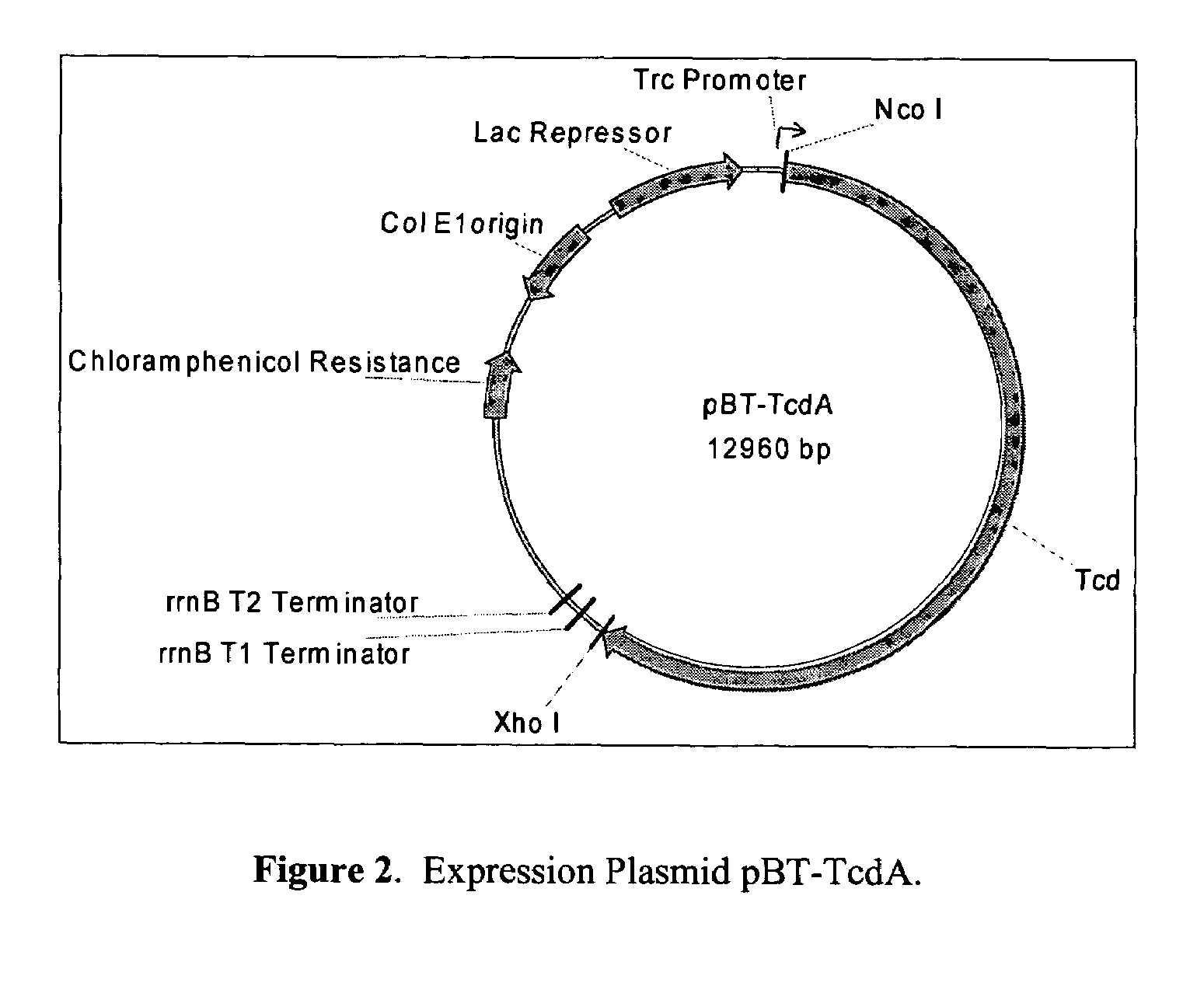
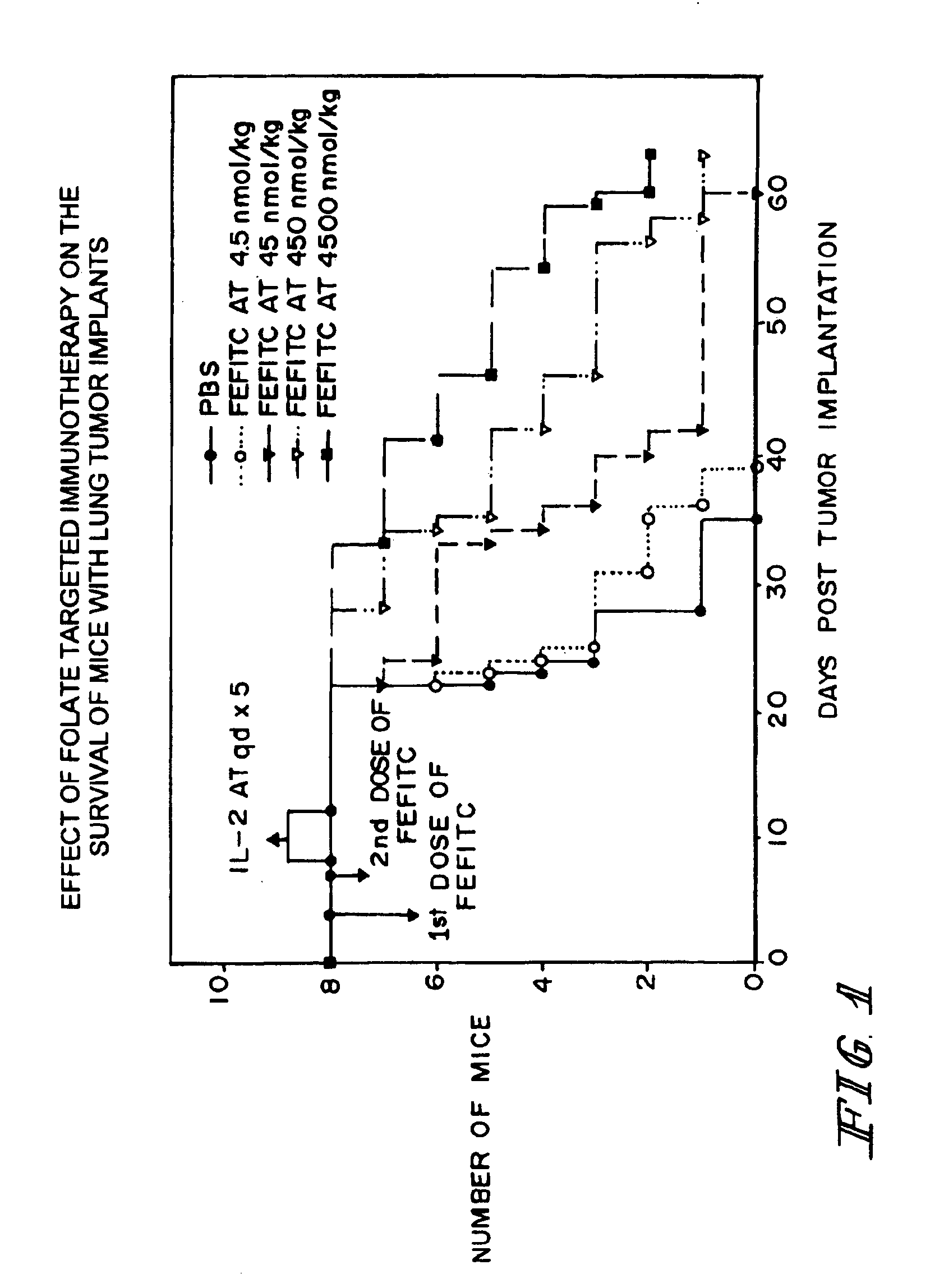
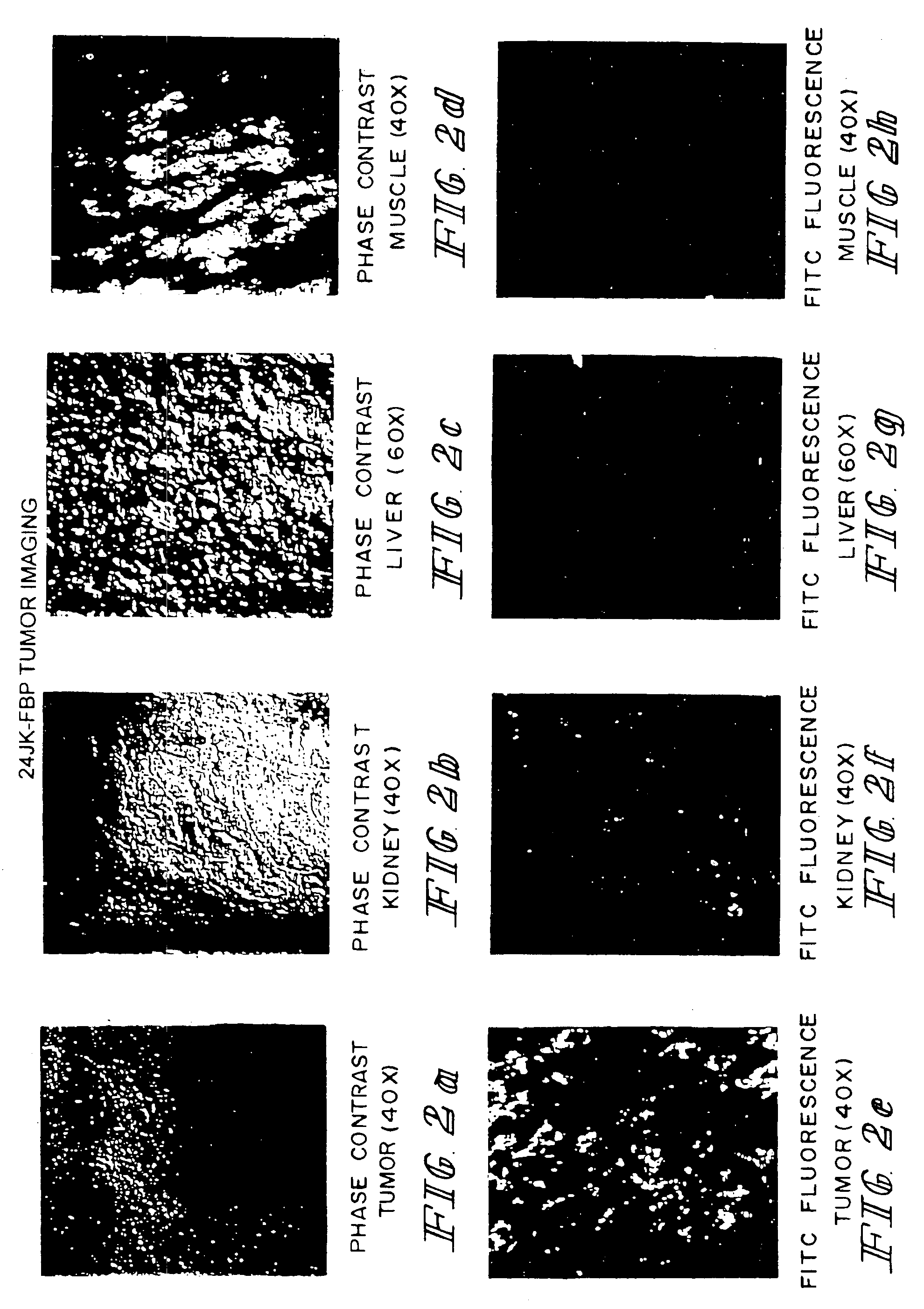
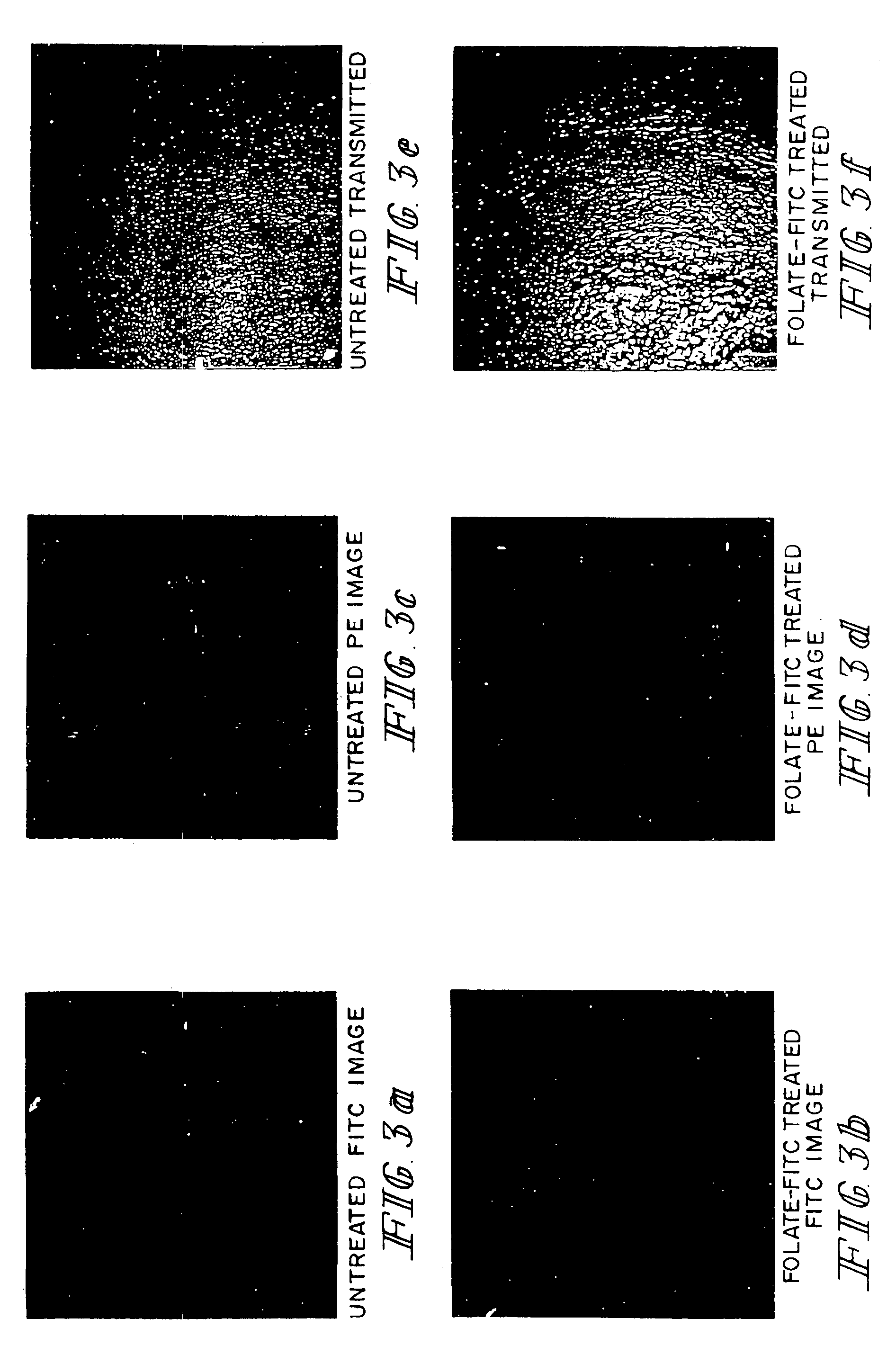
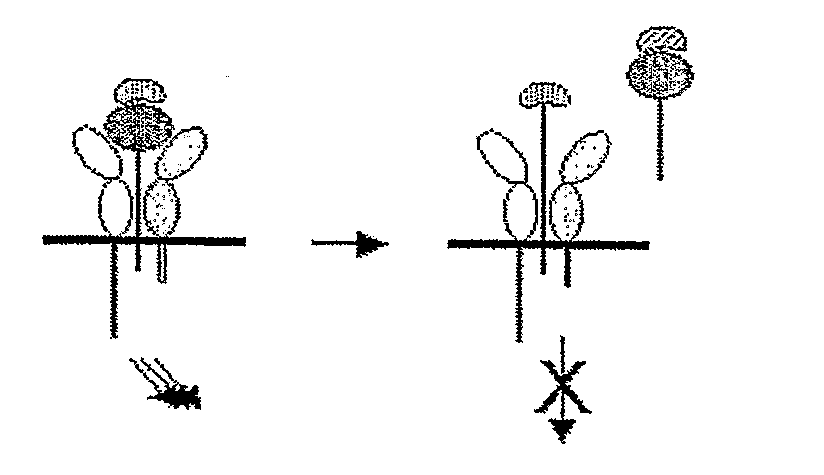
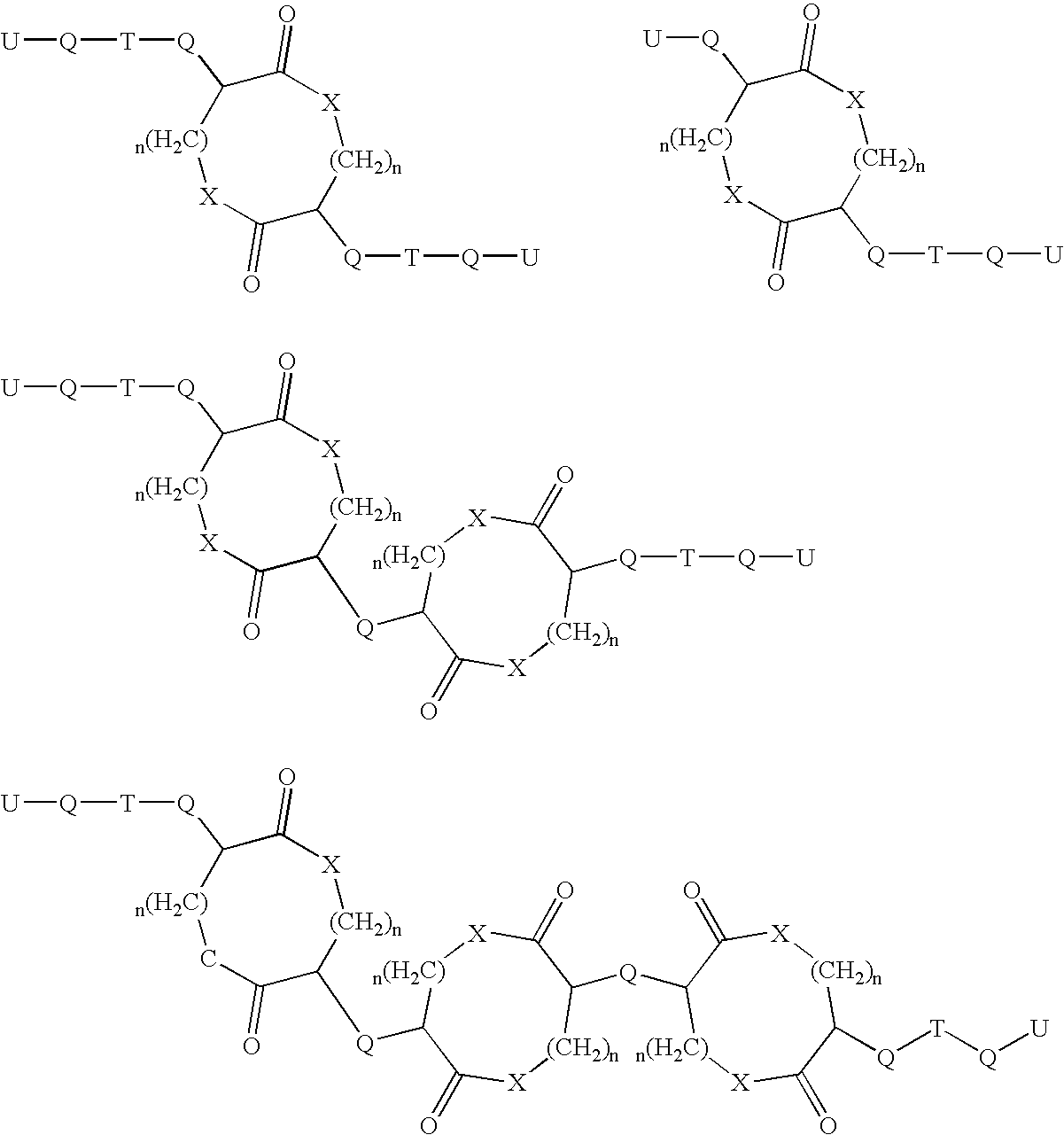
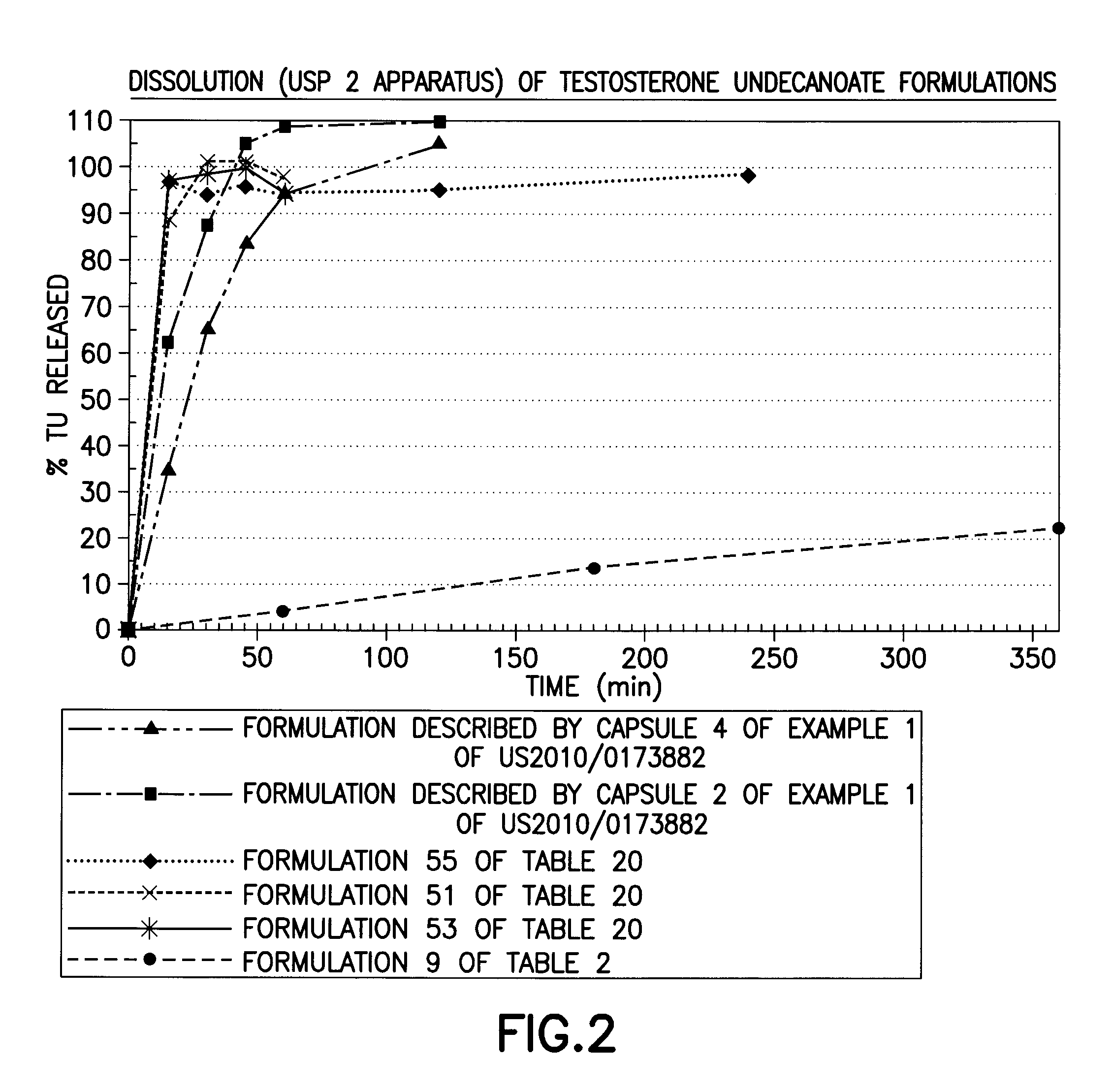
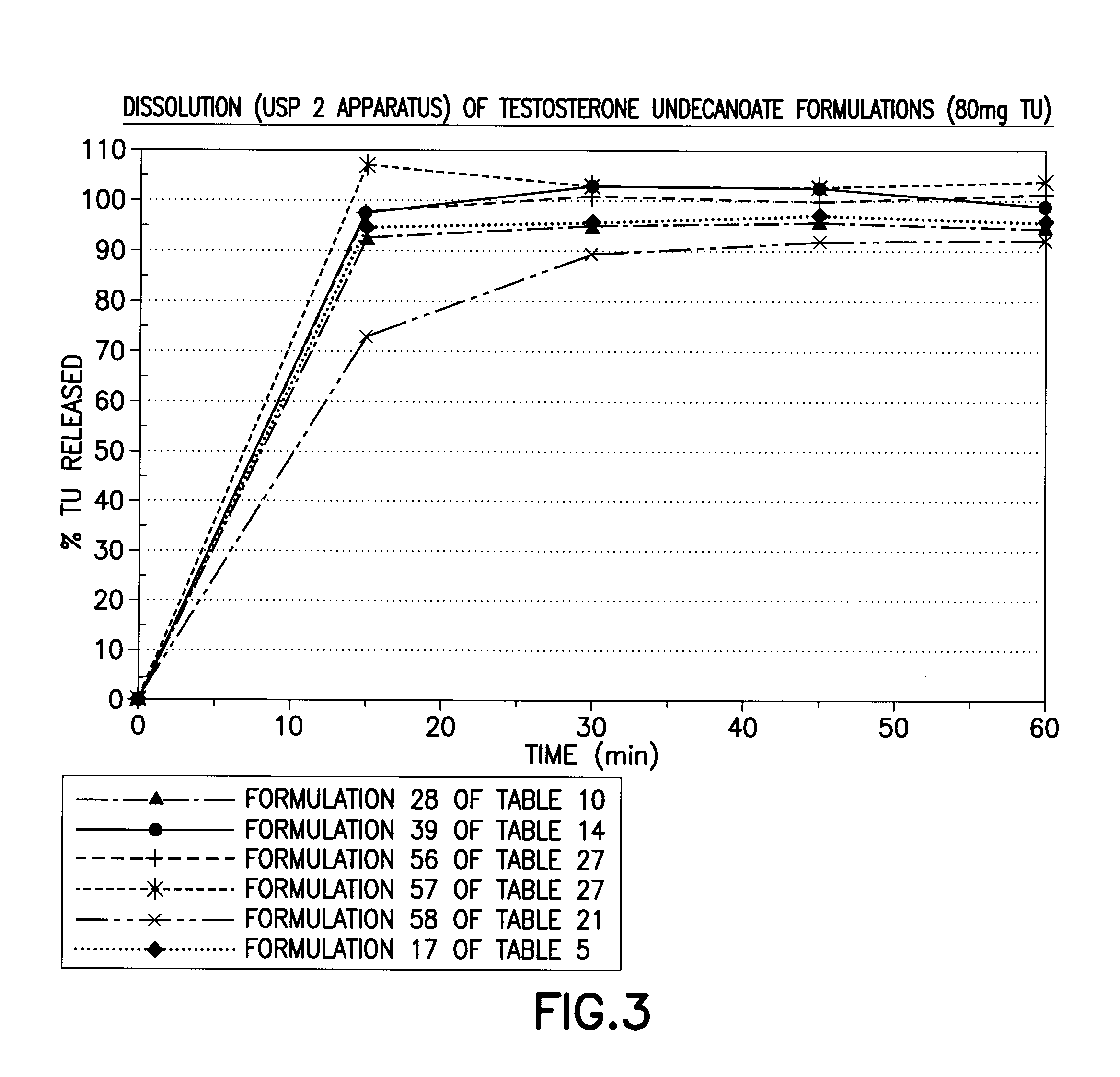
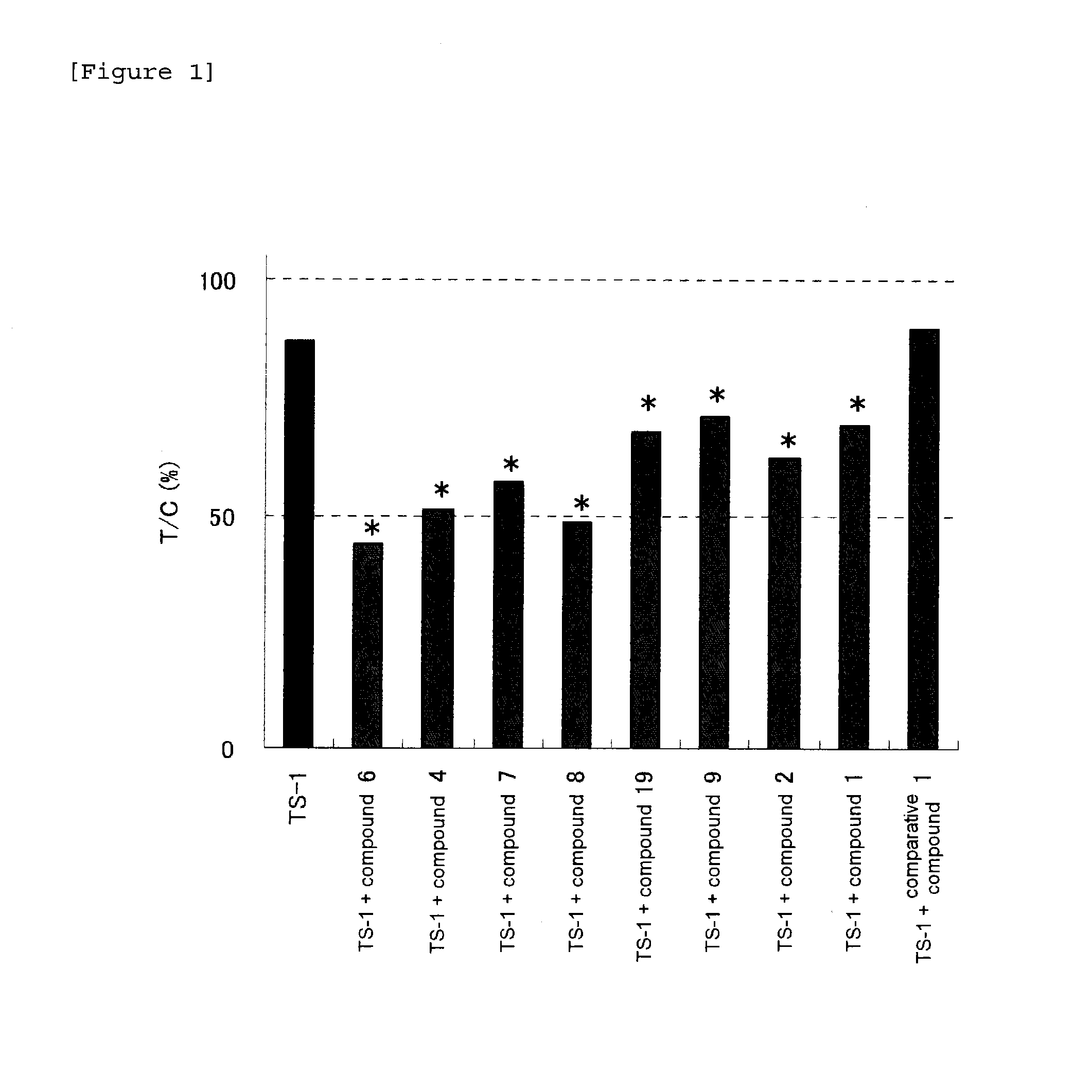
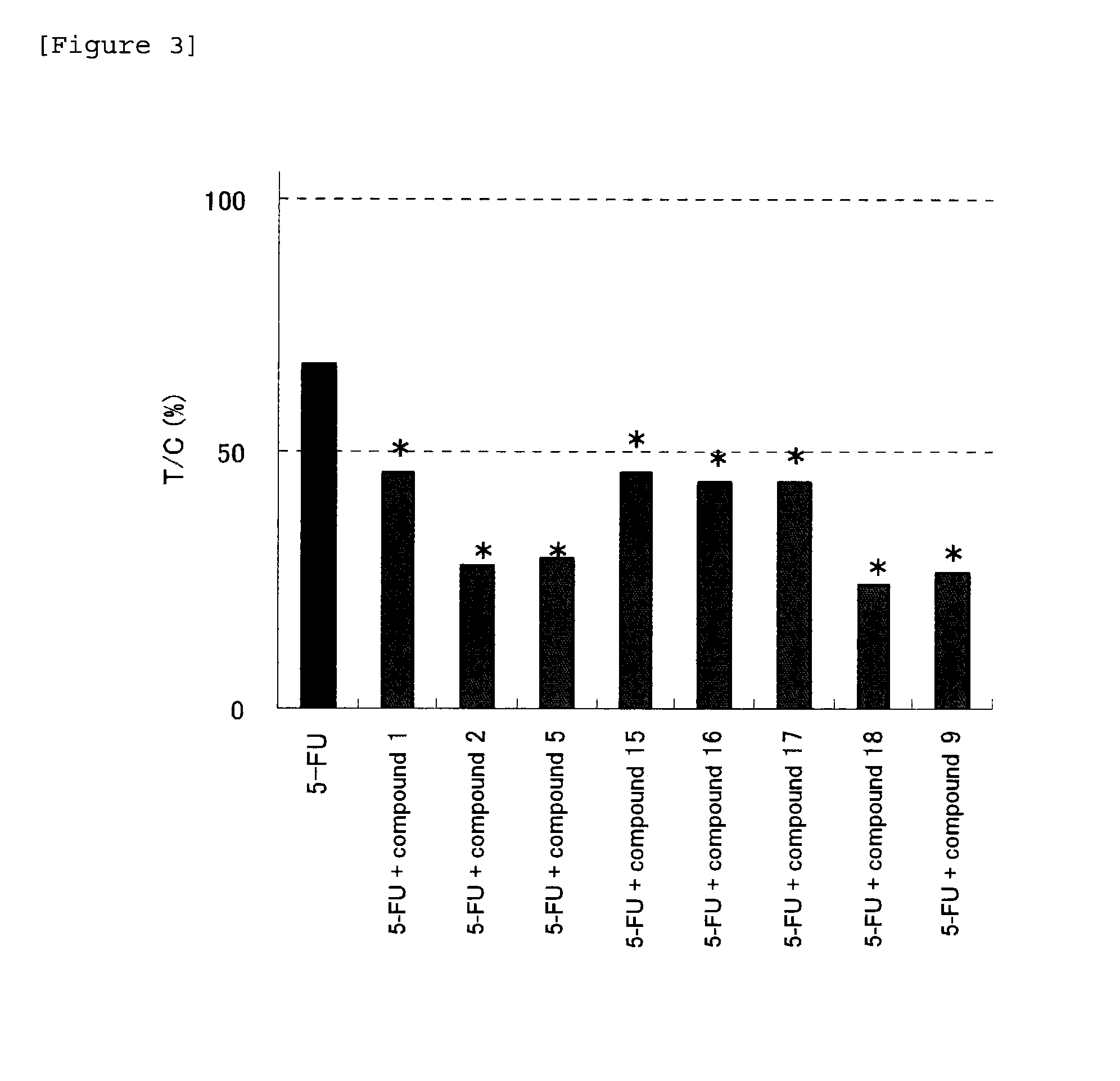
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
761 results about "Potentiator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In clinical terms, a potentiator is a reagent that enhances sensitization of an antigen. Potentiators are used in the clinical laboratory for performing blood banking procedures that require enhancement of Agglutination (biology) to detect the presence of antibodies or antigens in a patient's blood sample. Examples of potentiators include albumin, LISS (low ionic-strength saline) and PEG (polyethylene glycol). Potentiators are also known as enhancement reagents.
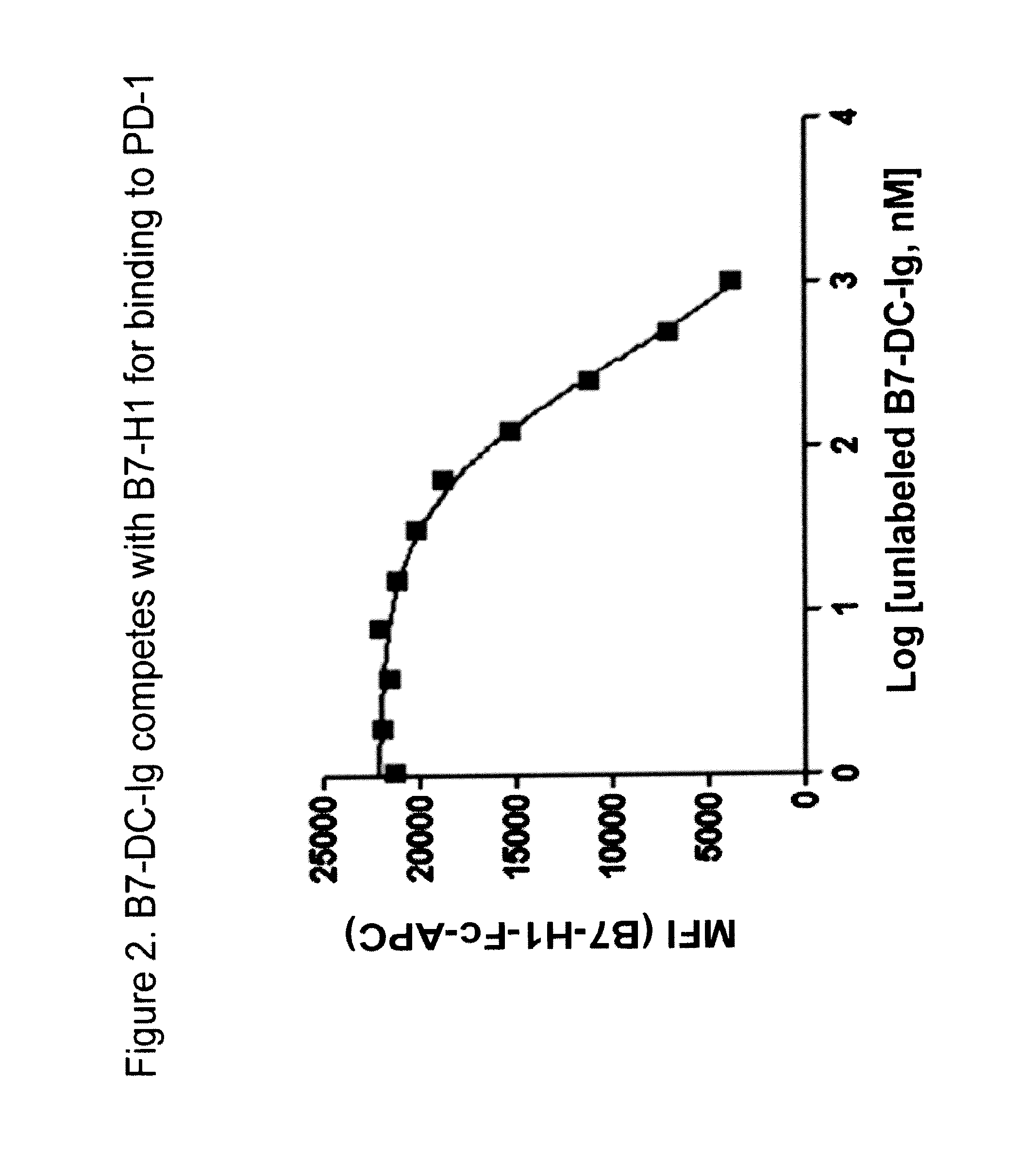
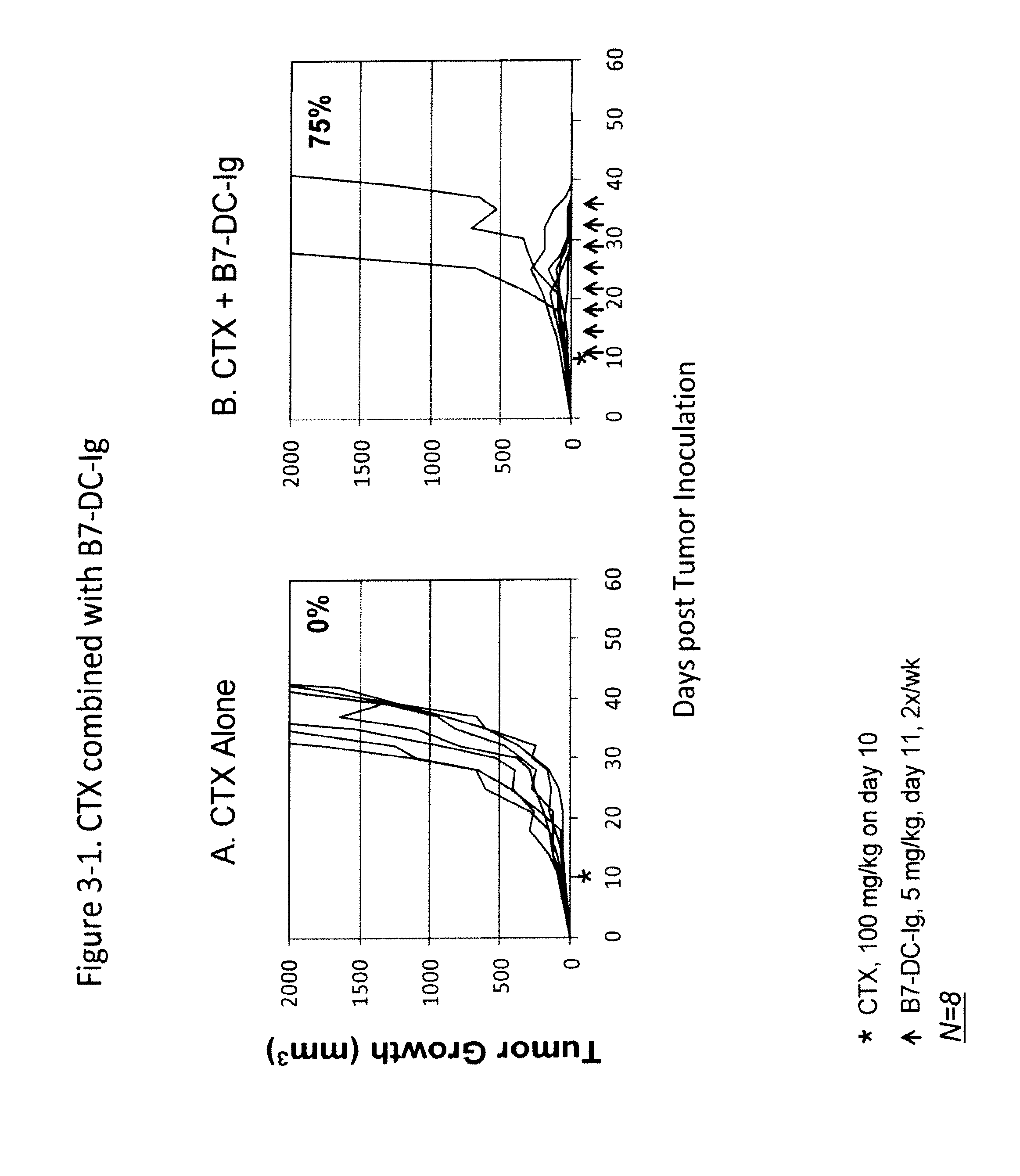
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
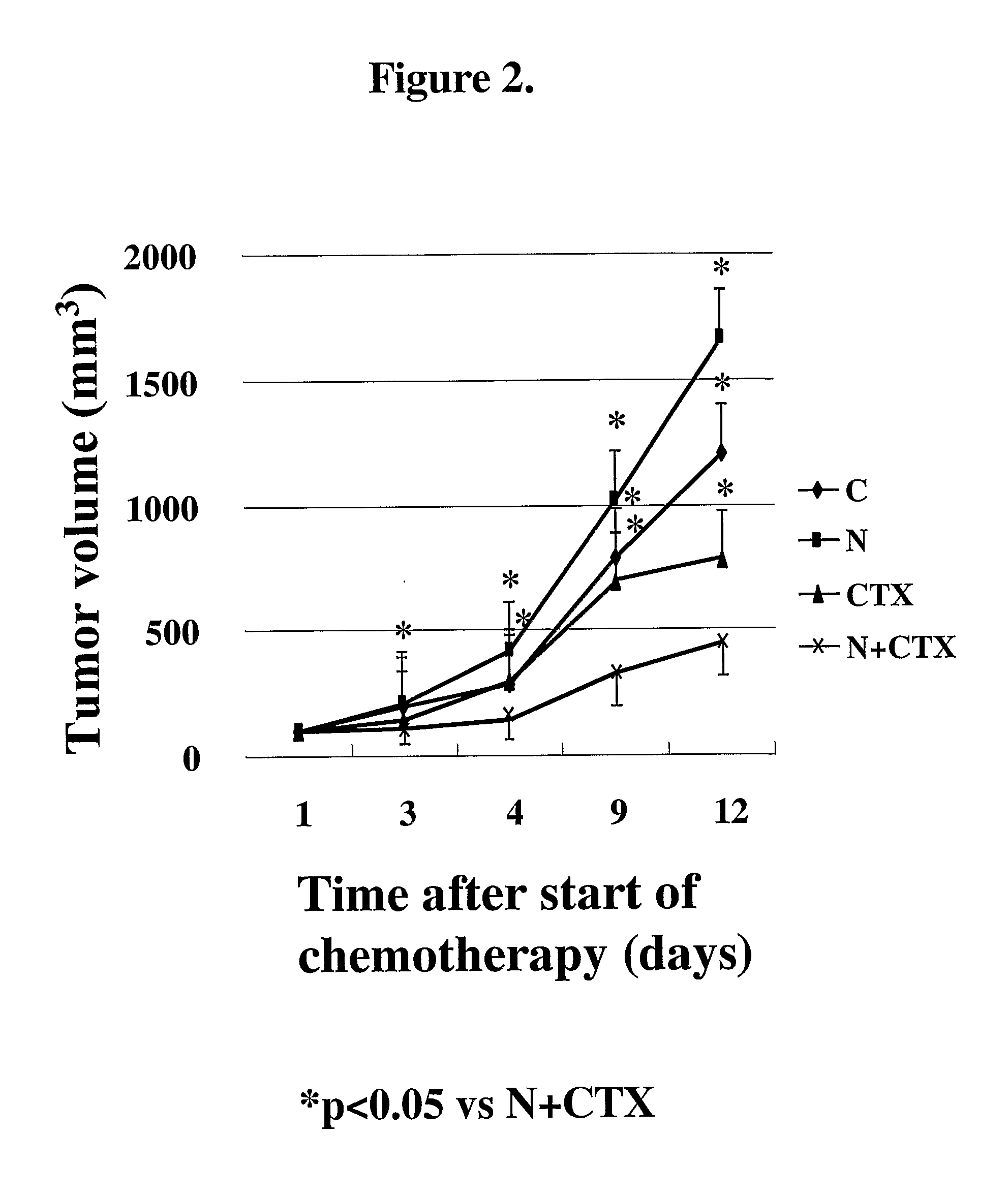
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
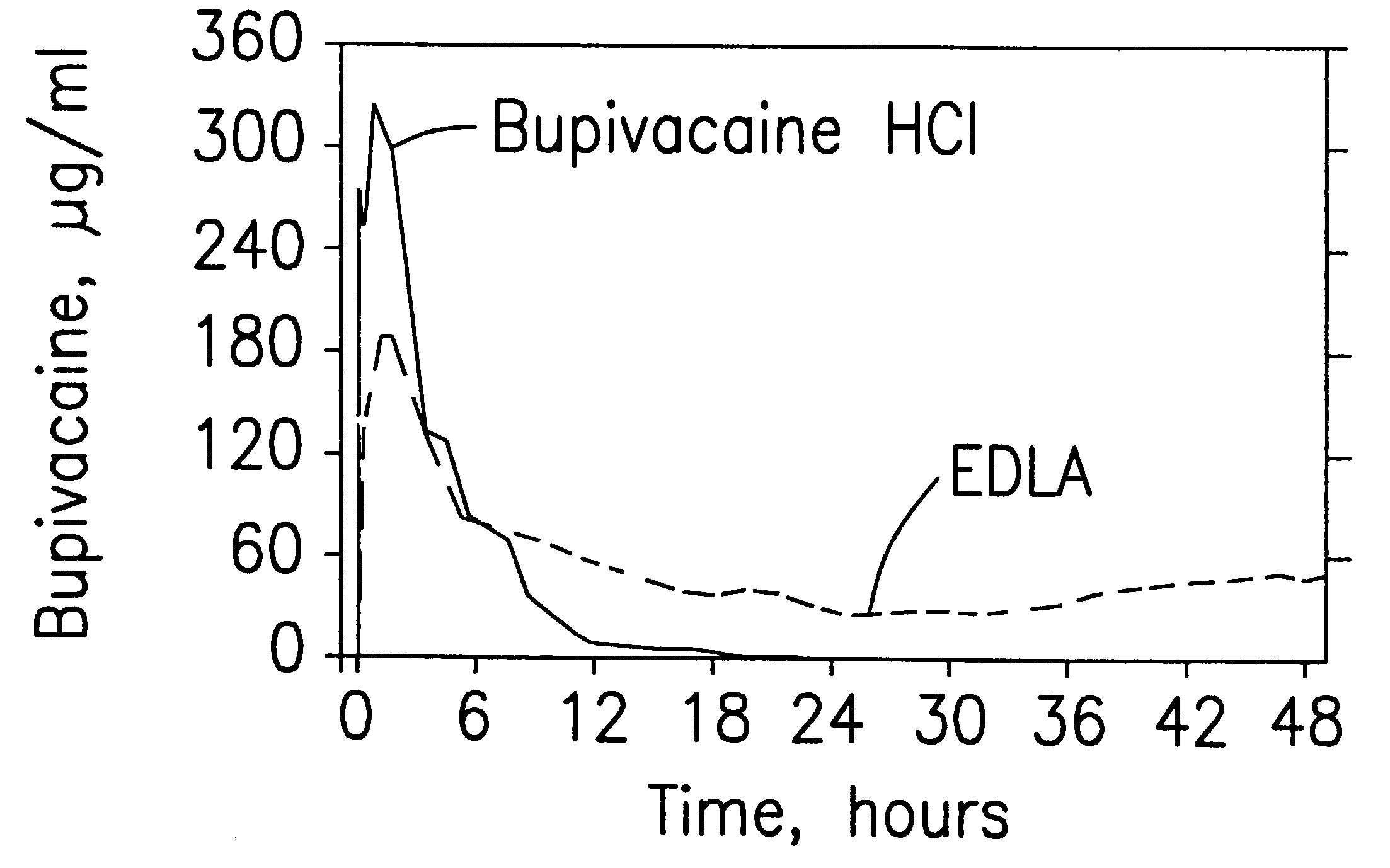
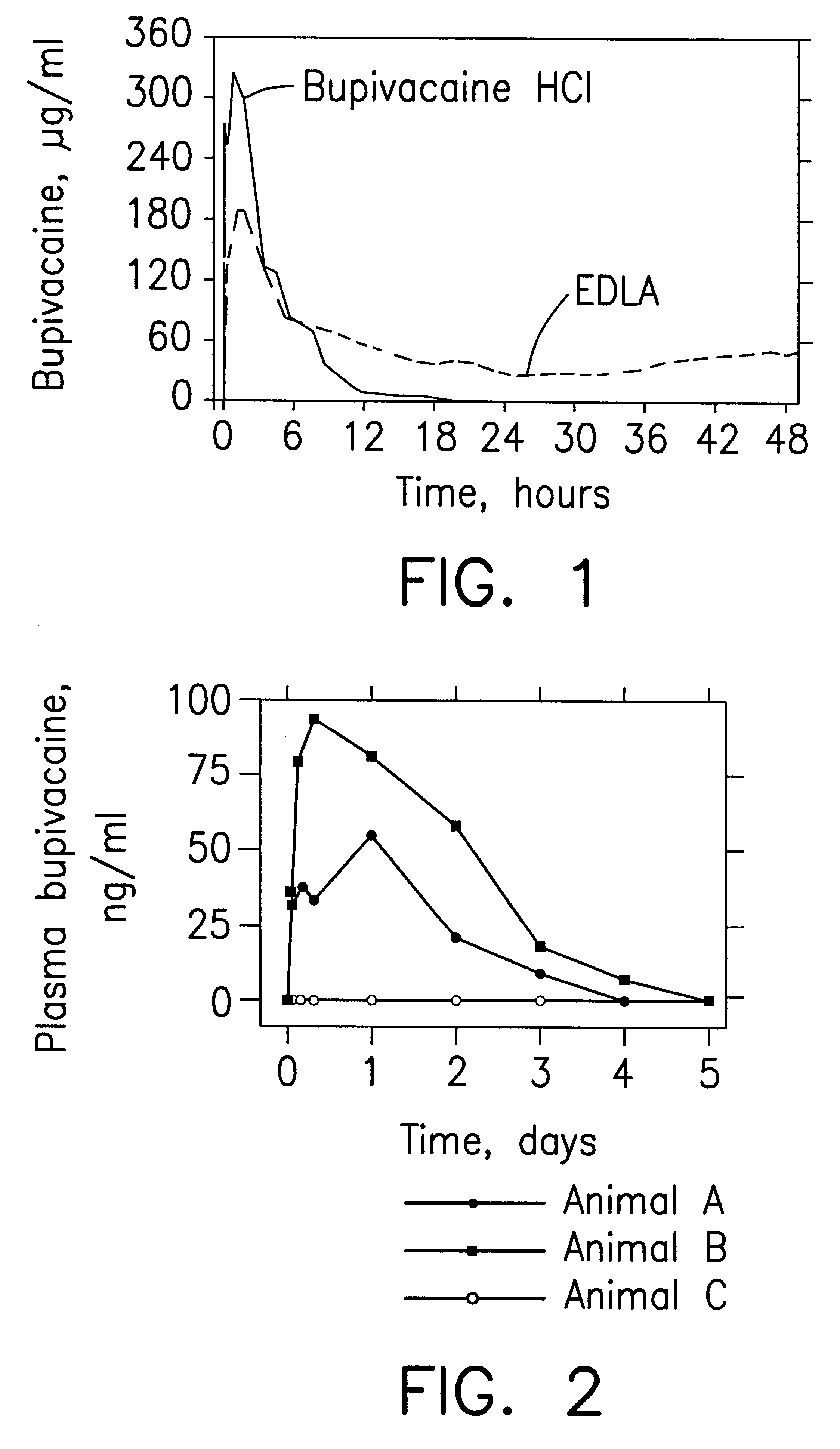
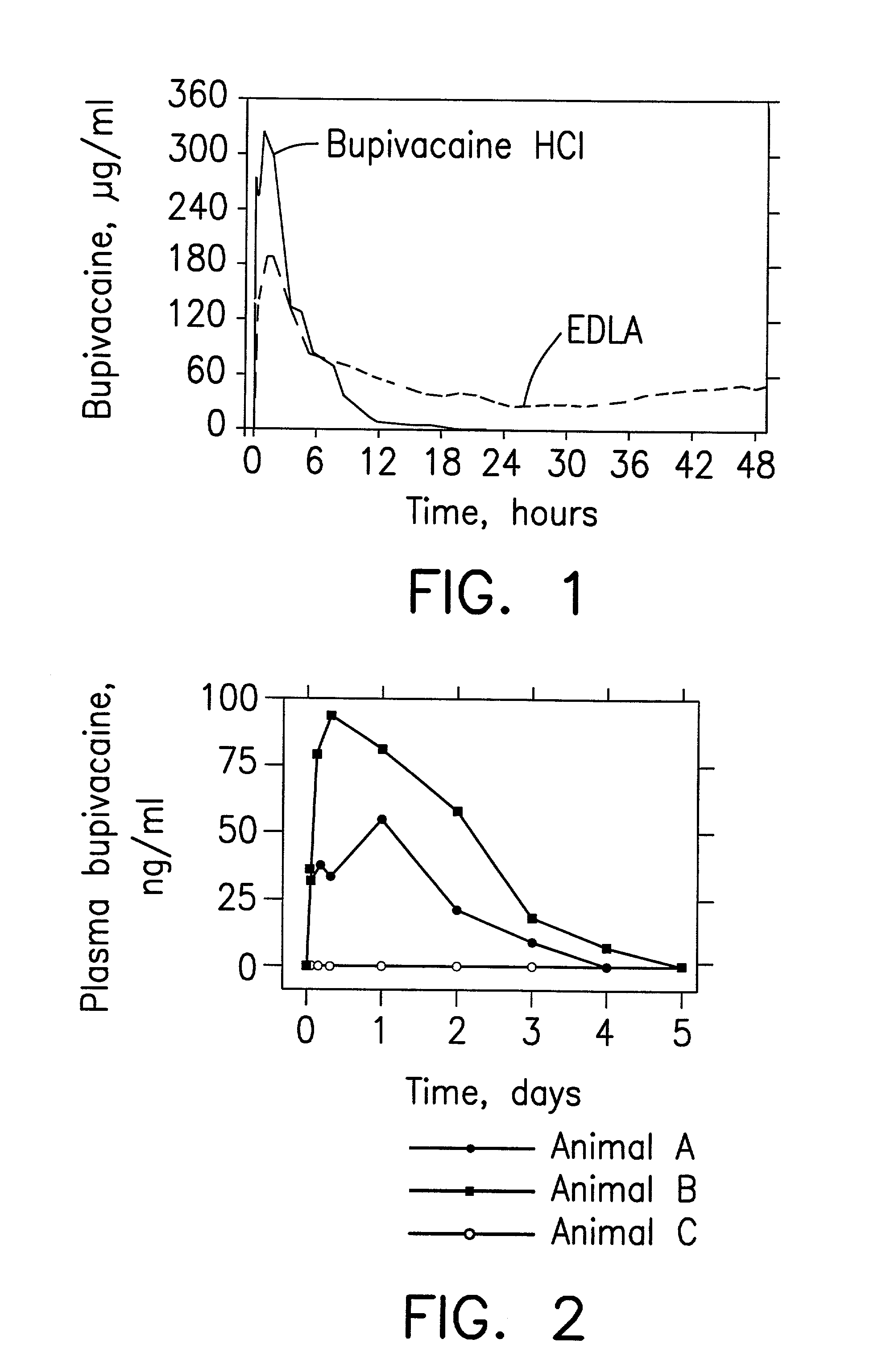
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Anti-infection augmentation foamable compositions and kit and uses thereof
Anti-infective foamable composition and kits include a foamable carrier; a therapeutically safe and effective concentration of an anti-infective agent; an augmenting agent selected from the group consisting of a keratolytic agent and a skin penetration enhancer; and a propellant. The composition is housed in a container and upon release is expandable to form a breakable foam. The foamable carrier is selected to generate a foam of good or excellent quality in the presence of the augmenting agent and anti-infective agent. Methods for treating, alleviating or preventing a disorder of the skin, a body cavity or mucosal surface, wherein the disorder involves a fungal, bacterial or viral infection as one of its etiological factors, is described.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Enhanced transport using membrane disruptive agents
Owner:UNIV OF WASHINGTON +1
Dimeric small molecule potentiators of apoptosis
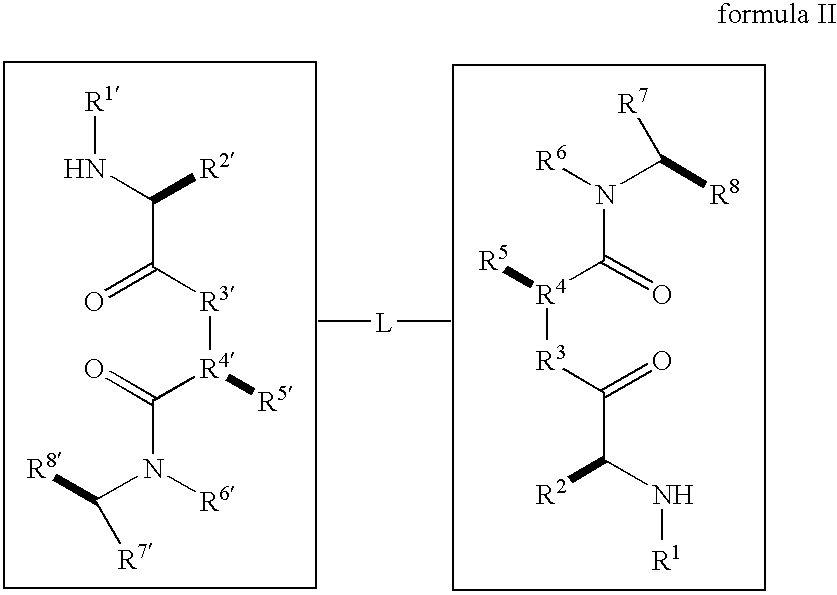
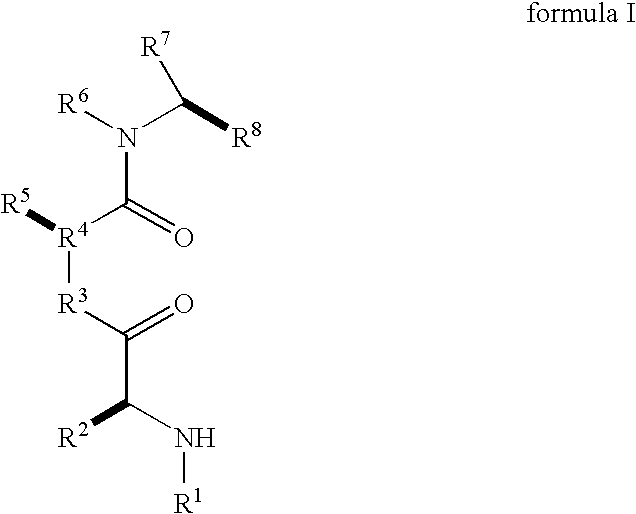
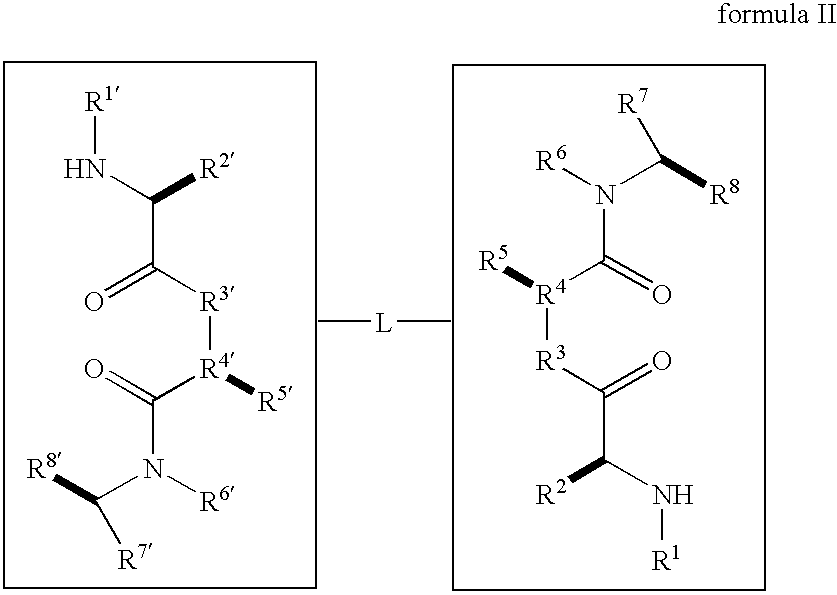
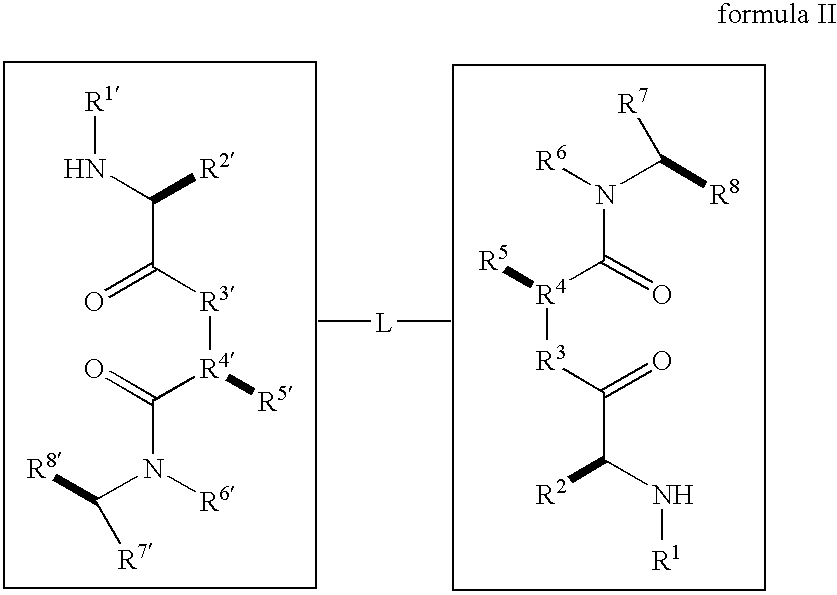
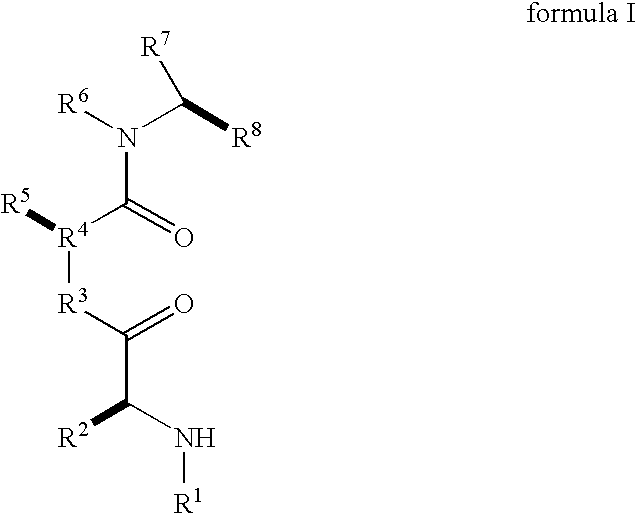
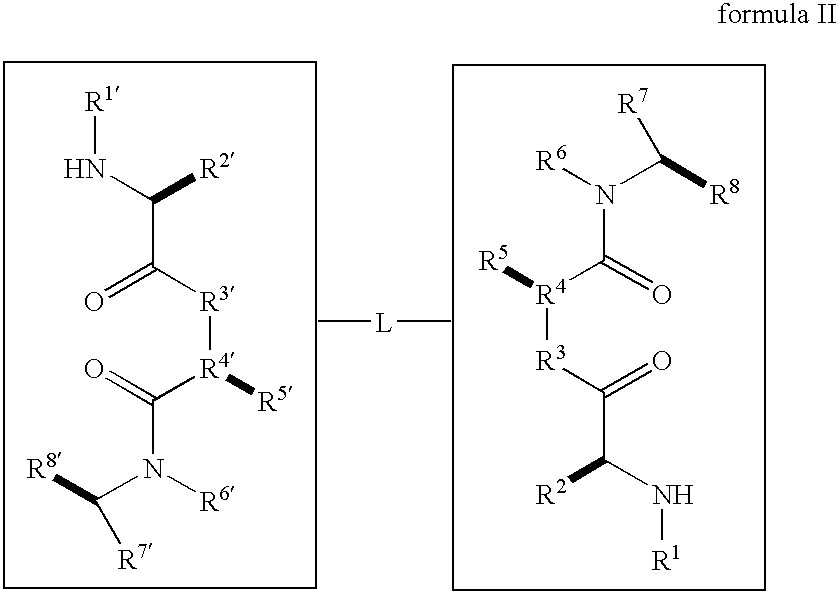
Caspase activity and apoptosis are promoted using active, dimeric Smac peptide mimetics of the general formula M1-M2, wherein moieties M1 and M2 are monomeric Smac mimetics and L is a covalent linker. Target cancerous or inflammatory cells are contacted with an effective amount of an active, dimeric Smac mimetic, and a resultant increase in apoptosis of the target cells is detected. The contacting step may be effected by administering to a pharmaceutical composition comprising a therapeutically effective amount of the dimeric mimetic, wherein the individual may be subject to concurrent or antecedent radiation or chemotherapy for treatment of a neoproliferative pathology.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Mixing and matching TC proteins for pest control
InactiveUS7491698B2High activityEffective control of widerBiocidePeptide/protein ingredientsHeterologousToxin protein
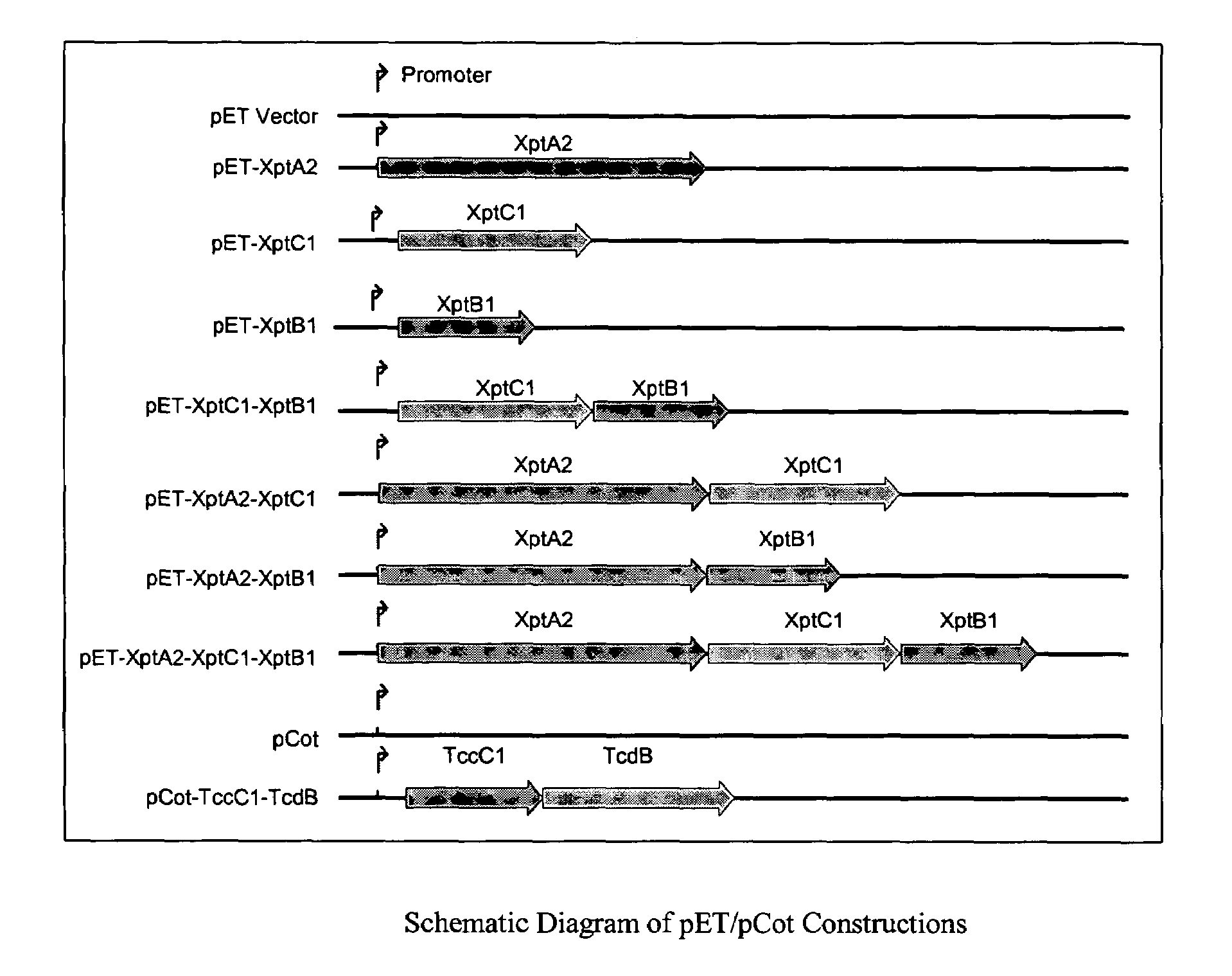
The subject invention relates to the surprising discovery that toxin complex (TC) proteins, obtainable from Xenorhabdus, Photorhabdus, and Paenibacillus, can be used interchangeably with each other. In particularly preferred embodiments of the subject invention, the toxicity of a “stand-alone” TC protein (from Photorhabdus, Xenorhabdus, or Paenibacillus, for example) is enhanced by one or more TC protein “potentiators” derived from a source organism of a different genus from which the toxin was derived. As one skilled in the art will recognize with the benefit of this disclosure, this has broad implications and expands the range of utility that individual types of TC proteins will now be recognized to have. Among the most important advantages is that one skilled in the art will now be able to use a single set of potentiators to enhance the activity of a stand-alone Xenorhabdus protein toxin as well as a stand-alone Photorhabdus protein toxin. (As one skilled in the art knows, Xenorhabdus toxin proteins tend to be more desirable for controlling lepidopterans while Photorhabdus toxin proteins tend to be more desirable for controlling coleopterans.) This reduces the number of genes, and transformation events, needed to be expressed by a transgenic plant to achieve effective control of a wider spectrum of target pests. Certain preferred combinations of heterologous TC proteins are also disclosed herein. Other objects, advantages, and features of the subject invention will be apparent to one skilled in the art having the benefit of the subject disclosure.
Owner:DOW AGROSCIENCES LLC
Penetration Enhancer Combinations for Transdermal Delivery
InactiveUS20070269379A1Easy to transportLess irritatingOrganic active ingredientsBiocideHigh-Throughput Screening MethodsIrritation
A high throughput screening and isolation system identifies rare enhancer mixtures from a candidate pool of penetration enhancer combinations. The combinations are screened for high penetration but low irritation potential using a unique data mining method to find new potent and safe chemical penetration enhancer combinations. The members of a library of chemical penetration enhancer combinations are screened with a high throughput device to identify “hot spots”, particular combinations that show higher chemical penetration enhancement compared to neighboring compositions. The irritation potentials of the hot spot combinations are measured to identify combinations that also show low irritation potential. A active component, such as a drug, is then combined with the combination in a formulation which is tested for the ability of the drug to penetrate into or through skin. It is then assessed whether the formulation can deliver the quantity of drug required, and animal tests are conducted to confirm in vivo the ability of the chemical penetration enhancer combinations to facilitate transport of sufficient active molecules across the skin to achieve therapeutic levels of the active molecule in the animal's blood. The invention provides specific unique and rare mixtures of chemical penetration enhancers that enhance skin permeability to hydrophilic macromolecules by more than 50-fold without inducing skin irritation, such as combinations of sodium laurel ether sulfate and 1-phenyl piperazine, and combinations of N-lauryl sarcosine and Span 20 / sorbitan monolaurate.
Owner:RGT UNIV OF CALIFORNIA
Method of treatment using ligand-immunogen conjugates
InactiveUS7033594B2Improve recognitionEnhance endogenous immune response-mediated eliminationAntibacterial agentsBiocideBinding siteCytotoxicity
A method and pharmaceutical composition are provided for enhancing the endogenous immune response-mediated elimination of a population of pathogenic cells in a host animal wherein the pathogenic cells preferentially express, uniquely express, or overexpress a binding site for a particular ligand. The invention comprises administering the ligand conjugated to an immunogen to a host animal harboring the population of pathogenic cells. Antibodies, preexisting or administered to the host animal to establish a passive immunity, directed against the immunogen bind to the ligand-immunogen conjugate resulting in elimination of the pathogenic cells by the host's immune response. At least one additional therapeutic factor is administered selected from the group consisting of a cell killing agent, a tumor penetration enhancer, a chemotherapeutic agent, antimicrobial agent, a cytotoxic immune cell, and a compound capable of stimulating an endogenous immune response wherein the compound does not bind to the ligand-immunogen conjugate.
Owner:PURDUE RES FOUND INC
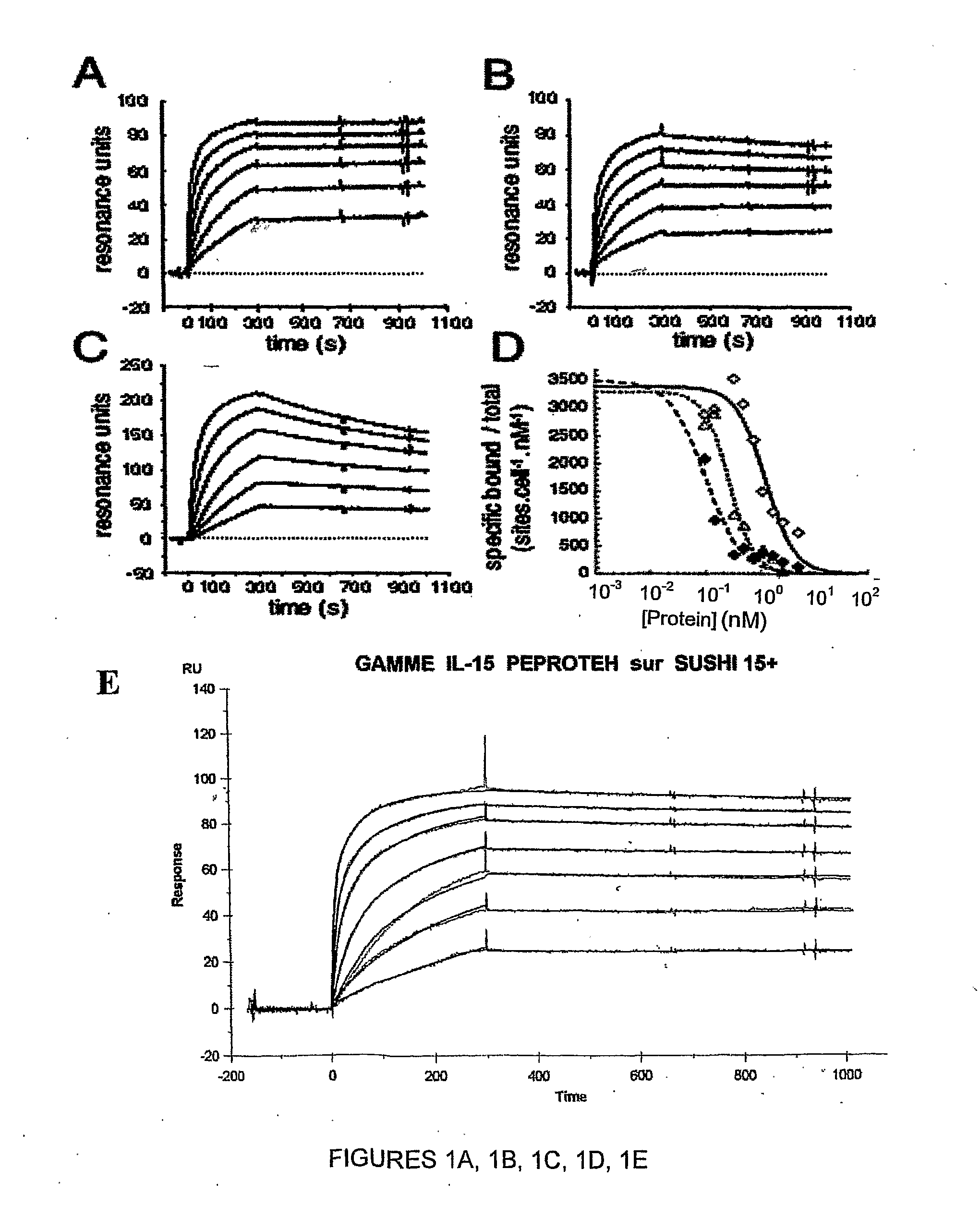
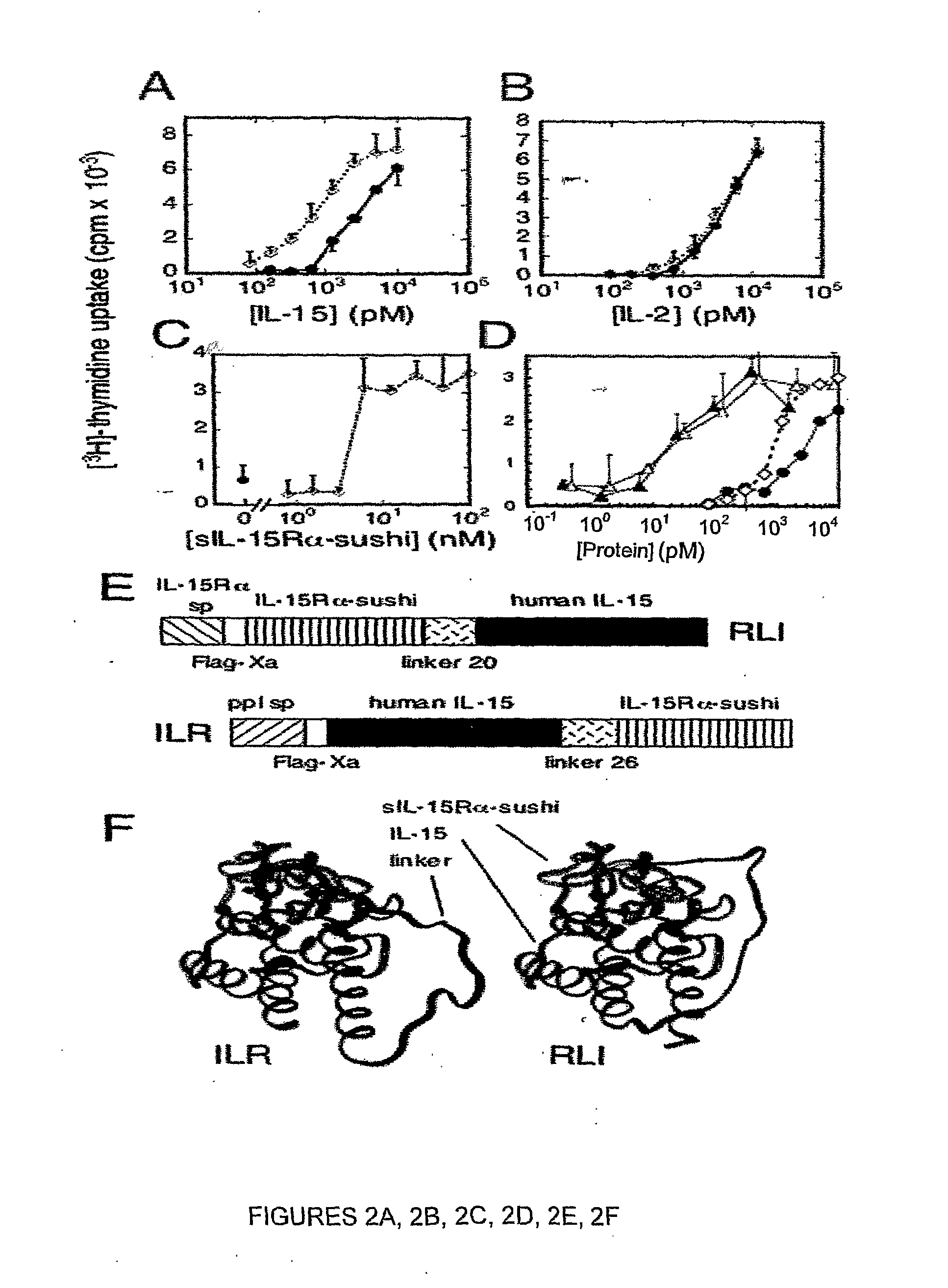
Il-15ralpha sushi domain as a selective and potent enhancer of il-15 action through il-15beta/gamma, and hyperagonist (il-15ralpha sushi - il-15) fusion proteins
ActiveUS20090238791A1Improve efficiencyAntibacterial agentsAntimycoticsSushi domainBiological activation
The present invention relates to the stimulation of the IL-15R beta / gamma signalling pathway, to thereby induce and / or stimulate the activation and / or proliferation of IL-15Rbeta / gamma-positive cells, such as NK and / or T cells. Appropriate compounds include compounds comprising at least one IL-15Rbeta / gamma binding entity, directly or indirectly linked by covalence to at least one polypeptide which contains the sushi domain of the extracellular region of an IL-15Ralpha.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Antiviral method
This invention provides a method of inactivating human noroviruses and other acid stable viruses. The method includes the step of contacting the virus with a virucidally-enhanced alcoholic composition that includes an alcohol, and an enhancer selected from cationic oligomers and polymers, chaotropic agents, and mixtures thereof.
Owner:GOJO IND INC
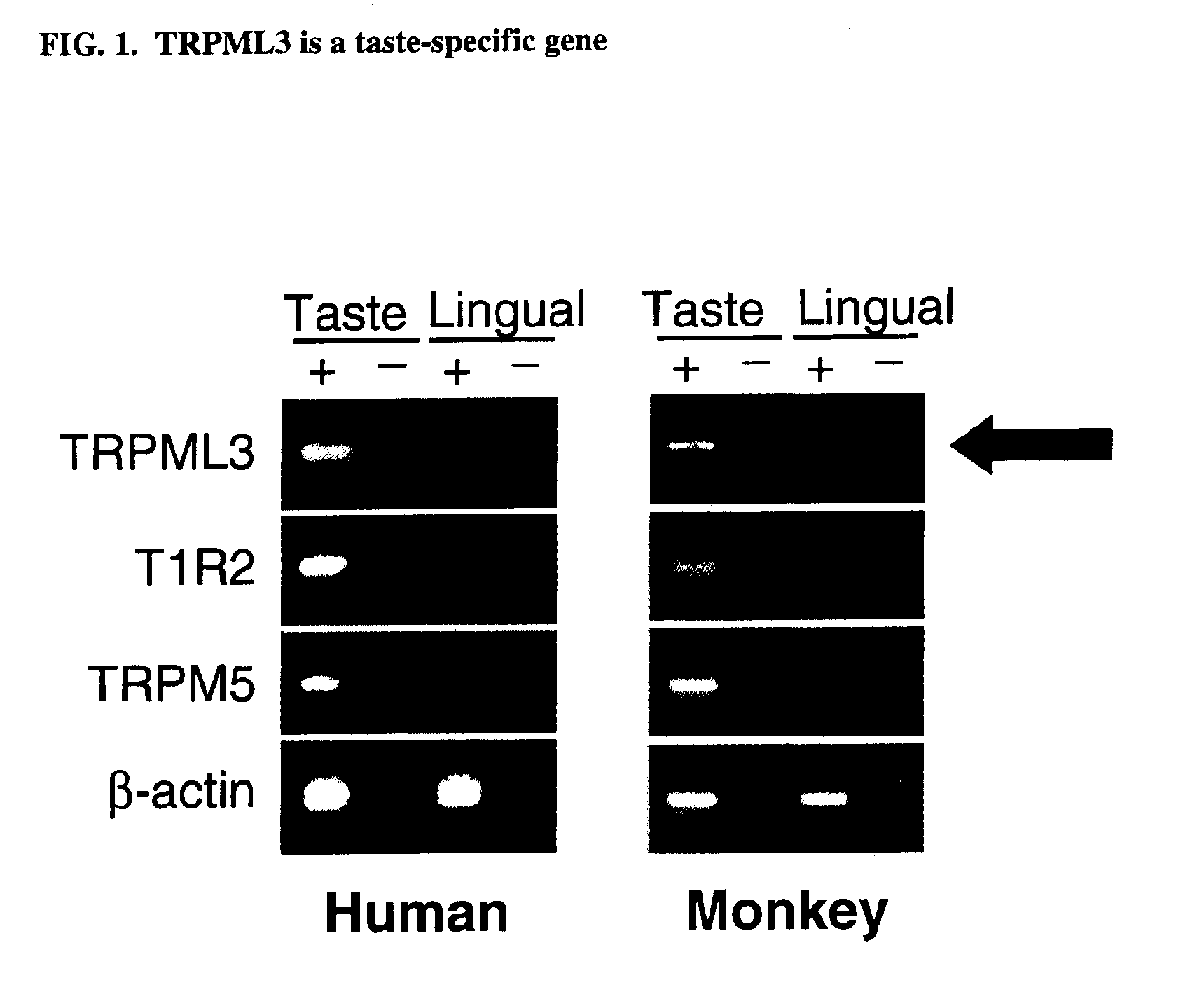
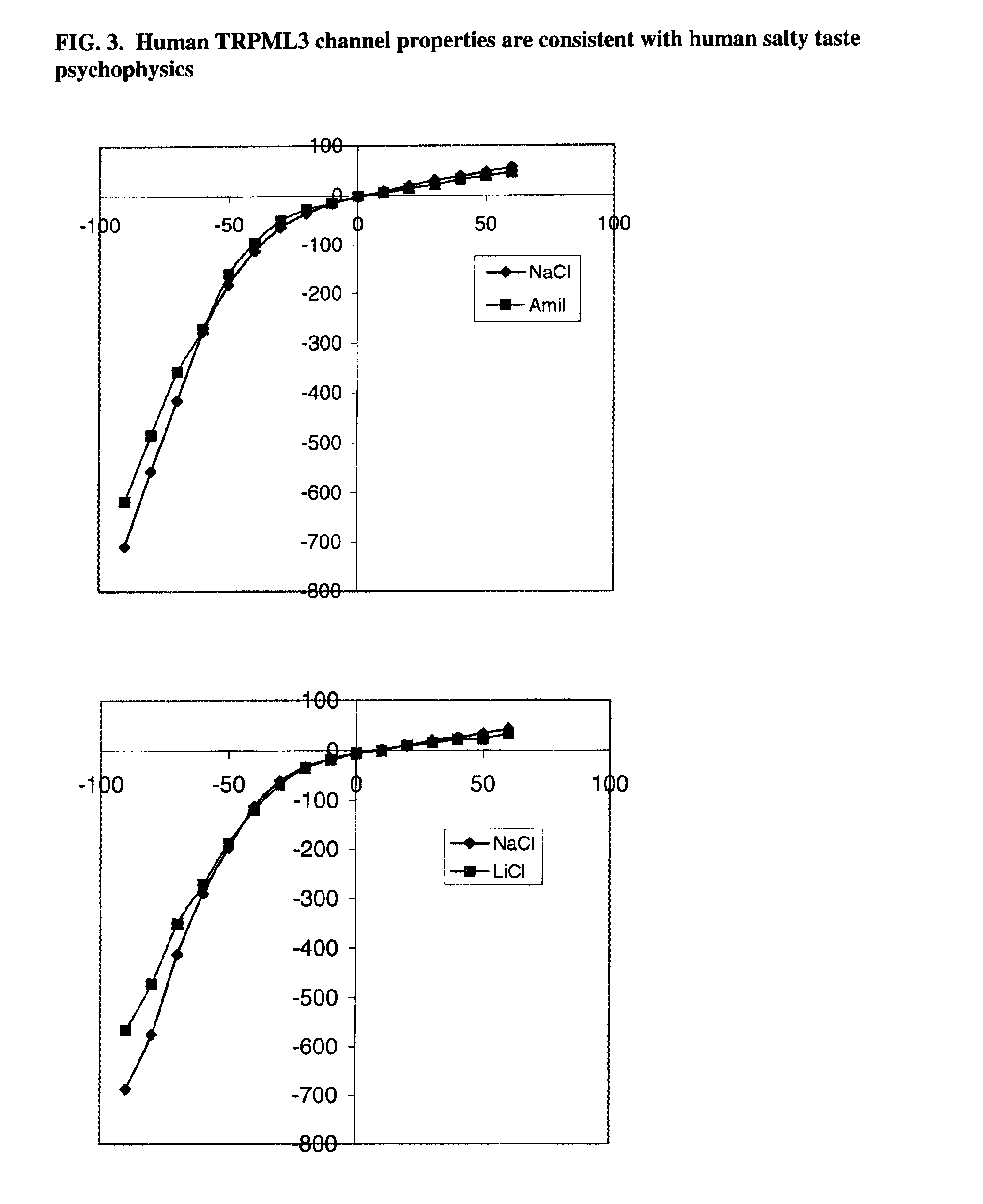
Identification of TRPML3 (MCOLN3) as a salty taste receptor and use in assays for identifying taste (salty) modulators and/or therapeutics that modulate sodium transport, absorption or excretion and/or aldosterone, and/or vasopressin production or release
InactiveUS20090210953A1Reduce volumeIncrease pressureCompound screeningApoptosis detectionSalty taste perceptionReceptor
The present invention relates to the elucidation that TRPML3 is involved in salty taste perception in primates including humans and likely other mammals and based thereon high-throughput mammalian and medium-throughput oocyte-based electrophysiological assays for identifying human TRPML3 modulators, preferably TRPML3 enhancers. Compounds that modulate TRPML3 function in the assay are expected to affect salty taste in humans. The inventive electrophysiological assays, such as the two-electrode voltage-clamp technique, facilitate the identification of compounds which specifically modulate human TRPML3. The assays of the invention provide a robust screen useful to detect compounds that facilitate (enhance) or inhibit TRPML3 function. Compounds that enhance or block TRPML3 channel activity should thereby modulate salty taste. In addition, these compounds may be used to regulate sodium excretion, urinary output and other biological functions relating to sodium levels and TRPML3 related functions.
Owner:SENOMYX INC
Dimeric small molecule potentiators of apoptosis
Caspase activity and apoptosis are promoted using active, dimeric Smac peptide mimetics of the general formula M1-L-M2, wherein moieties M1 and M2 are monomeric Smac mimetics and L is a covalent linker. Target cancerous or inflammatory cells are contacted with an effective amount of an active, dimeric Smac mimetic, and a resultant increase in apoptosis of the target cells is detected. The contacting step may be effected by administering to a pharmaceutical composition comprising a therapeutically effective amount of the dimeric mimetic, wherein the individual may be subject to concurrent or antecedent radiation or chemotherapy for treatment of a neoproliferative pathology.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Ultrasound imaging and treatment
InactiveUS7078015B2Low costSuitable for useUltrasonic/sonic/infrasonic diagnosticsCosmetic preparationsSound energyUltrasonic imaging
Methods of and apparatus for preparing temperature activated gaseous precursor-filled liposomes are described. Gaseous precursor-filled liposomes prepared by these methods are particularly usefull for example, in ultrasonic imaging applications and in therapeutic drug delivery systems.Gas, gaseous precursors and perfluorocarbons are presented as novel potentiators for ultrasonic hyper-thermia. The gas, gaseous precursors and perfluorocarbons which may be administered into the vasculature, interstitially or into any body cavity are designed to accumulate in cancerous and diseased tissues. When therapeutic ultrasonic energy is applied to the diseased region heating is increased because of the greater effectiveness of sound energy absorption caused by these agents.
Owner:IMARX THERAPEUTICS
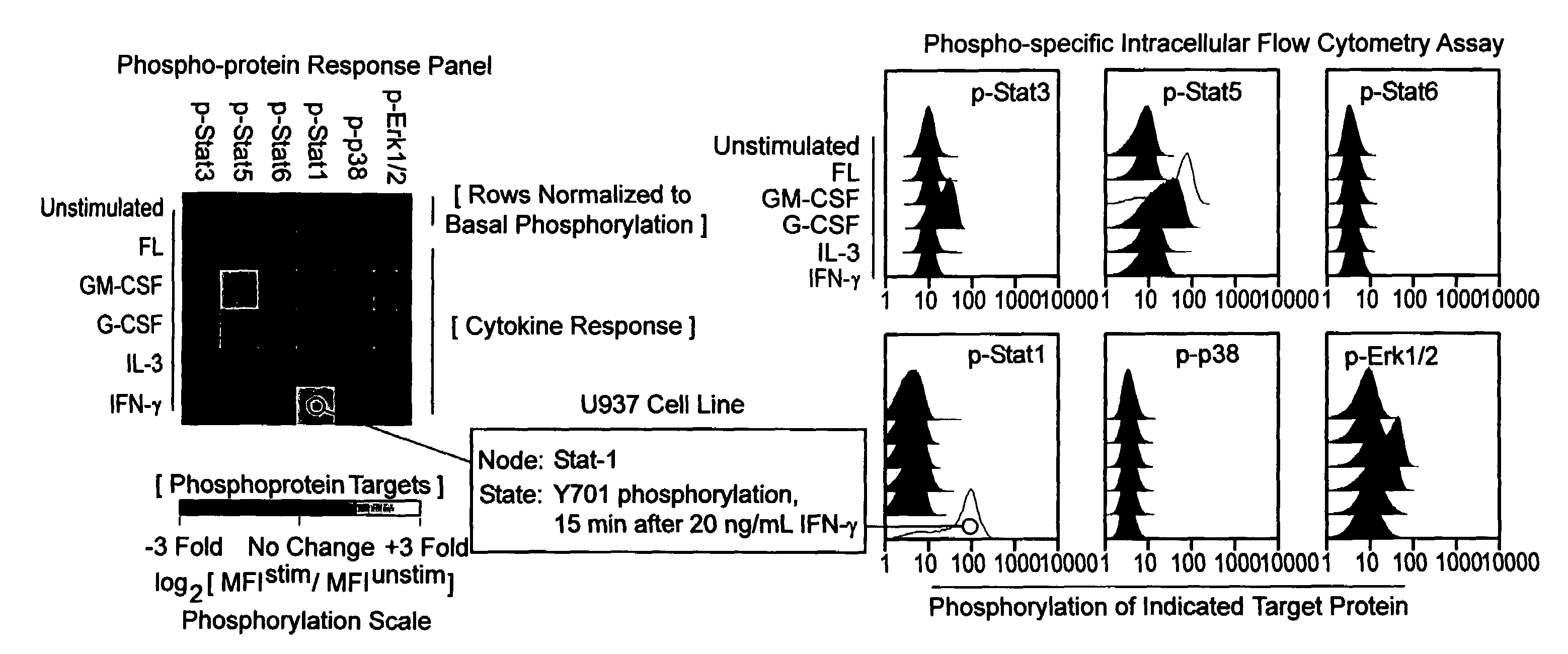
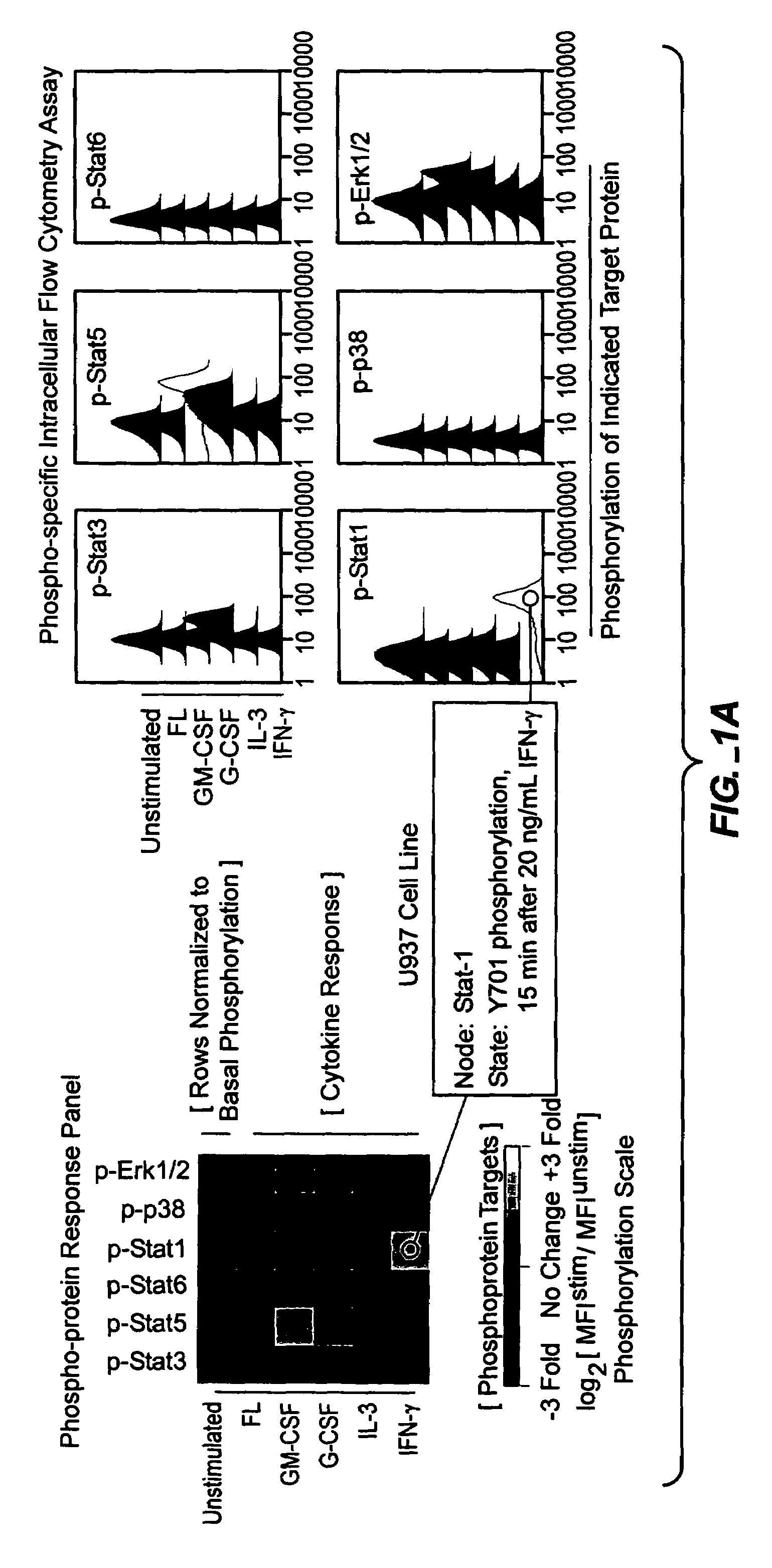
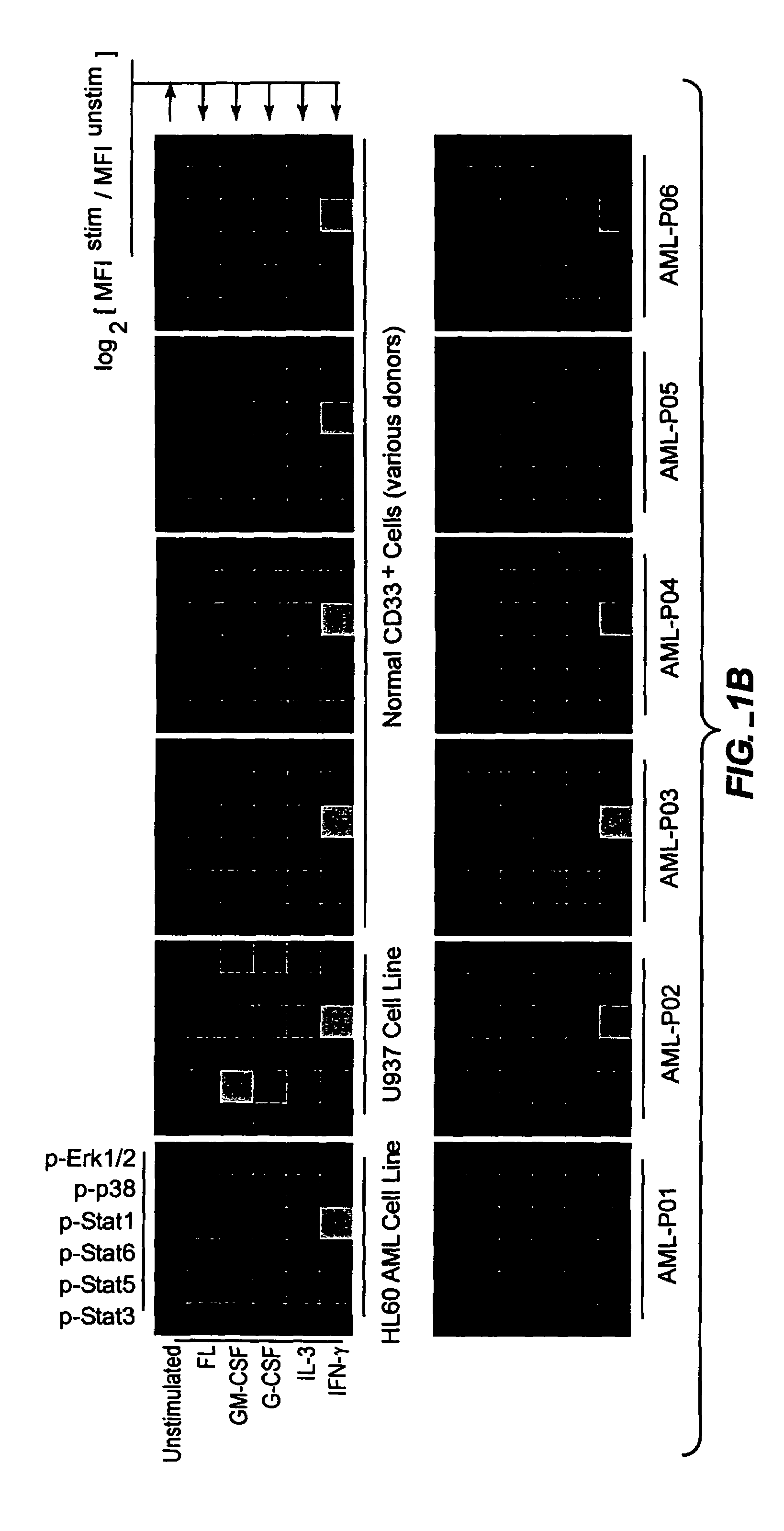
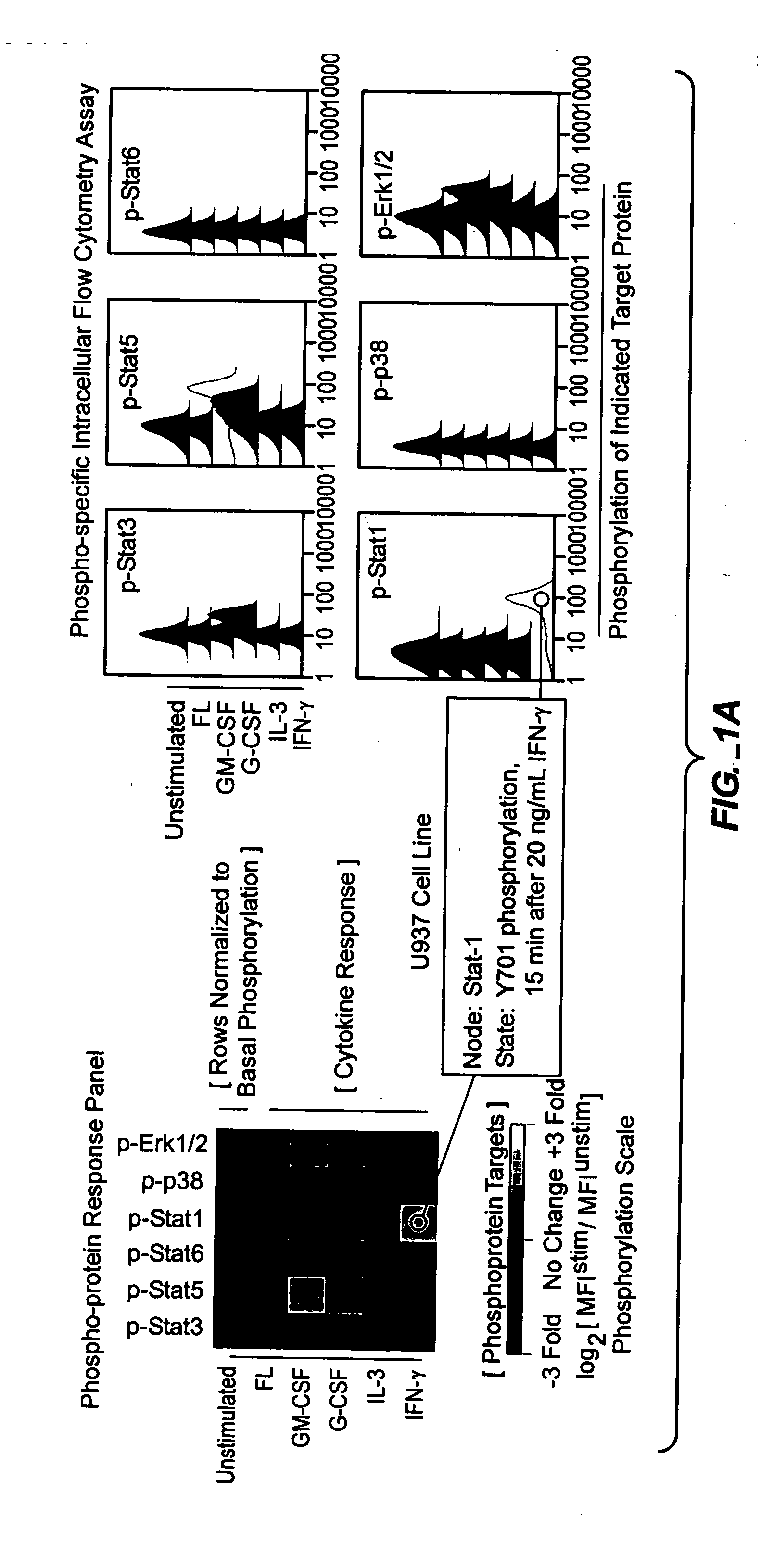
Methods and compositions for risk stratification
InactiveUS7393656B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCell subpopulationsPotentiator
The present invention provides an approach for the simultaneous determination of the activation states of a plurality of proteins in single cells. This approach permits the rapid detection of heterogeneity in a complex cell population based on activation states, and the identification of cellular subsets that exhibit correlated changes in activation within the cell population. Moreover, this approach allows the correlation of cellular activities or properties. In addition, the use of potentiators of cellular activation allows for characterization of such pathways and cell populations.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Anticancer Effect Enhancer
InactiveUS20080039521A1Good treatment effectBiocideUrinary disorderAnticarcinogenAdditive ingredient
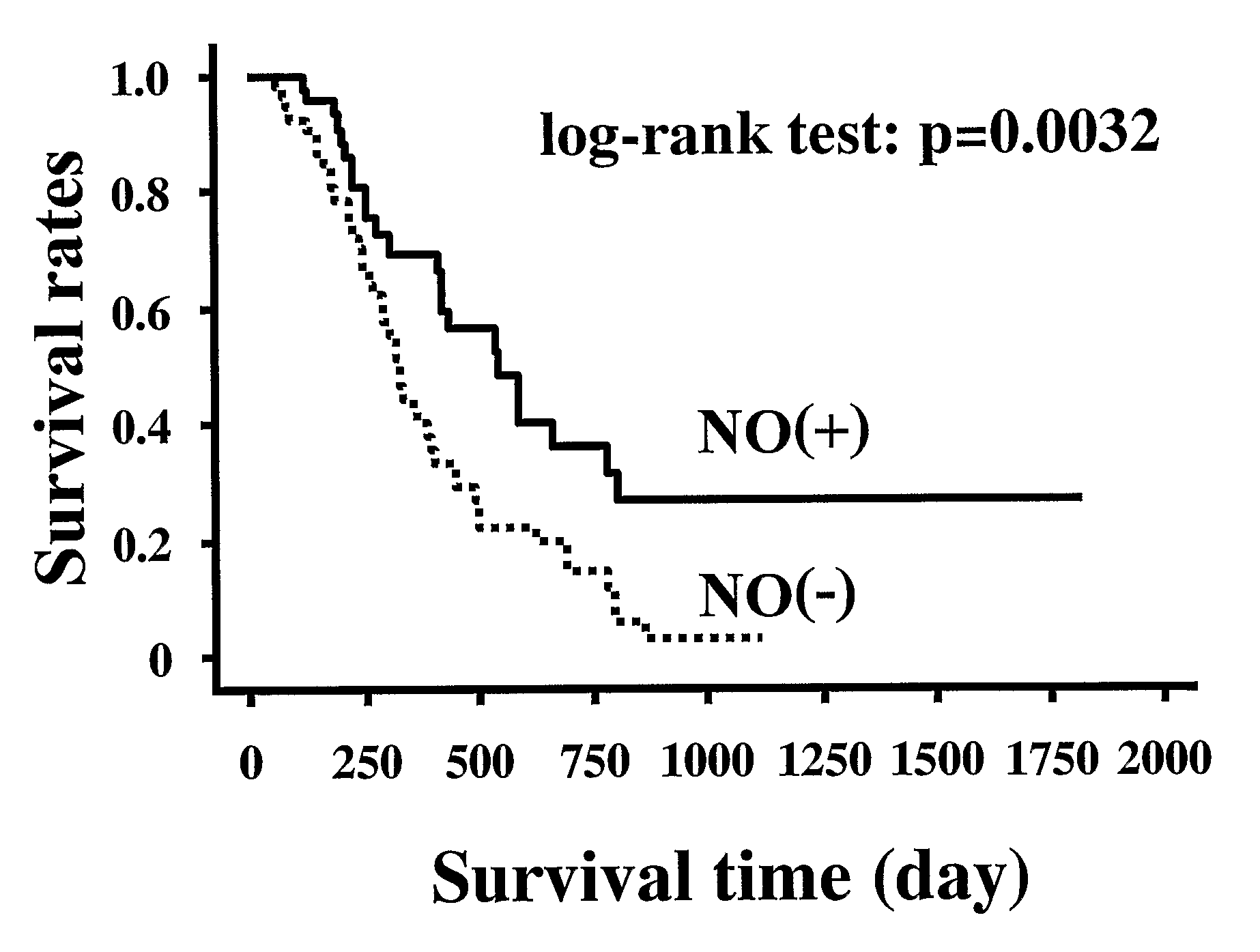
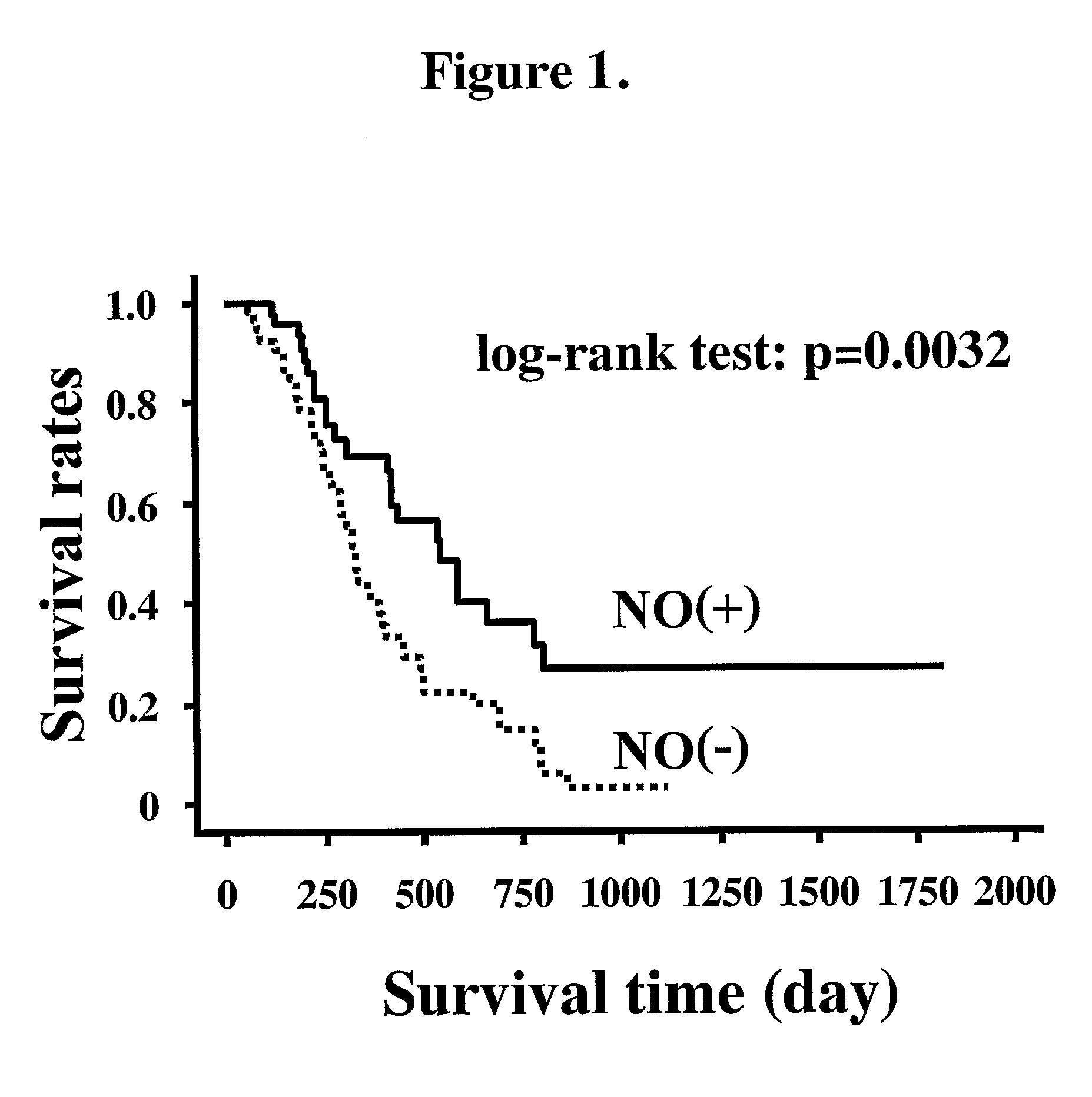
An object of the present invention is to provide an enhancing agent for effect of anticancer agent for achieving an excellent therapeutic effect on cancer. The enhancing agent for effect of anticancer agent according to the present invention which is a solving means therefor is characterized in that a nitric oxide donor is an effective ingredient. In accordance with the present invention, an excellent therapeutic effect is able to be achieved on non-small cell lung cancer which is still in such a state that no effective therapeutic method has been established yet for a progressive cancer which is not operable and is one of cancers where chemotherapy is most difficult to apply.
Owner:NIPPON KAYAKU CO LTD
Formulations and methods for providing prolonged local anesthesia
A formulation and methods for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a pharmaceutically acceptable, i.e., non-toxic, non-glucocorticoid augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent.
Owner:PURDUE PHARMA LP
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114648A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsMedicineT cell
Owner:MEDIMMUNE LLC
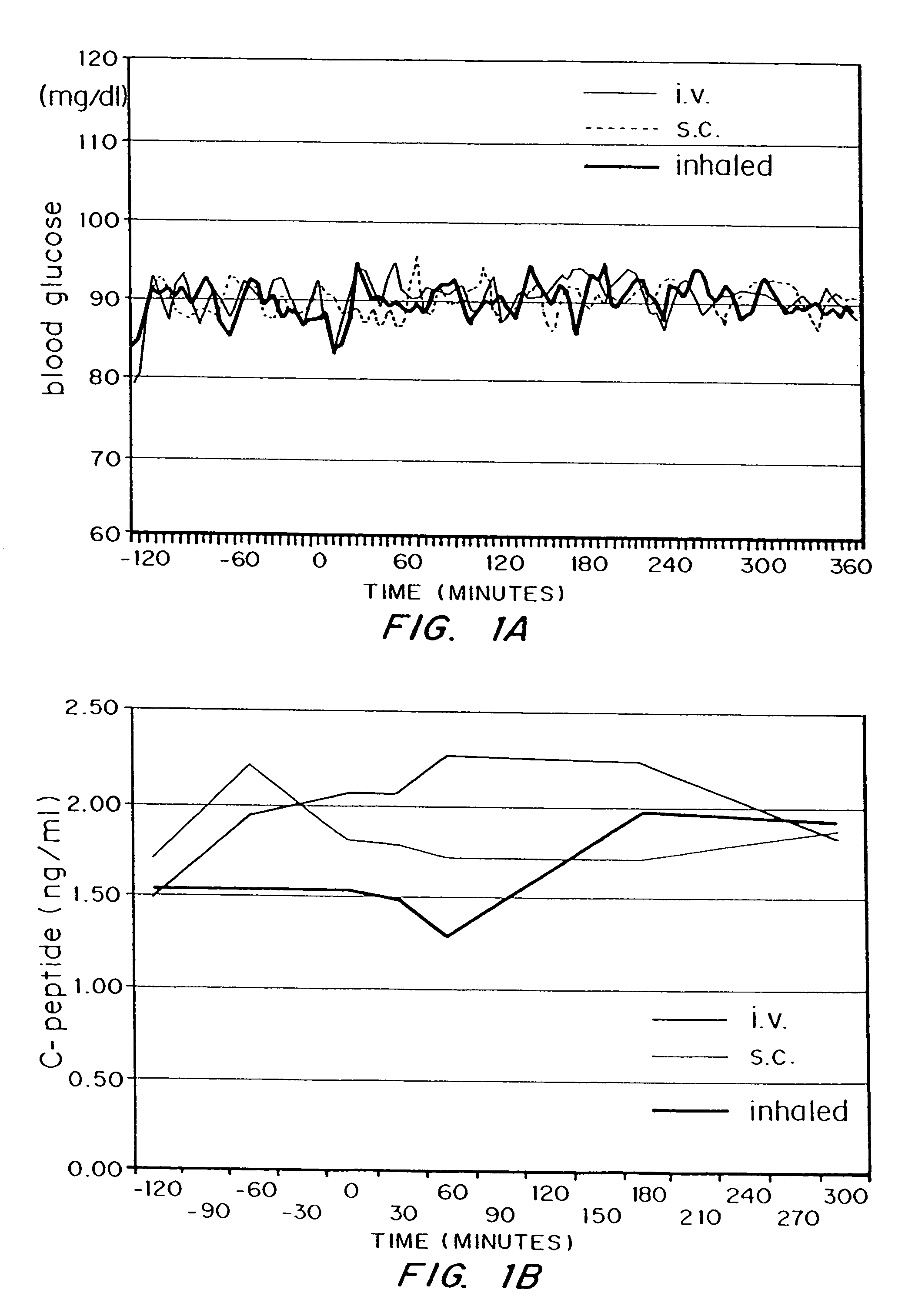
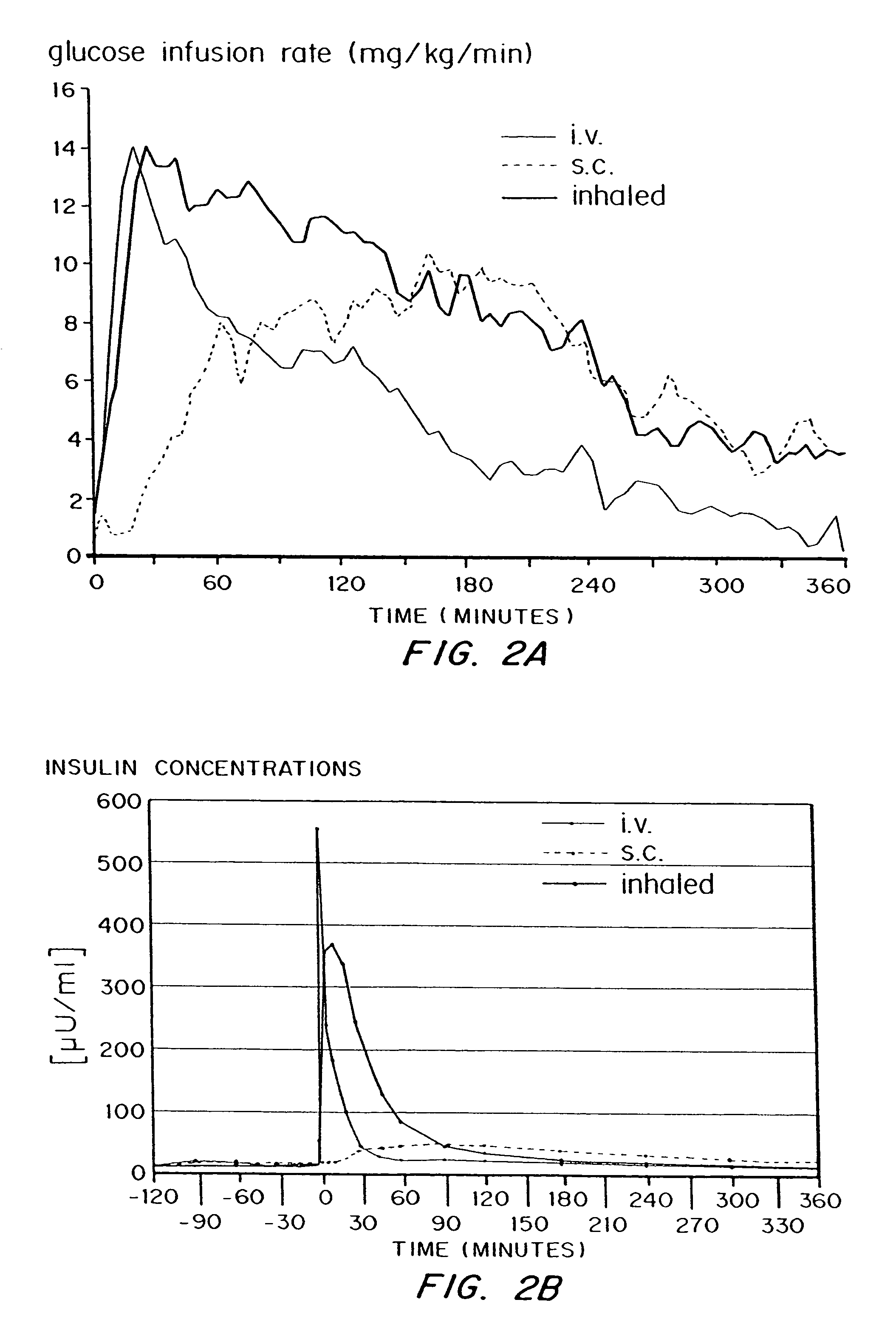
Method for delivery of monomeric or dimeric insulin complexed to diketopiperazine microparticles
InactiveUS7648960B2Rapid increase in blood agent concentrationEasy to transportPowder deliverySpray deliveryBlood insulinBlood agent
Methods are provided for purifying peptides and proteins by incorporating the peptide or protein into a diketopiperazine or competitive complexing agent to facilitate removal of one or more impurities from the peptide or protein. Formulations and methods also are provided for the improved transport of active agents across biological membranes, resulting for example in a rapid increase in blood agent concentration. The formulations include microparticles formed of (i) the active agent, which may be charged or neutral, and (ii) a transport enhancer that masks the charge of the agent and / or that forms hydrogen bonds with the target biological membrane in order to facilitate transport. In one embodiment insulin is administered via the pulmonary delivery of microparticles comprising fumaryl diketopiperazine and insulin in its biologically active form. This method of delivering insulin results in a rapid increase in blood insulin concentration that is comparable to the increase resulting from intravenous delivery.
Owner:MANNKIND CORP
Diagnosis and treatment system for reward deficiency syndrome (RDS) and related behaviors
InactiveUS6955873B1Reduce the amount requiredLow caloric dietBiocideHydroxy compound active ingredientsNervous systemAllelotype Analysis
The present invention relates to a kit and an intervenously administrable preparation, both, with a signal transmitter precursor, an enhancer of precursor uptake, and an inhibitor of neurotransmitter reuptake or signal transmitter catabolism. The kit also contains an appropriate swab for obtaining oral cells suitable for allelic analysis. The intervenous formulation contains similar materials and, in some cases, ethanol. Either the kit composition or the intervenous formulation may be used as guided by a subjects allelic analysis. Collections of particular alleles, especially those relating to neural system are comprehensible in terms of likelihood of success in the administration of an interveinous formulation or ingestion of components of the subject kit.
Owner:SYNAPTAMINE INC
Use of polymerized lipid diagnostic agents
InactiveUS6090408AImprove toleranceEliminate needUltrasonic/sonic/infrasonic diagnosticsNanotechDiseaseLipid formation
Polymerized liposome particles which are linked to a targeting agent and may also be linked to a contrast enhancement agent and / or linked to or encapsulating a treatment agent. The targeting imaging enhancement polymerized liposome particles interact with biological targets holding the image enhancement agent to specific sites providing in vitro and in vivo study by magnetic resonance, radioactive, x-ray or optical imaging of the expression of molecules in cells and tissues during disease and pathology. Targeting polymerized liposomes may be linked to or encapsulate a treatment agent, such as, proteins, drugs or hormones for directed delivery to specific biological sites for treatment.
Owner:NANOVALENT PHARMA +1
Low-temperature scouring and bleaching adjuvant composition and method for producing the same
ActiveCN101487184ARealize ecological dyeing and finishingMeet environmental protection requirementsFibre treatmentBleaching apparatusEthylene diamineDecomposition
The invention relates to a low-temperature scouring and bleaching promoter composition and a preparation method thereof. The composition comprises 2.5-5.5% of Tetra acetyl ethylene diamine (TAED), 0-0.5% of hydrogen peroxide decomposition catalyst, 5-15% of bleaching potentiator, 10-15% of chelating agent, 77-54.5% of carrier and 5-10% of surfactant, according to the weight percentage. The preparation method comprises the steps as follows: the carrier, the TAED, the hydrogen peroxide decomposition catalyst, the chelating agent and the bleaching potentiator are sequentially added in a powder mixer and mixed uniformly for 45-60min; subsequently, the surfactant is added and all mixtures are mixed for 15-30min and then discharged, thus obtaining the composition. The low-temperature scouring and bleaching promoter composition belongs to the environment-protective promoter composition, contains no phosphor and other substances harmful to the environmental protection, has no AOX problem, achieves the object that one promoter replaces more than four promoters, and facilitates processing and quality control.
Owner:NINGBO GUANG YUAN FABRIC
Novel small molecule potentiators of metabotropic glutamate receptors
ActiveUS20110245247A1Increase mGlu receptor responseFunction increaseBiocideNervous disorderDiseaseAryl
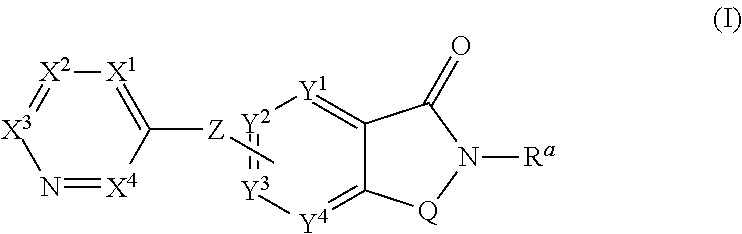
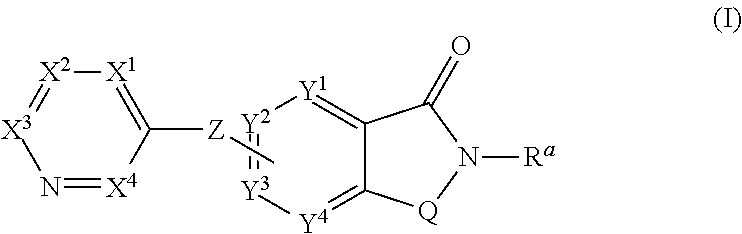
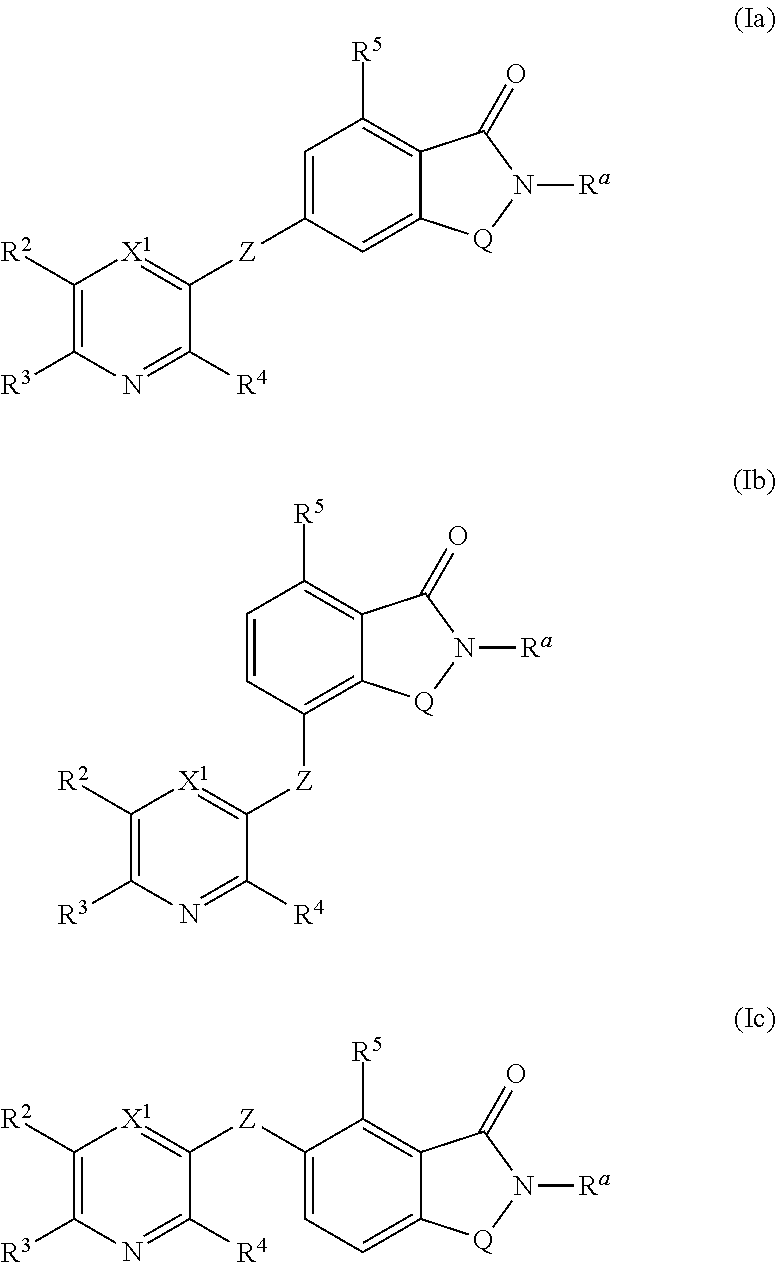
The present invention relates to small molecule potentiators of metabotropic receptors, in particular of the mGlu2 receptor. The present invention also relates to the use of these compounds for the prevention or treatment of neurological and psychiatric disorders associated with glutamate dysfunction and diseases in which metabotropic glutamate receptors are involved. The present invention thus provides compounds of formula Iwherein X1 is N or C—R1, X2 is N or C—R2, X3 is N or C—R3, X4 is N or C—R4 provided that none or one of X1, X2, X3 or X4 is N; Y1 is N, C or C—R5, Y2 is N, C or C—R6, Y3 is Y1, Y2, N, C or C—R7, Y4 is N, C or C—R8 provided that only the moiety Y1, Y2, Y3 or Y4 to which Z is bound is C and further provided at most one of Y1, Y2, Y3 or Y4 is N; Z is O, S, S(O), S(O)2 or NRZ; Q is CH2 or CH2CH2, where one or two of the hydrogen atoms in CH2 or CH2CH2 may be replaced by halogen, C1-C4-alkyl or C1-C4-haloalkyl; R1 is inter alia hydrogen, halogen, C1-C6-alkyl, C1-C6-haloalkyl, C1-C6-alkoxy, C1-C4-haloalkoxy, C3-C8-cycloalkyl, a radical NR1aR1b, C-bound 3- to 7-membered, saturated heterocyclyl having 1 or 2 nitrogen atoms and 0 or 1 heteroatoms, selected from O and S, as ring members, aryl, aryl-CH2, aryloxy, hetaryl, hetaryloxy or hetaryl-CH2, wherein the heterocyclyl, aryl and hetaryl rings ring in the last six radicals themselves are unsubstituted or carry 1, 2, 3, 4 or 5 identical or different radicals R1c; R2 has one of the meanings given for R1; R3 and R4 are, inter alia, selected from hydrogen, halogen, C1-C4-alkyl, C1-C4-haloalkyl, C3-C6-cycloalkyl, C1-C4-alkoxy, C1-C4-alkoxy-C1-C4-alkyl, phenyl, C1-C4-haloalkoxy, a radical (CH2)nNR′R″; R5, R6, R7, R8 are, independently of each other, selected from hydrogen, halogen, etc.; Ra is C3-C6-cycloalkyl, C1-C6-haloalkyl or C1-C6-alkyl, which is unsubstituted or carries one radical selected from C1-C4-alkoxy, C1-C4-haloalkoxy and a radical NRa1Ra2; and the N-oxides and the pharmaceutically acceptable salts thereof.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Dry storage stabilizing composition for biological materials
ActiveUS20120039956A1Improve biostabilityBenefit of stabilizingPowder deliveryVirusesBiotechnologyBiological materials
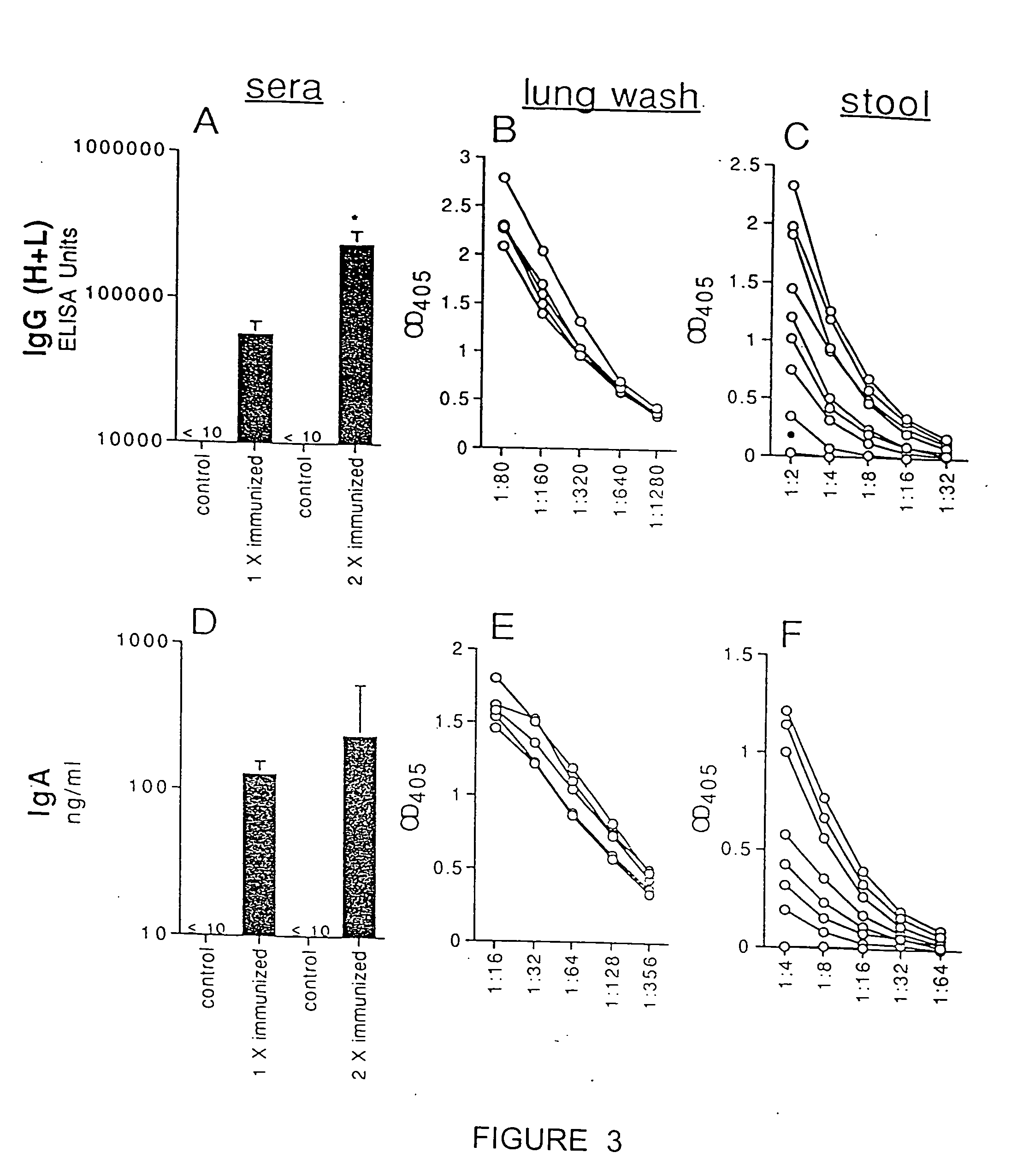
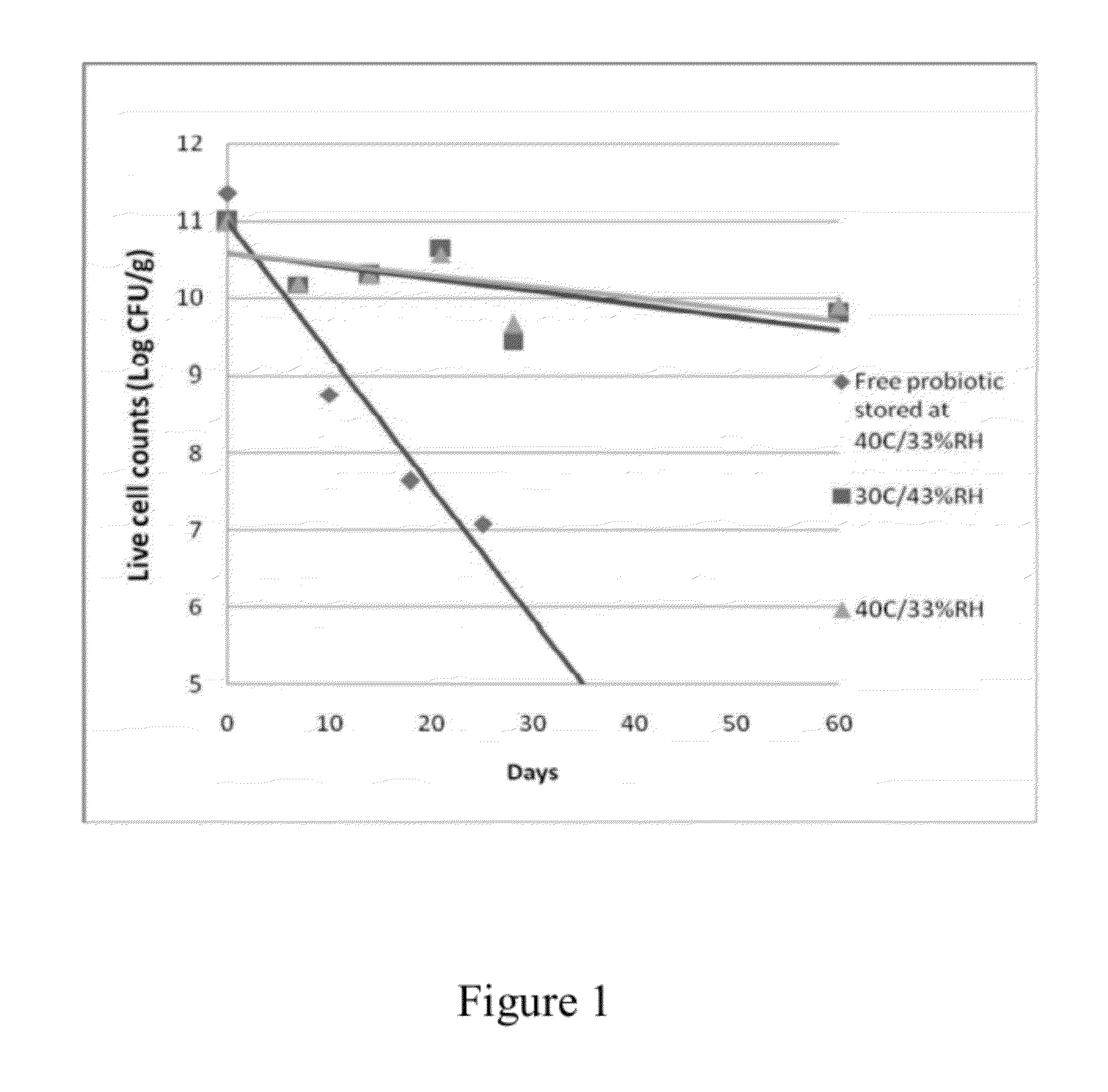
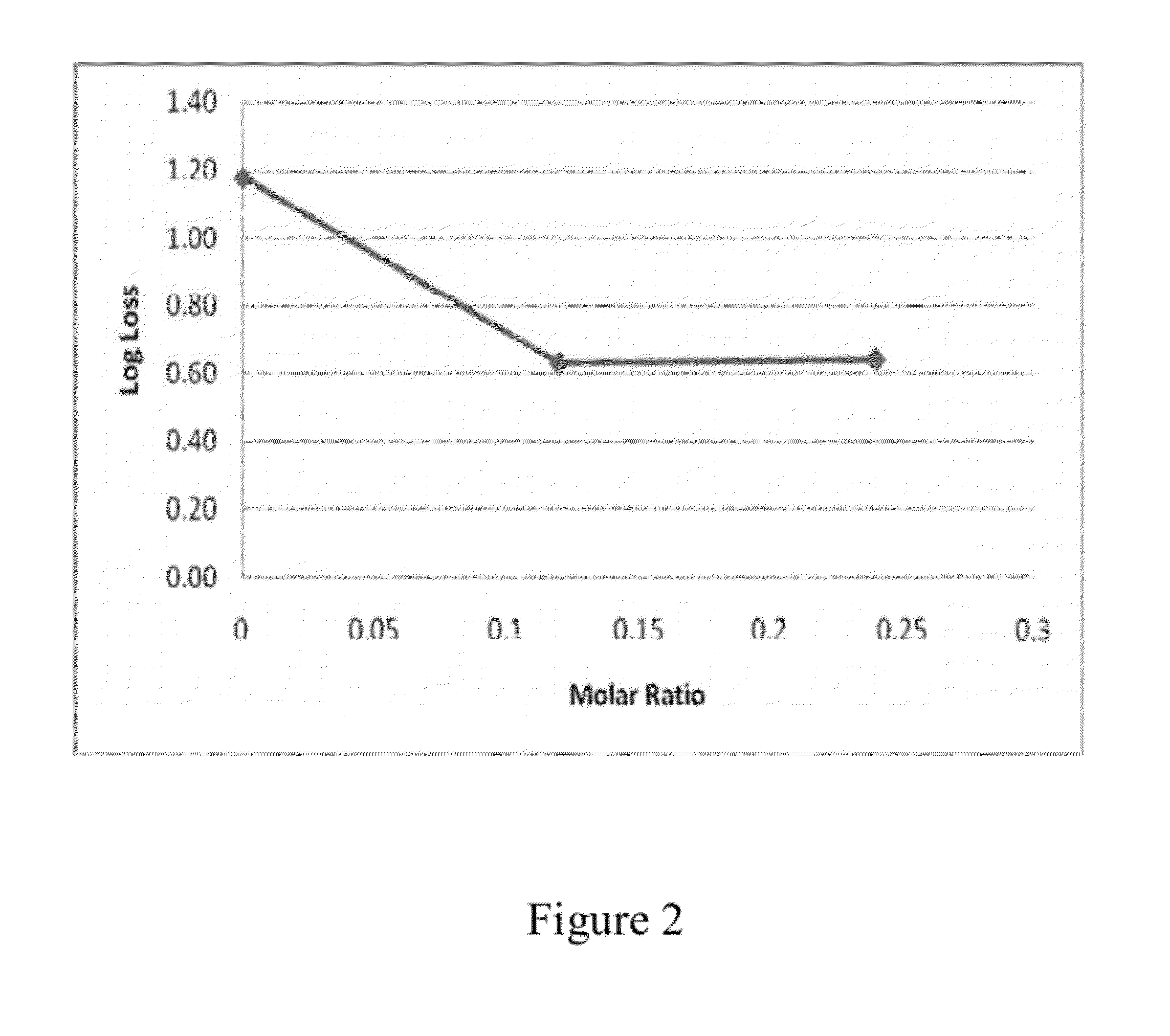
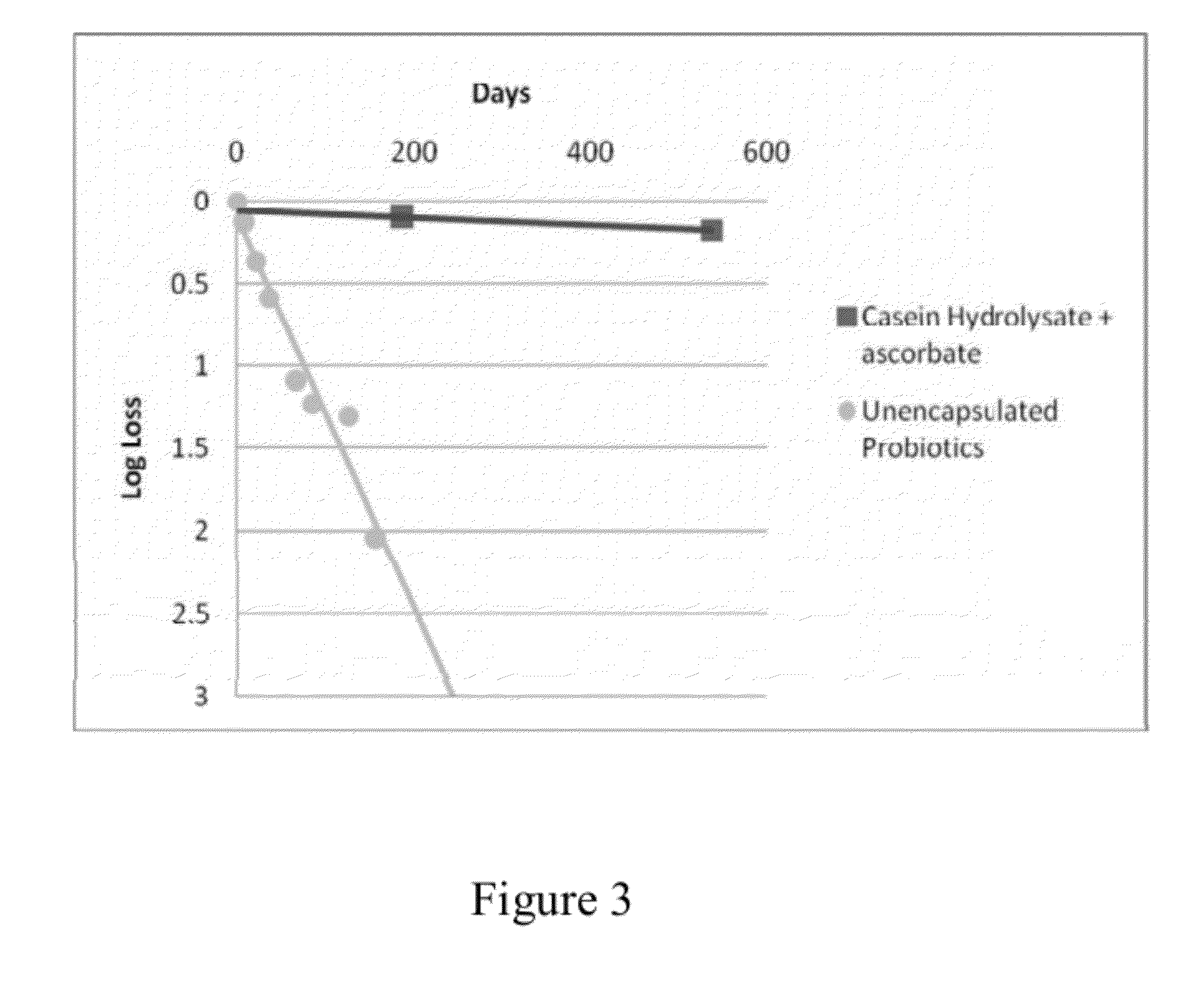
The present invention includes compositions and drying methods for preserving sensitive bioactive materials, such as peptides, proteins, hormones, nucleic acids, antibodies, drugs vaccines, yeast, bacteria (probiotic or otherwise), viruses and / or cell suspensions, in storage. The compositions include a carbohydrates component and a glass enhancer component, wherein the carbohydrate component includes a mixture of di-, oligo- and polysaccharides and the glass enhancer includes ions of organic acid and protein hydrolysates. The composition is prepared by dispersing all the solid components in a solution and then snap-frozen to form small beads, strings or droplets. The preferred drying method of the frozen beads, strings or droplets is initiated by a short purging and structure stabilizing step of the frozen particles under a vacuum pressure of less than <2000 mTORR followed by a primary drying step under vacuum pressure of more than >2000 mTORR and at a desired temperature. During the secondary and final drying step of the material a full vacuum pressure and elevated temperature are applied, to achieve a final desirable water activity of the dry material.
Owner:ADVANCED BIONUTRITION CORP
Topical anesthetic formulation
The topical medicament gel formulation of the present invention includes an anesthetic, an anti-microbial, an oxidant, a nutrient, a diuretic, an opioid, an anti-emetic, an anti-seizure drug, and a non-steroidal anti-inflammatory drug (NSAID), USP in a molecular, as opposed to a salt form, as the active ingredient. Additional constituents illustratively include a skin penetration enhancer and a gelling agent. This invention deals with problems commonly associated with topical application of local medicaments such as: slow onset of action; need for occlusion; and rapid loss of effect due to rapid systemic dispersion. The invention permits enhanced penetration of the medicament and thereby allows for a lesser total dosage of pharmaceutically active ingredient. The use of a lesser total dosage also decreases systemic toxicity.
Owner:WEPFER SCOTT
Pharmaceutical combination comprising an ibat inhibitor and a bile acid binder
InactiveUS20170182115A1Reduce generationEliminate the effects ofOrganic active ingredientsDipeptide ingredientsDipeptidyl peptidasePancreatic hormone
The present invention relates to a combination comprising a substance with inhibiting effect on the ileal bile acid transport system (I BAT) and at least one other active substance selected from an IBAT inhibitor; an enteroendocrine peptide or enhancer thereof; a dipeptidyl peptidase-IV inhibitor; a biguanidine; an incretin mimetic; a thiazolidinone; a PPAR agonist; a HMG Co-A reductase inhibitor; a bile acid binder; and a TGR5 receptor modulator; wherein the IBAT inhibitor compound and the at least one other active substance are adminstered simultaneously, sequentially or separately.
Owner:ALBIREO
Prolonged anesthesia in joints and body spaces
InactiveUS20020054915A1Enhance and prolong local anesthesiaGood effectPeptide/protein ingredientsAnaestheticsAnesthetic AgentMicroparticle
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Modulation of solubility, stability, absorption, metabolism, and pharmacokinetic profile of lipophilic drugs by sterols
InactiveUS20110160168A1Enhance biological absorptionGood metabolic stabilityBiocideMetabolism disorderSterol esterPharmaceutical drug
A formulation for drug delivery, providing enhanced modulation of solubility, stability, absorption, metabolism, and / or pharmacokinetic profile of a lipophilic therapeutic agent by formulation with sterols and / or sterol esters, resulting in higher bioavailability of a therapeutic agent administered to a subject in need of such therapeutic agent. The formulation contains a therapeutic agent and a sterol or sterol ester, and can, optionally, further contain a solubilizer and / or an enhancing agent. Also described are pharmaceutical compositions containing the formulations and methods of making and methods of using the formulations and pharmaceutical compositions. Formulations of the disclosure can be constituted to minimize the synthesis of dihydrotestosterone when the therapeutic agent includes testosterone or testosterone esters.
Owner:MARIUS PHARMA LLC
Anti-tumor effect potentiator
There is provided an agent for potentiating the effects of an anti-tumor agent.An anti-tumor effect potentiator containing, as an active ingredient, a uracil compound represented by the following formula (I) or a pharmaceutically acceptable salt thereof:wherein X represents a C1-5 alkylene group and one of methylene groups constituting the alkylene group is optionally substituted with an oxygen atom;R1 represents a hydrogen atom or a C1-6 alkyl group; R2 represents a hydrogen atom or a halogen atom; and R3 represents a C1-6 alkyl group, a C2-6 alkenyl group, a C3-6 cycloalkyl group, a (C3-6 cycloalkyl) C1-6 alkyl group, a halogeno-C1-6 alkyl group or a saturated heterocyclic group.
Owner:TAIHO PHARMA CO LTD
Methods and compositions for risk stratification
InactiveUS20050112700A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBiochemistryBiological activation
The present invention provides an approach for the simultaneous determination of the activation states of a plurality of proteins in single cells. This approach permits the rapid detection of heterogeneity in a complex cell population based on activation states, and the identification of cellular subsets that exhibit correlated changes in activation within the cell population. Moreover, this approach allows the correlation of cellular activities or properties. In addition, the use of potentiators of cellular activation allows for characterization of such pathways and cell populations.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com