Method for preparing purity astragaloside
A technology of astragaloside IV and pure product, applied in the field of medicine, can solve the problems of unsuitability for industrial production, difficult extraction and separation, high production cost, and achieve the effects of high product yield, reduction of environmental pollution and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
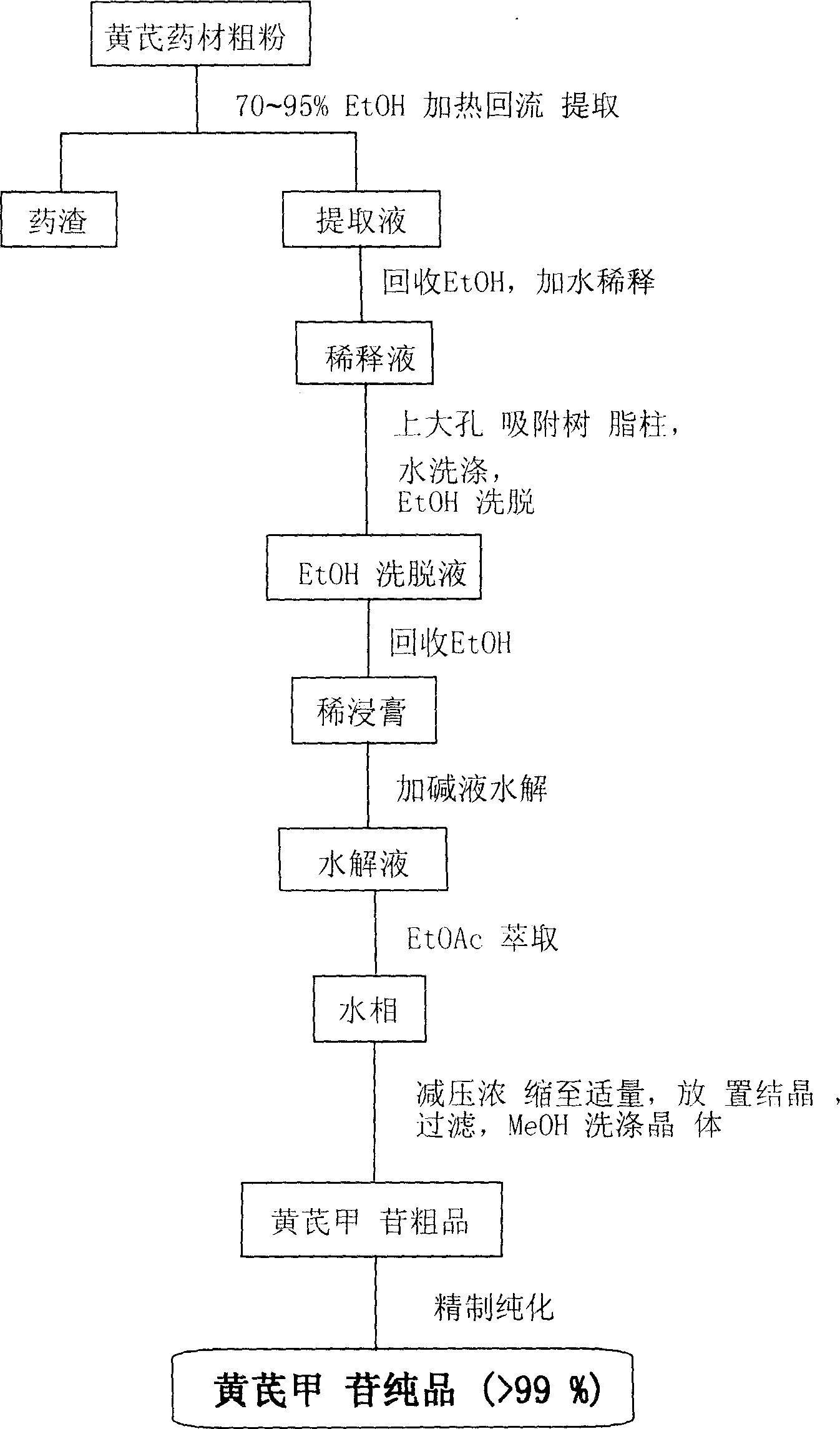
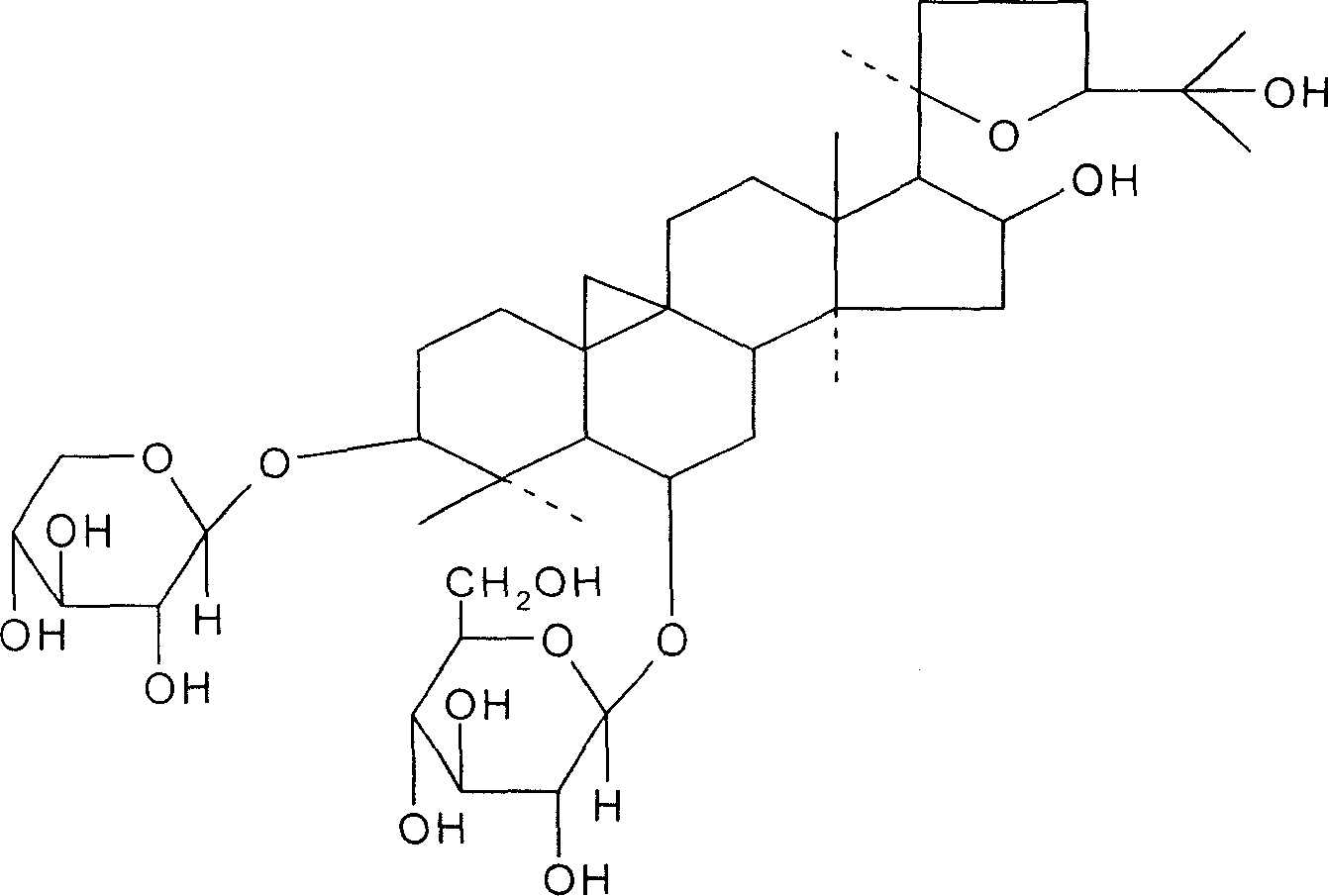
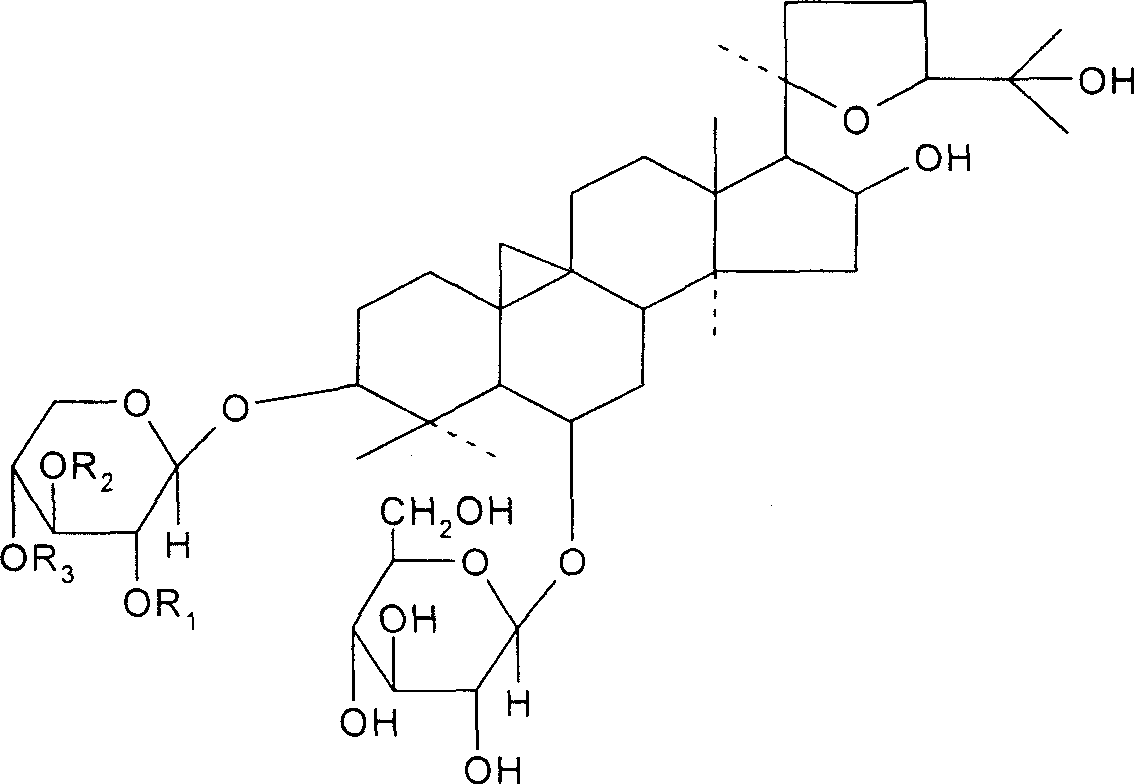
[0025] Take 10 kg of astragalus medicinal materials, pulverize it into coarse powder, add 70% ethanol to the reflux extraction tank, add 70% ethanol to heat and reflux for 3 times, the first 120 minutes, the second and third 90 minutes each, the extracts are combined, and the ethanol is recovered under reduced pressure. Add water to dilute, put the water on the D101 macroporous adsorption resin column (10kg), and the flow rate of the sample is 2~5BV / h. The 70% ethanol of the resin bed volume is eluted, the ethanol eluent is collected, the ethanol is recovered under reduced pressure to an appropriate amount (about 1.5 kg of the extract), and 10 g of NaOH solution is added for alkaline hydrolysis. Neutralized to neutrality, followed by extraction with ethyl acetate 3 times, the aqueous phase was concentrated under reduced pressure to an appropriate volume, left to crystallize, filtered, and washed with a small amount of methanol to obtain 9 g of astragaloside IV crude product, wh...
Embodiment 2
[0027] Take 20 kg of astragalus medicinal materials, pulverize it into coarse powder, add 80% ethanol to the reflux extraction tank, add 80% ethanol for heating and reflux extraction for 3 times, each time for 90 minutes, combine the extracts, recover the ethanol under reduced pressure, add water to dilute, and add HPD100 to the water. Porous adsorption resin column (20kg), the sample flow rate is 2-5BV / h, after the liquid is applied, wash with deionized water with 4 times the volume of the resin bed, and then wash with 80% ethanol with 4 times the volume of the resin bed Take off, collect the ethanol eluent, recover ethanol under reduced pressure to an appropriate amount (extract about 3.0kg), add 20g KOH solution to carry out alkali hydrolysis, hydrolysis temperature 50 ℃, after the hydrolysis is complete, neutralize with dilute acid to neutrality, then use Extracted with ethyl acetate 4 times, concentrated the aqueous phase to an appropriate volume under reduced pressure, le...
Embodiment 3
[0029] Take 100kg of astragalus medicinal materials, pulverize it into coarse powder, add 70% ethanol to the reflux extraction tank, add 70% ethanol, heat and reflux for 3 times, the first time is 120 minutes, the second and third times are 90 minutes each, the extracts are combined, and the ethanol is recovered under reduced pressure. Diluted with water, the water was placed on a D101 macroporous adsorption resin column (100kg), and the loading flow rate was 2-5BV / h. After the drug solution was applied, wash with deionized water with 4 times the volume of the resin bed, and then 5 times the volume of the resin bed. The 80% ethanol of the resin bed volume is eluted, the ethanol eluent is collected, the ethanol is recovered under reduced pressure to an appropriate amount (about 13 kg of the extract), and 150 g of KOH solution is added to carry out alkali hydrolysis. The hydrolysis temperature is 20 ° C. And to neutrality, followed by extraction with ethyl acetate 3 times, the aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com