Modulation of solubility, stability, absorption, metabolism, and pharmacokinetic profile of lipophilic drugs by sterols
a technology of sterols and solubility, which is applied in the field of formulations of therapeutic agents, can solve the problems of lowering systemic bioavailability of drugs, difficult to formulate, and poor water solubility, so as to enhance enhance biological absorption and/or metabolic stability, and enhance the effect of biological absorption or metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of TU Solubility with & without Sterols in Formulations of Varying Complexity
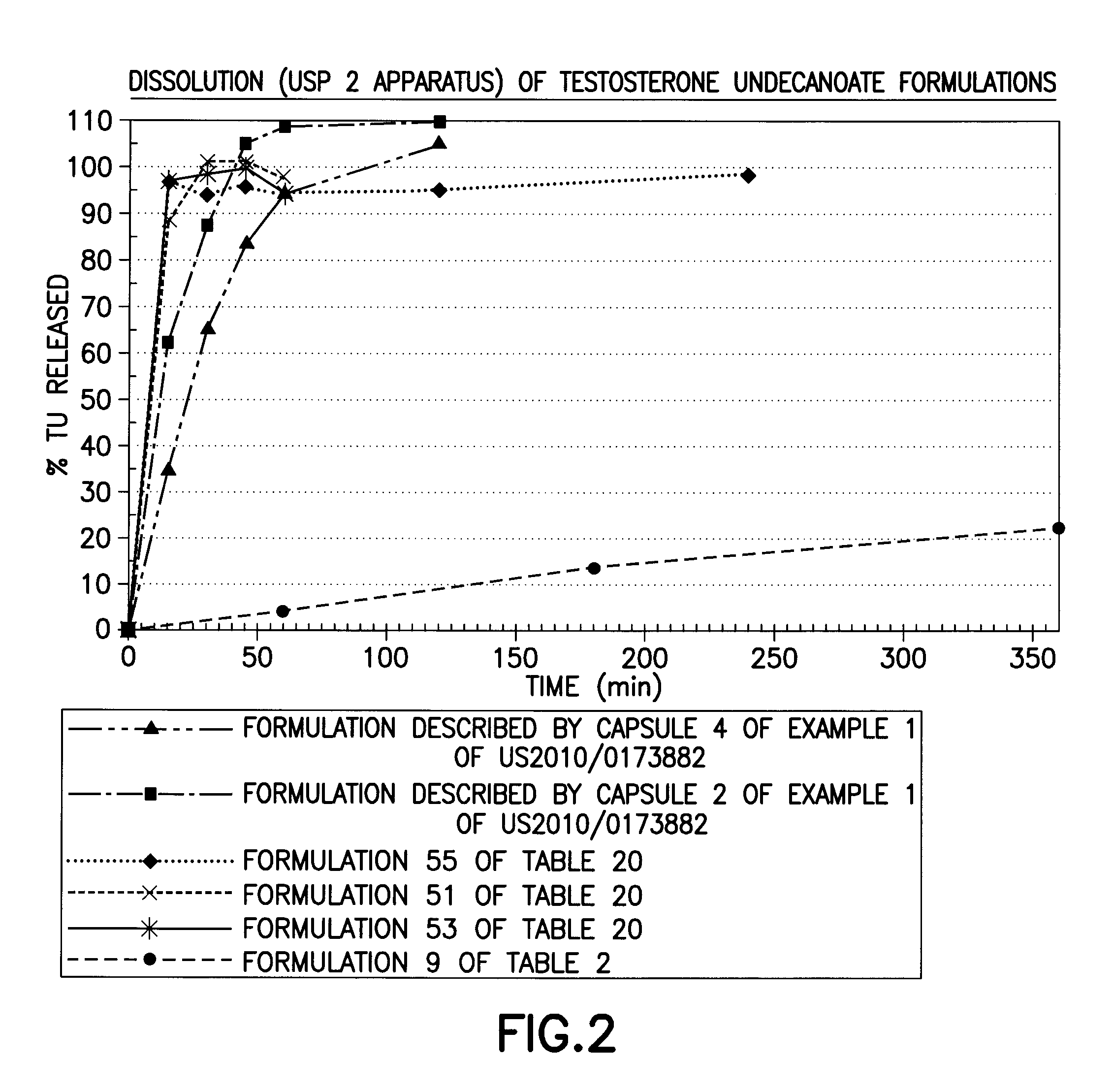
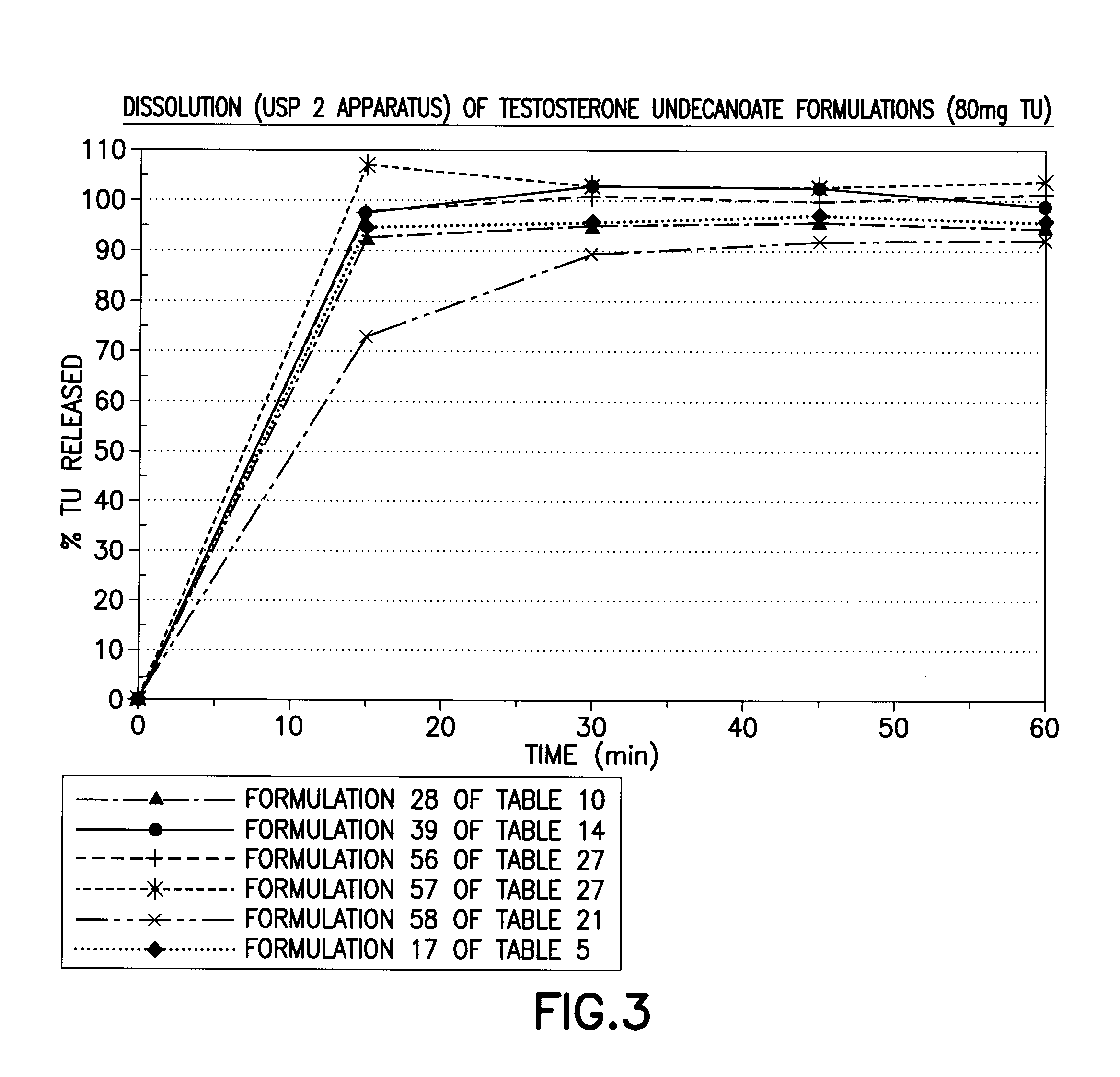
[0202]The solubility of TU in various solubilizers was determined using conventional techniques such as incrementally adding TU until the solubilizer could no longer solubilize additional material. Table 1 below lists experimentally measured solubilities of testosterone undecanoate (TU) in various excipients of interest. Formulations 1-50 below starting with simple one component to complex 4-6 components (Classes I through VII) were then prepared representing different categories of solubilizers. The solubility of TU and / or T was enhanced by sterols (phytosterols, cholesterol, sitostanol, and beta-sitosterol) by 1-40%. The extent of enhancement is governed by the properties of solubilizers, emulsifiers, and surfactants selected to form the formulation.
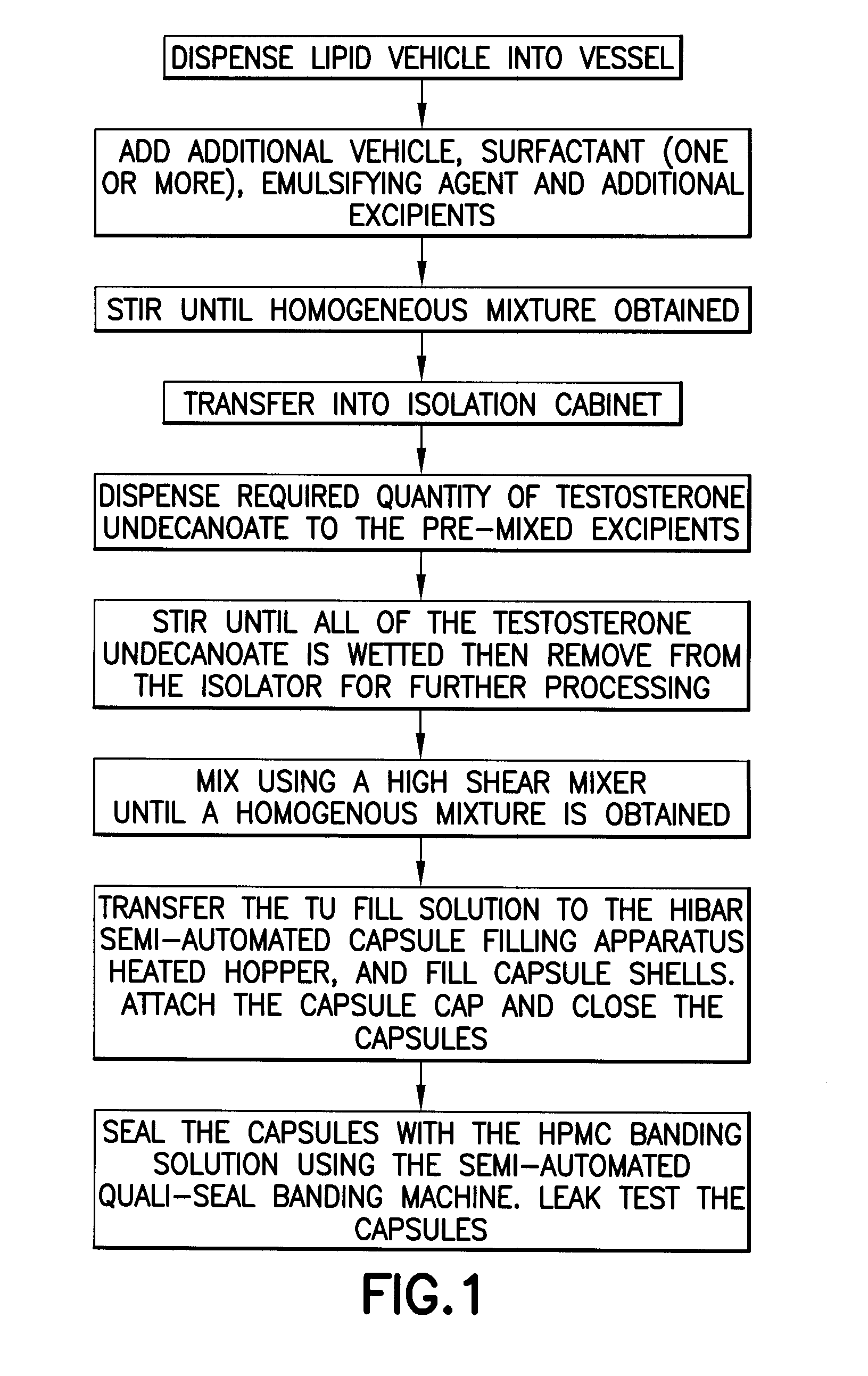
[0203]The formulations listed in Tables 1 to 20 were prepared by combining the excipients, except phytosterols, in the proportions given. The formu...
example 2
Preparation of Compositions Comprising Testosterone Undecanoate
[0208]Compositions comprising T, TU, and Phytosterol were prepared by weighing out the components in the described amount, placing the components into an appropriate container, mixing the components in an appropriate manner and, if necessary, heating to facilitate the solubilization of T, TU, and Phytosterols in the formulation. The formulations can be prepared by adding the components in any order. For example, T, TU, and phytosterols can be added to an individual component or into mixtures of two or more components. The composition can be prepared at room temperature or gently heated to 40-60° C. The composition can also be prepared by melting TU or Phytosterol and / or phytosterol esters at a temperature above the melting point, i.e., 64-66° C., followed by mixing it with other components. Traditional mixing techniques can be used, including, for example, mechanical agitation, stirring and sonication of the components. ...
example 3
Preparation of Compositions Comprising Testosterone Undecanoate and Phytosterols
[0213]The percentage of phytosterol in the phytosterol-saturated formulations ranges from 2% to 20%. Three formulations are described in Table 22 containing between 5.8% and 44.6% phytosterols. FIG. 4 describes the dissolution profiles of these three formulations. Dissolution was measured in 900 mL of 25 mM phosphate buffer at pH 7.0 containing 0.1% SLS, obtained at 200 rpm using USP 2 apparatus. Formulation 59 illustrates the dissolution of a formulation with the properties of remaining a liquid a room temperature, while Formulation 60 is a suitable formulation which is solid at room temperature. Phytosterols in excess of the amount soluble at 70° C. may be added to the composition to modulate the release rate, as illustrated by the dissolution profile of Formulation 61 in FIG. 4. Formulation 61 further has the desirable property of being a sufficiently hard material that it may be reduced to a powder, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com