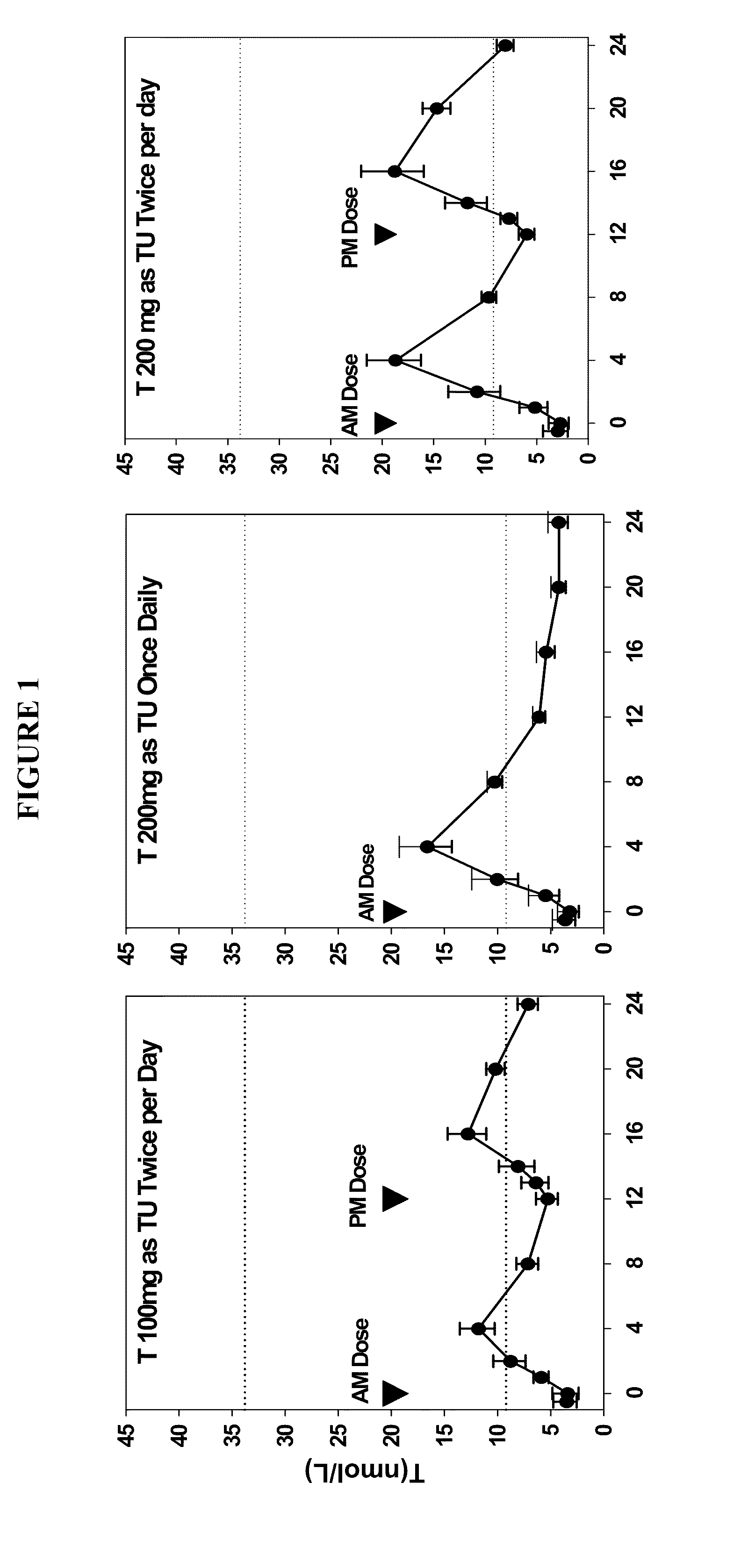
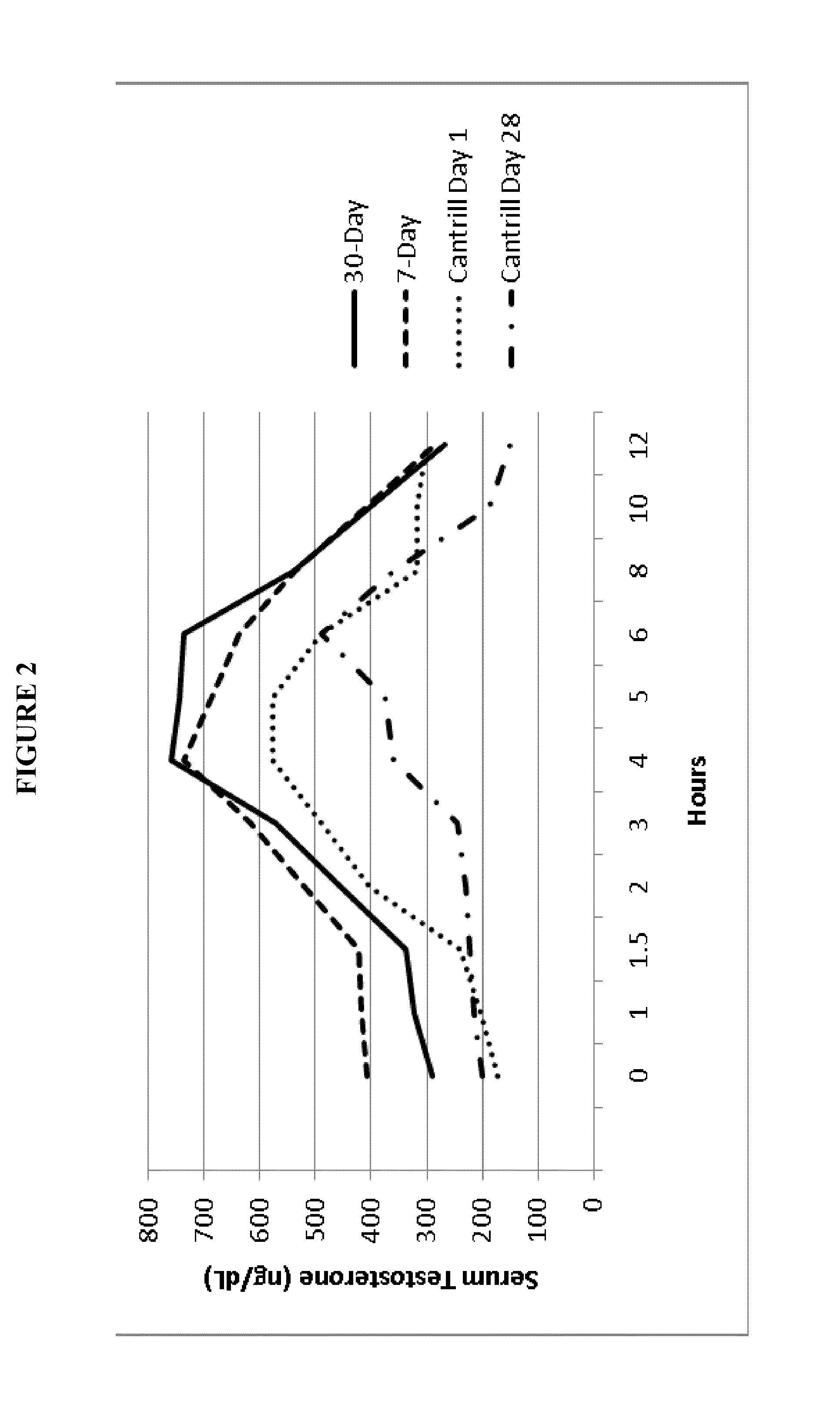
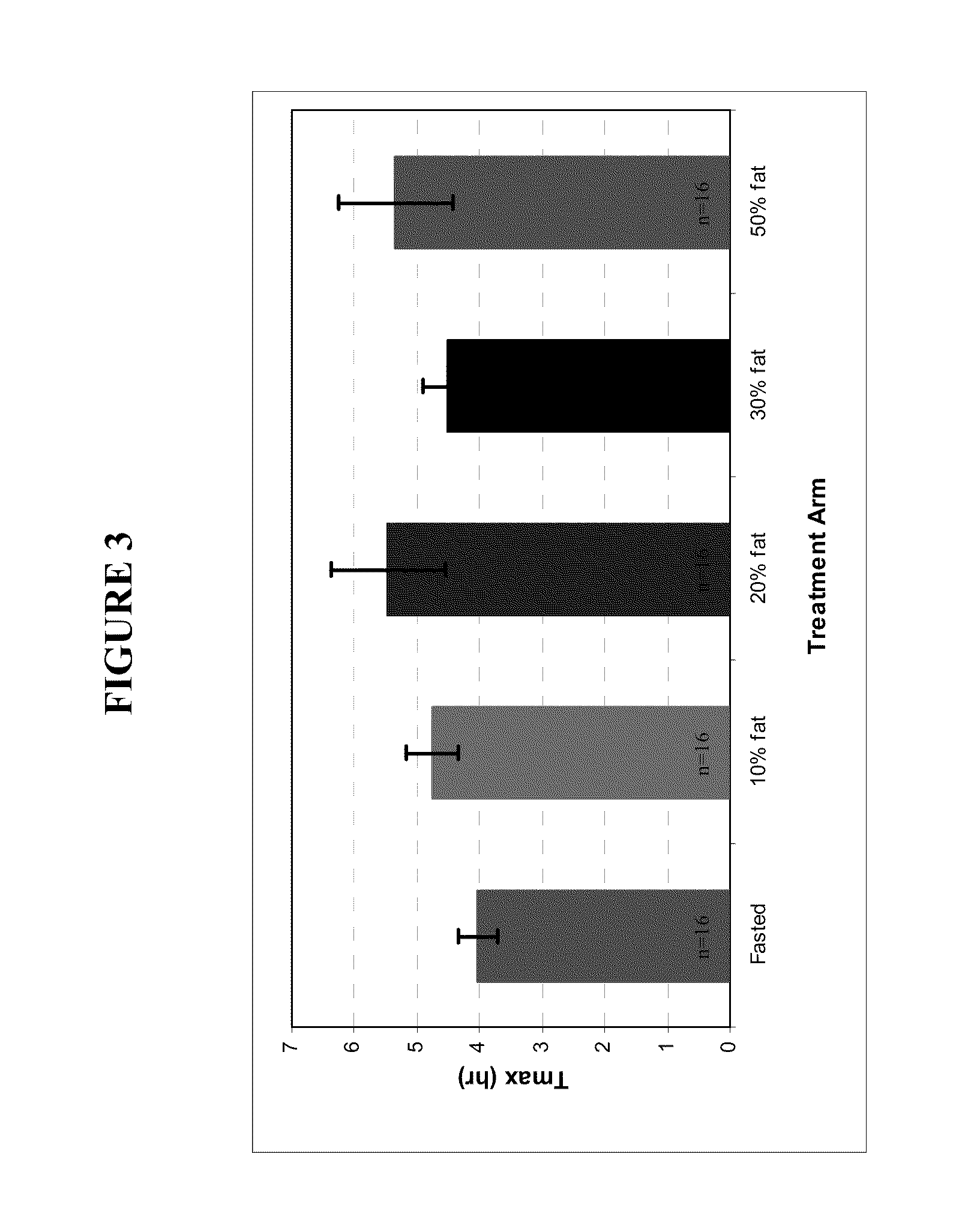
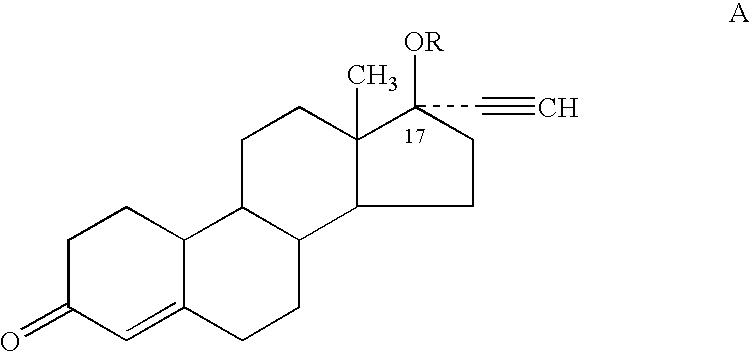
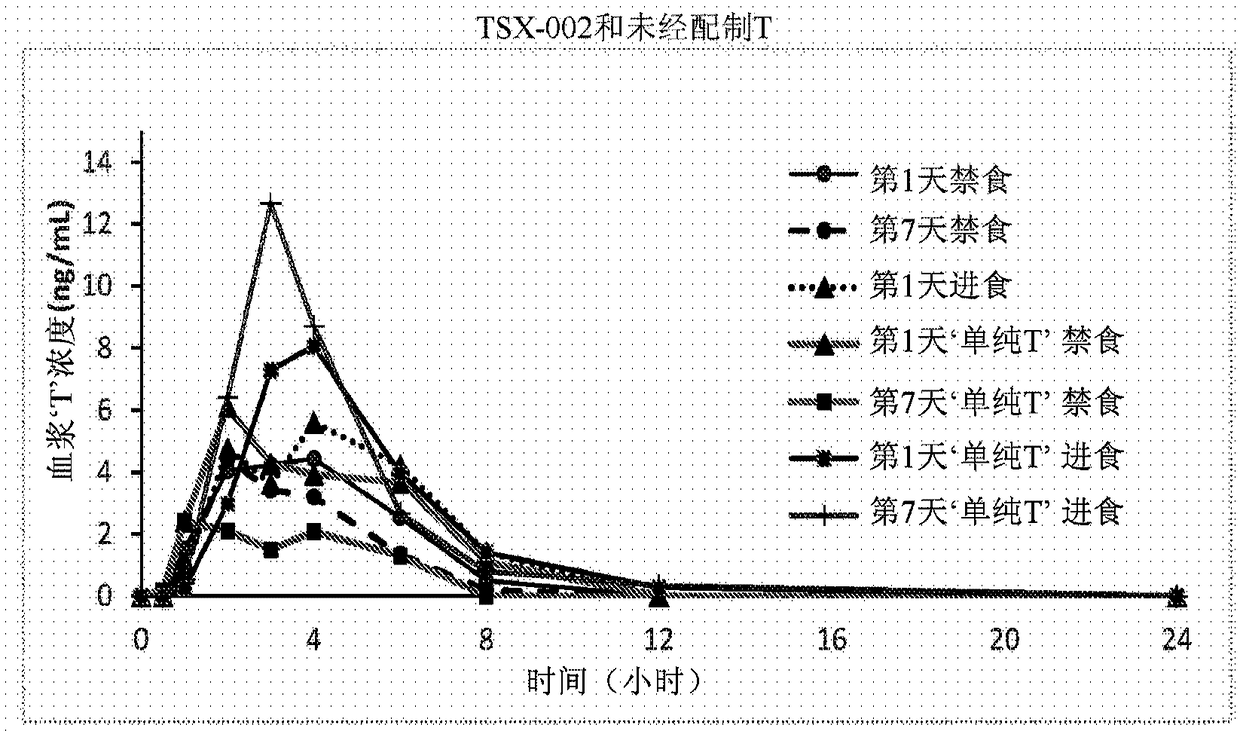
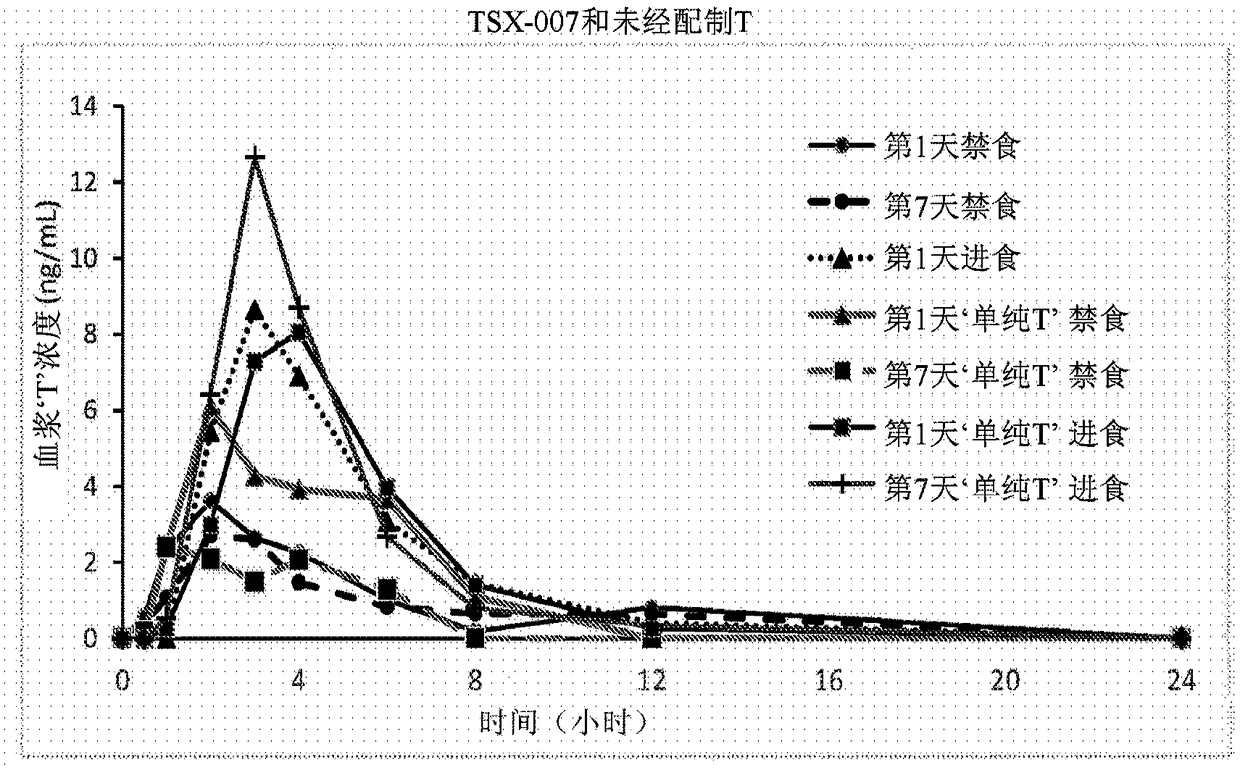
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Testosterone undecanoate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used in men who do not make enough of a natural substance called testosterone.
Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same
ActiveUS20110251167A1Organic active ingredientsDispersion deliveryPhysiologyPharmaceutical formulation
A pharmaceutical formulation of testosterone undecanoate is provided. Methods of treating a testosterone deficiency or its symptoms with the inventive formulations are also provided.
Owner:TOLMAR INC
Testosterone undecanoate compositions
InactiveUS20120148675A1Powder deliveryOrganic active ingredientsRadiation equivalent doseAqueous solution
The present disclosure is drawn to pharmaceutical compositions and oral dosage forms containing testosterone undecanoate, as well as related methods of treatment. In one embodiment, the oral dosage form can include a therapeutically effective amount of testosterone undecanoate and a pharmaceutically acceptable carrier. The dosage form can be formulated such that, when measured using a USP Type II apparatus in 1000 mL of 8 wt % Triton X-100 in water at 37° C. and 100 rpm, the oral dosage form releases at least 20% more testosterone undecanoate after the first 120 minutes than an equivalent dose testosterone undecanoate containing oral dosage form without the pharmaceutically acceptable carrier.
Owner:LIPOCINE
Method of preventing or treating benign gynaecological disorders
ActiveUS8071576B2Few side-effectsLow recurrence rateBiocideOrganic active ingredientsDiseaseGynecological disorders
The present invention relates to a method of preventing or treating benign estrogen sensitive gynecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:NIESCHLAG EBERHARD +5
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:BAYER SCHERING PHARMA AG
Methods and pharmaceutical compositions for reliable achievement of acceptable serum testosterone levels
The present invention relates to pharmaceutical compositions, formulated for injectable administration, which comprises a testosterone ester, in particularly testosterone undecanoate, in a vehicle comprising castor oil and a co-solvent. Upon injecting the compositions according to a particular administration scheme, reliable levels of testosterone in serum in the normal physiological range is achieved for a long period. This allows for the use of the compositions in hormone replacement therapy and male contraception without concomitant monitoring of testosterone levels in serum by a physician.
Owner:SCHERING AG +1
High-strength testosterone undecanoate compositions
ActiveUS9034858B2Improve performanceReduce loadOrganic active ingredientsCapsule deliveryOral medicationSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
Methods and pharmaceutical compositions for reliable achievement of acceptable serum testosterone levels
ActiveUS20050032762A1Maintain serum levelMaintain levelBiocideOrganic active ingredientsDosing regimenPhysiology
The present invention relates to pharmaceutical compositions, formulated for injectable administration, which comprises a testosterone ester, in particularly testosterone undecanoate, in a vehicle comprising castor oil and a co-solvent. Upon injecting the compositions according to a particular administration scheme, reliable levels of testosterone in serum in the normal physiological range is achieved for a long period. This allows for the use of the compositions in hormone replacement therapy and male contraception without concomitant monitoring of testosterone levels in serum by a physician.
Owner:SCHERING AG +1
High-strength testosterone undecanoate compositions
ActiveUS9358241B2Improve performanceReduce loadOrganic active ingredientsGranular deliveryOral medicationSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
Modulation of side effect profile of 5-alpha reductase inhibitor therapy
InactiveUS20110263552A1Modulate effectBiocideOrganic active ingredientsSide effectBenign prostatic hyperplasia (BPH)
Methods for modulating one or more side effects of 5-α-reductase inhibitor therapy are provided, where the method includes administering a 5-α-reductase inhibitor and testosterone undecanoate. Also provided are methods of maintaining or restoring a DHT serum level in an individual undergoing 5-α-reductase inhibitor therapy, where the method includes administering testosterone undecanoate in addition to 5-α-reductase inhibitor. Further provided are methods of treating or preventing a DHT-related condition, such as benign prostate hyperplasia (BPH) or prostate cancer by administering a 5-α-reductase inhibitor and testosterone undecanoate.
Owner:MARIUS PHARMA LLC
High-strength testosterone undecanoate compositions
InactiveUS20140309202A1Improve sexual symptomImproves and enhances physicalOrganic active ingredientsPowder deliverySerum igeSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
Nanonized testosteron formulations for improved bioavailability
InactiveUS20120135069A1Small sizeImproving BAPowder deliveryOrganic active ingredientsOral medicationBioavailability
Nanonized formulations of testosterone esters, especially testosterone undecanoate, and of testosterone are prepared which show an enhanced oral bioavailability compared to the existing oral products on the market. The drug is dissolved in a melted lipid phase, which is subsequently nanonized. The drug is associated with the lipid. The drug can also be nanonized without having lipid present yielding nanocrystals. The nanonized drug can be incorporated into tablets or capsules for oral administration, typically one unit is sufficient for delivery of a single dose.
Owner:PHARMASOL
Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same
ActiveUS20120309731A1Organic active ingredientsDispersion deliveryPhysiologyPharmaceutical formulation
A pharmaceutical formulation of testosterone undecanoate is provided. Methods of treating a testosterone deficiency or its symptoms with the inventive formulations are also provided.
Owner:TOLMAR INC
High-strength testosterone undecanoate compositions
ActiveUS20140303130A1Improve performanceIncrease loadPowder deliveryOrganic active ingredientsSerum igeOral medication
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
High-strength testosterone undecanoate compositions
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods of treatment. In one embodiment, the present invention provides for a pharmaceutical composition that includes a therapeutically effective amount of testosterone undecanoate and a solubilizer. The testosterone undecanoate is solubilized in the composition and is present in an amount such that it comprises about 14 wt % to about 35 wt % of the total composition.
Owner:LIPOCINE
Oral pharmaceutical products and methods of use combining testosterone esters with hypolipidemic agents
ActiveUS20160317553A1No decline in steady state serum testosterone responsePowder deliveryOrganic active ingredientsPhysiologyTestosterone deficiency
Pharmaceutical products comprising a hypolipidemic agent and a testosterone ester such as testosterone undecanoate are provided. Methods of safely treating a testosterone deficiency or its symptoms with the inventive pharmaceutical products are also provided.
Owner:TOLMAR INC
Male contraceptive formulation comprising norethisterone
A formulation for male contraception comprising a progestin possessing both estrogenic and androgenic properties is remarkably effective for spermatogenesis suppression in males. The progestin Norethisterone (NET), particularly its derivatives Norethisterone acetate and Norethisterone enanthate in sufficient doses induce oligozoospermia or azoospermia in males. Formulations further comprising an androgen, such as a testosterone derivative such as a testosterone ester, particularly testosterone undecanoate, are especially effective male contraceptive formulations.
Owner:BAYER SCHERING PHARMA AG
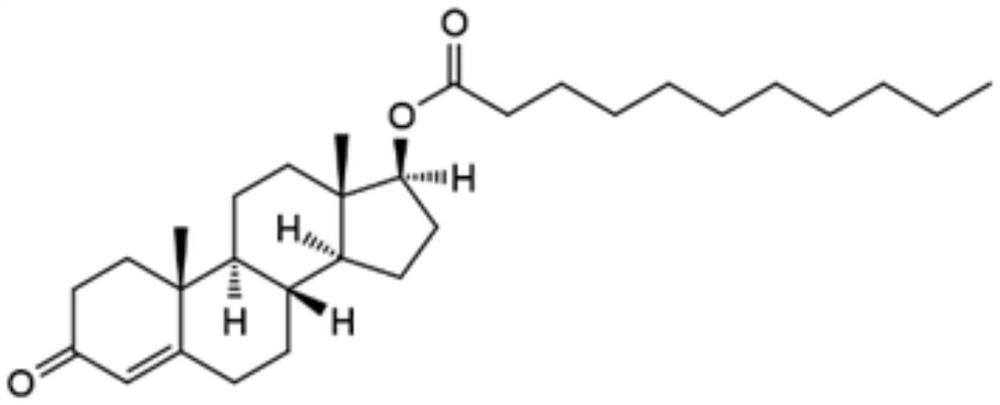
Hendecanoic acid testosterone self emulsified soft capsule composition and its preparation method
InactiveCN1686143AImprove stabilityImprove performanceOrganic active ingredientsCapsule deliveryCurative effectOil phase
A self-emulsifying composite softgel of testosterone undecanoate is proportionally prepared from testosterone undecanoate, oil phase and emulsifier through dissolving testosterone undecanoate in oil phase, adding oil phase, stirring, and loading in softcapsule. It can be automatically emulsified in stomach for high curative effect.
Owner:ZHEJIANG UNIV
Testosterone undecanoate compositions
ActiveUS20150018324A1Powder deliveryOrganic active ingredientsRadiation equivalent doseAqueous solution
The present disclosure is drawn to pharmaceutical compositions and oral dosage forms containing testosterone undecanoate, as well as related methods of treatment. In one embodiment, the oral dosage form can include a therapeutically effective amount of testosterone undecanoate and a pharmaceutically acceptable carrier. The dosage form can be formulated such that, when measured using a USP Type II apparatus in 1000 mL of 8 wt % Triton X-100 in water at 37° C. and 100 rpm, the oral dosage form releases at least 20% more testosterone undecanoate after the first 120 minutes than an equivalent dose testosterone undecanoate containing oral dosage form without the pharmaceutically acceptable carrier.
Owner:LIPOCINE
High-strength testosterone undecanoate compositions
ActiveUS20190365780A1Improve performanceReduce loadOrganic active ingredientsDisease diagnosisOral medicationSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
Oral pharmaceutical products and methods of use combining testosterone esters with hypolipidemic agents
Pharmaceutical products comprising a hypolipidemic agent and a testosterone ester such as testosterone undecanoate are provided. Methods of safely treating a testosterone deficiency or its symptoms with the inventive pharmaceutical products are also provided.
Owner:TOLMAR INC
Testosterone undecanoate slow-release medicine composition, preparation method thereof and application
ActiveCN109419771AImprove stabilityGood reconstitutionPowder deliveryOrganic active ingredientsIrritationMicrometer
The invention belongs to the technical field of medicines, and relates to testosterone undecanoate suspension, a preparation method thereof and an application, in particular to testosterone undecanoate suspension which comprises testosterone undecanoate, stabilizers and water. The content of the testosterone undecanoate is 10%-50%, the particle diameter D10 of the testosterone undecanoate is 0.2-0.5 micrometer, the particle diameter D50 of the testosterone undecanoate is 0.5-1.5 micrometers, the particle diameter D90 of the testosterone undecanoate is 2.2-6.0 micrometers, the stabilizers include first stabilizers and second stabilizers, the weight ratio of the first stabilizers to the second stabilizers is (1-10):(1-20), and the weight ratio of the testosterone undecanoate to the stabilizers is (1-20):(1-20). The testosterone undecanoate suspension is good in stability and high bioavailability and low in irritation, redissolved conditions after sedimentation are good, and in-vivo hormone level can be stably maintained for a long time.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method of preventing or treating benign gynaecological disorders
ActiveUS20050148559A1Improve actionIncreased proliferationBiocideOrganic active ingredientsDiseasePresent method
The present invention relates to a method of preventing or treating benign estrogen sensitive gynaecological disorders in a female mammal, wherein the method comprises the administration to said female mammal of a combination of progestogen and androgen in an amount that is therapeutically effective to prevent or reduce the symptoms of these disorders. The present method is particularly suitable for preventing or treating disorders selected from the group consisting of endrometriosis, adenomyosis, uterine fibroids, dysmenorrhoea, menorrhagia and metrorrhagia. Another aspect of the invention relates to a pharmaceutical kit comprising a plurality of oral dosage units which comprise a progestogen in an amount equivalent to 3-500 μg levonorgestrel and either 5 to 250 mg dehydroepiandrosterone or 1 to 50 mg testosterone undecanoate.
Owner:PANTARHEI BIOSCI
Stable pharmaceutical composition comprising testosterone undecanoate
PendingCN112638365AImprove ease of useImprove stabilityOrganic active ingredientsInorganic non-active ingredientsTestosterone deficiencyInternal medicine
The present invention relates to an injectable composition of testosterone ester for the treatment of testosterone deficiency. Particularly, the present invention relates to a pharmaceutical composition comprising testosterone undecanoate, which is capable of increasing the convenience for use in injection with the low viscosity and injection force, and has improved stability.
Owner:CHONG KUN DONG CORP
High-strength testosterone undecanoate compositions
InactiveUS20180153904A1Improve performanceReduce loadOrganic active ingredientsDisease diagnosisOral medicationSerum concentration
The present disclosure is drawn to pharmaceutical compositions and oral dosage capsules containing testosterone undecanoate, as well as related methods. The capsule includes a capsule shell and a capsule fill. The capsule fill can include a solubilizer and about 14 wt % to about 35 wt % testosterone undecanoate based on the total capsule fill. The oral dosage capsule is such that when a single oral administration to a male subject of one or more capsules with a total testosterone undecanoate daily dose of about 350 mg to about 650 mg it provides a ratio of serum testosterone Cmax to serum testosterone Cave of about 2.7 or less. In yet another embodiment, a method for providing a serum concentration of testosterone within a target serum testosterone concentration Cave range for a male subject is provided.
Owner:LIPOCINE
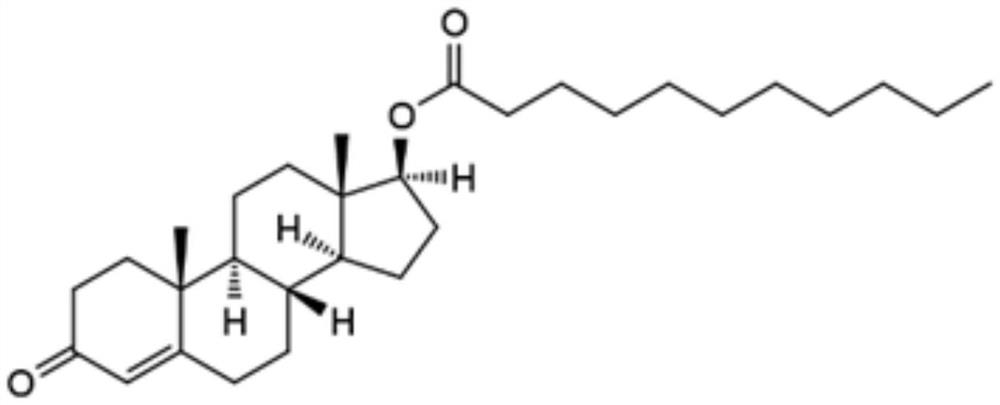
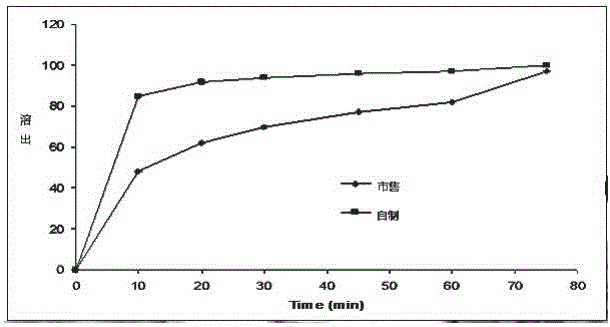
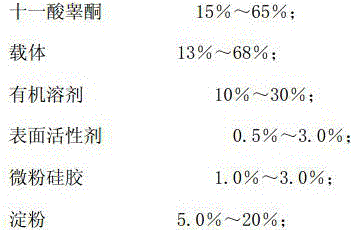
Testosterone undecanoate capsule and preparation method thereof
InactiveCN106727409AIncreased release rateImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsSide effectIn vivo
The invention discloses a testosterone undecanoate capsule. The testosterone undecanoate capsule comprises the following components in percentage by mass: 15-65% of testosterone undecanoate, 13-68% of carrier, 10-30% of organic solvent, 0.5-3.0% of surfactant, 1.0-3.0% of micropowder silica gel and 5.0-20% of starch. The invention also discloses a preparation method of the testosterone undecanoate capsule. According to the invention, the testosterone undecanoate active compound is innovatively dispersed in a molecular form in the carrier material based on a solid dispersion technology, so that no troubles in the aspects of crystal form and granularity exist, and the in vivo release rate of the drug is greatly increased, thereby improving the bioavailability of the product. Thus, as a male hormone supplement preparation having the minimal side effect, the testosterone undecanoate has huge market prospects in the present era with the problem of population ageing.
Owner:ZHEJIANG XIANJU PHARMA
Stable formulations of testosterone undecanoate
ActiveUS9925200B2Increase exposureAltered bioavailabilityOrganic active ingredientsCapsule deliveryPharmaceutical formulationTestosterone undecanoate
The present invention is directed to stable pharmaceutical formulations. More specifically, the present invention is directed to stable pharmaceutical formulations of testosterone undecanoate.
Owner:MERCK SHARP & DOHME BV
Preparation method of high-purity testosterone undecanoate
The invention discloses a preparation method of high-purity testosterone undecanoate, belonging to the technical field of steroid medicine preparation. According to the method, testosterone is used as a raw material, and esterification and purification are carried out, so the high-purity testosterone undecanoate with a high purity of larger than or equal to 99.5% is obtained; and a technical route is high in preparation efficiency, and the obtained product is good in quality and high in yield. The preparation method disclosed by the invention solves the problems that related raw and auxiliary materials are seriously polluted, production wastewater is difficult to treat, large-scale batch production is not facilitated, and the obtained testosterone undecanoate is low in purity and relatively more in impurities in the prior art.
Owner:湖北竹溪人福药业有限责任公司
Proliposomal testosterone undecanoate formulations
InactiveCN108601736AFacilitated releaseOrganic active ingredientsDigestive systemPhospholipidPhosphatidyl Cholines
Novel testosterone undecanoate (TU) formulations are disclosed in which TU is incorporated into proliposomal powder dispersions of TU and distearoyl phosphatidylcholine (DSPC). The proliposomal powderdispersions of the invention can also be combined with pharmaceutically acceptable excipients, and incorporated into enterically coated oral dosage forms that are useful for testosterone replacementtherapy.
Owner:WESTERN UNIV OF HEALTH SCI +1
Synthetic method of alkyl acid testosterone compound
A method for synthesizing the testosterone alkylate compound, comprising performing esterification reaction on the testosterone compound in the presence of a solvent and a base to obtain the testosterone alkylate compound. In the synthesis method of the alkylate testosterone compound provided by the present invention, the solvent is a water-soluble organic solvent, so after the reaction is completed, the product can be directly precipitated by adding water, which simplifies the overall process steps.
Owner:EVERLIGHT CHEMICAL INDUSTRIAL CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com