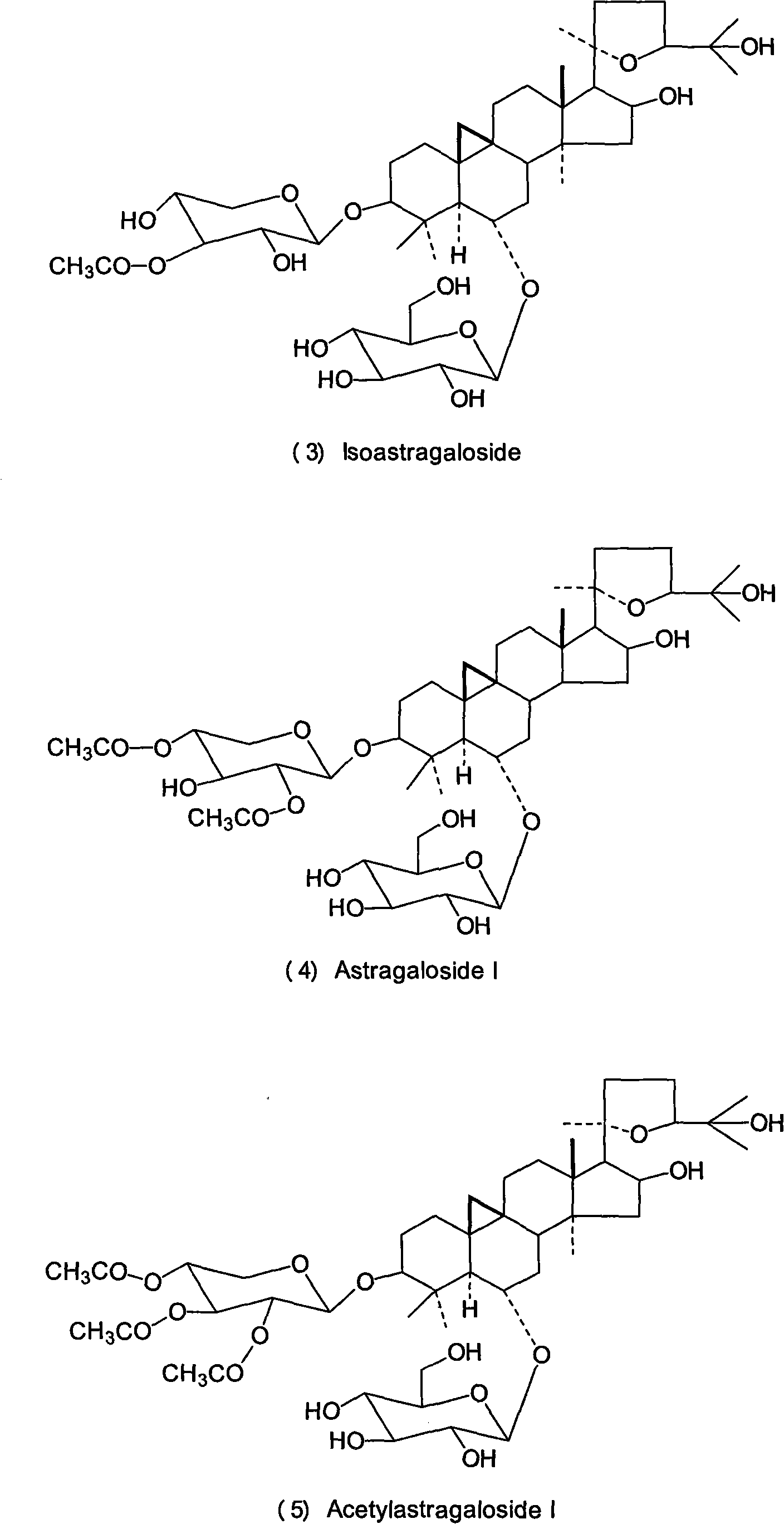
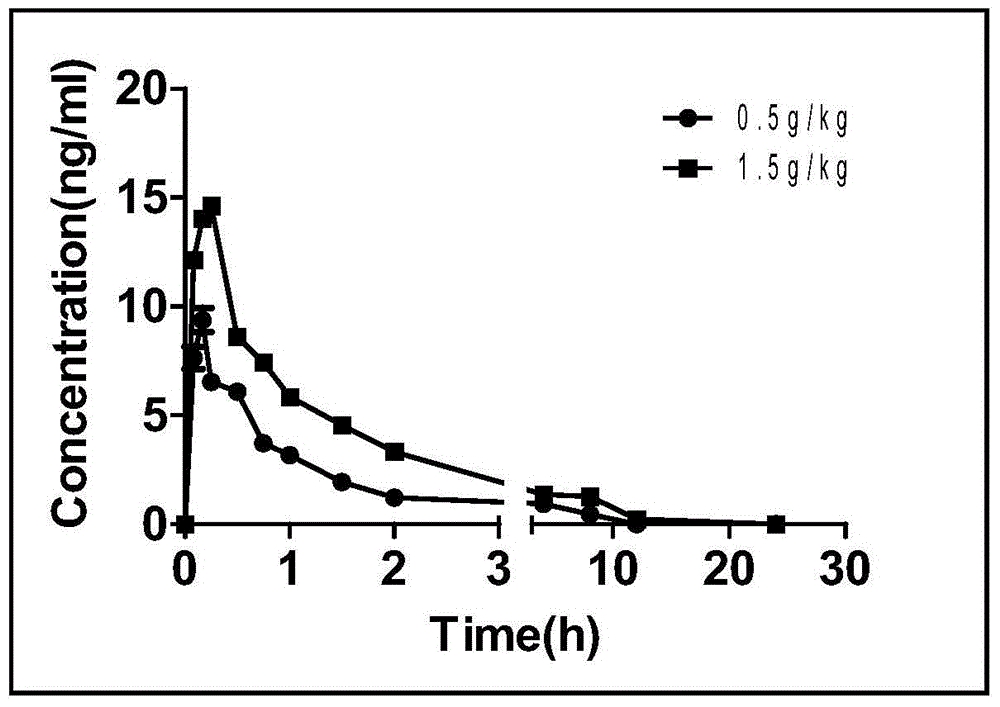
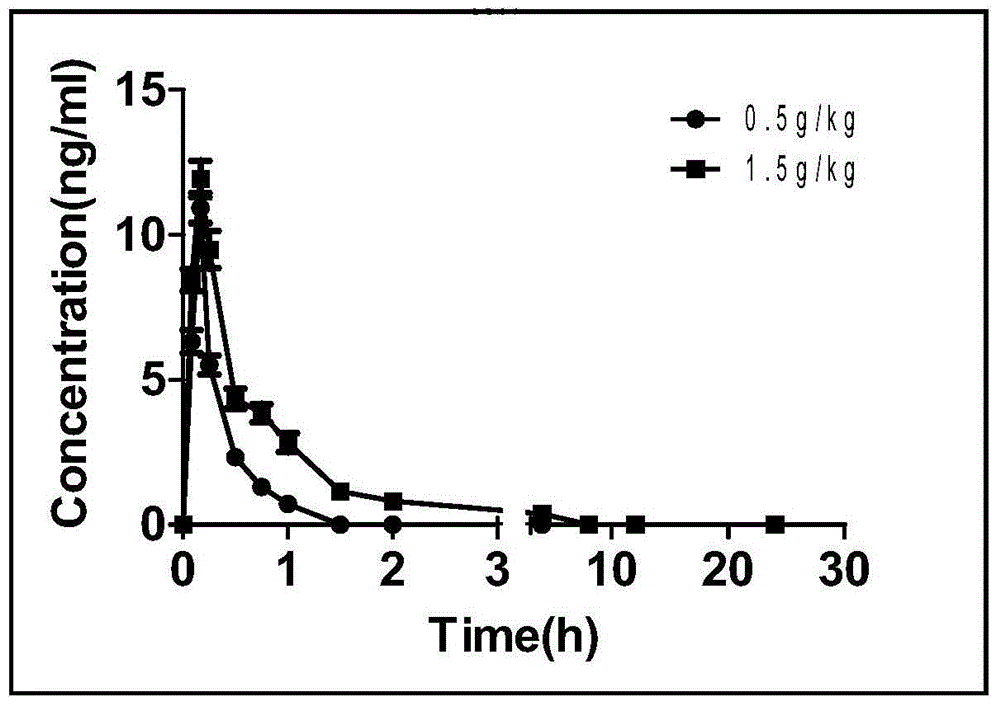
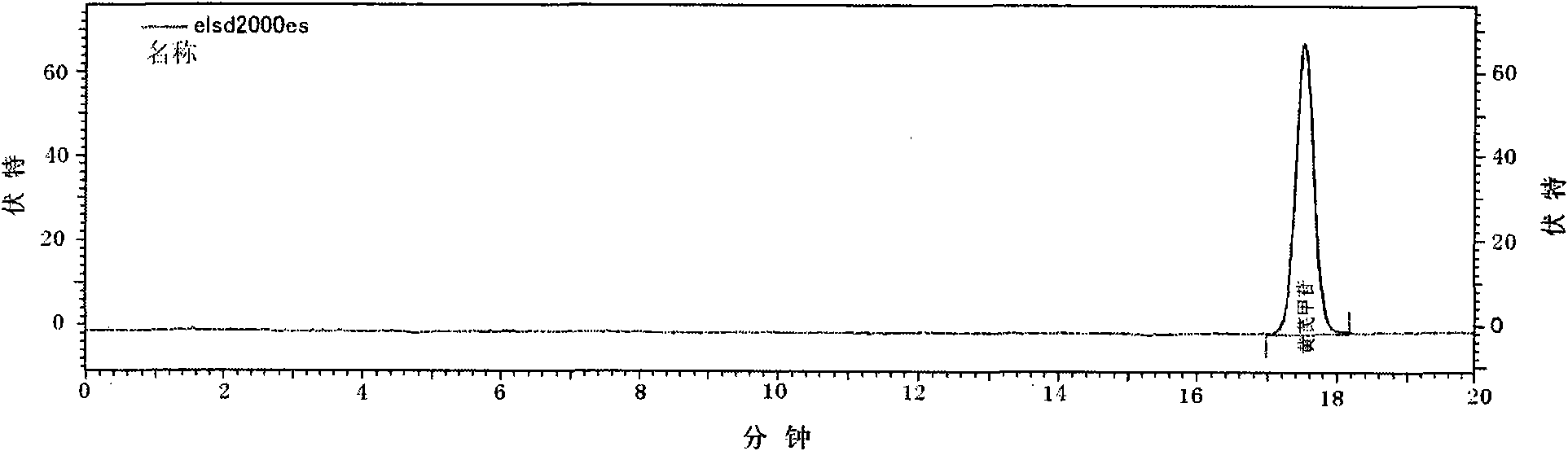
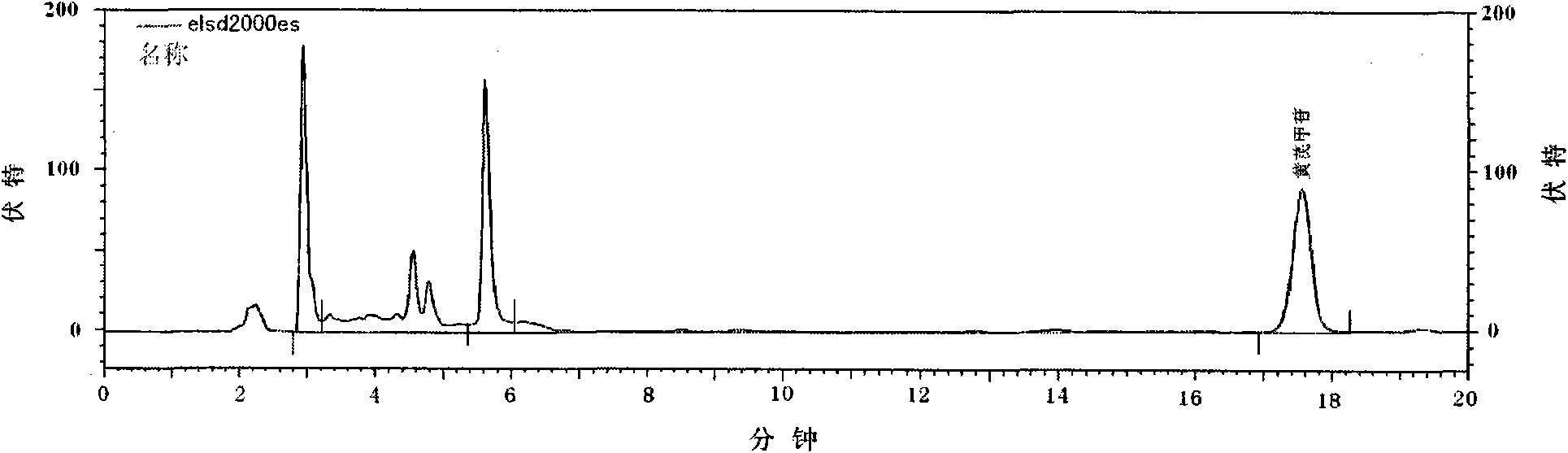
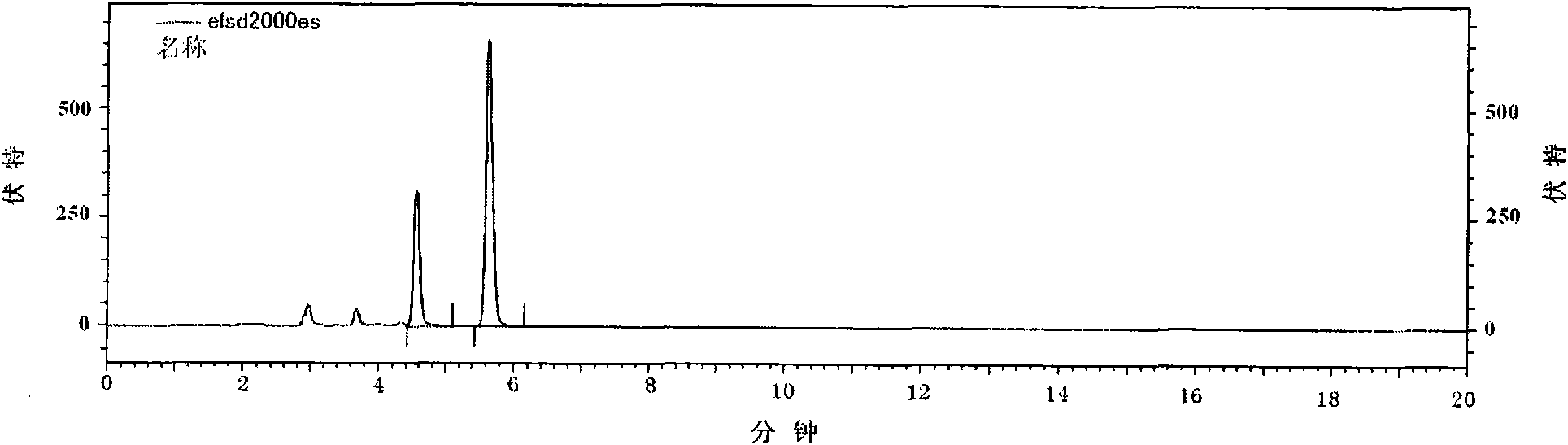
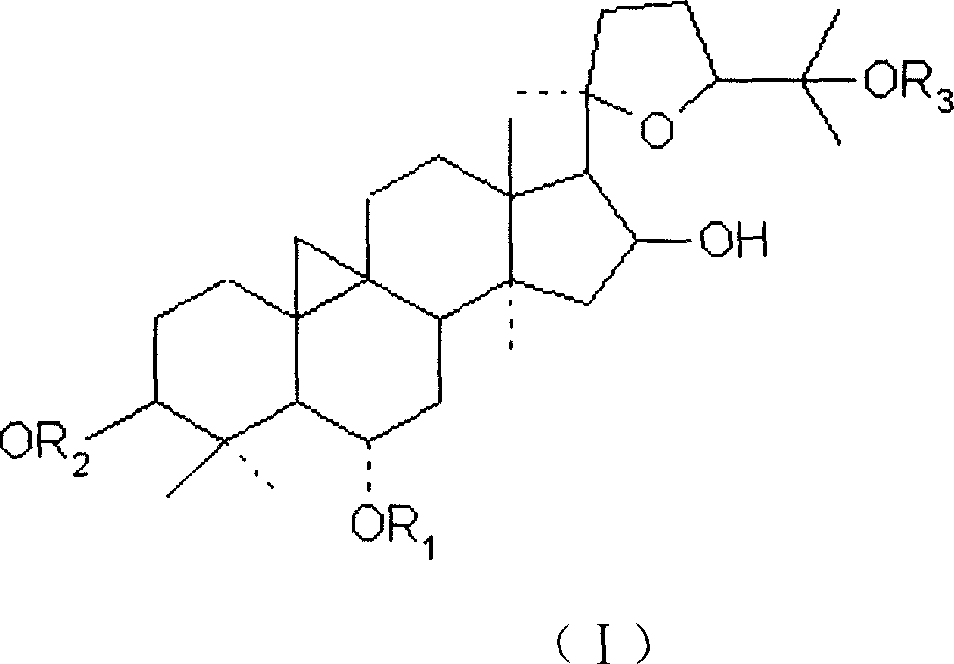
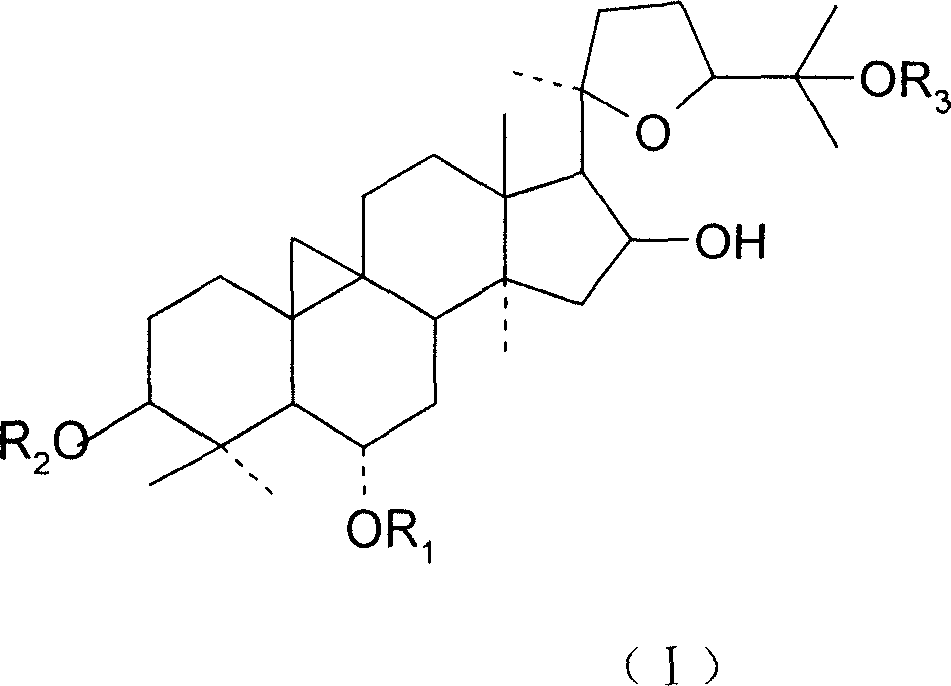
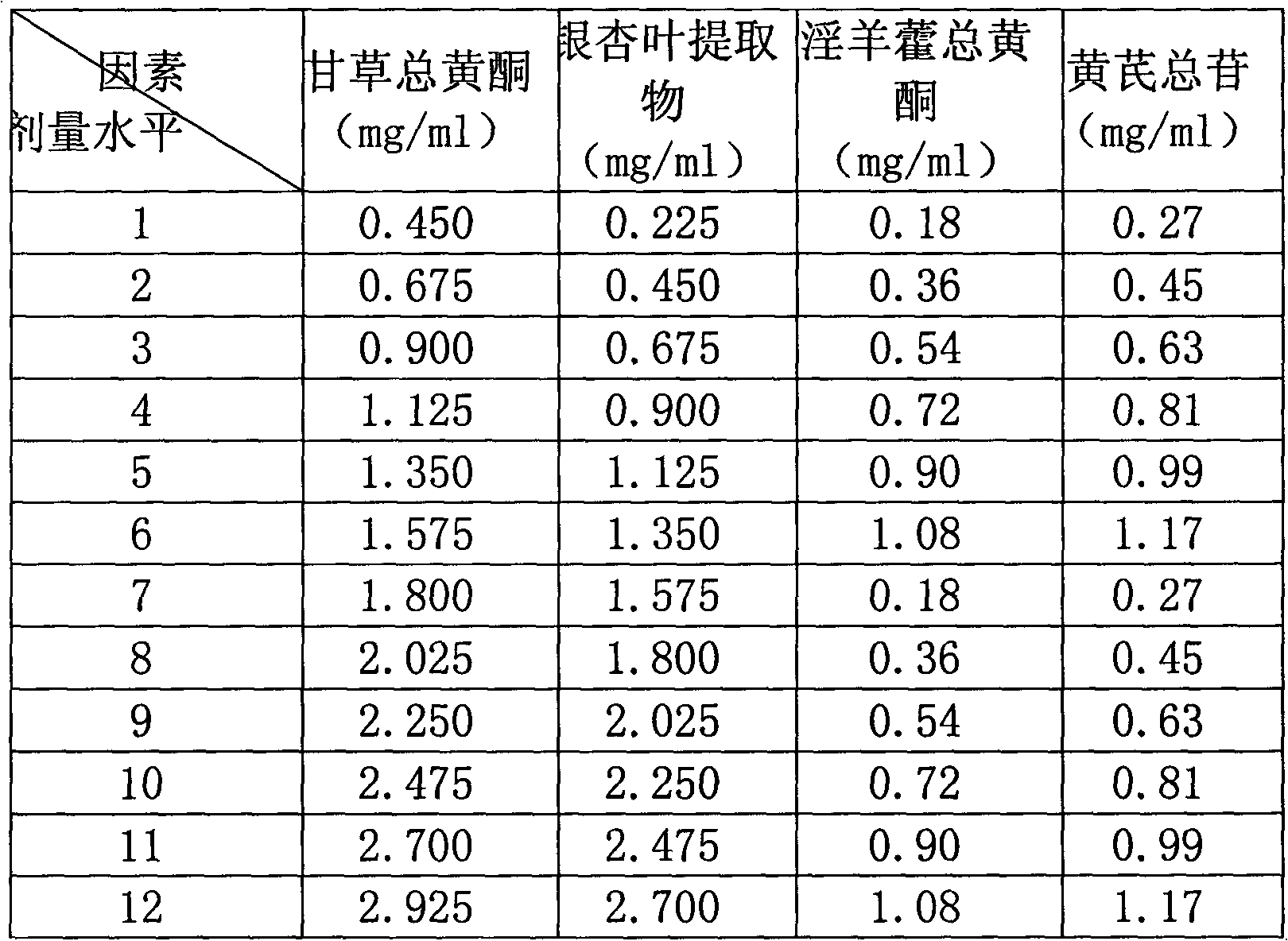
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
424 results about "Astragaloside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
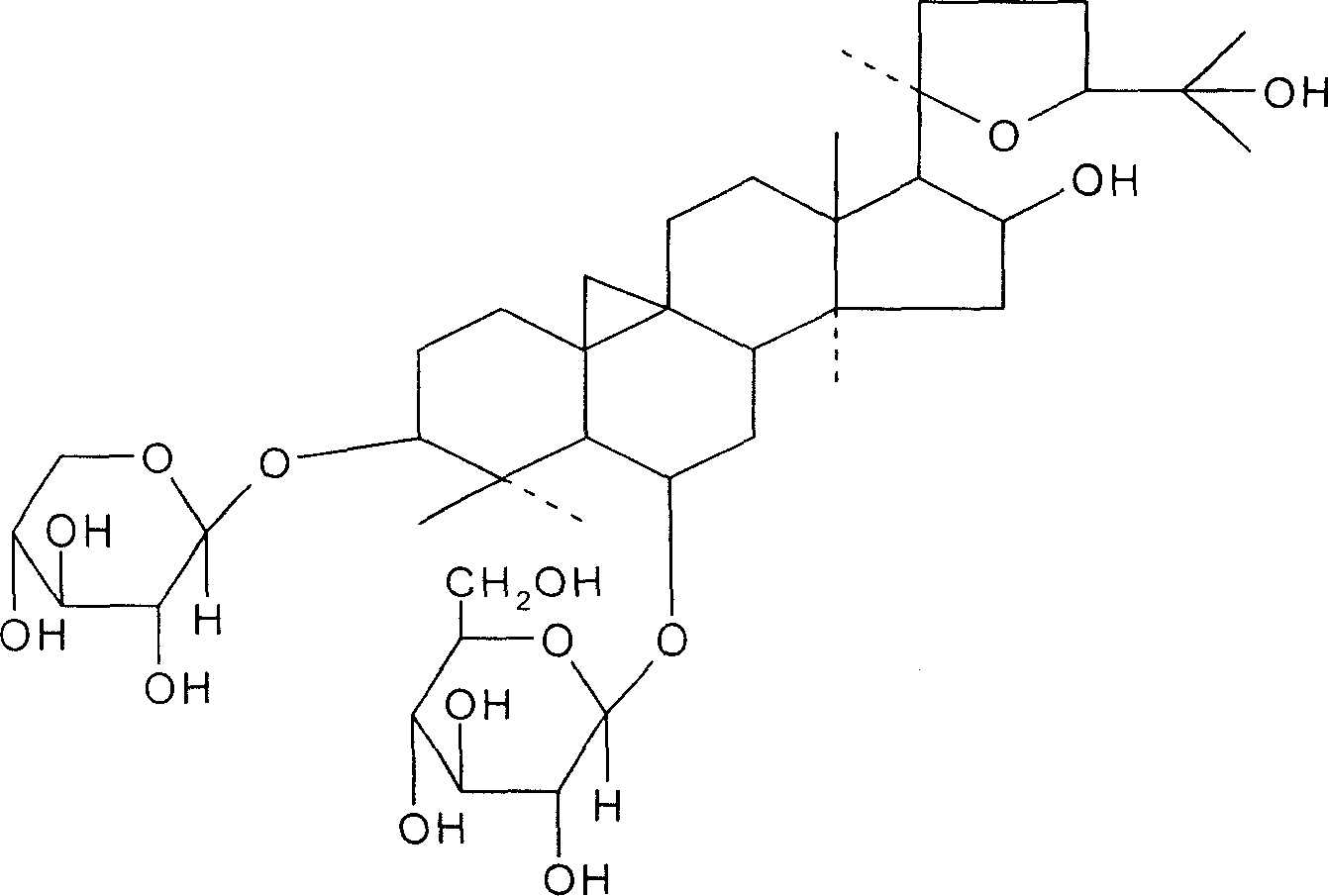
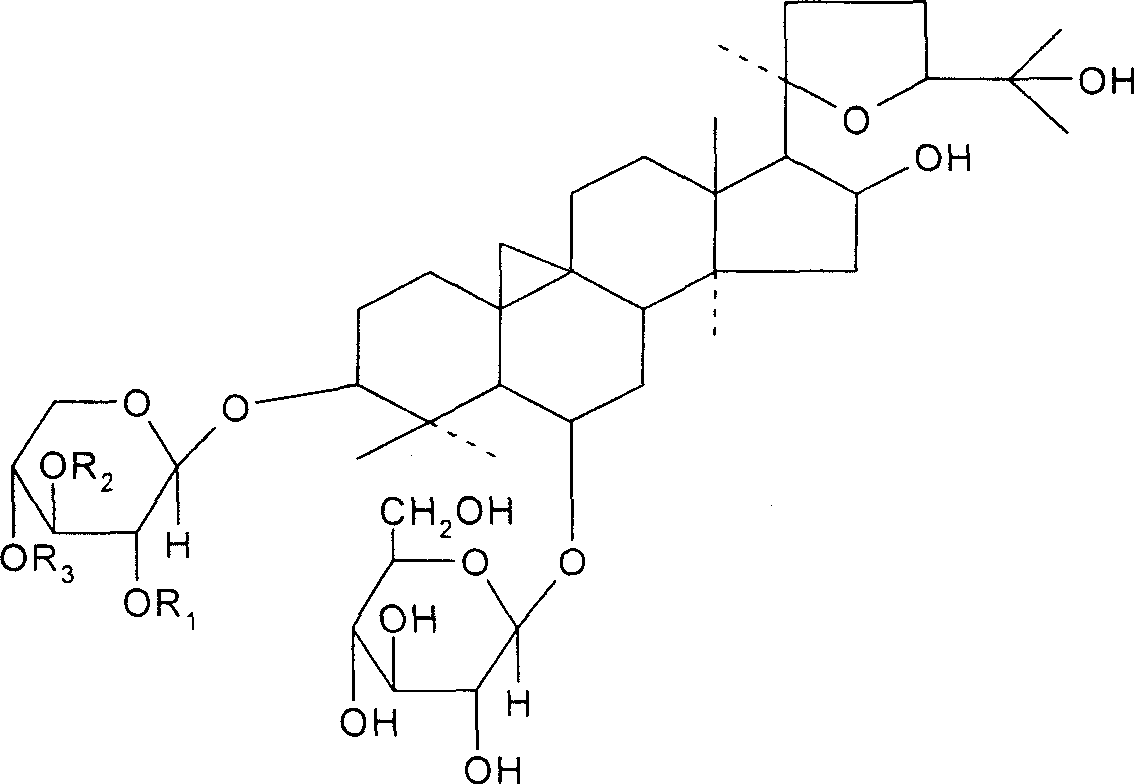
Astragalosides are a series of related chemical compounds isolated from Astragalus membranaceus.
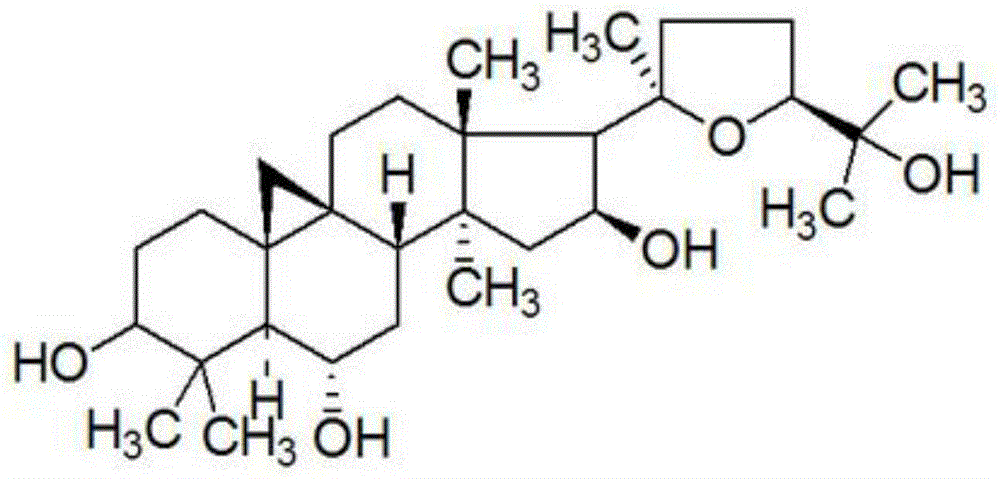
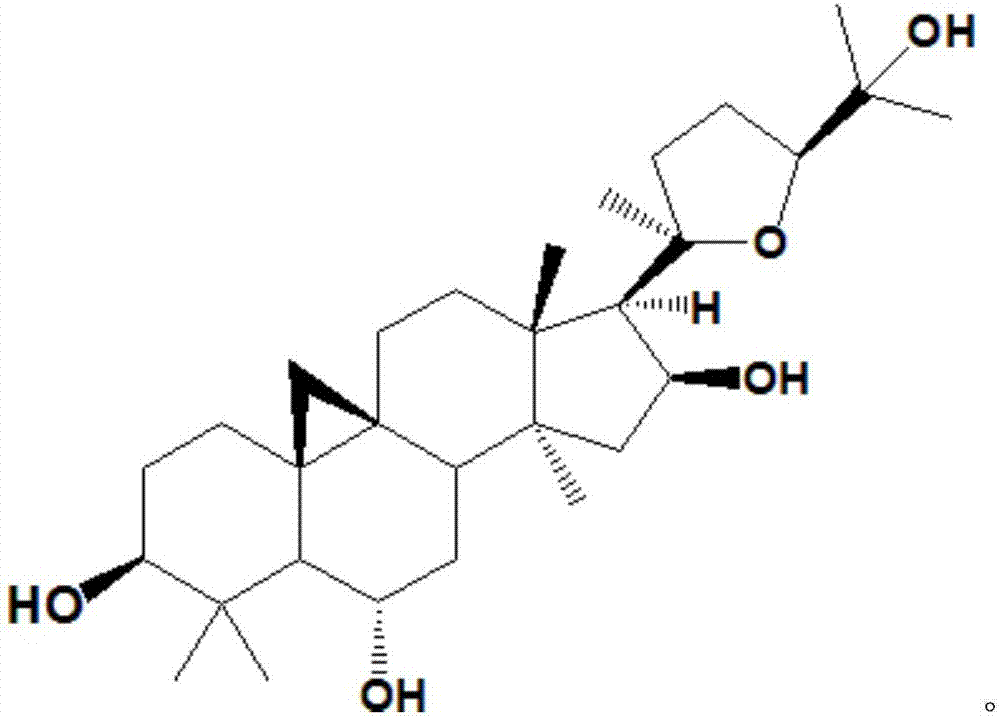
Preparation method and application of cycloastragenol
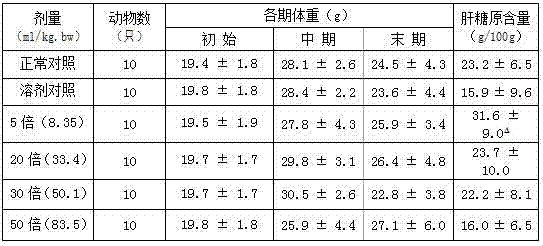
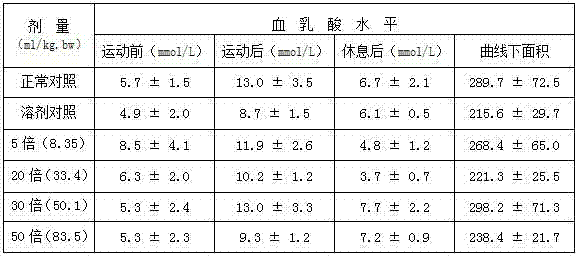
ActiveCN103880910AImprove qualityEfficient removalSteroidsAntineoplastic agentsAstragalosideAdjuvant
The invention discloses a preparation method and application of cycloastragenol. The preparation method of the cycloastragenol comprises the following steps: by using astragaloside or astrasieversianin as a raw material, oxidizing, reducing, hydrolyzing, extracting and purifying to obtain high-purity cycloastragenol. The method is simple to operate, gentle in condition, good in product quality and high in yield. The cycloastragenol can be applied to preparation of anticancer adjuvant therapeutic medicine. An anticancer adjuvane therapeutic medicine prepared by using the cycloastragenol as a pharmaceutical active ingredient has the anticancer adjuvant therapeutic effect of enhancing the anticancer therapeutic effect, reducing the toxicity of the anticancer medicine, preventing and treating the neutropenia caused by the anticancer medicine therapy.
Owner:SOUTHWEST JIAOTONG UNIV
Scald and burn dressing prepared by coaxial electrostatic spinning method and preparation method thereof
ActiveCN103893815AImprove mechanical propertiesHigh drug loadingAbsorbent padsNon-woven fabricsAstragalosideFiber
The invention discloses scald and burn dressing prepared by a coaxial electrostatic spinning method. The scald and burn dressing is a nanofiber membrane with a core / shell structure, wherein the shell is made of a natural biological high molecular material and the core is a degradable synthetic high molecular material loaded with astragaloside. The mass ratio of astragaloside to the degradable synthetic high molecular material is 1:(2-50). The invention further discloses a preparation method of the scald and burn dressing. The raw materials are easy to get and cheap, and the preparation process is simple, easy to operate and control and can be easily put into large-scale industrial production. The prepared scald and burn dressing has good flexibility and high mechanical strength, and can form a protective fiber felt on the surface of the wound to promote growth of normal skin tissues and reduce formation of scar tissues, thereby curing skin injury.
Owner:ZHEJIANG UNIV
Method for preparation of Cycloastragenol by sulfuric acid hydrolysis
The invention relates to a method for preparation of Cycloastragenol by sulfuric acid hydrolysis. The method provided by the invention utilizes sulfuric acid to hydrolyze the Chinese medicine astragalus crude extract astragaloside, and realizes conversion of astragaloside to Cycloastragenol under certain hydrolysis condition, then performs impurity separation on the Cycloastragenol crude extract obtained by acid hydrolysis by chloroform and n-butanol extraction, and further makes use of silica gel column chromatography purification to finally obtain high purity Cycloastragenol. The invention aims to provide the method that can overcome the disadvantages of complex production process, low yield, high cost and the like of Cycloastragenol production and is suitable for large-scale production of different purity Cycloastragenol.
Owner:BEIJING UNIV OF CHEM TECH
Astragaloside injection preparation and its preparing process
InactiveCN1543976AClear contentQuality is easy to controlOrganic active ingredientsPharmaceutical delivery mechanismAstragalosideFluid infusion
The invention discloses an astragaloside IV injection preparation (small injection, powder injection, glucose fluid infusion and sodium chloride fluid infusion), and the process for its preparation, wherein the auxiliary solvent and solvent assisting deflocculating agent are screened, which has proved that propylene glycol, ethanol, glycerin, polyethylene glycol, benzyl benzoate and acetic acid dimethylamide has solubilization function for astragaloside IV.
Owner:上海博泰医药科技有限公司
Preparation method for astragaloside
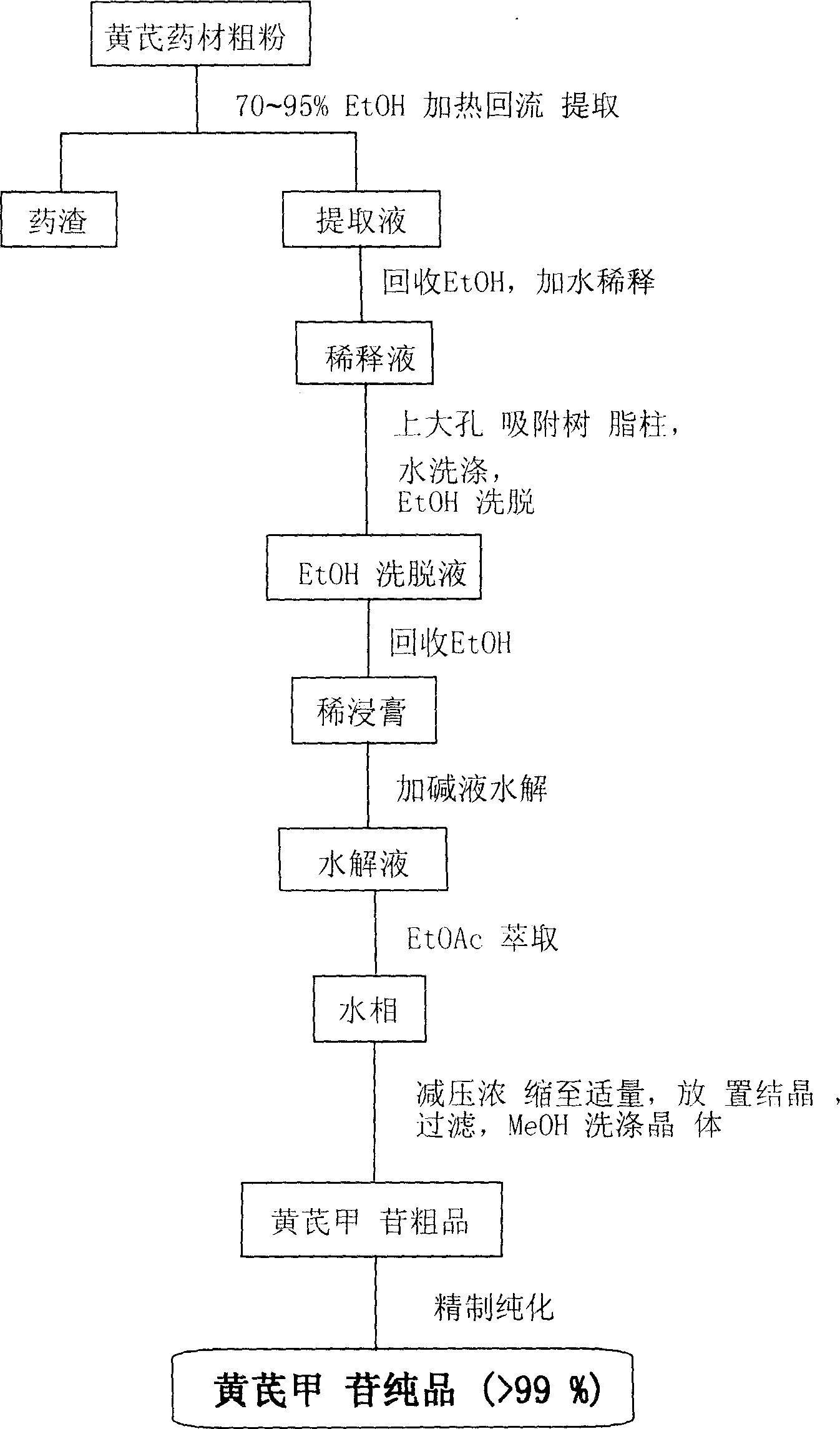
The invention aims to provide an Astragaloside IV preparation method with simple operation, high yield and high purity. In order to achieve the purposes of the method, the method adopts the specific technical proposal as follows: the preparation method of Astragaloside IV takes a traditional Chinese medicine of astragalus root as a raw material, and comprises the steps of water extraction, alkali treatment, adsorption by macroporous adsorptive resin, washing, rinsing by diluted alcohol liquid, elution of Astragaloside IV by alcohol liquid with rather high concentration, concentration and crystallization. The Astragaloside IV prepared by the method has the advantages of light color and high purity, and the preparation method has the advantages of high yield, low production cost, and simple, convenient and standard process.
Owner:SHANGHAI NORMAL UNIVERSITY
Method for simultaneously detecting main components of Naoxintong capsule in plasma
ActiveCN104614456AInhibit aggregationImprove neurological deficitsComponent separationAstragalosideSalvianolic acid B
The invention provides a method for simultaneously detecting main components of paeoniflorin, beta ecdysterone, laetrile, mulberroside A, caffeic acid, ferulic acid, salvianolic acid B, astragaloside, formononetin, cryptotanshinone and tanshinone IIA of a Naoxintong capsule in a plasma sample by a liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). In a liquid chromatogram, a mobile phase consists of acetonitrile and a formic acid aqueous solution of which the volume fraction is 0.1%, and gradient elution is used. A mass spectrum uses a quick positive and negative ions switching and analyzing mode and an MRM (Multiple Reaction Monitoring) scanning manner. After the Naoxintong capsule is taken, the situations of the changes of the blood-medicine concentration of several kinds of main components in the plasma of a rat are detected at the same time. The methodological survey results indicate that the established method conforms to determination requirements on biological samples in a body; the method is good in sensitivity, high in specificity, stable, reliable, and suitable for detecting substances with lower contents.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
Qi-nourishing and blood-activating medicinal composition and preparation method, detection method and application thereof
According to the invention, a medicinal composition consists of 13 raw material medicaments, such as astragalus, pilose asiabell root, root of red-rooted salvia, vinegar corydalis tuber and the like. A plurality of formulations are prepared by the steps of decocting, filtering, concentrating, depositing in alcohol and the like, wherein the medicinal composition in a dripping pill formulation has more obvious advantages. The medicinal composition has the effects of supporting healthy energy, nourishing Qi, activating blood, promoting vessels and relieving pain. The original quality standard is improved by the quality control method of the medicinal composition in the invention, the astragaloside content of the preparation is determined by a high performance liquid phase-evaporative light scattering detection method, and the determination method is studied, so that the controllability and stability of product quality are improved, and the quality control in industrialized production is facilitated.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
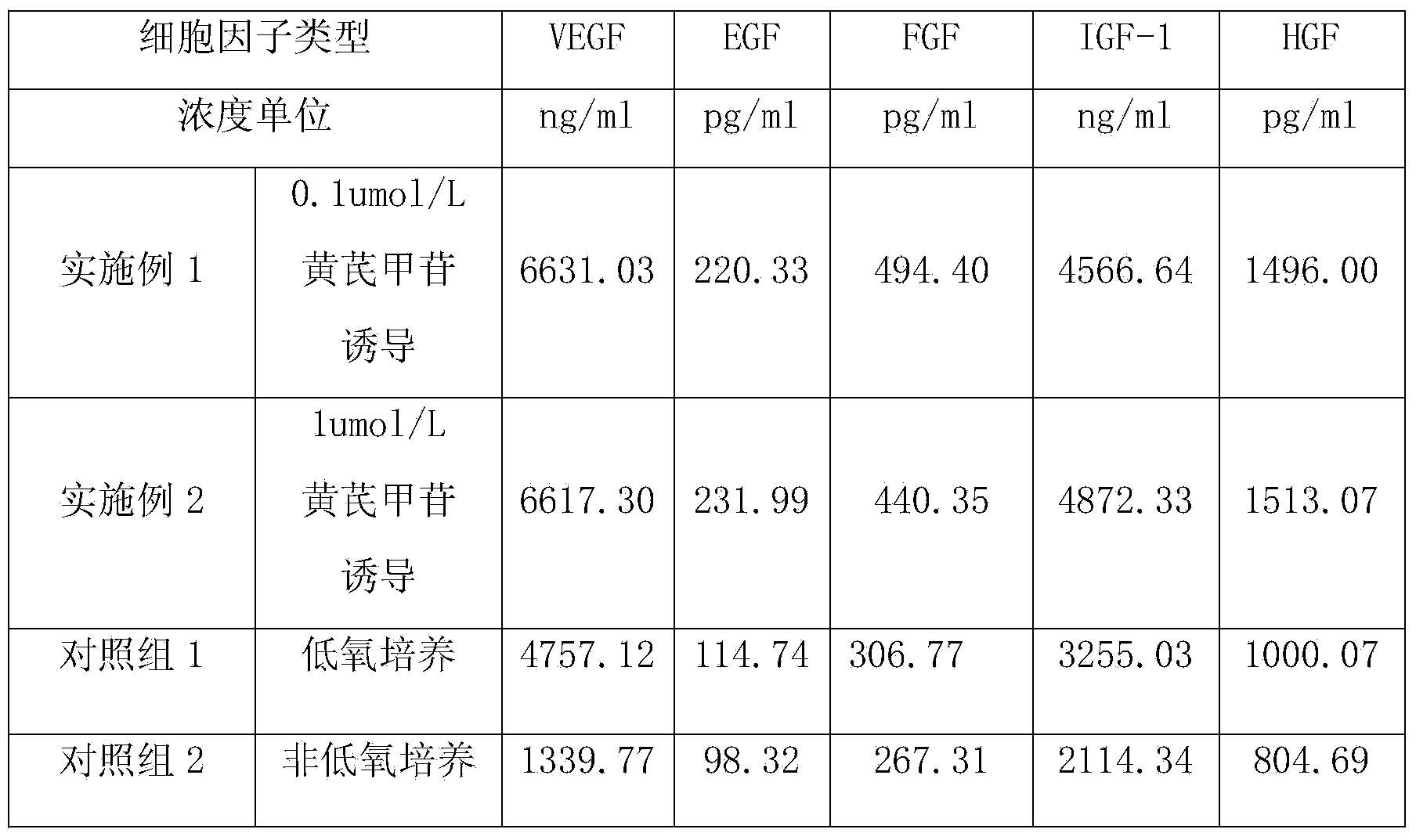
Conditioned medium preparation of mesenchymal stem cells used for skin aging restoration
ActiveCN104013644AAddress issues such as agingDoes not affect activityOrganic active ingredientsTripeptide ingredientsAstragalosideLiquid Change
The invention discloses a conditioned medium preparation of mesenchymal stem cells used for skin aging restoration. A preparing method of the conditioned medium preparation includes: culturing the mesenchymal stem cells under normal conditions until the growth density of the mesenchymal stem cells is 60-80%, performing liquid changing, culturing with a phenol red-free DMEM, adding astragaloside, performing induction culturing in an incubator for 24 h, removing cytokines having a molecular weight lower than 3000 after the culturing is finished, concentrating, detecting the cytokines in the medium, adding 0.01-0.6 mg / mL of reduced glutathione and 0.01-0.4% of hyaluronic acid into the concentrated culture medium solution. The conditioned medium preparation is rich in a plurality of the cytokines. The astragaloside can maintain the characteristics of the stem cell and stimulates the capability of secreting the plurality of the cytokines of the stem cells. The hyaluronic acid avoids the problems of short half-life period of the cytokines and short curative effect time, and enhances the skin restoration effects.
Owner:CHENGDU QINGKE BIOTECH
Medicine for treating and preventing immune abnormalism disease
InactiveCN101116667APrevention of Secondary LesionsEnhance anti-inflammatoryOrganic active ingredientsPharmaceutical delivery mechanismAstragalosideDisease cause
The invention relates to medical use of astragalus saponin adopting cyclo astragenol as aglycon, wherein astragaloside ó¶ and cyclo astragenol are representative compounds of astragalus saponin. Astragalus saponin can effectively cure and prevent abnormal immunity diseases such as asthma, allergic rhinitis, allergic dermatosis, systemic lupus erythematosus, rheumatoid arthritis and mesangial proliferative glomerulonephritis, etc. During being used in curing the diseases, astragalus saponin is characterized by less toxicity, definite curative effect and being suitable for long-term use, etc.; meanwhile, astragalus saponin can be made into different dosage forms with medically acceptable vehicle to cure prevent abnormal immunity diseases.
Owner:罗河生
Producing and refining method for high-purity cycloastragenol
InactiveCN105734109AFew reaction stepsImprove conversion rateSteroidsFermentationAstragalosideHydrolysis
The invention relates to a method for producing and refining cycloastragenol with a purity greater than or equal to 95%. Using astragaloside IV as a raw material, through two-phase enzymatic hydrolysis, extraction, and recrystallization, cycloastragenol products with a purity greater than or equal to 95% are obtained. Compared with the existing cycloastragenol production method, the present invention has simple production process, mild reaction conditions, high reaction conversion rate, is more suitable for large-scale production, and solves the problem of astragaloside IV being hydrolyzed under acid-base conditions. The easy ring opening leads to the difficulty of generating astragalol.
Owner:CHENGDU KING TIGER PHARM CHEM TECH CO LTD
Application of the effective part of Chinese medicinal material lindley eupatorium herb in preparing antiviral medicine
Lindley eupatorium herb total flavone and its pharmaceutically acceptable salt is used in preparing medicine for preventing and treating virus diseases, especially SARS. Lindley eupatorium herb total flavone is selected from quercetin, kaempferol, jaceidin, hyperoside, trifolirhizin, astragaloside or their mixture. Lindley eupatorium herb total flavone in the concentration of 100 microgram / ml has no toxicity to VeroE6 cell and complete supperssion on SARS virus.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
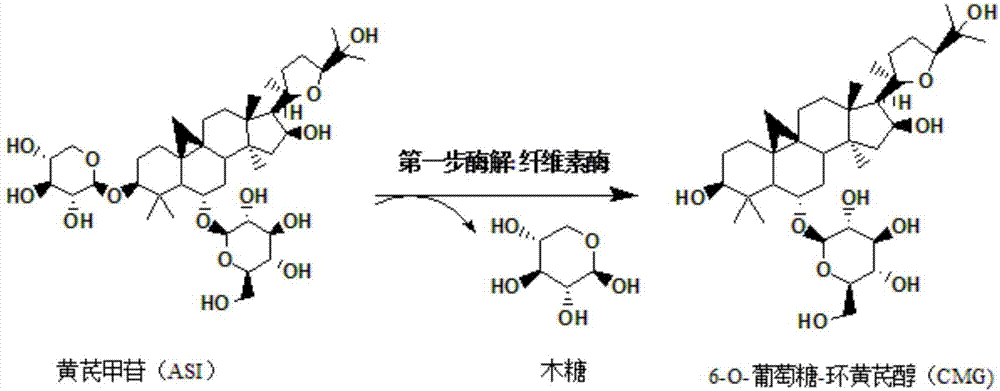
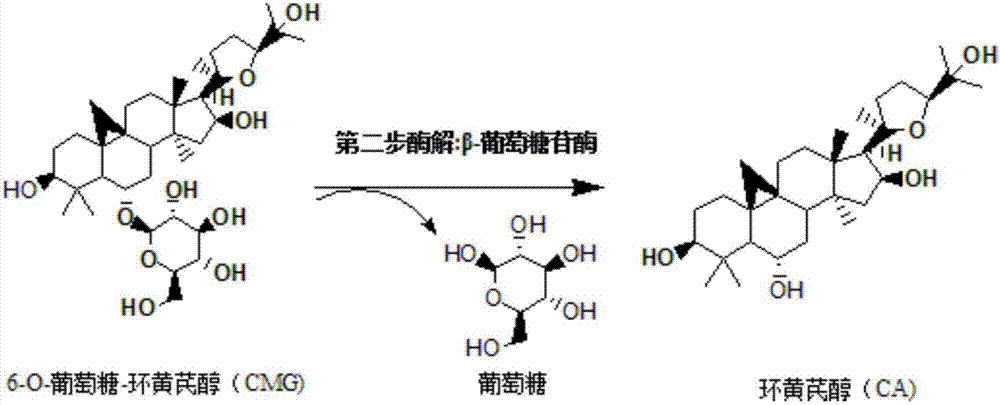
Method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis
The invention discloses a method for converting astragalosides to prepare cycloastragenol by two-step enzymolysis; the method comprises the specific steps of using astragalosides as a substrate, using two different hydrolases to hydrolyze and break beta-xyloside bond at site C3 of the astragalosides and beta-glucoside bond at the site C6 respectively to obtain aglycone cycloastragenol of the astragalosides, extracting, performing silica-gel column chromatography, recrystallizing with ethanol, and purifying to obtain the finished cycloastragenol up to 95% and higher in purity. The problem that damage of three-membered ring structure of astragalosides during the preparation of cycloastragenol by a chemical process causes massive byproducts is solved, the defects of traditional cycloastragenol preparation methods, such as low substrate conversion rate, step complexity and environmental pollution, are overcome. The method has the advantages that substrate specificity is high, the substrate astragalosides is completely converted, the steps are simple, the conditions are mild, the cost is low, and the method is a mild biological preparation method, has no environmental pollution and is suitable for industrial production.
Owner:BEIJING UNIV OF CHEM TECH
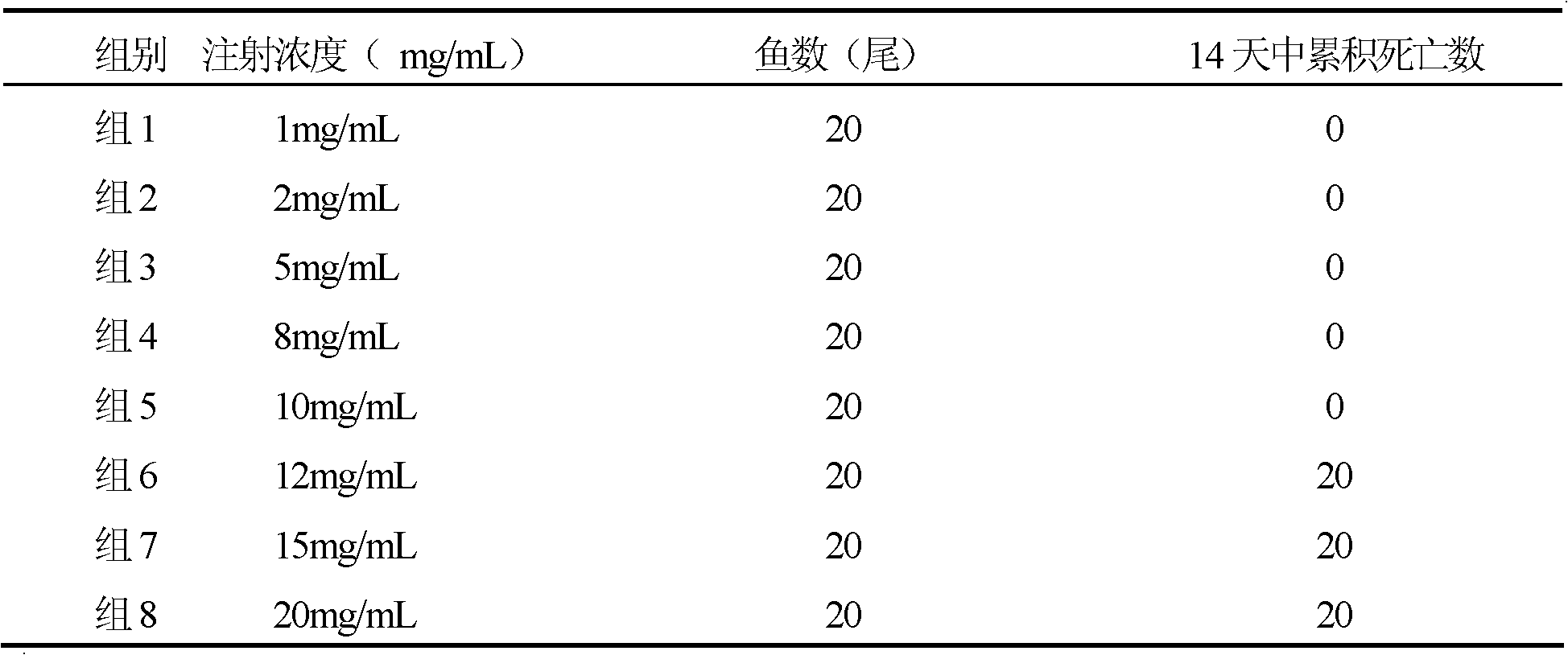
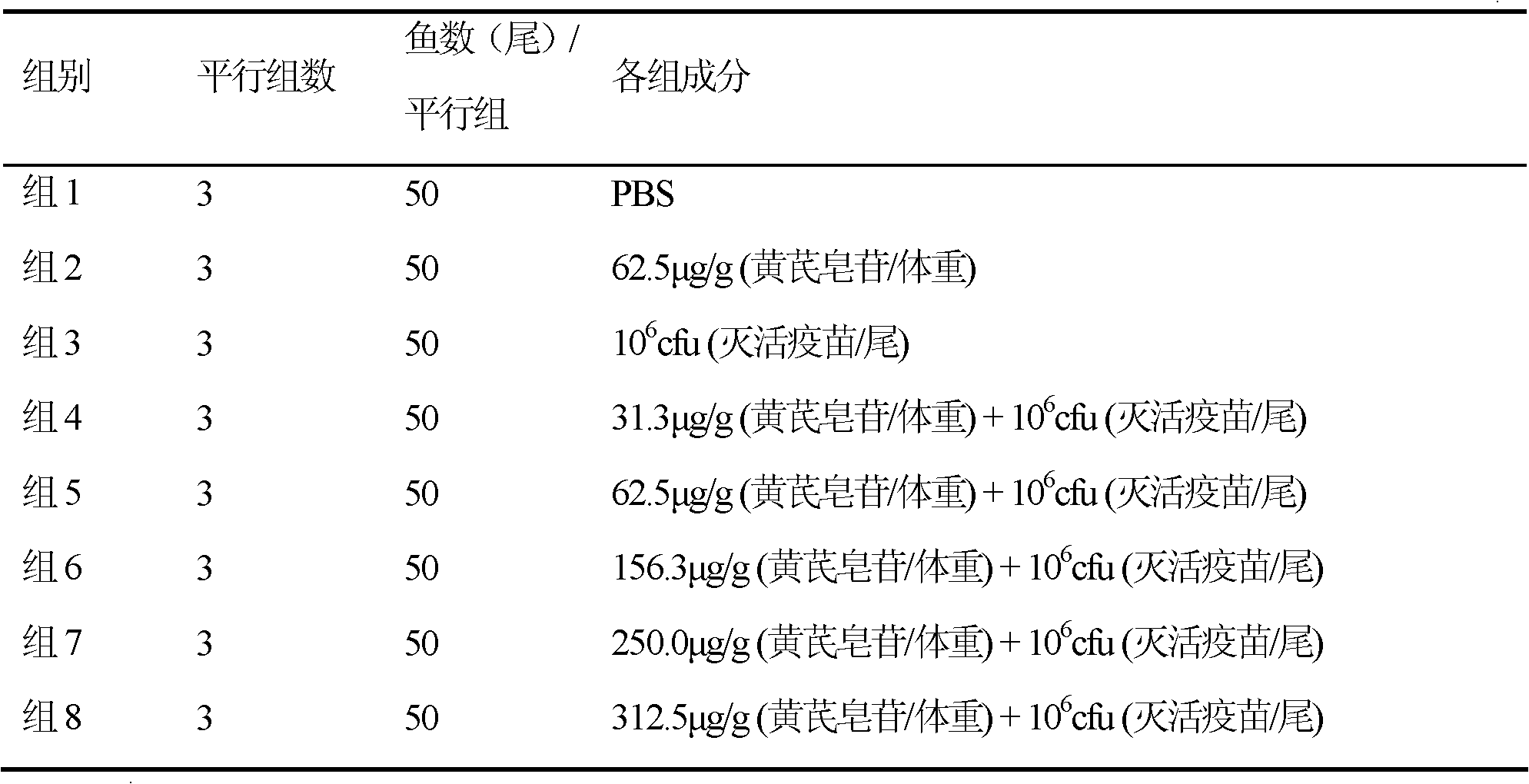
Adjuvant for enhancing fish vaccine immunization effect and application thereof
ActiveCN102430120AImprove featuresImprove protectionImmunological disordersAntibody medical ingredientsProtective antigenAstragaloside
The invention relates to an adjuvant for enhancing a fish vaccine immunization effect and an application thereof. The adjuvant for enhancing the fish vaccine immunization effect is characterized in that an immune potentiator is extracted from Astragalus mongholicus as a leguminous plant or effective components of the Astragalus mongholicus, comprising Astragaloside and Astragalus polysacharin, and natural products or manually modified products or manually synthetic products comprising the Astragaloside and the Astragalus polysacharin can be adopted to serve as the effective components. The adjuvant is capable of increasing the specific immune protection rate after vaccine component immunization is finished. When the adjuvant is applied, a vaccine can comprise the following components: any one or more than one of expression products of inactivated pathogens, bacterial ghost components, hypotoxic pathogens, attenuated pathogens, protective antigens, antigen subunits, antigen determinants or antigen gene expression vectors of bacteria, viruses and parasites. When in use, the adjuvant can be mixed with the components of the vaccine for application, also cannot be mixed with the components of the vaccine for application and also can be applied with the vaccine at different time.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Kindir leaf total flavone extract and its prepn and application
InactiveCN1931205ASignificant anti-ischemic effectClear certaintyOrganic active ingredientsSugar derivativesTrifolinAstragaloside
The present invention is kinder leaf total flavone extract and its preparation process and application, and features that the kinder leaf total flavone extract contains total flavone in 50-95 %, including rutin 0.2-30.0 %, hyperin 0.5-30.0 %, isoquercitrin 10.0-30.0 %, trifolin 3.0-20.0 %, astragaloside 2.0-5.0 %, 6''-O-acetyl isoquercitrin 1.0-6.0 %, quercitrin 0.5-4.0 %, trifolitin 0.2-3.0 %, etc. The kinder leaf total flavone extract is applied in preparing medicine for treating cardiac and cerebral ischemia.
Owner:周亚球 +1
Method for preparing purity astragaloside
A process for preparing high-purity astragaloside from astragalus root includes such steps as extracting, concentrating, removing impurities, hydrolytic conversion, extracting in solvent and purifying.
Owner:山西振东泰盛制药有限公司
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Improvement of method for preparing healthy qi strengthening, astragalus root and glossy privet fruit containing medicine
The present invention relates to the improved preparation process of preparing available medicine Zhenqi medicine for strengthening body's resistance. The technological process of preparing available medicine Zhenqi medicine includes the steps of: alcohol reflux extraction of coarse powder of astragalus root and privet fruit, decompression distillation of the extracted liquid to obtain alcohol extracted extractum, decocting the medicine dregs, filtering to obtain filtrate, concentration and drying, mixing with the alcohol extracted extractum, further decompression drying, and spray drying to obtain dry extractum powder. The improved technological process can obtain the effective component oleanolic acid and higher content of astrasgaloside A.
Owner:甘肃扶正药物科技股份有限公司
Quality control method of astragalus-leech capsules capable of regulating collaterals
InactiveCN103197027AControl drug qualityThe identification method is mature and feasibleComponent separationAstragalosideGround beetle
The invention discloses a quality control method of astragalus-leech capsules capable of regulating collaterals. The quality control method comprises the steps of: respectively differentiating leech, red peony root, astragalus root, ginseng, chuanxiong rhizome, red sage root, ground beetle, cinnamon, borneol and polygonum multiflorum in medicaments by using a thin-layer chromatography; determining the content of astragaloside in the medicaments by using a thin-layer scanning method; and determining the content of tanshinol and paeoniflorin in the medicaments by using a liquid-phase chromatography. According to the quality control method disclosed by the invention, the medicinal material differentiation method is mature, feasible, negative and non-interfering and is strong in specificity, and the content determination method is easy to operate, high in precision and good in reproducibility, so that the quality control method disclosed by the invention can be used for controlling the quality of the medicaments accurately and stably, thereby being adaptable to the industrial stable production of the medicaments.
Owner:山西振东五和堂制药有限公司
Method for extracting high-purity astragaloside from astragalus mongholicus
The invention discloses a method for extracting high-purity astragaloside from astragalus mongholicus, and belongs to the technical field of biological medicines. The method comprises the following steps: (1) taking a ground astragalus mongholicus root, adding water, carrying out reflux extraction for 2-4 times at normal temperature, combining water extraction solutions, concentrating until the density is 1.2-1.3, adding ethanol, stirring for depositing fully, centrifuging, filtering and collecting a supernatant solution; (2) placing the supernatant solution into a reaction kettle, adding 10% of alkali solution by mass concentration for adjusting the pH value to be 10, reacting for 8-12 h at the temperature of 40 DEG C, adding dilute acid for adjusting the pH value to be neutral, extracting by using normal butanol and concentrating; and (3) adding ethyl acetate into a normal butanol concentrated solution for extracting, and re-crystallizing by using methanol to obtain a white crystal, which is a target product-astragaloside. The method is simple and easy to implement, the yield of a product is more than 0.08%, the purity of the product reaches more than 98%, the product has no chemical reagent residues, and the extraction process is environment-friendly and low in requirement on reagent and equipment and is suitable for large-scale industrial production.
Owner:西安岳达生物科技股份有限公司
Method for detecting radix astragali
InactiveCN104198600AImprove accuracyThe method is simple and accurateComponent separationAstragalosidePesticide residue
The invention belongs to the field of detection of the quality of traditional Chinese medicinal materials and in particular relates to a method for detecting the quality of radix astragali. The method comprises the steps of determining the content of astragaloside and calycosin contained in radix astragali and determining the pesticide residues of radix astragali. The method conduces to solving the technical problems that identification methods of traditional physicochemical properties are complex to operate and have limitation and the quality is difficult to regulate and control, has high degree of accuracy, is simple, convenient and fast to operate, is low in cost and has good application prospect and economic benefits.
Owner:LONGXI ZHONGTIAN PHARM CO LTD
Preparation method of controlled-release hydrogel with photo-thermal treatment and wound repair functions
ActiveCN110384654AExcellent photothermal performanceFunction increaseOrganic active ingredientsEnergy modified materialsCross-linkAstragaloside
The invention relates to the field of medical polymers, and discloses a preparation method of a controlled-release hydrogel with photo-thermal treatment and wound repair functions. The method comprises the following steps of: firstly, modifying dopamine on alginic acid to ensure that an o-diphenol side group is arranged on a molecular chain of the alginic acid, and simultaneously preparing coppersulfide @ melanin-PEG @ dopamine nanoparticles with good photo-thermal performance and radiotherapy effect; and then adding the copper sulfide @ dopamine nanoparticles into a dopamine-modified alginicacid solution, adding astragaloside with the function of promoting wound healing into the solution at the same time, and forming gel through a catalytic cross-linking system of horseradish peroxidaseand hydrogen peroxide. The hydrogel has good controlled release performance and can play a role in reducing administration dosage and reducing or avoiding toxic and side effects. The hydrogel prepared by the method has good biocompatibility, can be used for treating cancer cells by utilizing photo-thermal performance and radiotherapy, and can be combined with the astragaloside for promoting postoperative wound healing.
Owner:ZHEJIANG SCI-TECH UNIV
Cigarette harm reduction additive and preparation method thereof
The invention discloses a cigarette harm reduction additive, which comprises a plant extract composition, wherein the plant extract composition comprises licorice flavonoids, folium ginkgo extracts, epimedium flavonoids and astragaloside, the addition proportion of tobacco shreds into the additive is 0.001 to 0.05 weight percent, the additive comprises 15 to 84 weight percent of licorice flavonoids, 15 to 84 weight percent of folium ginkgo extracts, 0.3 to 20 weight percent of epimedium flavonoids and 0.5 to 25 precent wt of astragaloside and can further comprise plant extracts of total saponin from ophiopogon japonicus. The additive can externally inhibit the generation of harmful smoke substances such as polycyclic aromatic hydrocarbon, free radicals and the like, so the harm of smoke to people is effectively reduced. In addition, the invention also provides the preparation method of the cigarette harm reduction additive.
Owner:CHINA TOBACCO GUANGXI IND
Method for extracting astragaloside and polysaccharide from traditional Chinese medicine astragalus and its capsule preparation
InactiveCN1557826AHigh purityHigh activityOrganic active ingredientsSugar derivativesAstragalosideMedicinal herbs
The present invention relates to the preparation process and capsule preparation of astragaloside an and astragalus polysaccharide. Medicine material astragalus root is processed through water decoction, alcohol precipitation to obtain supernatant, concentration, butyl alcohol or sodium hydroxide solution extraction of the concentrate and decompression recovering to obtain astragaloside A; the alcohol precipitate is dried, water dissolved and alcohol precipitated repeatedly or ethanol washing, and drying to obtain astragalus polysaccharide. The astragaloside an and the astragalus polysaccharide are compounded with or without supplementary material, and the mixture is prepared into capsule. The capsule has the functions of benefiting vital energy, enhancing body resistance and nourishing lung and spleen and is used mainly in treating chronic bronchitis and obstructive pneumonectasis.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for extraction preparation of astragaloside
ActiveCN1844132AReduce pollutionExtraction Total Time SavingsSugar derivativesSteroidsAstragalosideHydrolysate
This invention publisizes an extraction procedure for Astragalus membranaceous as followings: a.The raw astragalus roots would be soaked in water, refluxing extracted. The extract is condensed to dense concrete; b. precipitates the dense concrete with ethanol, recover the supernatant and adjust PH value to no more than 7, hydrolyze at 60-120deg C for 0.5-3 hours; c. filter the hydrolysate, precipitate ,wash to colorless with alkali, then add water to PH 7; d. Dry the sediment. The purity of Astragalus membranaceous with this invention is up to 90-98%. The recovery ratio reaches to 1.13-0.18%. The manipulation time is reduced by 30-50%.
Owner:SHINEWAY PHARMA GRP LTD
Stem cell preparation for skin beauty and preparation method thereof
InactiveCN106420390APassage multiple increaseSolve key problems such as small number and low factor contentCosmetic preparationsPeptide/protein ingredientsAstragalosideCulture cell
The invention provides a stem cell preparation for skin beauty and a preparation method thereof, wherein the method comprises: a) providing stem cells acquired from a mammal animal; b) subjecting the stem cells to primary culture; c) subjecting the stem cells to subculture in a stem cell medium with vitamin A; d) changing with phenol-red-free culture liquid with astragaloside, and continuously culturing under hypoxic condition; e) subjecting the stem cells and the culture liquid to ultrasonic disruption and centrifuging, collecting supernate, and filtering to remove bacteria; f) detecting total protein concentration of the supernate, and adjusting the total protein concentration of the supernate. By using the preparation method of the stem cell preparation for skin beauty, the key problems of conventional culture methods, such as low in-vitro culture cell quantity and low factor content are solved.
Owner:JILIN TUO HUA BIOTECH
Antifatigue healthcare composition and preparation method thereof
ActiveCN103404847ANo side effectsSafe to takeAntinoxious agentsImmunological disordersAMERICAN GINSENG ROOTAstragaloside
The invention relates to an antifatigue healthcare composition comprising the following active components in parts by weight: 20-50 parts of icariin, 5-20 parts of astragaloside, 20-40 parts of sealwort extractive and 10-30 parts of American ginseng extractive. The invention further discloses a preparation method of the composition. The antifatigue healthcare composition has the functions of improving exorbitance of brainwork and physical work, tonifying Qi, nourishing yin, tonifying spleen, moistening lung, remarkably refreshing brain, improving memory, boosting immunity, slowing down aging and the like.
Owner:GANSU YALAN PHARMA
<<Xiaokewan>> medicine quality control method for treating diabetes
ActiveCN1588037APerfect quality control methodQuality improvementComponent separationColor/spectral properties measurementsAstragalosidePharmacy
The invention provides a quantity control method for a kind of antidiabetic, which belongs to medication field. Concretely, It relates to the quantity control method of the combination of the Traditional Chinese medicine with the Westen medicine. It adds the following method to the original quantity control standard. (1): adopting thin layer chromatography, contrasting to to identify if the antidiabetics contain astragalus. (2) adopting thin layer chromatography to comparing the Wuweizisu A to schisandra chinenis positive to indetify if the antidiabetics contain kadsura longepedunculata (3) Adopting high performance chromatography to assay the percentage content of the weigh of the glyburide is 0.07%-0.11%. The invention improves the quantity control technique of the antidiaetics, increase the identification method of if the abtidiabetics with high sepcificity contain astragalus and schisandra and can make a quantitative assay for the glyburide content, so as to control the quantity of the products to secure the safety to human pharmacy.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
White spirit with anti-fatigue function and production method thereof
ActiveCN103937655AHigh content of active ingredientsAvoid complex ingredientsAntinoxious agentsAlcoholic beverage preparationAstragalosideBetaine
The invention provides white spirit with an anti-fatigue function, wherein 0.15g-0.16g of ginseng extract, 0.02g-0.03g of astragalus root extract, 0.10g-0.15g of horny goat weed extract and 2.0g-2.5g of wolfberry extract are added to 33-68%Vol white spirit per liter so that the content of total saponins of panax ginseng is greater than or equal to 60mg / L, the content of icariin is greater than or equal to 20mg / L, the content of astragaloside is greater than or equal to 5mg / L and the content of glycine betaine is greater than or equal to 490mg / L in the white spirit by Re. The white spirit with the anti-fatigue function has the advantages that ginseng, astragalus root, horny goat weed and wolfberry are taken as raw materials and the active ingredients total saponins of panax ginseng, astragaloside, icariin and glycine betaine of the raw materials are extracted and added to the white spirit, respectively, and therefore, the original flavor, color and luster as well as taste of the white spirit are remained, the white spirit is clear in ingredients and stable in content, and meanwhile, the white spirit has accurate anti-fatigue function; the white spirit with the anti-fatigue function is yellowish in color, aromatic and elegant, soft, lasting sweet and smooth, harmonious in various aromas, and smooth and clear in taste; judged by professional wine taster, the white spirit with the anti-fatigue function is close to common kaoliang spirit or pure grain wine in style.
Owner:JING BRAND
Quick-drying type plant scar removal, black removal and restoration membrane
ActiveCN104382766ATo promote metabolismImprove permeabilityCosmetic preparationsToilet preparationsCentella asiatica extractAstragaloside
The invention belongs to the field of light industry, in particular to a quick-drying type plant scar removal, black removal and restoration membrane which comprises the following raw materials in percentage by weight: 0.5-1% of chitosan quaternary ammonium salt, 1-2% of polyvinylpyrrolidone, 0.5-1% of centella asiatica extractive, 0.3-0.7% of astragaloside, 5-10% of an aloe extracting solution, 5-10% of a coix seed extracting solution, 3-5% of a portulaca oleracea extracting solution, 3-5% of VB3, 35-45% of ethyl alcohol, 6-10% of propylene glycol and the balance of water, wherein the sum of percentages by weight of the raw materials is 100%. According to the quick-drying type plant scar removal, black removal and restoration membrane, a continuous gas-permeable membrane having the property of positive ions is formed at the scars, so that the duration of stay of scar removing effective constituents is prolonged; the membrane has a certain pressure, so that effective constituents can easily permeate the skin, and the scar removing, black removal, restoration, antibacterial and itching relieving effects are obvious.
Owner:欧霖拱
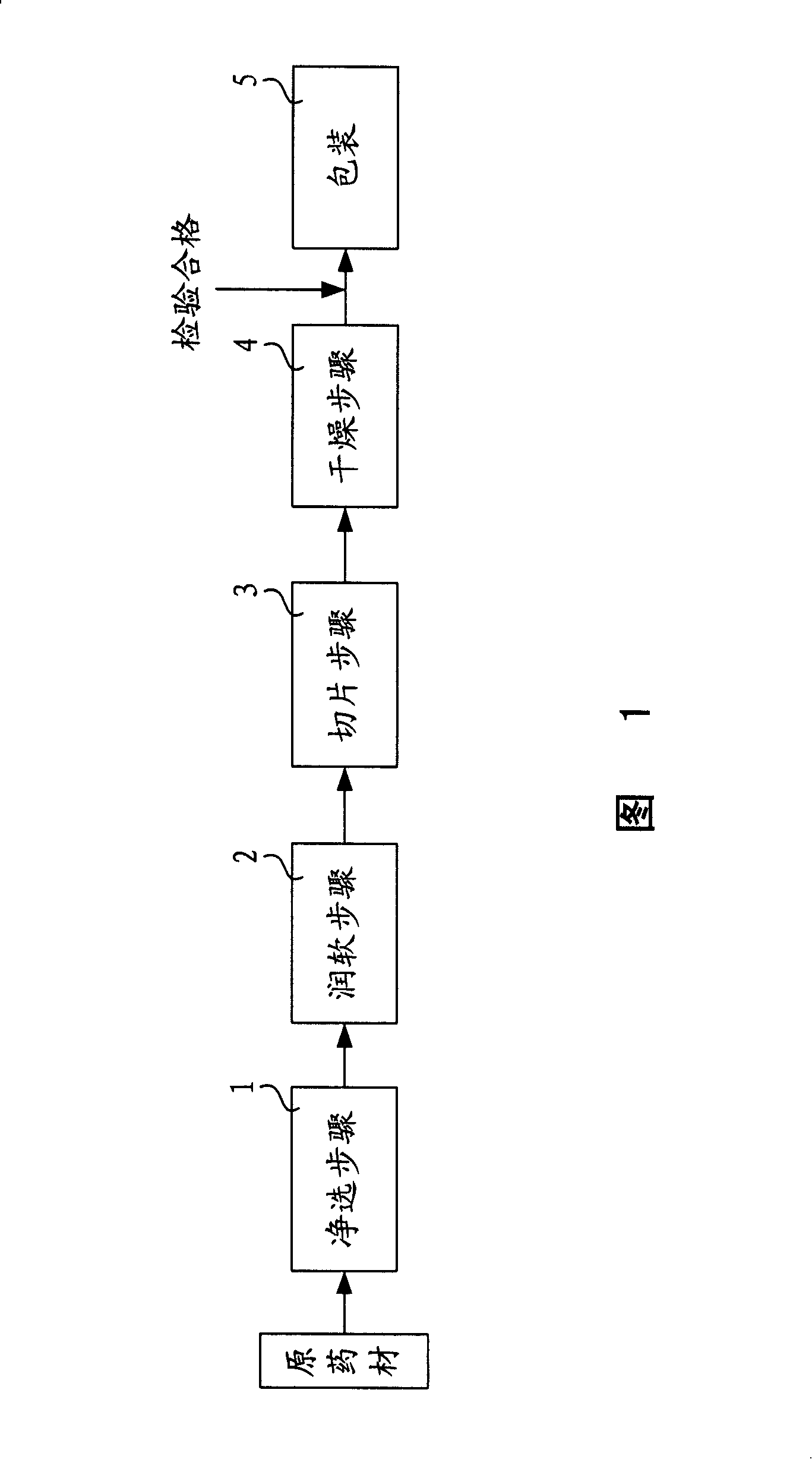
Technology of processing astragalus root decoction pieces
InactiveCN101229219AQuality improvementQuality assurancePlant ingredientsAstragalosideAstragalus Root
The invention discloses a processing technique of astragalus decoction pieces; the invention is characterized by comprising the following steps: cleaning and selecting step, smoothing and softening step by 2 to 24 hours, slicing step, and drying step under the temperature of 20 to 85 DEG C. By being proved by experiment and being unified in standard and parameter, the technique of the invention is steady and the processing quality is accordant, so the technique improves the content of astragaloside and strengthens the medical efficacy of astragalus.
Owner:上海德华国药制品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com