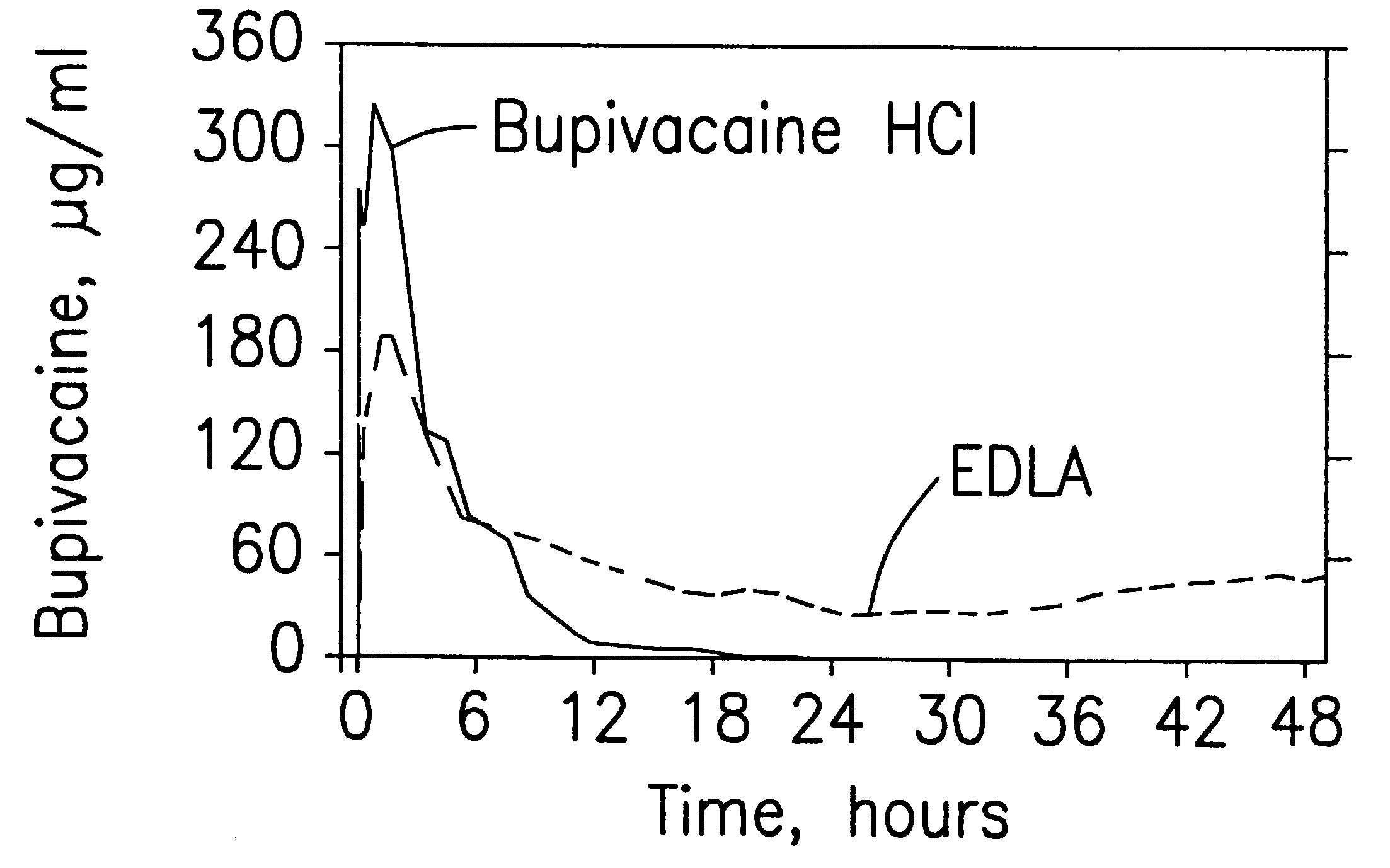
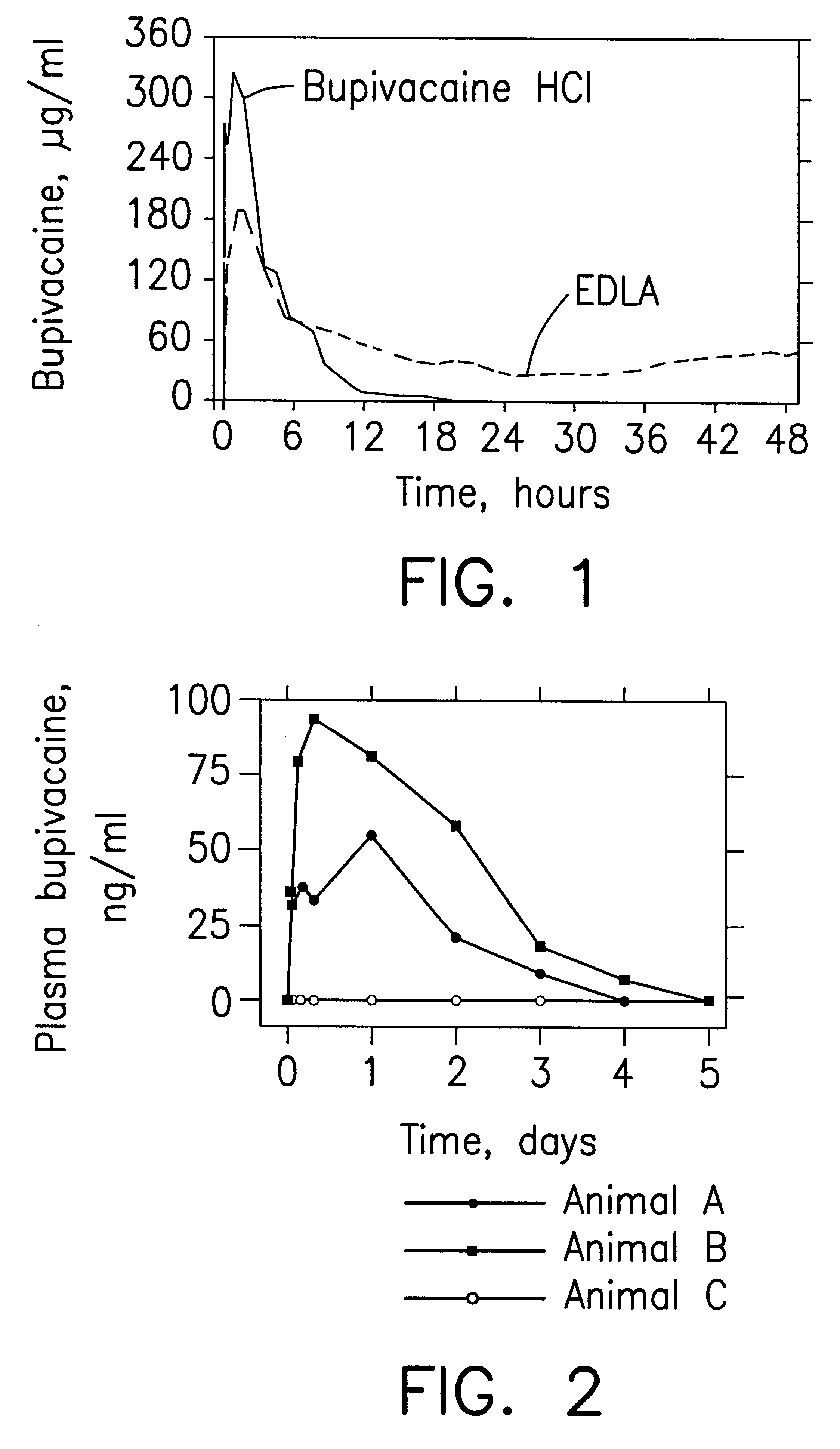
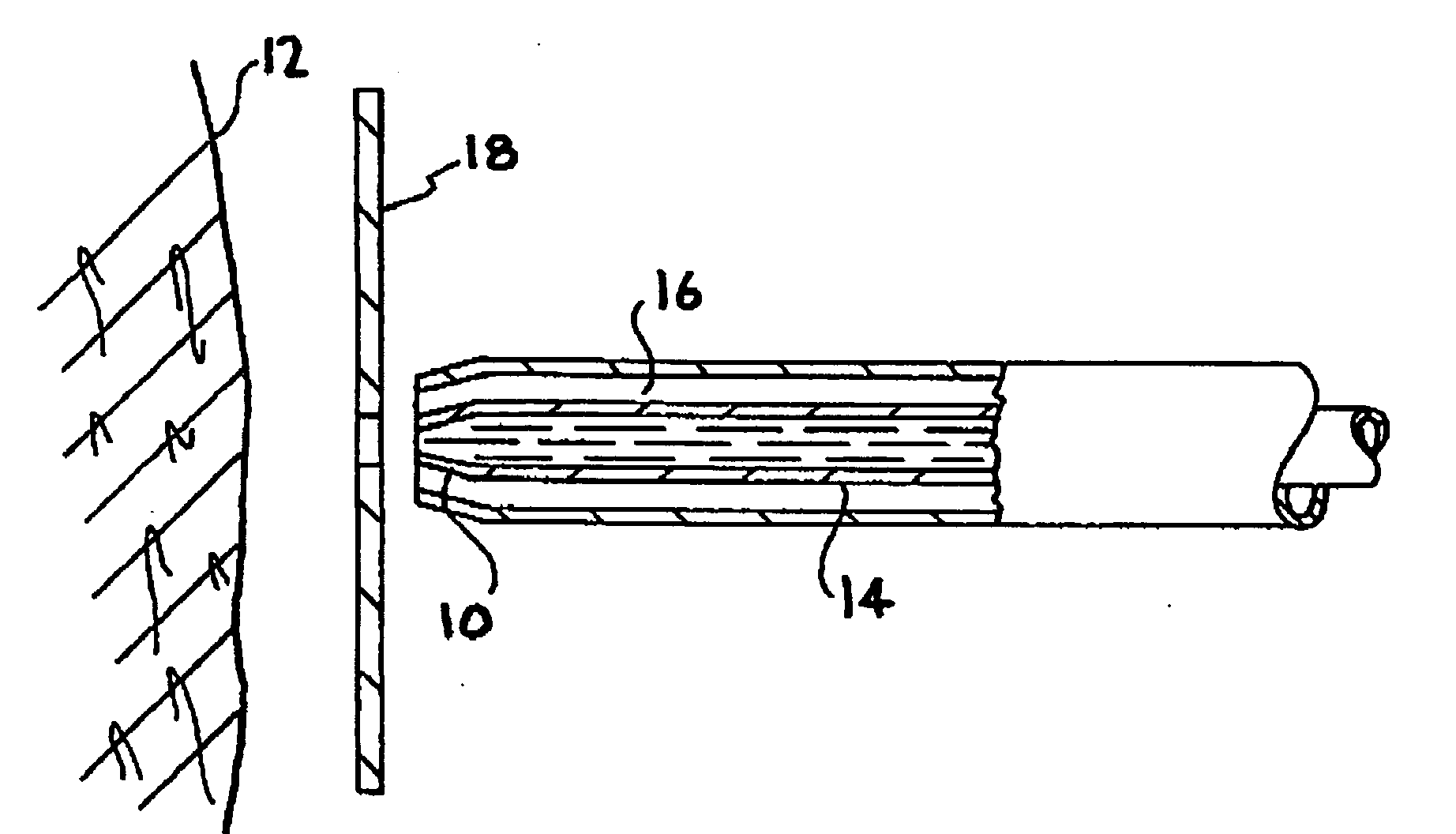
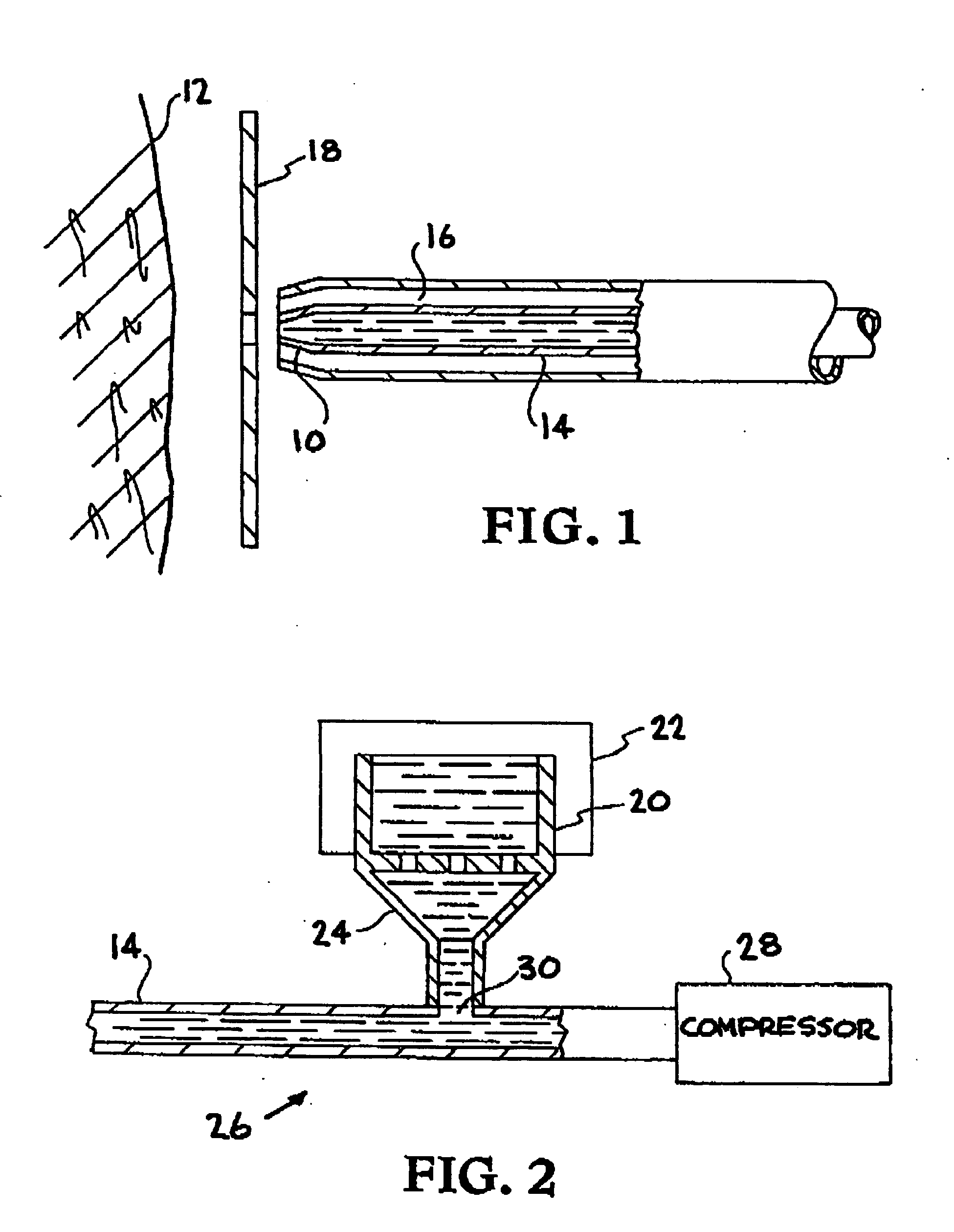
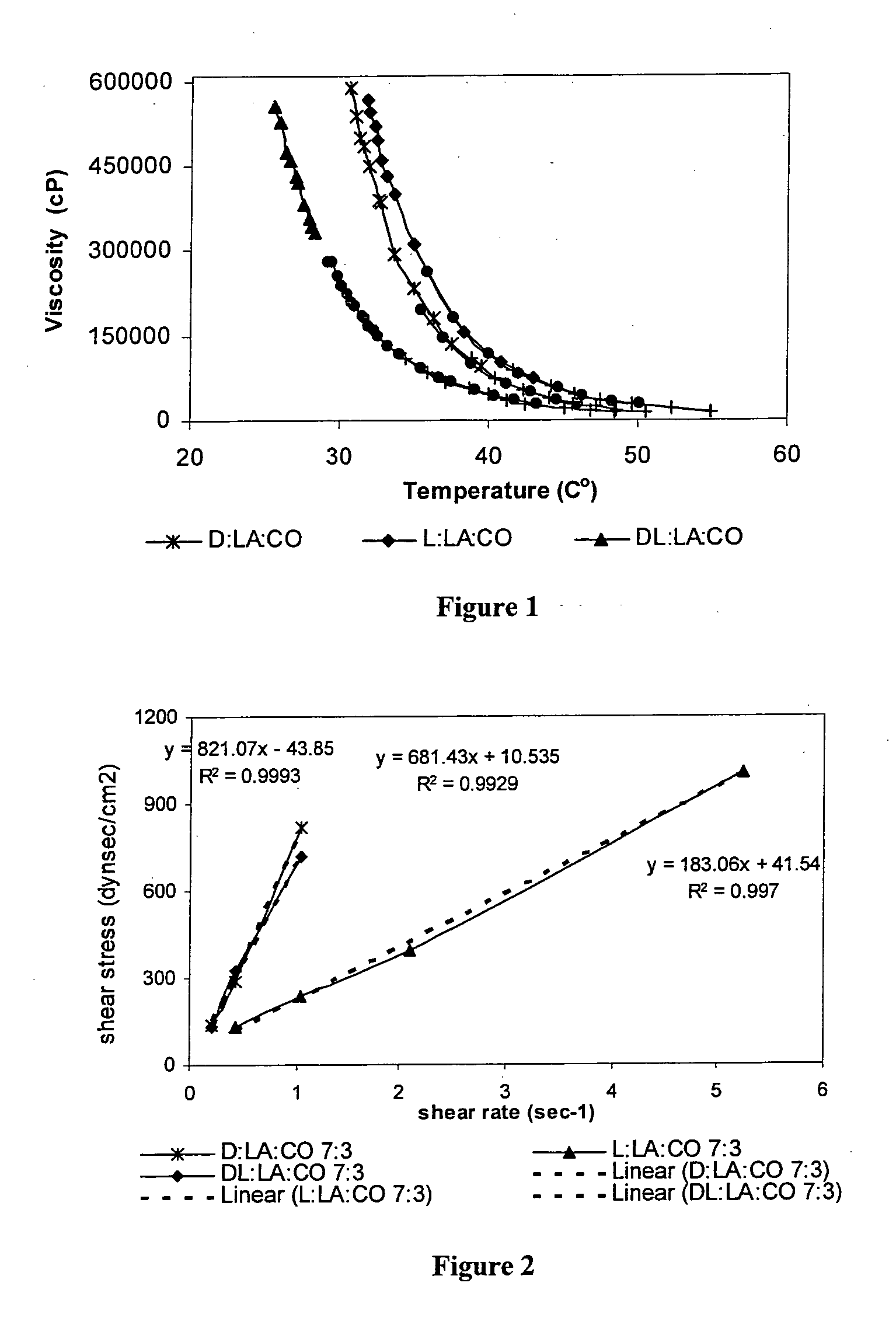
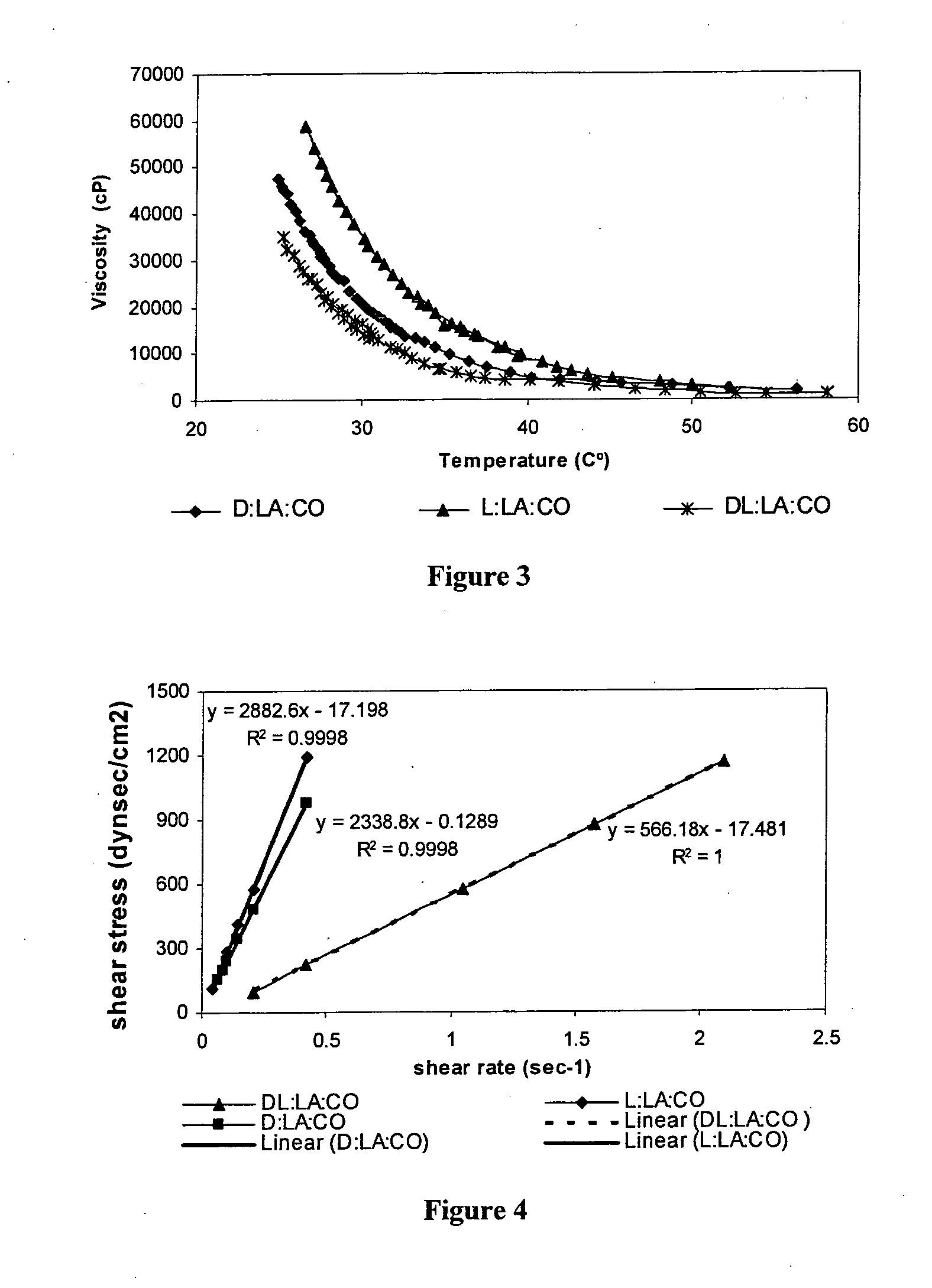
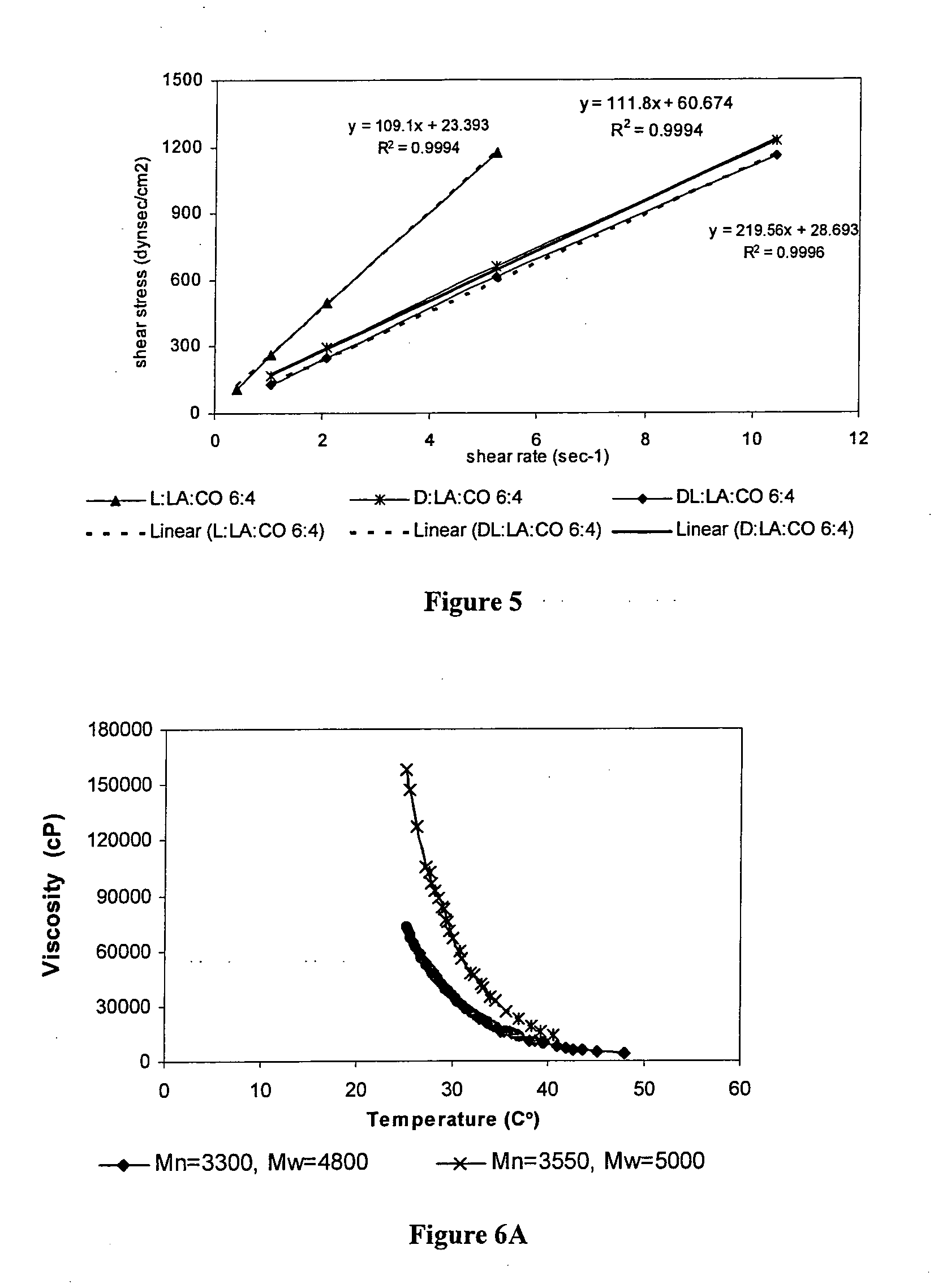
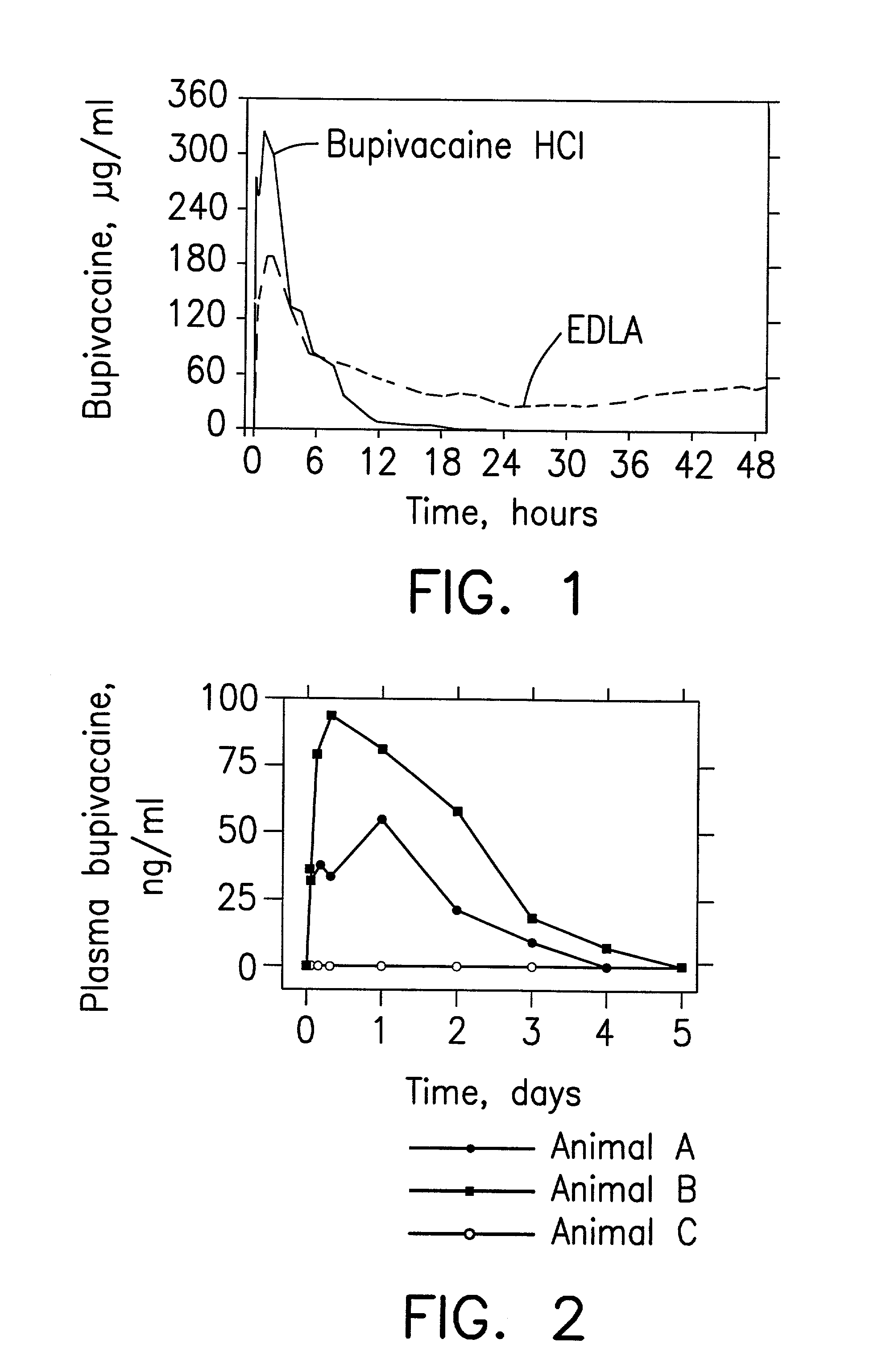
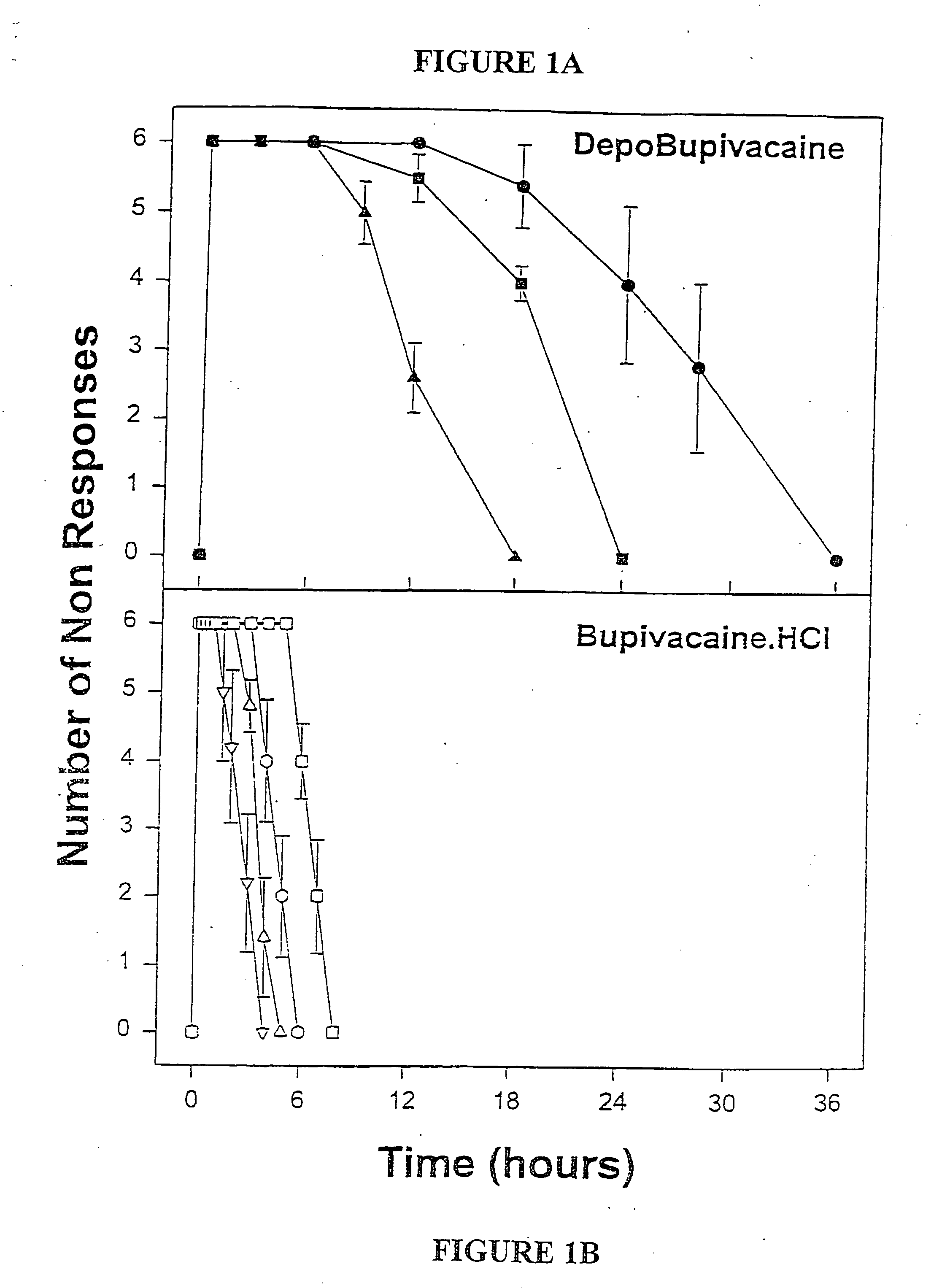
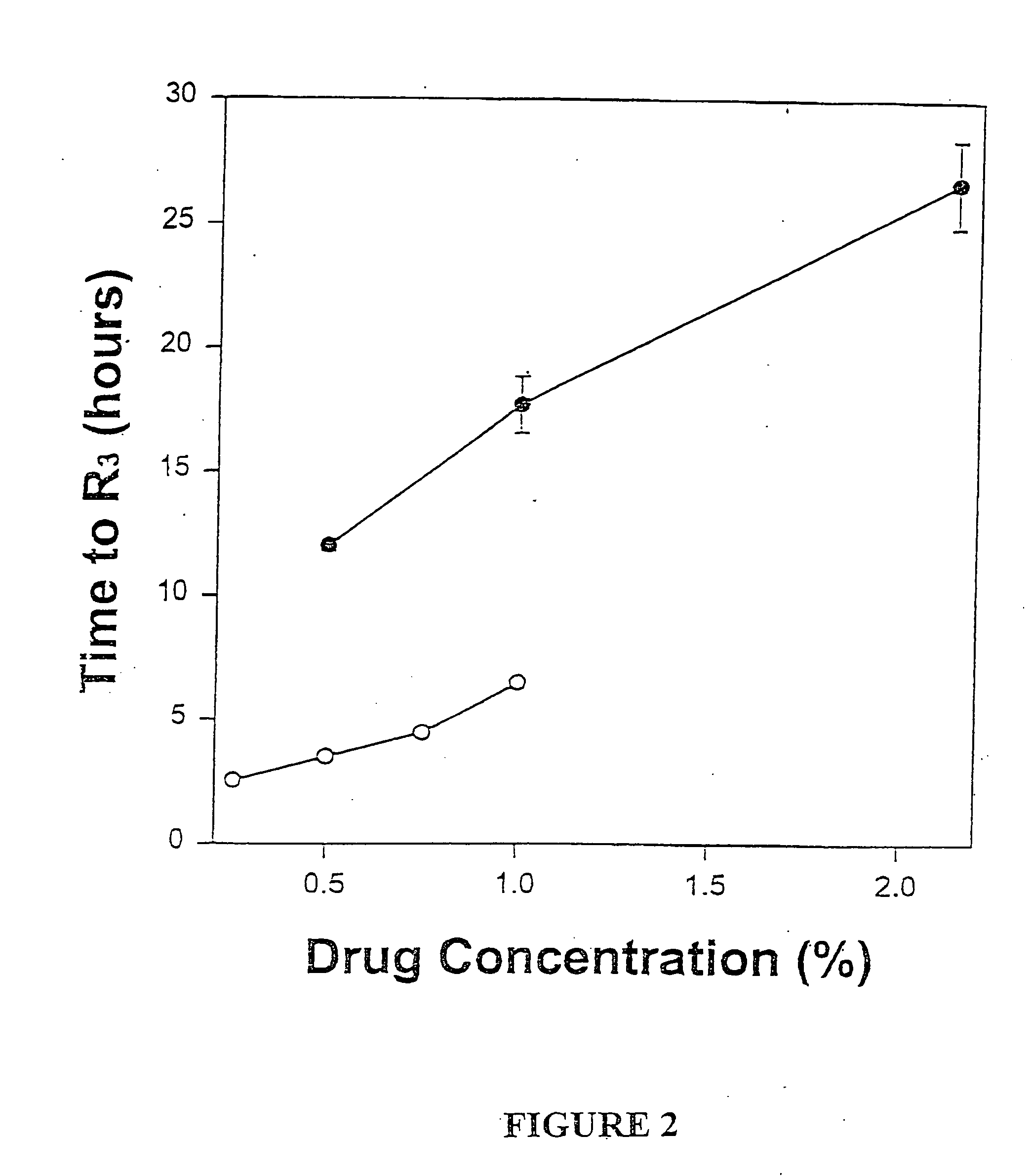
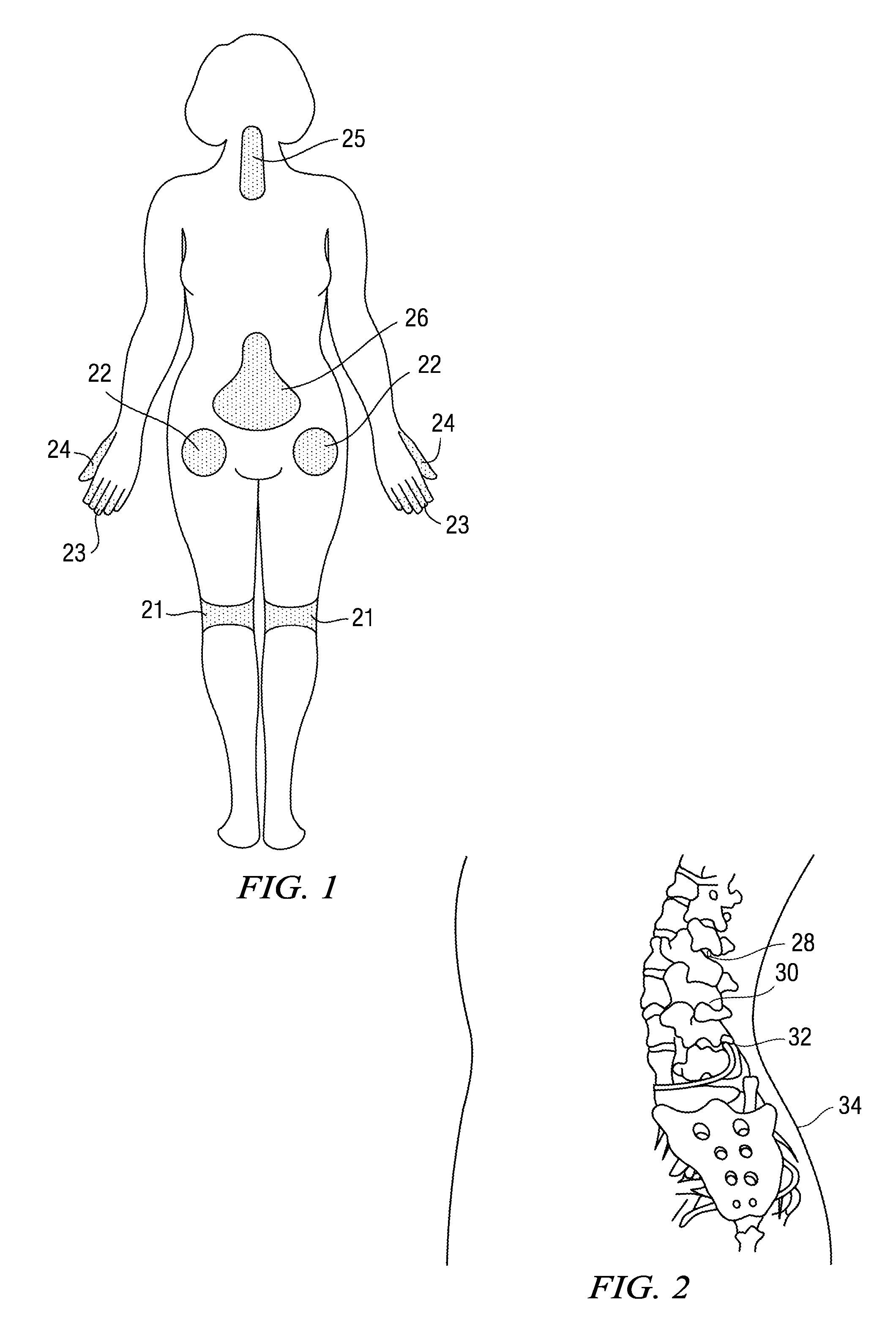
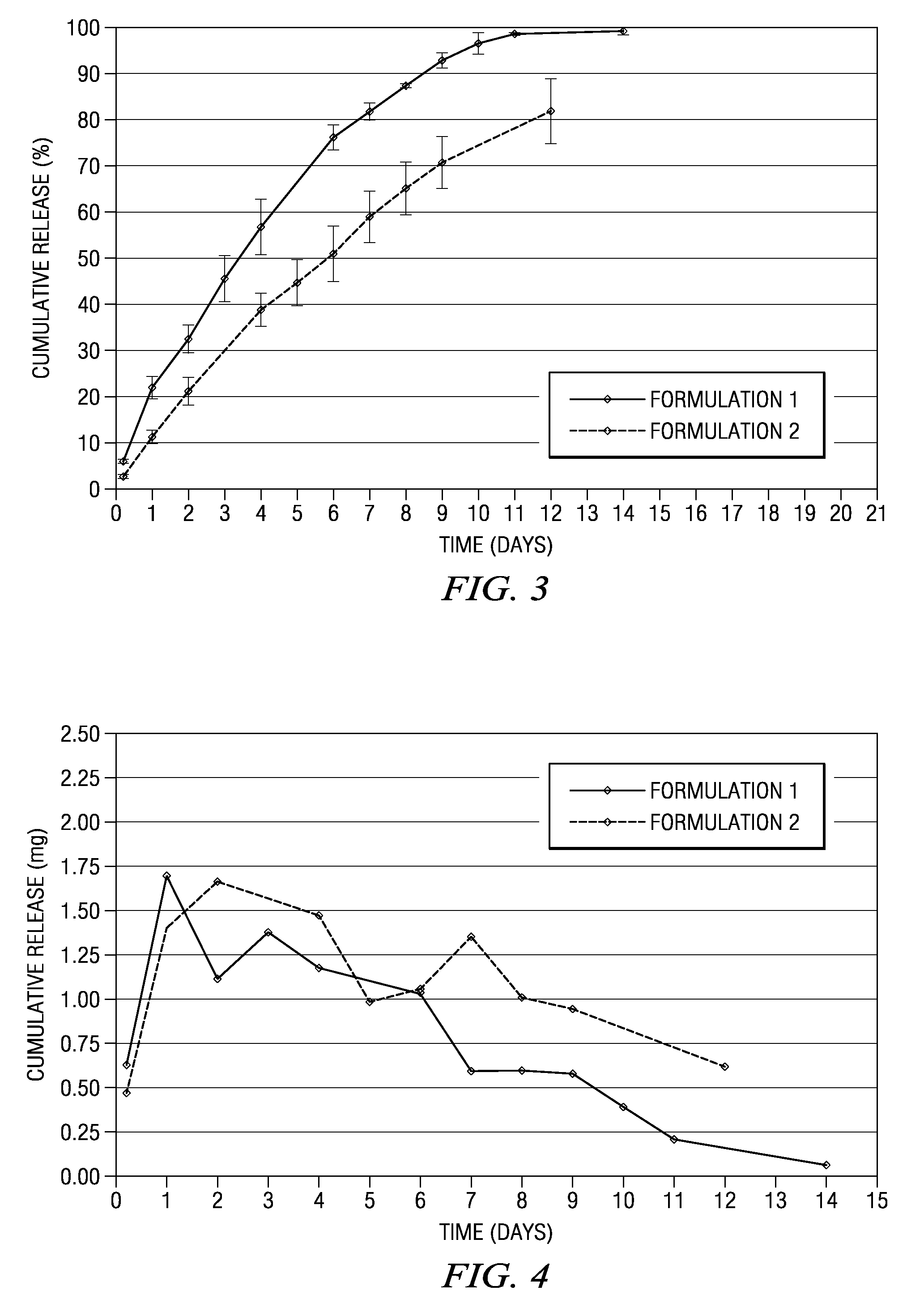
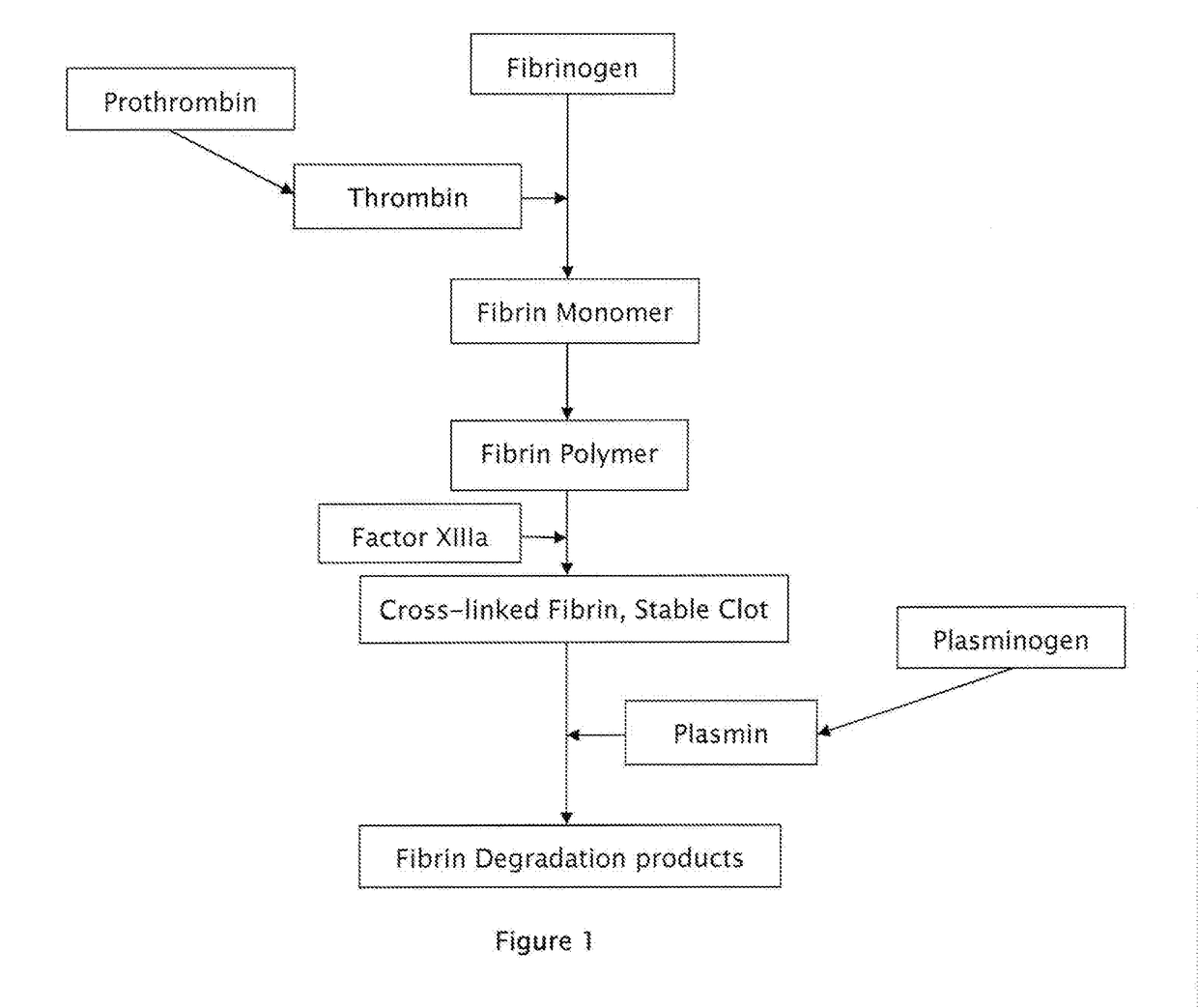
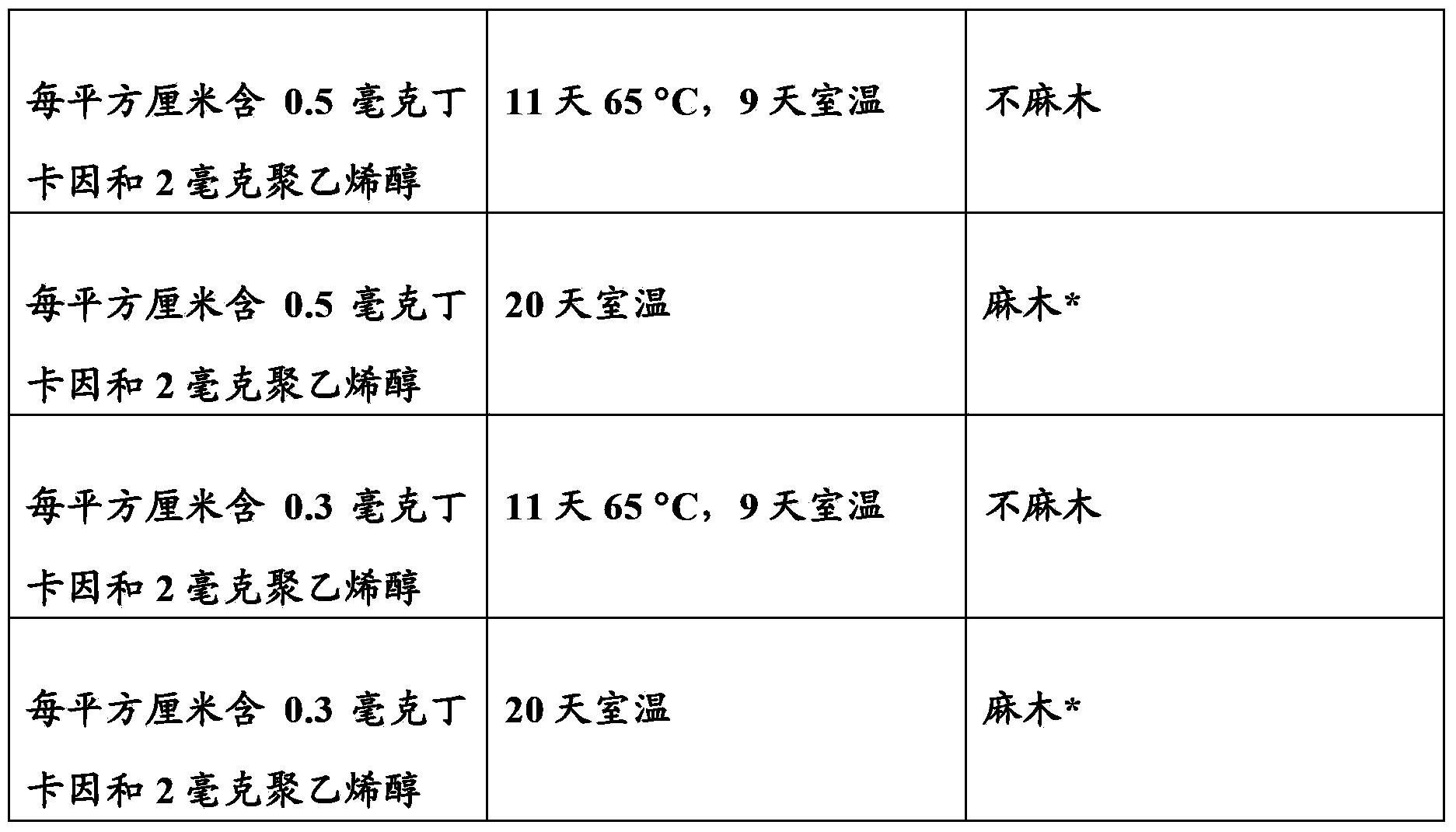
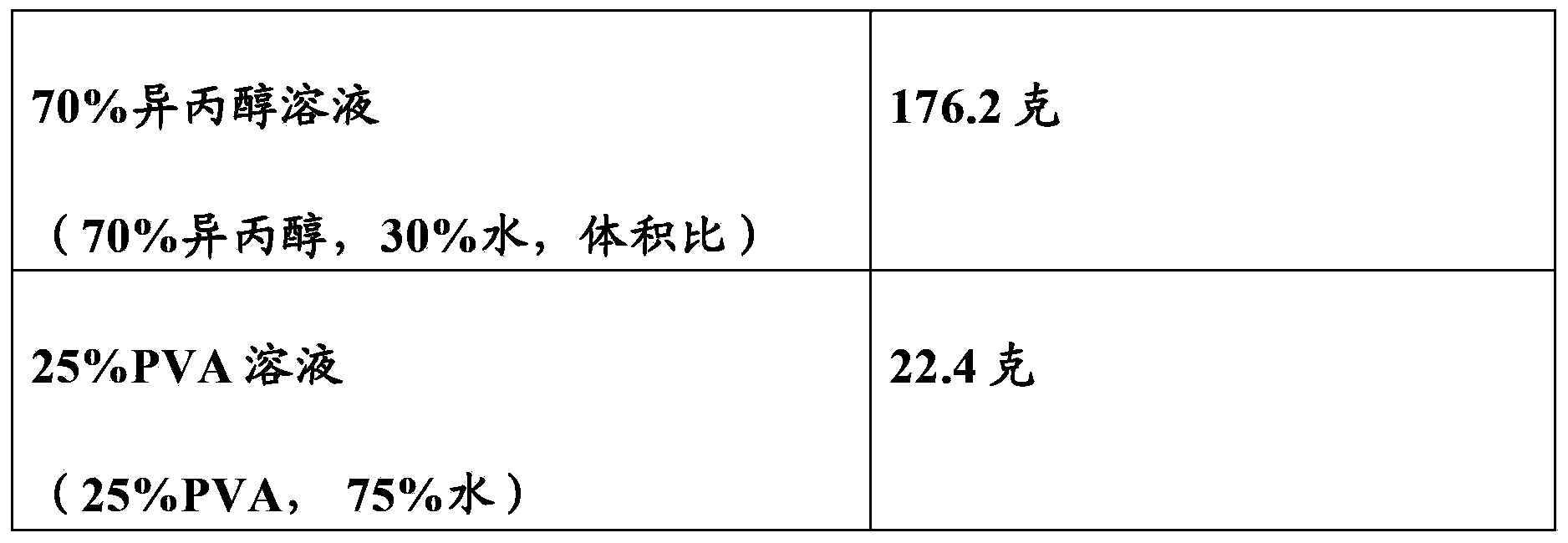
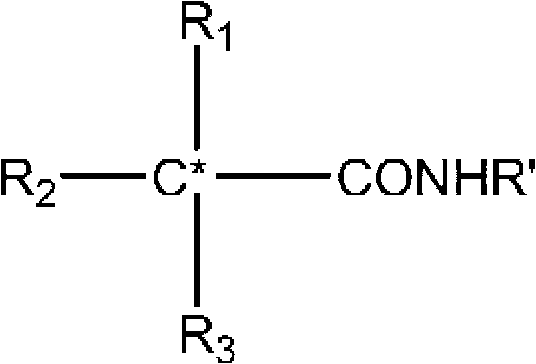
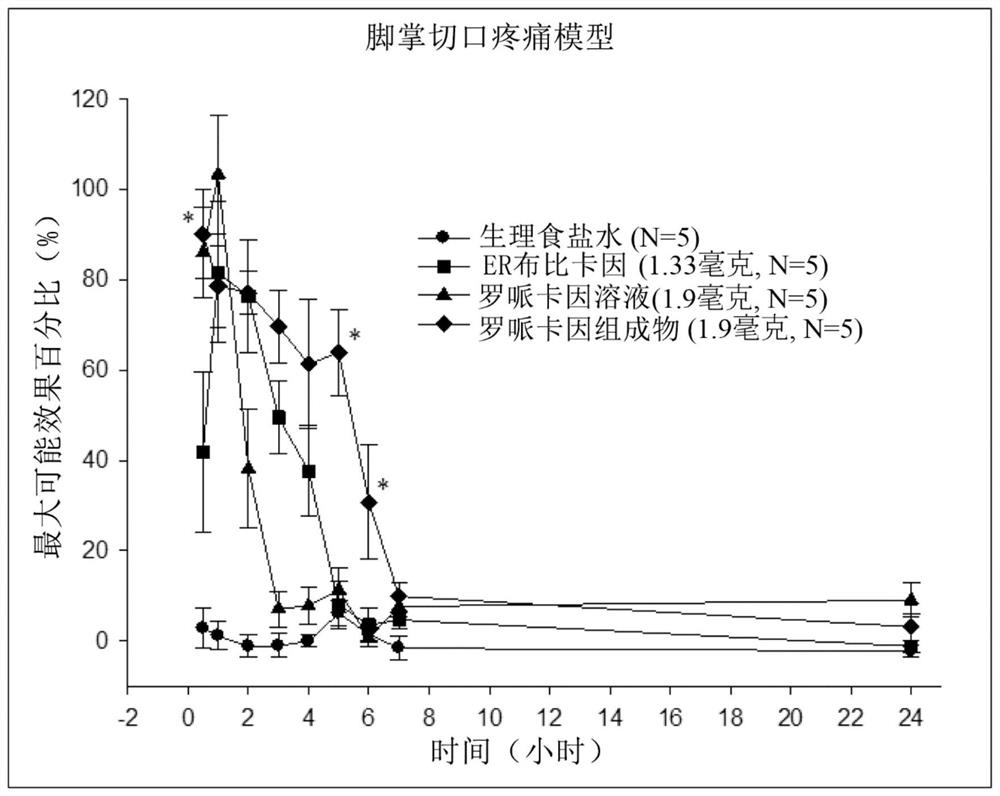
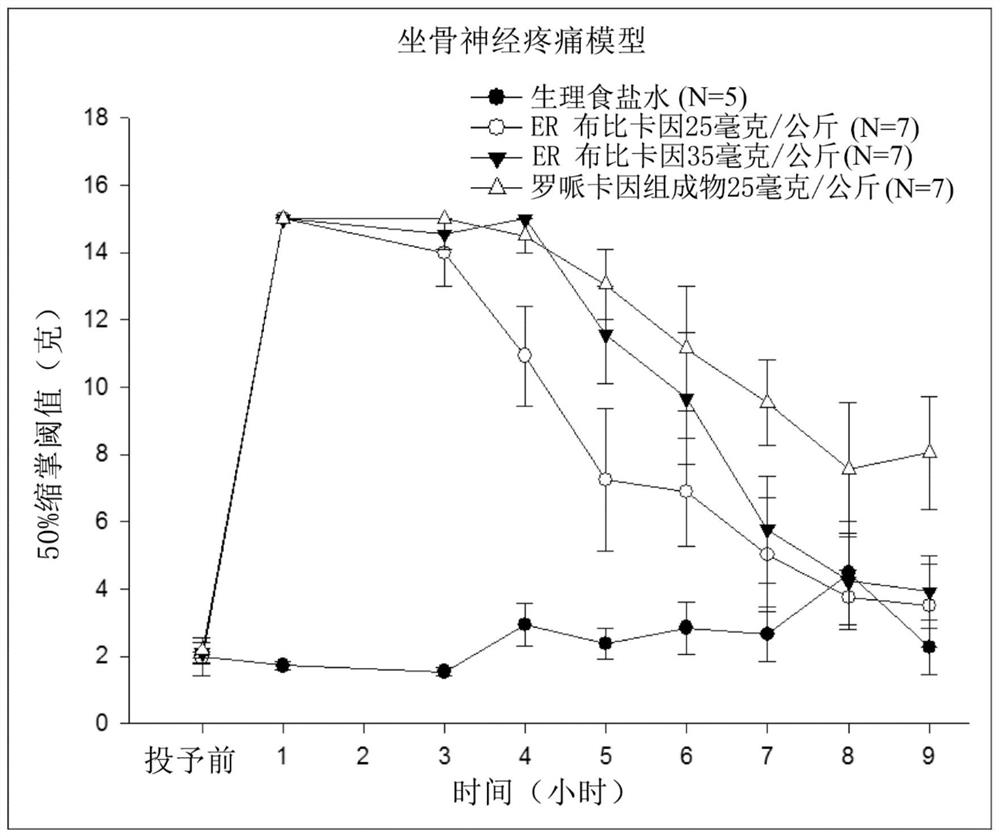
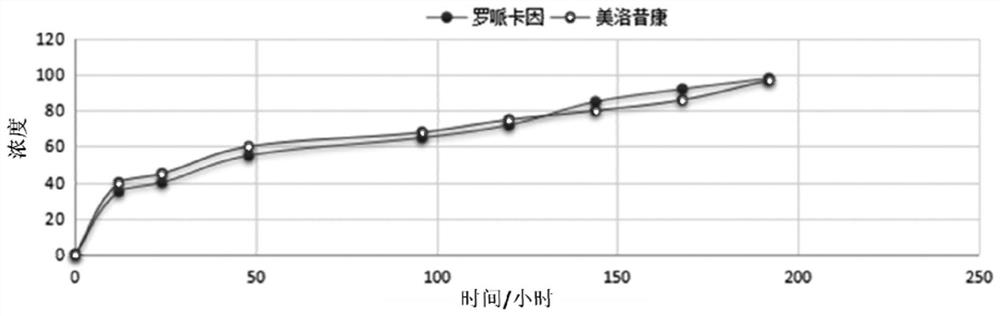
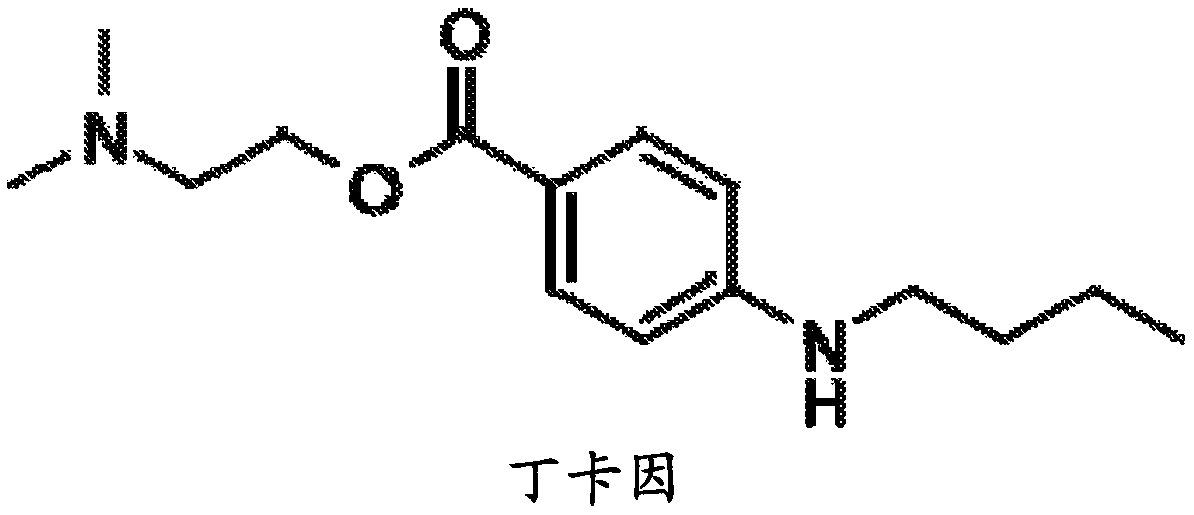
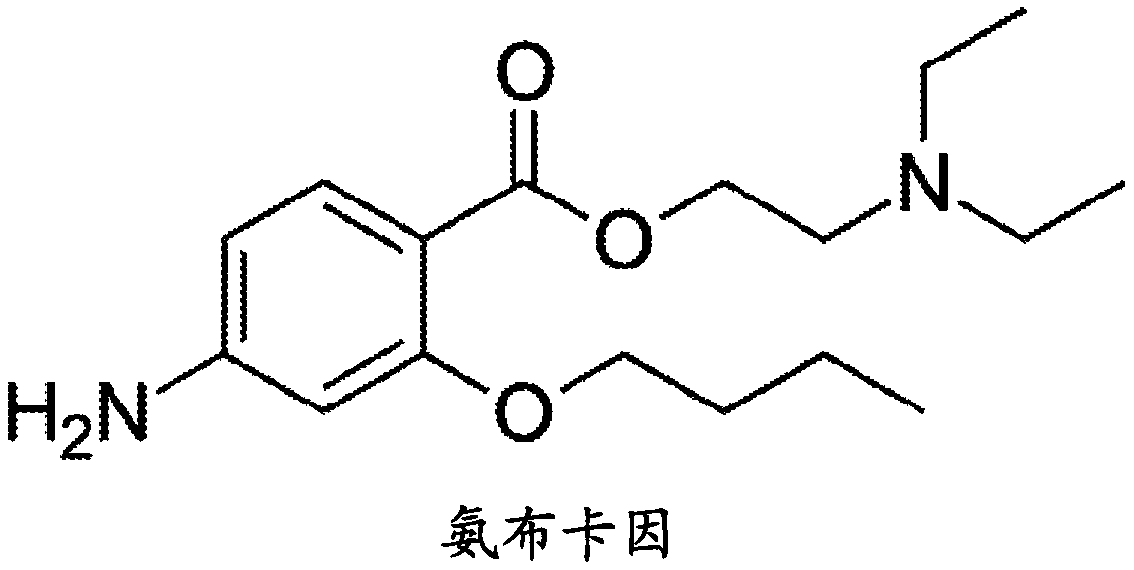
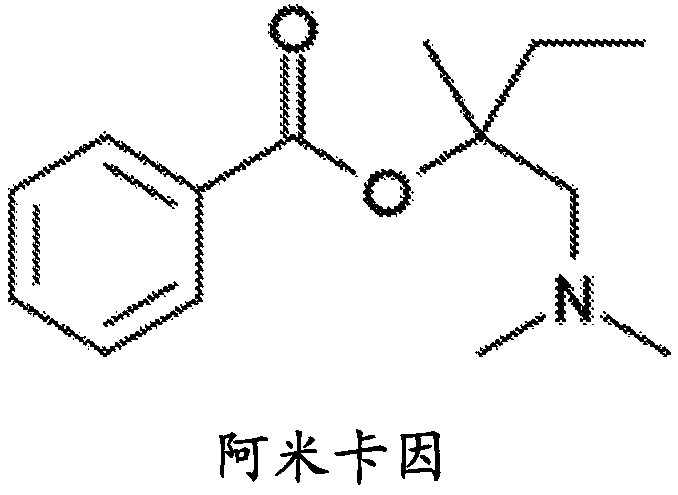
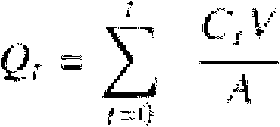Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Topical local anesthetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prolonged anesthesia in joints and body spaces
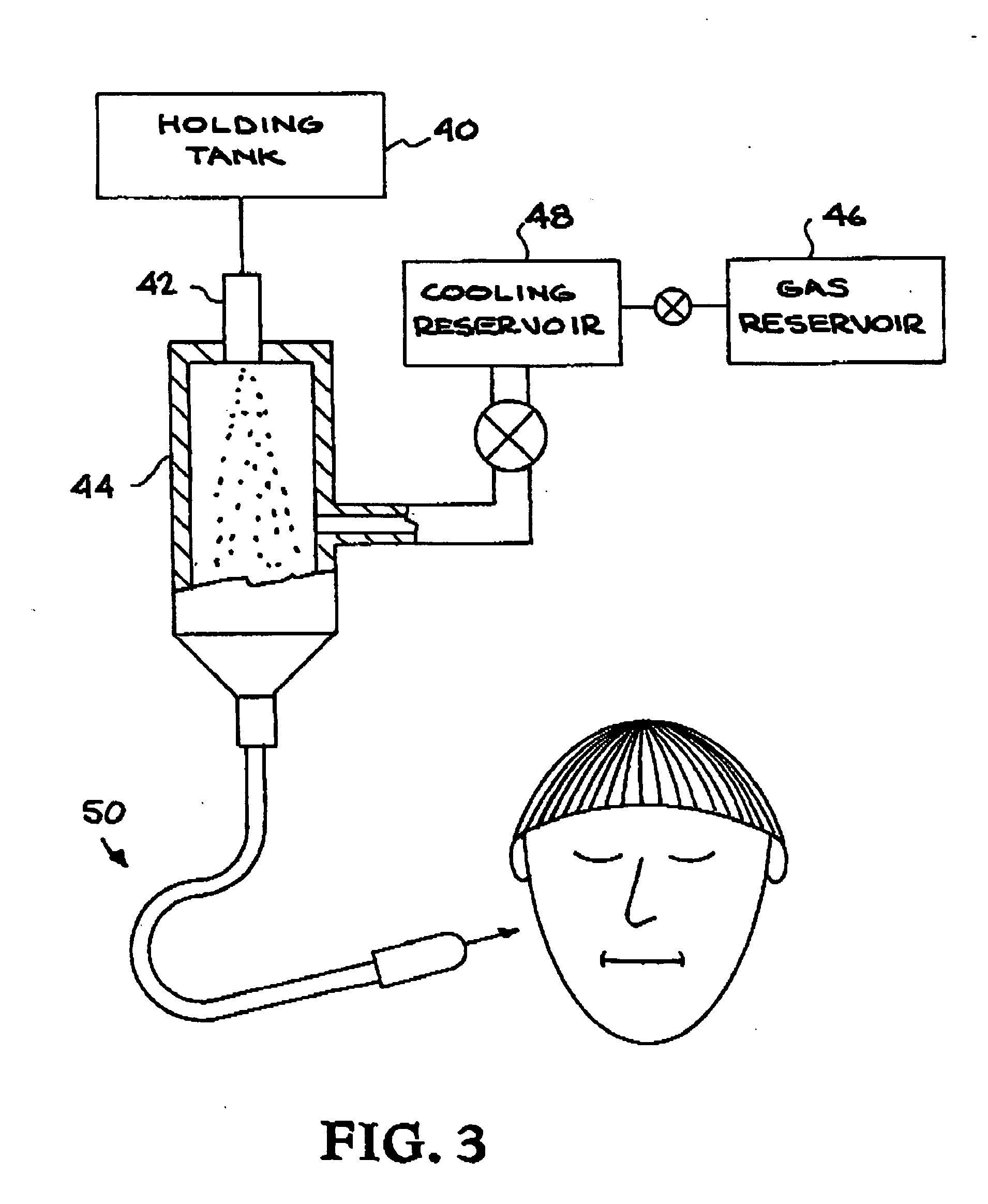
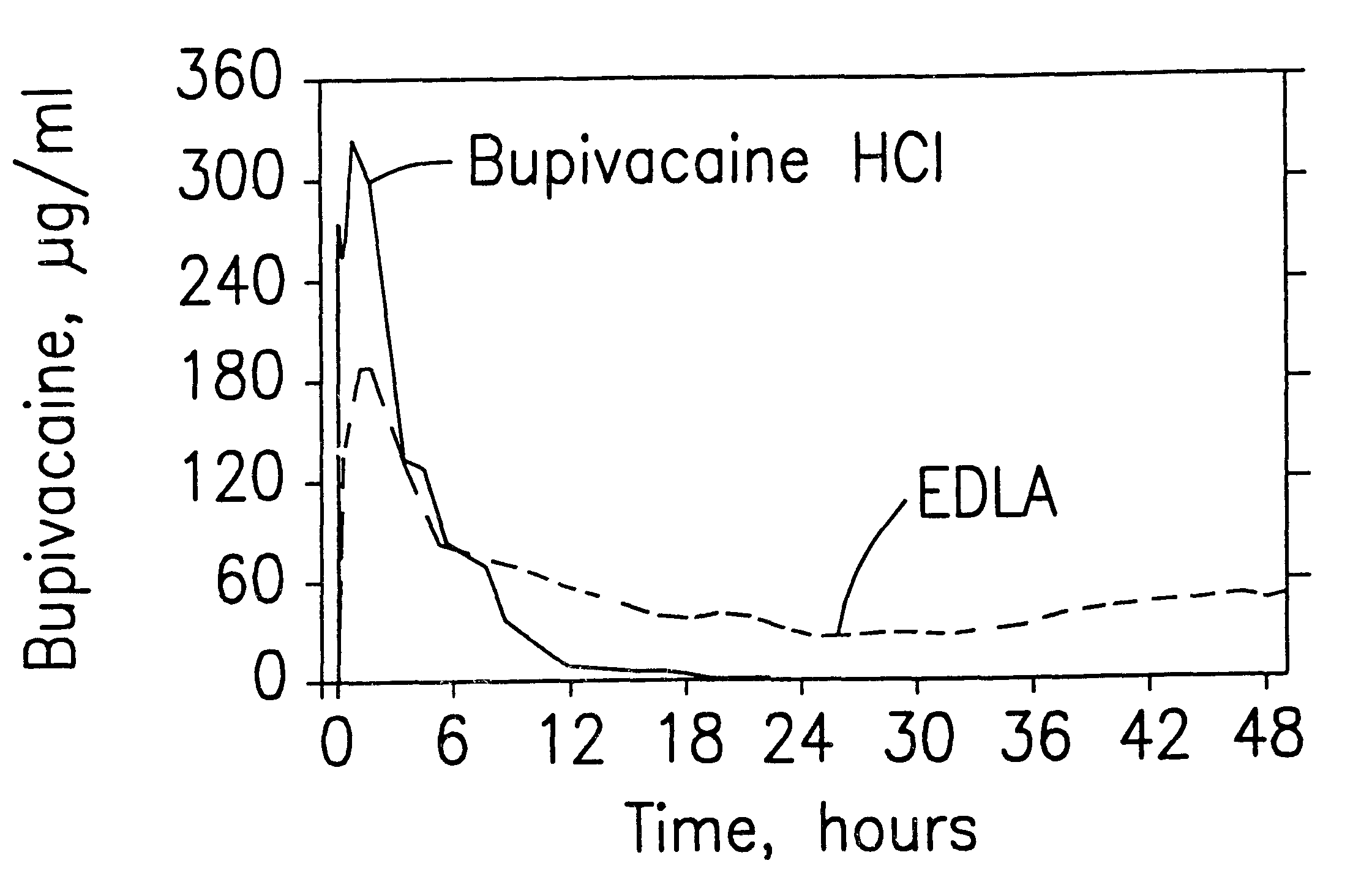
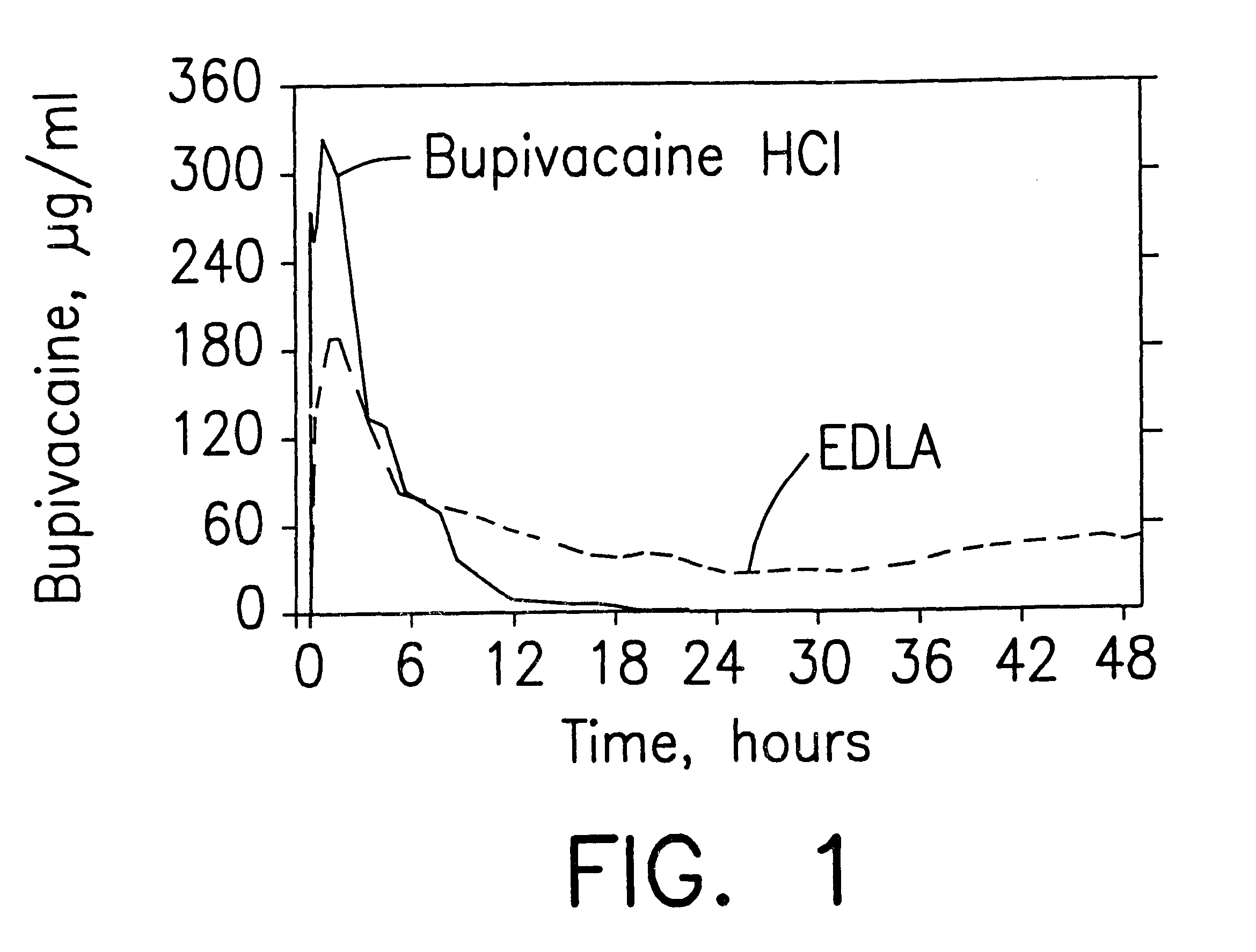
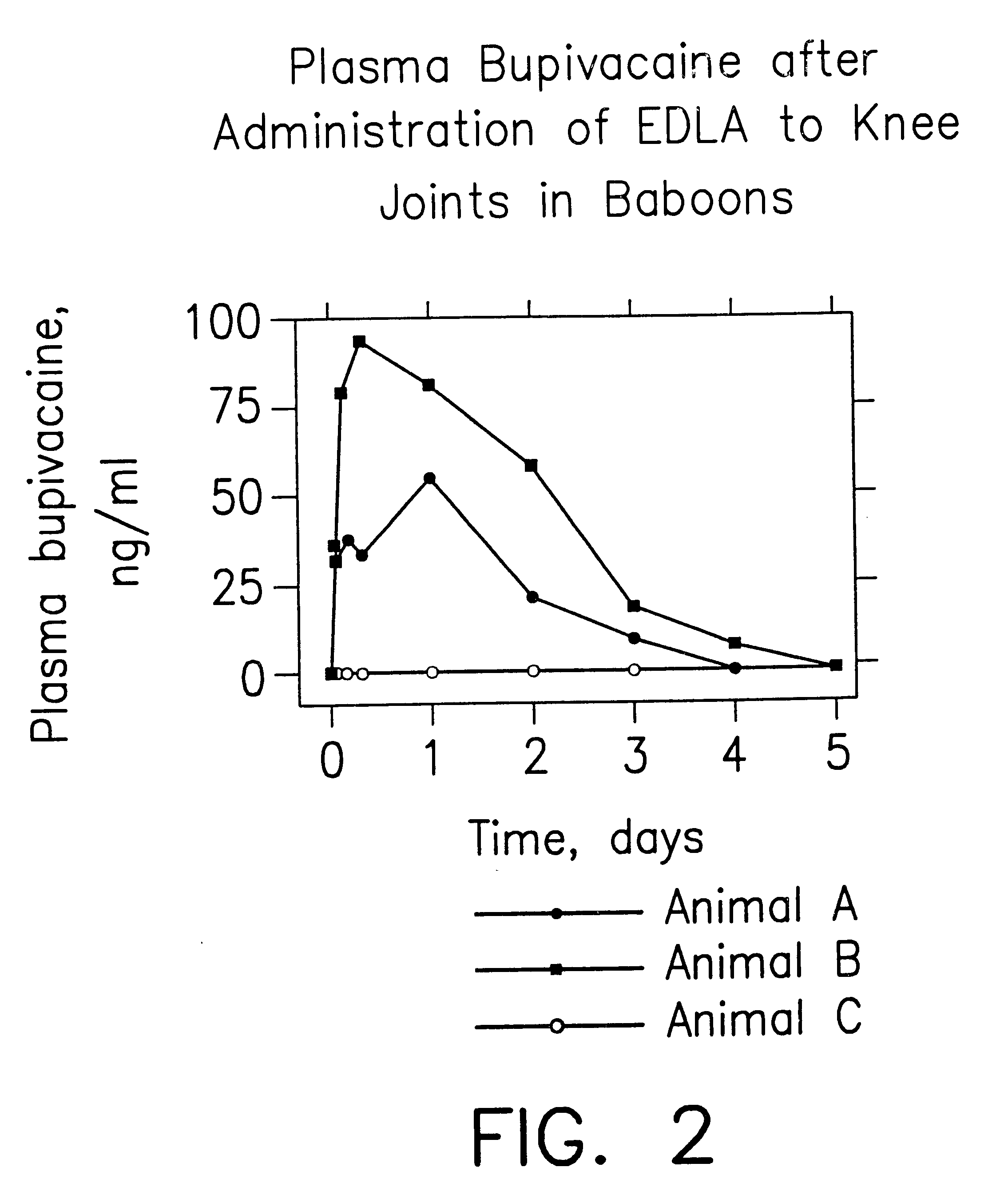
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
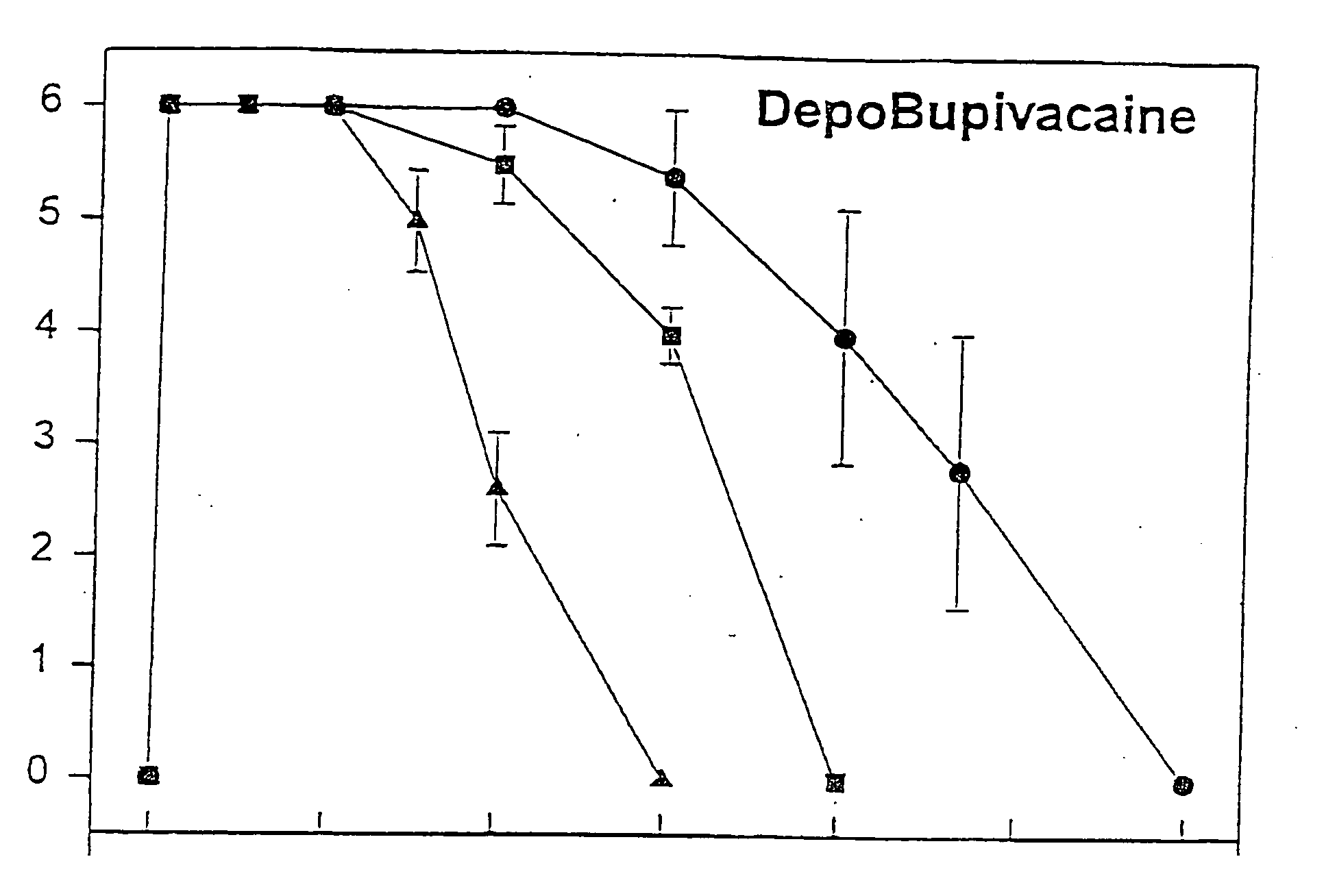
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Skin resurfacing and treatment using biocompatible materials
InactiveUS20050059940A1Eliminate the problemAvoid infectionSurgeryMedical devicesHuman bodyCarrier fluid
Biocompatible materials are propelled at the skin with sufficient velocity to cause desired resurfacing of skin layers to the desired penetration depth. The materials, such as dry ice or water ice, are harmonious with the human body and thus eliminate foreign body reactions. Various materials may be used in combination, including local anesthetics and vasoconstrictors in solid or liquid form. The biocompatible solid or liquid particles are suspended in a cold carrier fluid and propelled through an insulated delivery system to the surface of the skin. The treatment of diseased skin lesions may be accomplished using the present invention as a drug delivery system.
Owner:PEARL TECHNOLOGY HOLDINGS LLC
Formulations and methods for providing prolonged local anesthesia
InactiveUS6451335B1Slow in-vitro releaseRelease the local anestheticAnaesthesiaGranular deliveryControlled releaseAnesthetic Agent
A formulation for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a non-toxic augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent. In preferred embodiments, the controlled release material is a low molecular weight, acid-terminated polymer. A further aspect of the invention is directed to such formulations which release the local anesthetic in two phases, the first a rapid "bolus" to initiate anesthesia and a second, slower release to maintain anesthesia.
Owner:EURO-CELTIQUE SA
Formulations and methods for providing prolonged local anesthesia
InactiveUS6521259B1Good effectPain controlPowder deliveryNervous disorderControlled releaseGlucocorticoid
A formulation and methods for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when inplanted or injected in a patient, and a pharmaceutically acceptable, i.e., non-toxic, non-glucocorticoid augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent.
Owner:PURDUE PHARMA LP
Formulations and methods for providing prolonged local anesthesia
A formulation and methods for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a pharmaceutically acceptable, i.e., non-toxic, non-glucocorticoid augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent.
Owner:PURDUE PHARMA LP
Injectable biodegradable polymer compositions for soft tissue repair and augmentation
InactiveUS20100260703A1Pharmaceutical delivery mechanismUrinary disorderInjectable polymersActive agent
Methods for soft tissue repair and / or augmentation using injectable, biodegradable polymers are described herein. In one embodiment, the polymer compositions are liquid or pastes at room temperature. In a preferred embodiment, the polymer composition contains liquid or pasty hydroxy fatty acid-based copolyesters, polyester-anhydrides, or combinations thereof. The viscosity of the polymers increases upon contact with bodily fluid to form a solid or semisolid implant suitable for soft tissue repair and / or augmentation. In another embodiment, the polymer composition contains particles of a polymer stereocomplex. One or more active agents may be incorporated into the polymer compositions. Suitable classes of active agents include local anesthetics, anti-inflammatory agents, antibiotics, analgesics, growth factors and agents that induce and / or enhance growth of tissue within the filled cavity or control the growth of a certain type of tissue, and combinations thereof. The polymer compositions may also contain one or more additives or excipients that modify the physical and / or mechanical properties of the polymer. The polymer compositions are typically administered by injection. The injectable polymers can be used for a variety of soft tissue repair and augmentation procedures.
Owner:POLYGENE LTD
Prolonged anesthesia in joints and body spaces
InactiveUS20020054915A1Enhance and prolong local anesthesiaGood effectPeptide/protein ingredientsAnaestheticsAnesthetic AgentMicroparticle
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Systems and methods for providing gastrointestinal pain management
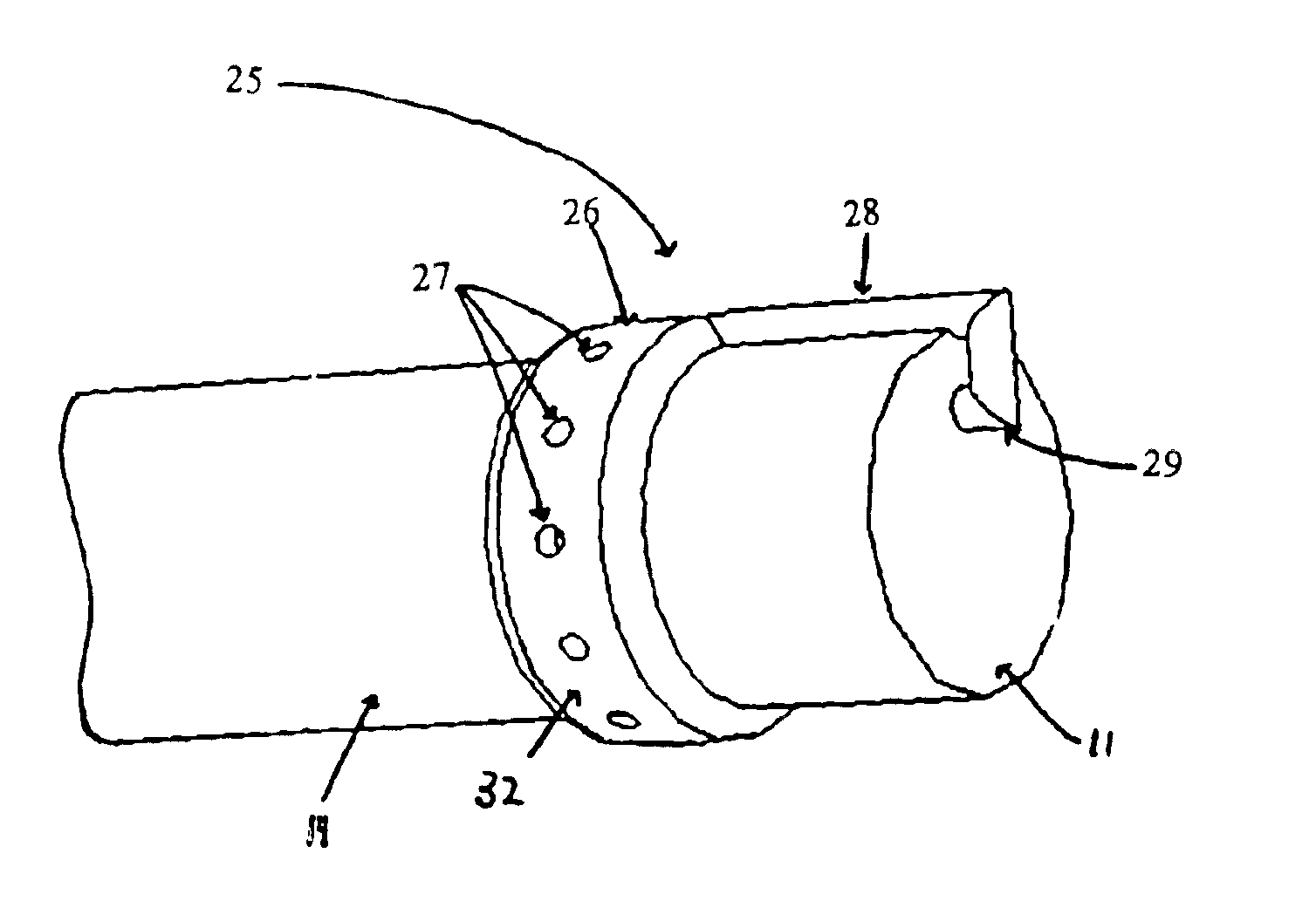
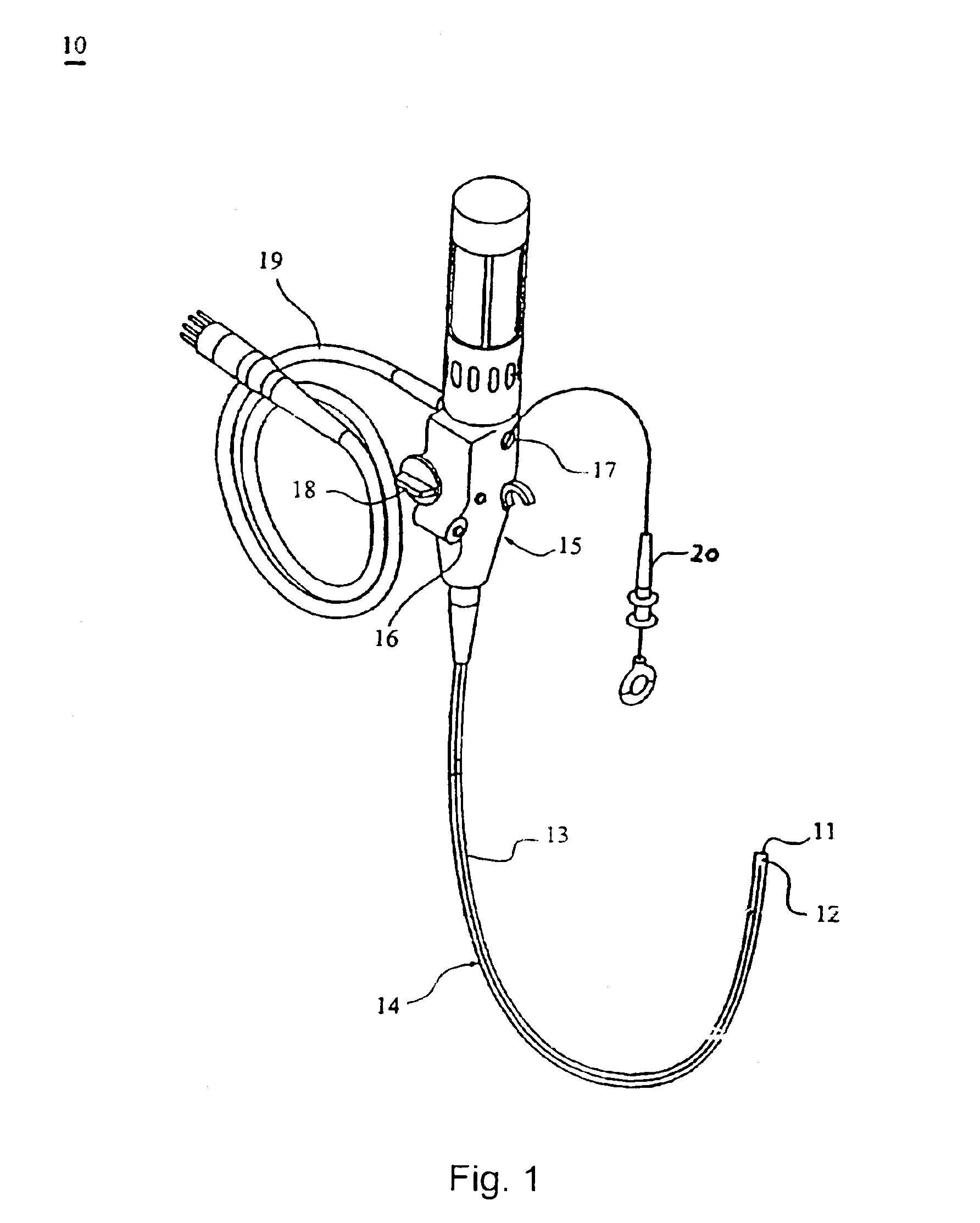
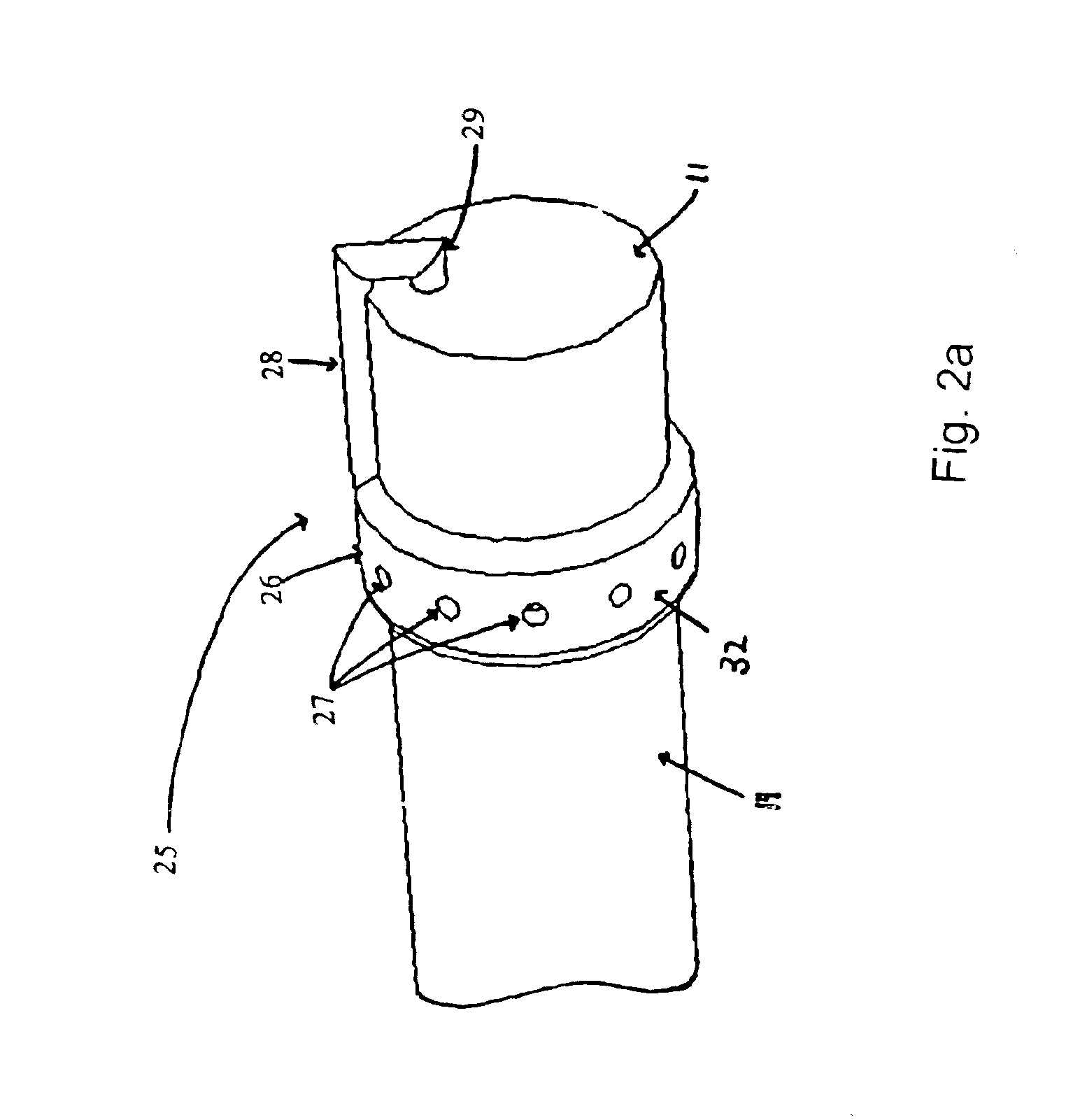
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
Sustained-release liposomal anesthetic compositions
InactiveUS20060078606A1High acceptabilityImprove encapsulationInorganic non-active ingredientsRotary piston pumpsHalf-lifeMaximum tolerated dose
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intracutaneous dose, the duration of anesthesia and half-life of the drug at the local injection site is increased as compared to injection of unencapsulated anesthetic. The maximum tolerated dose of the encapsulated anesthetic is also markedly increased in the liposomal formulation over injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
Owner:PACIRA PHARMA INC
Methods and compositions for treating post-operative pain comprising a local anesthetic
ActiveUS20090264472A1Ease the pain of treatmentReduce deliveryPowder deliveryBiocidePharmacyPharmaceutical drug
The present invention is directed to an implantable drug depot useful for reducing, preventing or treating post-operative pain in a patient in need of such treatment, the implantable drug depot comprising a polymer and a therapeutically effective amount of a local anesthetic or pharmaceutically acceptable salt thereof, wherein the drug depot is implantable at a site beneath the skin to reduce, prevent or treat post-operative pain, and the drug depot is capable of releasing (i) a bolus dose of the local anesthetic or pharmaceutically acceptable salt thereof at a site beneath the skin and (ii) a sustained release dose of an effective amount of the local anesthetic or pharmaceutically acceptable salt thereof over a period of at least 4 days.
Owner:COMPANION SPINE LLC +1
Long-acting polymeric delivery systems
ActiveUS9801945B2Enhance the imageEffective pain reliefPowder deliverySurgeryDelivery vehicleActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Methods for treating myofascial, muscle, and/or back pain
The present disclosure is drawn to methods for treating myofascial pain, muscle pain, back pain, or combinations of these pains. Specifically, a method for treating myofascial pain, muscle pain, back pain, or combinations thereof includes the application of an analgesic system to a skin surface of a subject experiencing the pain and maintaining the analgesic system on the skin surface for a period of time of at least 30 minutes. The analgesic system applied to the skin surface can include a heating component and a local anesthetic formulation which includes at least one local anesthetic. The heating component can be capable of heating the skin surface to a temperature of 36° C. to 42° C.
Owner:NUVO RES
Local anesthetic methods and kits
Methods of reversing local anesthesia are disclosed. The methods comprise administering a local anesthetic and alpha adrenergic receptor agonist to induce local anesthesia followed by reversing anesthesia with a low dose of an alpha adrenergic receptor antagonist. Also disclosed are kits comprising a local anesthetic, an alpha adrenergic receptor agonist and a low dose of an alpha adrenergic receptor antagonist.
Owner:SEPTODONT HLDG
Controlled release local anesthetic for post dental surgery and method of use
The present invention relates to a method of delivering local anesthetic after dental extraction surgery. The present invention relates to packing the tooth socket with a tinned release local anesthetic which is coordinated with an initial local anesthetic and which lasts up to 5 days. The socket can be surgically sealed or the implant can act as the sealing means.
Owner:RILENTO PHARMA LLC
Composition of novel powder formulations of tranexamic acid
InactiveUS20180116986A1Facilitate administrationFacilitate methodPowder deliverySpray deliveryHydrophilic polymersEngineering
Powder composition of Tranexamic acid have been provided for the treatment of wound and bleeding. The powder composition may also contain aprotinin and epsilon-aminocaproic acid as active antifibrinolytic agent. The composition may also contain antibiotic(s), anti-inflammatory agent(s), local anesthetic(s) and hydrophilic polymer(s). The powder composition in this patent application is applied to mucosal or non-mucosal surfaces, but it is not for an oral administration.
Owner:JOSHI HEMANT N +2
Controlled release local anesthetic for post dental surgery and method of use
The present invention relates to a method of delivering local anesthetic after dental extraction surgery. The present invention relates to packing the tooth socket with a tinned release local anesthetic which is coordinated with an initial local anesthetic and which lasts up to 5 days. The socket can be surgically sealed or the implant can act as the sealing means.
Owner:RILENTO PHARMA LLC
Sheet and liquid combination systems for dermal drug delivery
A dermal drug delivery system is provided which comprises at least two components, for example, a sheet of a solid and flexible material, and a vehicle liquid comprising a solvent and optionally other ingredients. A drug, which can be unstable in said solvent but needs the solvent for being delivered into the skin, can be impregnated in the sheet. Other ingredients, such as agents for fastening the drug on the sheet can also be impregnated in the sheet. These two components may be stored separately and joined either shortly before or at the time of application. To use the system, the vehicle liquid may be applied either on the target skin area or on the sheet, and the sheet may then be applied on the target skin area so that the vehicle liquid is positioned between the sheet and the skin and brought into contact with the ingredients impregnated in the sheet. After the sheet and the vehicle liquid are combined in this way, the ingredients in the sheet and in the vehicle liquid are joined to form a combined formulation that is capable of delivering a drug through the skin at a desired rate. The sheet may have low enough permeability to the solvent or its vapor to control the time it takes for the solvent to evaporate across the sheet. When an appropriate local anesthetic agent, such as a tetracaine, is the drug, some embodiments of the system can have wide applications in anesthesia and pain control.
Owner:张洁
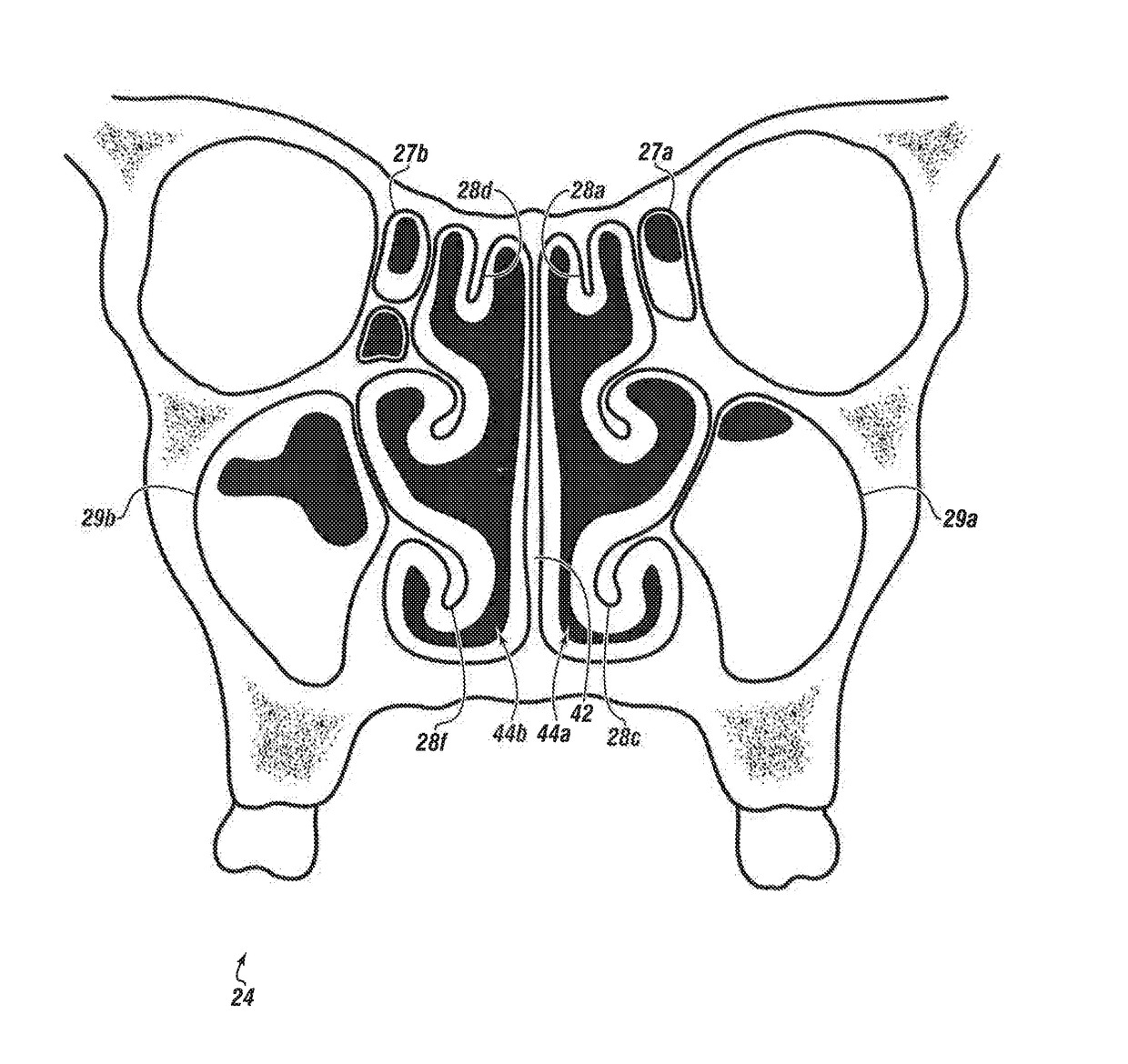
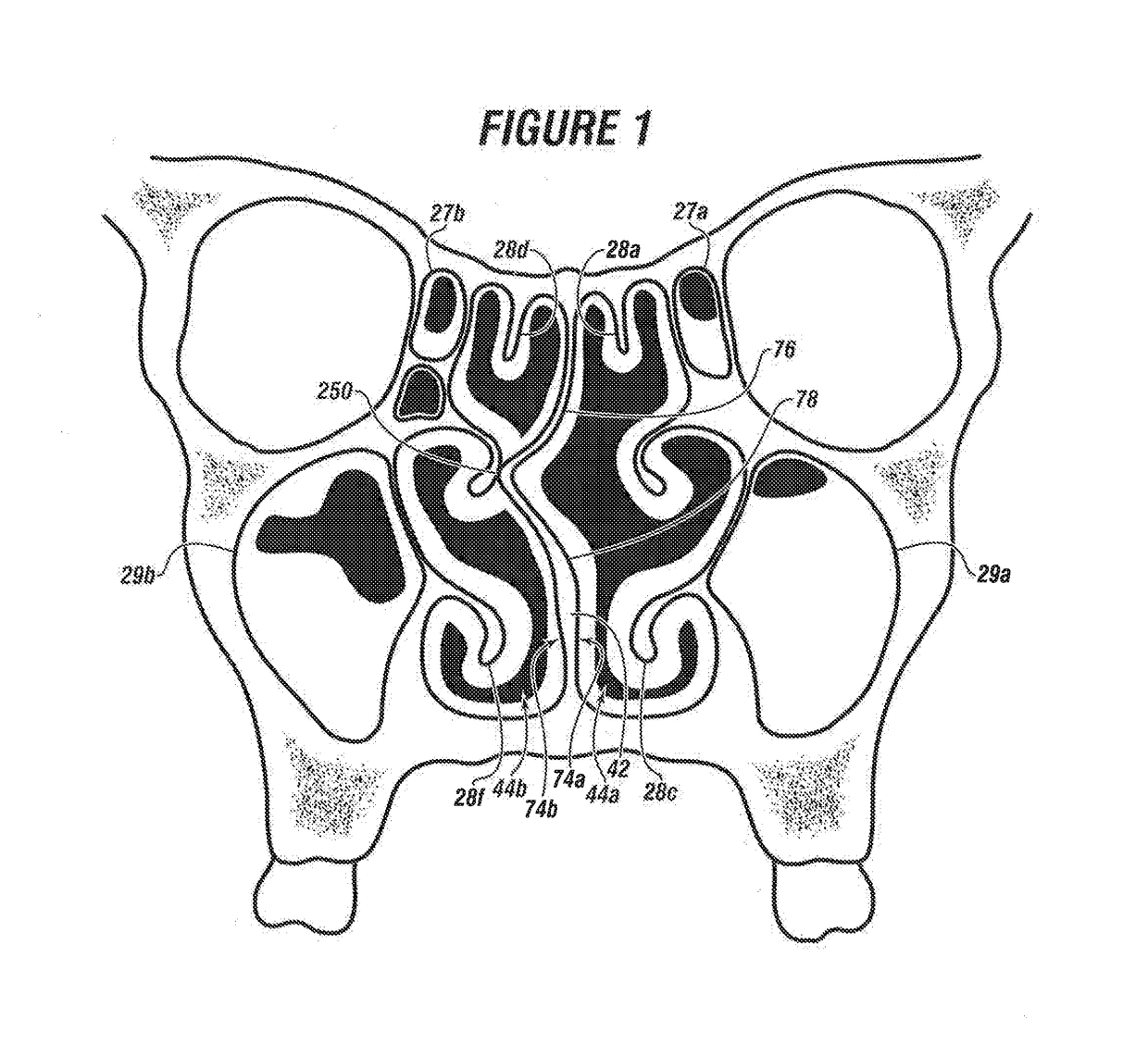
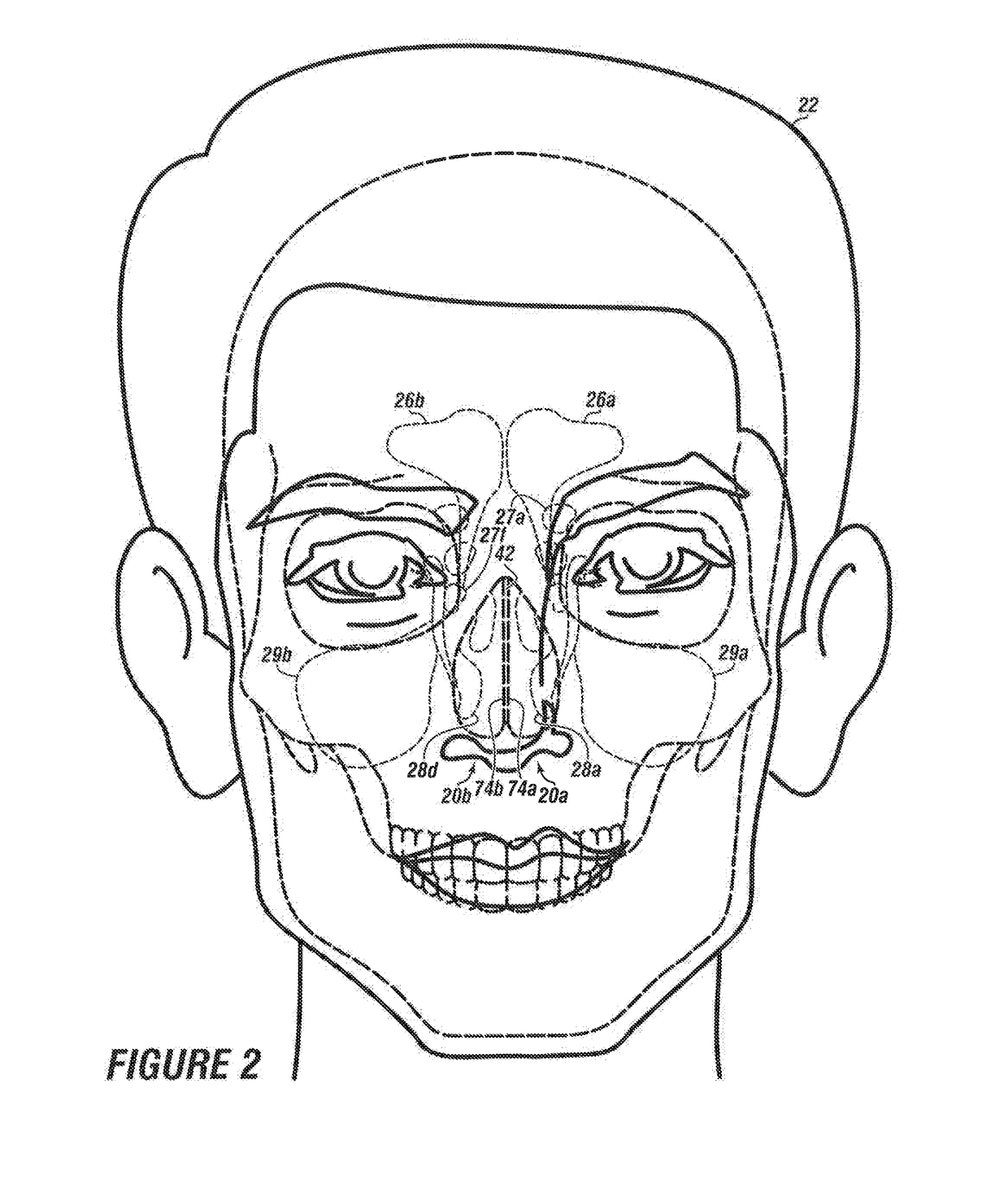
System & method for matching the results of a CT scan to a nasal-sinus surgery plan to treat migraine headaches
InactiveUS20170360511A1Reducing or eliminating the root cause of persistent, headachesLoss of productivityImage enhancementImage analysisNasal cavityHeadaches
A method and system to treat headaches in a patient by performing surgery via at least one nostril. Data from a computer tomography scan of at least one nasal cavity and one sinus cavity of the patient and a completed headache questionnaire are matched to at least one nasal / sinus surgery plan to operate on at least one of; a nasal septum, at least one sinus cavity and at least one turbinate of the patient. The surgery plan is executed by installing a topical local anesthetic and decongestant onto the at least one turbinate forming an anesthetized decongested nasal cavity; infusing an anesthetic into the anesthetized decongested nasal cavity of the patient; dilating the at least one sinus ostium; incising at least one of: a first mucosal flap or a second mucosal Hap of the nasal septum of the anesthetized decongested nasal cavity to expose deviated septal cartilage and bone; removing deviated cartilage and / or bone of the nasal septum; fracturing the at least one turbinate laterally away from the nasal septum; inspecting between the first mucosal flap and the second mucosal flap for a residual broken hone, a residual segment of cartilage or combinations thereof, surgically closing the first mucosal flap and the second mucosal flap of the nasal septum; and suctioning unwanted matter from the anesthetized decongested nasal cavity. An interactive system guides the surgery and provides a record thereof.
Owner:SMITH KEVIN RAYNARD
Methods of treating pains associated with neuroma, nerve entrapment, and other conditions
The present disclosure is drawn to methods for treating nerve entrapment pain; neuroma pain; headache associated with neuralgia; connective tissue pain such as iliotibial band pain, blood vessel pain, tendinopathy pain, medial tibial stress syndrome pain, bursitis, etc.; arthritis pain such as osteoarthritis pain or rheumatoid arthritis pain; pain associated with injury such as fracture, severance, break, sprain, strain, tear, point pain, (e.g., trigger point pain or hit point pain), focal pain, or bruise; or combinations of these pains. Specifically, a method for treating various types of pain includes the application of an analgesic system to a skin surface of a subject experiencing the pain and maintaining the analgesic system on the skin surface for a period of time of at least 30 minutes. The analgesic system applied to the skin surface can include a heating component and a local anesthetic formulation which includes at least one local anesthetic. The heating component can be capable of heating the skin surface to a temperature of 36° C. to 42° C.
Owner:NUVO RES
Method of manufacturing composition comprising local anesthetic, heparinoid, and buffer
PendingUS20140194380A1Maintains bioavailabilityMaintenanceBiocideOrganic active ingredientsAnesthetic AgentMedicine
An improved method for preparing a composition including a heparinoid, a local anesthetic, and a buffer for treatment of a lower urinary tract disease or condition can comprise either: (A) (i) providing a heparinoid in solid form or liquid form; (ii) providing a local anesthetic in solid form or liquid form; (iii) adding a liquid buffer to the heparinoid in solid form or liquid form; (iv) adding the local anesthetic to the mixture of the liquid buffer and the heparinoid; and (v) if necessary, adjusting the pH of the mixture of the liquid buffer, the local anesthetic, and the heparinoid so that a pH is achieved of from about 6.8 to about 8.3 is achieved without precipitation of the local anesthetic; or (B) (i)providing a heparinoid in solid form or liquid form; (ii) providing a local anesthetic in solid form or liquid form; (iii) mixing the heparinoid in solid form or liquid form and the local anesthetic in solid form or liquid form; (iv) adding a liquid buffer to the mixture of the heparinoid and the local anesthetic to form a mixture of liquid buffer, the heparinoid, and the local anesthetic; and (v) if necessary, adjusting the pH of the mixture of the liquid buffer, the local anesthetic, and the heparinoid so that a pH is achieved of from about 7.0 to about 7.8 is achieved without precipitation of the local anesthetic. The invention also encompasses a stable premixed liquid composition that avoids precipitation of the local anesthetic.
Owner:PARSONS LOWELL C
Nasal sinus spray for treatment of sinus headache and method of using same
Disclosed is a nasal spray containing the combination of a vasoconstrictor and a topical local anesthetic.
Owner:MANSFIELD LYNDON
Local anesthetic emulsion compositions and methods of making and using the same
Local anesthetic emulsion compositions are provided. The local anesthetic emulsion compositions may include: an oily phase comprising a eutectic mixture of a local anesthetic and an acyclic amide; a surfactant; and an aqueous phase. Also provided are methods of making and using the emulsions.
Owner:TEIKOKU PHARMA USA INC
Portable anesthesia combined device
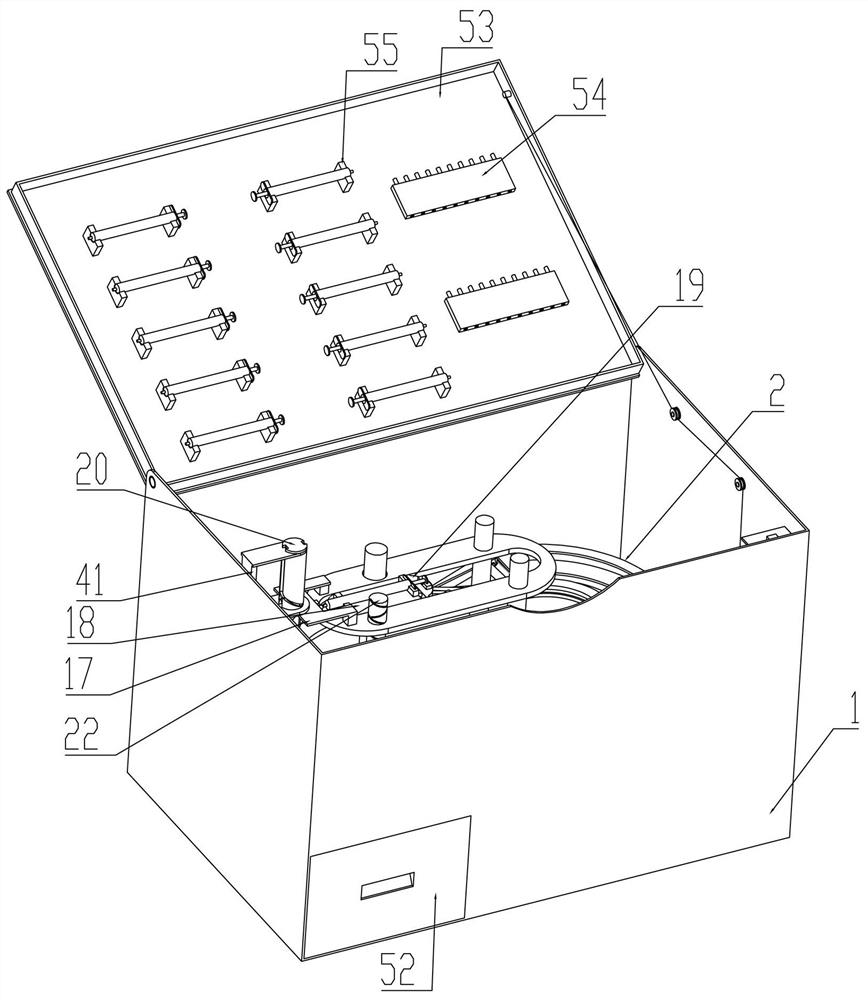
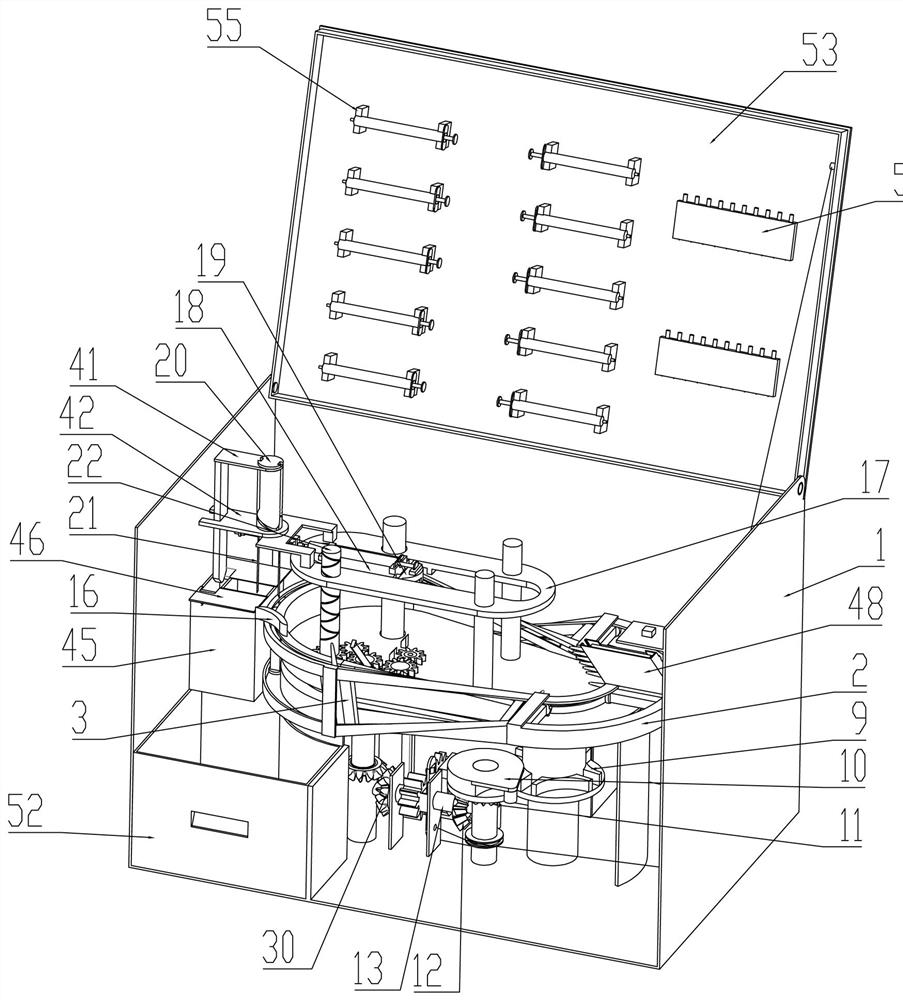
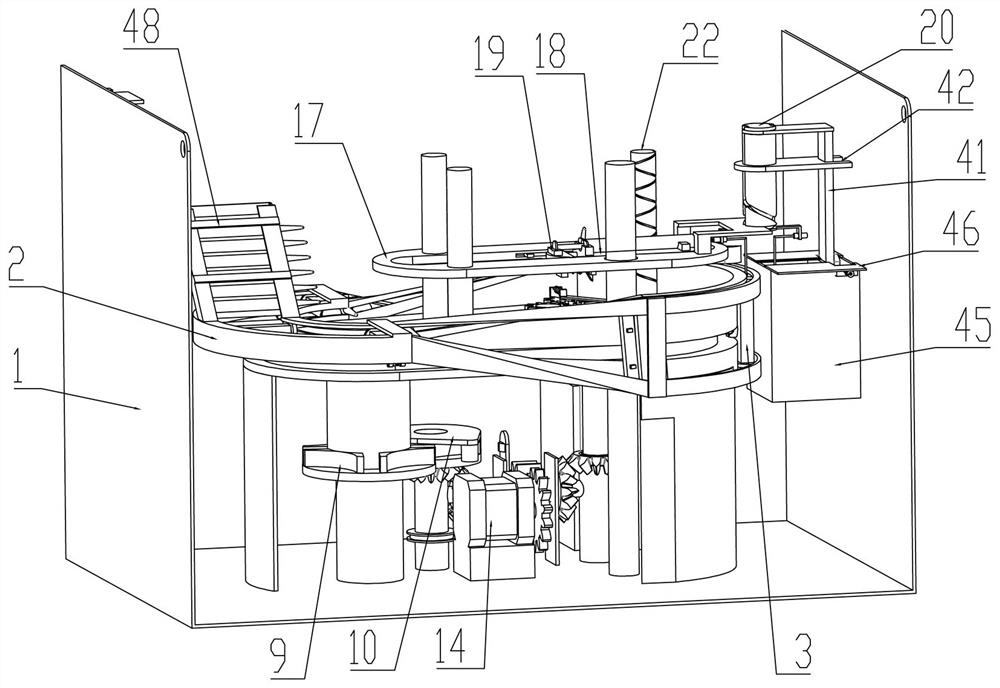
The invention relates to a portable anesthesia combined device and effectively solves a problem that a doctor helps to inject local anesthetics for patients one by one to influence treatment efficiency. The portable anesthesia combined device comprises a housing; the inside of the housing is fixedly connected with a ring-shaped medicine feeding rail; the inside of the ring-shaped medicine loadingrail is in sliding connection with a plurality of medicine frames; the medicine frames are rotationally connected with a conveying belt; the conveying belt is matched with a driving belt pulley; the driving belt pulley is connected with a driven belt pulley by a belt; the bottom of the driving belt pulley is coaxially and fixedly connected with a grooved wheel; the grooved wheel is matched with acam; the cam is coaxially and fixedly connected with a cam driven bevel gear; the cam driven bevel gear is meshed with a driving gear shaft; both the left and right parts of the driving gear shaft arein sliding connection to the bottom of the housing; the ring-shaped medicine feeding rail is in up-and-down sliding connection with a syringe fixed frame; the inside of the syringe fixed frame is inleft-and-right sliding connection with a syringe sliding plate; the syringe fixed frame is rotationally connected with a medicine application syringe needle fixed shaft; the medicine application syringe needle fixed shaft is fixedly connected with a medicine application syringe needle; and an effect of improving working efficiency is achieved.
Owner:HENAN PROVINCE HOSPITAL OF TCM THE SECOND AFFILIATED HOSPITAL OF HENAN UNIV OF TCM
Local anesthetic-containing acidic emulsion composition
InactiveUS20200030305A1Improve security levelStable storageAnaestheticsPharmaceutical non-active ingredientsLocal anaestheticGlycerol
An object of the present invention is to provide a local anesthetic-containing composition characterized by (1) exerting immediate and long-lasting medicinal effect without sustained release after administration, and / or (2) having storage stability.The present invention provides an acidic emulsion composition comprising a local anesthetic and a glyceride in which a fatty acid(s) having 6 to 12 carbon atoms is / are bound to glycerin via an ester bond(s).
Owner:MARUISHI PHARMACEUTICAL CO LTD
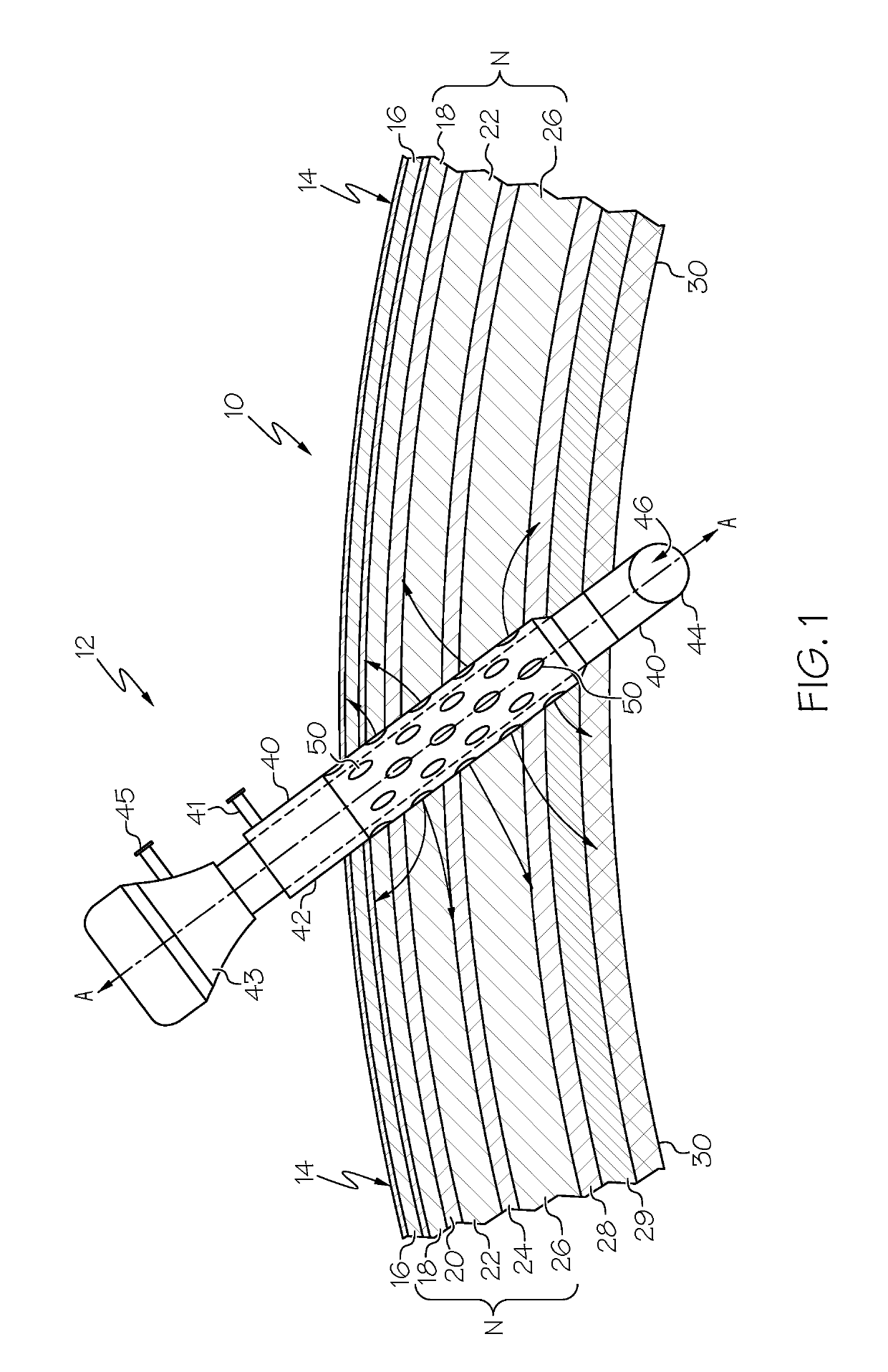
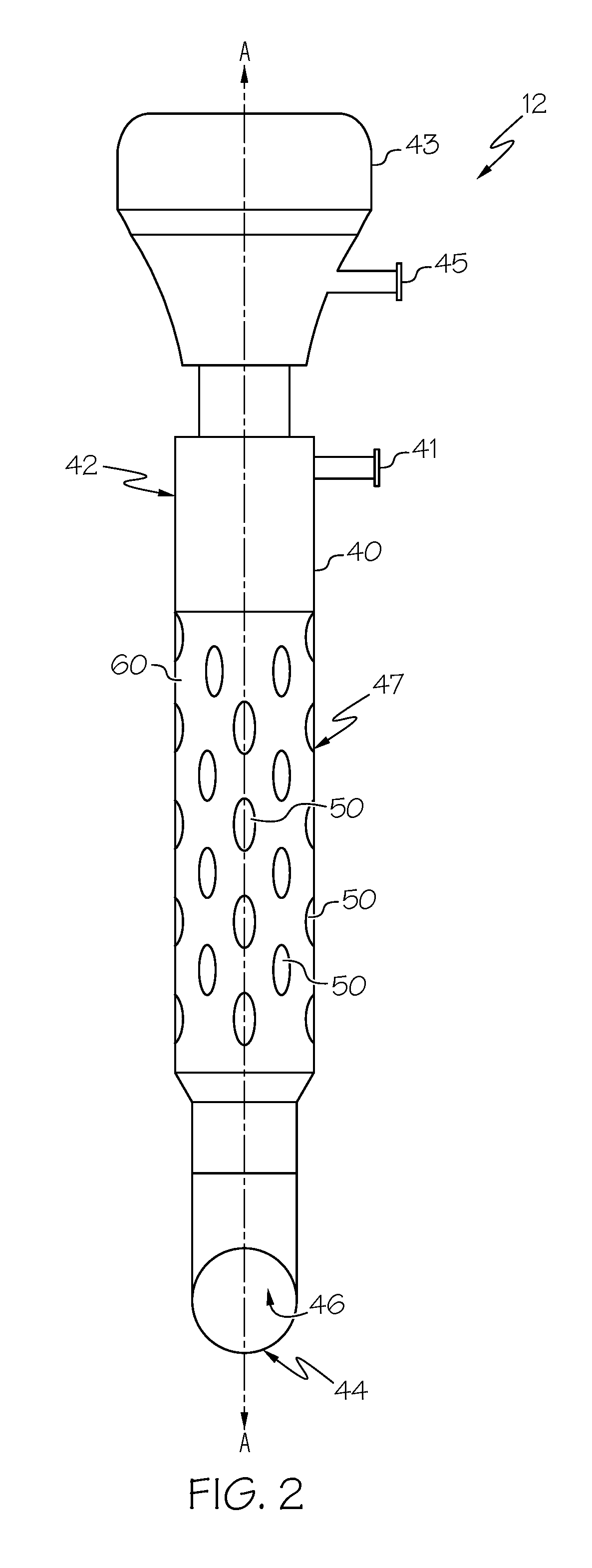
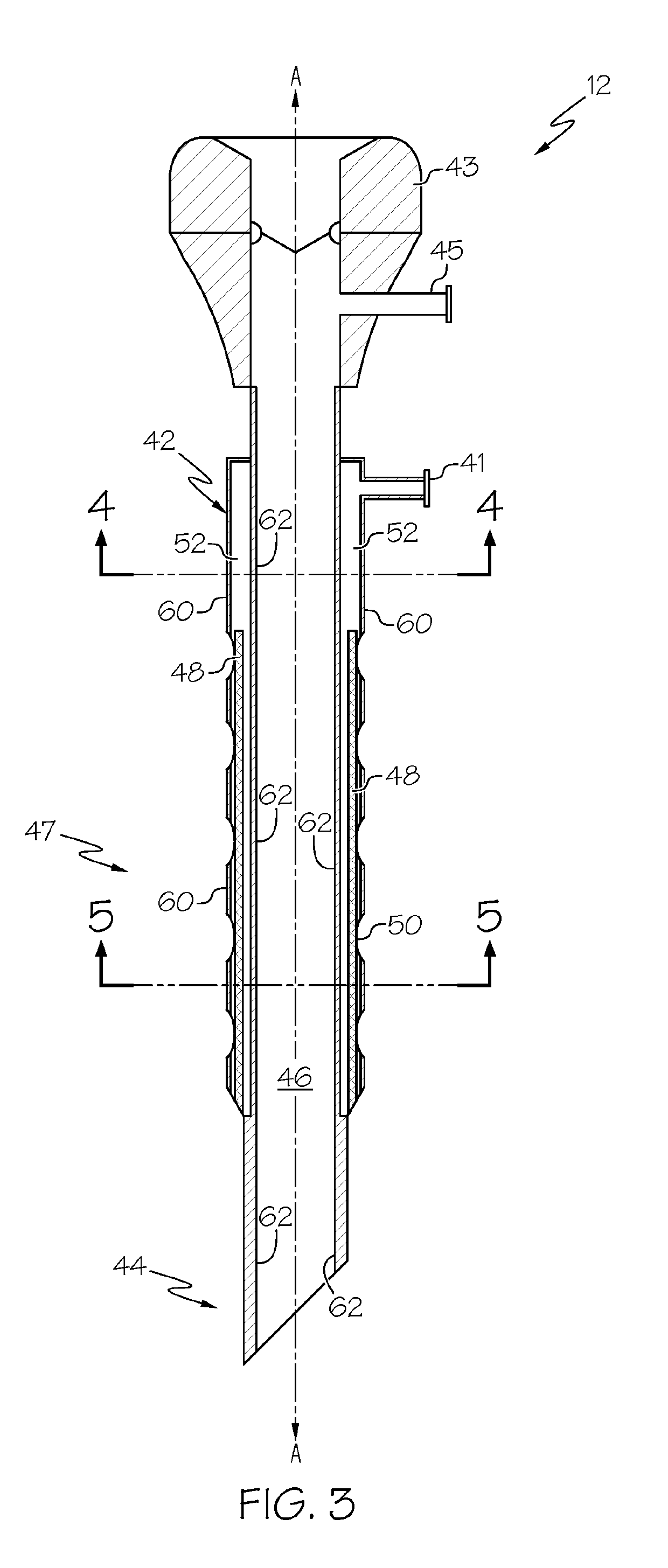
Medication sleeve for a trocar assembly
ActiveUS20190274726A1Uniformly and reliably and steadily introduceCannulasAnaesthesiaPort siteOperative laparoscopy
An obturator sleeve for a trocar assembly and method of its use are disclosed for infiltrating a liquid medication such as a local anesthetic into the tissue walls of a port site wound during a laparoscopic procedure. The sleeve includes an internal flow passage for receiving the liquid medication. A diffusion polymer, located within the internal flow passage, first absorbs the liquid medication and then releases the liquid medication through a plurality of apertures in the sleeve. When port site wound tissue contacts the diffusion polymer, the diffusion polymer within the sleeve steadily releases the absorbed medication through the plurality of apertures. The obturator sleeve provides a means to adequately infiltrate medication into the tissues of the port site wound, and can be used to block the free nerve endings of the port site wound as well as nearby sensory and motor nerves.
Owner:MODERN SURGICAL SOLUTIONS
Sustained-release anesthetic compositions and methods of preparation thereof
Provided is an anesthetic composition for locally administering a local anesthetic agent to a subject in need thereof. The anesthetic composition has a lipid based complex prepared by hydrating a lipid cake containing a local anesthetic agent and a lipid mixture with an aqueous buffer solution at a pH higher than 5.5. Also provided is a method to prepare an anesthetic composition using a simpler and more robust for large-scale manufacture and for providing a high molar ratio of local anesthetic agent to phospholipid content as compared to the prior art. This anesthetic composition has a prolonged duration of efficacy adapted to drug delivery.
Owner:TLC BIOPHARMACEUTICALS INC +1
Pain-relieving and anti-inflammatory compound sustained-release preparation
ActiveCN113616796AEasy to administerEasy to manageAntipyreticAerosol deliveryNon steroid anti inflammatory drugAntiinflammatory drug
The invention provides a pain-relieving and anti-inflammatory compound sustained-release preparation, which comprises a local anesthetic, a non-steroidal anti-inflammatory drug and a sustained-release preparation consisting of poloxamer 407 and poloxamer 188. The pain-relieving and anti-inflammatory compound sustained-release preparation disclosed by the invention is a non-opioid analgesic, is a dual-effect combined product composed of the local anesthetic and the low-dose non-steroidal anti-inflammatory drug, is specially used for treating postoperative pain and inflammation at an operative site through single administration, and has obvious advantages in efficacy, wherein the non-steroidal anti-inflammatory drug not only has an anti-inflammatory effect, but also can inhibit the formation of an acid environment and normalize the pH value, so that the permeation effect of the local anesthetic is recovered and enhanced, and the drug effect is as long as 4-5 days.
Owner:JIANGSU KANGHE BIOLOGICAL PHARMA
Extended duration local anesthetic formulation
An extended duration anesthetic includes a short duration local anesthetic in a dilute solution and a long duration local anesthetic. The long duration local anesthetic is maintained in a powdered form until the time of administration. Premeasured quantities of the dilute solution and powdered long duration local anesthetic in a kit allow for quick preparation of a solution with desired concentrations of both short duration local anesthetic and long duration local anesthetic at the time of administration.
Owner:VENTIS PHARMA
Adductor Canal Block Introducer
InactiveUS20200315657A1Pain reliefReduce catheter migrationCannulasAnaesthesiaAnesthetic AgentInitial dose
An adductor canal block introducer for introducing a catheter and also administering an initial dose of local anesthetic. The adductor canal block introducer generally includes a cannula having an elongated body with a distal end, a proximal end, and a slot extending along the elongated body, the slot having two sides and a lower surface. It also includes a cannulated trocar positioned in the slot of the cannula, the cannulated trocar having a distal end and a proximal end, wherein the trocar includes a discharge proximate to the distal end, wherein an inlet and the discharge are in fluid communication with each other. The introducer also includes a catheter positioned in the slot, held in place by the cannulated trocar. The catheter may also be positioned by a grasper introducer that holds the catheter and also includes a discharge at its distal end through which an anesthetic can be administered.
Owner:MATTHEWS DANIEL E
Analgesic formulations and methods for reduced postoperative nausea and vomiting and enhanced postoperative pain relief
A multimodal anti-emetic anesthetic / analgesic formulation for pain control not limited to postoperative pain control is described herein. The opioid-free / sparing anesthetic / analgesic formulation comprises a local anesthetic, an N-methyl-D-aspartate (NMDA) receptor antagonist, and a cyclooxygenase (COX) inhibitor such as Bupivacaine Hydrochloride, Ketamine Hydrochloride, and Ketorolac Tromethamine, which is effective to significantly reduce postoperative nausea and vomiting and enhance postoperative pain relief as compared to existing prior art anesthetics / analgesics. The formulation is administered to a mammal in need of anesthesia / analgesia and can be used as a preemptive and preventative multimodal analgesic. The formulation may have a buffer to enhance its shelf life and improve pharmacokinetics. The formulation may further comprise an alpha agonist, a steroid, a Transient Receptor Potential Channel agonist or antagonist, a beta-lactam antibiotic, a protein kinase inhibitor, a competitive or non-competitive glycine or glutamate antagonist, a glutamate or glycine inhibitor, a cyclooxygenase 3 inhibitor, or combinations thereof.
Owner:HUTCHISON HEALTH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com